Abstract
Objective: To assess how obesity, normal weight (NW) versus overweight/obese (OW/OB), impacts platelet-rich plasma’s (PRP) effectiveness during in vitro fertilization and how obesity affects platelets during the menstrual cycle. Methods: Endometrial mean thickness (EMT), embryo implantation, and clinical pregnancy were assessed using a self-controlled retrospective study that enrolled 59 patients with two failed cycles and treated with autologous PRP (three-dose scheme). The NHANES dataset was used to assess platelet changes during the menstrual cycle, using the mean platelet volume to platelet count ratio (MPR) index. The COSINOR packages for R were used to determine rhythmicity. Results: PRP treatments significantly improved the EMT (2.5 ± 1.4 mm, P<0.001), unaffected by obesity. After the PRP treatment, one patient spontaneously became pregnant; therefore, 58 patients underwent embryo transfer (62 cycles), of which in 39 cycles the embryos implanted (63.9%). This was a significant improvement from their previous cycle (vs. 22.6%, P<0.001). Clinical pregnancy also improved with the PRP treatment over the previous cycle (57.4% vs. 16.1%, P<0.001). When stratified by obesity, there was an appreciable decrease in embryo implantation and clinical pregnancy rates for the OW/OB group; nevertheless, the PRP treatment significantly improved embryo implantation and clinical pregnancy (P<0.05). A rhythm was observed with the MPR index (P<0.05) only for the NW group, suggesting that the platelets normally fluctuate during the menstrual cycle. Conclusion: PRP improved embryo implantation and clinical pregnancy rates; however, these beneficial effects were attenuated by obesity. PRP presumptively promoted a change in the uterine environment to mimic the normal findings associated with normal-weight women.
Keywords: Endometrial receptivity, IVF, intrauterine platelet-rich plasma, menstrual cycle, recurrent implantation failure, refractory thin endometrium
Introduction
A healthy human endometrium is crucial during embryo implantation, as it is like the soil that promotes the embryo’s growth [1]. It has been widely reported that embryo implantation depends on coordinated crosstalk between intrauterine factors and the embryo [2]. In addition, endometrial receptivity and thickness play an essential role in pregnancy outcomes, especially during assisted reproduction [3], in which platelet quality and quantity significantly affect the endometrium [4]. Under certain circumstances, the endometrium fails to reach an optimal condition [5], related to poor circulation and sub-optimal perfusion of the uterus [6]. This leads to low concentrations of cytokines and growth factors, which are secreted by endometrial epithelial and stromal cells during the window of implantation [7]. Interestingly, during the window of implantation, fluctuations concerning platelets have not been investigated; nevertheless, the administration of platelet-rich plasma (PRP) is a promising strategy for treating endometrium-associated infertility [8,9].
PRP is a concentrated sample of human platelets obtained from the patient’s blood (autologous) that is 5- to 10-fold higher than the physiologic concentration of thrombocytes in the whole blood [10]. PRP contains a variety of growth factors and bioactive molecules involved in clotting, inflammation, cell growth, cell adhesion, and host defense, among others [11]. The mechanism for PRP’s effect on endometrial tissue is unknown; nevertheless, the induction of cell proliferation, chemotaxis, regeneration, extracellular matrix synthesis, remodeling, angiogenesis, and epithelialization are the main pathways for PRP to affect female reproductive organs [8]. The critical role cytokines and growth factors play during embryo implantation has been well-described [2]. PRP contains many growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor-β, vascular endothelial growth factor (VEGF), and epidermal growth factor [11], that are present and are released by the blood during normal endometrial growth and embryo implantation [2].
The concept of an optimal endometrium is still under debate, and its characterization remains elusive; however, it is accepted that a minimum endometrial thickness (EMT) of 7 mm is required for embryo transfer, as it is the cutoff to define thin endometrium [12]. In vitro studies have shown that PRP exposure, as a single agent or a combination with growth factors, was associated with increased endometrial stromal and mesenchymal cell proliferation, increased expression of regenerative enzymes, and enhanced cell migration [13]. With in vivo animal studies, PRP treatments demonstrated a decrease in the expression of inflammatory and fibrotic markers. At the same time, there was an increase in the endometrial proliferation rate, expression of proliferative genes, and pregnancy rates [3,8]. This suggests that PRP would benefit infertile women, especially those suffering from thin endometrium and recurrent implantation failure. Indeed, intrauterine infusions of autologous PRP have been used in infertile women with recurrent implantation failure and thin endometrial lining [3]. The first study to show how effective an intrauterine infusion of PRP as a therapy for infertile women with thin endometrium was published in 2015, in which the PRP injections promoted endometrial growth and improved pregnancy outcomes in five patients [14]. A systematic review demonstrated that following intrauterine PRP, EMT was increased as did the embryo implantation rate, chemical pregnancy rate, and clinical pregnancy rate [9].
Clinical studies using PRP in women undergoing assisted reproductive technologies have conflicting results [9], as PRP preparation and application protocols for different therapeutic purposes have yet to be standardized. Moreover, especially in females, obesity, which has been shown to affect fertility [15], has also been shown to affect platelet concentration and activation [16,17]. Our study aimed to determine whether the intrauterine infusion of autologous PRP improved the EMT and enhanced embryo implantation and clinical pregnancy rates in patients with thin endometrium with a history of previously failed in vitro fertilization (IVF) cycles. Furthermore, we assessed whether the PRP effect is altered by obesity and how obesity affects the platelets during the menstrual cycle.
Methods
Study design and participants
A chart review for a retrospective self-controlled study was conducted from November 2019 to May 2023 following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [18]. The cohort pool consisted of patients who attended any of the seven centers of the Ingenes Institute and received a three-dose scheme intrauterine infusion of autologous PRP during an embryo transfer cycle. They had to fulfill the following inclusion criteria: 1) age >25 years, 2) have demonstrated a refractory thin endometrium (EMT<7 mm) at Day 10 during a gonadotropin-free estrogen-primed cycle of endometrial preparation for embryo transfer, 3) had a history of at least two failed IVF cycles with good quality embryos, 4) failed to achieve a pregnancy in the previous IVF cycle that used endometrial preparation with high-dose estradiol valerate, 5) as determined by hysteroscopy or hysterosonography, had a normal uterine cavity or had an abnormal uterine cavity but was corrected, and 6) had good quality embryos available for embryo transfer, either from donated oocytes or the patient’s ova. The patients were excluded if it was documented or suspected that: 1) the patient suffered from an auto-immune disease, thrombophilia, hematologic disorders, uncontrolled endocrine or other medical conditions, 2) congenital and untreated acquired uterine abnormalities, 3) couples with genetic or chromosomal abnormalities, or 4) only produced poor-quality embryos and were not willing to accept gamete donation. Once the potential records were identified, the patients were contacted, and of the patients who agreed, written informed consent was obtained according to the Declaration of Helsinki. The Ethics Committee of the Ingenes Institute approved this study (approval number: ISF201219).
IVF
All patients underwent controlled ovarian stimulation for 10 to 14 days with gonadotrophin-releasing hormone agonists and antagonists. On the second day of the menstrual cycle, recombinant human FSH (Corneumon, Corne, UPC: 7502242700449) was administered daily, in which the dose was adjusted by weight and antral follicle count according to an individualized protocol. Gonadotropin-releasing hormone antagonist, Cetrotide (Merk, UPC: 4054839325359, 0.25 mg/day), was administered from day 6 of ovarian stimulation until ovulation trigger was performed with 10,000 IU of human chorionic gonadotropin (hCG, Choriomon, Corne, UPC: 7680335240802). The ovarian response was assessed by measuring serum estradiol levels, and an ultrasound examination evaluated follicular development. Oocyte retrieval was conducted 36 hours after administering hCG. At the same time, the semen was prepared by density gradient centrifugation. The oocytes were inseminated by intracytoplasmic sperm injection, and fertilization was confirmed by the formation of two pronuclei 19 hours after insemination. Embryos were cultured in Global Total for Fertilization media (Cat # LGGT-30, Life Global) and incubated at 37°C, 8% CO2, 5% O2, and 87% N2. An embryologist monitored and recorded all information about the antral follicle count, fertilization rates, embryo development, and embryo morphology for each oocyte.
Autologous PRP preparation and intrauterine infusion
For preparing each PRP infusion, 3 ml of venous blood was drawn from patients using a 4.5 ml sample collection tube with 3.8% sodium citrate (BD vacutainer). Afterward, the samples were centrifuged at 1200 rpm for 12 minutes. Then, the plasma was obtained, transferred to a 15 ml conical tube (Falcon), and centrifuged at 3300 rpm for 7 minutes. 0.5-1 ml of PRP was collected and infused into the uterine cavity within 10 minutes to avoid protein degradation. Intrauterine autologous PRP administration was performed during an estrogen-primed cycle. Endometrial preparation with 6 mg of estrogen valerate (Primogyn UPC: 00770333115702) was started on Menstrual Cycle Day (MCD) 2 or 3. On MCD 10, EMT was measured by ultrasonography, and autologous PRP was infused into the uterine cavity using a Wallace Embryo Transfer Catheter (Sure View Soft 2, CE 123). The procedure was repeated on MCD 12 and 14 (Supplementary Figure 1).
