Abstract
There is no consensus on the optimal endpoint(s) in cancer cachexia trials. Endpoint variation is an obstacle when comparing interventions and their clinical value. The aim of this systematic review was to summarize and evaluate endpoints used to assess appetite and dietary intake in cancer cachexia clinical trials. A search for studies published from 1 January 1990 until 2 June 2021 was conducted using MEDLINE, Embase and Cochrane Central Register of Controlled Trials. Eligible studies examined cancer cachexia treatment versus a comparator in adults with assessments of appetite and/or dietary intake as study endpoints, a sample size ≥40 and an intervention lasting ≥14 days. Reporting was in line with PRISMA guidance, and a protocol was published in PROSPERO (2022 CRD42022276710). This review is part of a series of systematic reviews examining cachexia endpoints. Of the 5975 articles identified, 116 were eligible for the wider review series and 80 specifically examined endpoints of appetite (65 studies) and/or dietary intake (21 studies). Six trials assessed both appetite and dietary intake. Appetite was the primary outcome in 15 trials and dietary intake in 7 trials. Median sample size was 101 patients (range 40–628). Forty‐nine studies included multiple primary tumour sites, while 31 studies involved single primary tumour sites (15 gastrointestinal, 7 lung, 7 head and neck and 2 female reproductive organs). The most frequently reported appetite endpoints were visual analogue scale (VAS) and numerical rating scale (NRS) (40%). The appetite item from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) C30/C15 PAL (38%) and the appetite question from North Central Cancer Treatment Group anorexia questionnaire (17%) were also frequently applied. Of the studies that assessed dietary intake, 13 (62%) used food records (prospective registrations) and 10 (48%) used retrospective methods (24‐h recall or dietary history). For VAS/NRS, a mean change of 1.3 corresponded to Hedge's g of 0.5 and can be considered a moderate change. For food records, a mean change of 231 kcal/day or 11 g of protein/day corresponded to a moderate change. Choice of endpoint in cachexia trials will depend on factors pertinent to the trial to be conducted. Nevertheless, from trials assessed and available literature, NRS or EORTC QLQ C30/C15 PAL seems suitable for appetite assessments. Appetite and dietary intake endpoints are rarely used as primary outcomes in cancer cachexia. Dietary intake assessments were used mainly to monitor compliance and are not validated in cachexia populations. Given the importance to cachexia studies, dietary intake endpoints must be validated before they are used as endpoints in clinical trials.
Keywords: appetite, cachexia, cancer, dietary intake, endpoints, outcomes, trials
Introduction
Loss of appetite is a critical phenotypic feature of cancer cachexia and, combined with reduced dietary intake, drives weight loss. 1 The underlying biology of this is complex and attributable to disturbances of homeostatic mechanisms involved in the regulation of energy balance. 2 The hypothalamus is the key regulator of appetite and can be modulated by several factors including inflammatory cytokines, hormones, neurotransmitters, and sympathetic nerves and vagal afferent fibres. 3
Appetite loss is associated with impaired quality of life (QoL) in patients with cancer 4 and contributes to loss of muscle mass, decline in physical function and increased mortality as part of the cachexia syndrome. 5 Targeting appetite loss has been advocated as a therapeutic strategy to modify this maladaptive response to cancer and to improve QoL. Consequently, clinical trials have for decades aimed to improve appetite and thereby increase dietary intake and body weight in patients at risk of, or suffering from, cachexia. Although improving appetite may not always result in increased body weight, 6 it remains a valuable treatment objective, given its high prevalence and negative impact on patient QoL. One challenge in assessing appetite is that it can represent several symptoms including early satiety and a reduced desire to eat. This is often compounded by other nutritional impact symptoms (NIS) that may inhibit appetite and induce premature cessation of food intake. 7 Examples of NIS are alterations in taste and smell, dry mouth, pain and emotional status, and this means that dietary intake is not dependent on appetite alone. Many patients will also force themselves to eat despite a poor appetite. Thus, to fully understand why and how weight loss occurs, comprehensive assessments of appetite, other NIS, and energy and protein intake are necessary.
Currently, there are a multitude of methods for appetite and dietary intake assessment that may be relevant in cancer cachexia trials, and there is no consensus on which are preferable endpoints. Examples include patient‐reported outcome measures (PROMs), using various scales and time frames, and assessment of dietary intake through different methods of recall or prospective registrations.
