Abstract
Purpose
To investigate systemic and ocular toll-like receptor (TLR)-4 expression and its association with oxidative stress markers in ocular rosacea (OR)
Methods
This prospective study included 40 patients with rosacea with ocular involvement and 20 healthy volunteers. Tear break-up time (TBUT), Schirmer test, meibomoscore, and ocular surface disease index (OSDI) scores were estimated for all participants. TLR-4 expression in conjunctival epithelium and peripheral blood mononuclear cells was quantified using real-time polymerase chain reaction (RT-PCR). In the tears and serum samples of all participants, antioxidant status (TAS), total oxidant status (TOS), and arylesterase (ARE) activation levels were measured using a fully automated spectrophotometric method, and the oxidative stress index (OSI) was calculated.
Results
TLR-4 expression levels and oxidative stress status (TOS and OSI values) were significantly higher (p < 0.01), and antioxidant status (TAS and ARE values) were significantly lower (p < 0.01) in both ocular and blood samples of patients with OR compared with those in controls. A significant positive correlation was found between the ocular and blood values in all parameters (p < 0.05). According to the clinical associations of these results, we found negative correlations between TLR-4, OSI, and TBUT and between TLR-4 and Schirmer, whereas a positive correlation was observed between TLR-4, OSI, and meiboscore and between TLR-4, OSI, and OSDI (p < 0.05). No correlation was found between the OSI and Schirmer results (p = 0.92).
Conclusions
TLR-4 and oxidative stress both play important roles in OR pathophysiology and are closely related to clinical findings.
Introduction
Rosacea is a common chronic inflammatory disease that affects 10% of the population worldwide. It is characterized by facial flushing, papules, pustules, telangiectasia, and ocular manifestations [1]. Rosacea is a multifactorial disease that involves the dysregulation of inflammatory, immune, vascular, and neuronal systems, as well as genetic predisposition, ultraviolet radiation (UV), Demodex colonization, and oxidative stress [2-4]. Ocular rosacea (OR) occurs in 58%–72% of patients with skin rosacea. Characteristically, OR starts with eyelid and ocular surface inflammation, lid margin telangiectasia, blepharitis, meibomitis, and meibomian gland dysfunction. In advanced cases, it threatens vision by causing corneal infiltrates, corneal neovascularization, scleritis, sclerokeratitis, and corneal thinning and perforation [5,6].
The pathogenesis of OR shares many common mechanisms with skin rosacea [7]. In particular, dysregulations in the inflammatory system seem to play a pivotal role in its pathogenesis, as shown in previous studies [7]. At the onset of the inflammation cascade in OR, cell infiltration and the release of proinflammatory factors have been associated with high levels of toll-like receptor (TLR) expression (especially TLR-4) [8]. TLRs are membrane and intracellular receptors that recognize pathogen-associated molecular patterns, mediating the inflammatory response through the recruitment of specific adaptor molecules. TLRs recruit toll/IL-1 receptor (TIR) domain-containing adaptor proteins, such as MyD88 and TRIF, which initiate signal transduction pathways that activate NF-κB, interferon regulatory factors (IRFs), or mitogen-activated protein (MAP) kinases. These pathways induce the expression of cytokines, chemokines, and type I IFNs that protect the host from microbial infection. In addition, TLRs recognize infectious agents and endogenous molecules, such as lipids, lipoproteins, proteins, and nucleic acids, contributing to innate immunity. Saturated fatty acids can be recognized by the CD14-TLR-4-MD2 complex and trigger inflammation like lipopolysaccharide. In addition, oxidized LDL and oxidized phospholipids trigger a CD36-TLR-4-TLR6 inflammatory response through NF-κB [8]. In the skin, the expressions of TLR2, TLR-4, and iNOS are elevated in rosacea samples compared to normal skin controls [9]. TLRs are also activated by external stimuli, such as UV [7,10], which can contribute to the pathogenesis of rosacea through lipid oxidation [11,12]. However, the exact link between oxidative stress status, TLR-4, and OR is not clearly understood.
