Abstract
Breast cancer (BC) is the most prevalent malignancy in women globally. At time of diagnosis, premenopausal BC is considered more aggressive and harder to treat than postmenopausal cases. Cytochrome P450 (CYP) enzymes are responsible for phase I of estrogen metabolism and thus, they are prominently involved in the pathogenesis of BC. Moreover, CYP subfamily 2C and 3A play a pivotal role in the metabolism of taxane anticancer agents. To understand genetic risk factors that may have a role in pre-menopausal BC we studied the genotypic variants of CYP2C8, rs11572080 and CYP3A4, rs2740574 in female BC patients on taxane-based therapy and their association with menopausal status. Our study comprised 105 female patients with histologically proven BC on paclitaxel-therapy. They were stratified into pre-menopausal (n = 52, 49.5%) and post-menopausal (n = 53, 50.5%) groups. Genotyping was done using TaqMan assays and employed on Quantstudio 12 K flex real-time platform. Significant increased frequencies of rs11572080 heterozygous CT genotype and variant T allele were established in pre-menopausal group compared to post-menopausal group (p = 0.023, 0.01, respectively). Moreover, logistic regression analysis revealed a significant association between rs11572080 CT genotype and premenopausal BC. However, regarding rs2740574, no significant differences in genotypes and allele frequencies between both groups were detected. We reported a significant association between CYP2C8 genotypic variants and premenopausal BC risk in Egyptian females. Further studies on larger sample sizes are still needed to evaluate its importance in early prediction of BC in young women and its effect on treatment outcome.
Keywords: Breast cancer, Cytochrome P450, Taxane, Menopausal status
Subject terms: Cancer, Molecular biology
Introduction
Breast cancer (BC) is the most common cancer among women in Egypt causing 22 percent of all cancer-related female deaths1. In 2018, it constituted 24% of new cancer cases and 15% of deaths worldwide2. It is a hormone dependent cancer carrying a great heterogeneity in the outcomes of patients with similar clinical features. It is important to investigate breast cancer in the context of menopausal status due to differences in causes, risk factors, molecular features, and disease outcomes3. Early detection of premenopausal (pre-M) breast cancer constitutes a great burden in low- and middle-income countries3.
When diagnosed it is more advanced and challenging to manage than post-menopausal (post-M) cancer breast4. One of its risk factors is the longtime exposure to high levels of estrogen through estrogen signaling pathway and via the toxic effects of highly reactive metabolic compounds5,6. Cytochrome P450 (CYP) enzymes which belong to monooxygenase are a large family of heme proteins involved in the biosynthesis and oxidative metabolism of sex hormones. Gene polymorphisms of CYPs have been vigorously implicated for the risk and prognosis of breast cancer7–9.
Furthermore, they have effects on treatment outcomes and drug metabolism. These effects range from lack of treatment efficacy to adverse toxic reactions. Meta-analysis of thirty-one studies on chemotherapy-induced peripheral neuropathy (CIPN) involving 4179 patients on various neurotoxic chemotherapeutic agents demonstrated that the prevalence of CIPN was 48%10. They related the adverse effects upon taxane-based therapy to genetic variables in CYP enzymes especially CYP2C8 and CYP3A4 with inconclusive findings11.
CYP3A4 is the most abundant Cytochrome P450 enzyme (30%) in adults and is expressed predominantly in the liver. It is responsible for oxidative metabolism of endogenous and exogenous hormones12. CYP2C8 is responsible for most of paclitaxel elimination and correlates with exposure to paclitaxel13.
To our knowledge there have been no previous studies on genetic variables of these CYP450 enzymes in pre-menopausal versus post-menopausal cancer breast. To understand genetic risk factors that may have a role in pre-menopausal breast cancer we investigated variables of the two cytochrome P450 enzymes (CYP2C8, rs11572080 and CYP3A4, rs2740574) in female cancer breast patients on taxane-based therapy and we evaluated their variation based on menopausal status.
Patients and methods
In the current study one hundred and five female patients with histologically proven breast cancer have been enrolled from Baheya Centre for Early Detection and Breast Cancer Treatment between 2020 and 2022. All the assessed patients were diagnosed based on morphologic examination of the tumor tissues. Biopsy for histopathologic diagnosis and to perform hormonal receptors (ER, PR and HER2) was done for every patient. All participants were treated with neoadjuvant or adjuvant taxane-based chemotherapy (paclitaxel) as a single agent or combination therapy. Chemotherapy induced peripheral neuropathy (CIPN) was identified based on clinical and laboratory findings. All participants were informed about the study and its objectives before blood sampling. The study has been approved by both the Ethical Committee of the National Research Centre (no, 17-109) and Baheya-Research Ethics Committee (no.0317) in accordance with the ethical standards of the Declaration of Helsinki and written informed consents were obtained from all the patients.