This breadth of endpoints poses a significant obstacle in comparing interventions and understanding whether an intervention has clinical value. 8 The ideal endpoint should be meaningful for patients and healthcare professionals alike, reflect the mechanism of action of the intervention being tested, be easy to measure, and be sensitive and specific. 9 , 10 Achieving alignment on the assessment of appetite and dietary intake endpoints in cancer cachexia trials is fundamental for successful research outcomes, for comparing results between studies and for conducting meta‐analyses. The first step is to outline and evaluate the endpoints used in existing cachexia trials. This process is necessary for developing a standardized approach to nutritional endpoint selection.
Therefore, the aim of this systematic review is to summarize and evaluate the endpoints employed for assessment of appetite and dietary intake in clinical trials targeting cancer cachexia and to present a comprehensive overview of endpoints used, frequencies, results, and estimates of effect size and required sample size.
Methods
This systematic review is aligned with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 11 Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used to streamline the review process. The protocol is registered in the PROSPERO database (CRD42022276710 [http://www.crd.york.ac.uk/PROSPERO]).
Search strategy
The search for studies published from 1 January 1990 until 2 June 2021 was conducted by a research librarian using the databases MEDLINE (Ovid), Embase (Ovid) and Cochrane Central Register of Controlled Trials (see Appendix S1 for search strategy).
Study selection and data extraction
Eligible studies were controlled and examined interventions aiming to treat or attenuate cachexia in adult patients (>18 years) with cancer. Studies using pharmacological, nutritional, exercise and/or behavioural interventions were included, and there were no restrictions concerning the comparator(s).
Studies including endpoints assessing appetite and dietary intake were included if the outcome measure was clearly described, and the results were either presented numerically or the statistical significance of a difference between treatment arms was described. Studies using dietary intake outcome measures were included if they presented data on protein intake (g/day, g/kg/day) and/or energy intake (kcal/day, kcal/kg/day, % of estimated needs). Endpoints with a composite score based on additional items not directly related to dietary intake or appetite (e.g., Functional Assessment of Anorexia/Cachexia Therapy Anorexia/Cachexia Subscale [FAACT A/CS] 12 and Patient‐Generated Subjective Global Assessment [PG‐SGA] global score) 13 were not eligible unless they explicitly presented results pertaining to the appetite‐related items. Studies using both single items and studies using formalized questionnaires such as the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) C30, Edmonton Symptom Assessment Scale (ESAS) questionnaire or the MD Anderson Symptom Inventory (MDASI) with appetite as one of the several scales/items were included. Studies reporting other NIS (e.g., dysphagia, constipation, pain, and alterations of taste or smell), and anorexia only as an adverse event using the Common Terminology Criteria for Adverse Events (CTCAE), were excluded. Studies with <40 patients and/or an intervention duration shorter than 14 days were excluded. Eligible studies had to be published in full‐text format and written in English.
Using Covidence software, two independent reviewers (OFD and BJAL) performed a title‐based selection process for all identified trials. Another pair of independent reviewers (TSS and BJAL) reviewed and selected studies based on their abstracts. Any eligibility uncertainties were resolved through discussions to reach a consensus.
A data extraction table was developed, pilot‐tested and refined within the review group before use. Two independent review authors extracted the data. This systematic review is part of a comprehensive review collaboration covering six main groups of endpoints in cachexia: body composition, oncology, physical function, QoL, biomarkers and nutrition. Because most controlled trials in cachexia explore multiple endpoints, all papers were divided among the review teams, and each team extracted data for all endpoints.
Assessing risk of bias
Four different reviewers (JM, JS, OFD and BJAL) assessed the methodological quality of each study using the modified Downs and Black Checklist. 14 The tool assesses among other criteria, study design, external and internal validity, whether spread was reported and if outcome was defined (see Appendix S2 for scoring details).
Endpoints and data analysis
Data were reported narratively, describing the diversity and frequencies of endpoints. Concordance between endpoints of appetite and dietary intake and between appetite/dietary intake and weight loss was evaluated, whenever possible.
Where the mean difference in change of the endpoint (baseline to post‐intervention) and standard deviation of the difference (or standard error of the mean change) were reported, effect size was calculated using Hedge's g. Additionally, an estimate of what constituted a small, medium and large effect size was presented for each selected instrument based on the pooled standard deviation from two or more studies. An estimate of needed sample size to detect a small, medium and large effect size was calculated using the normal approximation to the two‐sample t test for independent samples.
