Abstract
Climate change is restructuring natural ecosystems. The direct impacts of these events on biodiversity and community structure are widely documented, but the impacts on the genetic variation of populations remains largely unknown. We monitored populations of Acropora coral on a remote coral reef system in northwest Australia for two decades and through multiple cycles of impact and recovery. We combined these demographic data with a temporal genetic dataset of a common broadcast spawning corymbose Acropora to explore the spatial and temporal patterns of connectivity underlying recovery. Our data show that broad-scale dispersal and post-recruitment survival drive recovery from recurrent disturbances, including mass bleaching and mortality. Consequently, genetic diversity and associated patterns of connectivity are maintained through time in the broader metapopulation. The results highlight an inherent resilience in these globally threatened species of coral and showcase their ability to cope with multiple disturbances, given enough time to recover is permitted.
Subject terms: Molecular ecology, Ecological genetics
Broad-scale dispersal and post-recruitment survival drive recovery and genetic stability of coral in the face of climate change-induced disturbances.
Introduction
The capacity of natural populations to recover from acute disturbances and maintain genetic variation is a key aspect of their resilience1–4. The process of recovery is primarily influenced by rates of survival, growth and immigration5,6. When mortality is high and immigration is low, recovery depends on the few individuals surviving locally, and declines in genetic diversity are expected7. In contrast, when immigration is high and impacts vary among sub-populations, areas least affected can serve as a source of recruits to aid in the recovery more widely. In these cases, genetic diversity remains stable8. On tropical coral reefs, acute heat stress events and associated bleaching have become routine disturbances and are now the primary cause for coral mortality globally9,10. Monitoring patterns of recovery and associated changes in coral cover have informed how climate change is impacting coral reefs11–13; however, information on how fluctuating coral populations influence genetic variation are also required.
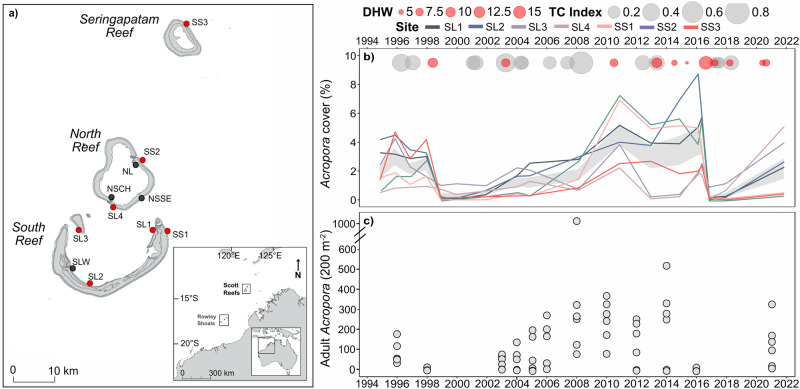
The isolated oceanic atolls of northwest Australia provide a unique opportunity to study coral populations through cycles of impact and recovery. The Scott system of three reefs is hundreds of kilometres from the mainland and neighbouring reefs, and is demographically isolated in space and time14–18. In recent decades, recurrent disturbances to the Scott reefs have caused dramatic shifts in coral population sizes and distribution11,19–21. From 1994 to 2021, moderate heat stress and tropical cyclones were frequent disturbances, reducing coral cover at one or more of our long-term monitoring (LTM) sites during 9 of the 27 years (Fig. 1). The most severe disturbances were mass bleaching events in 1998, and again in 2016, causing relative reductions in mean coral cover of ~75% following both events11. Cyclone Lua in 2012 also decreased mean cover by 8% across the reef system22, with other smaller disturbances (e.g. bleaching in 2010) causing less severe and more localised impacts. The reefs had largely recovered 12 years after the 1998 mass bleaching, but with local variation in recovery depending on subsequent exposure to disturbances and the resilience of different coral groups within communities, leading to long-term shifts in community composition11.
Fig. 1. Periodic disturbances and mortality on the Scott system of reefs.
a Permanent monitoring sites in which demographic (red symbols) and genetic variation (red and black symbols) were assessed through time. b Changes in the percentage cover (± se mean grey bar) of corymbose Acropora; and c density of adult Acropora colonies (with each grey circle representing an LTM site).