In this study, we investigated the expression of TLR-4 in the ocular surface cells and peripheral blood mononuclear cell (PBMC) of patients with OR and correlated it with oxidative stress markers and ocular phenotypes.
Methods
Patient population
In this prospective study, we performed ophthalmologic examinations on patients diagnosed with skin rosacea at the dermatology department to confirm ocular involvement. This study was approved by the local ethics committee and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants.
Inclusion and exclusion criteria
The study included patients with rosacea with mild ocular involvement (i.e., lid margin telangiectasia, blepharoconjunctivitis, and meibomian gland dysfunction) and age-matched healthy controls. Patients with severe ocular involvement (i.e., corneal or scleral involvement) or a history of other ocular or systemic diseases, pregnancy, use of ocular or systemic medication (including supplements), smoking, or alcohol consumption were excluded from the study.
Clinical examination and data collection
After a complete ophthalmological examination, we performed and recorded tear break-up time (TBUT) [13], Schirmer test (without anesthesia) [14], meibomoscore [15], and ocular surface disease index (OSDI) [16] scores for all participants.
Sample collection
Conjunctival epithelium collection
The conjunctival epithelium was collected non-invasively by compressing the conjunctiva with nitrocellulose membranes for 3–4 s. The samples were placed in 0.5 ml Eppendorf tubes containing 100 μl of phosphate-buffered saline (PBS). Each sample was immediately transferred to −80 °C after collection and then stored.
Peripheral blood mononuclear cell (PBMC) collection
Peripheral blood samples of the patients and controls were collected in whole blood collecting tubes following 8–10 h of fasting. PBMC were isolated from the whole blood by density centrifugation using Ficoll-Paque as the density-gradient medium [17]. Each sample was then immediately transferred to −80 °C and stored until further analysis.
Tear collection
Tear samples of the patients and controls were carefully collected (to prevent the stimulation of tear secretion) during the morning hours using Schirmer’s strips during the Schirmer test procedure without anesthesia. Only the right eye was used for tear sample collection. A 15-mm test result was considered sufficient for biochemical analysis (according to the literature [18] and our preliminary tests). To ensure equal sample collection in all patients, we cut the strips at the 15-mm level after the procedure, placed them in Eppendorf tubes, and diluted them with 200 μl of previously cooled PBS to prevent sample evaporation. If the amount of a participant’s tears was insufficient, the strips were kept on their lower eyelid for up to 10 min until a total of 15 mm of tears was collected. Each sample was immediately stored at −80 °C after collection until further analysis.
Serum collection
Blood samples of the patients and controls were collected following 8–10 h of fasting in clot activator tubes. The blood samples were immediately centrifuged at 3800 ×g for 10 min, and the serum portion was then stored in 1.5 ml Eppendorf tubes at −80 °C until further analysis.
RT-PCR analysis
Measurement of TLR-4 expression
Epithelial cell samples were centrifuged at 466 ×g for 3 min for analysis. First, RNA isolation was performed on the PBMC and epithelial cell samples. A Total RNA Extraction kit (ELK Biotechnology, China) was used for PBMC samples, whereas the RNeasy Mini kit (Qiagen, Germany) was used for epithelial cell samples. The presence and integrity of the 16S and 28S rRNA bands were demonstrated by agarose gel electrophoresis of the total RNA isolate. The ratio of A260 nm/A280 nm in RNA quantification with nanodrops ranged between 1.8 and 2.0. Second, using the so-obtained RNA samples, cDNA synthesis was performed using the EntiLink™ 1st Strand cDNA Synthesis kit (ELK Biotechnology, China). A Biorad T100-branded (Bio-Rad, Hercules, CA) thermal cycler was used for cDNA synthesis. By measuring ssDNA with a nanodrop (Biotek Synergy HTX), a purity ratio of 1.8–2.0 and an amount of more than 50 ng/ml were achieved.
