Abstract
Background
Both atrial fibrillation and venous thromboembolism (VTE) are highly prevalent among patients with chronic kidney disease (CKD). Until recently, warfarin was the most commonly prescribed oral anticoagulant. Direct oral anticoagulants (DOACs) have important advantages and have been shown to be noninferior to warfarin with respect to stroke prevention or recurrent VTE in the general population, with lower bleeding rates. This review article will provide available evidence on the use of DOACs in patients with CKD.
Summary
In post hoc analyses of major randomized studies with DOACs for stroke prevention in atrial fibrillation, in the subgroup of participants with moderate CKD, defined as a creatinine clearance (CrCl) of 30–50 mL/min, dabigatran 150 mg and apixaban were associated with lower rates of stroke and systemic embolism, whereas apixaban and edoxaban were associated with lower bleeding and mortality rates, compared with warfarin. In retrospective observational studies in patients with advanced CKD (defined as a CrCl <30 mL/min) and atrial fibrillation, DOACs had similar efficacy with warfarin with numerically lower bleeding rates. All agents warrant dose adjustment in moderate-to-severe CKD. In patients on maintenance dialysis, the VALKYRIE trial, which was designed initially to study the effect of vitamin K on vascular calcification progression, established superiority for rivaroxaban compared with a vitamin K antagonist (VKA) in the extension phase. Two other clinical trials using apixaban (AXADIA and RENAL-AF) in this population were inconclusive due to recruitment challenges and low event rates. In post hoc analyses of randomized studies with DOACs in patients with VTE, in the subgroup of participants with moderate CKD at baseline, edoxaban was associated with lower rates of recurrent VTE, whereas rivaroxaban and dabigatran were associated with lower and higher bleeding rates, respectively, as compared to warfarin.
Key Messages
DOACs have revolutionized the management of atrial fibrillation and VTE, and they should be preferred over warfarin in patients with moderate-to-severe CKD with appropriate dose adjustment. Therapeutic drug monitoring with a valid technique may be considered to guide clinical management in individualized cases. Current evidence questions the need for oral anticoagulation in patients on maintenance dialysis with atrial fibrillation as both DOACs and VKAs are associated with high rates of major bleeding.
Keywords: Direct oral anticoagulants, Warfarin, Pharmacokinetics, Atrial fibrillation, Venous thromboembolism, Chronic kidney disease
Introduction
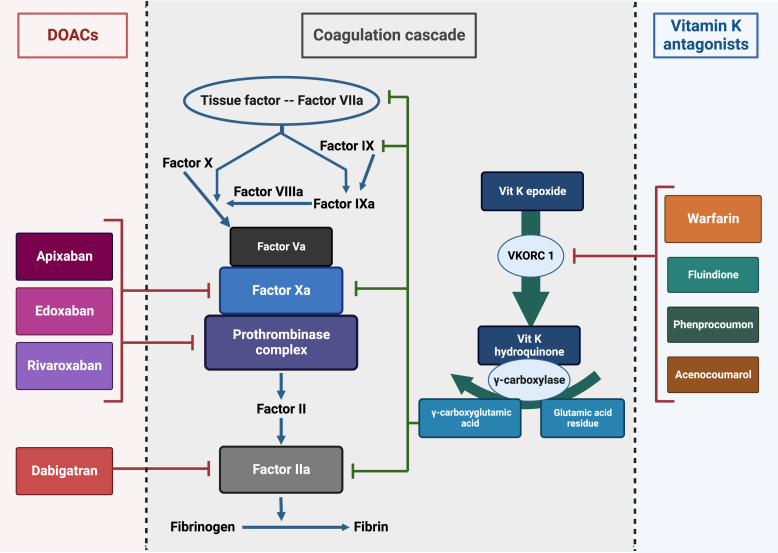
Oral anticoagulants can be classified into conventional vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs) (Table 1). The prototype drug among VKAs is warfarin, which until recently was the most commonly prescribed oral anticoagulant worldwide [1]. Acenocoumarol, fluindione, and phenprocoumon are the other less commonly used VKAs. Based on their mechanism of action, DOACs are classified into direct thrombin inhibitors (dabigatran) and direct factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban) (Fig. 1) [2].
Table 1.
Clinical pharmacology of oral anticoagulants
| Oral anticoagulant | Warfarin | Dabigatran etexilate | Apixaban | Rivaroxaban | Edoxaban |
|---|---|---|---|---|---|
| Mechanism of action | Vitamin K antagonist | Direct thrombin inhibitor | Free and clot-bound Xa inhibitor | Free and clot-bound Xa inhibitor | Free Xa inhibitor |
| Effector binding | Irreversible | Reversible | Reversible | Reversible | Reversible |
| Prodrug | No | Yes | No | No | No |
| Oral bioavailability, % | 99 | 6–7 | 34–88 | 80–100 (with food) | 50–62 |
| 66 (fasting) | |||||
| Kidney elimination, % | Nil | 80–85 | 27 | 35 | 50 |
| Extra-kidney metabolism | CYP2C9 | Hepatic glucuronidation | Oxidative metabolism, CYP3A4 | CYP3A4 | Carboxylesterase |
| Tmax, h | 2–6 | 1.5 | 1.5–3.5 | 2–4 | 1–5 |
| T½, h | 36–42 | 12–17 | 8–15 | 7–11 | 6–11 |
| Expected trough level, ng/mL* [3] | – | 60–91 | 63–103 | 26–44 | 19–36 |
| Expected peak level, ng/mL* [3] | – | 117–275 | 171–132 | 250–270 | 170–234 |
| Plasma protein binding, % | 97–99 | 26–28 | 87 | >90 | 55 |
| Drug clearance by dialysis, % | Negligible | 62–68 | Negligible | Negligible | Negligible |
| Drug interactions | |||||
| CYP3A4 modifiers | |||||
| − | − | + | + | − | |
| P-gp modifiers | − | + | + | + | + |
| Monitoring | PT/INR | Calibrated anti-IIa assay | Anti-Xa assay with specific calibrators | Anti-Xa assay with specific calibrators | Anti-Xa assay with specific calibrators |
| Antidote in case of major bleeding | Vitamin K | Idarucizumab | Andexanet alpha | Andexanet alpha | Andexanet alpha |
| FFP, PCC | Hemodialysis | PCC | PCC | PCC | |
APTT, activated partial thromboplastin time; BID, twice daily; CYP2C9, cytochrome P450 type 2C9; CYP3A4, cytochrome P450 type 3A4; FFP, fresh frozen plasma; INR, international normalized ratio; NVAF, nonvalvular atrial fibrillation; PCC, prothrombin complex concentrate; P-gp, permeability glycoprotein; QD, once daily; Tmax, time to peak drug concentration; PT, prothrombin time; T½, elimination half-life. *The expected trough and peak levels are provided for dabigatran 150 mg bid, apixaban 5 mg bid, rivaroxaban 20 mg qd, and edoxaban 60 mg qd.
Fig. 1.
Mechanism of action of oral anticoagulants. Modified from Mavrakanas and Bounameaux [4]. DOACs, direct oral anticoagulants; Vit K, vitamin K; VKORC 1, vitamin K epoxide reductase complex subunit 1.
As compared to VKAs, DOACs have certain pharmacokinetic (PK) and pharmacodynamic advantages. They have a rapid onset of action, more predictable anticoagulation profile, wider therapeutic window, no need for monitoring, and fewer food-drug and drug-drug interactions [4]. Several meta-analyses of large randomized controlled trials (RCTs) in patients with atrial fibrillation have shown that DOACs are superior (dabigatran 150 mg and apixaban) or noninferior (dabigatran 110 mg, rivaroxaban, and edoxaban) to VKAs in terms of stroke prevention, with similar (dabigatran 150 mg) or lower bleeding rates (dabigatran 110 mg, apixaban, rivaroxaban, and edoxaban) [5]. Current guidelines thus advocate DOACs as the first-choice oral anticoagulant except in certain situations, such as mechanical heart valves, patients with severe rheumatic mitral stenosis, antiphospholipid syndrome, or patients requiring a higher net anticoagulation (international normalized ratio [INR] >3) [6].
However, translation of these recommendations to patients with chronic kidney disease (CKD) is not straightforward. These patients form a unique subset at high risk of developing atrial fibrillation/venous thromboembolism (VTE) and have an increased risk of bleeding complications from the use of oral anticoagulants. Randomized trials comparing DOACs and warfarin have excluded patients with creatinine clearance (CrCl) below 30 mL/min. Lack of high-quality evidence in CKD has led to differences in recommendations by various professional bodies adding on to this confusion [7]. This has thus led to underutilization of DOACs in CKD patients [8]. In this article, we focus on the pharmacokinetics and current clinical evidence on the use of DOACs in patients with CKD.
Pharmacology of Oral Anticoagulants
Vitamin K Antagonists
VKAs act by inhibiting vitamin K epoxide reductase complex subunit 1, which converts oxidized vitamin K (vitamin K epoxide) to reduced vitamin K (vitamin K hydroquinone) (Fig. 1). Vitamin K hydroquinone is a cofactor for gamma carboxylase, which is essential for the post-translational modification of glutamic acid residues in molecules involved in coagulation (factors VII, IX, X, and II), anticoagulation (protein C, S, and Z), vascular calcification inhibition [9–12].
Direct Thrombin Inhibitors
Factor IIa or thrombin is involved in the terminal step of the coagulation cascade, which converts fibrinogen to fibrin (Fig. 1). Physiological substrates like fibrinogen, factor V, protein C, thrombomodulin, and protease-activated receptors have to bind to an anion-binding exosite to gain access to the active site [13]. Direct thrombin inhibitors either bind only to the active site (univalent) or to both the active site and the anion-binding exosite (bivalent) with similar clinical effects. Thrombin inhibition is thus either reversible or irreversible [14]. Dabigatran etexilate is a univalent, reversible, and potent direct thrombin inhibitor. It is a prodrug and is metabolized by a nonspecific esterase into the active metabolite dabigatran via two intermediate metabolites, BIBR 1087 (inactive) and BIBR 951 (active). Dabigatran is predominantly excreted by the kidneys (80%). The remaining 20% undergoes hepatic glucuronidation to form active glucuronides. Plasma protein binding of dabigatran is low (26–28%), and hence it can be removed by hemodialysis [15].
