Abstract
Rural women face an increased risk of cervical cancer diagnosis in comparison to women living in metropolitan areas. This review synthesized and critically evaluated cervical cancer screening interventions that target women living in rural communities in the USA. EBSCO, JSTOR, Medline, PsychINFO, Psychology and Behavioral Sciences Collection, PubMed, and Cochrane Library were searched using keywords related to cervical cancer screening, rural communities, and prevention interventions. Study eligibility included randomized controlled trials or quasi-experimental designs, a psychosocial or educational intervention targeting cervical cancer prevention, and implementation in a rural setting. Eleven articles met criteria for the systematic review and 6 of those included information sufficient for meta-analysis. Cochrane guidelines, CONSORT-Equity 2017, and PROGRESS-Plus were used to assess included studies. The systematic review encompassed 9720 participants who were involved in a variety of intervention types: social media campaigns, faith-based, and patient navigation with lay health advisors. None of the studies met all criteria for the health equity assessment. The meta-analysis found that women in the intervention groups were more likely to participate in cervical cancer screening than women in control groups (OR: 2.43, 95% CI: 1.49 to 3.97). The type of intervention mattered in increasing cervical cancer screening participation for women living in rural communities. Educational interventions in combination with patient navigation saw the most success in promoting cervical cancer screening. Further, health inequities focus is lacking robust consideration. Our results highlight a continued need to develop multicomponent interventions with a health equity focus to address barriers to screening and prevention.
Keywords: Cervical cancer screening, Cervical cancer prevention, Health promotion, Health equity
Introduction
Women in rural America are more likely to be diagnosed with cervical cancer, at all stages of the disease, and to die from cervical cancer than their counterparts in urban America (SEER, 2010–2014). This excess disease burden has many explanations, from differences in attitudes towards prevention, to lack of providers and access to specialized care [1, 2]. Interventions to address these disparities have been developed and tested but to date, there has been no synthesis or meta-analysis of this literature. To address this gap, we conducted a meta-analysis of cervical cancer prevention efforts in rural communities and draw attention to promising interventions.
Cervical cancer is caused by specific types of the human papillomavirus (primarily, HPV 16/18). Because HPV is a common infection transmitted most often during sexual activity, efforts to reduce its spread through vaccination become entangled with conservative and religious beliefs [3–5]. Regular gynecological examinations, including the Pap smear, are effective at detecting pre-cancerous lesions and early-stage cancer but require that abnormal results be followed up with more specialized providers. Advancements in HPV DNA testing and home-based self-sampling for HPV allow for less frequent screening and easier access, but the rollout of these tests is unequal across the world [6].
The studies selected for this meta-analysis were conducted in small towns across the USA and reflect the diversity of what it means to be “rural.” The US Department of Agriculture Economic Research Service identifies more than two dozen definitions of “rural.”7 Common among the definitions is that rural communities tend to be smaller, often surrounded by farm and grazing lands, with populations with less formal education, and lower household incomes. Importantly, medical and health interventions are often received with mistrust and wariness by rural dwellers.7, 8 Further, there exist many cultural variations among rural communities. Small towns in the desert west, whose residents may not speak English as their primary language and who draw their cultural norms from south and central America, may differ starkly from the small towns in Appalachia, which tend to be populated mostly by Caucasian descendants of Scotch-Irish who hold beliefs consistent with their ancestors. Indigenous territories, managed by the Indian Health Services, are chronically underfunded and understaffed [7], resulting in disparities among Native Americans compared to White counterparts in cancer control [8].
Public health interventions to reduce rural disparities in cervical cancer must be situated firmly within the communities where they are implemented and may not be effective outside of those communities [9]. Therefore, the context of the rural community needs to be considered when assessing the effectiveness of public health interventions to reduce disparities in cervical cancer. Community-based research designs that incorporate local representation in the study planning, implementation, and evaluation often achieve better engagement and trust by the local population. In some rural areas, such as Appalachia, where churches play large roles in the lives of the residents, interventions succeed to the extent in which they are endorsed by the local ministers and pastors [10]. The Community Prevention Services Task Force’s (CPSTF) Community Guide emphasizes the effectiveness of increasing cancer screening by involving patient navigation [11], community health workers [11], and multicomponent interventions [12]. The CPSTF proposes that effective client-oriented screening interventions use client reminders, one-on-one education, and small media campaigns. Provider-oriented interventions are effective when they use provider assessment and feedback strategies as well as provider reminder and recall systems. Multicomponent interventions consist of components that increase community demand, increase community access, and/or enhance provider delivery. Notably, these interventions are specifically designed to tackle structural barriers, contributing to their efficacy in promoting cancer screening and proving to be cost-effective.
Knowing that rural communities are hardly uniform, that several preventive methods exist, and that outcome measurements are imperfect, this study attempts to measure how successful recent efforts have been at increasing cervical cancer screening and prevention through pap smear screening adherence and HPV vaccination. In this paper, cervical cancer screening and prevention behavior include pap smear screening adherence and HPV vaccination initiation.
Research Aims
The overall purpose of the study is to systematically review health education and awareness interventions targeting cervical cancer prevention and detection efforts directed toward women living in rural communities. We have three aims: (1) to describe the characteristics of studies that evaluate effectiveness of health education programs for cervical cancer prevention in rural communities; (2) to compare the effectiveness of the interventions; and (3) to determine if the reporting of the studies adequately addressed health equity according to the CONSORT-Equity reporting standards.
Methods
This review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) [13].
Search Strategy
Eligible studies evaluated the impact of health education programs designed to promote cervical cancer screening and the HPV vaccination with women living in rural communities in the USA. Electronic databases (EBSCO, JSTOR, Medline, PsychINFO, Psychology and Behavioral Sciences Collection, PubMed, and The Cochrane Library) were searched using a combination of key terms: community-based intervention, HPV, cervical cancer, screening, psychosocial, control trial, randomized control, quasi-experimental, prevention, community-based workers, community-based participatory research, CBPR, HPV vaccine, vaccination, community, education intervention, rural, and pap smear in the abstracts. References to articles related to psychoeducational cervical cancer interventions in other systematic reviews were also searched. The search was restricted to studies published in peer-reviewed journals between 2000 and January 2023.
