Abstract
Objectives: To elucidate the expression levels and prognostic value of the Lipoyltransferase 2 (LIPT2) gene in a pan-cancer view. Methodology: Our study comprehensively investigated the role of LIPT2 in pan-cancer, combining bioinformatics analyses with experimental validations. Results: Analysis of LIPT2 mRNA expression across various cancers revealed a significant up-regulation in 18 tumor types and down-regulation in 8 types, indicating its diverse involvement. Prognostic assessment demonstrated a correlation between elevated LIPT2 expression and poorer outcomes in Overall Survival (OS) and Disease-Free Survival (DFS), particularly in Glioblastoma Multiforme (GBM), Liver Hepatocellular Carcinoma (LIHC), and Pheochromocytoma and Paraganglioma (PCPG). Protein expression analysis in GBM, LIHC, and PCPG affirmed a consistent increase in LIPT2 levels compared to normal tissues. Examining the methylation status in GBM, LIHC, and PCPG, we found reduced promoter methylation levels in tumor samples, suggesting a potential influence on LIPT2 function. Genetic mutation analysis using cBioPortal indicated a low mutation frequency (< 2%) in LIPT2 across GBM, LIHC, and PCPG. Immune correlation analysis unveiled a positive association between LIPT2 expression and infiltration levels of immune cells in GBM, LIHC, and PCPG. Single-cell analysis illustrated LIPT2’s positive correlation with functional states, including angiogenesis and inflammation. Enrichment analysis identified LIPT2-associated processes and pathways, providing insights into its potential molecular mechanisms. Drug sensitivity analysis demonstrated that elevated LIPT2 expression conferred resistance to multiple compounds, while lower expression increased sensitivity. Finally, RT-qPCR validation in HCC cell lines confirmed the heightened expression of LIPT2 compared to a control cell line, reinforcing the bioinformatics findings. Conclusion: Overall, our study highlights LIPT2 as a versatile player in cancer, influencing diverse aspects from molecular processes to clinical outcomes across different cancer types.
Keywords: LIPT2, pan-cancer, prognosis, biomarker, treatment
Introduction
Cancers pose significant global health challenges, impacting human well-being and quality of life [1-4]. As per World Health Organization statistics, cancers stand as a primary threat to human life [5]. The absence of universal pan-cancer biomarkers poses a significant challenge in oncology. While certain biomarkers show promise within specific cancer types, their applicability across diverse malignancies remains limited. Therefore, the identification of accurate key pan-cancer genes becomes imperative in unraveling the underlying mechanisms driving the onset and progression of diverse tumors.
Lipoyltransferase 2 (LIPT2) is an enzyme actively involved in the acyl transfer reaction within fatty acid metabolism, as described by Habarou et al. in 2017 [6]. Its primary role is to catalyze the combination of fatty acids with coenzyme A (CoA), a critical step in intracellular fatty acid metabolism [6]. LIPT2 exhibits broad distribution in the cytoplasm, with notable expression levels observed in the liver, muscle, and adipose tissues, as indicated by studies conducted by Tang et al. in 2023 and Bernardinelli et al. in 2017 [7,8]. LIPT2 plays a crucial role in governing fatty acid synthesis, oxidation, and storage, thereby contributing to the overall balance of fatty acid metabolism, as outlined by Habarou et al. in 2017 and Solmonson and DeBerardinis in 2018 [6,9]. Any defects or mutations in the LIPT2 gene may disrupt normal fatty acid metabolism, potentially leading to various related disorders. Existing studies propose an association between LIPT2 gene mutations and the development of conditions such as obesity, fatty liver, and metabolic syndrome [6,8,10]. Nevertheless, the current understanding of the potential mechanisms and biological implications of LIPT2 in cancer remains limited.
This investigation employed a combination of diverse bioinformatics methodologies and molecular approaches to delve into the intrinsic molecular mechanisms associated with LIPT2 in the development and clinical prognosis of various human cancers. Comprehensive analyses of LIPT2 expression profiles and corresponding survival outcomes were conducted using datasets from The Cancer Genome Atlas (TCGA). Concurrently, gene enrichment analyses shed light on the involvement of LIPT2-related molecules in tumorigenesis. Furthermore, this study explored the potential implications of LIPT2 in anti-tumor immune responses.
Methodology
mRNA expression analysis
The UCSC and UALCAN [11,12] platforms provided access to standardized TCGA data, allowing extraction of expression data for the ENSG00000175536 (LIPT2) gene. The data underwent log2(x+0.001) transformation and the R (3.6.4) program facilitated the comparison of mRNA expression between normal and cancer samples.
Prognostic analysis
Survival analysis across various cancers for LIPT2 was conducted using the GEPIA2 platform [13,14]. Cancer samples with follow-up durations less than 30 days and cancer types with fewer than 10 samples were excluded from the dataset obtained from GEPIA2. The log-rank test was employed to evaluate the association between LIPT2 expression and prognostic indicators, including Overall Survival (OS) and Disease-Free Survival (DFS) in pan-cancer patients.
Protein expression analysis
The Human Protein Atlas (HPA) database is a valuable resource offering insights into protein expression in various tissues and cancers [15,16]. It provides comprehensive immunohistochemistry-based data, aiding researchers in understanding the spatial distribution of proteins in normal and pathological conditions. In the present study, the HPA database was used to analyze the protein expression of LIPT2 across specified cancer types.
Genetic mutation analysis
The cBioPortal database was utilized to analyze mutation types, frequency, count, positions, and the three-dimensional (3D) structure of the LIPT2 protein in the TCGA datasets [17,18]. cBioPortal is a comprehensive web resource that facilitates the exploration of multidimensional cancer genomics data. Researchers can analyze genetic alterations, clinical outcomes, and pathways across various cancer types.