Direct Factor Xa Inhibitors
Factor Xa acts immediately upstream of thrombin in the coagulation cascade at the point of intersection of the intrinsic and extrinsic pathways (Fig. 1). Direct Xa inhibitors bind to factor Xa and inhibit its activity without the need for cofactors. Unlike indirect factor Xa inhibitors (heparin and fondaparinux), which inactivate only the fluid phase of factor Xa, direct Xa inhibitors inhibit both circulating and clot-bound forms of factor Xa [16]. The three direct factor Xa inhibitors that are commercially available in North America are apixaban, rivaroxaban, and edoxaban.
Apixaban metabolism involves both kidney (27%) and extra-kidney pathways. Extra-kidney metabolism includes oxidative pathways (O-demethylation by cytochrome P450 enzymes, hydroxylation, and sulfation of hydroxylated O-demethyl apixaban by sulfotransferases), biliary excretion, and direct intestinal excretion [17]. For rivaroxaban, two-thirds of the administered dose of the drug undergoes hepatic metabolism into inactive metabolites by cytochrome P450 enzymes (CYP3A4, CYP2J2, and CYP-independent mechanism). Half of these metabolites are excreted unchanged in the urine and the remaining half via the fecal route. The remaining one-third (35%) of the administered drug is excreted by the kidneys [18]. Edoxaban metabolism involves both kidney (50%) and extra-kidney (50%) pathways. Extra-kidney pathways include metabolism by carboxylestesterase-1 into an active metabolite M4 and cytochrome P450 enzymes (CYP3A4) into M5 (inactive), M6 (active), and M8 (active) metabolites. M4 is the most abundant edoxaban metabolite and accounts for 9% of edoxaban exposure [19]. All factor Xa inhibitors have high plasma protein binding and are thus minimally removed by hemodialysis [20–22] (Table 1).
Drug Interactions with DOACs
All four DOACs undergo direct intestinal excretion mediated by the permeability glycoprotein (P-gp) transporter. Coadministration of DOACs with potent P-gp modifiers will cause either increased (inhibitors) or inadequate exposure (inducers) [23]. P-gp inhibitors like ketoconazole, erythromycin, quinidine, verapamil, and amiodarone increased the area under plasma concentration-time curve (AUC) and maximum plasma concentration (Cmax) of edoxaban by 33–77% and 65–104%, respectively [24]. The PK analysis of the RE-LY trial showed that coadministration of verapamil, amiodarone, and diltiazem increased steady state exposure of dabigatran by 23, 12, and 8.4%, respectively [25]. Finally, apixaban and rivaroxaban undergo significant metabolism by cytochrome P450 (CYP3A4) and are thus additionally affected by CYP3A4 inhibitors and inducers [23] (Table 1).
A detailed review on drug interactions with DOACs is beyond the scope of this review. Use of DOACs is associated with fewer clinically relevant drug interactions than warfarin [26]. The most important drug interactions are listed below. Dronedarone, an antiarrhythmic agent, is a potent inhibitor of P-gp and CYP3A4. Coadministration with dabigatran is associated with a 2-fold increase in dabigatran AUC and a modest increase in the risk of gastrointestinal bleeding [27]. Dabigatran AUC increases by 143% when administered within 2 h with an immediate release formulation of verapamil [28]. Among antiplatelet agents, ticagrelor is the only one with P-gp inhibitory action and increases the exposure of dabigatran by 50% [26]. Rifampicin is one of the most potent inducers of P-gp and CYP3A4, and coadministration can lead to therapeutic failure with all DOACs with the exception of edoxaban that shows a compensatory increase of its active metabolites M4 and M6 [29]. Antiepileptic agents such as phenytoin, phenobarbital, and carbamazepine are also potent inducers of P-gp and CYP3A4, and their use is contraindicated in association with DOACs [30]. Valproic acid (mild P-gp inhibitor) and levetiracetam (no effect on P-gp) can be safely administered [31]. HIV protease inhibitors boosted with ritonavir or cobicistat are potent inhibitors of P-gp and CYP3A4 and their use is contraindicated with DOACs [32].
Pharmacodynamic Monitoring
An important advantage of DOACs over warfarin is that there is no need for regular pharmacodynamic monitoring. However, in certain clinical situations, the use of an anti-IIa assay for dabigatran or an anti-Xa assay calibrated for factor Xa-inhibitors may be required [33]. These clinical situations include the occurrence of major bleeding, need for emergent surgery, concerns about drug absorption or adherence, concomitant use of P-gp and CYP3A4 inhibitors or inducers, morbid obesity, and patients with advanced CKD [33–35]. A detailed description of anticoagulant reversal is beyond the scope of this manuscript [36–38].
PK of Oral Anticoagulants in Kidney Impairment
Most of the PK studies, observational studies, and randomized trials have used CrCl from the Cockcroft-Gault equation to estimate renal function. In a cross-sectional analysis of inpatients with documented renal function by the Cockroft-Gault, Modification of Diet in Renal Disease, and Chronic Kidney Disease Epidemiology (CKD-EPI) equations over 6 months, drug dosing discordance was evident in more than 20% of patients [39]. As per the European Medicines Agency, elimination capacity for drugs excreted by the kidney is not body size-adjusted and should be expressed as absolute glomerular filtration rate (GFR) in mL/min. As per US Food and Drug Administration, GFR estimation for drug dosing is to be calculated by multiplying the standardized GFR by the individual’s body surface area and then dividing by 1.73 and expressed in mL/min [40]. Although Kidney Disease: Improving Global Outcomes is in favor of estimated GFR calculation using the CKD‐EPI equation, there are no specific recommendations in the regulatory guidelines. Using the CKD‐EPI equation for measuring GFR in phase I studies may help in harmonizing population PK analyses and dosing by estimated renal function [41].
Warfarin
Warfarin is not eliminated by the kidneys. However, patients with CKD stage 3 and 4 are reported to require 10 and 20% dose reduction, respectively, to maintain INR in therapeutic range [42]. Patients with CKD also have greater fluctuations in INR and spend lesser time in therapeutic range (TTR) that can be as low as 44–50% in patients with end-stage kidney disease (ESKD) [43–45]. Risk of bleeding increases with the severity of CKD in patients treated with VKAs [46]. Patients with CKD stage 3 and 4 treated with warfarin have 2.2- and 5.8-fold higher risk of bleeding at supratherapeutic INRs, as compared to healthy controls [47].
Several PK studies have been conducted with DOACs in patients with CKD. However, in most of them, a single oral dose was given to study participants. This design precluded assessment of drug PK at steady state, and their results have to be interpreted with caution. A detailed description of these studies is outside the scope of this manuscript. We will focus on available PK data with DOACs at steady state.
Direct Oral Anticoagulants
Dabigatran
In the RE-LY trial, steady state PK measurements were collected at 4 weeks in 9,552 patients who received either 110 or 150 mg dabigatran twice daily. Factors affecting clearance of dabigatran included older age, female sex, heart failure, South Asian ancestry, and reduced CrCl. All factors with the exception of CrCl had only moderate effects on dabigatran exposure (<26%), warranting no dose modification. The effect of CrCl on the plasma area under the curve (AUC) was nonlinear; a decrease in CrCl from 100 to 80 mL/min resulted in 11% increase in AUC as compared to 51% increase with decrease in CrCl from 50 to 30 mL/min. The PK model predicted a similar exposure with 75 mg twice-daily dose in patients with CrCl <30 mL/min and 150 mg twice daily dose in patients with normal kidney function [25]. This was again confirmed in a steady-state PK study in 15 patients with stage 4 CKD, receiving 75 mg of dabigatran twice daily for 7.5 days. Mean AUCs were comparable to the predicted ones in the PK models [48].
Dabigatran exposure estimated from pharmacometric simulation modeling in dialysis patients was compared to that of a typical patient in the RE-LY trial. Twice daily regimens (75, 110, and 150 mg) resulted in a 1.5–3.3-fold increase in AUCs. A once daily 110 mg predialysis regimen resulted in 4.4% greater exposure, while a 75 mg once daily postdialysis regimen resulted in 13.3% lower exposure, compared with a typical RE-LY patient. The authors suggested using a 75 mg postdialysis once daily dosage in patients with ESKD to reduce the incidence of access bleeding from fistula cannulation [49]. However, this dose regimen has never been studied in clinical practice.
Apixaban
Steady-state PK analysis including data from patients in the second month of the ARISTOTLE trial, which recruited participants with a CrCl as low as 25 mL/min, showed considerable overlap of drug exposure with the 5 mg twice daily apixaban dose in patients with mild, moderate, and severe CKD [50]. However, in a PK analysis of 7 patients with ESKD on hemodialysis, Mavrakanas et al. showed that steady-state exposure on day seven with each apixaban dose was significantly higher compared with day one. Accumulation index was higher in ESKD (3.6) as compared to healthy controls (1.3–1.7). Steady-state exposure attained at the dose of 2.5 mg twice daily was comparable to the achieved with the 5 mg twice daily dose in healthy volunteers, whereas exposure in some patients with ESKD was supratherapeutic with the 5 mg twice daily dose. The authors recommended dose reduction of apixaban to 2.5 mg twice daily in patients with ESKD [22].
RENAL-AF was an RCT which compared apixaban to warfarin in patients with ESKD and nonvalvular atrial fibrillation at high risk for stroke [44]. Apixaban was given at the standard dose of 5 mg twice daily. The reduced dose (2.5 mg twice daily) was used in patients >80 years of age or with a body weight <60 kg. Exposure attained with the standard dose was higher than the exposure observed in patients from the ARISTOTLE trial, who had preserved kidney function (median steady state AUC in RENAL-AF was 2,475 ng/mL.h vs. 1,374 ng/mL.h in ARISTOTLE). Drug exposure with the reduced apixaban dose was comparable to patients with normal kidney function from the ARISTOTLE trial (median steady state AUC in RENAL-AF was 1,269 ng/ml.h vs. 1,374 ng/mL.h in ARISTOTLE) [44]. Hemodialysis had minimal effects on apixaban clearance.