Results
Figure 1 shows the flow chart for study selection. The literature search resulted in 5975 studies, and following the title and abstract screening, 369 articles were fully reviewed, 116 articles were eligible for the wider review series and 80 articles were eligible for this specific review.
Figure 1.
PRISMA flow chart.
Table 1 shows the key characteristics of eligible trials. The sample size ranged from 40 to 628 with a median of 101. Forty‐nine studies included multiple primary tumour sites, while 31 studies focused on single primary tumour sites (15 gastrointestinal [GI], 7 lung, 7 head and neck and 2 female reproductive organs). Pharmacological interventions were used in 43 (54%) studies, nutritional interventions in 25 (31%) studies, multimodal interventions in 9 (11%) studies and behavioural/exercise interventions in 3 (4%) studies.
Table 1.
Key characteristics of eligible studies
| First author | Year | Study type | N a | Quality | Cancer type | Cancer treatment | Cachexia inclusion criteria | Intervention | Comparator | Primary outcome | Nutrition outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kardinal 15 | 1990 | Double‐blind RCT | 293 | 8 | Other, GI, lung | No, chemotherapy/immunotherapy and/or radiotherapy | WL > 5 lb in 2 months or intake <20 kcal/kg BW/day | Cyproheptadine 8 mg t.i.d. | Placebo | BW | NCCTG (appetite questions) |
| Loprinzi 16 | 1990 | Double‐blind RCT | 133 | 9 | GI, lung, other | 60% received chemotherapy | WL > 5 lb in 2 months or intake <20 kcal/kg | MA 800 mg/day | Placebo | BW | NCCTG (appetite questions) |
| Feliu 17 | 1992 | Double‐blind RCT | 150 | 5 | Lung, other, gastric | No tumour‐directed treatment | WL > 10% and/or appetite <5 NRS | MA | Placebo | BW and appetite | Appetite VAS (SSA) |
| Downer 18 | 1993 | Double‐blind RCT | 60 | 1 | Other, lung, mesothelioma | 2/3 received chemotherapy | Loss of appetite, not further described | MPA | Placebo | Not defined | Appetite VAS |
| Loprinzi 19 | 1993 | RCT | 342 | 8 | Lung, GI, other | No, chemotherapy and/or radiotherapy in palliative intent | WL > 5 lb in 2 months or intake <20 kcal/kg | (1) MA 1280 mg, (2) 800 mg or (3) 400 mg/day | (4) MA 160 mg/day | BW | NCCTG (appetite questions) |
| Ovesen 20 | 1993 | Open‐label RCT | 137 | 8 | Ovarian, lung, breast | Chemotherapy | Life expectancy ≥2 months, ECOG ≥ 2 | Dietary counselling and multivitamin mineral tablet |
Standard care Including one daily multivitamin–mineral tablet |
BW and QoL | Food records |
| Lai 21 | 1994 | RCT | 52 | 5 | Cervical, endometrial, colorectal | Radiotherapy | Experiencing anorexia during RT | MA 160 mg/day or prednisolone 30 mg/day | Placebo | Not defined | Appetite study‐specific score |
| Goldberg 22 | 1995 | Double‐blind RCT | 70 | 8 | Lung, GI, other | WL > 5 lb in 2 months or intake <20 kcal/kg | Pentoxifylline 400 mg t.i.d. | Placebo | BW | NCCTG (appetite questions) | |
| Chen 23 | 1996 | RCT | 129 | 8 | H&N | Radiotherapy | NA | MA 40 mg q.i.d. or cisapride 5 mg t.i.d. | Placebo | BW and appetite | Scored by HCP |
| Gebbia 24 | 1996 | RCT | 122 | 6 | Lung, H&N, other | Refractory to prior chemotherapy | WL > 5% | MA 160 mg/day | MA 320 mg/day | NA | Appetite Symptom Distress Scale |
| Simons 25 | 1996 | Double‐blind RCT | 206 | 7 | NSCLC, GI, other | Concurrent chemotherapy 12% in both groups | NA | MPA | Placebo | Appetite, weight and QoL | Appetite NRS, EORTC QLQ C‐30 (appetite item) |