Methylation analysis
Methylation level of LIPT2 in tumor and normal tissues within the TCGA dataset was investigated using the UALCAN website [19-21]. UALCAN is known for its user-friendly interface, facilitates comprehensive analysis of gene expression differences and promoter methylation differences in 31 cancer types, contributing valuable insights to cancer research.
Single cell analysis
We investigated the correlation between LIPT2 expression at the single-cell level and diverse functional states of tumor cells utilizing the CancerSEA database [22]. The expression profile of LIPT2 in individual cells was visualized through T-SNE plots.
Enrichment analysis
We utilized the STRING website [23] to conduct a network analysis of experimentally determined LIPT2-binding proteins. Subsequently, the DAVID tool [24] was employed for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses.
Immune assessment
The associations among LIPT2 expression and immune cell infiltration were revealed using TIMER2.0 database [25]. TIMER2.0 is an updated and comprehensive resource for systematic analysis of immune infiltrates across diverse cancer types. It provides valuable insights into the tumor microenvironment by integrating multi-dimensional data, enabling users to explore associations between gene expression, clinical outcomes, and immune cell infiltration.
Drug sensitivity analysis
The Gene Set Cancer Analysis (GSCA) database is pivotal for drug sensitivity analysis, offering a curated collection of synthetic control arms for clinical trials [26]. In the present study, GSCA database was used to conduct drug sensitivity analysis of the LIPT2 gene.
Cell culture
Human hepatocellular carcinoma (HCC) cell lines (Huh7, HepG2, SMMC7721, and MHCC97H) and a control cell line (THLE-2) were obtained from the Cell Resources Bank at the Laboratory Animal Center, Sun Yat-sen University (Guangzhou, China). Following the manufacturer’s instructions, all cell lines were cultured in RPMI-1640 medium (Invitrogen, Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Gibco BRL, USA), 2 mmol/L glutamine, 100 mg/L penicillin, and 100 mg/L streptomycin. Incubation occurred in a humidified atmosphere with 5% CO2 at 37°C, and cells were harvested at 80% confluence for subsequent experiments.
Total RNA extraction and RT-qPCR
RNA was extracted from both HCC and control cells employing an RNA extraction kit (Qiagen, Hilden, Germany), followed by the synthesis of cDNA. The total cDNA was quantified using RT-qPCR with SYBRGreen (Enzynomics, Daejeon, South Korea). GAPDH served as an internal control, and the expression of LIPT2 was assessed using the 2-ΔΔCT method. The primer sequences are provided below, with GAPDH employed as the internal control.
GAPDH (forward): 5’-TGGACTCCACGACGTACTCAG-3’; GAPDH (reverse): 5’-CGGGAAGCTTGTCATCAATGGAA-3’; LIPT2 (forward): 5’-GTCTGGCTAGACGATCGCAAGA-3’; LIPT2 (reverse): 5’-GCACGATGTGCTCAAACCACGT-3’.
Statistics
All supplementary analyses were conducted using R software (version 3.6.3). Statistical significance was determined with a threshold of p-value less than 0.05. The Spearman test was applied for correlation analysis between two variables.
Results
LIPT2 mRNA expression in pan-cancer
We analyzed the mRNA expression of LIPT2 across various cancers using the TCGA dataset (Figure 1A, 1B). Our results demonstrated a notable up-regulation of LIPT2 in 18 tumor types, while it showed a down-regulation in 8 types of tumor tissues compared to the corresponding normal control tissues (Figure 1). According to the TCGA data, the variations in LIPT2 expression levels were found to be statistically significant (Figure 1A, 1B).
Figure 1.
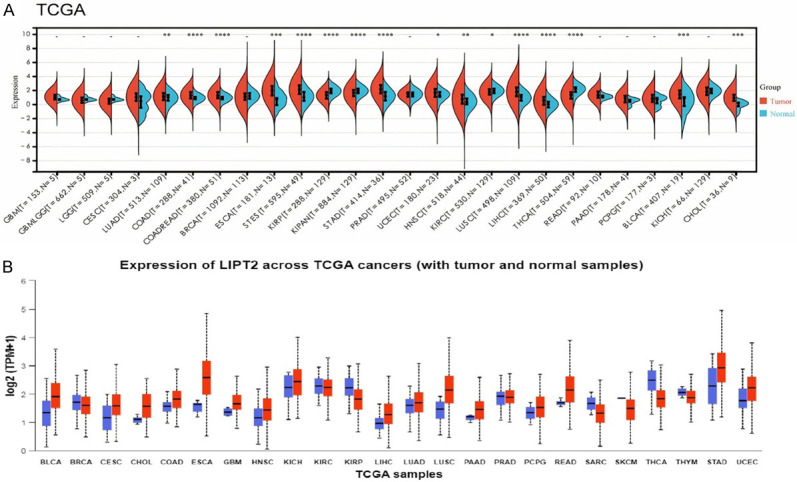
Pan-cancer analysis of LIPT2 mRNA expression. A. LIPT2 mRNA expression across diverse cancers in The Cancer Genome Atlas database. B. LIPT2 mRNA expression across various cancers in the UALCAN database. * = p-value < 0.05, ** = p-value < 0.01, *** = p-value < 0.001. LIPT2 = Lipoyltransferase 2.
Prognostic value of LIPT2 in pan-cancer
We explored the correlation between LIPT2 expression and survival prognosis across a spectrum of cancers. Utilizing Kaplan-Meier (KM) analysis on a pan-cancer scale, our investigation revealed statistically significant distinctions in both OS and DFS. Notably, patients exhibiting elevated LIPT2 expression levels experienced a significantly poorer outcome in specific types, including GBM, LIHC, and PCPG, as depicted in Figure 2A-C. These findings underscore a potential role for LIPT2 in influencing the clinical outcomes of individuals diagnosed with these three distinct cancer types.
Figure 2.