Embryo transfer
For fresh or frozen-thawed embryos, 1 to 3 good-quality embryos were transferred during an estrogen-primed cycle, free of gonadotropin stimulation. Clinical decisions about the number of embryos to transfer were determined by the physician, with the patient’s approval. Blastocysts were cryopreserved by vitrification. The uterine transfer occurred during a controlled endometrial development cycle with a daily dose of 6 mg of estrogen valerate (Primogyn UPC: 00770333115702) for ten days after menstruation. Embryo implantation was confirmed on Day 14 by serum β-hCG concentrations (>10 mUI/ml was considered positive) or by the presence of a fetal heartbeat using ultrasound at 6-8 weeks. Demographic data, IVF cycle information, embryo implantation rate, and IVF outcomes (pregnancies and miscarriages) were recorded by the physician. The primary outcomes were EMT, embryo implantation, and clinical pregnancy rates. The secondary outcome was the ongoing pregnancy/live birth rate.
Sample size
For this self-controlled study, the sample size was calculated using the SCCS package for R [19,20]. The inputs were based on the results from a similar study [21], in which the clinical pregnancy rate for the control and the treated group was 3.3% and 24.1%, respectively. With an alpha = 0.05 and power = 0.95, the calculated sample size was 15 subjects. However, using the inputs from another study [22], in which the control and treated group rates were 2.5% and 12.5%, respectively, the sample size was determined to be 25 subjects. Therefore, minimally, each group should contain between 15 and 25 participants.
National health and nutrition examination survey (NHANES) dataset
The National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention conducts the NHANES (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017). This large cross-sectional survey systematically gathers data on medical examinations, laboratory testing, and interviews for studying a range of variables of medical importance. The survey includes dietary, laboratory, body measurement examination, and demographic information. Before collection of the data, informed consent was obtained from each participant. The Ethics Review Board approved data gathering for the NCHS (https://www.cdc.gov/nchs/nhanes/irba98.htm), and the files were posted online for public use. NHANES fully describes the data collection procedures and methods (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?Cycle=2017-2018). All procedures were performed under the ethical standards of the institutional and national research committee and in agreement with the Declaration of Helsinki. All data generated and analyzed during this study are available on the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/default.aspx).
A set of eligibility criteria was constructed according to the patient population, intervention, comparison group, outcomes, and study design (PICOS) question scheme. The PICOS question was: in overweight/obese women, when compared to normal-weight women, does the platelet count, platelet volume, and the mean platelet volume to platelet count ratio (MPR) index, differ during the menstrual cycle, as determined using the NHANES cross-sectional dataset? The eligibility criteria reflected the PICOS components and the subsequent inclusion and exclusion criteria. In this study, the data came from the collection years between 1999 and 2006 (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017). To be included, the participants had to be 1) non-pregnant women aged ≥16 years, 2) body-mass index (BMI) of ≥18.5 kg/m2, and 3) had information about the number of days since their last period (Days 1 to 35). They were excluded for 1) having liver disease (Hepatitis B/C/D, autoimmune, or hepatocarcinoma), 2) having liver problems, 3) having thyroid problems, 4) having HIV, 5) taking insulin, 6) having/had cancer, 7) had a pregnancy/delivered within one year or were currently breastfeeding, 8) had a hysterectomy, ovariectomy, or endometrioses, or 9) taking hormones.
Key demographic variables collected were age, ethnicity, marital status, income, and poverty-to-income ratio. The age (years) was determined at the interview. NHANES categorizes ethnicity as Non-Hispanic White, Mexican American, Other Hispanics, Non-Hispanic Black, or Other Races (including multiracial). Anthropometric variables [weight (kg), height (m), BMI (kg/m2), waist circumference (cm), systolic and diastolic blood pressures (mmHg)] were collected according to a standardized protocol (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?Cycle=2017-2018). BMI was categorized into normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (30-39.9 kg/m2), according to the World Health Organization criteria (https://apps.who.int/iris/handle/10665/37003). For laboratory data, red and white blood cell count (n), platelet count (103/μL), mean platelet volume (fL), and luteinizing hormone (LH, IU/L) were analyzed according to a standardized protocol (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?Cycle=2017-2018). MPR was defined as mean platelet volume (fL)/platelet count (103/μL) × 100%.
Statistical analysis
All analyses were done with the Statistical Package for the Social Sciences software (SPSS, v. 22.0, Chicago, IL, USA) or with the R software [23]. The paired T-test was used for the clinical study to compare the pre-PRP and post-PRP EMT. The two-way repeated measures ANOVA determined differences between pre and post-PRP EMT when stratified by IVF outcome and obesity. The embryo implantation, clinical pregnancy, and live birth rates were analyzed using McNemar’s test. For the NHANES dataset, the normality of continuous variables was determined using the Kolmogorov-Smirnov test. Differences between categorical data were assessed with the Chi-Square test. Levene’s test was used to examine differences in the variances of continuous variables. Differences between groups for continuous variables were determined using either Student’s t-test for parametric data or the Mann-Whitney U test for non-parametric data. The COSINOR [24] and COSINOR2 [23] packages for R were used to determine rhythm. These packages fit a cosine curve with a free phase to data as well as calculate the MESOR (Midline Estimating Statistic Of Rhythm, a rhythm-adjusted mean), amplitude (half the predictable change within a cycle), and acrophase (time of highest value within a cycle) [24]. The cosinor models were adjusted by obesity [normal weight (BMI: 18.5-24.9 kg/m2) and overweight/obese (BMI: 25.0-39.9 kg/m2)] as a covariate. Including the obesity category in the cosinor model allows the MESOR, amplitude, and acrophase to change with respect to the binominal categories. The overall significance of the cosine model was established using the zero-amplitude test. Wald tests were conducted for differences in the amplitude and acrophase due to the covariates. Unless noted otherwise, data are represented as the frequency, percent, or mean ± standard deviation or standard error. P-values <0.05 (two-tailed) were considered significant.
Results
PRP treatment improved IVF
Over 300 patients who underwent intrauterine PRP infusion were identified during the study period. However, using the inclusion/exclusion criteria, 134 patient records were identified. After a thorough chart review and informed consent retrieval, 59 patients’ data (62 IVF cycles) were included in this study (Supplementary Figure 2). The ages of the patients ranged between 30 and 49 years, with over 56.5% of the cohort being overweight to obese (BMI = 26.8 ± 5.0 kg/m2, Table 1). The average duration of infertility was 4.9 ± 3.9 years, with an average of 3.0 ± 1.5 previous IVF attempts with embryo transfers at the Ingenes Institute. The most common reason for IVF was advanced age (49.2%), followed by polycystic ovary syndrome (PCOS) (15.3%), tubal factor (15.3%), and low ovarian response (10.2%). Male factor was suspected in 24 couples, and two patients had a female partner.
Table 1.
