ABSTRACT
Lenacapavir is a novel, first-in-class, multistage inhibitor of HIV-1 capsid function approved for the treatment of multidrug-resistant HIV-1 infection in combination with other antiretrovirals for heavily treatment-experienced people with HIV. Two Phase 1, open-label, parallel-group, single-dose studies assessed the pharmacokinetics (PK) of lenacapavir in participants with moderate hepatic impairment [Child–Pugh–Turcotte (CPT) Class B: score 7–9] or severe renal impairment [15 ≤ creatinine clearance (CLcr) ≤29 mL/min] to inform lenacapavir dosing in HIV-1-infected individuals with organ impairment. In both studies, a single oral dose of 300 mg lenacapavir was administered to participants with normal (n = 10) or impaired (n = 10) hepatic/renal function who were matched for age (±10 years), sex, and body mass index (±20%). Lenacapavir exposures [area under the plasma concentration–time curve from time 0 to infinity (AUCinf) and maximum concentration (Cmax)] were approximately 1.47- and 2.61-fold higher, respectively, in participants with moderate hepatic impairment compared to those with normal hepatic function, whereas lenacapavir AUCinf and Cmax were approximately 1.84- and 2.62-fold higher, respectively, in participants with severe renal impairment compared to those with normal renal function. Increased lenacapavir exposures with moderate hepatic or severe renal impairment were not considered clinically meaningful. Lenacapavir was considered generally safe and well tolerated in both studies. These results support the use of approved lenacapavir dosing regimen in patients with mild (CPT Class A: score 5–6) or moderate hepatic impairment as well as in patients with mild (60 ≤ CLcr ≤ 89 mL/min), moderate (30 ≤ CLcr ≤ 59 mL/min), and severe renal impairment.
KEYWORDS: hepatic impairment, renal impairment, pharmacokinetics, lenacapavir, long-acting, HIV-1
INTRODUCTION
Lenacapavir is a novel, first-in-class, multistage, selective inhibitor of HIV-1 capsid function approved for the treatment of multidrug-resistant HIV-1 in adults (1, 2). Currently, lenacapavir is also being investigated for the prevention of HIV-1 infection (3, 4). Lenacapavir is characterized by low human clearance and low aqueous solubility (5–8), which supports its every 6 months (Q6M) administration for treatment and prevention (9, 10). To date, lenacapavir has been evaluated in multiple clinical studies in both healthy participants and people with HIV-1 (PWH) and has been shown to be generally safe and well tolerated. The dosing regimen of lenacapavir used in the pivotal Phase 2/3 study and currently approved in highly treatment-experienced PWH includes 600 mg (2 × 300 mg tablets) oral dose on Days 1 and 2, 300 mg (1 × 300 mg tablet) oral dose on Day 8, and 927 mg (2 × 1.5 mL) subcutaneous (SC) injection Q6M starting from Day 15 to achieve effective plasma concentrations of lenacapavir (4).
Oral lenacapavir is rapidly absorbed with a median half-life (t1/2) of 10 to 12 days. Absolute bioavailability following oral administration of lenacapavir is low (~6–10%), and lenacapavir exposures increase less than dose proportionally over the dose range of 50 to 1,800 mg (4, 11). Lenacapavir administered subcutaneously is completely absorbed with peak plasma concentrations occurring ~84 days postdose. Lenacapavir exposures following subcutaneous injection increase dose proportionally over the dose range of 309 to 927 mg, with an apparent t1/2 of 8 to 12 weeks. Lenacapavir is a substrate of P-glycoprotein (P-gp), uridine diphosphate glucuronosyltransferase (UGT)1A1, and cytochrome P450 enzyme (CYP)3A and is highly bound to plasma proteins (>99%) (11).
People with HIV can have hepatic impairment due to hepatitis B and/or hepatitis C co-infection or a variety of other etiologies such as drug-induced hepatitis, chronic alcohol use, or toxin exposure (12). In a retrospective study by Qin et al., liver damage prevalence was 12.4% in approximately 2,100 PWH (13). Similarly, PWH may also be at risk of acute kidney injury, HIV-associated kidney disease, or chronic kidney disease. A meta-analysis showed that chronic kidney disease in PWH is common and its prevalence was approximately 4.8 to 12% (14). Both renal and hepatic impairment may alter the absorption, disposition, and elimination of drugs due to changes in metabolizing enzyme and/or drug transporter activity, hepatic blood flow, or drug–plasma protein binding, ultimately resulting in altered pharmacokinetics of the drug (15–17).
Evaluation of radiolabeled intravenous lenacapavir in a human mass balance study indicated that lenacapavir was majorly eliminated as an intact moiety, which was the predominant component in plasma (68.8% of total radioactivity) and feces (~33% of the administered dose and ~76% of the recovered dose). Lenacapavir is primarily eliminated via excretion into bile and intestinal secretion by P-gp with a minor contribution from metabolic pathways (CYP3A and UGT1A1). Negligible (0.24%) elimination of lenacapavir was observed in urine. Metabolites constituted a minor component of total radioactivity in plasma and feces (4, 18, 19). Based on this understanding of the elimination pathways of lenacapavir, a reduced design was utilized in this study to enable evaluation of the selected category of organ impairment rather than the entire range of severity.
The objective of this investigation was to assess the PK, safety, and tolerability of lenacapavir in participants with moderate hepatic impairment or severe renal impairment, following oral administration of a single 300 mg dose. Results from these studies were utilized to inform lenacapavir dosing recommendations in patients with impaired organ function.