Eligibility Criteria
To be included, studies had to meet the following criteria: (1) be a randomized controlled trial (RCT) or quasi-experimental design with a control group; (2) include a psychosocial or educational intervention focused on either cervical cancer screening or prevention; (3) include a sample of women above the age of 18 years old; (4) be implemented in a rural setting in the USA; and (5) be reported in a peer-reviewed journal. Since dissertations did not meet criterion 5, they were excluded. Intervention settings were determined by whether the articles’ authors self-reported the location as being a part of the rural USA.
The potential list of articles was divided among team members. Each member independently selected potentially eligible studies. During the initial stage of the review, articles were assessed on their titles and abstracts, full-text articles were evaluated after having passed the initial stage of eligibility. The team discussed any studies that members questioned eligibility.
The search yielded 5788 articles. Fig. 1 shows the flow of the study identification, retrieval, and the number of eligible articles. Five investigators independently reviewed the records (title and abstract) of 212 articles that were identified by the search and excluded 115 abstracts that did not meet the eligibility criteria. The remaining 97 articles were reviewed in their entirety and 11 studies were included in the sample.
Fig. 1.
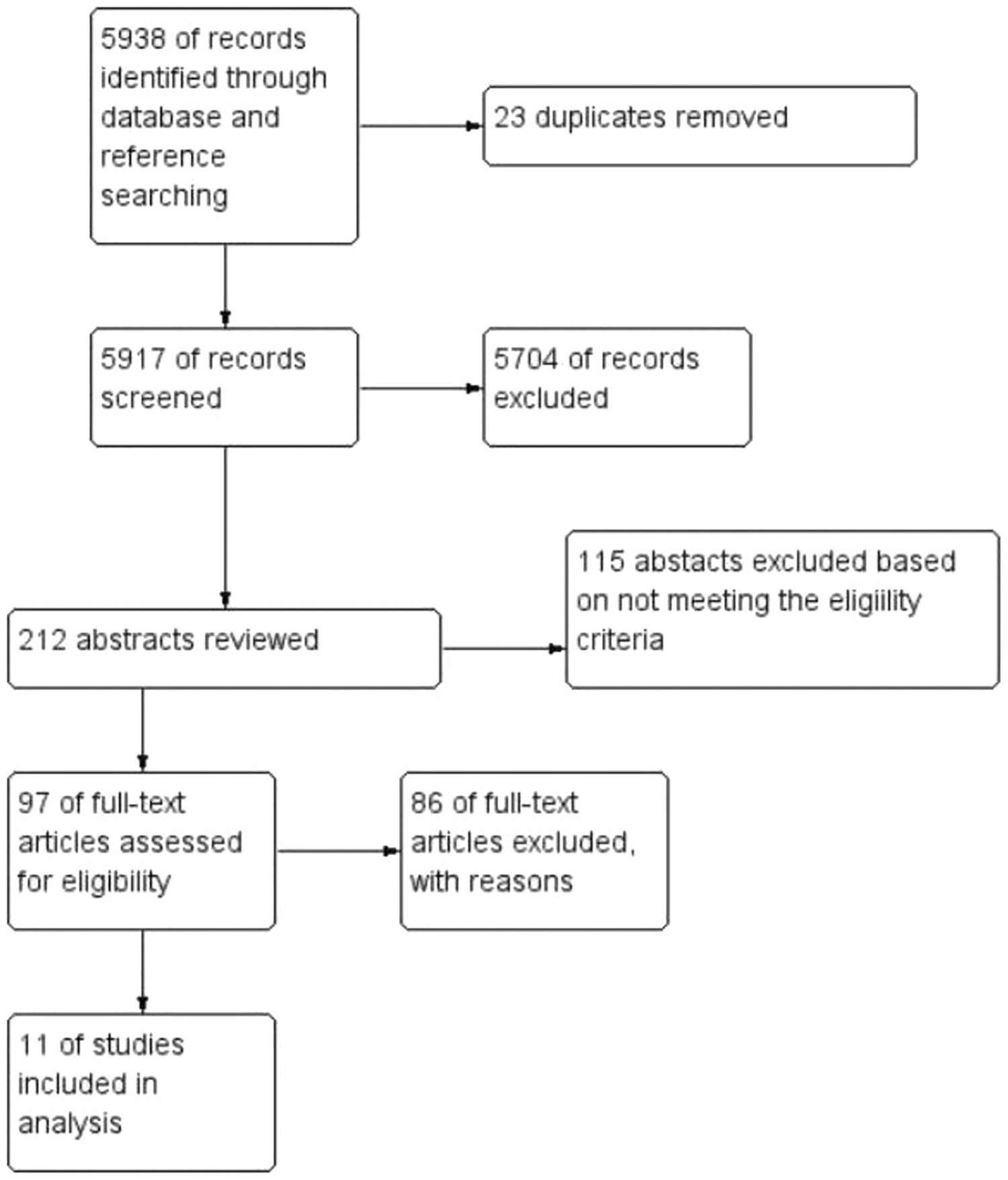
Study flow diagram of the 11 studies reviewed
Data Extraction
A data extraction sheet was developed and internally validated by team members who pre-tested the sheet on randomly chosen articles. All reviewers showed consistency and accuracy in the use of the data extraction sheet. Data extracted from the studies were demographics of the participants, setting, focus of study, theoretical framework, inclusion criteria, sampling method (i.e., probabilistic or non-probabilistic), dropout, attrition, data collection (self-reported or medical review), design, intervention, interventionist, measures, health outcomes, effect size, validity, reliability, and limitations or weaknesses of the studies.
Items related to the risk of bias in the studies were also entered in the data extraction sheet. Following Cochrane guidelines,19 the items included biases related to selection, performance, detection, attrition, and reporting. An example of potential bias from the Cochrane guidelines being the use of random sequence generation in assigning intervention or control status to participants. They were assessed for having low risk, unclear risk, and high risk of bias.
The data extraction sheet also included items of health equity which were based on CONSORT-Equity 2017.20 This guide builds on the original CONSORT 2010, which provides guidelines for presenting results of systematic reviews and meta-analyses. CONSORT-Equity 2017 guides users to capture social and demographic characteristics of participants and disadvantaged populations and to assess the adequacy of randomized trials in addressing health inequities in these populations. Table S1 (supplemental) lists the CONSORT-Equity items that were included in the data extraction.