Characteristics of the study participants
| Pt ID | Age (years) | BMI (kg/m2) | Infertility (years) | # Failed IVF cycles with ET | Parity | Hysteroscopic findings | Other medical history | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| T | P | A | L | |||||||
| 001 | 42 | 33.9 | 5 | 4 | 0 | 0 | 0 | 0 | Uterine septum (removed) | None indicated |
| 002a | 41 | 21.9 | 1 | 4 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 003 | 44 | 32.0 | 10 | 3 | 0 | 0 | 0 | 0 | Cervix hypoplastic, endometrial polyps (dissected), and ostium in central orientation | None indicated |
| 004 | 40 | 33.3 | 5 | 3 | 0 | 0 | 0 | 0 | Uterus arcuatus, polyps (removed) | None indicated |
| 005 | 44 | 23.6 | 5 | 4 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 006 | 34 | 25.1 | 2 | 1 | 1 | 0 | 0 | 1 | Normal | None indicated |
| 007 | 41 | 28.7 | 1 | 5 | 3 | 0 | 0 | 3 | Normal | None indicated |
| 008a | 48 | 35.4 | 12 | 3 | 0 | 0 | 0 | 0 | No permeable left ostium | Laparoscopy for tubal factor |
| 009 | 46 | 31.0 | 2 | 2 | 1 | 0 | 1 | 1 | Normal | None indicated |
| 010 | 32 | 21.2 | 5 | 4 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 011 | 49 | 39.0 | 1 | 6 | 0 | 0 | 0 | 0 | Uterus arcuatus (remodeled), vascularized uterine polyps (removed) | Complex endometrial hyperplasia |
| 012 | 45 | 21.1 | 10 | 1 | 0 | 0 | 0 | 0 | Stenosis was observed in the internal cervical orifice with access to a problematic cavity | None indicated |
| 013 | 46 | 23.0 | 5 | 1 | 0 | 0 | 2 | 0 | Normal | Myomectomy |
| 014 | 45 | 26.8 | 7 | 4 | 0 | 0 | 0 | 0 | Adhesions (removed) | None indicated |
| 015 | 36 | 28.8 | 4 | 5 | 0 | 0 | 0 | 0 | Uterus arcuatus and adhesions | None indicated |
| 016 | 30 | 22.3 | 6 | 1 | 0 | 0 | 0 | 0 | NR | Drug addictions, tobacco (G1, E1) |
| 017 | 42 | 32.2 | 3 | 5 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 018 | 44 | 39.0 | 4 | 3 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 019 | 41 | 27.9 | 1 | 4 | 2 | 0 | 0 | 2 | Normal | None indicated |
| 020 | 38 | 24.1 | 12 | 2 | 0 | 0 | 2 | 0 | Normal | None indicated |
| 021 | 42 | 21.0 | 4 | 3 | 0 | 0 | 1 | 0 | Normal | PCOS, endometriosis |
| 022a | 36 | 27.1 | 4 | 3 | 0 | 0 | 2 | 0 | Myoma (dissected, normal by pathology) | None indicated |
| 023 | 39 | 23.9 | 1 | 4 | 0 | 0 | 0 | 0 | Hyperplasia (normal by pathology) | None indicated |
| 024 | 45 | 22.6 | 4 | 3 | 0 | 0 | 0 | 0 | Adhesions (removed) | None indicated |
| 025 | 43 | 19.8 | 2 | 2 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 026 | 37 | 25.4 | 6 | 4 | 4 | 0 | 1 | 4 | Normal | None indicated |
| 027 | 38 | 27.8 | 10 | 2 | 0 | 0 | 0 | 0 | Normal | Moderate endometriosis |
| 028 | 43 | 21.5 | 1 | 1 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 029 | 39 | 21.7 | 2 | 2 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 030 | 41 | 33.3 | 19 | 3 | 0 | 0 | 4 | 0 | Endometrial and cervical polyps (normal by pathology) | Amenorrhea |
| 031 | 44 | 38.1 | 1 | 1 | 1 | 0 | 0 | 1 | Normal | None indicated |
| 032 | 46 | 25.7 | 7 | 4 | 0 | 0 | 0 | 0 | Normal | Polypus remotion |
| 033 | 46 | 24.2 | 6 | 1 | 0 | 0 | 0 | 0 | Normal | Polypus remotion |
| 034 | 40 | 26.4 | 5 | 1 | 0 | 0 | 0 | 0 | Polyps (removed) | None indicated |
| 035 | 36 | 23.1 | 5 | 2 | 0 | 0 | 0 | 0 | Unicorn uterus | Uterine adhesions, cyst salpingooforectomy, and adhesions treated by hysteroscopy |
| 036 | 30 | 27.6 | 2 | 1 | 0 | 0 | 2 | 0 | Endometrial hypotrophy (reactivation) | Salpingectomy |
| 037 | 49 | 23.7 | 7 | 7 | 3 | 0 | 2 | 3 | NR | Curettage |
| 038 | 39 | 22.1 | 3 | 6 | 0 | 0 | 1 | 0 | Normal | None indicated |
| 039 | 43 | 20.1 | <1 | 3 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 040 | 40 | 23.9 | 2 | 4 | 0 | 0 | 0 | 0 | Polyps (removed) | None indicated |
| 041 | 30 | 28.3 | 1 | 2 | 0 | 0 | 1 | 0 | Normal | Right oophorectomy |
| 042 | 38 | 32.1 | 8 | 5 | NR | NR | NR | NR | Normal | Hypertrophic uterus |
| 043 | 35 | 29.9 | 3 | 2 | 0 | 0 | 3 | 0 | Normal | None indicated |
| 044 | 39 | 32.7 | 2 | 2 | 0 | 0 | 3 | 0 | Polyps (removed) | None indicated |
| 045 | 37 | 24.7 | 3 | 2 | 0 | 0 | 0 | 0 | Asherman Syndrome | None indicated |
| 046 | 35 | 25.1 | 7 | 1 | 0 | 0 | 2 | 0 | Adhesions (removed) | PCOS |
| 047 | 35 | 28.9 | 10 | 6 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 048 | 39 | 22.6 | 6 | 2 | 1 | 0 | 2 | 1 | Normal | None indicated |
| 049 | 45 | 20.2 | 5 | 5 | 1 | 0 | 0 | 1 | Normal | Refractory endometrium |
| 050 | 49 | 28.8 | 5 | 2 | 0 | 0 | 1 | 0 | No visible right ostium-suspected unicorn uterus | None indicated |
| 051 | 36 | 25.4 | 3 | 4 | 0 | 0 | 1 | 0 | Normal | None indicated |
| 052 | 45 | 26.7 | 3 | 3 | 0 | 0 | 2 | 0 | Uterus arcuatus | None indicated |
| 053 | 42 | 22.6 | 3 | 2 | 0 | 0 | 0 | 0 | Normal | Atrophic endometrium |
| 054 | 39 | 20.8 | 1 | 2 | 0 | 0 | 0 | 0 | The left ostium is not permeable; a small cavity with a partition in the bottom | Severe endometriosis left ovarian wedge |
| 055 | 48 | 30.1 | 18 | 2 | 0 | 0 | 0 | 0 | Normal | Polypus remotion |
| 056 | 46 | 23.4 | 4 | 6 | 0 | 0 | 1 | 0 | Irregular hypersecretory endometrium; coronary villosities; ischemic necrosis | Curettage |
| 057 | 36 | 33.3 | 3 | 3 | 0 | 0 | 1 | 0 | Polyps (removed) | Myomectomy |
| 058 | 36 | 26.4 | 3 | 1 | 0 | 0 | 0 | 0 | Normal | None indicated |
| 059 | 38 | 23.8 | 4 | 3 | 0 | 0 | 0 | 0 | Normal | None indicated |
Abbreviations: BMI: body mass index; ET: embryo transfer; IVF: in vitro fertilization; NR: not recorded. Parity: T, Term; P, Preterm; A, Abortion; L, Live birth; PCOS, Polycystic Ovary Syndrome.
Patients underwent 2 IVF cycles of autologous platelet-rich plasma treatment and embryo transfer.
Before treatment, the average EMT was 5.6 ± 1.4 mm (minimum = 2.1 mm; maximum = 10.9 mm) for the cohort; however, after the PRP treatment, the average EMT was 8.1 ± 1.5 mm (minimum = 5.5 mm; maximum = 12.3 mm). This significantly improved in the EMT (2.5 ± 1.9 mm, P<0.001, Figure 1A). Interestingly, only six cycles did not achieve the ideal EMT of 7.0 mm (Table 2). When stratified by obesity, there was no difference in the net increase in the EMT (P = 0.354, Figure 1B), nor when stratified by IVF outcome (P = 0.574, Figure 1C). There was also no significant difference when the cohort was separated into patients who did attain embryo implantation versus those who did not (P = 0.909, Figure 1D), as well as with successful (live birth/ongoing) pregnancy (P = 0.499, Figure 1E). Of the 59 patients, 58 underwent embryo transfer (62 IVF cycles), and one became pregnant naturally. Between 1 and 3 embryos were transferred per patient and implanted in 39 cycles (63.9%). The results of each pregnancy are presented in Table 2. When the PRP-treated cycle outcomes were compared to the previous cycle (control), the implantation, clinical pregnancy, and ongoing pregnancy/live birth rates all significantly increased (P<0.01, Table 3). When stratified by obesity, there was an appreciable difference between normal-weight patients and overweight/obese patients, in which obesity was associated with lower rates of implantation, clinical pregnancy, and ongoing pregnancy/live birth. Nevertheless, compared to the previous cycle, even for overweight/obese patients, there was significant improvement due to the PRP treatment.
Figure 1.