RESULTS
Participant demographics and baseline characteristics
Twenty participants in total were enrolled in the hepatic impairment study (10 participants with moderate hepatic impairment and 10 healthy matched control participants with normal hepatic function), and 20 participants in total were enrolled in the renal impairment study (10 participants with severe renal impairment and 10 healthy matched control participants with normal renal function) (Table 1). All 20 participants in the hepatic impairment study, except for a healthy control participant who was lost to follow-up, received the study drug and completed the study. In the renal impairment study, all 20 participants received the study drug and completed the study. Additional parameters for hepatic or renal function are presented in (Table S4).
TABLE 1.
Demographics and baseline characteristicsa
| Moderate hepatic impairment (n = 10) |
Normal hepatic function (n = 10) |
Severe renal impairment (n = 10) |
Normal renal function (n = 10) |
|
|---|---|---|---|---|
| Sex (at birth), n (%) | ||||
| Male | 7 (70.0) | 7 (70.0) | 7 (70.0) | 7 (70.0) |
| Female | 3 (30.0) | 3 (30.0) | 3 (30.0) | 3 (30.0) |
| Age, median (range), years | 56 (39–71) | 55 (31–69) | 69 (18–77) | 63 (21–73) |
| Race, n (%) | ||||
| White | 10 (100.0) | 10 (100.0) | 9 (90.0) | 10 (100.0) |
| Black | 0 | 0 | 1 (10.0) | 0 |
| Ethnicity, n (%) | ||||
| Hispanic or Latinx | 7 (70.0) | 5 (50.0) | 7 (70.0) | 5 (50.0) |
| Not Hispanic or Latinx | 3 (30.0) | 5 (50.0) | 2 (20.0) | 5 (50.0) |
| Not provided | 0 | 0 | 1 (10.0) | 0 |
| ALT, median (range), units/L | 23 (11–312) | 17 (8–32) | 15 (8–30) | 18 (8–45) |
| AST, median (range), units/L | 33 (14–129) | 17 (11–19) | 16 (9–24) | 18 (12–27) |
| Serum creatinine, median (range), mg/dL | 0.83 (0.55–1.19) | 0.84 (0.55–1.05) | 3.24 (1.81–5.03) | 0.83 (0.51–1.05) |
| CLcr, median (range), mL/min | 113 (69.4–224) | 117 (90.4–193) | 21.9 (15.8–30.8) | 98.4 (90.0–130) |
| Weight, median (range), kg | 81.8 (54.8–127) | 93.0 (58.5–113.2) | 75.6 (41.4–114) | 78.8 (64–97.6) |
| Height, median (range), cm | 168.9 (151–188) | 174.5 (152-191) | 168.5 (145-185) | 173.5 (161-179) |
| BMI, median (range), kg/m2 | 31.9 (23.5–37.8) | 29.5 (25.0–36.1) | 26.6 (19.7–33.2) | 26.6 (23.7–30.5) |
| CPT score, n (%) | ||||
| 7 | 7 (70) | NA | NA | NA |
| 8 | 3 (30) | NA | NA | NA |
BMI, body mass index; CPT, Child–Pugh–Turcotte; ALT, alanine aminotransferase, AST, aspartate transaminase, NA, not available.
Pharmacokinetic results
Effect of hepatic impairment on lenacapavir pharmacokinetics
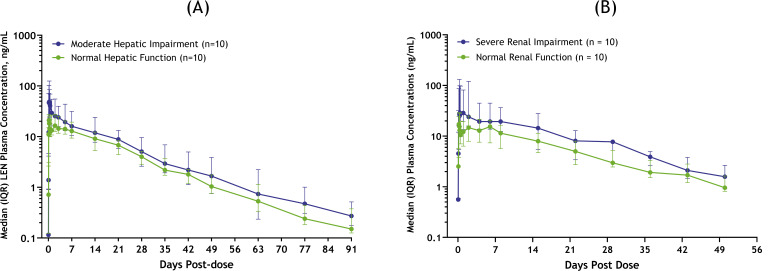
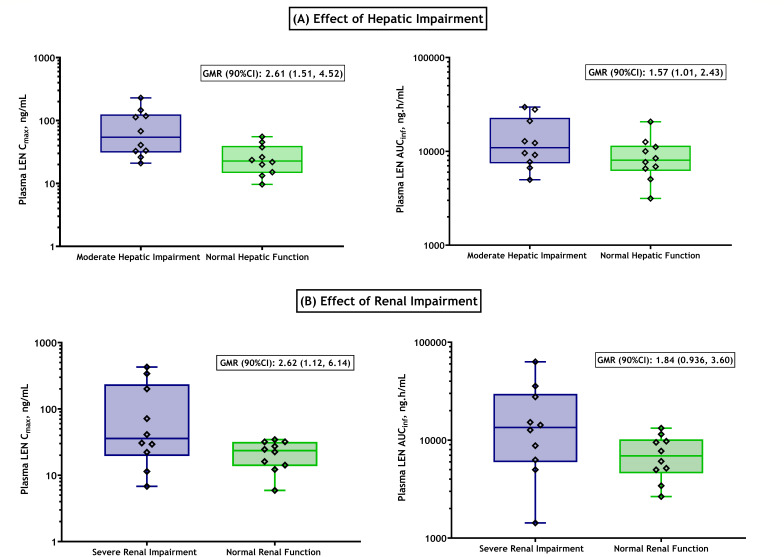
Following a single oral dose of 300 mg lenacapavir, plasma concentrations of lenacapavir increased in both groups, with a median Tmax (time to Cmax) of 6 and 4 hours in participants with moderate hepatic impairment and their healthy matched controls with normal hepatic function, respectively. Higher exposures were observed in participants with moderate hepatic impairment relative to healthy matched control participants with normal hepatic function (Fig. 1A); geometric mean Cmax were 61.1 and 23.4 ng/mL, respectively (Table 2). However, total lenacapavir plasma exposures (as assessed by AUCinf) increased marginally in the hepatic impairment group relative to healthy matched control participants with normal hepatic function; geometric mean AUCinf were 12,000 ng*h/mL and 8,180 ng*h/mL, respectively. Geometric least-squares means ratios (GMRs) for lenacapavir AUCinf and Cmax were approximately 1.47- and 2.61-fold higher, respectively, in participants with moderate hepatic impairment relative to participants with normal hepatic function (Fig. 2). Median t1/2 were 12.6 and 13.1 days for participants with moderate hepatic impairment and their healthy matched controls with normal hepatic function, respectively. Lenacapavir plasma protein binding was high (>99%) and similar between both groups. The relationship between lenacapavir exposures and hepatic function was explored using Spearman’s correlation analysis, and no significant relationships were observed when lenacapavir exposure parameters (AUC and Cmax) were plotted as a function of the CPT score and individual elements of CPT classification (e.g., albumin, total bilirubin, prothrombin time, or international normalized ratio). In the moderate hepatic impairment group, there were 5 participants with ascites and 2 participants with mild ascites. Most participants with ascites were on diuretics for treating fluid retention. As shown in Table 2, while a numeric difference was noted, the apparent volume of distribution for most (6 out of 7) participants in the moderate hepatic impairment group with ascites was within the range of those with normal hepatic function. Therefore, volume of distribution was not significantly affected in participants with moderate hepatic impairment. The mean apparent volume of distribution of lenacapavir in the moderate hepatic impairment group was numerically lower than that in healthy matched controls, which is likely due to increased bioavailability in participants with hepatic impairment.