Results and discussion
Through the regime of recent disturbances to the Scott system of reefs, the impacts of even the most severe event varied spatially, usually with a different group of sites worst affected by bleaching or by cyclones (Fig. 1b). Consequently, when Acropora cover decreased to very low levels (<1%) at some sites following disturbance, it was often higher (1–7%) at other sites across the reef system (Fig. 1b). This spatial variation in cover of Acropora reflected the changes in abundance of adult colonies (>20 cm) within the long-term monitoring sites (Fig. 1c).
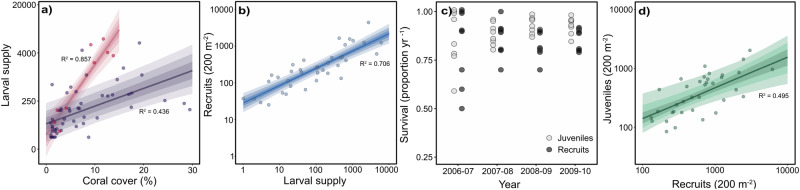
The loss of adult colonies caused a comparative reduction in reproductive output and larval supply to recruitment tiles across the reef system (R2 = 0.857; red points, Fig. 2a); however, this stock-recruitment relationship was much weaker at the scale of the individual sites (200 m2), with the abundance of adult colonies being a poor predictor of larval supply to settlement tiles locally (R2 = 0.438; purple points, Fig. 2a). Subsequent recovery at each site was then driven by local conditions, with the supply of Acropora larvae to settlement tiles predicting (R2 = 0.706) the number of new Acropora recruits (<2 cm) on natural substrata six to 12 months later (Fig. 2b). Survival of these recruits and the resulting juveniles (6–15 cm) was also high (Fig. 2c), with the number of recruits on substrata influencing the number of juvenile colonies within each site 12–24 months later (Fig. 2d).
Fig. 2. Broadscale dispersal and post-recruitment survival drives recovery.
a Stock-recruitment relationship for Acropora at each site (purple, 200 m2) and across the reef system (red, years). Larval supply is the number of settlers on tiles following mass-spawning. b Relationship between larval supply to recruitment tiles and density of new Acropora recruits (≤2 cm) on substrata, 6 to 12 months later, within each site (200 m2). c Annual survival of Acropora recruits (<5 cm) and juveniles (6–15 cm) at sites during years of rapid recovery (2006–2010) following mass bleaching (left). d Relationship between density of Acropora recruits and juveniles, 12 to 24 months later, within each site (200 m2). Dark lines are the median slope and coloured ribbons (darker to lighter) show 50%, 75%, and 95% posterior density intervals. Conditional R2 values are displayed for each model.
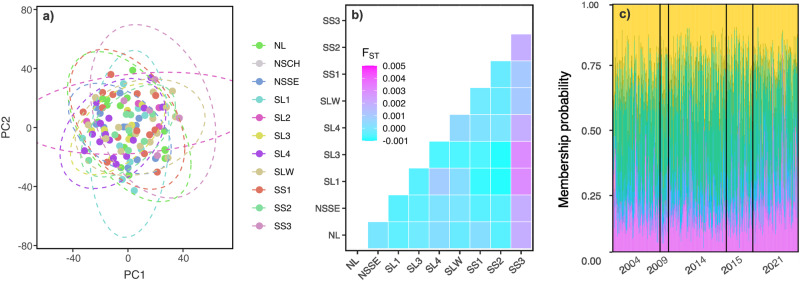
Demographic changes in Acropora assemblages provided indirect evidence of recovery at the Scott reefs being seeded by the supply of larvae from less affected sites. This mechanism of recovery was supported by population genetics of a common species of corymbose Acropora. Low-coverage whole genome re-sequencing of Acropora sp. (formerly Acropora tenuis23) revealed high gene flow and connectivity across the Scott system of reefs, with no clustering of samples by site using a genotype likelihood approach based on 8,329,442 variant sites (Fig. 3a). This pattern of high gene flow was confirmed using more stringent depth filters (29,462 single nucleotide polymorphisms (SNPs); Fig. S1). Mean FST was −0.0002 (Fig. S1) and most pairwise estimates between sites were negative and few were significant (Fig. 3b). The exception was the sample site from Seringapatam reef (SS3), which showed low (FST < 0.004) but significant levels of genetic differentiation with all other sites from North and South Scott (Fig. 3b, Supplementary Data 1). Together, the whole genome re-sequencing data pointed to broadscale dispersal and high gene flow across the reef system.