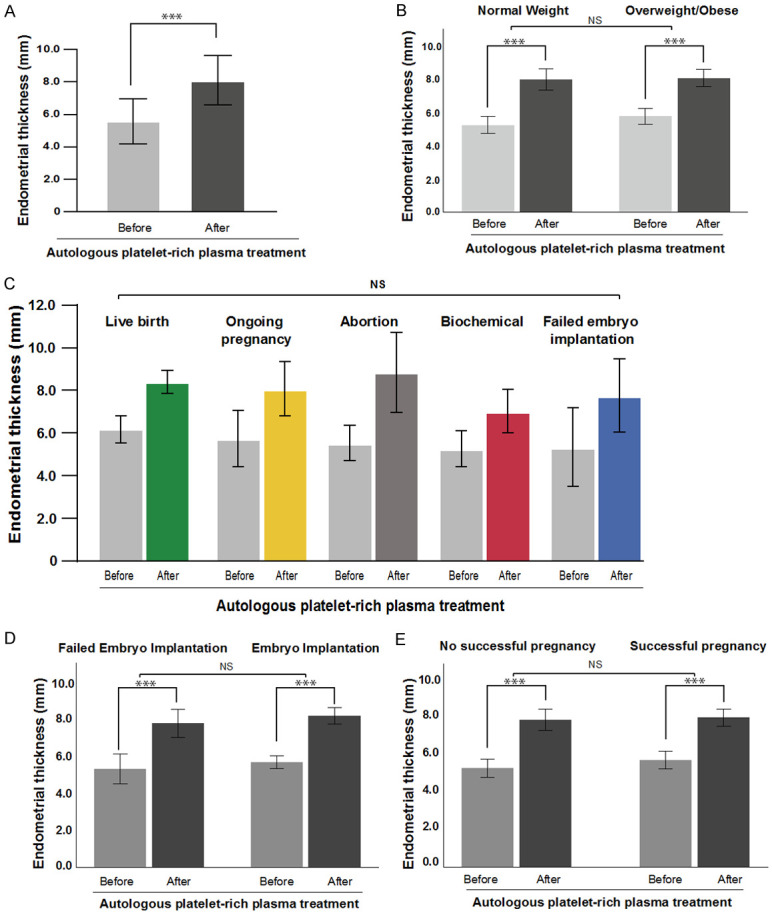
Endometrial mean thickness (EMT) improved after intrauterine infusion of platelet-rich plasma (PRP). (A) The EMT for each patient was graphed pre- and post-PRP treatment. EMT for pre- and post-PRP treatment was stratified by (B) normal weight and overweight/obese, (C) in vitro fertilization outcome, (D) embryo implantation result, and (E) successful pregnancy (live birth/ongoing). Column height represents the average, and bars represent the standard deviation. The results were compared using the two-way repeated measures ANOVA. NS: Not significant, *P<0.05, **P<0.01, ***P<0.001.
Table 2.
Results of autologous platelet-rich plasma treatment
| Obstetric result | EMT (mm) | Ova source | Sperm source | Frozen embryo | β-hCG (mUI/mL) | # Embryo transferred | Embryo Quality (stage/ICM quality + trophectoderm quality) | # Gest. sacs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Result | Week | Pre | Post | Diff | >7 | |||||||
| Live Birth | 36+6 | 6.4 | 9.2 | 2.8 | + | Patient | Partner | Yes | 229.4 | 3 | BE/BB | 1 |
| 36+6 | 6.0 | 8.9 | 2.9 | + | Donor | Partner | Yes | 321.2 | 2 | BE/AB | 1b | |
| 25+3 | 5.0 | 8.0 | 3.0 | + | Donor | Partner | No | 146.7 | 2 | BE/BB | 1 | |
| 38+6 | 6.9 | 7.8 | 0.9 | + | Donor | Donor | Yes | 179.8 | 2 | BE/BB | 1 | |
| 40+1 | 6.5 | 8.0 | 1.5 | + | Donor | Partner | Yes | NR | 2 | BE/BB | 1 | |
| 32+1 | 6.6 | 8.7 | 2.1 | + | Donor | Partner | Yes | 50.0 | 3 | BE/BB | 3 | |
| 38+3 | 5.9 | 8.2 | 2.3 | + | N/A | N/A | N/A | QTPC | N/A | N/A | 1c | |
| Ongoing | 30+0 | 5.6 | 9.0 | 3.4 | + | Donor | Partner | Yes | 1208.0 | 2 | BE/AB, BE/BB | 1b |
| 8+2 | 3.8 | 9.1 | 5.3 | + | Donor | Partner | Yes | 108.1 | 3 | BE/BC | 1 | |
| 26+3 | 4.0 | 7.0 | 3.0 | + | Donor | Partner | Yes | 131.1 | 2 | BE/BB, BE/BC | 1 | |
| 3+0 | 6.9 | 7.0 | 0.1 | + | Donor | Donor | Yes | 343.2 | 3 | BE/BB | 2 | |
| 6+4 | 4.3 | 7.2 | 2.9 | + | Donor | Partner | Yes | 243.7 | 2 | BE/BB, BE/BC | 2 | |
| 19+2 | 6.0 | 7.9 | 1.9 | + | Patient | Partner | Yes | 438.4 | 3 | BC/BC | 3 | |
| 9+1 | 4.0 | 7.5 | 3.5 | + | Donor | Donor | No | 766.9 | 3 | BE/BB | 3 | |
| 5+1 | 8.1 | 7.8 | -0.3 | + | Donor | Partner | Yes | 246.9 | 2 | BE/BC, BC/BC | 1 | |
| 2+1 | 6.3 | 7.3 | 1.0 | + | Donor | Partner | Yes | 435.0 | 3 | BE/BB | 1 | |
| 15+3 | 6.6 | 7.8 | 1.2 | + | Patient | Partner | Yes | 319.0 | 2 | BE/BC, BC/BC | 2 | |
| 16+3 | 4.9 | 7.9 | 3.0 | + | Donor | Partner | Yes | 158.0 | 2 | BE/BB | 1 | |
| 7+4 | 6.1 | 7.3 | 1.2 | + | Donor | Partner | No | 306.0 | 3 | BE/BB(2), BC/BB | 2 | |
| 20+4 | 5.0 | 7.0 | 2.0 | + | Patient | Partner | Yes | 355.1 | 2 | BE/BB | 1 | |
| 19+1 | 8.0 | 12.0 | 4.0 | + | Donor | Partner | Yes | 53.0 | 2 | BE/BB | 1 | |
| 16+5 | 7.0 | 7.2 | 0.2 | + | Donor | Partner | Yes | 1905.3 | 2 | BE/BB | 1 | |
| 10+4 | 4.5 | 9.7 | 5.2 | + | Donor | Partner | Yes | 1768.0 | 3 | BE/BB | 1 | |
| 12+2 | 6.0 | 8.7 | 2.7 | + | Patient | Partner | Yes | 186.0 | 2 | BE/BC | 1 | |
| 9+5 | 6.0 | 8.0 | 2.0 | + | Donor | Donor | Yes | 162.5 | 2 | BE/BB | 2 | |
| Aborted | 11+1 | 5.6 | 9.0 | 3.4 | + | Donor | Partner | No | 1208.0 | 2 | BE/BB | 1a |
| 13+5 | 6.9 | 10.6 | 3.7 | + | Donor | Donor | Yes | 201.5 | 3 | BC/BB | 1 | |
| 11+0 | 6.0 | 8.4 | 2.4 | + | Donor | Partner | No | 68.5 | 3 | BE/BA, BE/AB, BE/BB | 1a | |
| 27+3 | 6.1 | 7.8 | 1.7 | + | Donor | Donor | Yes | 638.3 | 2 | BE/BC | 2 | |
| 10+2 | 5.5 | 9.4 | 3.9 | + | Donor | Donor | Yes | 299.6 | 2 | BE/AA, BE/BB | 1 | |
| 14+2 | 4.0 | 11.2 | 7.2 | + | Donor | Partner | No | 57.0 | 3 | BE/BB(2), BE/BC(1) | 1 | |
| 5+6 | 5.1 | 7.0 | 1.9 | + | Patient | Partner | Yes | 150.5 | 2 | BE/BB | 1 | |
| NR | 4.6 | 12.3 | 7.7 | + | Donor | Partner | Yes | 1231.5 | 2 | BE/BB | 0 | |
| NR | 6.4 | 8.2 | 1.8 | + | Donor | Partner | Yes | 88.0 | 2 | BE/BB | NR | |
| 5+2 | 5.0 | 6.2 | 1.2 | - | Patient | Partner | Yes | 293.0 | 3 | BE/BB(1), BE/BC(2) | 1 | |
| 10+0 | 5.8 | 7.3 | 1.5 | + | Patient | Partner | Yes | 262.0 | 3 | BE/AA(1), BE/AB(2) | 1 | |
| Biochemical | 8+3 | 4.5 | 5.6 | 1.1 | - | Donor | Partner | Yes | 40.3 | 3 | BE/BB | 0 |
| 6+1 | 6.0 | 7.4 | 1.4 | + | Donor | Partner | Yes | 255.5 | 3 | BE/BB(1), BE/BC(2) | 1 | |
| 2+3 | 4.6 | 7.1 | 2.5 | + | Donor | Partner | Yes | 65.0 | 3 | BE/BB | 1b | |
| 3+2 | 6.0 | 8.0 | 2.0 | + | Donor | Partner | Yes | 167.0 | 3 | BE/BB | NR | |
| Not Pregnant | NA | 5.2 | 8.0 | 2.8 | + | Donor | Donor | Yes | 0 | 3 | BE/BB | 0a |
| NA | 5.4 | 7.1 | 1.7 | + | Donor | Partner | Yes | 0 | 2 | BE/AB | 0 | |
| NA | 4.5 | 7.0 | 2.5 | + | Donor | Donor | Yes | 0.1 | 2 | BE/BB | 0 | |
| NA | 6.3 | 12.3 | 6.0 | + | Donor | Partner | Yes | 0 | 2 | BE/BB, BE/BC | 0 | |
| NA | 2.1 | 6.2 | 4.1 | - | Patient | Partner | Yes | 0.1 | 3 | BC/BB, BE/BC, BE/BB | 0 | |
| NA | 6.3 | 7.6 | 1.3 | + | Patient | Partner | Yes | 0 | 2 | BC/BB | 0a | |
| NA | 4.0 | 7.0 | 3.0 | + | Patient | Partner | Yes | 1.7 | 1 | BE/BB | 0 | |
| NA | 6.6 | 9.0 | 2.4 | + | Donor | Donor | Yes | 0 | 3 | BE/BB(1), BE/BC(2) | 0 | |
| NA | 6.0 | 8.2 | 2.2 | + | Donor | Partner | No | 0 | 3 | BE/BB(2), BE/BC(1) | 0 | |
| NA | 5.5 | 8.7 | 3.2 | + | Donor | Partner | Yes | 0 | 2 | BE/AA | 0 | |
| NA | 5.0 | 7.0 | 2.0 | + | Donor | Partner | Yes | 0.1 | 3 | BE/CC | 0 | |
| NA | 10.9 | 7.3 | -3.6 | + | Donor | Donor | Yes | 0 | 2 | NR | 0 | |
| NA | 4.1 | 5.6 | 1.5 | - | Patient | Partner | Yes | 0 | 3 | BE/BC | 0 | |
| NA | 3.2 | 11.6 | 8.4 | + | Donor | Partner | Yes | 7.7 | 2 | BE/BB | 0 | |
| NA | 5.3 | 7.2 | 1.9 | + | Patient | Partner | Yes | 0 | 1 | BE/BC | 0 | |
| NA | 4.5 | 7.5 | 3.0 | + | Patient | Partner | Yes | 0 | 3 | BC/BB(1), BC/BB(2) | 0 | |
| NA | 4.5 | 7.2 | 2.7 | + | Donor | Partner | No | 0 | 3 | BC/BB(1), BE/BB(2) | 0 | |
| NA | 8.1 | 10.0 | 1.9 | + | Donor | Donor | Yes | 8.1 | 3 | NR | 0 | |
| NA | 6.0 | 6.2 | 0.2 | - | Donor | Donor | Yes | 0 | 3 | BE/BB | 0 | |
| NA | 6.7 | 8.6 | 1.9 | + | Donor | Donor | Yes | 0 | 2 | BE/BB | 0 | |
| NA | 4.5 | 5.5 | 1.0 | - | Donor | Partner | Yes | 0 | 3 | BE/BE | 0 | |
| NA | 3.0 | 7.3 | 4.3 | + | Donor | Donor | Yes | 0.16 | 2 | BE/BB, BE/BC | 0 | |
Abbreviations: EMT: endometrial mean thickness; Diff: difference in mm between pre and post-EMT; ICM: Inner Cell Mass; NA: not applicable; NR: not recorded; QTPC: Qualitative test for pregnancy confirmation; β-hCG: beta-fraction of human chorionic gonadotropin.