Fig 1.
Pharmacokinetics of lenacapavir in participants with (A) moderate hepatic impairment, (B) severe renal impairment, and respective healthy matched controls. IQR, interquartile range.
TABLE 2.
Summary of lenacapavir pharmacokinetic parameters in moderate hepatic impairment, severe renal impairment, and healthy matched control groups
| Pharmacokinetic parametera |
Cmax (ng/mL) |
Tmax (h) |
AUClast (h*ng/mL) |
AUCinf (h*ng/mL) |
CL/Fb (L/h) |
Vz/Fb (L) |
t1/2b (days) |
|---|---|---|---|---|---|---|---|
| Hepatic impairment | |||||||
| Moderate hepatic impairment (n = 10) | 61.1 (21.0, 229) |
6.00 (2.00, 48.0) |
11,900 (4940, 29,200) |
12,000 (4,990, 29,600) |
25.0 (10.1, 60.2) |
10,800 (4,060, 23,000) |
12.6 (9.74, 17.1) |
| Normal hepatic function (n = 10) | 23.4 (9.70, 55.2) |
4.00 (4.00, 12.0) |
7,590 (3,050, 20,500) |
8,180 (3,150, 20,700) |
36.6 (14.5, 95.3) |
17,000 (6,970, 49,800) |
13.1 (10.7, 17.0) |
| Moderate hepatic impairment/normal hepatic function; GMR (90% CI) | 2.61 (1.51, 4.52) |
NC | 1.57 (1.01, 2.43) |
1.47 (0.947, 2.27) |
NC | NC | NC |
| Renal impairment | |||||||
| Severe renal impairment (n = 10) | 51.5 (6.80, 427) |
8.00 (4.00, 48.0) |
11,500 (1,310, 62,400) |
12,100 (1,430, 63,000) |
24.8 (4.76, 210) |
8,560 (1,690, 46,000) |
9.73 (5.69, 16.6) |
| Normal renal function (n = 10) | 19.7 (5.90, 34.4) |
6.00 (4.00, 48.0) |
6,050 (2,420, 12,300) |
6,590 (2,660, 13,200) |
45.5 (22.6, 113) |
20,900 (10,000, 52,300) |
13.3 (11.0, 17.0) |
| Severe renal impairment/normal renal function; GMR (90% CI) | 2.62 (1.12, 6.14) |
NC | 1.89 (0.952, 3.77) |
1.84 (0.936, 3.60) |
NC | NC | NC |
Pharmacokinetic parameters presented to three significant figures. AUCinf, AUClast, Cmax, CL/F, and Vz/F presented as geometric mean (minimum, maximum); Tmax (time to maximal concentration) and t1/2 (half-life) presented as median (minimum, maximum). AUCinf, area under the plasma concentration–time curve from time 0 to infinity; AUClast, area under the plasma concentration–time curve from time 0 to the last quantifiable plasma concentration; CI, confidence interval; CL/F, apparent oral clearance; Vz/F, apparent volume of distribution; Cmax, maximum concentration; GMR, geometric least-squares means ratio; NC, not calculated; t1/2, half-life; Tmax, time to maximum concentration.
Nonparametric comparisons of PK parameters between hepatic and renal function groups are presented in (Table S5).
Fig 2.
Effect of hepatic (A) or renal (B) impairment on lenacapavir exposure parameters Cmax and AUCinf. Data summarized as individual points; horizontal lines of the box represent the median with its interquartile, and error bars represent the range. GMRs with their 90% CIs are indicated in individual plots.
Effect of renal impairment on lenacapavir pharmacokinetics
Following a single oral dose of 300 mg lenacapavir, plasma concentrations of lenacapavir were higher in participants with severe renal impairment relative to healthy matched controls with normal renal function. Lenacapavir was absorbed with a comparable median Tmax of 6 to 8 hours, but higher exposures were observed in participants with severe renal impairment compared to healthy matched control participants with normal renal function (Fig. 1B); geometric mean Cmax were 51.5 and 19.7 ng/mL, respectively (Table 2). Similarly, total lenacapavir plasma exposures (as assessed by AUCinf) were higher in the renal impairment group relative to healthy matched control participants with normal renal function; geometric mean AUCinf were 12,100 ng*h/mL and 6,590 ng*h/mL, respectively. GMRs for lenacapavir AUCinf and Cmax were approximately 1.84- and 2.62-fold higher, respectively, in participants with severe renal impairment relative to participants with normal renal function (Fig. 2). Median t1/2 were 9.75 and 13.3 days for participants with severe renal impairment and their healthy matched controls with normal renal function, respectively. Lenacapavir plasma protein binding was high (>99%) and was similar between both groups. No meaningful relationships were observed when lenacapavir exposure parameters (AUC and Cmax) were plotted as a function of baseline renal function.