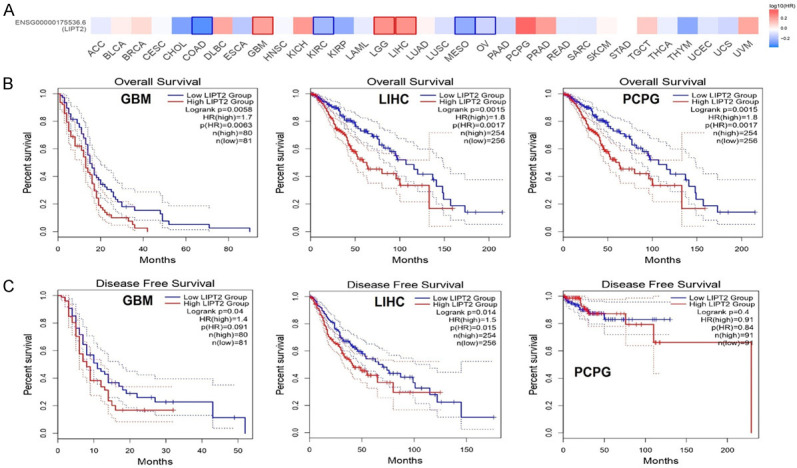
Survival map and Kaplan-Meier curves for Overall Survival (OS) and Disease-Free Survival (DFS) based on LIPT2 expression in the most significantly associated cancer. A. Survival map depicting LIPT2 across various cancers. B. OS curves illustrating LIPT2 expression in Glioblastoma Multiforme (GBM), Liver Hepatocellular Carcinoma (LIHC), and Pheochromocytoma and Paraganglioma (PCPG). C. DFS curves reflecting LIPT2 expression in GBM, LIHC, and PCPG. Statistical significance indicated by p-value < 0.05. LIPT2 = Lipoyltransferase 2.
Protein expression of LIPT2
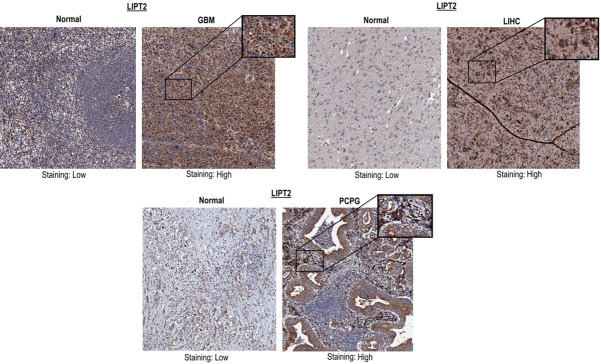
Expanding our inquiry beyond mRNA levels, we delved into the protein expression of LIPT2 in GBM, LIHC, and PCPG. Our analysis unveiled a notable up-regulation, indicated by high staining, of LIPT2 protein in the tissues of these specific cancer types when contrasted with normal tissues, which exhibited low staining (Figure 3). In summary, both LIPT2 mRNA and protein expression levels demonstrated a consistent increase across GBM, LIHC, and PCPG compared to normal control tissues.
Figure 3.
Immunohistochemical images from the Human Protein Atlas (HPA) depicting LIPT2 protein expression in Glioblastoma Multiforme (GBM), Liver Hepatocellular Carcinoma (LIHC), and Pheochromocytoma and Paraganglioma (PCPG) tissue samples, alongside corresponding normal control tissues. LIPT2 = Lipoyltransferase 2.
Promoter methylation analysis of LIPT2
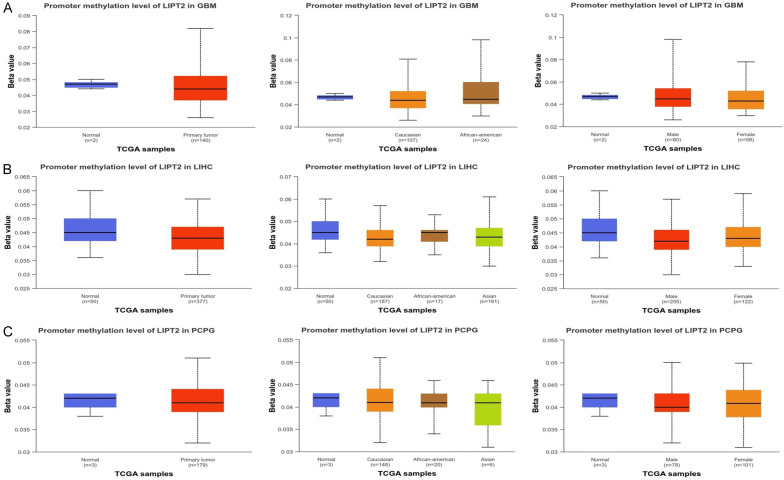
Prior research has highlighted the influence of tumor purity as a confounding factor, impacting gene expression, DNA methylation, and copy number alterations. These factors are interconnected with gene expression levels, tumor purity, and immune cell infiltration. To address this, we accessed the UALCAN database to examine the methylation expression levels of LIPT2 in GBM, LIHC, and PCPG samples. Our analysis revealed a significant reduction in the promoter methylation levels of LIPT2 in GBM, LIHC, and PCPG samples compared to normal tissues (Figure 4). These findings suggest that the observed alterations in methylation levels may play a role in influencing the function of LIPT2 in GBM, LIHC, and PCPG patients.
Figure 4.
Epigenetic alteration (promoter methylation) analysis of LIPT2 in Glioblastoma Multiforme (GBM), Liver Hepatocellular Carcinoma (LIHC), and Pheochromocytoma and Paraganglioma (PCPG) and normal control samples. A. Promoter methylation level of LIPT2 in GBM samples of different clinical variables paired with controls. B. Promoter methylation level of LIPT2 in LIHC samples of different clinical variables paired with controls. C. Promoter methylation level of LIPT2 in PCPG samples of different clinical variables paired with controls. Statistical significance indicated by p-value < 0.05. LIPT2 = Lipoyltransferase 2.