In a PK study with apixaban in patients on peritoneal dialysis, Fung et al. observed that a reduced dose apixaban regimen resulted in drug exposure twice as high than that achieved with a similar dose in patients on hemodialysis. No correlation was observed between drug exposure and transporter status, dialysis adequacy (Kt/V), or residual kidney function. The degree of dialyzate protein loss positively correlated with plasma AUCs. The majority of patients in the cohort were hypoalbuminemic with a mean serum albumin of 2.4 ± 0.5 g/dL and a very low body weight. The authors postulated that the loss of middle and low molecular weight proteins involved in oxidative metabolism of apixaban probably led to slower degradation and accumulation of apixaban in patients on PD [51]. However, these results should be interpreted with caution and have to be validated in a different cohort of patients before any recommendation with respect to apixaban dosing in PD can be made.
Rivaroxaban
In an observational study from China, 92 patients with CKD (stage 1–3) and atrial fibrillation received either 15 or 20 mg of rivaroxaban once daily for at least 1 week. Patients who received 15 mg of rivaroxaban were older, had lower eGFR, and higher CHA2DS2-VASc scores. Plasma trough levels were measured after a median of 13 months post-drug initiation. Rivaroxaban trough levels negatively correlated with eGFR and positively correlated with pharmacodynamic assays, such as prothrombin time and activated partial thromboplastin time. On multivariate analysis, trough levels were associated with bleeding events (odds ratio [OR] 1.02, 95% confidence interval [CI]: 1.00–1.04), but not with stroke/systemic embolism (SE) (OR 0.97, 95% CI: 0.93–1.02) [52].
De Vriese et al. performed a PK study using the reduced dose of 10 mg of rivaroxaban in 18 patients on hemodialysis. Steady-state AUC attained with this dose was comparable to drug exposure in patients from the ROCKET-AF trial who had moderate CKD (and received 15 mg of rivaroxaban daily) or preserved kidney function (and received 20 mg of rivaroxaban daily). Rivaroxaban was minimally cleared by hemodialysis [20].
Edoxaban
In a study from Japan, 93 participants with atrial fibrillation received either 15 mg of edoxaban (if they had a CrCl of 15–29 mL/min) or were randomized to receive 30 or 60 mg of edoxaban (if they had a CrCl >50 mL/min). A 50% dose reduction was applied in the group of participants with a CrCl >50 mL/min if they also had a body weight <60 kg or were treated with quinidine or verapamil [53]. Exposure at steady state with the 15 mg once daily dose in patients with stage IV CKD was comparable to drug exposure with the 30 and 60 mg once daily dose in patients with CrCl >50 mL/min. This study confirmed prior results from population PK modeling, suggesting 50% dose reduction for edoxaban in patients with advanced CKD [24].
Oral Anticoagulation for Stroke Prevention in Patients with Atrial Fibrillation and CKD
Burden of Atrial Fibrillation in Patients with CKD
Prevalence of atrial fibrillation is higher in patients with CKD as compared to the general population. In addition, patients with CKD and atrial fibrillation have five-fold increased risk of stroke and three-fold increased risk of congestive heart failure [54]. The incidence rate of atrial fibrillation in a large nation-wide database from Taiwan was 5.0, 7.3, and 12.1 events per 1000-patient years in the general population, the CKD, and the ESKD cohort, respectively. The risk of stroke or SE increased by 7% for every 10 mL/min decline in eGFR [55]. Despite the higher thrombotic risk, there are important limitations with anticoagulation in patients with CKD, namely, the higher rates of major bleeding [56]. In a retrospective analysis of 17,349 patients with nonvalvular atrial fibrillation, warfarin treatment was associated with increased hazards of bleeding in CKD stage 3 (hazard ratio [HR] 1.18, 95% CI: 1.07–1.31), stage 4 (HR 1.11, 95% CI: 0.87–1.42), and stage 5 (HR 2.01, 95% CI: 1.14–3.54), compared with no treatment [57]. Several studies have assessed the efficacy and safety of DOACs compared with warfarin in patients with CKD. These studies are summarized in Table 2 and will be briefly presented below.
Table 2.
DOACs versus VKAs in patients with nonvalvular atrial fibrillation and CKD
| Author | Type of trial | N | CrCl | Treatment arms | Age, years | Males, % | AntiPLT use, % | TTR for VKA | Median FU, years | Stroke/SE HR (95% CI) | Mortality HR (95% CI) | Major bleeding HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate CKD | ||||||||||||
| RE-LY investigators [58] | Post hoc RCT | 3,374 | 30–50 | Dabigatran 150 mg bid | 75±7 | 53 | 42 | – | 2.0 | 0.55 (0.34–0.89) | 1.03 (0.82–1.30) | 1.22 (0.95–1.58) |
| Dabigatran 110 mg bid | 0.78 (0.51–1.21) | 0.97 (0.77–1.24) | 1.02 (0.78–1.33) | |||||||||
| ARISTOTLE investigators [59] | Post hoc RCT | 3,017 | 25–50 | Apixaban 5/2.5 mg bid | 78±7 | 47 | 35 | – | 1.8 | 0.61 (0.39–0.94) | 0.78 (0.63–0.96) | 0.48 (0.37–0.64) |
| ROCKET AF investigators [60] | Post hoc RCT | 2,450 | 30–50 | Rivaroxaban 15 mg qd | – | – | – | – | 1.9 | 0.84 (0.57–1.23) | – | 0.98 (0.73–1.3) |
| J-ROCKET Investigators [61] | Post hoc RCT | 284 | 30–50 | Rivaroxaban10 mg qd | 78 (74–81) | 70 | 43 | – | 2.2 | 0.82 (0.25–2.69) | 1.04 (0.07–16.7) | 0.89 (0.36–2.18) |
| ENGAGE-AF TIMI 48 [62] | Post hoc RCT | 2,740 | 30–50 | Edoxaban 30 mg qd | 79 (75–83) | 46 | 29 | 67 | 2.8 | 0.87 (0.65–1.18) | 0.82 (0.69–0.97) | 0.76 (0.58–0.98) |
| Severe CKD | ||||||||||||
| Stanifer et al. [50] | Post hoc RCT | 269 | 25–30 | Apixaban 5/2.5 mg bid | 81 (76–85) | 40 | 39 | 55 | 1.8 (median) | 0.55 (0.20–1.51) | 1.02 (0.64–1.67) | 0.34 (0.14–0.80) |
| Ashley et al. [63] | Cohort | 1,514 | <30 | DOACs | – | – | – | – | – | 0.41 (0.20–0.82) | 0.75 (0.51–1.10) | 1 (0.29–3.45) |
| Welander et al. [64] | Cohort | 1,113 | <30 | DOACs | – | – | – | 67 | – | 1.01 (0.50–2.04) | 1.46 (1.11–1.91) | 0.61 (0.40–0.92) |
| Yao et al. [65] | Cohort | 917 | <30 | Apixaban | – | – | – | – | – | 0.83 (0.26–2.67) | 0.83 (0.45–1.54) | 1.03 (0.57–1.84) |
| Rivaroxaban | 0.25 (0.03–1.96) | 0.51 (0.16–1.68) | 0.81 (0.33–1.96) | |||||||||
| Dabigatran | – | 1.35 (0.37–4.94) | 0.62 (0.12–3.16) | |||||||||
| Weir et al. [66] | Cohort | 2,317 | <30 | Rivaroxaban | 80±8 | 40 | 51 | – | – | 0.93 (0.46–1.90) | 0.91 (0.65–1.28) | |
| Hsu et al. [67] | Cohort | 1,011 | <30 | Dabigatran | – | – | – | 40 | – | 0.36 (0.03–3.97) | – | 1.93 (0.31–11.9) |
| Rivaroxaban | 0.74 (0.13–4.33) | 1.79 (0.39–8.25) | ||||||||||
| Apixaban | – | 0.08 (0.01–1.55) | ||||||||||
| Edoxaban | 0.30 (0.04–2.47) | 0.34 (0.03–3.87) | ||||||||||
| Coleman et al. [68] | Cohort | 6,744 | <30 | Rivaroxaban | 72 (63–80) | 61 | 18 | – | 1.4 | 0.55 (0.27–1.10) | – | 0.68 (0.47–0.99) |
| 18 | ||||||||||||
| Hanni et al. [69] | Cohort | 861 | <25 | Apixaban 5/2.5 mg bid | 74±14 | 47 | 72 | – | 0.5 | 0% | 11.8% | 0.8% |
| Warfarin | 67±15 | 76 | 1.4% | 14.9% | 1.6% | |||||||
| Schafer et al. [70] | Cohort | 604 | <30 | Apixaban 5/2.5 mg bid | 74±12 | 50 | 61 | – | 0.7±0.3 | 0.7% | – | 1.5% |
| Warfarin | 71±14 | 0.8±0.3 | 0.3% | 8.4% | ||||||||
| Herndon et al. [71] | Cohort | 111 | <30 | Apixaban 5/2.5 mg bid | 71±8 | 100 | 53 | – | – | 2.0% | – | 7.0% |
| Warfarin | 72±8 | 0% | 14.0% | |||||||||
| Stanton et al. [72] | Matched cohort | 146 | <30 | Apixaban 5/2.5 mg bid | 79±12 | 40 | 64 | 68 | – | 7.5% | – | 9.6% |
| Warfarin | 79±14 | 49 | 7.5% | 17.8% | ||||||||
| End-stage kidney disease | ||||||||||||
| VALKYRIE investigators [43] | RCT | 132 | HD | Rivaroxaban 10 mg qd | 80 (74–84) | 67 | 33 | 48 | 1.8 (1–3.4) | 0.41 (0.25–0.68) | 65.2% | 0.39 (0.17–0.90) |
| Rivaroxaban 10 mg qd + vitamin K2 | 80 (73–83) | 41 | 0.34 (0.19–0.61) | 64.3% | 0.48 (0.22–1.08) | |||||||
| Warfarin | 80 (72–84) | 32 | 72.7% | |||||||||
| RENAL-AF investigators [44] | RCT | 154 | HD | Apixaban 5/2.5 mg bid | 69 (61–76) | 64 | 39 | 44 | 330 days | 3.0% | 26.0% | 1.20 (0.63–2.30) |
| Warfarin (reference) | 68 (61–73) | 47 | 340 days | 3.3% | 18.0% | |||||||
| AXADIA-AFNET 8 [45] | RCT | 97 | HD | Apixaban 2.5 mg bid Phenprocoumon | 75±8 | 70 | 34 | 51 | 429 days | 20.8% | 18.8 | 0.93 (0.53–1.65) |
| 506 days | 30.6% | 24.5% | ||||||||||
Warfarin is the reference arm for all studies, unless stated otherwise.
antiPLT, antiplatelet; BID, twice daily; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, stroke (doubled), vascular disease, age 65–74 years and sex category (female); C.I., confidence interval; CKD, chronic kidney disease; CrCl, creatinine clearance (in mL/min); DOAC, direct oral anticoagulant; FU, follow-up; HD, hemodialysis; HR, hazard ratio; ICH, intracranial hemorrhage; N, total number of patients; QD, once daily: RCT, randomized controlled trials; Retro, retrospective; SD, standard deviation; SE, systemic embolism, TTR, time in therapeutic range; VKA, vitamin K antagonist.