Safety
In both studies, a single oral dose of 300 mg lenacapavir was generally safe and well tolerated when administered to participants with impaired organ function and their healthy matched controls. No deaths, grade 4 adverse events (AEs), or AEs leading to premature discontinuation of the study drug or discontinuation of study participation were observed in either study.
In the hepatic impairment study, treatment-emergent AEs were reported in 6 of 20 participants [3/10 (30%) in the moderate hepatic impairment group and 3/10 (30%) in the normal hepatic function group]. All AEs were grade 1 or 2 in severity, and all AEs were reported as resolved. No serious AEs or grade 3 AEs were reported (Table S1 and S2).
In the renal impairment study, AEs were reported in 5 of 20 participants [4/10 (40%) in the severe renal impairment group and 1/10 (10%) in the normal renal function group]. The majority of the AEs were grade 1 or 2 in severity. One participant (10.0%) had a grade 3 AE (hypertension), which was not related to lenacapavir. All AEs were resolved. A serious AE (melena) occurred on Day 15 in one participant in the severe renal impairment group, which was considered unrelated to the study drug and resolved 6 days later (Table S1 and S3).
DISCUSSION
Comorbidities such as impaired liver and/or kidney function can be commonly found in PWH. As organ impairment is known to alter the disposition of drugs via multiple mechanisms, it is imperative to evaluate the pharmacokinetics and safety of investigational agents in participants with hepatic or renal impairment to inform appropriate dosing recommendations in these HIV-1 subpopulations (15–17). This is the first report summarizing the combined results of two separate studies that evaluated the effect of organ impairment on the pharmacokinetics, safety, and tolerability of lenacapavir.
Evaluation of single oral lenacapavir doses in a Phase 1 study and cross-study comparison demonstrated a significant overlap of exposures between 300 and 600 mg oral lenacapavir doses (20, 21). Additionally, a human mass balance study conducted with a single intravenous 20 mg dose of [14C]lenacapavir showed that the systemic clearance of lenacapavir was lower relative to hepatic blood flow (extraction ratio = 0.03), which suggests that the pre-systemic (first pass) clearance following oral absorption is minimal and studies conducted with oral lenacapavir can be readily extrapolated to SC administration (18). Furthermore, the long apparent t1/2 of 10–12 weeks for SC lenacapavir makes the conduct of these organ impairment studies with SC dosing impractical due to substantial clinical confinement time and burden on participants. Based on these considerations, a single oral 300 mg dose used in the hepatic impairment and renal impairment studies was deemed adequate to support the assessment of lenacapavir pharmacokinetics and safety for the approved clinical regimen.
Lenacapavir exposures in participants with moderate hepatic impairment (CPT Class B) or severe renal impairment were consistently higher from Day 1 through the end of PK collection compared to the respective healthy matched control participants. As the higher exposures observed with moderate hepatic impairment or severe renal impairment were not associated with significant changes in the elimination t1/2, the differences in exposures relative to healthy matched controls may be attributed to changes in lenacapavir absorption rather than altered elimination. Furthermore, higher variability in Cmax (as a result of altered absorption) was observed for both moderate hepatic impairment as well as severe renal impairment groups and may have contributed to the higher fold-change observed for Cmax, relative to AUC changes. The apparent volume of distribution of lenacapavir in the moderate hepatic impairment and severe renal impairment groups was numerically lower than that of respective healthy matched controls, which is also likely due to increased bioavailability in participants with organ impairment. While organ impairment can often impact the plasma protein binding of small molecules, additional analyses showed that the protein binding of lenacapavir was high (≥ 99%) and similar between the organ impairment groups and the respective healthy matched controls (2, 11).
Hepatic impairment is known to decrease intestinal P-gp and CYP3A4 enzyme expression, while UGT enzyme expression seems to be largely unchanged in cirrhosis (22). Stangier et al. evaluated the impact of moderate hepatic impairment (CPT-B) on the PK of dabigatran etexilate, a substrate for P-gp. Reported plasma exposures of dabigatran etexilate were relatively higher in participants with hepatic impairment compared to healthy controls, indicating a possible reduction in P-gp-mediated efflux (23). McConn II et al. reported that duodenal CYP3A expression and total midazolam hydroxylation were reduced by 47% and 34%, respectively, in participants with cirrhosis compared to healthy controls. Furthermore, greater decreases in gut CYP3A expression were associated with increasing severity of cirrhosis (24). Wang et al. evaluated the effect of liver failure on both intestinal P-gp expression and function using P-gp substrates (zidovudine and rhodamine) in a preclinical model. Hepatic failure significantly downregulated intestinal P-gp expression in rats (to 35% of that in the control rats) and therefore helped increase both absorption and bioavailability of these substrates (25). Collectively, these findings support the hypothesis that hepatic impairment may downregulate the expression of P-gp in the intestine and reduce the intestinal secretion of lenacapavir, which may result in increased bioavailability of orally administered lenacapavir. Various liver pathologies or disease types can lead to different degrees of hepatic impairment (CP-A, mild; CP-B, moderate; and CP-C, severe). Atilano-Roque et al. reviewed the effect of disease pathologies on canalicular P-gp expression and function. In advanced stages of primary biliary cirrhosis, protein expression was increased with unchanged mRNA expression, while in nonalcoholic steatohepatitis, increased mRNA expression was associated with increased protein expression. In hepatocellular carcinoma, protein expression was decreased with increased mRNA expression, and inconsistent mRNA expression was noted in hepatitis C (16). In another study, mRNA levels of canalicular MDR1 were reported to remain unchanged in cholestatic alcoholic hepatitis (26). Disease-related alterations of transporter protein expression may not always translate to the transporter activity (27). Given the discordance in these findings, it is unclear how altered canalicular P-gp expression in hepatic impairment would affect the biliary excretion of lenacapavir. As the contribution of CYP3A-mediated metabolism of lenacapavir is low, a significant change in exposures is not anticipated due to differences in intestinal and hepatic CYP3A.