TLR-4 gene expression was quantified using a real-time PCR (RT-PCR) device and Syber Green dye. For the RT-PCR analysis, an ABI 7500 Real-Time PC device (Applied Biosystem) and EnTurbo™ SYBR Green PCR SuperMix (ELK Biotechnology, China) kit were used. The synthesized cDNA samples were placed on the block at 4 °C for 10 min for further use. The GAPDH gene was used as the control (housekeeping). We used SYBR Green Master Mix, Primer F, Primer R, PCR Grade H2O, and cDNA for each sample in appropriate cycles with the ABI 7500 Real-Time PCR instrument. The PCR program consisted of an initial denaturation step at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 10 s, and extension at 72 °C for 10 s, with a final extension at 95 °C for 30 s.. The primer sequences are shown in Table 1. The ct values of the TLR-4 mRNA samples were analyzed using the 2-ΔΔCt method.
Table 1. List of primers used in the study.
| Gene | Primer sequences (5′-3′) |
|---|---|
| TLR-4 |
F: GTGCCTCCATTTCAGCTCTG |
| TLR-4 |
R: CAAAGATACACCAGCGGCTC |
| GAPDH |
F: GAAGGTGAAGGTCGGAGTCAAC |
| GAPDH | R: CAGAGTTAAAAGCAGCCCTGGT |
Oxidative stress analysis
All tear samples were centrifuged at 3800 ×g for 10 min before the analysis, and the liquid portion was transferred to a new Eppendorf tube. All tear and serum plasma samples were simultaneously analyzed. Oxidative stress parameters (total antioxidant status [TAS], total oxidant status [TOS], and arylesterase [ARE]) were measured using commercial kits (Rel Assay Kit Diagnostics, Turkey) and a fully automated spectrophotometer (Roche/Hitachi Modular, Mannheim, Germany).
Measurement of TAS [19]:
The tear and serum TAS levels were determined using an automated colorimetric measurement method based on the bleaching of the characteristic color of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) radical cation. Trolox, a water-soluble analog of vitamin E, was used as a calibrator. This method determines the antioxidant effect of the sample against strong free radical reactions initiated by the hydroxyl radical product. The results were expressed in mmol Trolox equivalents/L.
Measurement of TOS [20]:
The tear and serum TOS levels were determined using an automated colorimetric measurement method. Oxidants present in the sample oxidized ferrous o-dianisidine complexes into ferric ions, and the oxidation reaction was enhanced by glycerol molecules. The ferric ions formed a colored complex with xylenol orange, an acidic medium. Therefore, the color intensity, which was measured spectrophotometrically, was related to the total number of oxidant molecules present in the sample. Hydrogen peroxide (Sigma Aldrich, Germany) was used for calibration, and the results were expressed in micromolar hydrogen peroxide equivalent per liter (µmol H2O2 equiv/l).
Oxidative stress index (OSI) calculation [20]:
The ratio of TOS/TAS level was rendered as the OSI. The results were expressed as an “arbitrary unit” (AU) and calculated according to the following formula: OSI = TOS (μmol H2O2 equiv/l)/TAS(mmol Trolox equiv/L) × 10.
Measurement of ARE [21]:
The ARE activities of the tears and serum were measured using phenylacetate as the substrate. Enzymatic activity was calculated from the molar absorptivity coefficient (1,310 M−1•cm−1) of the produced phenol. One unit of ARE activity was defined as 1 μmol phenol generated/min under the above-defined assay conditions and expressed as kU/L serum.
Statistical analysis
SPSS Statistics 22.0 was used to calculate the means and standard deviations of the variables. Comparison plots were made using the Graphpad Prism 5 program (Graphpad Software, San Diego, CA). The Shapiro–Wilk test was used to determine the normality of the data. An independent sample t test or Kruskal–Wallis test was selected to compare the groups, considering the data normality. In addition, for correlation analysis, the Pearson test was used for normal values, and the Spearman test was used otherwise. A p value lower than 0.05 was considered statistically significant.