All patients have been subjected to full history, clinical examination, and metastatic workup, including chest radiograph, abdominal sonar, and bone scan. Laboratory examination including CBC and biochemical analyses including ALT, AST, urea, and creatinine were sequentially assessed for cancer breast patients within 48 h before chemotherapy.
Inclusion criteria included age (≥ 18 years), performance status less than 3 in accordance with the Eastern Cooperative Oncology Group criteria (ECOG)14. Patients with comorbid disease conditions like severe liver disease or renal failure prior to treatment, peripheral neuropathy or vascular complications from hypothyroidism, hypercholesterolemia, hypertension or diabetes, varicella zoster, peripheral vascular disease, and autoimmune disease with vasculitis were excluded. These conditions are known to be associated with the development of peripheral neuropathy15.
Blood sampling
Peripheral blood samples (10 mL) were withdrawn from all participants under complete aseptic conditions into plain and EDTA-containing vacutainer tubes for biochemical analysis, complete blood count and genomic DNA extraction.
Genotyping of rs11572080 and rs2740574
Genomic DNA was isolated from the whole blood by the QIA amp DNA blood mini kit (Qiagen, Germany) in accordance with the supplier’s instructions using Qia Cube® automated nucleic acid extractor (Qiagen, Germany). A Nano Drop spectrophotometer (Nano Drop Technologies Inc., DE, USA) was used for measuring DNA concentration and purity.DNA was adjusted at A260/280 ratio between 1.7 and 1.9 and normalized to the recommended working concentration at ~ 25 ng/μL.Then DNA yield was stored frozen at − 20 °C for all recruited samples until further use. Genotyping and allele frequencies using TaqMan assays from Thermo Fisher Scientific, Catalog number: 4362691wereemployed on Quant studio 12 K flex real-time PCR system; CYP2C8 gene, C_25625794_10, rs11572080 (CYP2C8*3, c.416G > A > CYP2C8*3, g.2130G > A); CYP3A4 gene, C_1837671_50, rs2740574 (CYP3A4*15B, g.-392A > G > CYP3A4*1B, g.-392A > G)16.
Sequence of primers
RS 11572080 Context Sequence [VIC/FAM]
CTCTTGAACACGGTCCTCAATGCTC [C/T]
TCTTCCCCATCCCAAAATTCCGCAA
RS2740574 Context Sequence [VIC/FAM]
TAAAATCTATTAAATCGCCTCTCTC [C/T]
TGCCCTTGTCTCTATGGCTGTCCTC
The Taqman probe principle relies on the 5′–3′ exonuclease activity of Taq polymerase to cleave a dual- labeled probe during hybridization to the complementary target sequence and fluorophore-based detection. The amplification condition consists of an initial 2 min at 50 °C for optimizing the UNG enzyme, 10 min denaturation at 95 °C followed by 40 cycles of 30 s of denaturation at 95 °C, 30 s of annealing at 52 °C and 60 s of extension at 65 °C. Data analysis of the genotyping results and allele frequencieswere carried out by TaqMan Genotyper software. Software tools enable converting the raw data into genotype calls (homozygotes and heterozygotes).
Statistical methods
All test data was converted and manipulated by using SPSS software program version 20.0. Data was analyzed, mean and standard deviation or standard error of mean and range were calculated for the quantitative data as age, tumor size, biochemical laboratory results. All categorical variables were summarized using frequencies and percentages. Comparison among studied cancer breast patients’ groups based on menopausal status was done by using the chi‐square test for categorical variables and using Student's t‐test for continuous data. P value was established to determine the statistically significant difference between them. The difference between groups were considered statistically significant when p < 0.05 and considered highly statistically significant when p < 0.01. Genotype frequencies found among all studied patients were compared with their expected frequencies under Hardy–Weinberg equilibrium using a χ2 test (P > 0.05). Logistic regression analysis was applied to test for association between rs11572080 CT genotype and premenopausal breast cancer expressed as categorical variables and odds ratios (OR) with 95% confidence.
Ethical approval
The study has been approved by both the Ethical Committee of the National Research Centre (no, 17-109) and Baheya-Research Ethics Committee (no.0317) in accordance with the ethical standards of the Declaration of Helsinki.
Informed consent
Informed written consent was obtained from all participants after the study objectives were explained and before blood sampling. Confidentiality of patient data was guaranteed.
Results
Patients’ age ranged between 27 and 73 years old. Patients were stratified according to their menopausal status into pre-menopausal (n = 52, 49.5%) and post-menopausal (n = 53, 50.5%) groups. PTX was administered on a weekly basis in a dose of 80 mg/m2 IV over 3 h as an adjuvant in 41%, neo-adjuvant in 48.5% or palliative in 4.7% of the studied patients. Six patients received hormonal therapy, some patients got PTX with other chemotherapies e.g. Epirubicin–Cyclophosphamide (EC), (n = 35, 33.3%), or doxorubicin hydrochloride (Adriamycin) and cyclophosphamide (AC), (n = 48, 45.7%).