Fig. 3. High genetic connectivity across the Scott system of reefs.
a Scatter plot of the first two principal components for samples collected in 2021 and based on 8,329,442 variant sites using a whole genome genotype likelihood approach. Each point represents a unique coral colony and is colour coded by site. Dashed lines represent 95% confidence ellipses for each site. b Tiled heatmap of genetic differentiation (pairwise FST Weir and Cockerham, 1984) among sites in 2021 using a reduced dataset of 29,462 single nucleotide polymorphisms with more strict depth filters (depth > 10). Warmer colours indicate higher values (Supplementary Data 1). c Genotype composition plot of assignment probabilities for individuals (vertical bars) to predefined groups based on year of collection (K = 5) using the temporal genetic dataset of microsatellite genotypes.
Our temporal genetic dataset of microsatellite genotypes (2004, 2009, 2014, 2015, 2021) revealed a consistent pattern of connectivity underlying recovery (Fig. 3c). Within each year, mean estimates of genetic differentiation were negative, and most pairwise values were low and non-significant (Fig. S2, Supplementary Data 2). The genetic patchiness that did arise within each timepoint was ephemeral and changed from year to year (Fig. S3). As a result, there were no significant differences in allele frequencies among sites nested within years (θST = −0.008, P = 0.752). Differences among years under the AMOVA framework accounted for only 1.6% of the total variation (θRT = 0.0192, P = 0.003) and we did not observe any clustering of samples by year in multi-dimensional space (Supplementary Data 3, Fig. 3c). This pattern of chaotic genetic patchiness is a common attribute of broadcast spawning marine species, where the complex interplay between biological and physical processes often drives unstable patterns of spatial genetic structure24,25. In turn, one generation of patches does not predict the next (Fig. S4). These levels of genetic differentiation among sites and years were outside the bounds of normality when we randomized site-time assignments and recalculated genetic differentiation (Fig. S5), suggesting that they more likely reflect stochastic biological processes than technical artefacts.
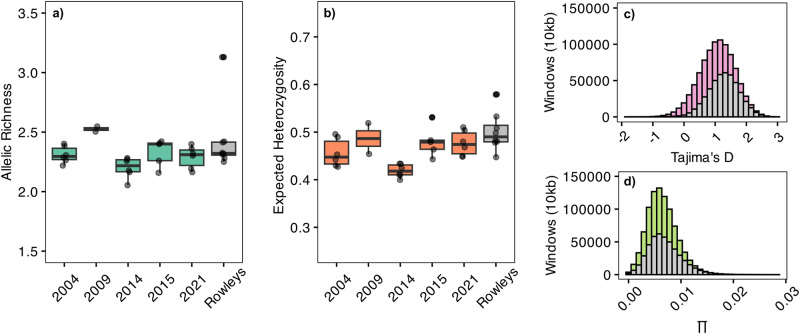
Despite recurrent disturbances and dramatic demographic changes in Acropora assemblages, the genetic diversity of the broader metapopulation at the Scott reefs has remained stable. We did not observe any changes in allelic richness or heterozygosity among our sampling timepoints (Fig. 4a, b; Supplementary Data 4). While site-level estimates seemed to vary slightly among years, genetic diversity in the broader population remained stable across our study period. Although we did not have samples from the Scott reefs that predated the 1999 mass bleaching event, we found that levels of genetic diversity were similar to samples collected from the neighbouring Rowley Shoals, an offshore oceanic atoll system 400 km to the south of the Scott reefs that has avoided widespread bleaching and mortality over similar timescales11. Indeed, microsatellite markers (Fig. 4a, b, Supplementary Data 4) and whole genome re-sequencing techniques (Fig. 4c, d) indicated that genetic diversity in the two systems are similar, despite strong genetic drift (genome-wide FST = 0.078, Fig. S6) and contrasting disturbance histories. Thus, despite severe declines in coral cover, our data suggest that there have been enough survivors at Scott system of reefs to facilitate recovery and maintain moderate levels of genetic diversity in the metapopulation.