Similar to hepatic impairment, chronic kidney disease has also been known to impact both uptake and efflux transporters as well as metabolic enzymes in the liver and gastrointestinal tract, as evidenced by decreased clearance for non-renally cleared drugs (17, 28, 29). While direct evidence of changes in enzyme and transporter expression in renal impairment is currently not available, uremic toxins that accumulate in the plasma as a result of impaired renal function are considered to be the cause of transcriptional, translational, or acute post-translational modifications in hepatic and intestinal drug transporters (17). As a result, impaired renal function may also increase the bioavailability of P-gp substrates by decreasing the activity of these efflux transporters in the intestine (17, 29). Tatosian et al. evaluated a micro-dose cocktail of CYP450 and drug transporter substrates in participants with different magnitudes of renal impairment (mild, moderate, and severe) and observed an impact on bioavailability via reduction in gut P-gp activity (by up to 40%) with increasing severity of renal impairment using dabigatran etexilate, a P-gp substrate (30). Similarly, Thomson et al. showed a significant increase (~3 fold) in plasma exposures for participants with chronic kidney disease relative to healthy controls with an another P-gp substrate, fexofenadine (31). In our study, the observed higher exposures of lenacapavir in participants with severe renal impairment compared to healthy matched controls may in part be explained by the broader effect of organ insufficiency resulting in the accumulation of uremic toxins affecting both intestinal P-gp and metabolic enzymes.
A limitation of the current study was that while participants with impaired organ function were evaluated, the assessment did not evaluate the impact in participants with the most advanced stage of hepatic or renal impairment, such as people with cirrhosis or end-stage renal disease requiring/not requiring hemodialysis. Nonetheless, lenacapavir is highly bound to plasma proteins (≥ 99%), and therefore hemodialysis is not expected to affect its circulating levels.
Although a modest increase in lenacapavir exposures was observed in participants with organ impairment, it is important to note that plasma exposures of up to 9-fold higher Cmax and up to 15-fold higher AUCtau relative to therapeutic exposures have been evaluated in prior clinical studies of lenacapavir. At these exposures, lenacapavir was well tolerated and was not associated with clinically significant safety concerns. The modest increase in exposures observed in participants with organ impairment is unlikely to represent a safety risk in the target patient population; therefore, no dose adjustment of lenacapavir in patients with moderate hepatic impairment or severe renal impairment was recommended. Furthermore, use of the unadjusted, approved dose of lenacapavir in participants with organ impairment provides convenience and ease of administration for this vulnerable subpopulation of PWH.
In conclusion, lenacapavir exposures were higher in participants with moderate hepatic impairment or severe renal impairment relative to their healthy matched controls. Based on cumulative safety data across clinical dosing regimens (SC and oral) of lenacapavir, the observed increase in lenacapavir exposures with moderate hepatic impairment or severe renal impairment was not deemed clinically significant and therefore do not warrant a dose adjustment for lenacapavir in the organ impaired population. Across each study, the single oral dose of 300 mg lenacapavir was generally safe and well tolerated. These results support the use of lenacapavir in patients with impaired hepatic function [mild (CPT Class A: score 5–6) and moderate (CPT Class B: score 7–9)] or impaired renal function [mild, moderate, and severe].
MATERIALS AND METHODS
Study design
Two separate studies assessed the pharmacokinetics of lenacapavir in participants with moderate hepatic impairment or severe renal impairment relative to healthy matched control participants (19, 32). The studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practices and the principles in the Declaration of Helsinki. The study protocols and informed consents were approved by the study centers’ institutional review board, and participants provided written consent before study participation.
These studies were Phase 1, open-label, parallel-group studies to evaluate the pharmacokinetics of a single oral dose of 300 mg lenacapavir in participants with impaired hepatic or renal function and healthy controls demographically matched for age (±10 years), sex, race, and body mass index (BMI; ±20%). Eligible participants included men and nonpregnant, nonlactating women between 18 and 79 years of age with a body mass index (BMI) of 18.0 to ≤40.0 kg/m2 and a creatinine clearance of ≥60 mL/min (hepatic impairment study; for participants with impaired and normal hepatic function) or ≥90 mL/min (renal impairment study; for participants with normal renal function). A total of 40 participants, 20 in each study (10 participants with moderate hepatic impairment, defined as a score of 7–9 on the CPT classification at screening, or 10 participants with severe renal impairment [15 ≤ CLcr ≤ 29 mL/min] and their healthy matched control participants) were enrolled based on US Food and Drug Administration (FDA) guidance (28, 33–35). Healthy matched control participants in both studies had alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values ≤ upper limit of normal.
Following screening and admission assessments, eligible participants were confined to the study center beginning on Day -1 and until the completion of assessments on Day 8. Following discharge on Day 8, participants returned for follow-up visits on Days 15, 22, 29, 36, 43, and 50. In the hepatic impairment study, additional follow-up visits occurred on Days 64, 78, 92, and 120.