Clinical and laboratory findings in both groups are presented in Table 1. No statistically significant differences were found in tumor characteristics, pathology type and hormonal receptors’ frequencies between both groups. Performance grade 2 was significantly associated with post-menopausal breast cancer; as shown in Fig. 1; and with presence of Taxane-based CIPN (p = 0.008 and 0.01 respectively).CIPN was significantly more encountered in post-menopausal breast cancer group than the pre-menopausal patients (69.8%, p = 0.047, Fig. 2). High grade CIPN (grade ≥ 2) association with ER (estrogen receptor) negative cases was near significance, p = 0.055 as shown in Fig. 3.
Table 1.
Clinical and laboratory findings in both pre- and post-menopausal patient groups.
| Variable | Pre-menopausal N = 52 N (%) |
Post-menopausal N = 53 N (%) |
Odds ratio (95% CI) |
P value |
|---|---|---|---|---|
| Age (y), mean ± SD | 45.52 ± 7.19 | 60.43 ± 5.9 | – | < 0.001** |
| Positive family history | 14 (26.9) | 12 (22.6) | – | 0.603 |
| Pathology type | ||||
| IDC | 49 (94.2) | 46 (86.8) | – | 0.071 |
| ILC | 0 | 5 (9.4) | ||
| Others | 3 (5.8) | 2 (3.8) | ||
| Tumor side | ||||
| Right | 21 (40.4) | 21 (39.6) | – | 0.932 |
| Left | 31 (59.6) | 32 (60.4) | ||
| Tumor size | ||||
| < 4 cm | 27 (51.9) | 22 (41.5) | 0.363 | |
| > 4 cm | 25 (48.1) | 31 (58.5) | ||
| ER+ | 41 (78.8) | 40 (75.5) | – | 0.368 |
| PR+ | 43 (82.7) | 46 (86.8) | – | 0.589 |
| HER2− | 46 (88.5) | 52 (98.1) | – | 0.287 |
| Pathology grade | ||||
| 1 | 3 (5.8) | 3 (5.7) | – | 0.343 |
| 2 | 41 (78.8) | 36 (67.9) | ||
| 3 | 8 (15.4) | 14 (26.4) | ||
| Clinical stage | ||||
| T (0–2) | 22 (42.3) | 17 (32.1) | 0.493 | |
| (3–4) | 30 (57.7) | 36 (67.9) | – | |
| N (0–1) | 37 (71.2) | 34 (64.2) | 0.704 | |
| (2–3) | 15 (28.8) | 19 (35.8) | ||
| M (0) | 46 (88.5) | 46 (86.8) | 0.537 | |
| − 1 | 6 (11.5) | 7 (13.2) | ||
| Performance status | ||||
| 1 | 50 (96.2) | 42 (79.2) | 1.944 (1.375–2.751) | 0.008* |
| 2 | 2 (3.8) | 11 (20.8) | ||
| No. of cycles of treatment Mean ± SD | 16.8 ± 10.3 | 17.0 ± 12.0 | 0.37 | |
| Peripheral Neuropathy | 26 (50.0) | 37 (69.8) | 2.273 (1.002–5.154) | 0.047* |
| Diarrhea | 8 (16.7%) | 12 (24.5%) | – | 0.341 |
| Gastritis | 9 (18.8%) | 8 (16.3%) | – | 0.754 |
| Fatigue | 9 (18.8%) | 11 (22.4%) | – | 0.653 |
| Skin rash | 2 (4.2%) | 1 (1.9%) | – | 0.545 |
| Nail Damage | 2 (4.2%) | 0 | – | 0.149 |
| Nausea | 10 (20.8%) | 7 (14.3%) | – | 0.396 |
| Vomiting | 10 (20.8%) | 5 (10.2%) | – | 0.148 |
| Stomatitis | 1 (2.1%) | 0 | – | 0.309 |
| Dyspnea | 4 (8.3%) | 3 (6.1%) | – | 0.674 |
| Bony pain | 27 (52%) | 26 (48%) | – | 0.752 |
*P < 0.05 is considered significant; **p < 0.001is highly significant.
N number, CI confidence interval, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, T from 0 to 4 (higher T numbers indicates a larger tumor and/or spread to tissues near the breast), N from 0 to 3 (higher N numbers indicates that the cancer has spread to lymph nodes M 0 or 1 indicates if the cancer has spread to distant organs e.g. liver, lungs, or bones, PR progesterone receptor, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, SD standard deviation.
Significant values are in [bold].
Figure 1.