After developing and testing the data extraction sheet, the articles were divided among 4 reviewers; two reviewers were assigned to each article. The two reviewers would discuss any discrepancies in entering the data and reach a consensus. If consensus was not reached, the discrepancy was resolved by a third reviewer.
Data Analysis
Demographics were extracted from the studies to describe the participants’ characteristics. For our meta-analysis, our primary outcomes of interest, as measured by self-report or medical record review, were cervical cancer screening adherence (yes or no) and HPV vaccination (yes or no). A meta-analysis of the outcomes was conducted when sufficient data could be extracted from the included studies, based on the number of women screened or vaccinated versus the control. We then entered the data into Review Manager (RevMan) software for meta-analysis [14].
Because of the dichotomous outcomes of screening or no screening, vaccination or no vaccination, odds ratio was deemed appropriate for interpreting the effect size of the interventions. Odds ratios allow for the interpretation of whether the intervention results in an increased likelihood of being screened or vaccinated versus the control groups. A random effects model was used when calculating and pooling the effect sizes of the included data. The random effects model was used for two reasons: based on the I2 statistic showing heterogeneity between the studies and because there was an estimated clinical diversity among the included studies. The random effects model assumes that the included studies are estimating different but related intervention effects. Since each included study had a different intervention, using the random effects model was the more conservative option.
Results
Sample
Figure. 1 displays the study flow of the literature search. A total of 5788 titles were initially retrieved, and 151 additional titles were identified through hand-searching reference lists and other cervical cancer systematic reviews. After removing duplicates, 5917 titles and abstracts were screened. From those 5917 titles, 212 abstracts were considered to either be a yes for further review or a maybe; conflicts were resolved between each reviewing team through discussion, with a third reviewer weighing in. Of the 212 abstracts that were reviewed, 115 were excluded for not meeting criteria, and 97 full articles were reviewed. Of the 97 full-text articles review, 86 were excluded for not meeting criteria leaving only 11 articles for our review.
Study Characteristics
Table 1 summarizes the characteristics of the 11 studies that met our eligibility criteria. There were 9720 participants in the studies. Participants’ ages ranging from 22 to 65, with the majority choosing some high school education as their highest educational attainment, and most participants were low to middle income. Over half of the studies included Hispanic women as the majority of participants [15–20], while five of the studies had non-Hispanic White women as the majority [9, 21–23]. None of the studies had a majority of Black women or American Indian/Alaskan Native women in their samples. Three studies used probabilistic sampling [17, 21, 22], and seven used non-probabilistic [15, 16, 18, 20, 23–26].
Table 1.
Summary table of studies reviewed
| Study (references) | Study type sample/location | Theoretical framework | Intervention | Primary outcomes and measures | Intervention effectiveness |
|---|---|---|---|---|---|
| Cates, Shafer, Diehl, Deal (2011) | 2-arm cluster; mothers with daughters ages 11–12 (n=225); North Carolina | Ecological Model | Social marketing campaign about HPV vaccination targeting mothers and healthcare practices | Campaign awareness; website traffic, hotline calls, media placement, and HPV immunization rates; cross-sectional survey | 82% of mothers heard or saw campaign messages or materials; 94% of providers used campaign brochures; HPV vaccination rates rose in 2 of the 4 intervention counties compared to 9 non-intervention counties; mothers who reported some campaign awareness were more likely to take action than mothers who were unaware (71% vs. 22%, p < .001) |
| Falk, Foley, Weaver, Jones, Cubbin (2022) | 3-arm Quasi-experimental; women ages 21–64 (n=6169); Texas | Psycho-educational; Not clearly identified | Education only; PN only; Both PN and education; PN included navigating participant barriers to screening such as cost and transportation | Self-reported receipt of pap test | Education only: English speaking Latina women had an 18% probability of receiving a Pap test; non-Latina White women had a 50% probability. Education and PN: Spanish speaking Latina women had the highest probability (70%) followed by non-Latina White (62%), non-Latina Black (60%), and English-speaking Latina women (50%). Women aged 21–39 had higher odds of screening (OR: 1.40, CI: 1.23–1.59) compared to older women; English-speaking Latina women (0R:0.51, CI 0.40–0.64) had lower odds compared to non-Latina White women |
| Kepka, Coronado, Rodriguez, Thompson (2011) | RCT; Hispanic parents of daughters aged 9–17 (n = 88); Washington | Community-based participatory research approach | Culturally tailored Spanish radionovela about HPV vaccine | Knowledge of HPV, cervical cancer, and HPV vaccine; attitudes, decision-making, and intention to receive HPV vaccine. Pre- and posttest participant questionnaire |
Parents who listened to the HPV radionovela scored higher on 6 knowledge and belief items than control group parents. The HPV vaccine radionovela improved HPV and vaccine knowledge and attitudes. Less acculturated parents in intervention arm improved in relation to self-efficacy for vaccine uptake (p < .05) |
| Krok-Schoen, Oliveri, Young, Katz, Tatum, Paskett (2016) | RCT; 90 women in need of Pap test randomized to lay health advisor (intervention arm) or usual care (control arm—letter from physician & brochure). 96.7% Caucasian Appalachia counties in Ohio |
Transtheoretical Model | 10-month lay health advisor (LHA) intervention with in-person visits, phone calls, and mailed postcards tailored to participant’s stage of change | Receipt of Pap test; self-report and medical record review; barriers to cervical cancer screening were measured by an author developed survey using eleven items based on previously reported barriers; stages of change for Pap testing was measured by participants’ self-reported responses to intention to receive a pap test | |
| Luque, Tarasenko, Reyes-Garcia, Alfonso, Suazo, Rebling, Ferris (2016) | 2-arm quasi-experimental feasibility study; Hispanic or Latina immigrant women aged 21–65 years and overdue for Pap test (n=90); Southeast Georgia | Social Cognitive Theory | Spanish language educational group session on cervical cancer led by promotoras; group activities included watching video, dialog about barriers to healthcare, resources, etc. | Cancer screening knowledge, cervical cancer beliefs, sources of health information, and self-efficacy; pre- and posttest surveys. Receipt of Pap test was self-reported | Twelve (32%) intervention group participants received Pap test compared to 10 (19%) control group participants (p = 0.178). Intervention participants were higher on both cervical cancer knowledge recall and retention than control group (p < 0.001) |
