Abstract
Ambulatory blood pressure (ABP) and home blood pressure (HBP) monitoring is currently recommended for management of hypertension. Nonetheless, traditional HBP protocols could overlook diurnal fluctuations, which could also be linked with adverse cardiovascular outcomes. In this observational study, we studied among a group of treated hypertensive patients (N = 62, age: 52.4 ± 10.4 years) by using out-of-office ABP and wearable HBP. They received one session of 24-h ABP measurement with an oscillometric upper-arm monitor, and totally three sessions of 7-day/6-time-daily wearable HBP measurement separated in each month with HeartGuide. Controlled hypertension is defined as an average BP <130/80 mmHg for both daytime ABP and HBP. There was substantial reliability (intraclass correlation coefficient, ICC 0.883–0.911) and good reproducibility (Cohen’s kappa = 0.600) for wearable HBP measurement, especially before breakfast and after dinner. Among all patients, 27.4% had both uncontrolled HBP and ABP, 30.6% had uncontrolled HBP only, while 6.5% had uncontrolled ABP only. Female gender and increased numbers of anti-hypertensive agents are correlated with controlled hypertension. Patients with uncontrolled hypertension had a significantly higher maximal daytime blood pressure, which was previously signified as an imperial marker for cardiovascular risk. In conclusion, wearable HBP monitoring in accordance with a dedicated daily-living schedule results in good reliability and reproducibility. Patients with an uncontrolled wearable HBP should benefit from repeated HBP or ABP measurement for risk stratification.
Keyword: Wearable device, Home blood pressure, Uncontrolled hypertension
Introduction
Out-of-office blood pressure (BP) monitoring is more emphasized by recently updated hypertension guidelines [1–4]. Uncontrolled hypertension detected by out-of-office modalities correlates with target organ damage more accurately than office readings [5, 6]. Both ambulatory blood pressure (ABP) and home blood pressure (HBP) modalities are complementary for hypertension management, considering their assessment in different BP profiles and inherent technical limitations. On one hand, ABP is measured frequently under various settings of activity including work, diet and sleeping, but it remains rather costly and bothersome to patients [7]. On the other, HBP is taken under standardized timing, which is only reliable under correct manual recordings and regular measurements [8, 9]. Diagnostic disagreement for uncontrolled hypertension between HBP and ABP varied from 8.2% to 18.3% [10, 11]. Meanwhile, the reproducibility of detecting uncontrolled hypertension by HBP is limited. The predictors of disagreement included anti-hypertensive treatment and office normotension [12].
In addition to average readings, daytime BP fluctuation is also an important issue. The consistent BP control is important, as more time spent in hypertensive range may be related to increased incidence of cardiovascular events [13]. Recently, more patients are tracking their health behaviors and vital readings on a regular basis with wearable devices [14]. Accumulating evidence revealed that smartwatches may assist to uncover subclinical cardiovascular disorders in ambulatory settings [15]. HeartGuide stands among the latest developed and validated wearable devices suitable for both out-of-office BP measurement and telemedicine applications [16]. In spite of the fact that wearable HBP readings measured by HeartGuide is in good agreement with ABP, reports of wearable HBP to detect control status of hypertension is scarce [17].
The following objectives are to be covered in this study. First, we studied the control status by wearable HBP and inspected predictors associated with controlled hypertension detected by wearable HBP. We also compared the status of BP fluctuation in between patients with or without controlled hypertension by HBP. Second, we investigated the reliability of wearable HBP measurement and the reproducibility to detect an elevated SBP at different daytime periods.
Methods
Study population
Patients actively receiving antihypertensive therapy with a routine office blood pressure (ROBP) <140/90 mmHg were recruited at the cardiovascular clinics at the National Taiwan University Hospital Hsin-chu Branch (Hsin-chu City, Taiwan). We excluded patients with terminal illness, end-stage renal disease, impaired performance status (ECOG ≥2), active pregnancy, resistant hypertension (actively taking ≥4 kinds of antihypertensive drugs), or known persistent arrhythmia. All participating patients provided written informed consent. This study was approved by the Institutional Review Board of the National Taiwan University Hospital Hsin-chu Branch (109-029-E). The study protocol was also registered at ClinicalTrials.gov (NCT04863508).