Genetic mutation analysis of LIPT2
The well-established understanding posits that the accumulation of genetic mutations is a primary driver of human cancer [27]. In light of this, we undertook a thorough analysis of genetic alterations in LIPT2 across GBM, LIHC, and PCPG utilizing the cBioPortal platform. The analysis of the overall mutation count for LIPT2 revealed a relatively low genetic alteration frequency, approximately < 2% (Figure 5). In summary, these results indicate that the LIPT2 gene does not undergo frequent mutations in GBM, LIHC, and PCPG patients.
Figure 5.
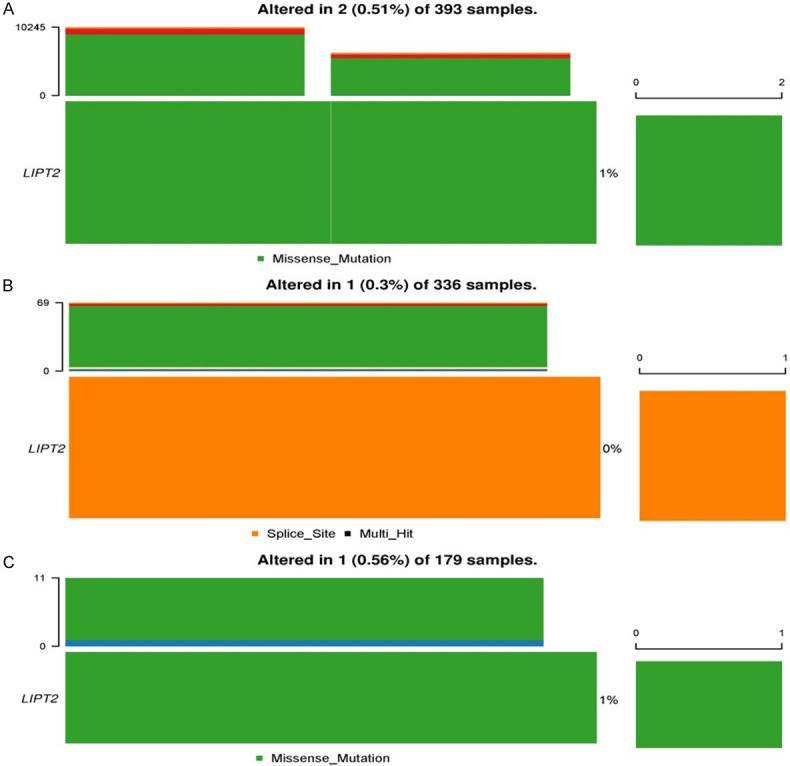
Genetic mutation analysis of LIPT2 in Glioblastoma Multiforme (GBM), Liver Hepatocellular Carcinoma (LIHC), and Pheochromocytoma and Paraganglioma (PCPG) tissue samples via the cBioPortal. A. LIPT2 mutational status in GBM samples. B. LIPT2 mutational status in LIHC samples. C. LIPT2 mutational status in PCPG samples. LIPT2 = Lipoyltransferase 2.
Immune assessment
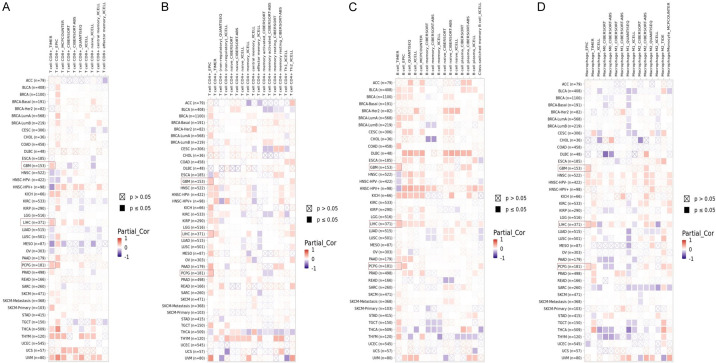
We explored the correlation between LIPT2 levels and immune infiltration across diverse cancer types using the TIMER2.0 database. The findings from TIMER2.0 demonstrated a statistically significant positive correlation (p-value < 0.05) between LIPT2 expression and the infiltration levels of CD8+ T cells, CD4+ T cells, B cells, and macrophages in GBM, LIHC, and PCPG (Figure 6A-D). This suggests a potential interplay between LIPT2 expression and the immune response within the tumor microenvironment across GBM, LIHC, and PCPG.
Figure 6.
Correlation analysis between LIPT2 expression and CD8+ T cells, CD4+ T cells, B cells, and macrophages immune cell infiltration in Glioblastoma Multiforme (GBM), Liver Hepatocellular Carcinoma (LIHC), and Pheochromocytoma and Paraganglioma (PCPG) using TIMER2.0. A. Correlation between LIPT2 expression and CD8+ T cells infiltration in GBM, LIHC, and PCPG. B. Correlation between LIPT2 expression and CD4+ T cells infiltration in GBM, LIHC, and PCPG. C. Correlation between LIPT2 expression and B cells infiltration in GBM, LIHC, and PCPG. D. Correlation between LIPT2 expression and macrophages infiltration in GBM, LIHC, and PCPG. Statistical significance indicated by p-value < 0.05. LIPT2 = Lipoyltransferase 2.
Single cell analysis
We elucidated the relationship between LIPT2 and 14 distinct functional states across various cancers. Our investigation unveiled a positive correlation between LIPT2 expression and key functional states, including angiogenesis, differentiation, and inflammation in cancer (Figure 7A). The T-SNE plot provided a comprehensive view of LIPT2 expression profiles at the single-cell level (Figure 7B). These findings underscore the potential regulatory role of LIPT2 expression levels in shaping the diverse biological behaviors exhibited by cancer cells.
Figure 7.