Results
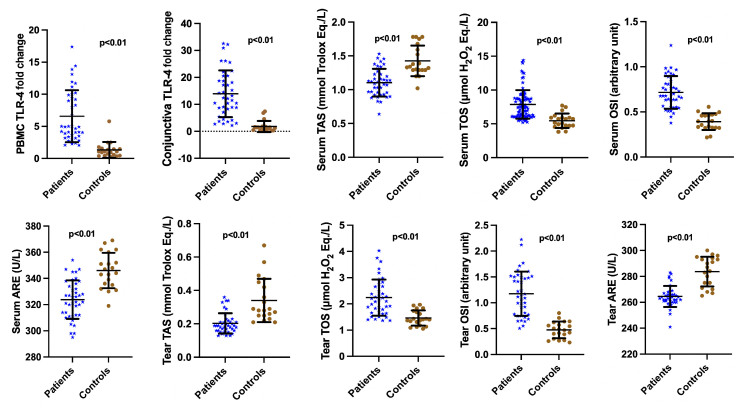
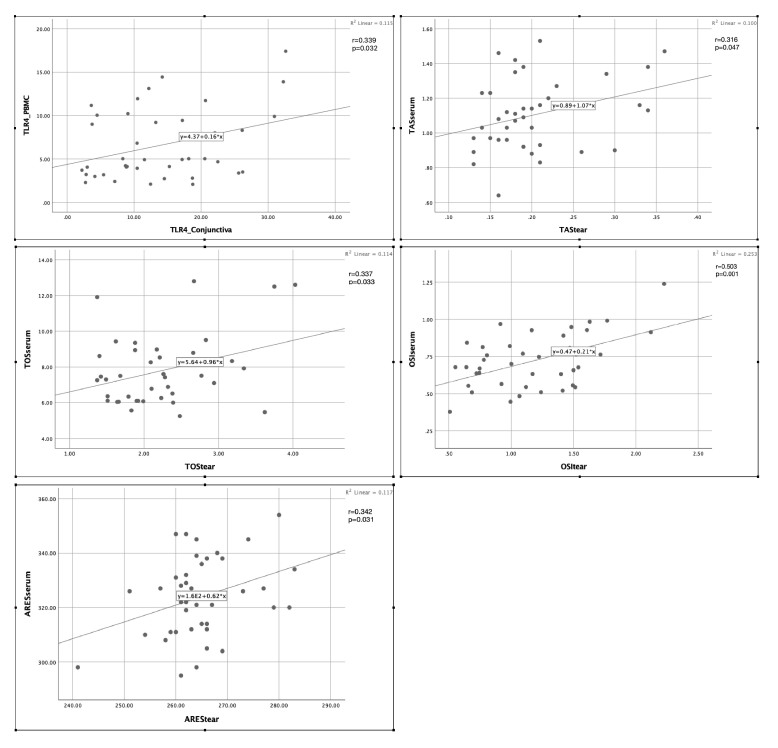
This study included 40 patients with OR (21 females, 19 males) who met the inclusion and exclusion criteria and 20 healthy controls (11 females, 9 males; p = 0.54). The mean ages were not significantly different between the patients (52.64 ± 8.27 years) and controls (52.90 ± 7.48 years; p = 0.91). A comparison of the TLR-4 expression levels (obtained using RT-PCR) between the patients and the controls is shown in Figure 1. TLR-4 expression in conjunctival epithelium and in PBMC was higher in patients with OR than in healthy controls (p < 0.01). A significant positive correlation was found between TLR-4 expression in the conjunctival epithelium and in PBMC (p < 0.05; Figure 2).
Figure 1.
Comparison of toll-like receptor-4 (TLR-4), total antioxidant status (TAS), total oxidant status (TOS), oxidative stress index (OSI), arylesterase (ARE) levels between patients and controls. Conjunctival epithelium and PBMC TLR-4 levels were significantly higher in patients with OR compared with the controls (p < 0.001). Serum and tear TAS, TOS, OSI, and ARE levels were significantly different between patients with OR and the controls (p < 0.001).
Figure 2.
Correlation of TLR-4, TAS, TOS, OSI, and ARE levels between ocular and blood samples. Significant positive correlations were found between conjunctival epithelium and PBMC TLR-4 levels and between serum and tear TAS, TOS, OSI, and ARE levels of patients with OR (p < 0.05).