Demographic data, medications and laboratory data of all recruited participants were acquired from electronic health records. Echocardiography (EPIQ7 or IE33, GE Healthcare, Chicago, Illinois, United States) was performed by certified cardiologists. Left ventricular mass was calculated using the American Society of Echocardiography (ASE) formula [18, 19]. Left ventricular mass index (LVMI) was corrected by body surface area (BSA). Left ventricular hypertrophy (LVH) was defined as a female with an LVMI ≥95 g/m2 or a male with an LVMI ≥115 g/m2.
Blood pressure measurement and variability index
The ROBP was obtained by using an automated oscillometric upper arm BP monitor while attended by registered nurses. BP measurement was taken with patients in a seated position after at least a 5-min rest before measurement. During a routine clinic visit, one ROBP reading each was required at 1st and 3rd month of the study respectively. Final report of the ROBP were obtained by the average of both readings.
ABP was measured for 24 h within the first month after recruitment with a validated oscillometric upper-arm BP monitor (BP3MZ1-1, Microlife Corporation, Taipei, Taiwan). Patients received measurements 30 min apart within the patient-defined awake/daytime period, and 60 min apart within the patient-defined asleep/nighttime period. Final report of 24-h, daytime and nighttime ABP were the average of all BP readings taken at respective timeframe.
Wearable HBP was measured by using HeartGuide (HEM-6411T-MAE; Omron Healthcare, Kyoto, Japan), a wrist-worn oscillometric BP monitor. Patients were instructed to wear the watch-type BP monitor at the non-dominant hand with positioning mark aligned to middle finger. They were required to sit quietly for 5 min beforehand, and to hold the device to heart level and two inches in front of the chest. Automatic BP measurement will then be taken at the appropriate position, which is confirmed by the built-in algorithm and notified with vibration of the device. One session is defined by HBP measurement by HeartGuide at six prespecified time periods daily for one week in each month. The session is repeated consecutively for 3 months. The prespecified periods include within 1 h after wakeup and before breakfast in the morning, 1 h before and after lunch, 1 h before and after dinner, and within 1-h before bedtime (Fig. 1). A total of 42 HBP readings is expected for each monthly session. All HBP readings were automatically accessed by the study team via BlueTooth transmission. The average wearable HBP was calculated for all monthly sessions (described as overall wearable HBP in the paragraph below), for each individual monthly session and for each individual measurement period.
Fig. 1.
Measurement protocol for different modalities
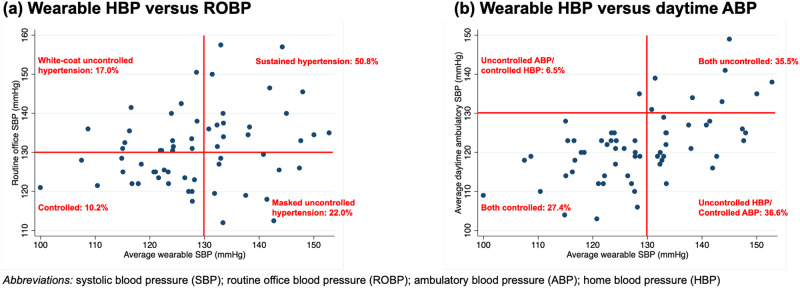
The 2020 Taiwan Society of Cardiology/Taiwan Hypertension Society (TSOC/THS) Consensus on HBP recommended an ROBP <140/90 mmHg but HBP ≥135/85 mmHg be regarded as masked uncontrolled hypertension [1]. The 2022 TSOC/THS guideline had recently proposed a universal BP target <130/80 mmHg for ROBP, daytime ABP and wearable HBP, which is in agreement with the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline [2, 4, 20]. Therefore, recruited patients with an ROBP <130/80 mmHg but an overall wearable HBP ≥130/80 mmHg is categorized as masked uncontrolled hypertension, and those with an ROBP ≥130/80 mmHg but an overall wearable HBP <130/80 mmHg is categorized as white-coat uncontrolled hypertension. Meanwhile, those with both ROBP and overall wearable HBP <130/80 mmHg are defined as sustained controlled hypertension, and those with both ROBP and overall wearable HBP ≥130/80 mmHg are defined as sustained uncontrolled hypertension.
The level of peak blood pressure was calculated as either the average of all wearable home SBP readings above 90th percentile or the highest three readings. The BP variability were also calculated, as defined by coefficient of variation (CV) or average real variability (ARV).