The patient had multiple IVF cycles. These are the data for the first cycle.
The patient has multiple IVF cycles. These are the data for the second cycle.
After PRP and before assisted reproduction, the patient was able to get pregnant spontaneously.
Table 3.
Comparison of outcomes between the treatment and the previous cycles
| Category | Previous Cycle | Treatment Cycle | p-valuea |
|---|---|---|---|
| Cycle implantation rate (%) | 22.6 (14/62) | 63.9 (39/62) | <0.001*** |
| Normal weight | 30.8 (8/26) | 71.1 (19/26) | 0.001*** |
| Overweight/obese | 17.1 (6/35) | 57.1 (20/35) | 0.001*** |
| Clinical pregnancy rate (%) | 16.1 (10/62) | 57.4 (35/62) | <0.001*** |
| Normal weight | 19.2 (5/26) | 69.2 (18/26) | 0.001*** |
| Overweight/obese | 14.3 (5/35) | 48.6 (17/35) | 0.002* |
| Ongoing pregnancy/live birth rate (%) | 0.0 (0/62) | 40.3 (25/62) | <0.001*** |
| Normal weight | 0.0 (0/27) | 51.9 (14/27) | <0.001*** |
| Overweight/obese | 0.0 (0/35) | 31.4 (11/35) | 0.001* |
p-value was calculated using the McNemar test.
Significant results (two-tailed) are indicated as follows:
P<0.05;
P<0.001.
Platelets significantly fluctuate during the menstrual cycle
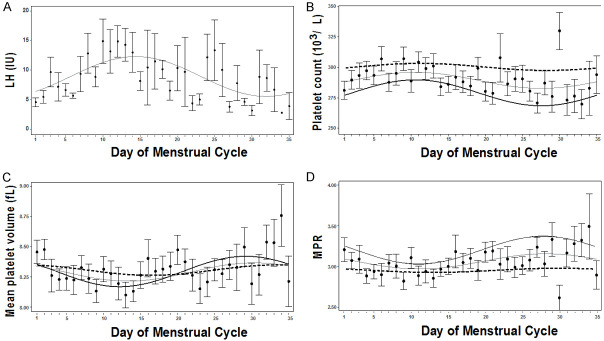
Using the NHANES dataset, 41,474 participants were identified (Supplementary Figure 3). 48.9% of the cohort were men, and 36.5% of the cohort did not have acceptable information on the number of days since the end of their period; therefore, these participants were excluded. Additionally, 3,132 participants were removed for having BMIs or ages outside the defined acceptable range, and 983 participants were removed for having conditions that could affect their menstrual cycle or their platelet concentration. Lastly, 88 participants were removed for lacking complete CBCs, resulting in 1,854 participants being included in this analysis (Table 4). The total cohort presented an average BMI of 26.5 ± 0.2 kg/m2; however, most of the cohort were overweight or obese (51.7%). When the cohort was divided into two groups (“normal weight” and “overweight/Obese”), there were no clinical differences regarding age, systolic or diastolic blood pressure or LH. The platelet count was significantly higher in the normal weight group, which resulted in the MPR index scores being significantly higher in this group as well (P<0.05).
Table 4.
Characteristics of the study participants from the NHANES dataset
| Category | Totala | NWa | OW/OBa | p-valueb |
|---|---|---|---|---|
| Sample (n) | 1854 | 831 | 1023 | - |
| Age (years) | 32.7 ± 0.3 | 31.7 ± 0.4 | 33.5 ± 0.4 | 0.001** |
| Weight (kg) | 70.5 ± 0.7 | 59.0 ± 0.3 | 81.2 ± 0.8 | <0.001*** |
| Height (m) | 163.1 ± 0.3 | 163.6 ± 0.4 | 162.7 ± 0.3 | 0.075 |
| Body-mass Index (kg/m2) | 26.5 ± 0.2 | 22.0 ± 0.1 | 30.6 ± 0.2 | <0.001*** |
| Normal weight (%) | 48.2 ± 2.1 | 100 | - | - |
| Overweight (%) | 26.0 ± 1.6 | - | 50.3 ± 2.6 | |
| Obese (%) | 25.7 ± 1.7 | - | 49.7 ± 2.6 | |
| Waist circumference (cm) | 88.29 ± 0.55 | 78.6 ± 0.3 | 97.3 ± 0.7 | <0.001*** |
| Systolic blood pressure (mmHg) | 111 ± 1 | 108 ± 1 | 114 ± 1 | <0.001*** |
| Diastolic blood pressure (mmHg) | 68 ± 1 | 67 ± 1 | 69 ± 1 | 0.001** |
| Blood counts | ||||
| Red blood cell (n) | 4.49 ± 0.19 | 4.45 ± 0.02 | 4.52 ± 0.02 | 0.002** |
| White blood cell (n) | 7.32 ± 0.07 | 7.07 ± 0.08 | 7.57 ± 0.11 | 0.001* |
| Platelet count (103/mL) | 293.7 ± 2.3 | 284.6 ± 4.7 | 302.2 ± 3.1 | 0.008* |
| Mean platelet volume (fL) | 8.16 ± 0.02 | 8.14 ± 0.05 | 8.18 ± 0.04 | 0.553 |
| MPR | 2.98 ± 0.03 | 3.08 ± 0.06 | 2.89 ± 0.04 | 0.025 |
| Laboratory results | ||||
| Follicle stimulating hormone (mIU/mL) | 7.7 ± 0.5 | 8.1 ± 0.6 | 7.3 ± 0.6 | 0.262 |
| Luteinizing hormone (mIU/mL) | 9.7 ± 0.9 | 10.6 ± 1.1 | 9.0 ± 1.2 | 0.260 |
| Days since the last period | 15.1 ± 0.3 | 15.3 ± 0.4 | 14.9 ± 0.4 | 0.480 |
Abbreviations: NW: normal weight group; MPR: the mean platelet volume to platelet count ratio; OW/OB: overweight/obese group.
Data are presented as mean or frequency ± standard error.
p-value corresponds to the difference between the NW and OW/OB groups determined by either the Chi2 test, Student’s t-Test, or Mann-Whitney U test.
Significant results (two-tailed) are indicated as follows:
P<0.05;
P<0.01;
P<0.001.