| Nuno, Martinez, Harris, Garcia (2011) | RCT; Hispanic women 50 or older, medically underserved (n=381); Yuma county, AZ | Social Cognitive Theory | Trained promotoras led classes about breast and cervical cancer prevention and community resources for screening | Self-reported mammogram and Pap smear screening Baseline survey identified barriers |
Women in intervention group were 1.5 times more likely to report having a Pap smear within the last year when compared with usual care group (although this was not statistically significant). Secondary analysis indicated a stronger effect on women who had not had a Pap smear within the past year at baseline |
| Paskett, Krok-Schoen, Pennell, et al. (2016) | Group-randomized trial; parents of daughters age 9–17 who had not received HPV vaccine (n=337), providers (n=119); Appalachian Ohio | Health Belief Model; Theory of Planned Behavior; Organizational Developmental Theory | Multi-level: clinic educational information; educational session for providers; packet with brochure and DVD video mailed to parents | Primary outcome: Medical record-confirmed receipt of first HPV dose 3 months after intervention. Secondary outcomes: receipt of first HPV vaccine dose by 6 months and changes in provider knowledge |
Ten (7.7%) daughters of intervention participants received first dose of HPV vaccine within 3 months compared to 4 (3.2%) daughters of comparison group (p = 0.061). By 6 months, 17 (13.1%) daughters in intervention group received first HPV vaccine compared to 8 (6.5%) daughters in comparison group (p = 0.002). Provider knowledge about HPV increased (p = 0.001) |
| Studts, Tarasenko, Schoenberg, Shelton, Hatcher-Keller, Dignan (2012) | RCT; women aged 40–64 and overdue for screening (N = 345); Appalachian Kentucky | Precede-Proceed | Luncheon with educational program on cervical cancer screening and prevention; LHAs home visits; newsletters addressing barriers to screening. | Primary outcome: Self-reported receipt of Pap test | Treatment group participants (17.6% screened) had over twice the odds of wait-list controls (11.2% screened) of reporting Pap test receipt post-intervention. Regardless of group, recently screened participants (1–5 years ago) had significantly higher odds of obtaining screening than rarely or never screened participants |
| Thompson, Coronado, Chen, Islas (2006) | Randomized group design (N =1962), Eastern Washington | Community-based Participatory Research (CBPR) | Comprehensive: community activities, organizational, small group and individual levels; health fairs, block parties, festivals, fun runs, education presentations; schools, religious organizations, worksites; wellness van; local clinics; home health parties | Cross-sectional surveys and in-person interviews measured: healthcare access, smoking behavior, eating patterns, cancer screening behavior, awareness of cancer events, questions about screening, acculturation | Few significant changes in use of Pap test between intervention and control communities; more awareness of and participation in intervention activities in the treatment communities than control communities |
| Thompson, Carosso, Jhingan, et al. et al (2017) | RCT; 443 Latina women (n=443); Yakima Valley in Washington State | CBPR | Two arms: (1) Spanish-language video; (2) Promotora-led educational session in home; watching video with promotora, making commitment and appointment for Pap test; sheet of resources for overcoming barriers; reminder magnet, appointment card | Knowledge and attitudes towards cervical cancer screening and risk factors assessed pre- and post-intervention; receipt of cervical cancer screening assessed by review of electronic medical records. Cost-effectiveness was measured by ratio comparing additional cost per participant screened with usual-care group |
Seven months after randomization, significantly more women in the promotora intervention received a Pap test (53.4%) compared to the video group (38.7%; p < .001) and usual care arm (34%; p < .01) |
| Vanderpool, Cohen, Crosby, Jones, Bates, Casey, Collins (2013) | RCT; women ages 18–26, not previously vaccinated against HPV (n=344); Appalachian Kentucky | Theory of Planned Behavior ; Motivation, behavioral skills model | After receiving first dose of HPV vaccine, participants in intervention group watched 13-minute educational DVD | Intent to complete vaccination series measured by questionnaire; Vaccine series completion rate measured by medical record review | Participants assigned to the intervention were 2.44 times more likely than women in the usual care group to complete the series. Women’s beliefs that all 3 doses reduced cancer risk predicted intent and completion. Intention predicted completion |
Outcomes for the studies included cervical cancer screening behaviors [16–20, 26] and HPV vaccination awareness and knowledge, and HPV vaccination uptake [15, 22, 23]. Both cervical cancer screening and HPV vaccination uptake were measured by self-reported measures [9, 15–17, 19] or medical record review [18, 22, 23].
Types of Interventions
Types of interventions included social media campaigns [25], novella health education [15], education led by lay health advisors/promotoras [17, 19, 21], a faith-based intervention [26], and an educational DVD [23]. One study had a multi-level program that included educational sessions for providers and parents [16], and one had comprehensive activities at the level of small groups, individuals, and community organizations [22]. The largest RCT involved three arms: education only, education and patient navigation by lay health advisors, and patient navigation only [20].
The goals of the interventions were primarily to focus on improving HPV vaccination uptake [15, 22, 23, 25] and improving cervical cancer screening rates [16–21, 26]. One study included other cancer prevention behaviors such as smoking, eating patterns, healthcare access, and cancer screening (breast and colorectal cancers) [19].
There were a variety of theoretical frameworks for the interventions: extended parallel process model [22], transtheoretical model [21], health belief model [21, 22, 25, 26], theory of planned behavior [23,] theory of reasoned action [22], organizational developmental [22], precede-proceed [21, 26], community-based participatory research [15, 18, 19], and social cognitive theory [16–19, 21, 26]. Many of these theories attempt to explain how social interactions and experiences impact heath behaviors; thus, suggesting that changing knowledge through education-based interventions will result in changed behavior.
Study Designs and Methods
Most of the studies randomized participants to either an intervention group or a control group/waitlist [15, 19, 21, 26] and one used a comparison intervention [18]. Two studies used randomized cluster designs in which counties or communities were matched and randomly assigned to an intervention or control arm [16, 22]. Another study allowed participants to choose which intervention arm they would participate in between education only, education and patient navigation by lay health advisors, and patient navigation only [20].