Statistical analysis
Between-group differences were verified using Student’s t test for continuous variables and Chi-square test for categorical variables. A stepwise multiple logistic regression analysis was performed for predictors associated with the presence of controlled hypertension by wearable HBP measurement. Additionally, the performance of wearable HBP measurement at different daytime periods was validated with inter-session reliability and reproducibility of detecting uncontrolled BP. Statistical significance was considered if a P value is <0.05.
The relatively reliability was shown by intra-class correlation coefficient (ICC) index, which is calculated by using one-way random-effects model of absolute agreement. An ICC index ≥0.75 indicates good reliability [21]. The absolute reliability was shown by Bradley-Blackwood test (F index) and repeatability coefficient (RC) [21, 22]. A nonsignificant F index means concordance of both means and variances among different monthly sessions. Meanwhile, RC demonstrates the precision of HBP measurement for all sessions, for which a lower RC value means a higher precision. Finally, the reproducibility of detecting an elevated SBP is shown by both percentage agreement and Cohen’s kappa value.
All analyses were carried out using Stata/MP (StataCorp, College Station, Texas, United States) and corresponding modules for calculating reliability (icc, blandaltman, repeatability) and reproducibility (kappaetc).
Results
Patient characteristics
Patients treated for hypertension were recruited between November 2020 and October 2021. Overall, 76 patients initially provided informed consent, and 11 withdrew consent during the study period. An additional 3 patients were found to have uncontrolled ROBP or were unable to complete the study protocol, and therefore were excluded. Finally, 62 patients were included (44 men and 18 women) (Fig. 2). The average age was 52.4 ± 10.4 years, the average body mass index (BMI) was 26.5 ± 3.8 kg/m2, the average LVMI was 112.6 ± 24.7 g/m2. As for comorbidities, 12.9% of all patients had type 2 diabetes mellitus, 19.4% had coronary artery disease confirmed by angiography, and 3.2% had cerebrovascular disease. All patients used an average of 1.8 ± 0.7 anti-hypertensive drugs, with angiotensin receptor blockers (ARB) being most widely prescribed (79.0%), followed by calcium channel blockers (CCB) (45.2%) and beta blockers (37.1%).
Fig. 2.
Flowchart for study participants
Blood pressure readings
All patients had an average count of 201.6 ± 95.3 wearable HBP readings within 3 months, and an average count of 30.7 ± 7.3 daytime ABP readings taken in first month. The average SBP was 130.9 ± 9.8 mmHg (ROBP), 118.6 ± 9.3 mmHg (24-h ABP), 121.6 ± 8.9 mmHg (daytime ABP), 112.6 ± 11.1 mmHg (nighttime ABP), and 128.6 ± 11.3 mmHg (overall wearable HBP) respectively. There is an average SBP difference of 10.0 ± 9.5 mmHg between 24-h ABP and overall wearable HBP, whereas the difference between daytime ABP and overall wearable HBP being 7.0 ± 9.7 mmHg (Supplementary Fig. 1a).
Meanwhile, the average DBP was 79.7 ± 7.7 mmHg (ROBP), 74.1 ± 6.9 mmHg (24-h ABP), by 76.0 ± 7.0 mmHg (daytime ABP), 70.3 ± 7.5 mmHg (nighttime ABP), and 78.9 ± 7.7 mmHg (overall wearable HBP). There is an average DBP difference of 4.8 ± 6.0 mmHg between 24-h ABP and overall wearable HBP, and the difference between daytime ABP and overall wearable HBP being 2.9 ± 6.4 mmHg (Supplementary Fig. 1b).
Consistency of wearable HBP at different time periods
The reproducibility of detecting controlled hypertension by wearable HBP in each individual month was moderate (Cohen’s kappa = 0.600 between all 3 months of wearable HBP). The Bradley-Blackwood test (F index) showed concordance of HBP measurement at every pre-specified daytime period. The ICC was higher for HBP measurement taken in the morning, after dinner and before bedtime amongst all periods. The HBP measurement taken in morning, after dinner and before bedtime also had the lowest repeatability coefficient, which further suggested the relative consistency of readings at the above time periods. There is a higher percentage agreement and reproducibility regarding classification of elevated blood pressure based on readings taken in the morning and after dinner (Table 2).