The COSINOR analysis was used to assess obesity’s effect on platelet fluctuations during the menstrual cycle. For this cohort, to verify that the model was predictive, the well-characterized hormone LH, that oscillates during the menstrual cycle was examined (Figure 2A). As expected, LH presented a significant rhythm, as confirmed with the zero-amplitude test (P<0.05). A significant MESOR and amplitude (MESOR = 8.7 mg/dL, amplitude = 3.5 mg/dL) was observed, and the cosine fit conformed to the expected cycle, suggesting that the cohort represents a typical menstrual cycle. For the platelet count and the MPR index, in the overall cohort, a rhythm was observed (P<0.05, Figure 2B-D and Table 5), with platelet volume almost achieving significance (P = 0.056). However, when the cohort was stratified into normal weight and overweight/obese, the model presented with a significant rhythm (P<0.05). Upon further investigation, only the normal weight group presented with a significant amplitude and acrophase (P<0.05). Even though the MESORS were significant for platelet count, platelet volume, and the MPR index, the MESORs for platelet count were significantly higher in the overweight/obese group than in the normal weight group (P<0.001). However, the MESORs for the MPR index, the normal weight group, presented a higher score (P<0.001). Overall, this suggests that obesity affects platelet fluctuations during the menstrual cycle.
Figure 2.
Platelets fluctuate during the menstrual cycle, which is affected by obesity. Means (dot) and standard error (bars) plots with cosine waves were constructed to demonstrate the changes for (A) luteinizing hormone (LH, IU/L), (B) platelet count (103/μL), (C) mean platelet volume (fL), and (D) the mean platelet volume to platelet count ratio index (MPR) during the menstrual cycle. Cosinor model fits are shown for all participants (grey line), normal-weight participants (black line), and overweight/obese participants (dashed line).
Table 5.
Fluctuations in platelet count, platelet volume, and the mean platelet volume to platelet count ratio index during the menstrual cycle, stratified into normal weight and overweight/obese participants
| Group | Na | MESORb | Amplitude | Acrophaseb | pZero-ampc |
|---|---|---|---|---|---|
| Platelet count | |||||
| Overall | 1854 | 291 (288-294), <0.001* | 7 (2-11), 0.006* | -1.4 (-2.1--0.7), <0.001* | 0.022* |
| Normal weight | 831 | 280 (275-285), <0.001* | 11 (4-18), 0.003* | -1.4 (-2.0--0.7), <0.001* | <0.001*** |
| Overweight/obese | 1023 | 301 (290-313), <0.001* | 3 (-4-9), 0.377 | -1.3 (-3.3-0.8), 0.238 | |
| pMESORd<0.001* | pAMPe = 0.108 | pACRf = 0.907 | |||
| Platelet volume | |||||
| Overall | 1854 | 8.30 (8.27-8.35), <0.001* | 0.07 (0.01-0.14), 0.016* | -0.8 (-1.6--0.1), 0.022* | 0.056 |
| Normal weight | 831 | 8.30 (8.24-8.36), <0.001* | 0.12 (0.03-0.21), 0.007* | -1.1 (-1.7--0.4), 0.002* | 0.012* |
| Overweight/obese | 1023 | 8.31 (8.17-8.46), <0.001* | 0.04 (-0.03-0.12), 0.302 | -0.3 (-2.1-1.5), 0.739 | |
| pMESORd = 0.792 | pAMPe = 0.184 | pACRf = 0.419 | |||
| MPR index | |||||
| Overall | 1854 | 3.06 (3.01-3.10), <0.001* | 0.09 (0.03-0.16), <0.001*** | -1.9 (-2.0--0.6), <0.001* | 0.022* |
| Normal weight | 831 | 3.19 (3.12-3.26), <0.001* | 0.17 (0.07-0.27), <0.001*** | -1.3 (-1.8--0.7), <0.001* | <0.001*** |
| Overweight/obese | 1023 | 2.94 (2.78-3.10), <0.001* | 0.03 (-0.07-0.12), 0.580 | -0.9 (-4.1-2.2), 0.569 | |
| pMESORd<0.001* | pAMPe = 0.034* | pACRf = 0.818 |
Abbreviations: MESOR: Midline Estimating Statistic of Rhythm; MPR: the mean platelet volume to platelet count ratio.
Number of participants.
MESOR, amplitude, and acrophase were determined using the COSINOR and COSINOR2 packages for R. Values are estimated, as well as a 95% confidence interval and p-values.
p-value was calculated using the cosinor. Detect function of the COSINOR2 package, which detects rhythm (also called the zero-amplitude test) and tests the overall significance of the cosinor model.
p-value is for the difference in MESORS and was calculated using the summary function of the COSINOR package. The result assessed the difference between the second and primary categories.
p-value is for the difference in amplitude and was calculated using the test_cosinor function of the COSINOR package.
p-value is for the difference in acrophase and was calculated using the test_cosinor function of the COSINOR package.
Significant results (two-tailed) are indicated as follows:
P<0.05;
P<0.001.
Discussion
In this study, the implantation and clinical pregnancy rates improved in women who underwent intrauterine infusion of PRP. Patients who achieved a live birth or had an ongoing pregnancy showed an increase in their Endometrial thickness (EMT); however, patients in whom the IVF cycle resulted in a failed implantation, biochemical pregnancy, or an abortion also had similar increases. Thus, this suggests that, even though PRP did improve EMT, other factors should still be considered, such as obesity. Indeed, obesity was associated with low rates of embryo implantation and clinical pregnancy. Here, we also show that obesity is associated with loss of platelet fluctuations during the menstrual cycle.
EMT is postulated to be critical in determining an augmented probability of achieving successful embryo implantation [25]. Indeed, in 90% of the cycles included in our cohort, the patients’ EMT was >7 mm after PRP treatment, and a higher portion had their embryos implanted (63.9%). Furthermore, it has been demonstrated that both clinical pregnancy and live birth rates decreased significantly when EMT was <7 mm, independent of whether the embryo transfer was fresh or frozen-thawed [5]. The mechanisms for thin endometrium-impaired receptivity have not yet been elucidated; nevertheless, the balance between the pro- and anti-inflammatory cytokines and their associated pathways are speculated to be key for endometrium receptivity [7,26,27]. Three proposed mechanisms involved in PRP’s contribution to endometrial adequateness are angiogenesis, metabolic health, and the Th1/Th2 cell ratio.
Endometrial angiogenesis is crucial for endometrial regeneration. Insufficient vascularization may be a plausible cause of thin or inadequate endometrium, as uterine blood flow is essential for endometrial growth and vascular development [25]. PRP application has been demonstrated to improve reproductive outcomes due to increased tissue vascularization in mice [28]. Recently, it has been proposed that the therapeutical feature of PRP in endometrial repair for infertile patients is mediated by growth factors contained in platelet granules released in response to activating stimuli. PDGFs facilitate angiogenesis, cytoskeletal plasticity, and cell migration by exerting mitogenic action on cells of mesenchymal origin. At the same time, VEGF promotes angiogenesis by supporting tissue homeostasis and promoting accelerated repair in the case of damage. Even though highly variable in concentration, VEGF is a critical factor associated with endometrial receptivity [29]. Immunochemical characterization of PRP injected into patients with endometrial atrophy, who presented a significant increase in EMT, revealed that higher levels of PDGF-BB (2.8 times) and VEGF (2.4 times) are present in PRP when compared to ordinary plasma [30].
Within our cohort, many of the patients also suffered from other forms of infertility, affecting the endometrium’s receptivity. Metabolic disorders, such as insulin resistance, PCOS, or Type 2 Diabetes Mellitus, are more frequent in overweight/obese patients. Recently, our group demonstrated that correcting insulin resistance in PCOS patients, which was also associated with weight loss, improved EMT and endometrial receptivity for successful ongoing pregnancies [31]. Recently, in PCOS patients, a significant increase in EMT was observed after PRP infusions; however, none of the patients were able to conceive. Although not significant, the increase in EMT for the PCOS group was less when compared to patients with other non-metabolic factors of infertility (tubal or diminished ovarian reserve) [32]. This may explain the observed differences between the normal-weight and the overweight/obese patients observed here, in which obesity was associated with lower rates of implantation, clinical pregnancy, and ongoing pregnancy/live birth. Therefore, future work should evaluate how metabolic disorders influence the effect PRP has on endometrial health.
Successful embryo implantation requires the immune system to tolerate the semi-allogeneic embryo by altering the balance of pro- and anti-inflammatory cytokines secreted by Th1 and Th2 cells, respectively [33]. Th1 dominance is associated with implantation failure after embryo transfer, potentially due to the toll-like receptor 4 (TLR4) [34], whereas anti-inflammatory cytokines from the Th2 cells favor implantation [33]. It has been demonstrated that repeated implantation failure and recurrent pregnancy loss have been linked to a Th1/Th2 cell ratio, favoring Th1 cells [35]. Therefore, a shift favoring Th2 cytokines would be beneficial for IVF. PRP downregulates the expression of TLR4, a receptor that protects against non-autologous antigens [36]. Using PRP alters the endometrium’s inflammatory cytokine profile. Thus, it is postulated that PRP may act by promoting Th2 cytokines and diminishing Th1 cytokines. This would improve endometrial immune tolerance of endometrial decidualization and improve embryo implantation. Future studies should consider the concentration of cytokines within the PRP isolate and the patient’s cytokine profile at the endometrium level.