Hepatic impairment study
Participants in the hepatic impairment group were selected based on a diagnosis of chronic (> 6 months), stable hepatic impairment with no clinically significant changes within 2 months prior to study drug administration and a score of 7 to 9 on the CPT scale [moderate hepatic impairment (CPT Class B)] at screening (35). Additional key inclusion criteria for participants with hepatic impairment included ALT and AST values ≤ 10 times the upper limit of normal, platelets ≥ 25,000 /mm3, hemoglobin ≥8 g/dL, and α-fetoprotein < 50 ng/mL.
Renal impairment study
Participants in the renal impairment group were selected based on the classification of severe renal impairment that was considered stable (no clinically significant changes during the 3 months prior to randomization) and not requiring dialysis or anticipated to require dialysis within 90 days of study entry. Additional key inclusion criteria for participants with renal impairment included ALT and AST values ≤ 5 greater than the upper limit of normal.
Lenacapavir pharmacokinetic sampling
For both studies, intensive pharmacokinetic plasma sampling was performed relative to administration of the single oral 300 mg lenacapavir dose on Day 1 at predose (≤5 minutes predose) and 0.5, 1, 2, 4, 6, 8, 12, 24, and 48 hours postdose. Single anytime plasma samples were collected on Days 4, 6, 8, 15, 22, 29, 36, 43, and 50. In the hepatic impairment study, additional plasma samples were collected on Days 64, 78, 92, and 120.
For plasma protein binding evaluation, additional plasma samples were collected on Day 1, and plasma protein binding was determined by equilibrium dialysis using blood samples collected predose from participants with impaired or normal organ function for both studies.
Lenacapavir bioanalysis
Lenacapavir concentrations in plasma samples were determined using fully validated high-performance liquid chromatography–tandem mass spectroscopy bioanalytical methods. The sample volume was 50 µL, and sample preparation was done using supported liquid extraction and Hamilton Star automation. All samples were analyzed within the time frame supported by frozen stability storage data. The chromatographic analysis was performed on a binary Shimadzu Prominence LC-20AD, with an autosampler and a degasser (Shimadzu Corp., Canby, OR), using a Phenomenex Gemini NX-Cl8 column with 110 Å pore size, 50 × 2.0 mm column size, and 5 µm particle size (Part Number 00B-4454-B0) and optimized mobile phases consisting of water: formic acid = 100: 0.1 (Mobile Phase A) and acetonitrile: formic acid = 100:0.1 (Mobile Phase B). Gradient elution with an initial composition of Mobile Phase A:Mobile Phase B of 55:45 (v:v) to a final composition of 3:97 (v:v) was used to chromatographically separate lenacapavir and lenacapavir-d6 (internal standard) from other endogenous interferences of the biological matrix. Ionization and detection of lenacapavir and lenacapavir-d6 were performed on an API-5000 triple quadrupole mass spectrometer (AB Sciex LLC, Framingham, MA, USA), equipped with Turbo Ion Spray MS/MS detection. Positive [M + H]+ ions were monitored for both lenacapavir and lenacapavir-d6 in multiple reaction monitoring mode. Quantitation was performed using parent → product ion (m/z) transitions of 968.4 → 869.3 for lenacapavir and 974.4 → 875.3 for lenacapavir-d6. The calibration ranges were 0.1 to 100 ng/mL for the hepatic impairment study and 0.5 to 500 ng/mL for the renal impairment study. The interassay precision range (%CV) was 2.8 to 8.5, and the interassay accuracy range (%RE) was −6.5 to −4.6 for the hepatic impairment study. The interassay precision range (%CV) was 4.5 to 8.7, and the interassay accuracy range (%RE) was −4.8 to 4.6 for the renal impairment study. The method validation and bioanalysis were performed at Labcorp (Madison, WI, USA).
Statistical analyses
Noncompartmental analyses using Phoenix WinNonlin Professional Version 7.0 (Certara USA Inc., Princeton, NJ, USA) were performed to estimate the pharmacokinetic parameters of lenacapavir. PK parameters included the area under the concentration–time curve (AUC) calculated from time 0 to the last quantifiable plasma concentration (AUClast), AUC from time 0 to infinity (AUCinf), observed peak plasma concentration (Cmax), time to reach peak plasma concentration (Tmax), terminal elimination half-life (t1/2), apparent oral clearance (CL/F), and apparent volume of distribution (Vz/F). The statistical comparisons of the natural log-transformed Cmax, AUClast, or AUCinf for lenacapavir between each organ impaired group and the respective healthy matched controls with normal organ function were based on the matched PKp analysis set for lenacapavir. All participants in each matched PK analysis set for lenacapavir were included in the analysis model.
Lenacapavir plasma concentrations and PK parameters were summarized using descriptive statistics (by group), and a parametric (normal theory) analysis of variance was fitted to the natural log-transformed values of each of the single-dose PK parameters under evaluation as prespecified. For each study, geometric least-squares mean ratios and the corresponding 90% CIs were calculated [(hepatic impairment)/ (healthy control)] or [(renal impairment)/ (healthy control)] for lenacapavir AUCinf, AUClast, and Cmax. Nonparametric analyses for the PK parameters CL/F, Vz/F, and t1/2 were performed using the Wilcoxon rank-sum test for parallel design between impaired organ function and healthy matched control groups. In the hepatic impairment study, the relationship between lenacapavir exposure parameters (AUCinf, AUClast, and Cmax) and overall CPT score or laboratory components of CPT score (e.g., albumin, total bilirubin, prothrombin time, and international normalized ratio) was explored using Spearman’s correlation analysis and examined graphically. In the renal impairment study, the relationship between lenacapavir exposure parameters (AUCinf, AUClast, and Cmax) and baseline renal function was explored using Spearman’s correlation analysis.