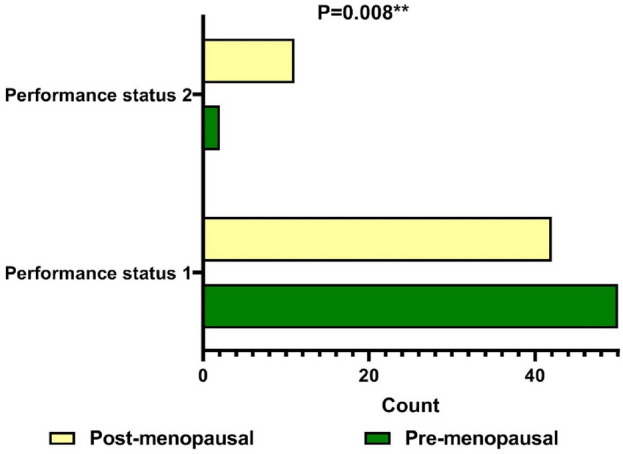
Peripheral neuropathy in the studied groups based on menopausal status.
Figure 2.

Performance status in the studied groups based on menopausal status.
Figure 3.
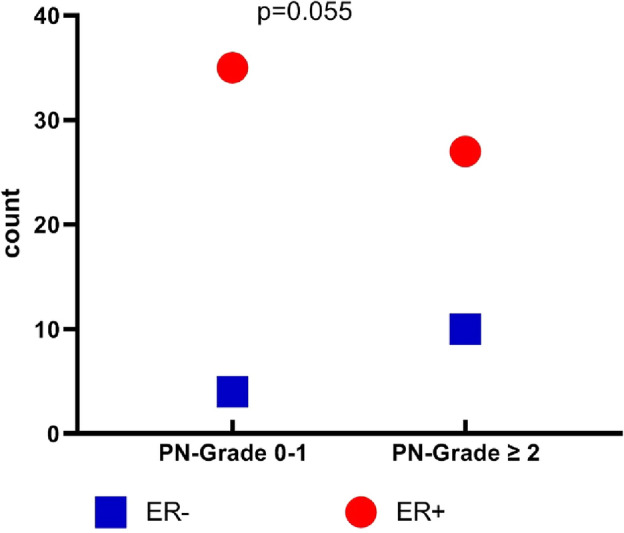
Estrogen receptor frequency in the studied groups based on peripheral neuropathy grades.
Biochemical analysis results are shown in Table 2 with statistically higher urea and creatinine levels in the post-menopausal group. Genotypes and allele frequencies of all the studied patients are shown in Table 3. Genotypes’ frequencies are complying with Hardy–Weinberg equilibrium (p > 0.05). Increased frequency of rs11572080 heterozygous CT genotype was a significant characteristic finding in pre-menopausal breast cancer group (42.3%, p = 0.023) with variant T allele frequency of 21.2% vs. 8.5% in post-menopausal group as demonstrated in Table 4 and Fig. 4, (p = 0.01). Logistic regression analysis revealed a significant association between rs11572080 CT genotype and premenopausal breast cancer, p = 0.023.
Table 2.
Biochemical findings in the studied patients (n = 105).
| Variable | Pre-menopausal N = 52 Mean ± SD |
Post-menopausal N = 53 Mean ± SD |
P value |
|---|---|---|---|
| HB; g/dL | 12.2 ± 1.27 | 12.5 ± 1.07 | 0.172 |
| TLC; × 103/µL | 6.8 ± 2.1 | 6.9 ± 1.8 | 0.943 |
| PLT; × 103/µL | 296.0 ± 64.4 | 287.2 ± 69.8 | 0.521 |
| Urea; mg/dL | 23.2 ± 7.1 | 31.2 ± 11.1 | < 0.001** |
| Creatinine; mg/dL | 0.76 ± 0.13 | 0.84 ± 0.18 | 0.025* |
| AST; U/l | 20.8 ± 10.1 | 22.0 ± 10.3 | 0.570 |
| ALT; U/L | 21.1 ± 14.3 | 20.5 ± 11.4 | 0.819 |
*P < 0.05 is considered significant; **p < 0.001 is highly significant.
Hb hemoglobin, TLC total leukocytic count, PLT platelet count, AST aspartate transaminase, ALT alanine transaminase, SD standard deviation.
Significant values are in [bold].
Table 3.
Frequencies of studied Genes among studied patients (n = 105).
| Gene | Call rate (%) | Genotype/Allele | No. (%) | MAF, Global (1000genomes/our cohort | H.W.E |
|---|---|---|---|---|---|
| P value | |||||
| RS 2740574 | 100 | CC | 4 (3.8) | – | 0.202 |
| CT | 18 (17.1) | ||||
| TT | 83 (79.0) | ||||
| C | 26 (12.38) | C = 0.2308/0.1238 | |||
| T | 184 (87.6) | T = 0.7692/0.8762 | |||
| RS 11572080 | 95.8 | CC | 73 (69.5) | 0.352 | |
| CT | 32 (30.5) | ||||
| C | 178 (84.7) | C = 0.9543 /0.8476 | |||
| T | 32 (15.2) | T = 0.0457/0.1524 |
MAF minor allele frequency; risk allele is presented in bold; H.W.E. Hardy–Weinberg equilibrium.