In vitro studies have shown that prolonged exposure to PRP (three to seven days) leads to endometrial stromal and mesenchymal cell migration and increased proliferation in a time-dependent manner [13]. Therefore, our study proposed a three-dose scheme to mimic this effect. This is because multiple intra-articular injections of PRP in damaged tissues are more effective than a single application, as seen for knee osteoarthritis [37,38]. This is supported by other studies where the clinical pregnancy rate was higher when at least two doses were applied over a single dose [21,39,40]. Therefore, it is posited that a multi-dose treatment should be considered for future studies.
The embryo’s successful implantation depends on platelet activation [41,42]. Platelet activation, which consists of the adhesion and aggregation of platelets, is part of primary hemostasis [43]. This activity not only occurs in epithelial lesions but is also activated by inflammatory processes or blood flow alterations, some of which thin the thickness of the endometrium. If platelet activation is inhibited, the body will suffer from hypofunction of its tissues [44]. Platelet count assesses platelet activation, and mean platelet volume is a marker of platelet size and function [45]. These last two hematologic parameters must be maintained at adequate levels to achieve excellent physiological reproduction [44,46,47]. Platelet activation causes changes in the female reproductive tract, such as increasing the motility of the cilia in the uterine tubes and improving the zygote’s transport to the uterine cavity. If platelet activation fails or is diminished, early implantations that are not viable for the embryo can occur in the uterine tubes. Decreased platelets in mice affected the rate of uterine ectopic pregnancies [42]. In the same way, platelet activation causes vasodilation and angiogenesis at the endometrial level to favor embryo implantation [42,48]. Here, it was observed that the platelet count, the platelet volume, and the MPR index cycled for the normal weight group but were planar for the obese group. Adiposity has been associated with thrombotic changes by promoting platelet activation [49]. However, other studies have not established whether elevated platelet counts in obese individuals are associated with platelet activation [16]. Obesity directly affects the menstrual cycle, as shown by studies where obesity decreases the transport and production of female sex hormones due to altering the hypothalamus-pituitary-ovarian axis. In obese women, gonadotropin-releasing hormone levels are affected, altering the temporospatial production of FSH and LH, which are vital for the menstrual cycle [50,51]. Due to the presence of estrogen receptors on the platelets, platelet function is affected by the concentration of LH and FSH [52]; therefore, it was expected that obesity would affect platelet function. In normal-weight patients, platelet function should be increased more during the luteal phase than in the follicular phase [53,54]. Here, we observed that platelet function increased during the luteal phase in patients in the normal weight group and decreased during the follicular phase. It was found that the MPR index in overweight/obese women was arhythmic, which is why platelet activity is expected to be decreased in these patients. Therefore, the effect observed in the clinical study presented here is most likely due to the PRP injections mimicking the normal increase seen for normal-weight participants.
Our study has a few limitations. First, the quality of the PRP was not measured. The lack of a consensus among researchers about the optimal procedure for PRP preparation may affect the clinical outcomes, as the quality and contents of the isolate can vary significantly between each study, between the patient’s IVF cycles, and each subsequent PRP preparation for the same patient with an IVF cycle [55]. However, the PRP was obtained and applied three times, and the sample was minimally degraded. Second, the self-control study design uses a repeated measure methodology instead of including a control group. With IVF, the probability of an embryo implanting decreases with each additional cycle. Therefore, the likelihood of an embryo implanting is low after two failed IVF cycles. Concerning the NHANES study, first, only women who presented information about days since their last period were utilized. This assessment did not consider the average number of days for each participant’s cycle or whether there were any abnormalities associated with their cycle. Second, diseases affecting platelet count and quality, such as congenital pathologies, especially hematological diseases (hemophilia, hemoglobinopathies, von Willebrand disease, Wiskott-Aldrich syndrome), were not assessed. Moreover, patients taking certain medications, such as heparins, NSAIDs, or antiepileptics, that could directly affect the normal physiology of platelets were not excluded. Therefore, the potential effects of these conditions should be considered and assessed in future studies. Third, the results shown here were not adjusted. Platelet counts and volumes are affected by endometriosis, diet, and lifestyle. These factors should be considered in future studies.
In conclusion, we show that an intrauterine infusion of autologous PRP improved the embryo implantation rate and the clinical pregnancy rate of women suffering from refractory thin endometrium, most likely promoting a change in the uterine environment. In addition, we demonstrate that the platelets fluctuate during the menstrual cycle, which can be lost when subjects become obese. Therefore, intrauterine infusions of PRP are suspected to mimic the normal changes associated with normal-weight women, who are typically more fertile.
Acknowledgements
We want to express our gratitude to this study’s participants and the IVF and medical staff at Ingenes, especially to Abril Romero. The research was supported by the Ingenes Institute for materials; however, they did not have a role in the design, collection, analysis, and interpretation of data or in writing the manuscript. DHM received support from the National Council of Science and Technology of Mexico (CONACYT, scholarship number 790971).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.López-Luna A, Hernández-Melchor D, Ramírez-Martínez L, López-Bayghen E. The genetic and biochemical blueprint of endometrial receptivity: past, present, and future factors involved in embryo implantation success. In: Gomy E, editor. Modern Medical Genetics and Genomics. IntechOpen; 2018. [Google Scholar]
- 2.Bos-Mikich A, Ferreira MO, de Oliveira R, Frantz N. Platelet-rich plasma or blood-derived products to improve endometrial receptivity? J Assist Reprod Genet. 2019;36:613–620. doi: 10.1007/s10815-018-1386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouanness M, Ali-Bynom S, Jackman J, Seckin S, Merhi Z. Use of intra-uterine injection of platelet-rich plasma (PRP) for endometrial receptivity and thickness: a literature review of the mechanisms of action. Reprod Sci. 2021;28:1659–1670. doi: 10.1007/s43032-021-00579-2. [DOI] [PubMed] [Google Scholar]
- 4.Karateke A, Kaplanoglu M, Baloglu A. Relations of platelet indices with endometrial hyperplasia and endometrial cancer. Asian Pac J Cancer Prev. 2015;16:4905–4908. doi: 10.7314/apjcp.2015.16.12.4905. [DOI] [PubMed] [Google Scholar]
- 5.Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40000 embryo transfers. Hum Reprod. 2018;33:1883–1888. doi: 10.1093/humrep/dey281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura H, Hosono T, Minato K, Hamasaki T, Kumasawa K, Kimura T. Importance of optimal local uterine blood flow for implantation. J Obstet Gynaecol Res. 2014;40:1668–1673. doi: 10.1111/jog.12418. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Sengupta J, Kriplani A, Roy KK, Ghosh D. Profiles of cytokines secreted by isolated human endometrial cells under the influence of chorionic gonadotropin during the window of embryo implantation. Reprod Biol Endocrinol. 2013;11:116. doi: 10.1186/1477-7827-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajipour H, Farzadi L, Latifi Z, Keyhanvar N, Navali N, Fattahi A, Nouri M, Dittrich R. An update on platelet-rich plasma (PRP) therapy in endometrium and ovary related infertilities: clinical and molecular aspects. Syst Biol Reprod Med. 2021;67:177–188. doi: 10.1080/19396368.2020.1862357. [DOI] [PubMed] [Google Scholar]
- 9.Maleki-Hajiagha A, Razavi M, Rouholamin S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: a systematic review and meta-analysis. J Reprod Immunol. 2020;137:103078. doi: 10.1016/j.jri.2019.103078. [DOI] [PubMed] [Google Scholar]
- 10.Dawood AS, Salem HA. Current clinical applications of platelet-rich plasma in various gynecological disorders: an appraisal of theory and practice. Clin Exp Reprod Med. 2018;45:67–74. doi: 10.5653/cerm.2018.45.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlovic V, Ciric M, Jovanovic V, Stojanovic P. Platelet rich plasma: a short overview of certain bioactive components. Open Med (Wars) 2016;11:242–247. doi: 10.1515/med-2016-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranisavljevic N, Raad J, Anahory T, Grynberg M, Sonigo C. Embryo transfer strategy and therapeutic options in infertile patients with thin endometrium: a systematic review. J Assist Reprod Genet. 2019;36:2217–2231. doi: 10.1007/s10815-019-01576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghajanova L, Houshdaran S, Balayan S, Manvelyan E, Irwin JC, Huddleston HG, Giudice LC. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J Assist Reprod Genet. 2018;35:757–770. doi: 10.1007/s10815-018-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, Liang X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286–1290. [PMC free article] [PubMed] [Google Scholar]