| Beller 26 | 1997 | Double‐blind RCT | 240 | 4 | GI, lung, other | 50% received chemotherapy | BW ≥ 5% below ideal or WL > 5% | (1) MA high dose |
(2) MA low dose (3) Placebo |
QoL, combined | Appetite VAS (LASA) |
| Catalina 27 | 1998 | Double‐blind RCT | 150 | 5 | Lung, other, colorectal | Majority without chemotherapy | WL > 5% during illness |
(1) MA low dose (2) MA high‐dose group |
(3) Placebo group | NA | Appetite VAS |
| De Conno 28 | 1998 | RCT | 42 | 6 | Lung, other, GI | BSC | Diminished or absent appetite | MA | Placebo | Appetite | NRS appetite |
| Simons 29 | 1998 | Double‐blind RCT | 54 | 6 | NSCLC, GI, other | No chemotherapy | NA | MPA 500 mg b.i.d. | Placebo | Energy and protein intake, body composition | Dietary history |
| Loprinzi 30 | 1999 | RCT | 496 | 8 | Lung, GI, other | WL > 5 lb in 2 months or intake <20 kcal/kg |
(1) MA 800 mg/day (2) Dexamethasone 0.75 mg q.i.d. |
(3) Fluoxymesterone 10 mg b.i.d. | Appetite | NCCTG (appetite questions) | |
| McMillan 31 | 1999 | Double‐blind RCT | 73 | 7 | GI (67% pancreatic) | BSC | WL > 5% | MA + ibuprofen | MA + placebo | BW | Appetite VAS, EORTC QLQ C‐30 (appetite item) |
| Westman 32 | 1999 | Double‐blind RCT | 255 | 7 | Colorectal, lung, other | Treatment with palliative intent | Progressive, symptomatic cancer, preferably (not necessarily) anorexia and/or WL | MA | Placebo | QoL | EORTC QLQ C‐30 (appetite item) |
| Erkurt 33 | 2000 | RCT | 100 | 5 | Lung, H&N, other | Chemotherapy and/or radiotherapy | Increasing anorexia, smell and taste change and WL due to RT | MA 480 mg/day | Placebo | BW | Scored by HCP |
| Jatoi 34 | 2002 | RCT | 469 | 10 | Lung, GI, other | 70% received chemotherapy | WL 5 lb in 2 months or intake <20 kcal/kg |
(1) MA 800 mg/day + dronabinol 5 mg/day (2) Dronabinol 5 mg/day + placebo |
(3) MA 800 mg/day + placebo | BW and appetite | NCCTG (appetite questions) |
| Persson 35 | 2002 | Open‐label RCT | 137 | 6 | Colorectal, gastric | No, chemotherapy and/or radiotherapy in palliative or curative intent | NA |
(1) Individual dietary and psychological support (IS) (2) IS + group rehabilitation (GR) |
(3) SOC (4) GR |
Not defined | EORTC QLQ C‐30 (appetite item) |
| Ulutin 36 | 2002 | RCT | 119 | 9 | NSCLC | None | WL > 10% in 6 months | MA 160 mg/day | MA 320 mg/day | BW and appetite | Symptom Distress Scale |
| Bruera 37 | 2003 | Double‐blind RCT | 91 | 7 | Other, GI, urogenital | Palliative treatment | Anorexia (>3 on VAS) plus WL > 5% | Fish oil capsules (EPA, DHA, vitamin E) | Placebo | Appetite | Appetite VAS, food records |
| Fearon 38 | 2003 | Double‐blind RCT | 200 | 8 | Pancreatic | No previous treatment in the last 4 weeks. Ongoing treatment in trial not described | >5% WL in 6 months | Protein and energy‐dense n‐3 PUFA‐enriched ONS | Oral supplement (without n‐3 PUFAs) | BW | Food records |
| Jatoi 39 | 2004 | Double‐blind RCT | 421 | 8 | Other, lung, GI | With/without chemotherapy | WL > 5 lb in 2 months or intake <20 kcal/kg BW/day | EPA | MA | BW | NCCTG (appetite questions), FAACT (appetite question) |
| Lundholm 40 | 2004 | Open‐label RCT | 309 | 5 | Other, colorectal, gastric | No antitumour treatment, palliative treatment | WL 3–5% over 3 months, ongoing WL due to disease or no tumour therapy available | COX inhibitor, EPO, dietary counselling and total PN (TPN) | COX inhibitor, EPO | BW, energy and protein intake, body composition, exercise capacity | Food records |