Fig. 4. Genetic diversity is stable through time.
a Boxplots of allelic richness and b expected heterozygosity at each timepoint based on the microsatellite genotype data. Each black point represents a site value averaged across five loci. The boxes represent the interquartile range (IQR) of the data distribution, and the thick black line inside the box represents the median. Data from the Rowley Shoals are also provided for regional context. c Histograms of Tajima’s D and d nucleotide diversity (∏) for Scott Reef (coloured) and Rowley Shoals (grey) based on whole genome re-sequencing data using a genotype likelihood approach.
The persistence of coral populations depends largely on their connectivity and larval supply, and their capacity to absorb and adapt to emerging pressures. Our results suggest that a high dispersal capacity and post-recruitment survival in Acropora are key mechanisms of resilience to climate change. This is in contrast to species with a lower capacity to disperse across a group of reefs, such as some brooding corals or those reliant on vegetative propagation26. However, our data also highlight that recovery depends on spatial variation in impacts and relatively high abundances of survivors at several locations across the reef system, in addition to considerable time between severe disturbances. Under these conditions, population abundances and genetic diversity of Acropora corals may be maintained, but with an increasing severity and frequency of disturbances through global heating, longer term degradation is likely, particularly at these isolated coral reefs. This study underscores the importance of long-term monitoring to understand the impacts of climate change on coral reef ecosystem health. More importantly, it highlights an inherent resilience in broadcast spawning coral populations on remote reefs, and offers a glimmer of hope to coral reef ecosystems more broadly against a backdrop of rapid environmental change.
Methods
Demographic changes in Acropora
In 1994, permanent transects (250 metres) were established at 21 reef slope sites replicated across seven locations and three reefs27. At each location (Fig. 1a), three sites were separated by approximately 300 metres and consisted of 250 metres of permanent transects marked at 10-m intervals. Surveys were conducted annually between 1994 and 1999, and then in 2003, 2004, 2005, 2008, 2010, 2012, 2014, 2016, 2017, and 2021. In 2016, additional surveys were conducted in January, April and October 2016, before, during and after the mass bleaching, and from 2016 only the first site at each location was surveyed to allow for expansion into additional habitats (not included here). During each survey, a tape was laid along the permanent transect and images of the benthic community captured from a distance between 30 and 50 cm from the substrata. Images were analysed using point sampling technique and benthic groups identified to the lowest taxonomic resolution achievable by each observer28. These data were then divided among benthic groups according to taxa (e.g., family, genus) and growth form (e.g. encrusting, foliose, massive, branching).
Larval supply and recruitment
Larval supply of the most diverse and abundant coral genus, the Acropora, was quantified using terracotta settlement tiles along the permanent transects at 18 sites at six of the reef slope locations, during nine years between 1996 to 201321 (1996–1999, 2002, 2003, 2008, 2010, 2011). At each of the sites, groups of six settlement tiles (10 cm × 10 cm) were deployed at distances of 30–100 cm apart, spaced at 60 m along the permanent transect. Settlement tiles (n = 108 tiles total) were deployed and collected one month either side of the primary mass coral spawning29. Size-frequency distributions of Acropora colonies (excluding staghorn and hispidose growth forms) were quantified along the permanent transects (200 m) at the first site at each of six reef slope locations. Colony sizes (longest linear dimension) were recorded to the nearest centimetre along ten 20 m transects spaced at 10 m intervals, within a width of 25 cm for colonies <10 cm (50 m2) and 1 m for colonies ≥10 cm (200 m2). Density at each site was adjusted to 200 m2. Growth and survival of colonies was quantified in a species of corymbose Acropora (nominally Acropora spicifera) at two of the permanent sites at four reef slope locations over four years (2006–2010). Locations were chosen to be representative of the primary habitat for corymbose Acropora across the reef system. Colonies (n = 3692; of all sizes were tagged and resurveyed annually. Given issues identifying juvenile coral to species, an assemblage of Acropora species were tagged for colonies <10 cm in size. Size and growth were quantified from photographs taken directly above the colony, or perpendicular to the maximum length when first tagged. Colonies were digitized and their size defined by the maximum diameter through the ellipse of best fit.