DOACs versus VKAs in Patients with Atrial Fibrillation and Moderate CKD (CrCl 30–50 mL/min)
Post hoc analysis of the four major RCTs comparing DOACs with warfarin (RE-LY, ARISTOTLE, ROCKET-AF, and ENGAGE AF-TIMI 48) consistently showed that the rates of stroke/SE, mortality, and major bleeding increased with declining kidney function [58–60, 62]. Dabigatran 150 mg and apixaban were associated with lower rates of stroke/SE in patients with moderate CKD [58, 59]. Rates of stroke/SE with dabigatran 110 mg, rivaroxaban, and edoxaban were comparable to warfarin in patients with moderate CKD [58, 60, 62] (Table 2). An exploratory analysis in patients with CrCl >95 mL/min showed a trend toward lower efficacy with rivaroxaban (HR 1.47, 95% CI: 0.81–2.68) and edoxaban (HR 1.36, 95% CI: 0.88–2.10) in preventing stroke/SE [60, 62]. Apixaban and edoxaban were also associated with lower mortality rates, as compared to warfarin (apixaban – HR 0.78, 95% CI: 0.63–0.96 and edoxaban – HR 0.83, 95% CI: 0.69–0.97) [59, 62].
Apixaban and edoxaban were associated with lower rates of major bleeding, whereas both doses of dabigatran and rivaroxaban were associated with similar rates of major bleeding, as compared to warfarin (Table 2). Both doses of dabigatran were actually associated with lower bleeding rates, as compared to warfarin at CrCl >80 mL/min. With decreasing CrCl, bleeding rates increased in both warfarin and dabigatran groups. The curves of dabigatran 150 mg and 110 mg crossed the warfarin curve at a CrCl of 50 and 40 mL/min, respectively, and surpassed warfarin rates at lower levels of CrCl [58]. In an individual patient level meta-analysis of 71,683 patients (COMBINE AF) from the four RCTs where kidney function was assessed as a continuous variable, Harrington et al. [73] classified patients into three groups: standard dose DOAC, low dose DOAC, and warfarin. Standard dose DOACs, as compared to warfarin, had lower hazards for stroke/SE, mortality, and intracranial hemorrhage (ICH) and similar rates of major bleeding. The hazards for stroke/SE, mortality, and ICH with standard dose DOACs compared with warfarin dropped by 4.8, 2.1, and 6.2%, respectively, for every 10 mL/min reduction in CrCl. Patients treated with low-dose DOACs as compared to warfarin had a lower hazard of bleeding, death, and ICH across CrCl values above 35, 56 mL/min, or all CrCl values, respectively.
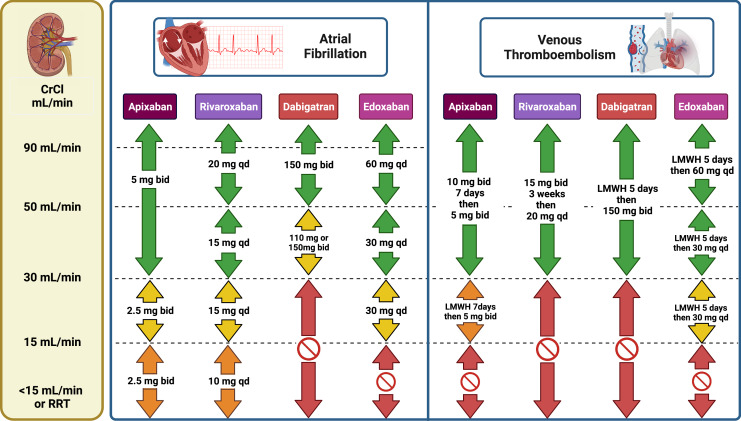
Based on these data, DOACs are preferable to warfarin for patients with moderate CKD. Dosing recommendations are shown in Figure 2.
Fig. 2.
Dosing recommendations based on available data and expert opinion for DOACs in patients with CKD. Color guide: green – recommended; yellow – suggested; orange – very limited data, use with caution after considering risks and benefits; red – no data or formal contraindication, do not use. bid, twice daily; CrCl, creatinine clearance; LMWH, low-molecular-weight heparin; qd, once daily; RRT, renal replacement therapy.
DOACs versus VKAs in Patients with Atrial Fibrillation and Severe CKD (CrCl <30 mL/min)
Apixaban is the most commonly studied DOAC in CKD stage 4 and 5. In a post hoc analysis from the ARISTOTLE trial, 269 patients with CrCl of 25–30 mL/min received either apixaban (2.5/5 mg) or warfarin. Apixaban was associated with lower rates of stroke/SE (HR 0.55, 95% CI: 0.20–1.51) and major bleeding (HR 0.34, 95% CI: 0.14–0.80), in concordance with primary study findings [50].
There are no data from other RCTs with DOACs in patients with advanced CKD. However, several retrospective cohort studies have been conducted in this population and are briefly presented in Table 2 [50, 63–72]. Most of available data are suggestive of a similar efficacy of direct factor Xa inhibitors compared with warfarin for stroke prevention in this population and of a numerically lower incidence of major bleeding. No major safety issues have been identified with direct factor Xa inhibitors in this population so far. However, observational studies are confounded by indication, and in spite of a large body of literature in this area, significant uncertainty remains and calls for the need of randomized trials in patients with severe CKD.
An important consideration is appropriate drug dosing in these patients. Clinical outcomes stratified by DOAC dosage have recently been reported for apixaban [74]. In an observational study including 4,313 new users of apixaban with atrial fibrillation and nondialysis dependent CKD stage 4 or 5, the two apixaban dose regimens were compared using inverse probability weighting. There was no difference between apixaban 2.5 and 5 mg twice daily with respect to the incidence of stroke or SE. In contrast, the incidence of bleeding-related hospitalizations was higher among patients treated with the 5 mg dose with an absolute risk difference of 3.1% at 2 years. The results were similar for the outcome of major bleeding [74]. Recommendations from professional societies are conflicting in this patient subgroup [75–79]. In appropriately selected cases, therapeutic drug monitoring with a valid technique may be considered to guide clinical management (Table 1). Dosing recommendations based on the available data and expert opinion are summarized in Figure 2.
Oral Anticoagulation in Patients with Atrial Fibrillation and ESKD
Atrial fibrillation has a high prevalence in patients with ESKD and is associated with significant morbidity and mortality [80]. VKAs have been considered for a long time the agent of choice for the prevention of thromboembolic events in this population but no RCTs have ever been performed. Recently, several large observational studies have compared warfarin to no anticoagulation in patients with atrial fibrillation on maintenance hemodialysis. They have recently been summarized in a systematic review and meta-analysis by Randhawa et al. [81]. In these studies, the incidence of ischemic stroke was similar with warfarin compared with no treatment: HR 0.96 (95% CI: 0.82–1.13). However, the incidence of hemorrhagic stroke and of major bleeding was higher in warfarin-treated participants: HR of 1.46 (95% CI: 1.05–2.04) and HR of 1.20 (95% CI: 0.99–1.47), respectively. In addition to bleeding complications, use of warfarin has been shown to increase the risk of calciphylaxis by a factor of 3–13 in this population, a complication associated with significant morbidity and mortality [82]. Given the limitations of warfarin, DOACs have recently drawn significant attention as a possible substitute in ESKD. Apixaban and rivaroxaban are the most studied agents, and the results from these studies will be presented below.
Rivaroxaban: The VALKYRIE Trial
The VALKYRIE trial was initially designed to assess the effect of vitamin K status on vascular calcification progression [83]. A total of 132 adult patients on maintenance hemodialysis with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of ≥2 were randomized to three groups (VKAs alone; rivaroxaban 10 mg once daily; and rivaroxaban 10 mg once daily plus a vitamin K2 supplement, menaquinone-7, at the dose of 2,000 mg three times per week post-dialysis) in a 1:1:1 ratio. Patients who completed this study were kept on the same treatment and were subsequently followed for at least an additional 18 months (Valkyrie-extension study) [43]. In the VKA arm, the target INR was 2–3 and mean TTR was 48.0% during the first 6 months, but this increased progressively over the course of the study. The primary end point, a composite of fatal cardiovascular disease and nonfatal stroke, cardiac events, and other vascular events, occurred in 35/44 patients in the VKA arm, in 23/46 patients in the rivaroxaban arm, and in 17/42 patients in the rivaroxaban and vitamin K2 arms. The competing risk-adjusted HR was 0.37 (95% CI: 0.24–0.58) in the pooled rivaroxaban groups compared with the VKA group. The incidence of stroke (both ischemic and hemorrhagic) was similar across the treatment groups, while the use of VKAs was associated with higher rates of symptomatic limb ischemia [43].
The safety profile of rivaroxaban was deemed to be better than that of the VKAs as both rivaroxaban groups were associated with a lower incidence of life-threatening and major bleeding episodes: HR of 0.44 (95% CI: 0.23–0.85) in the pooled rivaroxaban groups, compared with the VKA arm. Overall, the net clinical benefit, expressed as the ratio of the incidence rate of the primary endpoint and of life-threatening or major bleeding, was in favor of rivaroxaban: 0.45 (95% CI: 0.29–0.69) [43].
The VALKYRIE trial was the first and the only study to establish superiority for a DOAC compared with a VKA in the ESKD population. Study design was based on solid PK data, and results are compelling and clinically relevant. However, the event rate in the VKA arm (63.8 events per 100 person-years) was higher as compared to other trials. Whether the benefit was due to deleterious effect of VKAs or protective action of rivaroxaban is debatable. However, the absence of benefit from the addition of vitamin K supplements supports the theory that vitamin K status alone does not explain the difference between the warfarin and rivaroxaban groups.