| Bauer 41 | 2005 | Double‐blind RCT | 200 | 8 | Pancreatic | Surgery, chemotherapy or radiotherapy | WL > 5% in the previous 6 months | ONS with EPA | Isocaloric ONS, 2 cans a day | Body composition, energy and protein intake, QoL | Food records |
| Dias 42 | 2005 |
Non‐RCT Grouped according to nutritional needs |
64 | 1 | H&N | Radiotherapy | Not described | Dietary counselling for a pureed food |
Group 1: EN Group 2: Oral diet enriched with alimentary supplement |
Energy and protein intake | 24‐h recall |
| Ravasco 43 | 2005 | RCT | 75 | 7 | H&N | Preoperative radiochemotherapy | NA |
(1) Dietary counselling (2) ONS |
(3) Intake ad lib | Energy and protein intake | EORTC QLQ C‐30 (appetite item), dietary history, 24‐h recall |
| Fearon 44 | 2006 | Double‐blind RCT | 518 | 8 | GI, lung | >4 weeks after chemotherapy/radiotherapy or surgery | ≥5% of pre‐illness stable weight |
(1) EPA 2 g (2) EPA 4 g |
(3) Placebo | BW | EORTC QLQ C‐30 (appetite item) |
| Strasser 45 | 2006 | Double‐blind RCT | 243 | 8 | GI, urogenital, other | 50% received chemotherapy | WL > 5% in 6 months | (1) THC + CBD |
(2) THC (3) Placebo |
Appetite | Appetite VAS |
| Jatoi 46 | 2007 | Double‐blind RCT | 63 | 7 | Lung, GI, other | No, chemotherapy and/or radiotherapy | WL > 5 lb in 2 months or intake <20 kcal/kg BW/day | Etanercept 25 mg twice weekly | Placebo | BW | NCCTG (appetite questions) |
| Beijer 47 | 2009 | Open‐label RCT | 100 | 8 | Lung, other, colon | No curative treatment, details not available | Incurable cancer, life expectancy 1–6 months, WHO PS ≤ 2, one of the following: Fatigue, WL > 5% in the last 6 months, anorexia | Weekly ATP infusion, 8–10 h, dose up to 50 μg/kg/min | Standard nutritional advice | BW, energy and protein intake, body composition, survival | Food records |
| Jatoi 48 | 2009 | Double‐blind RCT | 61 | 7 | NSCLC | >3 weeks after chemotherapy/radiotherapy or surgery | NA | Infliximab | Placebo | BW | NCCTG (appetite questions) |
| Mantovani 49 | 2010 | RCT | 332 | 7 | Lung, breast, colorectal | Concomitant palliative chemotherapy in ~80% | WL > 5% of ideal or pre‐illness in the previous 3 months |
(1) MPA or MA (2) EPA‐enriched ONS (3) l‐carnitine (4) Thalidomide |
(5) Combination: MPA or MA + EPA‐enriched ONS + l‐carnitine + thalidomide | LBM, REE, fatigue | Appetite VAS |
| Navari 50 | 2010 | Open‐label RCT | 80 | 7 | Lung, GI | >4 weeks after chemotherapy/radiotherapy or surgery | WL ≥ 5% and appetite loss (not defined) | MA 800 mg/day + olanzapine 5 mg/day | MA 800 mg/day | BW and appetite | Appetite VAS (MDASI) |
| Baldwin 51 | 2011 | Open‐label RCT | 358 | 8 | Colorectal, other, lung | Palliative chemotherapy | Any WL in the last 3 months |
(1) Dietary counselling (increase intake 600 kcal/day) (2) Supplement (3) Dietary counselling + supplement |
(4) SOC | OS | Appetite EORTC QLQ C‐30 (appetite item) |
| Silander 52 | 2011 | RCT | 134 | 6 | H&N | Radiochemotherapy or surgery and adjuvant radiotherapy in curative intent | WL > 10% during the last 6 months | EN | SOC | Not defined | EORTC QLQ C‐30 (appetite item) |
| Wen 53 | 2012 | RCT | 102 | 5 | Other, lung, gastric/breast | Treatment with palliative intent | WL > 5% of pre‐illness or ideal BW | MA + thalidomide | MA | BW, fatigue, EORTC QLQ and safety | Appetite NRS |
| Maccio 54 | 2012 | RCT | 144 | 8 | Ovarian, endometrial, cervical | Palliative chemotherapy | WL ≥ 5% in 3 months | Lipoic acid + carbocysteine + l‐carnitine + celecoxib + MA | MA | LBM | Appetite VAS |