Microsatellite genotyping
Tissue samples were collected from 982 colonies of a corymbose Acropora (Acropora sp.) at five timepoints (2004, 2009, 2014, 2015, and 2021; Supplementary Data 5) spanning 17 years. At each site, samples were taken from colonies separated by at least 1.5 m and not from loose fragments or colonies directly down-slope from another colony. Genomic DNA was extracted using a Qiagen DNeasy kit or using a high-throughput membrane-based DNA extraction protocol30 and genotyped across at seven microsatellite loci16,31 in 10 µL multiplex PCR reactions using the Qiagen multiplex PCR kit. Fragment analysis was carried out on a GE Healthcare MegaBACE 1000 capillary sequencer or an Applied Biosystems 3730 capillary sequencer. Electropherograms were visualised and scored using the software GeneMarker v1.91 (SoftGenetics). To facilitate comparison of allele sizes among collections that were analysed on different platforms, 19 control samples from earlier collections that exhibited a representative range of allele sizes were run alongside the new samples and allele sizes calibrated accordingly. To further minimise genotyping errors, automated scorings of alleles in all microsatellite analyses were checked manually, and uncertainties were cleared by re-amplification and comparison. Of the seven loci originally trialled, two loci scored in 2004 and 2009 (Amil2_006 and Amil5_028) could not be consistently scored and/or calibrated on the Applied Biosystems sequencer, and these loci were removed.
Cryptic lineages and clonality
Previous data from the Scott reefs have shown cryptic lineages are widespread in Acropora and reflect divergence in reproductive timing, with the main spawning period during austral autumn and a secondary smaller event in austral spring29,32. Sympatric colonies of Acropora sp. display strong genetic divergence, suggesting pronounced reproductive boundaries and limited gene flow29,33–35. Before analysing patterns of genetic variation through space and time, an initial screening of all samples using microsatellite data was conducted to remove the less abundant spring spawning lineage to focus our analyses on a single gene pool. To do this, we utilized data from 2009 that tracked a subset of colonies (n = 68) across multiple years with a consistent seasonal spawning phenotype29. These samples were used to assign samples from all timepoints to one of the two lineages using model-based Bayesian clustering in Structure36 (Fig. S7). We ran structure with the no prior model at K = 2 based on the panel of five common microsatellites. Analyses were run ten times using the admixture model with independent allele frequencies and with a burn-in period of 100,000, followed by 500,000 MCMC replications for each run. These model conditions were implemented because the genetic differentiation between spawning groups is large and initial investigations showed that runs with correlated allele frequencies model overestimated K and individuals lacked strong assignment29. After removing the spring spawning lineage and poor-quality samples that failed to amplify, we were left with 506 autumn spawning colonies (321 multi-locus genotypes) with greater than 0.75 probability of assignment (mean assignment was 0.96 +/− 0.05 S.D.). Genotypic richness in the targeted species at the Scott reefs is high (>0.9615) with little evidence of clonal propagation via vegetative fragmentation; however, replicate multi-locus genotypes were common in our temporal dataset when we restricted analyses to the five microsatellite loci that could be scored across all years. The 2004 and 2009 datasets included two additional loci15 and showed that the replicate MLGs in the temporal dataset were indeed unique individuals, and so we proceeded with a dataset of all 506 multi-locus genotypes for downstream analyses. We also clone corrected at the population level using poppr and confirmed patterns identified with the larger dataset held true when focussing on unique MLGs.