Safety evaluation
Safety and tolerability were monitored throughout the hepatic and renal impairment studies by assessment of clinical laboratory tests, electrocardiogram, periodic physical examinations (including vital signs at various time points during the study), and collection/documentation of AEs were coded according to MedDRA Version 23.1.
Safety results were summarized using descriptive statistics for both the organ impaired groups and healthy matched controls, including treatment-emergent AEs (TEAEs), serious AEs, discontinuation due to AEs or death, and laboratory abnormalities.
ACKNOWLEDGMENTS
We thank all the participants and clinical research unit staff who participated in both studies. We also acknowledge all the principal investigators of both studies.
Funding for this project and involved analyses was provided by Gilead Sciences, Inc.
Contributor Information
Vamshi Jogiraju, Email: vamshi.jogiraju@gilead.com.
Miguel Angel Martinez, IrsiCaixa Institut de Recerca de la Sida, Badalona, Spain.
DATA AVAILABILITY
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01344-23.
Tables S1 to S5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. US Food and Drug Administration . 2023. FDA approves new HIV drug for adults with limited treatment options. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-hiv-treatment-patients-limited-treatment-options. Retrieved 17 Jan 2023.
- 2. European Medicines Agency . Sunlenca. Available from: https://www.ema.europa.eu/en/documents/product-information/sunlenca-epar-product-information_en.pdf. Retrieved 17 Jan 2023.
- 3. Bester SM, Wei G, Zhao H, Adu-Ampratwum D, Iqbal N, Courouble VV, Francis AC, Annamalai AS, Singh PK, Shkriabai N, Van Blerkom P, Morrison J, Poeschla EM, Engelman AN, Melikyan GB, Griffin PR, Fuchs JR, Asturias FJ, Kvaratskhelia M. 2020. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science 370:360–364. doi: 10.1126/science.abb4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dvory-Sobol H, Shaik N, Callebaut C, Rhee MS. 2022. Lenacapavir: a first-in-class HIV-1 capsid inhibitor. Curr Opin HIV AIDS 17:15–21. doi: 10.1097/COH.0000000000000713 [DOI] [PubMed] [Google Scholar]
- 5. Daar ES, McDonald C, Crofoot G, Ruane P, Sinclair G, Patel H, Sager J, Liu YP, Brainard DM, Hyland RH, Rhee M. 2019. Safety and antiviral activity over 10 days following a single dose of subcutaneous GS-6207, a first-in-class, long-acting HIV capsid inhibitor in people living with HIV International AIDS Society (IAS) Conference on HIV Science, July 21 to 24, 2019; Mexico City, Mexico [Google Scholar]
- 6. Link JO, Rhee MS, Tse WC, Zheng J, Somoza JR, Rowe W, Begley R, Chiu A, Mulato A, Hansen D, et al. 2020. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 584:614–618. doi: 10.1038/s41586-020-2443-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stepan A, Villasenor G, Jin AG, Nicolas D, Ahmadyar AM, Ram S, Somoza RR, Singer E, Wong M, Xu Y, Link JO, Cihlar T, Link JO, Tse WC. 2019. “GS-6207, a potent and selective first-in-class long-acting HIV-1 capsid inhibitor” Conference on Retroviruses and Opportunistic Infections (CROI), March 4 to 7, 2019; Seattle, WA [Google Scholar]
- 8. Zheng J, Yant SR, Ahmadyar S, Chan TY, Chiu A, Cihlar T, Link JO, Lu B, Mwangi J, Rowe W, Schroeder SD, Stepan GJ, Wang KW, Subramanian R, Tse WC. 2018. 539. GS-CA2: a novel, potent, and selective first-in-class inhibitor of HIV-1 capsid function displays nonclinical pharmacokinetics supporting long-acting potential in humans. Open Forum Infect Dis 5:S199–S200. doi: 10.1093/ofid/ofy210.548 [DOI] [Google Scholar]
- 9. Begley R, Lutz J, Rhee M, Dvory-Sobol H, Chiu A, West SK, Corpus J, Ling J, German P. 2020. “GS-6207 sustained delivery formulation supports 6-month dosing interval” International AIDS Conference, July 6 to 10, 2020; Virtual [Google Scholar]
- 10. Sager JE, Begley R, Rhee M, West SK, Ling J, Schroeder SD, Tse WC, Mathias A. 2019. “Safety and PK of subcutaneous GS-6207, a novel HIV-1 capsid inhibitor” Conference on Retroviruses and Opportunistic Infections (CROI), March 4 to 7, 2019; Seattle, WA [Google Scholar]
- 11. US Food and Drug Administration . Sunlenca. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215973s000lbl.pdf. Retrieved 11 Dec 2023.