| Madeddu 55 | 2012 | RCT | 60 | 7 | Other, H&N, lung | Concomitant palliative chemotherapy in ~80% | WL ≥ 5% of ideal or pre‐illness BW in the previous 6 months (associated with proinflammatory cytokines and/or CRP) | l‐carnitine + celecoxib + MA | l‐carnitine + celecoxib | LBM and total daily physical activity | Appetite VAS |
| Del Fabbro 56 | 2013 | Double‐blind RCT | 73 | 10 | GI, lung | NA | Appetite loss ≥4 (ESAS) and WL ≥ 5% over 6 months | Melatonin | Placebo | Appetite | Appetite NRS (ESAS) |
| Dobrila‐Dintinjana 57 | 2013 | Non‐RCT with historical controls | 628 | 7 | Colorectal | Chemotherapy | NA | Dietary counselling + MA and EPA‐enriched ONS | SOC | Nutritional status (not defined), survival | Appetite NRS |
| Kanat 58 | 2013 | RCT | 62 | 8 | Lung, other, pancreatic | No, chemotherapy/hormone therapy and/or radiotherapy in palliative or curative intent | WL > 5% over 3 months | MA + meloxicam |
(1) MA + meloxicam + EPA‐enriched supplement (2) EPA + meloxicam |
BW and LBM | Appetite VAS |
| Bourdel‐Marchasson 59 | 2014 | RCT | 341 | 10 | Other, colon, pancreatic | First‐line chemotherapy (>80%) | Mini Nutritional Assessment score <17 | Dietary counselling | SOC | Mortality | Food records |
| Pottel 60 | 2014 | RCT | 91 | 8 | H&N | Radiotherapy | WL > 5% or >2% and BMI < 20 kg/m2 | Echium oil 7.5 mL b.i.d. during treatment (omega‐3) | Placebo (oil same amount without omega‐3) | BW | EORTC QLQ C‐30 (appetite item) |
| Poulsen 61 | 2014 | Open‐label RCT | 61 | 5 | Gastric, oesophageal, gynaecological | No, chemotherapy and/or radiotherapy in palliative or curative intent | NA | Dietary counselling + protein supplement with EPA | SOC | Not defined | EORTC QLQ C‐30 (appetite item) |
| Focan 62 | 2015 | RCT | 53 | 7 | Breast, other, GI | NR | WL 2% 1 month or 5% 6 months, BMI < 20, low Alb/pre‐Alb, elevated CRP | Dietetic and psychological mindfulness workshops | SOC | Not defined | EORTC QLQ C‐30 (appetite item) |
| Gavazzi 63 | 2016 | Open‐label RCT | 79 | 7 | GI (56% gastric) | Planned surgery, additional chemotherapy in 30 patients | NRS2002 ≥ 3 | EN (night). Usual food (daytime) | Dietary advice and ONS. EN if WL ≥ 5% after 2 months | BW | Dietary history |
| Jatoi 64 | 2016 | RCT | 141 | 8 | Lung, GI, other | No, chemotherapy and/or radiotherapy | WL > 5 lb in 2 months or intake <20 kcal/kg BW/day | White wine twice a day | Nutritional supplement | Appetite | NCCTG (appetite questions) |
| Takayama 65 | 2016 | Double‐blind RCT | 181 | 8 | NSCLC | (Not high‐emetic risk) palliative chemotherapy | WL ≥ 5% in 6 months; two of the following: Anorexia, fatigue, malaise, decreased muscle strength, arm muscle circumference <10th percentile. One of the following: CRP > 5.0 mg/L, Hb < 12 g/dL or Alb < 3.2 g/dL |
(1) Anamorelin 50 mg (2) Anamorelin 100 mg |
(3) Placebo | LBM and grip strength | Appetite QoL‐ACD |
| Woo 66 | 2016 | RCT | 67 | 9 | Pancreatic | Chemotherapy, radiotherapy or no treatment. | WL 5–10% in the last 3 months | Pancreatic exocrine replacement therapy | Placebo | BW | EORTC QLQ C‐30 (appetite item), food records |
| Jatoi 67 | 2017 | Double‐blind RCT | 263 | 8 | Other, lung, GI | With/without chemotherapy | WL > 5 lb in 2 months or intake <20 kcal/kg BW/day | Creatine | Placebo | BW | NCCTG (appetite questions) |
| Kapoor 68 | 2017 | Open‐label RCT | 63 | 8 | Female genitourinary, other, breast | Not described |