A comparison of oxidative stress parameters between the patients and the controls is shown in Figure 1. The tear and serum TOS and OSI levels were significantly higher (p < 0.01), whereas TAS and ARE values were significantly lower (p < 0.01) in patients with OR than in healthy controls. A positive correlation was found between the tear and serum TAS, TOS, OSI, and ARE values (p < 0.05; Figure 2).
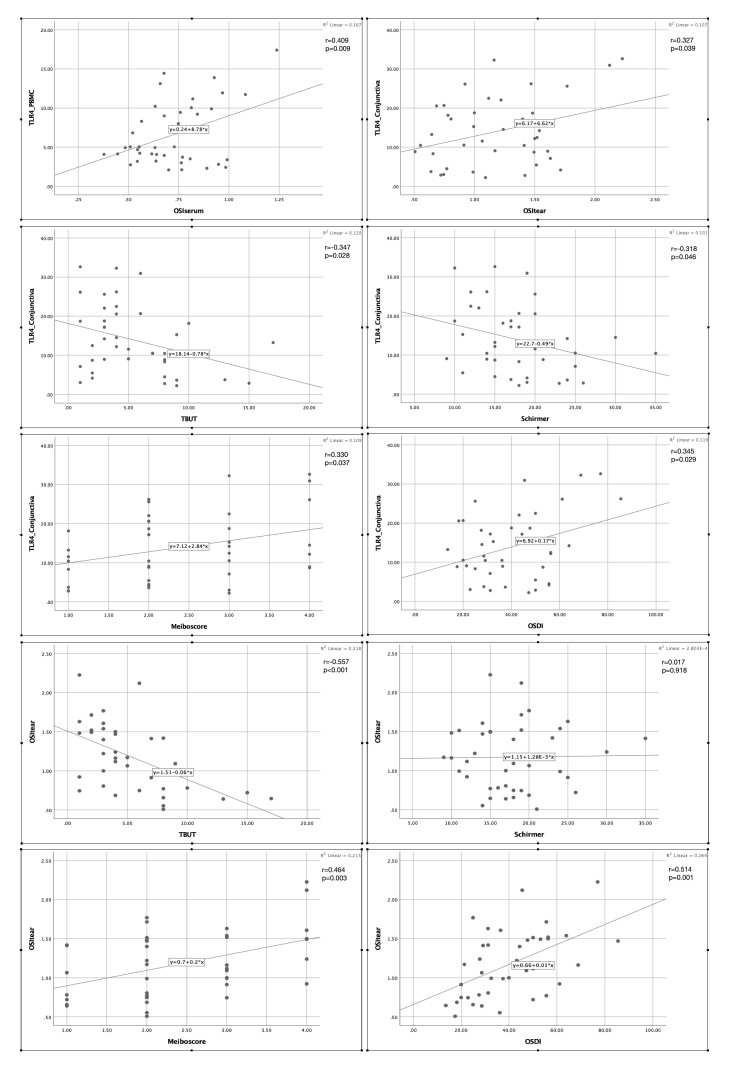
When investigating the relationship between TLR-4 expression levels and oxidative stress status, we identified strong positive correlations between TLR-4 expression and OSI values in both ocular and blood samples (p < 0.05; Figure 3). In addition, a significant negative correlation was found between TLR-4, OSI, and TBUT and between TLR-4 and Schirmer score, and a significant positive correlation was determined between OSI, TLR-4, and meiboscore and between TLR-4, OSI, and OSDI (p < 0.05; Figure 3). No correlation was found between OSI and Schirmer score.
Figure 3.
Correlation of TLR-4 levels with OSI levels and ocular surface parameters. Significant positive correlations were found between conjunctival epithelium TLR-4, tear OSI, and meiboscore and between conjunctival epithelium TLR-4, tear OSI, and OSDI score, whereas negative correlations were observed between conjunctival epithelium TLR-4, tear OSI, and TBUT and between conjunctival epithelium TLR-4 and Schirmer (p < 0.05). No correlation was noted between tear OSI and Schirmer (p = 0.92).