Genetic differentiation and diversity
Using our temporal dataset of microsatellite genotypes for the autumn spawning lineage, we calculated pairwise estimates of FST among sites with stammp37 and adjusted for multiple comparisons using a False Discovery Rate in R (p.adjust). We used prcomp (R Core Team, 2021) to carry out principal components analyses and used Discriminant Analyses of Principal Components (DAPC) with adegenet38 to estimate group assignment probabilities under a Bayesian framework. To explore hierarchical patterns of genetic structure, we carried out an analysis of molecular variance (AMOVA) with sample sites nested within timepoints. To measure levels of genetic diversity, we calculated allelic richness and expected heterozygosity for each site and timepoint comparison using hierfstat39. We then used a Bayesian generalised linear mixed effects model with a gaussian distribution to test for differences in genetic diversity among years. Sampling site was included as a random effect to account for the lack of spatial independence. The model was based on three chains with 3000 iterations, including 1000 iterations to warm-up and a thinning interval of 5. All Bayesian models were performed in Stan (Stan Development Team, 2021) using the “brms” package40. We ran posterior predictive checks and visually inspected all models for violations for statistical assumptions using the ‘dharma’ package (Hartig, 2022). Finally, to compare these estimates of genetic diversity with a pristine reef system, we genotyped conspecific colonies of Acorpora sp. from the Rowley Shoals using the same panel of microsatellite markers (n = 196 colonies) and whole genome re-sequencing techniques (n = 10 colonies, below).
Whole genome re-sequencing
We re-sequenced the genomes of samples (n = 111 colonies) collected in 2021 to approximately 8X coverage (Fig. S8) using Covaris library preparation via sonication and sequenced on a NovaSeq at Genomics WA. Raw sequence files were mapped with bwa mem (Supplementary Note 1) to the A. tenuis pseudo-chromosome-scale assembly (aten.chr.fasta)33. We used samtools41 to sort and index and picardtools (http://broadinstitute.github.io/picard) to mark and remove duplicate reads. Samtools41 was used to calculate sequencing depth of each sample at each position across the genome (Supplementary Note 2, Fig. S10). We called single nucleotide polymorphisms (SNPs) using mpileup in bcftools42 and filtered using samtools and based on a minor allele frequency of 0.05, a call rate of 0.95, and a mean read depth of 10x per sample (Supplementary Note 3). This produced a variant call file of 29,462 biallelic SNPs that was used to estimate pairwise FST among sites (minimum of 5 individuals per site) using stamp. We also explored genetic structure among sites using a genotype likelihood approach in angsd43 and estimated genome-wide allele frequencies to carry out principal components analyses using pcangsd44 (Supplementary Note 4). We masked sites with a minQ < 20, minMapQ < 30, sites with less than 1/3 and greater than 2x the mean read depth and removed loci with missing data in more than half of the samples. We only retained variable sites (snp_pval < 1e−6) and those with a minor allele frequency greater than 0.05. This filtering criteria produced 8,329,442 variant sites used for clustering analysis. We also re-sequenced a subset of genomes from the Rowley Shoals (n = 10) using Nextera Flex Library Preparation Kits (Illumina) and sequenced on a NovaSeq.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This research was funded by the Australian Institute of Marine Science and Woodside Energy Ltd as Operator for and on behalf of the Browse Joint Venture (BJV). We would like to thank the crew of the RV Solander for their professionalism and hard work. We gratefully acknowledge the support from the Australian Cancer Research Foundation for the Centre for Advanced Cancer Genomics which has made available the Illumina Novaseq6000 systems for the use of Genomics WA. We also thank Helix Solutions for their support with microsatellite genotyping. This manuscript was written on Whadjuk Noongar boodja. We acknowledge the Whadjuk people as traditional owners of this land and pay respect to their elders past and present.
Author contributions
J.G., L.T., and J.U. designed the study, collected samples, analysed the data. D.S., S.D., N.R. and C.G collected samples and analysed data. A.S. and W.J.K. analysed data. All authors wrote the manuscript.
Peer review
Peer review information
Communications Biology thanks Chris Langdon, Mikhail Matz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Luciano Matzkin and Luke R. Grinham. A peer review file is available.
Data availability
All of the data to support the findings of this study can be found on the Open Science Framework 10.17605/OSF.IO/2VBHW (https://osf.io/2vbhw/)45.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06100-0.