Advanced cancer, WL > 5% pretreatment weight, BMI < 20, Hb < 12 g/dL, energy intake <1500 kcal/day Dietary advice |
Dietary counselling + Improved Atta (IAtta) (flour mix) | Dietary advice | BW, body composition and QoL | EORTC QLQ C‐30 (appetite item), 24‐h recall |
| Leedo 69 | 2017 | Open‐label RCT | 40 | 8 | Lung | Chemotherapy and/or radiotherapy or no treatment | NRS2002 ≥ 3, life expectancy >12 weeks | Dietary counselling | SOC | QoL | Dietary history |
| Werner 70 | 2017 | Double‐blind RCT | 60 | 7 | Pancreatic | In both groups, 12 received chemotherapy and one was treated with radiation | WL > 5% since diagnosis | Capsules: Marine omega‐3 fatty acids as phospholipids; 6.9% EPA and 13.6% DHA | Capsules: Marine omega‐3 fatty acids as triglycerides; 6.9% EPA and 13.6% DHA | BW and appetite | EORTC QLQ C‐30 (appetite item) |
| Zietarska 71 | 2017 | RCT | 95 | 6 | Colorectal | Chemotherapy | Asymptomatic pre‐cachexia according to SCRINIO Working Group | ONS | SOC | Chemotherapy‐related toxicity | Appetite VAS |
| Katakami 72 | 2018 | Double‐blind RCT | 174 | 8 | NSCLC | No, chemotherapy/TKI therapy and/or radiotherapy |
WL ≥ 5% in 6 months, anorexia and two of the following: fatigue, muscle weakness, arm muscle circumference <10th percentile And one of the following: Alb < 3.2 g/dL, CRP > 5 mg/L, Hb < 12 g/dL |
Anamorelin | Placebo | LBM | Appetite QoL‐ACD |
| Kouchaki 73 | 2018 | Double‐blind RCT | 90 | 8 | GI (52% gastric) | No, prior surgery and/or concomitant chemotherapy | WL ≥ 5% past 6 months or premorbid stable weight or BMI < 20 kg/m2 | MA + celecoxib | MA + placebo | BW | Appetite VAS, EORTC QLQ C‐30 (appetite item) |
| Schink 74 | 2018 | Non‐randomized | 131 | 9 | Other, colon, lung | Systemic therapy, radiotherapy or combinations | NA | Exercise + dietary counselling | Nutritional advice only | Skeletal muscle mass | EORTC QLQ C‐30 (appetite item) |
| Turcott 75 | 2018 | Double‐blind RCT | 47 | 7 | NSCLC | Chemotherapy | FAACT ACS | Nabilone | Placebo | FAACT ACS | Appetite VAS, EORTC QLQ C‐30 (appetite item) |
| Uster 76 | 2018 | RCT | 58 | 9 | Lung, GI, other | Not described | NA | Dietary counselling + exercise | SOC | QoL | EORTC QLQ C‐30 (appetite item), food records |
| Britton 77 | 2019 | Cluster RCT | 307 | 8 | H&N | (Chemo)radiotherapy, primary or (neo)adjuvant | NA | Motivational interview and cognitive behavioural therapy | SOC | PG‐SGA | EORTC QLQ C‐30 (appetite item) |
| Cereda 78 | 2019 | RCT | 166 | 8 | Lung, pancreatic, other | First‐line chemotherapy (>90%) | NA | Dietary counselling with whey protein isolate | Nutritional counselling | Phase angle | 24‐h recall, food records |
| Laviano 79 | 2019 | Double‐blind RCT | 55 | 8 | NSCLC | Chemotherapy | WL 2.5–11% in the last 12 months | n‐3 PUFAs (2 g), 25‐hydroxy vitamin D3 and high‐quality whey protein | Isocaloric drink based on milk and sunflower oil | Safety and tolerability | Appetite NRS (Council on Nutrition and Appetite) |
| Obling 80 | 2019 | RCT | 47 | 7 | GI (58% pancreatic) | Palliative chemotherapy (>90%) | NRS 2002 ≥ 2 | Supplemental home PN | Dietary advice to reach 75% of estimated energy and protein needs | Fat‐free mass | 24‐h recall |
| Bouleuc 81 | 2020 | RCT | 148 | 7 | Other, GI, lung | Ongoing chemotherapy 45% | Median WL 8.2 kg in the previous 6 months, mean food intake 40% | PN | ONS | QLQ C15 PAL (overall QoL, physical function and fatigue) | EORTC QLQ C15 PAL (appetite item) |