Table 4.
Frequencies of studied Genes among patients’ groups based on menopausal status.
| Gene | Pre-menopausal N = 52 N (%) |
Post-menopausal N = 53 N (%) |
Odds ratio (95% CI) |
P value |
|---|---|---|---|---|
| rs11572080 genotypes/alleles | ||||
| CC | 30 (57.7) | 44 (83.0) | ||
| CT | 22 (42.3) | 9 (17.0) | 2.199 (1.071–4.513) | 0.023* |
| TT | 0 | 0 | ||
| C allele | 82 (78.8) | 97 (91.5) | ||
| T allele | 22 (21.2) | 9 (8.5) | 2.491 (1.204–5.154) | 0.01* |
| rs2740574 genotypes | ||||
| TT | 40 (76.9) | 42 (79.2) | – | |
| CT | 9 (17.3) | 8 (15.1) | ||
| CC | 3 (5.8) | 3 (5.7) | 0.961 | |
| T allele | 89 (85.6) | 92 (88.5) | ||
| C allele | 15 (14.4) | 14 (13.5) | ||
Risk allele is presented in bold; CI confidence interval.
* < 0.05 is considered significant.
Figure 4.
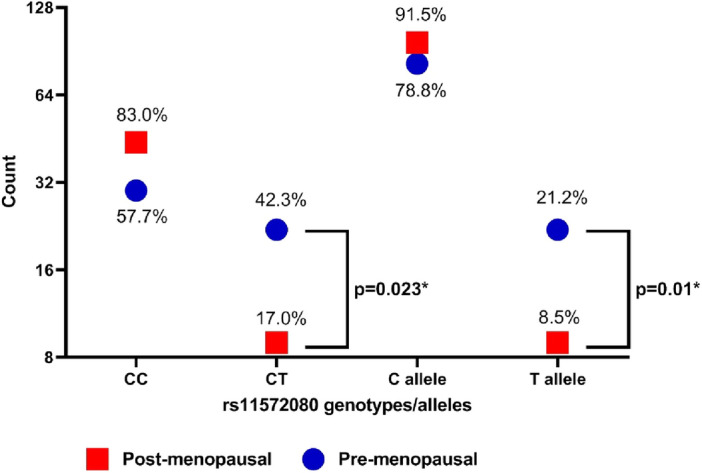
Frequencies of rs11572080 genotypes and alleles in the studied groups based on menopausal status.
Discussion
Breast cancer is the most diagnosed malignancy in women and the most common cancer overall, and its health and economic burden has been rising over the past decades in many parts of the world17. Breast cancer in pre-M women is frequently associated with worse prognosis compared to post-M women as it is more often diagnosed at a later stage of the disease18. Taxane-based chemotherapy regimens (e.g., paclitaxel and docetaxel) have been used as the first line of treatment in early-stage19. A frequent side effect is chemotherapy induced peripheral neuropathy CIPN, that occur in up to 70% of all treated patients and impacts the quality of life during and after treatment20.
Some factors such as increased dosage and age, are known to be associated with increased susceptibility of developing CIPN21. Moreover, there is a large interindividual variability independent of known risk factors suggesting that there could be an underlying genetic basis for susceptibility. Some single-nucleotide polymorphisms (SNPs) and other genetic variants may aid in predicting individual predisposition22. In the current study we aimed to verify genotypic variations in two cytochrome P450 enzymes involved in Paclitaxel metabolism in breast cancer patients and to identify their association with menopausal status.
In our cohort study, performance grade 2 and CIPN were significantly more prevalent in postmenopausal cancer breast patients than the premenopausal group. Hormonal fluctuations could be the reason for the adverse effects observed in post-menopausal cancer breast patients. The lower circulating progesterone level detected in such patients was one of the suggested mechanisms by Akshita et al.23 and was also confirmed by Sing and Su who have reported that progesterone exerts a neuro-protective effect through both genomic and non-genomic pathways24.
Progesterone has been authenticated as a neuroprotective hormone with beneficial effects on both central and peripheral nervous systems comprising promoting myelination, myelin repair and improving injuries of spinal cord and brain. Moreover, in an experimental study by Roglio et al.25 the use of progesterone reduced docetaxel-induced peripheral neuropathy in rats and prevented adverse changes in nerve conduction and consequently, it was considered as neuroprotective steroid in peripheral nerves26. Similarly, Ekici and Balkaya demonstrated that the protective effect of progesterone in rat model27. Prabhu et al.28 raised attention to a neuroprotective effect of premenopausal status, possibly related to higher circulating levels of progesterone. They hypothesized that progesterone administration prior to taxane-chemotherapy might protect against CIPN.