Overview and diagnostic agreement of control status
Thirty-six patients had uncontrolled hypertension by overall wearable HBP (58.1%). Patients with or without controlled hypertension by HBP had similar prevalence rates of major comorbidities and left ventricular hypertrophy, while those with controlled hypertension tended to be female and to be under more anti-hypertensive agents. Patients with controlled hypertension also had significantly lower 24-hour, daytime and nighttime ABP (Supplementary Table 1).
When comparing the control status between both ROBP and wearable HBP measurement, 38.7% had sustained hypertension, 19.4% had masked uncontrolled hypertension, 27.4% had white-coat hypertension and finally 14.5% with sustained controlled hypertension (Fig. 3a). Meanwhile, 27.4% of all patients had uncontrolled hypertension for both daytime ABP and wearable HBP, 35.5% with controlled hypertension by both modalities, 30.6% with controlled daytime ABP but uncontrolled wearable HBP and 6.5% with controlled wearable HBP but uncontrolled daytime ABP (Fig. 3b).
Fig. 3.
Comparison of control status between a wearable HBP versus ROBP; and b wearable HBP versus daytime ABP
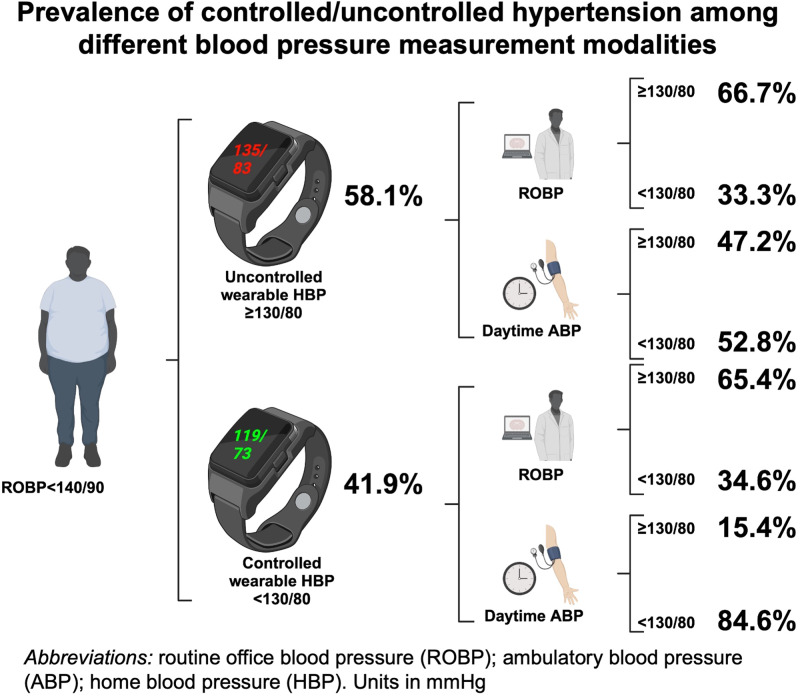
In terms of control status, the overall agreement between daytime ABP and wearable HBP was good (the average of all HBP readings in overall sessions [percentage agreement: 62.9%] or the separate average of HBP readings in each individual monthly session [percentage agreement: 63.9–65.6%]). Most of patients (84.6%) with controlled wearable HBP also had controlled daytime ABP. Meanwhile, only half of all patients (47.2%) with uncontrolled wearable HBP also had uncontrolled daytime ABP (Fig. 4).
Fig. 4.
There is substantial agreement (84.6%) between wearable HBP and daytime ABP in terms of controlled hypertension. Female and those with more prescribed antihypertensive drugs are associated with controlled wearable HBP
Predictors of controlled hypertension with wearable HBP
Multiple logistic regression analysis was performed to identify predictors of uncontrolled hypertension by wearable HBP. The regression analysis was based on 2 models, first on all attributable clinical variables (model 1) and then focusing on variables with a P value <0.2 (model 2). All attributable clinical variables included age (<65 years old as reference), gender (male as reference), obesity (body mass index per 5 kg/m2 increase, with BMI <25 kg/m2 as reference), left ventricular hypertrophy, active smoking, history of coronary artery disease, history of diabetes, and number of prescribed anti-hypertensive drug. Amongst all, variables including gender, obesity, active smoking, history of coronary artery disease, history of diabetes, and number of prescribed anti-hypertensive drugs (monotherapy as reference) had P values below 0.2. These variables were analyzed in model 2. Female and more use of anti-hypertensive drugs are important predictors for controlled hypertension by wearable HBP. There was also a tendency towards controlled hypertension for patients without obesity, and those with diabetes (Table 3).