- 12. Kaspar MB, Sterling RK. 2017. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol 4:e000166. doi: 10.1136/bmjgast-2017-000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin F, Jiang J, Qin C, Huang Y, Liang B, Xu Y, Huang J, Xu Z, Ning C, Liao Y, Zang N, Lai J, Wei W, Yu J, Ye L, Qin X, Liang H. 2019. Liver damage in patients living with HIV on antiretroviral treatment with normal baseline liver function and without HBV/HCV infection: an 11-year retrospective cohort study in Guangxi, China. BMJ Open 9:e023140. doi: 10.1136/bmjopen-2018-023140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ekrikpo UE, Kengne AP, Bello AK, Effa EE, Noubiap JJ, Salako BL, Rayner BL, Remuzzi G, Okpechi IG. 2018. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS One 13:e0195443. doi: 10.1371/journal.pone.0195443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams RL, Mamelok RD. 1980. Hepatic disease and drug pharmacokinetics. Clin Pharmacokinet 5:528–547. doi: 10.2165/00003088-198005060-00002 [DOI] [PubMed] [Google Scholar]
- 16. Atilano-Roque A, Roda G, Fogueri U, Kiser JJ, Joy MS. 2016. Effect of disease pathologies on transporter expression and function. J Clin Pharmacol 56:S205–S221. doi: 10.1002/jcph.768 [DOI] [PubMed] [Google Scholar]
- 17. Nolin TD, Naud J, Leblond FA, Pichette V. 2008. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 83:898–903. doi: 10.1038/clpt.2008.59 [DOI] [PubMed] [Google Scholar]
- 18. Weber E, Subramanian R, Rowe W, Graupe M, Ling J, Shen G, Begley R, Sager J, Wolckenhauer S, Rhee M, Palaparthy R, Singh R. 2024. Pharmacokinetics, disposition, and biotransformation of [14C]lenacapavir, a novel, first-in-class, selective inhibitor of HIV-1 capsid function, in healthy participants following a single intravenous infusion. Clin Pharmacokinet 63:241–253. doi: 10.1007/s40262-023-01328-1 [DOI] [PubMed] [Google Scholar]
- 19. Jogiraju V, Begley R, Hindman J, West SK, Ho E, Ling J, German P. 2021. “Pharmacokinetics of lenacapavir, an HIV-1 capsid inhibitor, in hepatic impairment” Conference on Retroviruses and Opportunistic Infections (CROI), March 6 to 10, 2021; Virtual [Google Scholar]
- 20. Begley R, Rhee M, West SK, Corpus J, Ling J, German P. 2020. “PK, food effect, and safety of oral GS-6207, a novel HIV-1 capsid inhibitor” Conference on Retroviruses and Opportunistic Infections (CROI), March 8 to 11, 2020; Boston, MA [Google Scholar]
- 21. Jogiraju V, Graham H, West S, Ling J, Cuvin J, Rhee M, Palaparthy R, Singh R. 2022. Pharmacokinetics of a simplified subcutaneous lenacapavir regimen versus Phase 2/3 regimen International AIDS Conference, July 29 to August 2, 2022; Montreal, Canada [Google Scholar]
- 22. Dietrich CG, Götze O, Geier A. 2016. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol 22:72–88. doi: 10.3748/wjg.v22.i1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stangier J, Stähle H, Rathgen K, Roth W, Shakeri-Nejad K. 2008. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol 48:1411–1419. doi: 10.1177/0091270008324179 [DOI] [PubMed] [Google Scholar]
- 24. McConn DJ, Lin YS, Mathisen TL, Blough DK, Xu Y, Hashizume T, Taylor SL, Thummel KE, Shuhart MC. 2009. Reduced duodenal cytochrome P450 3A protein expression and catalytic activity in patients with cirrhosis. Clin Pharmacol Ther 85:387–393. doi: 10.1038/clpt.2008.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang F, Miao MX, Sun BB, Wang ZJ, Tang XG, Chen Y, Zhao KJ, Liu XD, Liu L. 2017. Acute liver failure enhances oral plasma exposure of zidovudine in rats by downregulation of hepatic UGT2B7 and intestinal P-gp. Acta Pharmacol Sin 38:1554–1565. doi: 10.1038/aps.2017.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pauli-Magnus C, Meier PJ. 2006. Hepatobiliary transporters and drug-induced cholestasis. Hepatology 44:778–787. doi: 10.1002/hep.21359 [DOI] [PubMed] [Google Scholar]
- 27. Chu X, Prasad B, Neuhoff S, Yoshida K, Leeder JS, Mukherjee D, Taskar K, Varma MVS, Zhang X, Yang X, Galetin A. 2022. Clinical implications of altered drug transporter abundance/function and PBPK modeling in specific populations: an ITC perspective. Clin Pharmacol Ther 112:501–526. doi: 10.1002/cpt.2643 [DOI] [PubMed] [Google Scholar]
- 28. US Food and Drug Administration . Pharmacokinetics in patients with impaired renal function — study design, data analysis, and impact on dosing. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-renal-function-study-design-data-analysis-and-impact-dosing-and. Retrieved 14 Jun 2022.
- 29. Sun H, Frassetto L, Benet LZ. 2006. Effects of renal failure on drug transport and metabolism. Pharmacol Ther 109:1–11. doi: 10.1016/j.pharmthera.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 30. Tatosian DA, Yee KL, Zhang Z, Mostoller K, Paul E, Sutradhar S, Larson P, Chhibber A, Wen J, Wang YJ, Lassman M, Latham AH, Pang J, Crumley T, Gillespie A, Marricco NC, Marenco T, Murphy M, Lasseter KC, Marbury TC, Tweedie D, Chu X, Evers R, Stoch SA. 2021. A microdose cocktail to evaluate drug interactions in patients with renal impairment. Clin Pharmacol Ther 109:403–415. doi: 10.1002/cpt.1998 [DOI] [PubMed] [Google Scholar]
- 31. Thomson BKA, Nolin TD, Velenosi TJ, Feere DA, Knauer MJ, Asher LJ, House AA, Urquhart BL. 2015. Effect of CKD and dialysis modality on exposure to drugs cleared by nonrenal mechanisms. Am J Kidney Dis 65:574–582. doi: 10.1053/j.ajkd.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 32. Weber EJ, Graham H, West SK, Ling J, Rhee M, Palaparthy R. 2022. “Pharmacokinetics of lenacapavir in participants with severe renal impairment” Conference on Retroviruses and Opportunistic Infections (CROI), February 12 to 16, 2022; Virtual [Google Scholar]
- 33. US Food and Drug Administration . Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-hepatic-function-study-design-data-analysis-and-impact-dosing-and. Retrieved 14 Jun 2022.
- 34. Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 35. Tsoris A, Marlar CA. 2023. Use of the child pugh score in liver disease. In StatPearls. StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S5.
Data Availability Statement
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.