Blood estrogen levels dramatically decrease through peri menopause and further decrease for several years after menopause. It is likely that estrogen plays a role in neuroprotection or prevention of excessive neuronal excitability, thus, the decreased estrogen levels may be associated with the accelerated development of peripheral neuropathy. A clinical retrospective study by Miyamoto et al.29 showed that postmenopausal estrogen decline in female BC patients was considered a risk factor for therapy related peripheral neuropathy, and such a high-risk patient group, particularly, might require pharmacological intervention, except if the anti-cancer effect of paclitaxel is interfered. This was further supported by their preclinical study showing that ovariectomy in mice induced a somatic and visceral hyperalgesic state that could be reversed by estrogen.
Other mechanisms previously encountered in CIPN were explained by Starobova et al., including immune-mediated processes as loss of peripheral fibers, demyelination, axon degeneration, altered retrograde and anterograde transport, and mitochondrial dysfunction30. Increased incidence of CIPN with increasing age was demonstrated by Goreishi et al. who reported that advanced age is a significant risk factor for incidence and severity of neurotoxicity induced by chemotherapeutic agents including paclitaxel in particular26. In addition, Luclie et al.31 has stated that patients treated with paclitaxel appeared to be more at risk of developing persistent clinically significant CIPN especially if they were older than 75 and other potential factors were insignificant as regards being an ER positive or negative breast tumor.
Although genomic and molecular alterations play a significant role in breast cancer biology, studies that address the unique molecular changes in pre-M and post-M are limited32. Some studies have targeted differences in gene expression between pre-M and post-M breast cancer which were found exclusively in ER + breast cancer and suggested that the majority of differences was driven by altered hormonal levels33. Anders et al. analyzed microarray data from 784 early-stage breast cancers to discover gene sets able to distinguish breast tumors arising in younger women from tumors of older women34.
Prior studies have investigated somatic mutation analysis identifying 5 genes (CDH1, GATA3, MLL3, GPS2, and PI3KCA) for which mutation rates were significantly different between pre-M and post-M patients, where the overall mutation rates were lower in pre-M than post-M cases and that was likely a general effect of oxidative damage during aging rather than endocrine response35,36.
In our study, genotypes, and allele frequencies of all the studied patients showed significant increased frequency of rs11572080 heterozygous CT genotype in pre-menopausal breast cancer group with variant T allele frequency of 21.2 vs. 8.2% in post-menopausal group. Moreover, logistic regression analysis revealed a significant association between rs11572080 CT genotype and premenopausal breast cancer. In our opinion, this is a very interesting and promising finding as it reveals the association of this genotyping variation in early breast cancer patients and its importance in early prediction of cancer breast in young women.
Limitation
The study limitation was that the relatively small sample size (150 BC patients) due to limited budget.
Conclusions
CYP2C8 genotypic variants were significantly associated with premenopausal BC risk among Egyptian females. Larger scale genetic studies including large number of participants are still needed to elucidate the role of CYP2C8 gene polymorphism in the development and early prediction of BC in young women, in addition to its effect on treatment outcome.
It is very important to study attributable molecular risk factors that increase the chance of acquiring Breast cancer in young females, thus establishing prediction and screening programs which aims to offer appropriate drug regimens with improved patients' life outcome and emotional wellbeing.
Acknowledgements
The authors gratefully acknowledge the Science, Technology & Innovation Funding Authority through Capacity Building Program under grant (4880). The authors give special thanks to the Baheya Research Centre coordinator, Doaa Elsayed Mostafa Abo-kresha, for her efforts to provide us with all important data of the patients and make the path clear for us to implement our project.
Abbreviations
- BC
Breast cancer
- pre-M
Premenopausal
- CYP
Cytochrome P450
- CIPN
Chemotherapy-induced peripheral neuropathy
- ER
Estrogen receptor
- SNPs
Some single-nucleotide polymorphisms
- PTX
Paclitaxel
- PN
Peripheral neuropathy
- ECOG
Eastern Cooperative Oncology Group criteria
- EC
Epirubicin-cyclophosphamide
- AC
Adriamycin and cyclophosphamide
- CIPN
Chemotherapy induced peripheral neuropathy
Author contributions
H.R.M.A. contributed to the project preparation, study design, submission for funding and writing draft of the manuscript. H.R.M.A., M.H.I. were responsible for the management of purchasing tasks and schedules. M.H.I. coordinated specimen collection and transport and implemented a quality policy throughout the laboratory analysis workflow. M.H.I., D.F.A., S.H.A.E., M.M.A.W., S.N.A.E.F. and M.A.A.M. contributed to laboratory analysis. Appropriate patient selection and data collection were performed and supervised by A.H. and M.M.K. Statistical analysis of data and tabulation of final results were accomplished by T.M.R. All authors have read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was financially supported by the National Research Centre (NRC), Egypt through project grant no. E120507.