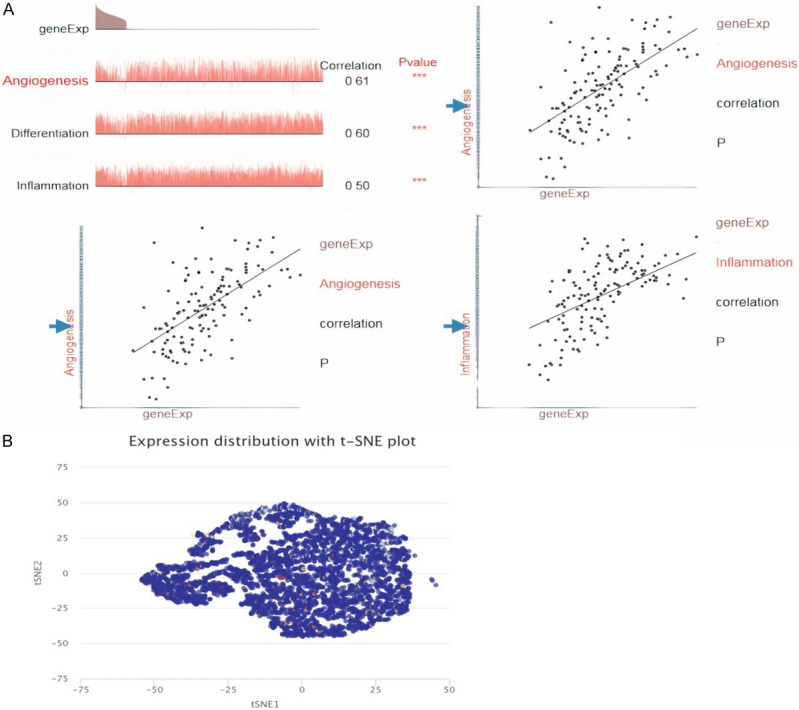
LIPT2 expression at the single-cell level. A. Analysis on the CancerSEA website examining the correlation between LIPT2 expression at the single-cell level and various functional states of tumor cells. B. T-SNE map illustrating LIPT2 expression in tumor cells. * = p-value < 0.05, ** = p-value < 0.01, *** = p-value < 0.001. LIPT2 = Lipoyltransferase 2.
Enrichment analysis
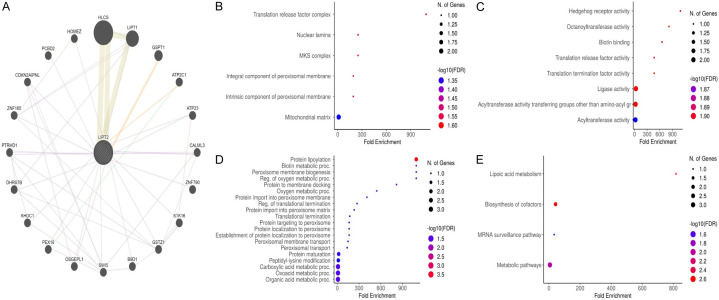
To delve deeper into the potential molecular mechanisms underlying LIPT2 in cancer initiation and progression, we conducted an enrichment analysis focusing on proteins that interact with LIPT2. Employing the STRING tool, we identified 19 binding proteins associated with LIPT2. The network visualization in Figure 8A illustrates the intricate interconnections among these proteins. Results of the gene enrichment analysis showed that LIPT2 genes are associated with “Translation release factor complex, nuclear lamina, and MSK complex” etc. CC terms (Figure 8B), “Hedgehog receptor activity, octanoyltransferase activity, and biotin binding” etc. MF terms (Figure 8C), “Protein lipoylation, biotin metabolic proc., and peroxisome membrane biogenesis” etc. BP terms (Figure 8D), and “Lipoic acid metaboloism, biosynthesis of cofactors, mRNA surveillance pathway, and metabolic signaling pathways etc. (Figure 8E)”.
Figure 8.
Gene Enrichment Analysis of LIPT2-associated proteins using the DAVID Tool. A. PPI network. B. Cellular Component (CC) terms. C. Biological Process (BP) terms. D. Molecular Function (MF) terms. E. Kyoto Encyclopedia of Genes and Genomes (KEGG) terms. Statistical significance indicated by p-value < 0.05. LIPT2 = Lipoyltransferase 2.
Drug sensitivity analysis
The drug sensitivity analysis of LIPT2 revealed that elevated expression of this gene renders tumor cells resistant to a spectrum of compounds, including 17-AAG, AZD6482, BEZ235, Belomycin (50 uM), Bortezomib, CHIR-99021, FTI-277, GSK269962A, MG-132, Midostaunin, PD325901, Piperlongumine, RDEA119, SB216763, and Trametinib (Figure 9). Conversely, lower expression of LIPT2 was associated with increased sensitivity of cancer cells to AT-7519, CUDC-101, EKB-569, GSK1070916, GSK690693, KIN001-055, KIN001-266, MPS-1-IN-1, NPK76-II-72-1, HSC-207895, SB52334, TAK-715, TPCA-1, and WZ3105 (Figure 9).
Figure 9.
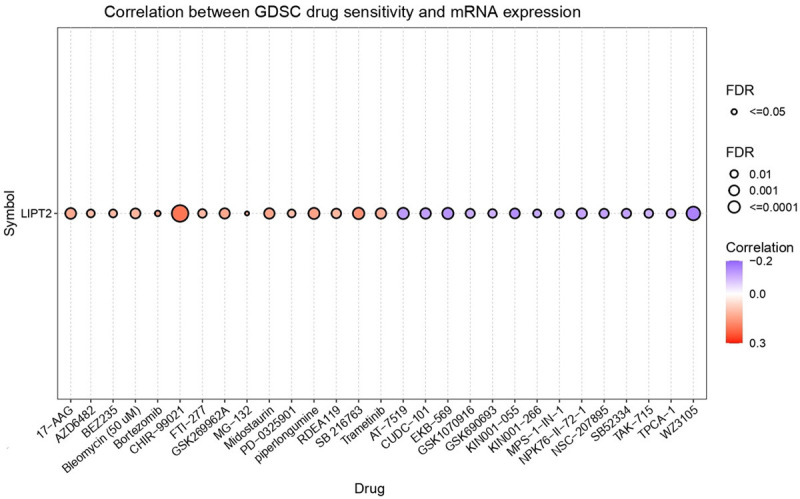
Correlations between LIPT2 expression and sensitivity of various drugs retrieved from the Gene Set Cancer Analysis (GSCA) database. LIPT2 = Lipoyltransferase 2.