Blood pressure variability by wearable HBP
The coefficient of variation for SBP was similar regardless of control status (controlled v. uncontrolled: 9.7 ± 2.3% v. 10.6 ± 2.2%, P = 0.131). There was also no difference in terms of average real variability for SBP (controlled v. uncontrolled: 11.9 ± 3.4 mmHg v. 11.6 ± 3.0 mmHg, P = 0.637). However, patients with uncontrolled hypertension had significantly higher peaked BP as measured by either the average SBP of all above 90th percentile (controlled v. uncontrolled: 136.0 ± 9.4 mmHg v. 152.6 ± 9.8 mmHg, P < 0.001) or highest three readings (controlled v. uncontrolled: 150.8 ± 10.2 mmHg v. 166.6 ± 12.6 mmHg, P < 0.001).
Discussion
To the author’s knowledge, this is the first study to investigate the prevalence of controlled hypertension by using a wearable BP device. Wearable HBP revealed good reliability for measurement and reproducibility in detecting the control status. Detecting the prevalence of an elevated SBP is more reproducible when HBP is measured in morning and after dinner than other time periods. Meanwhile, most of patients (84.6%) with well-controlled wearable HBP (<130/80 mmHg) also presented had well-controlled daytime ABP (<130/80 mmHg). Female gender and increased numbers of anti-hypertensive agents are associated with well-controlled status. Patients with uncontrolled hypertension had significantly higher peak daytime blood pressure.
Our study reported a relatively high reliability and good reproducibility of wearable HBP measurement over a relatively short time interval, particularly in the morning and after dinner. The preceding reports upon the performance of HBP monitoring are rather contradictory [23–25]. Some suggested that the reproducibility for HBP to unmask hypertension is suboptimal [26]. Specifically, post-prandial and bedtime BP readings are known to be influenced by meal content and bathing [27, 28]. Nonetheless, post-prandial BP is critical considering the magnitude of meal-related reduction is often associated with higher incidence of lacunar infarctions [29]. Repeated post-prandial HBP measurement may boost the reliability [30]. There are two unique features for this study. First, the study protocol requested to control the dosing regimen, dosing interval and measurement timing. Second, the dietary content for a typical dinner among most Taiwanese people could be generally consistent, while the content itself is highly influenced by socioeconomic background and dietary belief [31].
The percentage of agreement for controlled hypertension between wearable HBP and daytime ABP is higher than previous reports, though a nonnegligible difference of 7.0 ± 9.7/2.9 ± 6.4 mmHg exist between two modalities [11, 32–34]. The agreement in between ABP and HBP in each individual month remains similar. It is understandable as more daytime time-windows are considered by our protocol. Increased detection of uncontrolled hypertension by wearable HBP may be explainable due to sympathetic activation during daytime, and significantly fewer daily recordings compared with daytime ABP [35, 36]. Daytime BP, typically unveiled by ABP and often cloaked by conventional HBP, can predict the risk of cerebrovascular accidents [37]. Notably, around one-fifth of patients had changed controlled status in between monthly sessions, which basically reflects the inadequate reproducibility of out-of-office monitoring in a short time interval [38]. The importance of monthly measurement with wearable devices should therefore be emphasized. An increased number of anti-hypertensive medications is associated with better control, which is congruent with that most hypertensive patients require combination therapy to attain control [39]. A higher proportion of female patients showed adherence to medications, which could explain the superior control status among women patients compared with men [40].
Patients with an uncontrolled wearable HBP also had significantly higher peak daytime BP. In the presence of stiffened arteries, daytime peak BP may be more aggravated by stimuli such as work stress, smoking or temperature [41]. It was shown that a higher maximal daytime ABP is possibly associated with plaque rupture and ensuing stroke events [42, 43]. The findings from our study suggested the applicability of wearable HBP devices in guiding personalized anti-hypertensive treatment, specifically with the target of ameliorating BP fluctuation [44].