Effectiveness of Interventions
Six studies reported enough information for odds ratios to be calculated, comparing their intervention strategy against a control group. For some studies, there was not a clear definition of usual care, while one study used a waitlisting design which allowed participants to eventually receive the intervention [26]. Overall, when comparing the health education intervention with usual care, women in the intervention group were twice as likely to report engaging in screening practices (OR: 2.43, 95% CI: 1.49 to 3.97; I2 =94%). Fig. 2 shows the studies that had enough information to calculate effectiveness. One study[19] analyzed Hispanic/Latina and Caucasian participants separately. Hispanic/Latina women in a multi-level intervention were more likely to participate in pap smear screening than the usual care group (OR: 4.86, 95% CI: 2.80 to 8.42) [19]. Faith-based health navigation intervention was effective at increasing cervical cancer screening participation when compared with a wait-listed control group (OR: 2.26, 95% CI: 0.45 to 1.2) [26]. In Falk, Foley (20), the women who received both patient navigation and education were more likely to be screened than those participants who only received the education intervention (OR: 6.16, 95% CI: 5.22 to 7.27).
Fig. 2.
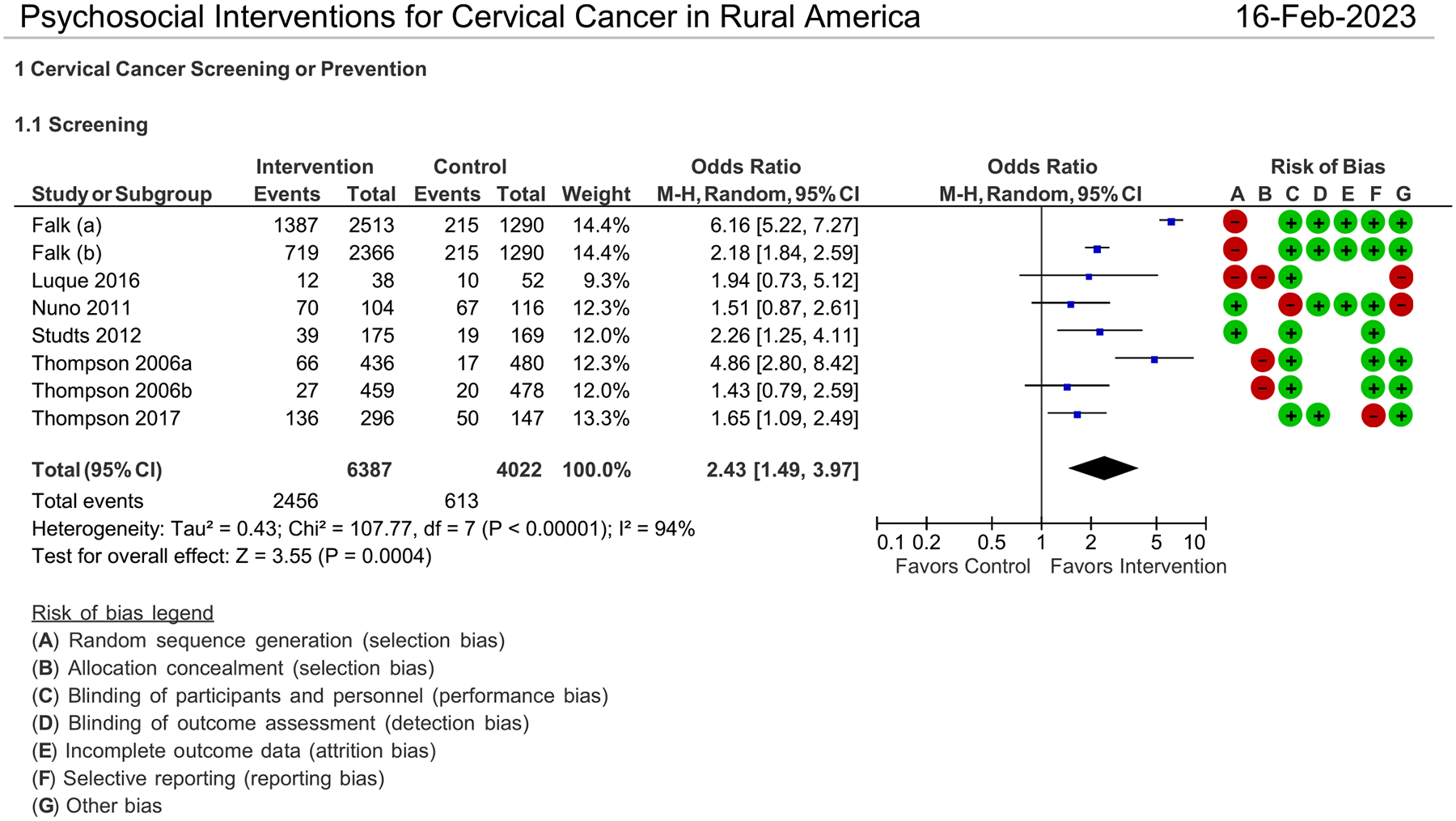
Psychosocial Interventions for Cervical Cancer in Rural America
Only two studies had sufficient information to compare the effectiveness of a prevention intervention, on HPV vaccine uptake among rural residents. However, neither study had significant findings.
Risk of Bias
Figure. 2 shows the risk of bias for each study, with green indicating minimal risk and red indicating high risk. Studies were generally low risk for bias, except for selection bias. Most studies did not randomly assign intervention or control groups which makes them susceptible to selection bias [27].
Reporting of Health Equity Factors
Table 2 presents the 22 items that were used to assess the reporting of health equity factors in the review articles. Most articles (90–100%) covered 9 of the 22 items in the reporting of the studies. These items included (1) a rational for focusing on health equity, (2) an objective with reference to health equity, (3) a proposed trial design to answer equality questions, (4) the context and relationship to health equity, (5) details of partnerships with populations and communities, (6) methods of recruitment to reach populations across relevant PROGRESS-Plus characteristics, (7) limitations related to assessing effects on health equity, (8) applicability related to the population of interest across PROGRESS-Plus characteristics, and (9) details of partnerships with populations and communities. Less than half of the studies (0–36%) reported (1) how the intervention compared to the best standard of care, (2) outcomes relevant and important to population(s) across PROGRESS-Plus, (3) whether the sample size was powered to detect statistical differences between groups, (4) whether randomization was stratified by geographical region and quality of care, (5) an analysis of losses and exclusions after randomization, (6) details of implementation in each trial arm relevant to the PROGRESS-Plus characteristics, and (7) whether there were any unintended inequities caused by the intervention.