Validation of LIPT2 expression in HCC cell lines
In this phase of our investigation, we verified the expression of the LIPT2 gene in four HCC cell lines (Huh7, HepG2, SMMC7721, and MHCC97H) and one control cell line (THLE-2) using RT-qPCR. The results demonstrated a significant up-regulation of LIPT2 gene expression in HCC cell lines compared to the control cell line (Figure 10). Collectively, the RT-qPCR findings corroborated the bioinformatics results, affirming the heightened expression of LIPT2 in the present study.
Figure 10.
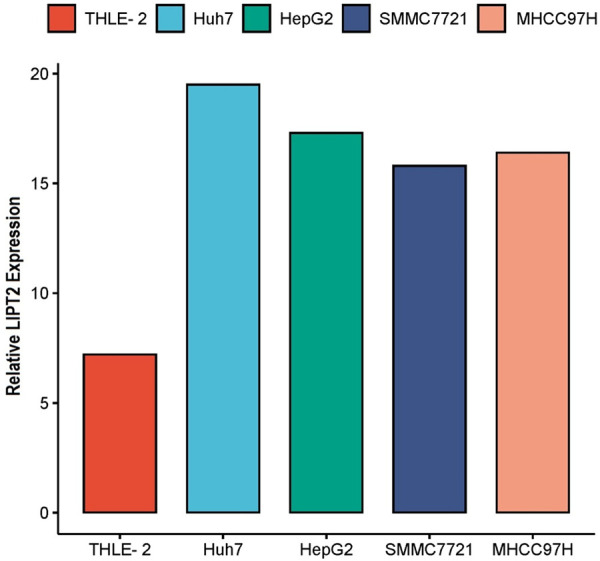
Verification of LIPT2 expression in Hepatocellular Carcinoma (HCC) cell lines (Huh7, HepG2, SMMC7721, and MHCC97H) and a normal control cell line (THLE-2) using RT-qPCR. LIPT2 = Lipoyltransferase 2.
Discussion
Recent investigations indicate a close association between cuproptosis and the onset, advancement, and prognostic outcomes across various human cancer types [28-31]. This study extensively assessed the significance of the cuproptosis-related gene LIPT2 in pan-cancer using a combination of diverse bioinformatics platforms and molecular experiments.
Analyzing the pan-cancer expression profiles obtained from TCGA, HPA databases, and cell lines, our findings revealed a significant up-regulation of LIPT2 in 18 tumor types, juxtaposed with a down-regulation in 8 types of tumor tissues compared to their respective normal control tissues. These results suggest that LIPT2 may serve as a novel cancer biomarker. Moreover, the expression levels of LIPT2 play a role in influencing the prognosis of cancer patients. Particularly in GBM, LIHC, and PCPG, LIPT2 emerges as a risk factor, where heightened expression predicts a poor prognosis for patients. These findings underscore the considerable potential of LIPT2 as a prognostic biomarker for individuals diagnosed with GBM, LIHC, and PCPG, offering valuable insights for future research directions.
Tumor development is frequently linked to genetic mutations, as highlighted in previous studies [32]. Mutations in Mismatch Repair (MMR) genes can compromise the stability of the normal cell genome, resulting in genomic instability [33]. The process of DNA methylation, a crucial epigenetic modification, has substantial impacts on gene expression and cellular function [34]. Notably, mutations in MMR genes and alterations in DNA methylation play a pivotal role in the progression of tumors, forming a robust connection to the tumorigenesis process [35-37].
Surprisingly, the results of LIPT2 mutation analysis revealed a relatively low genetic alteration frequency, amounting to approximately less than 2% across these specific cancer types. This finding suggests that the LIPT2 gene experiences infrequent mutations in patients diagnosed with GBM, LIHC, and PCPG. Such a low mutation frequency may imply that alternative mechanisms, beyond genetic alterations, could be contributing to the dysregulation of LIPT2 in these cancers. Moreover, our analysis uncovered a substantial reduction in the promoter methylation levels of LIPT2 in GBM, LIHC, and PCPG samples when compared to normal tissues. These findings suggest that LIPT2 plays a crucial role in the initiation of tumors, operating at both the genetic and epigenetic levels.
Results from single-cell sequencing indicate that LIPT2 might govern different biological processes in cancer, including angiogenesis, differentiation, and inflammation. Functional enrichment analysis unveils the potential molecular pathways through which LIPT2 may contribute to the initiation and progression of cancer.
Immune cells are essential in identifying cancer cells and controlling tumor growth [38]. B cells, renowned for their production of antibodies (IgE, IgG, IgA, and IgM), hold a prominent position in this regard [39,40]. Cancer-Associated Fibroblasts (CAFs) play a crucial role in promoting cancer cell growth and metastasis [41]. Regulatory T cells (Tregs) contribute to immune homeostasis through diverse pathways [42]. In our investigation, we explored the relationship between LIPT2 expression levels and immune infiltration across GBM, LIHC, and PCPG tumors. The results unveiled a statistically significant positive correlation (p-value < 0.05) between LIPT2 expression and the infiltration levels of key immune cell subsets, including CD8+ T cells, CD4+ T cells, B cells, and macrophages in GBM, LIHC, and PCPG. These findings suggest a potential interplay between LIPT2 expression and the immune response within the tumor microenvironment across GBM, LIHC, and PCPG.