Apixaban: The RENAL-AF and AXADIA Trials
The RENAL-AF trial compared the safety and efficacy of apixaban with warfarin in patients on maintenance hemodialysis with documented nonvalvular atrial fibrillation and a CHA2DS2-VASc score ≥2 [44]. Study participants were randomly allocated to apixaban 5 mg twice daily or dose-adjusted warfarin to achieve a target INR of 2–3. The reduced dose of apixaban (2.5 mg twice daily) was used if participants were ≥80 years old or had a body weight ≤60 kg. The initial target size was 762 patients but the lower than anticipated recruitment rate led to the premature interruption of the study after enrollment of 154 participants. The median follow-up was 11 months. The median age was 68 years, 36% of participants were women, and 58% had diabetes. The median CHA2DS2-VASc score was 4; 25% of participants had a history of stroke and the median time since dialysis initiation was 3 years. In the warfarin group, TTR was 44% with patients being 3 times more likely to be subtherapeutic than supratherapeutic. The 1-year incidence of the primary outcome (major or clinically relevant nonmajor bleeding) was 31.5% in the apixaban arm and 25.5% in the warfarin arm (HR 1.20; 95% CI: 0.63–2.30). With regards to the secondary outcomes, the 1-year event rates for stroke or SE were 3.0 and 3.3% in the apixaban and warfarin groups, respectively, while 21 deaths occurred in the apixaban group (26%) versus 13 in the warfarin group (18%). The study was not adequately powered but failed to establish noninferiority or superiority of apixaban compared with warfarin in ESKD.
The AXADIA-AFNET 8 was a RCT that compared the reduced dose of apixaban with VKA therapy in patients on maintenance hemodialysis with atrial fibrillation and a CHA2DS2-VASc score ≥2 [45]. The trial had an initial target sample size of 222 participants but finally enrolled only 97 patients. The median time on dialysis was 2.7 years. Participants were randomly allocated to apixaban (2.5 mg twice daily) or dose-adjusted phenprocoumon, a long-acting VKA (target INR of 2–3). The median age was 77 years, 30% of participants were women, and the median CHA2DS2-VASc score was 5. TTR was 51% for patients on phenprocoumon. The median follow-up time was 14 months in the apixaban arm and 17 months in the phenprocoumon arm. The incidence of the primary outcome (a composite endpoint of death from any cause, major bleeding, or clinically relevant nonmajor bleeding) was 36.1 and 36.6 events per 100 patient-years in the apixaban and the phenprocoumon arms, respectively (HR 0.93, 95% CI: 0.53–1.65). One ischemic stroke occurred in the phenprocoumon group and none was observed in the apixaban group. The incidence of cardiovascular events (cardiovascular death, myocardial infarction, ischemic stroke, deep vein thrombosis, or pulmonary embolism) was similar in both study arms. Nine patients died in the apixaban group (19%) and 12 patients died in the phenprocoumon group (25%) [45].
Although both underpowered, several conclusions can be drawn from these studies. First, in both trials with apixaban, as well as in VALKYRIE, ischemic events were significantly less frequent than major bleeding events. Second, in this population, optimal anticoagulation appears to be difficult to achieve with VKAs, with patients nearly 50% of time being outside the therapeutic range. Third, recruiting participants for trials on anticoagulation in hemodialysis can prove to be very challenging [84].
Unresolved Questions
Additional important questions remain to be answered. In a position paper summarizing the existing evidence, An de Vriese and Gunnar Heine suggest that patients on dialysis with atrial fibrillation may have a lower attributable risk for ischemic stroke than the general population [85]. This could be explained either by a high prevalence of subclinical atrial fibrillation in the “no atrial fibrillation” arm in observational studies or by the fact that in these patients, many episodes of paroxysmal atrial fibrillation may occur during hemodialysis while receiving parenteral anticoagulation.
Optimal discrimination between individuals who will have an event or not is another major issue. The CHA2DS2-VASc score may overestimate the risk of stroke and cut-off values currently used might explain, at least in part, poor model performance [85]. Therefore, there is an unmet need for development of a dialysis-specific predictive model to accurately identify patients at high risk of stroke.
Another important consideration is whether anticoagulation can really prevent the occurrence of new strokes in patients on maintenance dialysis with atrial fibrillation. The pathophysiology of stroke seems to be different in this population. It has been shown that the relative incidence of hemorrhagic stroke increases as the kidney function declines [86]. Taking this observation into consideration, as well as the failure of VKAs to reduce the incidence of new strokes as mentioned above, it seems reasonable that novel agents, such as DOACs, should be compared with no anticoagulation instead of standard VKA treatment in patients with ESKD. There is only one retrospective observational study comparing apixaban with no treatment in this population. Mavrakanas et al. used the US Renal Data System to create a propensity score-matched cohort including 2,082 patients (521 on apixaban and 1,561 without any anticoagulant prescription) [87]. The incidence of the composite outcome of new ischemic or hemorrhagic stroke, transient ischemic attack, or systemic thromboembolism was similar in the apixaban and the no treatment group (HR 1.24; 95% CI: 0.69–2.23). However, the incidence of fatal or intracranial bleeding events was significantly higher in the apixaban group (HR 2.74; 95% CI: 1.37–5.47). This result was driven by a significantly higher incidence of fatal or intracranial bleeding in patients treated with the standard apixaban dose (5 mg twice daily). The risk of bleeding seems to be even higher in patients who were also treated with a P2Y12 inhibitor without any benefit on the risk of stroke [88].
Ongoing Trials
DANWARD is a Danish nationwide open label randomized controlled trial (NCT03862859) comparing warfarin with no anticoagulation in dialysis patients (N = 718). The SACK randomized trial (NCT05679024) is the first clinical trial comparing apixaban at the reduced dose of 2.5 mg twice daily with no anticoagulation and is currently recruiting participants. Results are expected in 2028 (N = 1,400). The SAFE-D study (NCT03987711) is a pilot, phase 2, open label randomized trial comparing warfarin, apixaban 5 mg twice daily (2.5 mg twice daily in selected patients), and no anticoagulation in patients with atrial fibrillation on dialysis (N = 150). The study has been completed and results are eagerly awaited.
Kidney Outcomes in Patients with Atrial Fibrillation Treated with VKAS or DOACs
An unexpected acute rise in serum creatinine has been described in patients on VKAs with supratherapeutic INR levels [89]. This condition has been labeled warfarin-related nephropathy or, most recently, anticoagulant-related nephropathy [90]. In a retrospective analysis of 4,006 warfarin-treated patients with INR >3 and serum creatinine measured within a week of INR measurement, presumed anticoagulant-related nephropathy occurred in 17% and 33% of patients with and without CKD, respectively, and was associated with increased 1-year mortality rates [89].
Mechanisms of warfarin-mediated kidney injury appear to be multifactorial. These include glomerular hemorrhage with filling up of distal tubules and Bowman’s space with red blood cells and red cell casts, as shown in biopsy studies, kidney microvascular calcification, allergic interstitial nephritis, spontaneous atheroembolic kidney disease, and pelvis/ureteral hematoma [91].
It was subsequently hypothesized that DOACs may be associated with a lower incidence of anticoagulant-related nephropathy, compared with VKAs, due to their more predictable anticoagulation profile. This would manifest as a lower incidence of acute kidney injury or CKD progression.
In the RE-LY trial, warfarin-treated patients had a greater decline in eGFR as compared to dabigatran-treated patients at 30 months of follow-up (−3.7 ± 0.2 mL/min/1.73 m2 with warfarin vs. −2.6 ± 0.2 mL/min/1.73 m2 with dabigatran 110 mg vs. −2.5 ± 0.2 mL/min/1.73 m2 with dabigatran 150 mg; p < 0.001 for both comparisons). The decline was more pronounced in patients with diabetes, past VKA experience, and supratherapeutic anticoagulation [92]. In contrast, in the ROCKET-AF trial, decline in CrCl while on treatment was slightly higher in patients randomized to rivaroxaban (−4.3 ± 14.6 mL/min), as compared to warfarin (−3.5 ± 15.1 mL/min; p < 0.001). However, the percentage of patients with a drop in CrCl of at least 20% from baseline while on treatment was similar in both groups (27% with rivaroxaban vs. 26% with warfarin) [93]. Finally, in the ARISTOTLE trial, worsening kidney function while on treatment occurred in 13.6% of patients. Patients at risk for worsening kidney function were elderly, had low hematocrit, diabetes, past VKA experience, congestive heart failure, and vascular disease. At 12 months, patients randomized to apixaban had a greater decline in eGFR, as compared to warfarin (−1.4 ± 10.1 vs. −0.9 ± 10.3 mL/min/1.73 m2) [94]. The results from the observational studies that addressed the same question are summarized in Table 3 [91, 95–101].
Table 3.