Discussion
The inflammatory component is one of the hallmarks in the pathogenesis of rosacea, although the exact triggering factors and their sequential roles remain to be understood [7]. TLRs, a family of pattern-recognition receptors triggered by pathogen-derived and tissue damage-related ligands, are not only expressed in immune cells but are also ubiquitously expressed in cells, including corneal and conjunctival cells [22]. Stimulation of TLRs by UV (a potential activator of reactive oxygen species [ROS]), oxidized lipids, saturated fatty acids [8], and S100A9 [23,24] activates various inflammatory pathways, including NF-κB, leading to the production of cytokines, such as IL-1α, IL-1β, IL-18, and IL-33, and antimicrobial peptides, such as β-defensins [25,26]. In ocular tissues, TLR-2 and -4 are constitutively expressed in the corneal, limbic, and conjunctival epithelial cells and are associated with inflammation [27,28]. In the eyelid biopsies of patients with OR, TLR-4 expression is significantly higher compared with that in normal subjects [29]. In the skin samples of patients with rosacea, TLR-2, which is activated by TLR-4, is overexpressed, and IL-8, IL-1β, and TNF-α production is increased [30]. In our current study, based on this extensive information and assuming that ROS/oxidized lipids can trigger the TLR-4-mediated inflammatory and vascular degeneration cascade [23], we examined the expression level of TLR-4 in both the conjunctival epithelium and PBMC of patients with OR and its relationship with oxidative stress status. We found that the expression of TLR-4 has increased in both types of cells from patients with OR and determined a good correlation between TLR-4 expression levels and oxidative stress status. This study cannot determine whether TL4 expression is caused by or a consequence of oxidative damage. However, notably, both TLR-4 overexpression and prooxidative status are observed at early disease stages and can thus contribute to disease pathogenesis. Indeed, ROS cause vascular changes and inflammation in rosacea and are associated with the oxidative modification of proteins and lipids, changes in lipid balance, alterations in antimicrobial peptides, such as cathelicidin LL-37, and the release of cytokines and other inflammatory mediators, such as IL-1 and TNF-α [31,32]. Particularly in skin rosacea, the levels of ROS and lipid peroxidation products are elevated, and the levels of antioxidant enzymes are reduced [33-35]. Our results are consistent with previous findings showing that both systemic and local deregulations occur in the OR.
Recent works have shown that among TLR-4-activating ligands, S100A8/9 proteins play an important role in keratinocytes and inflammatory cells, mediating the release of cytokines [24]. The expression of S100A8 and S100A9 is increased in human meibomian gland dysfunction, indicating that they may be early activators of the TLR-4 pathway in OR [36]. In aging endothelial cells, the S100A9/TLR-4 pathway is involved in vascular senescence [23], which can also contribute to the vascular features of OR. Interestingly, S100A8 is induced by corticoids in inflammatory cells [37], and we showed that S100A8/A9 are upregulated by the activation of the mineralocorticoid pathway [38], creating a possible mechanistic link between corticoids and OR. Among other triggering factors of OR, meibomian gland dysfunction is well recognized with changes in fatty acid composition, which can also activate TLR-4 [8]. Infectious agents and UV exposure also contribute to OR and activate TLR-4. Once activated, TLR-4 signaling leads to the production of proinflammatory cytokines and free oxygen species, amplifying the vicious cycle.
The early pathogenic role of oxidized components/TLR-4 cascade is supported by the good correlation found in our cohort among TLR-4 expression (both in conjunctiva and PBMC), oxidative stress markers, and ocular surface parameters, such as TBUT, OSDI, and meiboscore, which are also markers of OR severity. These results support the role of TLR-4 as a potential interventional target in OR. Further interventional studies, such as targeting TLR-4 or its upstream activators, are necessary to confirm this hypothesis.
Acknowledgments
This work was supported by Gazi University Scientific Research Projects Unit (project number: TDK-2022-7379) and by TÜBİTAK-1002 Fast Support Program (project number: 222S346) for the conduct of the biochemical and RT-PCR analysis.