Data availability
All data and materials are available and can be submitted when needed, Corresponding Author is responsible person who should be contacted if someone wants to request the data from this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], and ICF International. Egypt Health Issues Survey 2015. Ministry of Health and Population and ICF International, Cairo, Egypt and Rockville, Maryland, USA (2015).
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health. 2020;8(8):e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 4.Phillips KA, Milne RL, Friedlander ML, Jenkins MA, McCredie MR, Giles GG, Hopper JL. Prognosis of premenopausal breast cancer and childbirth prior to diagnosis. J. Clin. Oncol. 2004;22(4):699–705. doi: 10.1200/JCO.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Rod NH, Hansen AM, Nielsen J, Schnohr P, Gronbaek M. Low-risk factor profile, estrogen levels, and breast cancer risk among postmenopausal women. Int. J. Cancer. 2009;124:1935–1940. doi: 10.1002/ijc.24136. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Lin YC. Transformation of MCF-10A human breast epithelial cells by zeranol and estradiol-17 beta. Breast J. 2004;10:514–521. doi: 10.1111/j.1075-122X.2004.21410.x. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DJ, Riboli E, Haiman CA, Albanes D, Altshuler D, Chanock SJ, Haynes RB, Henderson BE, Kaaks R, Stram DO, Thomas G, Thun MJ, Blanché H, Buring JE, Burtt NP, Calle EE, Cann H, Canzian F, Chen YC, Colditz GA, Cox DG, Dunning AM, Feigelson HS, Freedman ML, Gaziano JM, Giovannucci E, Hankinson SE, Hirschhorn JN, Hoover RN, Key T, Kolonel LN, Kraft P, Le Marchand L, Liu S, Ma J, Melnick S, Pharaoh P, Pike MC, Rodriguez C, Setiawan VW, Stampfer MJ, Trapido E, Travis R, Virtamo J, Wacholder S, Willett WC, National Cancer Institute Breast and Prostate Cancer Cohort Consortium A candidate gene approach to searching for low-penetrance breast and prostate cancer genes. Nat. Rev. Cancer. 2005;5(12):977–985. doi: 10.1038/nrc1754. [DOI] [PubMed] [Google Scholar]
- 8.Johnson N, Walker K, Gibson LJ, Orr N, Folkerd E, Haynes B, Palles C, Coupland B, Schoemaker M, Jones M, Broderick P, Sawyer E, Kerin M, Tomlinson IP, Zvelebil M, Chilcott-Burns S, Tomczyk K, Simpson G, Williamson J, Hillier SG, Ross G, Houlston RS, Swerdlow A, Ashworth A, Dowsett M, Peto J, Dos Santos SI, Fletcher O. CYP3A variation, premenopausal estrone levels, and breast cancer risk. J. Natl. Cancer. Inst. 2012;104(9):657–669. doi: 10.1093/jnci/djs156. [DOI] [PubMed] [Google Scholar]
- 9.Mcdaniel DO, Thurber T, Lewis-Traylor A, Berry C, Barber WH, Zhou X, Bigler S, Vance R. Differential association of cytochrome P450 3A4 genotypes with onsets of breast tumors in African American versus Caucasian patients. J. Investig. Med. 2011;59:1096–1103. doi: 10.2310/JIM.0b013e3182277e3b. [DOI] [PubMed] [Google Scholar]
- 10.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Hertz DL. Germline pharmacogenetics of paclitaxel for cancer treatment. Pharmacogenomics. 2013;14(9):1065–1084. doi: 10.2217/pgs.13.90. [DOI] [PubMed] [Google Scholar]
- 12.Keshava C, McCanlies EC, Weston A. CYP3A4 polymorphisms–potential risk factors for breast and prostate cancer: A HuGE review. Am. J. Epidemiol. 2004;160(9):825–841. doi: 10.1093/aje/kwh294. [DOI] [PubMed] [Google Scholar]
- 13.Hertz DL, Walko CM, Bridges AS, Hull JH, Herendeen J, Rollins K, Watkins PB, Dees EC. Pilot study of rosiglitazone as an in vivo probe of paclitaxel exposure. Br. J. Clin. Pharmacol. 2012;74(1):197–200. doi: 10.1111/j.1365-2125.2012.04165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5(6):649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 15.National Institute of Neurological Disorders and Stroke: Peripheral neuropathy fact sheet. http://www.ninds.nih.gov/disorders/peripheralneuropathy/detail_peripheralneuropathy.htm.