Table 2.
Percentage of studies reporting health equity factors according to CONSORT-Equity 2017
| Extension for health equity relevant trials | % (n=11) | |
|---|---|---|
| 1 | Rationale for focus on health equity | 100 |
| 2 | Methods of recruitment to reach populations across relevant PROGRESS-Plus characteristics | 100 |
| 3 | Objective with reference to health equity | 91 |
| 4 | Report any limitations related to assessing effects on health equity | 91 |
| 5 | Applicability related to population of interest across PROGRESS-Plus characteristics | 91 |
| 6 | Trial design to answer equity questions | 91 |
| 7 | Population eligibility criteria across relevant PROGRESS-Plus1 characteristics | 91 |
| 8 | Context and relationship to health inequity | 91 |
| 9 | Details of partnerships with populations and communities | 91 |
| 10 | Participants who were assigned, received, and who were analyzed across relevant PROGRESS-Plus characteristics | 63 |
| 11 | Numbers of participants randomly assigned, received, and were analyzed across relevant PROGRESS-Plus characteristics | 63 |
| 12 | Baseline characteristics across relevant PROGRESS-Plus characteristics | 63 |
| 13 | Details of ethical clearance and informed consent | 54 |
| 14 | Additional analyses focused on health equity | 45 |
| 15 | Additional analytic approaches related to equity objectives distinguishing pre-specified from exploratory | 45 |
| 16 | Outcomes relevant and important to population(s) across PROGRESS-Plus | 36 |
| 17 | Losses and exclusions after randomization across relevant PROGRESS-Plus characteristics with reasons | 36 |
| 18 | Randomization was stratified by geographical region and quality of care | 36 |
| 19 | Details of implementation (coverage, intensity) in each trial arm across relevant PROGRESS-Plus characteristics | 27 |
| 20 | Analyses focused on health equity objectives are powered to detect differences | 18 |
| 21 | Comparator intervention as standard of care and whether it has equity implications | 0 |
| 22 | Whether inequities caused by the intervention (such as unintended effects) were assessed | 0 |
PROGRESS-Plus characteristics are place of residence, race/ethnicity/culture/language, occupation, gender/sex, religion, education, social capital, socioeconomic status, personal characteristics associated with discrimination, features of relationships, and time-dependent relationships
Discussion
Our goal was to systematically review studies on psychosocial and educational interventions for promoting cervical cancer screening and HPV prevention among women living in rural communities. Eleven research articles from 2000 through 2023 met our inclusion criteria of using an RCT or quasi-experimental design and implementing an educational intervention with a sample of adult women living in rural communities. Most studies were successful in recruiting samples of women with low to middle incomes and/or women of color, especially Hispanic/Latinas. The interventions were primarily educational using a variety of methods such as social media, novella, lay health advisors, patient navigators, and educational DVDs. None used mobile technology (e.g., mHealth) which includes using apps or text messaging with smart phones as an intervention delivery method. Nor did they use self-sampling kits which are a growing avenue in cervical cancer screening intervention. Although self-sampling kits have yet to be widely adopted in clinical practice and our currently not FDA approved, promising results have shown that self-sampling can positively impact the screening rates for under screened and hard to reach populations [28–32]. Both self-sampling and mHealth are newer intervention strategies that can potentially address the access issues encountered in rural healthcare.
Our findings suggest that educational interventions are effective in encouraging cervical cancer screening and prevention behavior. When paired with patient navigation services or lay health advisors, educational interventions are even more effective in promoting cervical cancer screening and prevention behavior as evidenced by two of the more successful studies [20, 26]. While there were improvements in women’s knowledge and beliefs about cervical cancer screening, screening and prevention behaviors (pap smear adherence and vaccination) did not produce comparable results. There may be practical barriers that are preventing access to screening. For example, transportation and costs are practical barriers that women in rural settings have been previously reported [9]. Between 2010 and 2021, 136 rural hospitals have closed. With the closure of rural medical centers and increasing shortage in qualified medical providers in rural areas, transportation and access to care issues become further exacerbated [33]. Transportation issues have been a consistent barrier to care for rural residents [34–37].
The relationship between patient and provider is both a barrier and facilitator to cervical cancer screening and HPV vaccination. While lack of recommendation by a healthcare provider is a known barrier to HPV vaccination [38], only one study in this review addressed provider recommendation. Additionally, the lack of regular healthcare provider, the lack of insurance and the lack of knowledge about cervical cancer tests have also been found to be barriers to cervical cancer screening [39].
Interventions that were most effective in our review were those who worked with community members or community health workers to educate and encourage screening. Community health workers and community engagement models have been effective in other populations for increasing cervical cancer screening participation and have been recommended by CPSTF [11]. Our findings reiterate their importance. In addition, the use of self-sampling kits was an effective strategy in increasing cervical cancer screening participation, reflecting similar findings in other priority populations as well [40, 41]. In fact, self-sampling kits have been recently promoted as a cost-effective measure for addressing under screened women [29, 42]. Self-sampling kits are one cost-effective way to address the barriers of access and health provider availability that rural women have encountered.
Unique to this review is the use of the health equity assessment, CONSORT-Equity 2017 PROGRESS-Plus. Most of the reviewed studies addressed health equity in their introductions, aims, population eligibility criteria, and recruitment of participants. However, few used rigorous analyses to address health equity objectives and outcomes. This is an area for improvement in future intervention research focused on addressing health inequities. Given that most of the studies did not report rigorous methods and may have had numerous risks of bias, we cannot reach any definitive conclusions about whether inequities were reduced.