References
- 1.Gunderson LH. Ecological resilience-in theory and application. Annu. Rev. Ecol. Syst. 2000;31:425–3. doi: 10.1146/annurev.ecolsys.31.1.425. [DOI] [Google Scholar]
- 2.O’Neill RV. Recovery in complex ecosystems. J. Aquat. Ecosyst. Stress Recover. 1999;6:181–187. doi: 10.1023/A:1009996332614. [DOI] [Google Scholar]
- 3.Holling CS. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973;4:1–23. doi: 10.1146/annurev.es.04.110173.000245. [DOI] [Google Scholar]
- 4.Nyström M, Folke C, Moberg F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 2000;15:413–417. doi: 10.1016/S0169-5347(00)01948-0. [DOI] [PubMed] [Google Scholar]
- 5.Davies ID, Cary GJ, Landguth EL, Lindenmayer DB, Banks SC. Implications of recurrent disturbance for genetic diversity. Ecol. Evol. 2016;6:1181–1196. doi: 10.1002/ece3.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks SC, et al. How does ecological disturbance influence genetic diversity? Trends Ecol. Evol. 2013;28:670–679. doi: 10.1016/j.tree.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Beheregaray LB, et al. Genes record a prehistoric volcano eruption in the Galápagos. Science. 2003;302:75. doi: 10.1126/science.1087486. [DOI] [PubMed] [Google Scholar]
- 8.Spear SF, Crisafulli CM, Storfer A. Genetic structure among coastal tailed frog populations at Mount St. Helens is moderated by post-disturbance management. Ecol. Appl. 2012;22:856–869. doi: 10.1890/11-0627.1. [DOI] [PubMed] [Google Scholar]
- 9.Hughes T, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 10.Hughes T, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 11.Gilmour, J. P. et al. A tale of two reef systems: local conditions, disturbances, coral life histories, and the climate catastrophe. Ecol. Appl. 10.1002/eap.2509 (2021). [DOI] [PubMed]
- 12.Hoegh-guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 13.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 14.Moore JaY, et al. Unprecedented mass bleaching and loss of coral across 12° of latitude in Western Australia in 2010-11. PLoS ONE. 2012;7:e51807. doi: 10.1371/journal.pone.0051807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Underwood JN. Genetic diversity and divergence among coastal and offshore reefs in a hard coral depend on geographic discontinuity and oceanic currents. Evol. Appl. 2009;2:222–233. doi: 10.1111/j.1752-4571.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Underwood JN, Smith LD, van Oppen MJH, Gilmour JP. Ecologically relevant dispersal of corals on isolated reefs: implications for managing resilience. Ecol. Appl. 2009;19:18–29. doi: 10.1890/07-1461.1. [DOI] [PubMed] [Google Scholar]
- 17.Adam, A. A. S. et al. Diminishing potential for tropical reefs to function as coral diversity strongholds under climate change conditions. Divers. Distrib. 27, 2245–2261 (2021).
- 18.Zhang J, et al. Evolutionary responses of a reef-building coral to climate change at the end of the last glacial maximum. Mol. Biol. Evol. 2022;39:1–18. doi: 10.1093/molbev/msac201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith LD, Gilmour JP, Heyward AJ. Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching. Coral Reefs. 2007;27:197–205. doi: 10.1007/s00338-007-0311-1. [DOI] [Google Scholar]
- 20.Gilmour JP, et al. The state of Western Australia’s coral reefs. Coral Reefs. 2019;38:651–667. doi: 10.1007/s00338-019-01795-8. [DOI] [Google Scholar]
- 21.Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS. Recovery of an isolated coral reef system following severe disturbance. Science. 2013;340:69–71. doi: 10.1126/science.1232310. [DOI] [PubMed] [Google Scholar]
- 22.Puotinen M, et al. Towards modelling the future risk of cyclone wave damage to the world’s coral reefs. Glob. Chang. Biol. 2020;26:4302–4315. doi: 10.1111/gcb.15136. [DOI] [PubMed] [Google Scholar]
- 23.Bridge, T. C. L. et al. Original Article A tenuis relationship: traditional taxonomy obscures systematics and biogeography of the ‘Acropora tenuis’ (Scleractinia: Acroporidae) species complex. Zool. J. Linn. Soc. 27, 1–24 (2023).