- 16.Espindola LMT, López MJC, Flores AU, Espinosa LR, Granados J, Pacheco JLC, García MP, Cervantes MTR, Méndez VCB, Doño SH, Ruíz Gómez D. Genetic polymorphism of CYP3A4 is associated with poor response to ifosfamide treatment in children with solid embryonic tumors. Arch. Med. Sci. 2021;17(6):1766–1771. doi: 10.5114/aoms.2019.86648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu J, Wu L, Xu T, Li D, Ying M, Jiang M, Jiang T, Fu W, Wang F, Du J. Young-onset breast cancer: A poor prognosis only exists in low-risk patients. J Cancer. 2019;10(14):3124–3132. doi: 10.7150/jca.30432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Škubník J, Pavlíčková V, Ruml T, Rimpelová S. Current perspectives on taxanes: Focus on their bioactivity, delivery and combination therapy. Plants. 2021;10(3):569. doi: 10.3390/plants100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson KB, Sharma A, Henry NL, Wei M, Bie B, et al. Genetic variations that influences paclitaxel pharmacokinetics and intracellular effects that may contribute to chemotherapy-induced neuropathy: A narrative review. Front. Pain Res. 2023;4:1139883. doi: 10.3389/fpain.2023.1139883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speck RM, Sammel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, DeMichele A. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 2013;9(5):e234–e240. doi: 10.1200/JOP.2012.000863. [DOI] [PubMed] [Google Scholar]
- 22.Di Francia R, Atripaldi L, Di Martino S, Fierro C, Muto T, Crispo A, Rossetti S, Facchini G, Berretta M. Assessment of pharmacogenomic panel assay for prediction of taxane toxicities: Preliminary results. Front. Pharmacol. 2017;8:797. doi: 10.3389/fphar.2017.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akshita S, Nair NS, Gupta S, Parmar V, Prabhu A, Hawaldar R, Badwe RA. Effect of menopausal status on chemotherapy-induced peripheral neuropathy: Single-institution retrospective audit. Ind. J. Med. Paediatr. Oncol. 2022 doi: 10.1055/s-0042-1742660. [DOI] [Google Scholar]
- 24.Singh M, Su C. Progesterone and neuroprotection. Horm. Behav. 2013;63(2):284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roglio I, Bianchi R, Camozzi F, Carozzi V, Cervellini I, Crippa D, Lauria G, Cavaletti G, Melcangi RC. Docetaxel-induced peripheral neuropathy: protective effects of dihydroprogesterone and progesterone in anexperimental model. J Peripher. Nerv. Syst. 2009;14(1):36. doi: 10.1111/j.1529-8027.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghoreishi Z, Keshavarz S, Asghari Jafarabadi M, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18(1):958. doi: 10.1186/s12885-018-4869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekici M, Balkaya M. Protective effects of oxytocin and progesterone on paclitaxel-induced neuropathy in rats. Neurol. Sci. Neurophysiol. 2021;38(4):262–270. doi: 10.4103/nsn.nsn_113_21. [DOI] [Google Scholar]
- 28.Prabhu AL, Singh A, Kaushik RV, Nair NS, Hawaldar RW, Vanmali V, Parmar V, Gupta S, Badwe RA. Does progesterone protect from chemotherapy-induced peripheral neuropathy? A retrospective audit of breast cancer patients. J. Clin. Oncol. 2012;30(15):e11552. doi: 10.1200/jco.2012.30.15_suppl.e11552. [DOI] [Google Scholar]
- 29.Miyamoto T, Hiramoto S, Kanto A, Tsubota M, Fujitani M, Fukuyama H, Hatanaka S, Sekiguchi F, Koizumi Y, Kawabata A. Estrogen decline is a risk factor for paclitaxel-induced peripheral neuropathy: Clinical evidence supported by a preclinical study. J. Pharmacol. Sci. 2021;146:49–57. doi: 10.1016/j.jphs.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pabst L, Velten M, Fischbach C, Kalish M, Pflumio C, Pivot X, Petit T. Persistent taxane-induced neuropathy in elderly patients treated for localized breast cancer. Breast J. 2020;26(12):2376–2382. doi: 10.1111/tbj.14123. [DOI] [PubMed] [Google Scholar]
- 32.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yau C, Fedele V, Roydasgupta R, Fridlyand J, Hubbard A, Gray JW, Chew K, Dairkee SH, Moore DH, Schittulli F, Tommasi S, Paradiso A, Albertson DG, Benz CC. Aging impacts transcriptomes but not genomes of hormone-dependent breast cancers. Breast Cancer Res. 2007;9(5):R59. doi: 10.1186/bcr1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J. Clin. Oncol. 2008;26(20):3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 35.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van’t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome Initiative. ICGC Breast Cancer Consortium. ICGC MMML-Seq Consortium. ICGC PedBrain. Zucman- Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asselin-Labat ML, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, Breslin K, Ward T, Shi W, Bath ML, Deb S, Fox SB, Smyth GK, Lindeman GJ, Visvader JE. Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14. Mol. Cell Biol. 2011;31(22):4609–4622. doi: 10.1128/MCB.05766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available and can be submitted when needed, Corresponding Author is responsible person who should be contacted if someone wants to request the data from this study.