Limitations
Our review has limitations, primarily due to problematic definitions of outcomes. For studies with outcomes that include adherence to Pap smear guidelines, adherence over the past 3 years is often self-recalled; errors in memory and knowledge for which gynecological procedures were conducted can lead to inaccuracies in reporting [43]. A more reliable but costly approach is to rely on reviews of medical charts [44]. Evaluation of vaccination is challenged by the number of doses needed (2 or 3) and time frame (up to 1 year from initiation). Many studies report the numbers of persons who intend to vaccinate rather than the number of those who have initiated or finished the vaccine series. The intention-behavior gap is problematic for estimating vaccination rates [45].
A common concern for the meta-analysis process is publication bias. Studies with non-significant findings are often excluded from the literature. Our study catchment excluded “gray literature” — studies that have not been published in peer-review journals. We intentionally left out gray literature, with the belief that the peer-review process, including revise and resubmit, leads to higher quality articles. However, this is an assumption that may need further empirical validation. We feel a strength of this review and our methodology is the use of the risk of bias assessment which provided details on the biases of each article reviewed.
Lastly, narrow definitions of geographic location and intervention strategy limit the generalization of our findings. However, the rigor of the inclusion/exclusion criteria and evaluation methods allow for insights to be drawn that can help future interventionists.
Future Research
The landscape for cervical cancer and HPV interventions continues to change and there are several future directions in which research and intervention development can expand. In 2022, American Indian/Alaskan Native women replaced Hispanic/Latina women in having the highest incidence of cervical cancer [46]. None of the studies in our review focused on this population, nor did they focus on African American/Black women who have the highest mortality rate, while being third for incidence [47]. In recent years, rural America has seen growing racial/ethnic diversity in their population, which could translate to an increased need to address cervical cancer health disparities in this population and setting. As we mentioned previously, education alone did not lead to higher likelihood of women being screened. Practical barriers (e.g., access to care) and strong recommendations from providers may be more important than knowledge and awareness for this setting. Future research and interventions can address accessibility barriers by using self-sampling kits. With the decreasing accessibility of healthcare services in rural America, self-sampling kits could increase women’s access to screening. Access to HPV vaccination could be improved by allowing pharmacists, school nurses, and other health professionals outside of primary care providers to administer the vaccines.
Conclusion
Cervical cancer continues to be a source of morbidity and mortality. This review assesses the current landscape of psychosocial and educational interventions to promote cervical cancer screening and HPV vaccination. We show that education-only interventions are not as effective as multi-level interventions. Patient navigators plus education, for example, increases the number of women screened and the frequency of screening. Rural and minority women who are at higher risk of not receiving timely healthcare require additional supports to allow them to receive the life-saving benefits afforded by regular cancer screening and HPV vaccination.
Supplementary Material
Funding
Dr. Randall received research support from the National Cancer Institute institutional training grant T32-CA-236621. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Cancer Institute.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s13187-023-02385-7.
Conflict of Interest The authors declare no competing interests.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
- 1.Caldwell JT, Ford CL, Wallace SP, Wang MC, Takahashi LM (2016) Intersection of living in a rural versus urban area and race/ethnicity in explaining access to health care in the United States. Am J Public Health 106(8):1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paskett ED, Tatum C, Rushing J, Michielutte R, Bell R, Foley KL et al. (2004) Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer: Interdisciplinary International Journal of the American Cancer. Society 101(11):2650–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco M, Mazzucca S, Padek M, Brownson RC (2019) Going beyond the individual: how state-level characteristics relate to HPV vaccine rates in the United States. BMC Public Health 19(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gollust SE, Attanasio L, Dempsey A, Benson AM, Fowler EF (2013) Political and news media factors shaping public awareness of the HPV vaccine. Womens Health Issues 23(3):e143–e151 [DOI] [PubMed] [Google Scholar]
- 5.Shelton RC, Snavely AC, De Jesus M, Othus MD, Allen JD (2013) HPV vaccine decision-making and acceptance: does religion play a role? J Relig Health 52(4):1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Palmer C, Bik EM, Cardenas JP, Nuñez H, Kraal L et al. (2018) Self-sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health 6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burhansstipanov L (2000) Urban Native American health issues. Cancer 88(S5):1207–1213 [DOI] [PubMed] [Google Scholar]
- 8.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS (2014) Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health 104(Suppl 3):S377–S387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studts CR, Tarasenko YN, Schoenberg NE (2013) Barriers to cervical cancer screening among middle-aged and older rural Appalachian women. J Community Health 38(3):500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plunkett R, Leipert B, Olson JK, Ray SL (2014) Understanding women’s health promotion and the rural church. Qual Health Res 24(12):1721–1731 [DOI] [PubMed] [Google Scholar]
- 11.Force CPST (2023) Guide to Community Preventive Services. In: Community Preventive Services Task Force Findings for Cancer Prevention and Control updated January 24 https://www.thecommunityguide.org/pages/task-force-findings-cancer-prevention-and-control.html [Google Scholar]
- 12.Mohan G, Chattopadhyay SK, Ekwueme DU, Sabatino SA, Oka-sako-Schmucker DL, Peng Y et al. (2019) Economics of multicomponent interventions to increase breast, cervical, and colorectal cancer screening: a community guide systematic review. Am J Prev Med 57(4):557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(RevMan Computer Program) RM. 5.4 The Cochrane Collaboration; 2020. https://documentation.cochrane.org/revmankb/cite-revman-web-in-a-reference-list-110242006.html