Our study has some limitations. First of all, the number of recruited participants by our initial plan was 90 to prove the reproducibility of detecting uncontrolled hypertension. The course of this study was hampered by the strike of COVID pandemic. Nonetheless, substantial agreement between HBP sessions was still observed. Secondly, ABP and wearable HBP were not performed on the concurrent timing. The comparison between both modalities may be flawed by unadjusted lifestyle factors. However, most enrolled patients were office workers, and their daily-living schedule may remain unaltered during the study period. Third, drinking habit could affect the evening HBP, but was not included in our collected demographic information. Nonetheless, patients with habitual drinking are actually expected to be minority. Only 5.1% of all Taiwanese adults reported habitual drinking to the inquiry questionnaire during May Measurement Month campaign in 2017 [9]. Finally, this was a cross-sectional study in which unidentified confounders could likely occur. Still, the prevalence rates of all identified demographic factors and comorbidities were similar between those with and without controlled HBP.
In conclusion, wearable HBP yielded good reliability and reproducibility for out-of-office monitoring, especially with measurements taken in morning and after dinner. Female gender and higher number of anti-hypertensive agents are associated with uncontrolled hypertension. There is significant agreement between wearable HBP and daytime ABP with a BP level <130/80 mmHg. Patients with an elevated wearable HBP should receive repeated out-of-office monitoring for diagnostic confirmation.
Supplementary information
Acknowledgements
Omron Healthcare supplied the HeartGuide devices during the study period. Blood pressure data were curated and analyzed independently by all authors.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Heng-Yu Pan, Chih-Kuo Lee
Supplementary information
The online version contains supplementary material available at 10.1038/s41440-023-01539-w.
References
- 1.Lin HJ, Wang TD, Yu-Chih Chen M, Hsu CY, Wang KL, Huang CC, et al. 2020 Consensus Statement of the Taiwan Hypertension Society and the Taiwan Society of Cardiology on Home Blood Pressure Monitoring for the Management of Arterial Hypertension. Acta Cardiol Sin. 2020;36:537–561. doi: 10.6515/ACS.202011_36(6).20201106A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TD, Chiang CE, Chao TH, Cheng HM, Wu YW, Wu YJ, et al. 2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension. Acta Cardiol Sin. 2022;38:225–325. doi: 10.6515/ACS.202205_38(3).20220321A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5.Sivén SS, Niiranen TJ, Langén VL, Puukka PJ, Kantola IM, Jula AM. Home versus office blood pressure: longitudinal relations with left ventricular hypertrophy: the Finn-Home study. J Hypertens. 2017;35:266–271. doi: 10.1097/HJH.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 6.Pierdomenico SD, Pierdomenico AM, Coccina F, Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of masked uncontrolled. Hypertension. 2018;72:862–869. doi: 10.1161/HYPERTENSIONAHA.118.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kario K, Hoshide S, Chia YC, Buranakitjaroen P, Siddique S, Shin J, et al. Guidance on ambulatory blood pressure monitoring: a statement from the HOPE Asia Network. J Clin Hypertens. 2021;23:411–421. doi: 10.1111/jch.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimbo D, Artinian NT, Basile JN, Krakoff LR, Margolis KL, Rakotz MK, et al. Self-measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association. Circulation. 2020;142:e42–e63. doi: 10.1161/CIR.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 9.Pan HY, Lin HJ, Chen WJ, Wang TD. Prevalence, treatment, control and monitoring of hypertension: a nationwide community-based survey in Taiwan, 2017. Acta Cardiol Sin. 2020;36:375–381. doi: 10.6515/ACS.202007_36(4).20191220A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anstey DE, Muntner P, Bello NA, Pugliese DN, Yano Y, Kronish IM, et al. Diagnosing masked hypertension using ambulatory blood pressure monitoring, home blood pressure monitoring, or both? Hypertension. 2018;72:1200–1207. doi: 10.1161/HYPERTENSIONAHA.118.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomitani N, Hoshide S, Kario K. Diagnostic agreement of masked uncontrolled hypertension detected by ambulatory blood pressure and home blood pressure measured by an all-in-one BP monitoring device: the HI-JAMP study. Hypertens Res. 2023;46:157–164. doi: 10.1038/s41440-022-01073-1. [DOI] [PubMed] [Google Scholar]