- 24.Johnson M, Black R. Chaotic genetic patchiness in an intertidal limpet, Siphonaria sp. Mar. Biol. 1982;164:157–164. doi: 10.1007/BF00397680. [DOI] [Google Scholar]
- 25.Johnson M, Black R. Pattern beneath the chaos: the effect of recruitment on genetic patchiness in an intertidal limpet. Evolution. 1984;38:1371–1383. doi: 10.2307/2408642. [DOI] [PubMed] [Google Scholar]
- 26.Thomas L, et al. Contrasting patterns of genetic connectivity in brooding and spawning corals across a remote atoll system in northwest Australia. Coral Reefs. 2020;39:55–60. doi: 10.1007/s00338-019-01884-8. [DOI] [Google Scholar]
- 27.Heyward, A. et al. Characterisation of Scott Reef Lagoon Biota – Fish and Macrobenthos (1999).
- 28.Jonker, M., Johns, K. & Osborne, K. Surveys of Benthic Reef Communities Using Underwater Digital Photography and Counts of Juvenile Corals. Long-term Monitoring of the Great Barrier Reef, Vol. 10 (2008).
- 29.Gilmour JP, Underwood JN, Howells EJ, Gates E, Heyward AJ. Biannual spawning and temporal reproductive isolation inacropora corals. PLoS ONE. 2016;11:1–14. doi: 10.1371/journal.pone.0150916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanova NV, Dewaard JR, Hebert PDN. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes. 2006;6:998–1002. doi: 10.1111/j.1471-8286.2006.01428.x. [DOI] [Google Scholar]
- 31.van Oppen MJH, Underwood JN, Muirhead A, Peplow L. Ten microsatellite loci for the reef-building coral Acropora millepora (Cnidaria, Scleractinia) from the Great Barrier Reef. Aust. Mol. Ecol. 2007;7:436–438. [Google Scholar]
- 32.Gilmour JP, Smith LD, Brinkman RM. Biannual spawning, rapid larval development and evidence of self-seeding for scleractinian corals at an isolated system of reefs. Mar. Biol. 2009;156:1297–1309. doi: 10.1007/s00227-009-1171-8. [DOI] [Google Scholar]
- 33.Thomas L, et al. Spatially varying selection between habitats drives physiological shifts and local adaptation in a broadcast spawning coral on a remote atoll in Western Australia. Sci. Adv. 2022;8:abl9185. doi: 10.1126/sciadv.abl9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosser NL, et al. Phylogenomics provides new insight into evolutionary relationships and genealogical discordance in the reef-building coral genus Acropora. Proc. R. Soc. B Biol. Sci. 2017;284:20162182. doi: 10.1098/rspb.2016.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosser NL, Edyvane K, Malina AC, Underwood JN, Johnson MS. Geography and spawning season drive genetic divergence among populations of the hard coral Acropora tenuis from Indonesia and Western Australia. Coral Reefs. 2020;39:989–999. doi: 10.1007/s00338-020-01923-9. [DOI] [Google Scholar]
- 36.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pembleton LW, Cogan NOI, Forster JW. StAMPP: an R package for calculation of genetic differentiation and structure of mixed-ploidy level populations. Mol. Ecol. Resour. 2013;13:946–952. doi: 10.1111/1755-0998.12129. [DOI] [PubMed] [Google Scholar]
- 38.Jombart T. Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 39.Goudet J. HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes. 2005;5:184–186. doi: 10.1111/j.1471-8286.2004.00828.x. [DOI] [Google Scholar]
- 40.Bürkner, P. C. Bayesian Item Response Modeling in R with brms and Stan. J. Stat. Softw. 100, 1–54 (2021).
- 41.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: analysis of next generation sequencing data. BMC Bioinforma. 2014;15:1–13. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meisner J, Albrechtsen A. Inferring population structure and admixture proportions in low-depth NGS data. Genetics. 2018;210:719–731. doi: 10.1534/genetics.118.301336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, L. Resilience to periodic disturbances and the long-term genetic stability in Acropora coral. Open Sci. Framew. 10.17605/OSF.IO/2VBHW (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All of the data to support the findings of this study can be found on the Open Science Framework 10.17605/OSF.IO/2VBHW (https://osf.io/2vbhw/)45.