- 15.Kepka D, Coronado GD, Rodriguez HP, Thompson B (2011) Evaluation of a radionovela to promote hpv vaccine awareness and knowledge among hispanic parents. J Community Health 36(6):957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luque JS, Tarasenko YN, Reyes-Garcia C, Alfonso ML, Suazo N, Rebing L et al. (2017) Salud es Vida: a cervical cancer screening intervention for rural latina immigrant women. J Cancer Educ 32(4):690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuño T, Martinez ME, Harris R, García F (2011) A Promotora-administered group education intervention to promote breast and cervical cancer screening in a rural community along the U.S.-Mexico border: a randomized controlled trial. Cancer Causes Control 22(3):367–374 [DOI] [PubMed] [Google Scholar]
- 18.Thompson B, Carosso EA, Jhingan E, Wang L, Holte SE, Byrd TL et al. (2017) Results of a randomized controlled trial to increase cervical cancer screening among rural Latinas. Cancer 123(4):666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson B, Coronado G, Chen L, Islas I (2006) Celebremos La Salud! A community randomized trial of cancer prevention (United States). Cancer Causes Control 17(5):733–746 [DOI] [PubMed] [Google Scholar]
- 20.Falk D, Foley K, Weaver KE, Jones B, Cubbin C (2022) An evaluation of breast and cervical cancer screening outcomes in an education and patient navigation program in rural and border Texas. J Cancer Educ 37(4):1043–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krok-Schoen JL, Oliveri JM, Young GS, Katz ML, Tatum CM, Paskett ED (2016) Evaluating the stage of change model to a cervical cancer screening intervention among Ohio Appalachian women. Women Health 56(4):468–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paskett ED, Krok-Schoen JL, Pennell ML, Tatum CM, Reiter PL, Peng J et al. (2016) Results of a multi-level intervention trial to increase human papillomavirus (HPV) vaccine uptake among adolescent girls. Cancer Epidemiol Biomarkers Prev 25(4):593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderpool RC, Cohen EL, Crosby RA, Jones MG, Bates W, Casey BR et al. (2013) “1–2-3 pap” intervention improves HPV vaccine series completion among Appalachian women. J Commun 63(1):95–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson HS, Valdimarsdottir HB, Winkel G, Jandorf L, Redd W (2004) The group-based medical mistrust scale: psychometric properties and association with breast cancer screening. Prev Med 38(2):209–218 [DOI] [PubMed] [Google Scholar]
- 25.Cates JR, Shafer A, Diehl SJ, Deal AM (2011) Evaluating a county-sponsored social marketing campaign to increase mothers’ initiation of HPV vaccine for their preteen daughters in a primarily rural area. Soc Mark Q 17(1):4–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studts CR, Tarasenko YN, Schoenberg NE, Shelton BJ, Hatcher-Keller J, Dignan MB (2012) A community-based randomized trial of a faith-placed intervention to reduce cervical cancer burden in Appalachia. Prev Med 54(6):408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP (2019) Assessing risk of bias in a non-randomized study. Cochrane Handbook Syst Rev Interv:621–641 [Google Scholar]
- 28.Serrano B, Ibáñez R, Robles C, Peremiquel-Trillas P, de Sanjosé S, Bruni L (2022) Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med 154:106900. [DOI] [PubMed] [Google Scholar]
- 29.Malone C, Barnabas RV, Buist DSM, Tiro JA, Winer RL (2020) Cost-effectiveness studies of HPV self-sampling: A systematic review. Prev Med 132:105953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozar T, Nagvekar R, Rohrer C, Dube Mandishora RS, Ivanus U, Fitzpatrick MB (2021) Cervical cancer screening postpandemic: self-sampling opportunities to accelerate the elimination of cervical cancer. International. J Womens Health 13(null):841–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pretsch PK, Spees LP, Brewer NT, Hudgens MG, Sanusi B, Rohner E et al. (2023) Effect of HPV self-collection kits on cervical cancer screening uptake among under-screened women from low-income US backgrounds (MBMT-3): a phase 3, open-label, randomised controlled trial. Lancet Public Health 8(6):e411–ee21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JS, Des Marais AC, Deal AM, Richman AR, Perez-Heydrich C, Yen-Lieberman B et al. (2018) Mailed human papillomavirus self-collection with papanicolaou test referral for infrequently screened women in the United States. Sex Transm Dis 45(1):42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn S, Wooster M, Valente C, Moshier E, Meng R, Pisapati K et al. (2018) Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol 25(10):2979–2986 [DOI] [PubMed] [Google Scholar]
- 34.Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, Perin J (2005) The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Serv Res 40(1):135–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arcury TA, Preisser JS, Gesler WM, Powers JM (2005) Access to transportation and health care utilization in a rural region. J Rural Health 21(1):31–38 [DOI] [PubMed] [Google Scholar]
- 36.Ratnapradipa KL, Jadhav S, Kabayundo J, Wang H, Smith LC (2023) Factors associated with delaying medical care: cross-sectional study of Nebraska adults. BMC Health Serv Res 23(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfe MK, McDonald NC, Holmes GM (2020) Transportation barriers to health care in the United States: findings from the national health interview survey, 1997–2017. Am J Public Health 110(6):815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newcomer SR, Caringi J, Jones B, Coyle E, Schehl T, Daley MF (2020) A mixed-methods analysis of barriers to and facilitators of human papillomavirus vaccination among adolescents in Montana. Public Health Rep 135(6):842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senkomago V, Greek A, Jackson JE, Thomas CC, Richardson LC, Benard VB (2021) Learning from cervical cancer survivors: an examination of barriers and facilitators to cervical cancer screening among women in the United States. J Prim Care Community Health 12:21501327211041862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daponte N, Valasoulis G, Michail G, Magaliou I, Daponte AI, Garas A et al. (2023) HPV-based self-sampling in cervical cancer screening: an updated review of the current evidence in the literature. Cancers (Basel) 15(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mekuria SF, Timmermans S, Borgfeldt C, Jerkeman M, Johansson P, Linde DS (2023) HPV self-sampling versus healthcare provider collection on the effect of cervical cancer screening uptake and costs in LMIC: a systematic review and meta-analysis. Syst Rev 12(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meenan RT, Troja C, Buist DSM, Tiro JA, Lin J, Anderson ML et al. (2023) Economic evaluation of mailed home-based human papillomavirus self-sampling kits for cervical cancer screening. JAMA Network Open 6(3):e234052–e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard M, Agarwal G, Lytwyn A (2009) Accuracy of self-reports of Pap and mammography screening compared to medical record: a meta-analysis. Cancer Causes Control 20(1):1–13 [DOI] [PubMed] [Google Scholar]
- 44.Rolnick SJ, Parker ED, Nordin JD, Hedblom BD, Wei F, Kerby T et al. (2013) Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine 31(37):3928–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheeran P, Webb TL (2016) The intention–behavior gap. Soc Personal Psychol. Compass 10(9):503–518 [Google Scholar]
- 46.Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33 [DOI] [PubMed] [Google Scholar]
- 47.Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