- 12.Ntineri A, Niiranen TJ, McManus RJ, Lindroos A, Jula A, Schwartz C, et al. Ambulatory versus home blood pressure monitoring: frequency and determinants of blood pressure difference and diagnostic disagreement. J Hypertens. 2019;37:1974–1981. doi: 10.1097/HJH.0000000000002148. [DOI] [PubMed] [Google Scholar]
- 13.Fatani N, Dixon DL, Van Tassell BW, Fanikos J, Buckley LF. Systolic blood pressure time in target range and cardiovascular outcomes in patients with hypertension. J Am Coll Cardiol. 2021;77:1290–1299. doi: 10.1016/j.jacc.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postel-Vinay N, Bobrie G, Savard S, Persu A, Amar L, Azizi M, et al. Home blood pressure measurement and digital health: communication technologies create a new context. J Hypertens. 2018;36:2125–2131. doi: 10.1097/HJH.0000000000001860. [DOI] [PubMed] [Google Scholar]
- 15.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens. 2019;21:853–858. doi: 10.1111/jch.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kario K, Shimbo D, Tomitani N, Kanegae H, Schwartz JE, Williams B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J Clin Hypertens. 2020;22:135–141. doi: 10.1111/jch.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizukoshi K, Takeuchi M, Nagata Y, Addetia K, Lang RM, Akashi YJ, et al. Normal values of left ventricular mass index assessed by transthoracic three-dimensional echocardiography. J Am Soc Echocardiogr. 2016;29:51–61. doi: 10.1016/j.echo.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Cheng HM, Lin HJ, Wang TD, Chen CH. Asian management of hypertension: current status, home blood pressure, and specific concerns in Taiwan. J Clin Hypertens. 2020;22:511–514. doi: 10.1111/jch.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez MM, Binkowitz BS. Guidelines for measurement validation in clinical trial design. J Biopharm Stat. 1999;9:417–38. doi: 10.1081/BIP-100101185. [DOI] [PubMed] [Google Scholar]
- 22.Kuwabara M, Hamasaki H, Tomitani N, Shiga T, Kario K. Novel triggered nocturnal blood pressure monitoring for sleep apnea syndrome: distribution and reproducibility of hypoxia-triggered nocturnal blood pressure measurements. J Clin Hypertens. 2017;19:30–37. doi: 10.1111/jch.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barochiner J, Posadas Martínez ML, Martínez R, Giunta D. Reproducibility of masked uncontrolled hypertension detected through home blood pressure monitoring. J Clin Hypertens. 2019;21:877–883. doi: 10.1111/jch.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viera AJ, Lin FC, Tuttle LA, Olsson E, Stankevitz K, Girdler SS, et al. Reproducibility of masked hypertension among adults 30 years or older. Blood Press Monit. 2014;19:208–15. doi: 10.1097/MBP.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiwara T, Hoshide S, Kanegae H, Nishizawa M, Kario K. Reliability of morning, before-dinner, and at-bedtime home blood pressure measurements in patients with hypertension. J Clin Hypertens. 2018;20:315–323. doi: 10.1111/jch.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabe H, Saito I. Reproducibility of masked hypertension determined from morning and evening home blood pressure measurements over a 6-month period. Hypertens Res. 2007;30:845–51. doi: 10.1291/hypres.30.845. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara T, Hoshide S, Nishizawa M, Matsuo T, Kario K. Difference in evening home blood pressure between before dinner and at bedtime in Japanese elderly hypertensive patients. J Clin Hypertens. 2017;19:731–739. doi: 10.1111/jch.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teunissen-Beekman KF, Dopheide J, Geleijnse JM, Bakker SJ, Brink EJ, de Leeuw PW, et al. Blood pressure decreases more after high-carbohydrate meals than after high-protein meals in overweight adults with elevated blood pressure, but there is no difference after 4 weeks of consuming a carbohydrate-rich or protein-rich diet. J Nutr. 2013;143:424–9. doi: 10.3945/jn.112.166744. [DOI] [PubMed] [Google Scholar]