The drug sensitivity analysis conducted on LIPT2 provided intriguing insights into its role in influencing the responsiveness of tumor cells to various compounds. Elevated expression of LIPT2 was found to confer resistance to a diverse array of substances, encompassing 17-AAG, AZD6482, BEZ235, Belomycin (50 uM), Bortezomib, CHIR-99021, FTI-277, GSK269962A, MG-132, Midostaunin, PD325901, Piperlongumine, RDEA119, SB216763, and Trametinib.
Conversely, lower expression of LIPT2 was correlated with heightened sensitivity of cancer cells to AT-7519, CUDC-101, EKB-569, GSK1070916, GSK690693, KIN001-055, KIN001-266, MPS-1-IN-1, NPK76-II-72-1, HSC-207895, SB52334, TAK-715, TPCA-1, and WZ3105. These results underscore the dual role of LIPT2 in modulating drug sensitivity, indicating its potential as a predictive biomarker for therapeutic responsiveness. This information may have clinical implications, guiding personalized treatment strategies by considering the LIPT2 expression profile in cancer patients. Future studies focusing on the underlying mechanisms of LIPT2 in mediating drug resistance or sensitivity could provide additional insights into its potential as a therapeutic target or predictive biomarker in cancer treatment.
Conclusion
In summary, our study illuminates LIPT2’s role in cancer, spanning genetic and epigenetic dimensions. LIPT2 has emerged as a potential diagnostic and prognostic biomarker for GBM, LIHC, and PCPG. Further exploration promises insights into LIPT2’s function and clinical applications in diverse cancer contexts.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R376) King Saud University, Riyadh, Saud Arabia.
Disclosure of conflict of interest
None.
References
- 1.Lippi L, de Sire A, Folli A, Turco A, Moalli S, Marcasciano M, Ammendolia A, Invernizzi M. Obesity and cancer rehabilitation for functional recovery and quality of life in breast cancer survivors: a comprehensive review. Cancers (Basel) 2024;16:521. doi: 10.3390/cancers16030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullah L, Hameed Y, Ejaz S, Raashid A, Iqbal J, Ullah I, Ejaz SA. Detection of novel infiltrating ductal carcinoma-associated BReast CAncer gene 2 mutations which alter the deoxyribonucleic acid-binding ability of BReast CAncer gene 2 protein. J Cancer Res Ther. 2020;16:1402–1407. doi: 10.4103/jcrt.JCRT_861_19. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Sahar AM, Li C, Chaudhary A, Yousaf I, Saeedah MA, Mubarak A, Haris M, Nawaz M, Reem MA, Ramadan FA, Mostafa AAM, Feng W, Hameed Y. A detailed multi-omics analysis of GNB2 gene in human cancers. Braz J Biol. 2022;84:e260169. doi: 10.1590/1519-6984.260169. [DOI] [PubMed] [Google Scholar]
- 4.Hameed Y, Ejaz S. TP53 lacks tetramerization and N-terminal domains due to novel inactivating mutations detected in leukemia patients. J Cancer Res Ther. 2021;17:931–937. doi: 10.4103/jcrt.JCRT_536_19. [DOI] [PubMed] [Google Scholar]
- 5.Ali A, Manzoor MF, Ahmad N, Aadil RM, Qin H, Siddique R, Riaz S, Ahmad A, Korma SA, Khalid W, Aizhong L. The burden of cancer, government strategic policies, and challenges in Pakistan: a comprehensive review. Front Nutr. 2022;9:940514. doi: 10.3389/fnut.2022.940514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habarou F, Hamel Y, Haack TB, Feichtinger RG, Lebigot E, Marquardt I, Busiah K, Laroche C, Madrange M, Grisel C, Pontoizeau C, Eisermann M, Boutron A, Chrétien D, Chadefaux-Vekemans B, Barouki R, Bole-Feysot C, Nitschke P, Goudin N, Boddaert N, Nemazanyy I, Delahodde A, Kölker S, Rodenburg RJ, Korenke GC, Meitinger T, Strom TM, Prokisch H, Rotig A, Ottolenghi C, Mayr JA, de Lonlay P. Biallelic mutations in LIPT2 cause a mitochondrial lipoylation defect associated with severe neonatal encephalopathy. Am J Hum Genet. 2017;101:283–290. doi: 10.1016/j.ajhg.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Ren X, Huang T, Miao Y, Ha W, Li Z, Yang L, Mi D. Prognostic and immunological significance of the molecular subtypes and risk signatures based on cuproptosis in hepatocellular carcinoma. Mediators Inflamm. 2023;2023:3951940. doi: 10.1155/2023/3951940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardinelli E, Costa R, Scantamburlo G, To J, Morabito R, Nofziger C, Doerrier C, Krumschnabel G, Paulmichl M, Dossena S. Mis-targeting of the mitochondrial protein LIPT2 leads to apoptotic cell death. PLoS One. 2017;12:e0179591. doi: 10.1371/journal.pone.0179591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solmonson A, DeBerardinis RJ. Lipoic acid metabolism and mitochondrial redox regulation. J Biol Chem. 2018;293:7522–7530. doi: 10.1074/jbc.TM117.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucci S, Alatibi KI, Wehbe Z. Altered metabolic flexibility in inherited metabolic diseases of mitochondrial fatty acid metabolism. Int J Mol Sci. 2021;22:3799. doi: 10.3390/ijms22073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675–678. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usman M, Hameed Y, Ahmad M. Does human papillomavirus cause human colorectal cancer? Applying Bradford Hill criteria postulates. Ecancermedicalscience. 2020;14:1107. doi: 10.3332/ecancer.2020.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thul PJ, Lindskog C. The human protein atlas: a spatial map of the human proteome. Protein Sci. 2018;27:233–244. doi: 10.1002/pro.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan M, Hameed Y. Discovery of novel six genes-based cervical cancer-associated biomarkers that are capable to break the heterogeneity barrier and applicable at the global level. J Cancer Res Ther. 2023;2023:9000. [Google Scholar]