References
- 1.Gold LM, Draelos ZD. New and Emerging Treatments for Rosacea. Am J Clin Dermatol. 2015;16:457–61. doi: 10.1007/s40257-015-0156-2. [DOI] [PubMed] [Google Scholar]
- 2.Rainer BM, Kang S, Chien AL. Rosacea: Epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:e1361574. doi: 10.1080/19381980.2017.1361574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo YR, Lim JH, Cho DH, Park HJ. Rosacea: molecular mechanisms and management of chronic cutaneous inflammatory condition. Int J Mol Sci. 2016;17:156. doi: 10.3390/ijms17091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, Mess C, Cevikbas F, Rivier M, Carlavan I, Déret S, Rosignoli C, Metze D, Luger TA, Voegel JJ. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2–11. doi: 10.1038/jidsymp.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavassoli S, Wong N, Chan E. Ocular manifestations of rosacea: A clinical review. Clin Exp Ophthalmol. 2021;49:104–17. doi: 10.1111/ceo.13900. [DOI] [PubMed] [Google Scholar]
- 6.Redd TK, Seitzman GD. Ocular rosacea. Curr Opin Ophthalmol. 2020;31:503–7. doi: 10.1097/ICU.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues-Braz D, Zhao M, Yesilirmak N, Aractingi S, Behar-Cohen F, Bourges JL. Cutaneous and ocular rosacea: Common and specific physiopathogenic mechanisms and study models. Mol Vis. 2021;27:323–53. [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211–5. doi: 10.1016/j.atherosclerosis.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Moura AKA, Guedes F, Rivitti-Machado MC, Sotto MN. Inate immunity in rosacea. Langerhans cells, plasmacytoid dentritic cells, Toll-like receptors and inducible oxide nitric synthase (iNOS) expression in skin specimens: case-control study. Arch Dermatol Res. 2018;310:139–46. doi: 10.1007/s00403-018-1806-z. [DOI] [PubMed] [Google Scholar]
- 10.Wladis EJ, Carlson JA, Wang MS, Bhoiwala DP, Adam AP. Toll-like receptors and vascular markers in ocular rosacea. Ophthalmic Plast Reconstr Surg. 2013;29:290–3. doi: 10.1097/IOP.0b013e318293764c. [DOI] [PubMed] [Google Scholar]
- 11.Tisma VS, Basta-Juzbasic A, Jaganjac M, Brcic L, Dobric I, Lipozencic J, Tatzber F, Zarkovic N, Poljak-Blazi M. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270–6. doi: 10.1016/j.jaad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Sundaram C, Köster W, Schallreuter KU. The effect of UV radiation and sun blockers on free radical defence in human and guinea pig epidermis. Arch Dermatol Res. 1990;282:526–31. doi: 10.1007/BF00371948. [DOI] [PubMed] [Google Scholar]
- 13.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Muhafız E, Aslan Bayhan S, Bayhan HA, Çölgeçen E, Gürdal C. Evaluation of Meibomian Glands in Cutaneous Rosacea. Med J SDU. 2021;28:621–6. [Google Scholar]
- 16.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 17.Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol 2009; Chapter 7:7.1.1–7.1.8. [DOI] [PubMed] [Google Scholar]
- 18.Balmus IM, Alexa AI, Ciuntu RE, Danielescu C, Stoica B, Cojocaru SI, Ciobica A, Cantemir A. Oxidative stress markers dynamics in keratoconus patients’ tears before and after corneal collagen crosslinking procedure. Exp Eye Res. 2020;190:107897. doi: 10.1016/j.exer.2019.107897. [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Haagen L, Brock A. A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem. 1992;30:391–5. doi: 10.1515/cclm.1992.30.7.391. [DOI] [PubMed] [Google Scholar]