- 29.Tabara Y, Okada Y, Uetani E, Nagai T, Igase M, Kido T, Ochi N, Ohara M, Takita R, Kohara K, Miki T. Postprandial hypotension as a risk marker for asymptomatic lacunar infarction. J Hypertens. 2014;32:1084–90. doi: 10.1097/HJH.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 30.Alfie J. Utility of home blood pressure monitoring to evaluate postprandial blood pressure in treated hypertensive patients. Ther Adv. Cardiovasc Dis. 2015;9:133–9. doi: 10.1177/1753944715593444. [DOI] [PubMed] [Google Scholar]
- 31.Lin YH, Hsu HC, Bai CH, Wu WC. Dietary patterns among older people and the associations with social environment and individual factors in Taiwan: a multilevel analysis. Int J Environ Res Public Health. 2022;19:3982. doi: 10.3390/ijerph19073982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WY, Thijs L, Zhang ZY, Asayama K, Boggia J, Hansen TW, et al. Evidence-based proposal for the number of ambulatory readings required for assessing blood pressure level in research settings: an analysis of the IDACO database. Blood Press. 2018;27:341–350. doi: 10.1080/08037051.2018.1476057. [DOI] [PubMed] [Google Scholar]
- 33.Verberk WJ, Kroon AA, Lenders JW, Kessels AG, van Montfrans GA, Smit AJ, et al. Self-measurement of blood pressure at home reduces the need for antihypertensive drugs: a randomized, controlled trial. Hypertension. 2007;50:1019–25. doi: 10.1161/HYPERTENSIONAHA.107.094193. [DOI] [PubMed] [Google Scholar]
- 34.Groenland EH, Vendeville JAC, Bemelmans RHH, Monajemi H, Bots ML, Visseren FLJ, et al. Smartphone application-assisted home blood pressure monitoring compared with office and ambulatory blood pressure monitoring in patients with hypertension: the AMUSE-BP study. Hypertension. 2022;79:2373–2382. doi: 10.1161/HYPERTENSIONAHA.122.19685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natsis M, Antza C, Doundoulakis I, Stabouli S, Kotsis V. Hypertension in obesity: novel insights. Curr Hypertens Rev. 2020;16:30–6. doi: 10.2174/1573402115666190415154603. [DOI] [PubMed] [Google Scholar]
- 36.lmeida TP, Cortés M, Perruchoud D, Alexandre J, Vermare P, Sola J, Shah J, Marques L, Pellaton C. Aktiia cuffless blood pressure monitor yields equivalent daytime blood pressure measurements compared to a 24-h ambulatory blood pressure monitor: Preliminary results from a prospective single-center study. Hypertens Res. 2023;46:1456–1461. doi: 10.1038/s41440-023-01258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara A, Tanaka K, Ohkubo T, Kondo T, Kikuya M, Metoki H, Hashimoto T, Satoh M, Inoue R, Asayama K, Obara T, Hirose T, Izumi S, Satoh H, Imai Y. Ambulatory versus home versus clinic blood pressure: the association with subclinical cerebrovascular diseases: the Ohasama Study. Hypertension. 2012;59:22–8. doi: 10.1161/HYPERTENSIONAHA.111.174938. [DOI] [PubMed] [Google Scholar]
- 38.Mancia G, Facchetti R, Cuspidi C, Bombelli M, Corrao G, Grassi G. Limited reproducibility of MUCH and WUCH: evidence from the ELSA study. Eur Heart J. 2020;41:1565–1571. doi: 10.1093/eurheartj/ehz651. [DOI] [PubMed] [Google Scholar]
- 39.Mancia G, Rea F, Corrao G, Grassi G. Two-drug combinations as first-step antihypertensive treatment. Circ Res. 2019;124:1113–1123. doi: 10.1161/CIRCRESAHA.118.313294. [DOI] [PubMed] [Google Scholar]
- 40.Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine. 2017;96:e5641. doi: 10.1097/MD.0000000000005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kario K. Evidence for the surge blood pressure resonance hypothesis as a trigger for cardiovascular disease events. Hypertens Res. 2023;46:2065–2069. doi: 10.1038/s41440-023-01346-3. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 43.Hoshide S, Tomitani N, Kario K. Maximum ambulatory daytime blood pressure and risk of stroke in individuals with higher ambulatory arterial stiffness index: the JAMP study. Hypertens Res. 2023;46:84–90. doi: 10.1038/s41440-022-01048-2. [DOI] [PubMed] [Google Scholar]
- 44.Huang HC, Pan HY, Wang TD. Renal nerve stimulation predicted blood pressure-lowering responses to percutaneous renal denervation. Circ Cardiovasc Inter. 2023;16:e012779. doi: 10.1161/CIRCINTERVENTIONS.122.012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.