- 17.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hameed Y. Decoding the significant diagnostic and prognostic importance of maternal embryonic leucine zipper kinase in human cancers through deep integrative analyses. J Cancer Res Ther. 2023;19:1852–1864. doi: 10.4103/jcrt.jcrt_1902_21. [DOI] [PubMed] [Google Scholar]
- 19.Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, Creighton CJ, Varambally S. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. doi: 10.1016/j.neo.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang G, Cho M, Wang X. OncoDB: an interactive online database for analysis of gene expression and viral infection in cancer. Nucleic Acids Res. 2022;50:D1334–D1339. doi: 10.1093/nar/gkab970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad M, Khan M, Asif R, Sial N, Abid U, Shamim T, Hameed Z, Iqbal MJ, Sarfraz U, Saeed H. Expression characteristics and significant diagnostic and prognostic values of ANLN in human cancers. Int J Gen Med. 2022:1957–1972. [Google Scholar]
- 22.Yuan H, Yan M, Zhang G, Liu W, Deng C, Liao G, Xu L, Luo T, Yan H, Long Z, Shi A, Zhao T, Xiao Y, Li X. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019;47:D900–D908. doi: 10.1093/nar/gky939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T, Chang W. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update) Nucleic Acids Res. 2022;50:W216–W221. doi: 10.1093/nar/gkac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CJ, Hu FF, Xie GY, Miao YR, Li XW, Zeng Y, Guo AY. GSCA: an integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief Bioinform. 2023;24:bbac558. doi: 10.1093/bib/bbac558. [DOI] [PubMed] [Google Scholar]
- 27.Takeshima H, Ushijima T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis Oncol. 2019;3:7. doi: 10.1038/s41698-019-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Huo Z, Qi X, Zuo T, Wu Z. Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicine (Lond) 2022;17:303–324. doi: 10.2217/nnm-2021-0374. [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Zhou S, Tóth J, Hajdu A. Cuproptosis-related gene index: a predictor for pancreatic cancer prognosis, immunotherapy efficacy, and chemosensitivity. Front Immunol. 2022;13:978865. doi: 10.3389/fimmu.2022.978865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Tan L, Li Y, Lyu Y, Zheng X, Jiang H, Zhang X, Wen H, Feng C. Cuproptosis identifies respiratory subtype of renal cancer that confers favorable prognosis. Apoptosis. 2022;27:1004–1014. doi: 10.1007/s10495-022-01769-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Zhang Y, Wang L, Zhang N, Xu W, Zhou J, Zhao Y, Zhu W, Zhang T, Wang L. Development and experimental verification of a prognosis model for cuproptosis-related subtypes in HCC. Hepatol Int. 2022;16:1435–1447. doi: 10.1007/s12072-022-10381-0. [DOI] [PubMed] [Google Scholar]
- 32.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015;349:1483–1489. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- 34.Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76:3446–3450. doi: 10.1158/0008-5472.CAN-15-3278. [DOI] [PubMed] [Google Scholar]
- 35.Mäki-Nevala S, Valo S, Ristimäki A, Sarhadi V, Knuutila S, Nyström M, Renkonen-Sinisalo L, Lepistö A, Mecklin JP, Peltomäki P. DNA methylation changes and somatic mutations as tumorigenic events in Lynch syndrome-associated adenomas retaining mismatch repair protein expression. EBioMedicine. 2019;39:280–291. doi: 10.1016/j.ebiom.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasir M, Nawaz A, Ghazanfar S, Okla MK, Chaudhary A, Al WH, Ajmal MN, AbdElgawad H, Ahmad Z, Abbas F, Wadood A, Manzoor Z, Akhtar N, Din M, Hameed Y, Imran M. Anti-bacterial activity of essential oils against multidrug-resistant foodborne pathogens isolated from raw milk. Braz J Biol. 2022;84:e259449. doi: 10.1590/1519-6984.259449. [DOI] [PubMed] [Google Scholar]
- 37.Khalil T, Okla MK, Al-Qahtani WH, Ali F, Zahra M, Shakeela Q, Ahmed S, Akhtar N, AbdElgawad H, Asif R, Hameed Y, Adetunji CO, Farid A, Ghazanfar S. Tracing probiotic producing bacterial species from gut of buffalo (Bubalus bubalis), South-East-Asia. Braz J Biol. 2022;84:e259094. doi: 10.1590/1519-6984.259094. [DOI] [PubMed] [Google Scholar]
- 38.Talty R, Olino K. Metabolism of innate immune cells in cancer. Cancers (Basel) 2021;13:904. doi: 10.3390/cancers13040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SS, Sumner WA, Miyauchi S, Cohen EEW, Califano JA, Sharabi AB. Role of B cells in responses to checkpoint blockade immunotherapy and overall survival of cancer patients. Clin Cancer Res. 2021;27:6075–6082. doi: 10.1158/1078-0432.CCR-21-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Li H, Hameed Y, Abdel-Maksoud MA, Almutairi SM, Mubarak A, Aufy M, Alturaiki W, Alshalani AJ, Mahmoud AM, Li C. Elucidating the clinical and immunological value of m6A regulator-mediated methylation modification patterns in adrenocortical carcinoma. Oncol Res. 2023;31:819–831. doi: 10.32604/or.2023.029414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Min KW, Kim DH, Noh YK, Son BK, Kwon MJ, Moon JY. Cancer-associated fibroblasts are associated with poor prognosis in solid type of lung adenocarcinoma in a machine learning analysis. Sci Rep. 2021;11:16779. doi: 10.1038/s41598-021-96344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Chen W, Kang FB, Zhang YH, Qi LL, Zhang YZ. Blood transfusion practices affect CD4(+) CD25(+) FOXP3(+) regulatory T cells/T helper-17 cells and the clinical outcome of geriatric patients with hip fracture. Aging (Albany NY) 2021;13:21408–21420. doi: 10.18632/aging.203479. [DOI] [PMC free article] [PubMed] [Google Scholar]