- 22.Erdinest N, Aviel G, Moallem E, Anteby I, Yahalom C, Mechoulam H, Ovadia H, Solomon A. Expression and activation of toll-like receptor 3 and toll-like receptor 4 on human corneal epithelial and conjunctival fibroblasts. J Inflamm (Lond) 2014;11:3. doi: 10.1186/1476-9255-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B, Yu J, Luo Y, Xie M, Qu C, Shi Q, Wang X, Zhao X, Kong L, Zhao Y, Guo Y. Deficiency of S100 calcium binding protein A9 attenuates vascular dysfunction in aged mice. Redox Biol. 2023;63:102721. doi: 10.1016/j.redox.2023.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Möller A, Jauch-Speer SL, Gandhi S, Vogl T, Roth J, Fehler O. The roles of toll-like receptor 4, CD33, CD68, CD69, or CD147/EMMPRIN for monocyte activation by the DAMP S100A8/S100A9. Front Immunol. 2023;14:1110185. doi: 10.3389/fimmu.2023.1110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou A, Tin MQ, Tong L. Toll-like receptor 2-mediated NF-kappa B pathway activation in ocular surface epithelial cells. Eye Vis (Lond) 2017;4:17. doi: 10.1186/s40662-017-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp Eye Res. 2011;92:209–20. doi: 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Shen J, Beuerman RW. Expression of toll-like receptors in human limbal and conjunctival epithelial cells. Mol Vis. 2007;13:813–22. [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed I, Said DG, Dua HS. Human antimicrobial peptides in ocular surface defense. Prog Retin Eye Res. 2017;61:1–22. doi: 10.1016/j.preteyeres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Wladis EJ, Carlson JA, Wang MS, Bhoiwala DP, Adam AP. Toll-like receptors and vascular markers in ocular rosacea. Ophthalmic Plast Reconstr Surg. 2013;29:290–3. doi: 10.1097/IOP.0b013e318293764c. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, Cogen AL, Gallo RL. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–97. doi: 10.1038/jid.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DA. Rosacea, reactive oxygen species, and azelaic Acid. J Clin Aesthet Dermatol. 2009;2:26–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Oztas MO, Balk M, Ogüs E, Bozkurt M, Ogüs IH, Ozer N. The role of free oxygen radicals in the aetiopathogenesis of rosacea. Clin Exp Dermatol. 2003;28:188–92. doi: 10.1046/j.1365-2230.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 33.Tisma VS, Basta-Juzbasic A, Jaganjac M, Brcic L, Dobric I, Lipozencic J, Tatzber F, Zarkovic N, Poljak-Blazi M. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270–6. doi: 10.1016/j.jaad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Baz K, Cimen MY, Kokturk A, Aslan G, Ikizoglu G, Demirseren DD, Kanik A, Atik U. Plasma reactive oxygen species activity and antioxidant potential levels in rosacea patients: correlation with seropositivity to Helicobacter pylori. Int J Dermatol. 2004;43:494–7. doi: 10.1111/j.1365-4632.2004.02137.x. [DOI] [PubMed] [Google Scholar]
- 35.Sundaram C, Köster W, Schallreuter KU. The effect of UV radiation and sun blockers on free radical defence in human and guinea pig epidermis. Arch Dermatol Res. 1990;282:526–31. doi: 10.1007/BF00371948. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Richards SM, Lo K, Hatton M, Fay A, Sullivan DA. Changes in gene expression in human meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2727–40. doi: 10.1167/iovs.10-6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu K, Passey RJ, Endoh Y, Rahimi F, Youssef P, Yen T, Geczy CL. Regulation of S100A8 by glucocorticoids. J Immunol. 2005;174:2318–26. doi: 10.4049/jimmunol.174.4.2318. [DOI] [PubMed] [Google Scholar]
- 38.Canonica J, Mehanna C, Bonnard B, Jonet L, Gelize E, Jais JP, Jaisser F, Zhao M, Behar-Cohen F. Effect of acute and chronic aldosterone exposure on the retinal pigment epithelium-choroid complex in rodents. Exp Eye Res. 2019;187:107747. doi: 10.1016/j.exer.2019.107747. [DOI] [PubMed] [Google Scholar]