Kidney outcomes of patients with nonvalvular atrial fibrillation on VKAs versus DOACs
| Author | Database | N | Treatment arm | Follow-up | Acute kidney injury HR (95% CI) | Annual decline in GFR | CKD progression HR (95% CI) | Progression to kidney failure HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Fordyce et al. [93] | ROCKET-AF trial | 12,612 | Rivaroxaban | – | – | −4.3±14.6 | – | – |
| Warfarin | −3.5±15.1 | |||||||
| Hijazi et al. [94] | ARISTOTLE trial | 16,869 | Apixaban | – | – | −1.4±10.1 | – | – |
| Warfarin | −0.9±10.3 | |||||||
| Böhm et al. [92] | RE-LY trial | 16,490 | Dabigatran 110 | 30 months | 0.81 (0.69–0.96) | −3.7±0.2 | 0.75 (0.62–0.92) | – |
| Dabigatran 150 | 0.66 (0.54–0.81) (reference) | −2.5±0.2 | 0.66 (0.54–0.81) | |||||
| Warfarin | −3.7±0.2 | (reference) | ||||||
| Yao et al. [91] | Optum Labs Warehouse (USA) | 9,769 | Dabigatran | 11±10 months | – | – | 0.45 (0.13–1.59) | |
| Apixaban | 1.02 (0.45–2.31) | |||||||
| Rivaroxaban | 0.63 (0.35–1.15) | |||||||
| All DOACs | 0.68 (0.58–0.81) | |||||||
| Warfarin | (reference) | (reference) | ||||||
| Hernandez et al. [96] | US IBM Market Scan | 21,682 | Rivaroxaban | 1.7 y (0.8–3.2) | 0.83 (0.74–0.92) | – | – | 0.82 (0.70–0.96) |
| Warfarin | (reference) | (reference) | ||||||
| Trevisan et al. [100] | SCREAM project | 32,699 | DOACs | 3.8 y | 0.88 (0.80–0.97) | 0.87 (0.78–0.98) | 0.43 (0.25–0.73) | |
| Warfarin | (reference) | (reference) | (reference) | |||||
| Chan et al. [95] | Taiwan National Registry | 19,932 | Dabigatran | 0.69 y | 0.56 (0.46–0.69) | – | – | – |
| Warfarin | 0.79 y | (reference) | ||||||
| Lin et al. [99] | Taiwan multi-center database | 2,382 | DOAC | 2.3±2.1 y | – | – | 0.75 (0.64–0.87) | 0.94 (0.78–1.12) |
| Warfarin | 2.6±2.3 y | (reference) | (reference) | |||||
| Wang et al. [101] | Taiwan single center database | 2,203 | Dabigatran | – | 0.94 (0.56–1.56) | – | 0.69 (0.50–0.95) | 0.56 (0.27–1.18) |
| Rivaroxaban | 1.06 (0.76–1.47) | 1.04 (0.86–1.30) | 0.83 (0.55–1.25) | |||||
| Edoxaban | 1.05 (0.64–1.72) | 1.2 (0.92–1.57) | 0.81 (0.42–1.55) | |||||
| Warfarin | (reference) | (reference) | (reference) | |||||
| Wetmore et al. [98] | US Medicare beneficiaries | 12,816 | Apixaban | 466±374 d | – | – | 0.90 (0.82–0.99) | – |
| Warfarin | 664±374 d | (reference) | ||||||
| Pastori et al. [97] | Italy | 1,667 | Dabigatran | 1 y | – | −0.3 (−9.0 to +4.5) | 0.70 (0.50–0.97) | – |
| Apixaban | −1.2 (−9.9 to +4.0) | 0.59 (0.42–0.82) | ||||||
| Rivaroxaban | −1.3 (−8.7 to +3.9) | 0.59 (0.43-0.81) | ||||||
| Warfarin | (reference) | (reference) |
CI, confidence interval; CKD, chronic kidney disease; d, days; DOACs, direct oral anticoagulants; GFR, glomerular filtration rate (in mL/min/1.73 m2/year); HR, hazard ratio; N, total number of patients; y, years.
In conclusion, the exact prevalence, mechanisms, and clinical significance of anticoagulant-related nephropathy remain unclear. In addition, there is no convincing evidence that DOACs are clearly associated with a lower incidence of kidney function decline compared with VKAs.
Management of VTE in Patients with CKD
Burden of VTE in CKD
Patients with CKD are at high risk of VTE due to prothrombotic risk factors that are highly prevalent in CKD and additional pathophysiological mechanisms that are not fully understood [102, 103]. Proteinuria and kidney dysfunction, as manifested by a lower eGFR, are independent risk factors of VTE [104]. In the National Inpatient Sample database, the incidence of pulmonary embolism has been estimated at 66, 204, and 527 events per 100,000 persons with normal kidney function, nondialysis-dependent CKD of any stage, and ESKD, respectively [105]. In an older dataset from the US Renal Data System, the incidence rate of pulmonary embolism was estimated at 150 events per 100,000 persons with ESKD, compared with 25 events per 100,000 persons in the general population [106].
VTE is associated with significant morbidity in CKD. Among patients hospitalized with acute pulmonary embolism, the length of stay was longer by 1 day in patients with CKD and by 2 days in patients with ESKD, compared with patients with preserved kidney function. Similarly, in-hospital mortality rates were higher in CKD and ESKD: odds ratio (OR) of 1.57 (95% CI: 1.27–1.93) and of 1.92 (95% CI: 1.17–3.15), respectively [105]. Finally, in the Global Anticoagulant Registry in the Field-Venous Thromboembolism, patients with moderate-to-severe CKD had higher hazards for recurrent VTE (HR 1.40, 95% CI: 1.10–1.77), mortality (HR 1.44, 95% CI: 1.21–1.73), and major bleeding (HR 1.40, 95% CI: 1.03–1.90), compared with patients with an eGFR ≥60 mL/min/1.73 m2 [107].
In the RE-COVER trial, VTE and VTE-related deaths decreased with declining kidney function: HR 0.75 (95% CI: 0.31–1.84) for patients with CrCl of 30–50 mL/min versus >80 mL/min. This reduction was predominantly seen in those randomized to receive dabigatran and was postulated to be related to the increase in drug exposure [108]. However, in a post hoc analysis of the EINSTEIN-DVT and EINSTEIN-PE trials comparing rivaroxaban with warfarin, the recurrent VTE rates for both treatments combined increased with declining kidney function from 1.8% (CrCl ≥80 mL/min) to 2.8% (CrCl 50–79 mL/min) and 3.3% (CrCl 30.49 mL/min) [109]. In regard to bleeding event rates, they increased with declining kidney function in both treatment arms in the RECOVER trial but only in the VKA arm in the EINSTEIN trials [108, 109].
Principles of Anticoagulation in VTE
Anticoagulation treatment in VTE is divided into three phases: acute (5–10 days), chronic (≤3 months), and extended anticoagulation phase (>3 months). The goal of the acute phase treatment is thrombus stabilization with the prevention of thrombotic extension and development of high risk or fatal pulmonary embolism [4]. Conventionally, it involves parenteral anticoagulation with unfractionated or low-molecular-weight heparin or fondaparinux for at least 5 days [110]. Dabigatran, edoxaban, and VKAs require parenteral anticoagulation in the acute phase, whereas apixaban and rivaroxaban can be initiated at higher doses (1 week for apixaban and 3 weeks for rivaroxaban), bypassing the need for parenteral anticoagulation [7]. The purpose of the maintenance phase is to complete treatment of the acute event, providing secondary prevention for recurrent VTE. In this phase, DOACs are given at a fixed dose, whereas VKAs are adjusted to maintain an INR of 2–3. The extended anticoagulation phase (beyond 3 months) is recommended in patients at high risk of recurrence [110]. With the exception of edoxaban, kidney-adjusted doses for DOACs have not been used in VTE.
VTE prophylaxis in patients with CKD is beyond the scope of this review. However, thromboprophylaxis in advanced CKD or ESKD requires dose adjustment of low-molecular-weight heparins or use of unfractionated heparin.
DOACs versus VKAs for the Treatment of VTE in Patients with CKD
Most DOACs have only been used at the standard dose in VTE. The only agent that was used at a reduced dose in patients with CKD was edoxaban. In the HOKUSAI VTE trial, patients with a CrCl of 30–50 mL/min, weight <60 kg, or those who were on potent P-gp inhibitors received edoxaban 30 mg once daily, instead of 60 mg. 36% of patients who received the reduced edoxaban dose had moderate CKD [111].
None of the large RCTs with DOACs in VTE has enrolled participants with a CrCl <30 mL/min. Therefore, post hoc analyses comparing DOACs with warfarin are only available for patients with moderate CKD. The efficacy and safety of the three factor Xa inhibitors among patients in this population was similar to that of warfarin, although the number of events in this subgroup was small and no definite conclusions can be drawn (Table 4) [108, 109, 111–113]. Edoxaban use was associated with a lower incidence of recurrent VTE and rivaroxaban use with a lower incidence of major bleeding among patients with CKD, compared with warfarin [111, 114]. In contrast, dabigatran use was associated with a significantly higher incidence of major bleeding [108]. Dosing recommendations based on the available data and expert opinion are summarized in Figure 2. There are no randomized data and no high-quality observational studies on the use of DOACs in patients with advanced, nondialysis-dependent CKD. In the ESKD population, Wetmore et al. [113] retrospectively identified 12,206 patients with VTE who received either warfarin or apixaban, using the USRDS dataset. Apixaban treatment was associated with lower rates of recurrent VTE (HR 0.58; 95% CI: 0.43–0.77) or major bleeding (HR 0.78; 95% CI: 0.62–0.98) and similar mortality rates (HR 1.04; 95% CI: 0.94–1.16), compared with warfarin. However, there was no information available on the dosage of apixaban that was used. Therefore, no definite conclusions can be made with respect of apixaban use in ESKD for the treatment of VTE [110].
Table 4.
DOACs versus VKAs for the treatment of VTE in patients with CKD
| Author | Database | CrCl, mL/min | N | Treatment arms | Age, years | Males, % | Recurrent VTE HR (95% CI) | Major bleeding HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Agnelli et al. [112] | AMPLIFY | 30–50 | – | Apixaban vs. Warfarin (reference) | – | – | 0.93 (0.34–2.61)* | 0.52 (0.18–1.51)* |
| Goldhaber et al. [108] | RE-COVER | 30–50 | 237 | Dabigatran vs. Warfarin (reference) | – | – | – | 6.71 (3.34–13.48) |
| Bauersachs et al. [109] | EINSTEIN | 30–50 | 636 | Rivaroxaban | 79 (75–83) | 34 | 1.05 (0.44–2.47) | 0.23 (0.06–0.81) |
| Warfarin (reference) | 80 (75–84) | |||||||
| Verhamme et al. [111] | HOKUSAI-VTE | 30–50 | 268 | Edoxaban vs. Warfarin (reference) | 60±19 | 33 | 0.38 (0.15–0.98) | 0.66 (0.37–1.18)* |
| Wetmore et al. [113] | USRDS cohort | ESKD | 12,206 | Apixaban vs. Warfarin (reference) | 63±14 | 47 | 0.58 (0.43–0.77) | 0.78 (0.62–0.98) |
CI, confidence interval; CrCl, creatinine clearance; HR, hazard ratio; N, total number of patients; USRDS, US Renal Data System; VTE, venous thromboembolism.
*Relative risk reported.
Conclusions and Future Directions
DOACs have revolutionized the management of patients with atrial fibrillation or VTE. These agents can also be used in patients with mild or moderate CKD, after appropriate dose adjustment when needed. However, they have not been adequately studied in patients with advanced CKD and they have to be used with caution in this subgroup, especially for the treatment of VTE (Fig. 2).
Future research should focus on appropriate risk stratification of patients with ESKD to identify those who may benefit from oral anticoagulation, develop better tools to predict high risk of bleeding, and determine optimal management strategies in VTE, especially with respect to dose adjustment for factor Xa inhibitors or in patients with catheter-related thrombosis. Novel agents that are currently under development may offer additional treatment options to the CKD population. After more than a decade of exciting progress in the field of thrombosis for patients with CKD, the best is yet to come.