- 15.Dag ZO, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16:111–117. doi: 10.5152/jtgga.2015.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samocha-Bonet D, Justo D, Rogowski O, Saar N, Abu-Abeid S, Shenkerman G, Shapira I, Berliner S, Tomer A. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm. 2008;2008:834153. doi: 10.1155/2008/834153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 19.Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med. 2006;25:2618–2631. doi: 10.1002/sim.2477. [DOI] [PubMed] [Google Scholar]
- 20.Farrington P, Whitaker H, Ghebremichael Weldeselassie Y. Self-Controlled Case Series Studies, A Modelling Guide with R. Chapman and Hall/CRC. 2018. [Google Scholar]
- 21.Morad A, Elgendy H, Assar T. Autologous platelet-rich plasma to prevent a thin endometrium in patients undergoing clomiphene citrate therapy: a pilot prospective self-controlled trial. Evidence Based Women’s Health Journal. 2021;11:10–16. [Google Scholar]
- 22.Zargar M, Pazhouhanfar R, Najafian M, Choghakabodi PM. Effects of intrauterine autologous platelet-rich plasma infusions on outcomes in women with repetitive in vitro fertilization failures: a prospective randomized study. Clinical and Experimental Obstetrics & Gynecology. 2021;48:179–184. [Google Scholar]
- 23.Ripley B. The R Project in Statistical Computing. Msor Connection. The newsletter of the LTSN Maths, Stats & OR Network. 2001;1:23–25. [Google Scholar]
- 24.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eftekhar M, Tabibnejad N, Tabatabaie AA. The thin endometrium in assisted reproductive technology: an ongoing challenge. Middle East Fertility Society Journal. 2018;23:1–7. [Google Scholar]
- 26.Rajaei S, Zarnani AH, Jeddi-Tehrani M, Tavakoli M, Mohammadzadeh A, Dabbagh A, Mirahmadian M. Cytokine profile in the endometrium of normal fertile and women with repeated implantation failure. Iran J Immunol. 2011;8:201–208. [PubMed] [Google Scholar]
- 27.Boomsma CM, Kavelaars A, Eijkemans MJ, Amarouchi K, Teklenburg G, Gutknecht D, Fauser BJ, Heijnen CJ, Macklon NS. Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod Biomed Online. 2009;18:85–94. doi: 10.1016/s1472-6483(10)60429-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10:61. doi: 10.1186/s13287-019-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosny N, Goubran F, BadrEldin Hasan B, Kamel N. Assessment of vascular endothelial growth factor in fresh versus frozen platelet rich plasma. J Blood Transfus. 2015;2015:706903. doi: 10.1155/2015/706903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Efendieva Z, Vishnyakova P, Apolikhina I, Artemova D, Butov K, Kalinina E, Fedorova T, Tregubova A, Asaturova A, Fatkhudinov T, Sukhikh G. Hysteroscopic injections of autologous endometrial cells and platelet-rich plasma in patients with thin endometrium: a pilot randomized study. Sci Rep. 2023;13:945. doi: 10.1038/s41598-023-27982-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palafox-Gomez C, Ortiz G, Madrazo I, Lopez-Bayghen E. Adding a ketogenic dietary intervention to IVF treatment in patients with polycystic ovary syndrome improves implantation and pregnancy. Reprod Toxicol. 2023;119:108420. doi: 10.1016/j.reprotox.2023.108420. [DOI] [PubMed] [Google Scholar]
- 32.Dogra Y, Singh N, Vanamail P. Autologous platelet-rich plasma optimizes endometrial thickness and pregnancy outcomes in women with refractory thin endometrium of varied aetiology during fresh and frozen-thawed embryo transfer cycles. JBRA Assist Reprod. 2022;26:13–21. doi: 10.5935/1518-0557.20210037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng SC, Gilman-Sachs A, Thaker P, Beaman KD, Beer AE, Kwak-Kim J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am J Reprod Immunol. 2002;48:77–86. doi: 10.1034/j.1600-0897.2002.01105.x. [DOI] [PubMed] [Google Scholar]
- 34.Lan Y, Li Y, Yang X, Lei L, Liang Y, Wang S. Progesterone-induced blocking factor-mediated Th1/Th2 balance correlates with fetal arrest in women who underwent in vitro fertilization and embryo transfer. Clin Immunol. 2021;232:108858. doi: 10.1016/j.clim.2021.108858. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda K, Nakagawa K, Horikawa T, Moriyama A, Ojiro Y, Takamizawa S, Ochiai A, Matsumura Y, Ikemoto Y, Yamaguchi K, Sugiyama R. Increasing number of implantation failures and pregnancy losses associated with elevated Th1/Th2 cell ratio. Am J Reprod Immunol. 2021;86:e13429. doi: 10.1111/aji.13429. [DOI] [PubMed] [Google Scholar]
- 36.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 37.Subramanyam K, Alguvelly R, Mundargi A, Khanchandani P. Single versus multi-dose intra-articular injection of platelet rich plasma in early stages of osteoarthritis of the knee: a single-blind, randomized, superiority trial. Arch Rheumatol. 2021;36:326–334. doi: 10.46497/ArchRheumatol.2021.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chouhan DK, Dhillon MS, Patel S, Bansal T, Bhatia A, Kanwat H. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a Guinea pig model of early knee osteoarthritis. Am J Sports Med. 2019;47:2300–2307. doi: 10.1177/0363546519856605. [DOI] [PubMed] [Google Scholar]
- 39.Dzhincharadze LG, Abubakirov AN, Mishieva NG, Bakuridze EM, Bystrykh OA. Effectiveness of intrauterine administration of autologous platelet-rich plasma for the preparation of the “thin” endometrium for the program of defrosted embryo transfer. Obstetrics and Gynegology. 2021;2:90–95. [Google Scholar]
- 40.Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. 2018;57:810–813. doi: 10.1016/j.tjog.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Hu CY. The role of platelet-activating factor (PAF) in fertilization and implantation. Shengzhi Yu Biyun. 1991;11:7–10. [PubMed] [Google Scholar]
- 42.O’Neill C. The role of paf in embryo physiology. Hum Reprod Update. 2005;11:215–228. doi: 10.1093/humupd/dmi003. [DOI] [PubMed] [Google Scholar]
- 43.Saad J, Asuka E, Schoenberger L. Physiology, Platelet Activation. In: StatPearls. Treasure Island (FL) ineligible companies: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023. [Google Scholar]
- 44.Bodis J, Papp S, Vermes I, Sulyok E, Tamas P, Farkas B, Zambo K, Hatzipetros I, Kovacs GL. “Platelet-associated regulatory system (PARS)” with particular reference to female reproduction. J Ovarian Res. 2014;7:55. doi: 10.1186/1757-2215-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colkesen Y, Muderrisoglu H. The role of mean platelet volume in predicting thrombotic events. Clin Chem Lab Med. 2012;50:631–634. doi: 10.1515/CCLM.2011.806. [DOI] [PubMed] [Google Scholar]
- 46.Moser G, Guettler J, Forstner D, Gauster M. Maternal platelets-friend or foe of the human placenta? Int J Mol Sci. 2019;20:5639. doi: 10.3390/ijms20225639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill C, Gidley-Baird AA, Pike IL, Porter RN, Sinosich MJ, Saunders DM. Maternal blood platelet physiology and luteal-phase endocrinology as a means of monitoring pre- and postimplantation embryo viability following in vitro fertilization. J In Vitro Fert Embryo Transf. 1985;2:87–93. doi: 10.1007/BF01139339. [DOI] [PubMed] [Google Scholar]
- 48.Roudebush WE, Wininger JD, Jones AE, Wright G, Toledo AA, Kort HI, Massey JB, Shapiro DB. Embryonic platelet-activating factor: an indicator of embryo viability. Hum Reprod. 2002;17:1306–1310. doi: 10.1093/humrep/17.5.1306. [DOI] [PubMed] [Google Scholar]
- 49.Bukhari IA, Habib SS, Alnahedh A, Almutairi F, Alkahtani L, Alareek LA, Assiri GA. Relationship of body adiposity with platelet function in obese and non-obese individuals. Cureus. 2020;12:e6815. doi: 10.7759/cureus.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itriyeva K. The effects of obesity on the menstrual cycle. Curr Probl Pediatr Adolesc Health Care. 2022;52:101241. doi: 10.1016/j.cppeds.2022.101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeung EH, Zhang C, Albert PS, Mumford SL, Ye A, Perkins NJ, Wactawski-Wende J, Schisterman EF. Adiposity and sex hormones across the menstrual cycle: the BioCycle study. Int J Obes (Lond) 2013;37:237–243. doi: 10.1038/ijo.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupuis M, Severin S, Noirrit-Esclassan E, Arnal JF, Payrastre B, Valera MC. Effects of estrogens on platelets and megakaryocytes. Int J Mol Sci. 2019;20:3111. doi: 10.3390/ijms20123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feuring M, Christ M, Roell A, Schueller P, Losel R, Dempfle CE, Schultz A, Wehling M. Alterations in platelet function during the ovarian cycle. Blood Coagul Fibrinolysis. 2002;13:443–447. doi: 10.1097/00001721-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Alzahrani F, Hassan F. Modulation of platelet functions assessment during menstruation and ovulatory phases. J Med Life. 2019;12:296–300. doi: 10.25122/jml-2019-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, Borojevic R. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.