Acknowledgments
The figures were created using the BioRender software.
Conflict of Interest Statement
Dr. Elenjickal received salary support from an educational grant by Pfizer. Dr. Travlos has nothing to disclose. Dr. Marques received salary support from an educational grant by Janssen. Dr. Mavrakanas received speaker honoraria from BMS Canada, Janssen, Astra Zeneca, and Pfizer and has served on advisory boards for Böhringer Ingelheim, Bayer, GSK, and Servier outside the submitted work. He has also received an unrestricted research grant from Astra Zeneca and operational grants from the Kidney Foundation of Canada, the Heart & Stroke foundation of Canada, and the Canadian Institute of Health Research. He is receiving salary support from the Fonds de Recherche Quebec Santé (Junior 1 Clinician Scholar Award # 298742) and is supported by a KRESCENT New Investigator Award.
Funding Sources
This work was supported by an operational grant from the Fonds de Recherche Quebec Santé (# 309790).
Author Contributions
E.J.E. and C.T. wrote the first draft. P.M. designed the figures and critically revised the manuscript. T.A.M. devised the proof outline, critically revised the manuscript, and provided supervision.
Funding Statement
This work was supported by an operational grant from the Fonds de Recherche Quebec Santé (# 309790).
References
- 1. Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy. 2018;38(9):907–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain N, Reilly RF. Clinical pharmacology of oral anticoagulants in patients with kidney disease. Clin J Am Soc Nephrol. 2019;14(2):278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et al. International council for standardization in haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–50. [DOI] [PubMed] [Google Scholar]
- 4. Mavrakanas T, Bounameaux H. The potential role of new oral anticoagulants in the prevention and treatment of thromboembolism. Pharmacol Ther. 2011;130(1):46–58. [DOI] [PubMed] [Google Scholar]
- 5. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. [DOI] [PubMed] [Google Scholar]
- 6. Chen A, Stecker E, A Warden B. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mavrakanas TA, Charytan DM, Winkelmayer WC. Direct oral anticoagulants in chronic kidney disease: an update. Curr Opin Nephrol Hypertens. 2020;29(5):489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emanuel S, Kaba RA, Delanerolle G, Field BCT, Lip GYH, De Lusignan S. Correct dosing, adherence and persistence of DOACs in atrial fibrillation and chronic kidney disease: a systematic review and meta-analysis. Open Heart. 2023;10(2):e002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsh J, Fuster V, Ansell J, Halperin JL; American, Heart Association, American College of Cardiology Foundation . American heart association/American college of cardiology foundation guide to warfarin therapy. Circulation. 2003;107(12):1692–711. [DOI] [PubMed] [Google Scholar]
- 10. Kuruvilla M, Gurk-Turner C. A review of warfarin dosing and monitoring. Proc. 2001;14(3):305–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morán-Mariños C, Corcuera-Ciudad R, Velásquez-Rimachi V, Nieto-Gutierrez W. Systematic review of warfarin-induced skin necrosis case reports and secondary analysis of factors associated with mortality. Int J Clin Pract. 2021;75(12):e15001. [DOI] [PubMed] [Google Scholar]
- 12. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6):160s–98s. [DOI] [PubMed] [Google Scholar]
- 13. Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005;3(8):1861–72. [DOI] [PubMed] [Google Scholar]
- 14. Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353(10):1028–40. [DOI] [PubMed] [Google Scholar]
- 15. Ganetsky M, Babu KM, Salhanick SD, Brown RS, Boyer EW. Dabigatran: review of pharmacology and management of bleeding complications of this novel oral anticoagulant. J Med Toxicol. 2011;7(4):281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng W, Dai X, Xu B, Tian W, Shi J. Discovery and development of factor Xa inhibitors (2015-2022). Front Pharmacol. 2023;14:1105880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byon W, Garonzik S, Boyd RA, Frost CE. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kreutz R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam Clin Pharmacol. 2012;26(1):27–32. [DOI] [PubMed] [Google Scholar]
- 19. Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55(6):641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Vriese AS, Caluwé R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B, et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66(1):91–8. [DOI] [PubMed] [Google Scholar]
- 21. Parasrampuria DA, Marbury T, Matsushima N, Chen S, Wickremasingha PK, He L, et al. Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb Haemost. 2015;113(4):719–27. [DOI] [PubMed] [Google Scholar]
- 22. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28(7):2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mavrakanas T, Samer C, Fontana P, Perrier A. Direct oral anticoagulants: efficacy and safety in patient subgroups. Swiss Med Wkly. 2015;145:w14081. [DOI] [PubMed] [Google Scholar]
- 24. Yin OQ, Tetsuya K, Miller R. Edoxaban population pharmacokinetics and exposure-response analysis in patients with non-valvular atrial fibrillation. Eur J Clin Pharmacol. 2014;70(11):1339–51. [DOI] [PubMed] [Google Scholar]
- 25. Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE‐LY trial. J Thromb Haemost. 2011;9(11):2168–75. [DOI] [PubMed] [Google Scholar]
- 26. Ferri N, Colombo E, Tenconi M, Baldessin L, Corsini A. Drug-drug interactions of direct oral anticoagulants (DOACs): from pharmacological to clinical practice. Pharmaceutics. 2022;14(6):1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandhi SK, Reiffel JA, Boiron R, Wieloch M. Risk of major bleeding in patients with atrial fibrillation taking dronedarone in combination with a direct acting oral anticoagulant (from a U.S. Claims database). Am J Cardiol. 2021;159:79–86. [DOI] [PubMed] [Google Scholar]
- 28. Härtter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa[®]) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75(4):1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendell J, Chen S, He L, Desai M, Parasramupria DA. The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin Drug Investig. 2015 2015/07/01;35(7):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stöllberger C, Finsterer J. Interactions between non-vitamin K oral anticoagulants and antiepileptic drugs. Epilepsy Res. 2016;126:98–101. [DOI] [PubMed] [Google Scholar]
- 31. Mathy F-X, Dohin E, Bonfitto F, Pelgrims B. Drug–drug interaction between levetiracetam and non-vitamin K antagonist anticoagulants. Eur Heart J. 2019;40(19):1571–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathias AA, German P, Murray BP, Wei L, Jain A, West S, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010;87(3):322–9. [DOI] [PubMed] [Google Scholar]
- 33. Wieland E, Shipkova M. Pharmacokinetic and pharmacodynamic drug monitoring of direct-acting oral anticoagulants: where do we stand? Ther Drug Monit. 2019;41(2):180–91. [DOI] [PubMed] [Google Scholar]
- 34. Dunois C. Laboratory monitoring of direct oral anticoagulants (DOACs). Biomedicines. 2021;9(5):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harenberg J, Schreiner R, Hetjens S, Weiss C. Detecting anti-IIa and anti-xa direct oral anticoagulant (DOAC) agents in urine using a DOAC dipstick. Semin Thromb Hemost. 2019;45(3):275–84. [DOI] [PubMed] [Google Scholar]
- 36. Ross B, Miller MA, Ditch K, Tran M. Clinical experience of life-threatening dabigatran-related bleeding at a large, tertiary care, academic medical center: a case series. J Med Toxicol. 2014;10(2):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pollack CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511–20. [DOI] [PubMed] [Google Scholar]
- 38. Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–24. [DOI] [PubMed] [Google Scholar]
- 39. McConachie SM, Shammout L, Martirosov DM. Clearance confusion: an exploratory analysis of inpatient dosing discordances between renal estimating equations. Ann Pharmacother. 2020;54(11):1102–8. [DOI] [PubMed] [Google Scholar]
- 40. Donker EM, Bet P, Nurmohamed A, Serné E, Burchell G, Friedman AN, et al. Estimation of glomerular filtration rate for drug dosing in patients with very high or low body mass index. Clin Transl Sci. 2022;15(9):2206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ravenstijn P, Chetty M, Manchandani P. Design and conduct considerations for studies in patients with impaired renal function. Clin Transl Sci. 2021;14(5):1689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Limdi NA, Limdi MA, Cavallari L, Anderson AM, Crowley MR, Baird MF, et al. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis. 2010;56(5):823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Vriese AS, Caluwé R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32(6):1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pokorney SD, Chertow GM, Al-Khalidi HR, Gallup D, Dignacco P, Mussina K, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146(23):1735–45. [DOI] [PubMed] [Google Scholar]
- 45. Reinecke H, Engelbertz C, Bauersachs R, Breithardt G, Echterhoff H-H, Gerß J, et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2023;147(4):296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harder S. Renal profiles of anticoagulants. J Clin Pharmacol. 2012;52(7):964–75. [DOI] [PubMed] [Google Scholar]
- 47. Limdi NA, Nolin TD, Booth SL, Centi A, Marques MB, Crowley MR, et al. Influence of kidney function on risk of supratherapeutic international normalized ratio–related hemorrhage in warfarin users: a prospective cohort study. Am J Kidney Dis. 2015;65(5):701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kooiman J, van der Hulle T, Maas H, Wiebe S, Formella S, Clemens A, et al. Pharmacokinetics and pharmacodynamics of dabigatran 75 mg b.i.d. in patients with severe chronic kidney disease. J Am Coll Cardiol. 2016;67(20):2442–4. [DOI] [PubMed] [Google Scholar]
- 49. Liesenfeld KH, Clemens A, Kreuzer J, Brueckmann M, Schulze F. Dabigatran treatment simulation in patients undergoing maintenance haemodialysis. Thromb Haemost. 2016;115(3):562–9. [DOI] [PubMed] [Google Scholar]
- 50. Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141(17):1384–92. [DOI] [PubMed] [Google Scholar]
- 51. Fung WW-S, Cheng PM-S, Ng JK-C, Chan GC-K, Chow KM, Li PK-T, et al. Pharmacokinetics of apixaban among peritoneal dialysis patients. Kidney Med. 2023;5(8):100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sin CF, Wong KP, Wong HM, Siu CW, Yap DYH. Plasma rivaroxaban level in patients with early stages of chronic kidney disease-relationships with renal function and clinical events. Front Pharmacol. 2022;13:888660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koretsune Y, Yamashita T, Kimura T, Fukuzawa M, Abe K, Yasaka M. Short-Term safety and plasma concentrations of edoxaban in Japanese patients with non-valvular atrial fibrillation and severe renal impairment. Circ J. 2015;79(7):1486–95. [DOI] [PubMed] [Google Scholar]
- 54. Nelson SE, Shroff GR, Li S, Herzog CA. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in medicare patients. J Am Heart Assoc. 2012;1(4):e002097–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liao J-N, Chao T-F, Liu C-J, Wang K-L, Chen S-J, Lin Y-J, et al. Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int. 2015;87(6):1209–15. [DOI] [PubMed] [Google Scholar]
- 56. Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol. 2019;74(17):2204–15. [DOI] [PubMed] [Google Scholar]
- 57. Bonde AN, Lip GYH, Kamper A-L, Fosbøl EL, Staerk L, Carlson N, et al. Renal function and the risk of stroke and bleeding in patients with atrial fibrillation: an observational cohort study. Stroke. 2016;47(11):2707–13. [DOI] [PubMed] [Google Scholar]
- 58. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129(9):961–70. [DOI] [PubMed] [Google Scholar]
- 59. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33(22):2821–30. [DOI] [PubMed] [Google Scholar]
- 60. Lindner SM, Fordyce CB, Hellkamp AS, Lokhnygina Y, Piccini JP, Breithardt G, et al. Treatment consistency across levels of baseline renal function with rivaroxaban or warfarin: a ROCKET AF (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) analysis. Circulation. 2017;135(10):1001–3. [DOI] [PubMed] [Google Scholar]
- 61. Hori M, Matsumoto M, Tanahashi N, Momomura S-I, Uchiyama S, Goto S, et al. Safety and efficacy of adjusted dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation: subanalysis of J-ROCKET AF for patients with moderate renal impairment. Circ J. 2013;77(3):632–8. [DOI] [PubMed] [Google Scholar]
- 62. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134(1):24–36. [DOI] [PubMed] [Google Scholar]
- 63. Ashley J, McArthur E, Bota S, Harel Z, Battistella M, Molnar AO, et al. Risk of cardiovascular events and mortality among elderly patients with reduced GFR receiving direct oral anticoagulants. Am J Kidney Dis. 2020;76(3):311–20. [DOI] [PubMed] [Google Scholar]
- 64. Welander F, Renlund H, Dimény E, Holmberg H, Själander A. Direct oral anticoagulants versus warfarin in patients with non-valvular atrial fibrillation and CKD G3–G5D. Clin Kidney J. 2023;16(5):835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yao X, Inselman JW, Ross JS, Izem R, Graham DJ, Martin DB, et al. Comparative effectiveness and safety of oral anticoagulants across kidney function in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2020;13(10):e006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weir MR, Ashton V, Moore KT, Shrivastava S, Peterson ED, Ammann EM. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J. 2020;223:3–11. [DOI] [PubMed] [Google Scholar]
- 67. Hsu C-C, Chen C-C, Chou C-Y, Chen K-H, Wang S-F, Chang S-L, et al. Effectiveness and safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation and advanced kidney disease. J Thromb Thrombolysis. 2023;56(4):518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132(9):1078–83. [DOI] [PubMed] [Google Scholar]
- 69. Hanni C, Petrovitch E, Ali M, Gibson W, Giuliano C, Holzhausen J, et al. Outcomes associated with apixaban vs warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11):2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and efficacy of apixaban versus warfarin in patients with advanced chronic kidney disease. Ann Pharmacother. 2018;52(11):1078–84. [DOI] [PubMed] [Google Scholar]
- 71. Herndon K, Guidry TJ, Wassell K, Elliott W. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a veterans affairs hospital. Ann Pharmacother. 2020;54(6):554–60. [DOI] [PubMed] [Google Scholar]
- 72. Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37(4):412–9. [DOI] [PubMed] [Google Scholar]
- 73. Harrington J, Carnicelli AP, Hua K, Wallentin L, Patel MR, Hohnloser SH, et al. Direct oral anticoagulants versus warfarin across the spectrum of kidney function: patient-level network meta-analyses from COMBINE AF. Circulation. 2023;147(23):1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu Y, Chang AR, Inker LA, McAdams-DeMarco M, Grams ME, Shin JI. Associations of apixaban dose with safety and effectiveness outcomes in patients with atrial fibrillation and severe chronic kidney disease. Circulation. 2023;148(19):1445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121–201. [DOI] [PubMed] [Google Scholar]
- 76. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J. 2018;39(24):2314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–32. [DOI] [PubMed] [Google Scholar]
- 78. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42(5):373–498. [DOI] [PubMed] [Google Scholar]
- 79. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27(10):3816–22. [DOI] [PubMed] [Google Scholar]
- 81. Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G, et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(4):e202175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Galloway PA, El-Damanawi R, Bardsley V, Pritchard NR, Fry AC, Ojha SK, et al. Vitamin K antagonists predispose to calciphylaxis in patients with end-stage renal disease. Nephron. 2015;129(3):197–201. [DOI] [PubMed] [Google Scholar]
- 83. De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the valkyrie study. J Am Soc Nephrol. 2020;31(1):186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mavrakanas TA. Apixaban for stroke prevention in hemodialysis patients with nonvalvular atrial fibrillation. Kidney Int. 2023;103(6):1014–7. [DOI] [PubMed] [Google Scholar]
- 85. De Vriese AS, Heine G. Anticoagulation management in haemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant. 2022;37(11):2072–9. [DOI] [PubMed] [Google Scholar]
- 86. Zamberg I, Assouline-Reinmann M, Carrera E, Sood MM, Sozio SM, Martin PY, et al. Epidemiology, thrombolytic management, and outcomes of acute stroke among patients with chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2022;37(7):1289–301. [DOI] [PubMed] [Google Scholar]
- 87. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus No anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. 2020;15(8):1146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mavrakanas TA, Charytan DM. Apixaban versus No anticoagulation by P2Y12 inhibitor prescription status in dialysis patients with atrial fibrillation. Kidney360. 2022;3(10):1769–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29(12):2787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–32. [DOI] [PubMed] [Google Scholar]
- 92. Böhm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. 2015;65(23):2481–93. [DOI] [PubMed] [Google Scholar]
- 93. Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, et al. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134(1):37–47. [DOI] [PubMed] [Google Scholar]
- 94. Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1(4):451–60. [DOI] [PubMed] [Google Scholar]
- 95. Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, et al. Acute kidney injury in asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol. 2016;68(21):2272–83. [DOI] [PubMed] [Google Scholar]
- 96. Hernandez AV, Bradley G, Khan M, Fratoni A, Gasparini A, Roman YM, et al. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):301–7. [DOI] [PubMed] [Google Scholar]
- 97. Pastori D, Ettorre E, Lip GYH, Sciacqua A, Perticone F, Melillo F, et al. Association of different oral anticoagulants use with renal function worsening in patients with atrial fibrillation: a multicentre cohort study. Br J Clin Pharmacol. 2020;86(12):2455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wetmore JB, Yan H, Herzog CA, Weinhandl E, Reyes JL, Roetker NS. CKD progression in medicare beneficiaries with nonvalvular atrial fibrillation treated with apixaban versus warfarin. Am J Kidney Dis. 2021;78(2):180–9. [DOI] [PubMed] [Google Scholar]
- 99. Lin Y, Chao TF, Tsai ML, Tseng CJ, Wang TH, Chang CH, et al. Cardiovascular and renal outcomes in patients with atrial fibrillation and stage 4-5 chronic kidney disease receiving direct oral anticoagulants: a multicenter retrospective cohort study. J Thromb Thrombolysis. 2023. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 100. Trevisan M, Hjemdahl P, Clase CM, de Jong Y, Evans M, Bellocco R, et al. Cardiorenal outcomes among patients with atrial fibrillation treated with oral anticoagulants. Am J Kidney Dis. 2023;81(3):307–17.e1. [DOI] [PubMed] [Google Scholar]
- 101. Wang YT, Chen JH, Liao SF, Chen YJ, Lip GYH, Yeh JS. Cardiac and renal outcomes of direct oral anticoagulants in patients with atrial fibrillation. Eur J Clin Invest. 2023:e14086. [DOI] [PubMed] [Google Scholar]
- 102. Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19(1):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mahmoodi BK, Gansevoort RT, Næss IA, Lutsey PL, Brækkan SK, Veeger NJ, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation. 2012;126(16):1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kumar G, Sakhuja A, Taneja A, Majumdar T, Patel J, Whittle J, et al. Pulmonary embolism in patients with CKD and ESRD. Clin J Am Soc Nephrol. 2012;7(10):1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tveit DP, Hypolite IO, Hshieh P, Cruess D, Agodoa LY, Welch PG, et al. Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. 2002;39(5):1011–7. [DOI] [PubMed] [Google Scholar]
- 107. Goto S, Haas S, Ageno W, Goldhaber SZ, Turpie AGG, Weitz JI, et al. Assessment of outcomes among patients with venous thromboembolism with and without chronic kidney disease. JAMA Netw Open. 2020;3(10):e2022886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Goldhaber S, Schulman S, Eriksson H, Feuring M, Fraessdorf M, Kreuzer J, et al. Dabigatran versus warfarin for acute venous thromboembolism in elderly or impaired renal function patients: pooled analysis of RE-COVER and RE-COVER II. Thromb Haemost. 2017;117(11):2045–52. [DOI] [PubMed] [Google Scholar]
- 109. Bauersachs RM, Lensing AW, Prins MH, Kubitza D, Pap ÁF, Decousus H, et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J. 2014;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mavrakanas TA. Treatment options for venous thromboembolism in patients receiving dialysis. Clin J Am Soc Nephrol. 2022;17(5):623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Verhamme P, Wells PS, Segers A, Ageno W, Brekelmans MPA, Cohen AT, et al. Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind HOKUSAI VTE trial. Thromb Haemost. 2016;116(4):747–53. [DOI] [PubMed] [Google Scholar]
- 112. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. [DOI] [PubMed] [Google Scholar]
- 113. Wetmore JB, Herzog CA, Yan H, Reyes JL, Weinhandl ED, Roetker NS. Apixaban versus warfarin for treatment of venous thromboembolism in patients receiving long-term dialysis. Clin J Am Soc Nephrol. 2022;17(5):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Daiichi Sankyo I. Cardiovascular and renal drugs advisory committee briefing document. FDA Advisory Committee Briefing Document; 2014. p. 114–33. [Google Scholar]