| Dehghani 82 | 2020 | Single‐blind RCT | 43 | 7 | Gastric | All treated with chemotherapy | BMI < 18 kg/m2 and muscle wasting | Captopril | Placebo | QLQ C30, not specified item/scale | EORTC QLQ C‐30 (appetite item) |
| Famil‐Dardashti 83 | 2020 | Double‐blind RCT | 47 | 8 | GI, lung, other | NA | WL ≥ 5% within 2 months, ≥3 months of life expectancy | Herbal combination (fenugreek, fennel, chicory) + MA | Placebo + MA | BW | Appetite NRS (ESAS) |
| Hong 84 | 2020 | Quasi‐RCT | 204 | 9 | GI (27% colon) | Chemotherapy or concomitant radiotherapy | NA | Resistance exercise | Relaxation exercise | Physical function | EORTC QLQ C‐30 (appetite item) |
| Movahed 85 | 2020 | Open‐label RCT | 100 | 8 | Oesophageal | Chemoradiation | Newly diagnosed oesophageal cancer, candidate for radiotherapy or chemoradiation |
Individualized nutritional plan based on needs ONS when needs not met |
General dietary advice | PG‐SGA score | 24‐h recall |
| Qiu 86 | 2020 | RCT | 96 | 6 | Oesophageal | NA | NA | Whole‐course nutrition management | SOC | Not defined | EORTC QLQ C‐30 (appetite item) |
| Storck 87 | 2020 | RCT | 52 | 10 | Lung, other, pancreatic | Palliative breast and prostate cancer received chemotherapy | NA | Physical exercise (3 × week endurance + strength), whey protein supplement | SOC | Short Physical Performance Battery | EORTC QLQ C‐30 (appetite item), food records |
| Bargetzi 88 | 2021 | Open‐label RCT | 506 | 8 | Other, lung, haematological | Ongoing cancer treatment (>86%) | NRS 2002 ≥ 3 | Early individual nutritional support | SOC | Mortality | Food records |
| Currow 89 | 2021 | Double‐blind RCT | 190 | 6 | Lung, other, colorectal | NA | Appetite ≤4 NRS |
(1) MA (2) Dexamethasone |
(3) Placebo | Appetite NRS | Appetite NRS (MSAS) |
| Hunter 90 | 2021 | Double‐blind RCT | 120 | 7 | Other, pancreatic, breast | With and without chemotherapy | WL > 5% over 6 months or >2% over 6 months and BMI < 20 kg/m2 | Mirtazapine | Placebo | Appetite | Appetite NRS |
| Izumi 91 | 2021 | RCT | 81 | 6 | Renal, other | Systemic therapy, radiotherapy, surgery | NA | Testosterone | SOC | Appetite NRS (ESAS) | |
| Ko 92 | 2021 | RCT | 40 | 8 | NA | >1 month after chemotherapy | Appetite VAS ≥ 4 | Yukgunja‐Tang + dietary counselling | Nutritional counselling | FAACT ACS | Appetite VAS |
| Molassiotis 93 | 2021 |
Pilot RCT Two trials |
74 | 9 | GI, other, urological | Not described | Stage III or IV, ECOG ≤ 2, at risk of malnutrition (MST) | Family‐centred nutritional intervention with a dietitian | SOC | PG‐SGA (SF), energy and protein intake, FAACT and QoL | Food records |
| Kutz 94 | 2022 | RCT | 69 | 7 | H&N | (Chemo)radiotherapy, primary or adjuvant | NA | Dietary counselling | SOC | Phase angle | EORTC QLQ C‐30 (appetite item) |
Abbreviations: Alb, albumin; ATP, adenosine‐5′‐triphosphate; b.i.d., bis in die (twice a day); BMI, body mass index; BSC, best supportive care; BW, body weight; CBD, cannabidiol; COX, cyclooxygenase; CRP, C‐reactive protein; DHA, docosahexaenoic acid; ECOG, Eastern Cooperative Oncology Group; EN, enteral nutrition; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EPA, eicosapentaenoic acid; EPO, erythropoietin; ESAS, Edmonton Symptom Assessment System; FAACT ACS, Functional Assessment of Anorexia/Cachexia Therapy Anorexia/Cachexia Subscale; GI, gastrointestinal; H&N, head and neck; Hb, haemoglobin; HCP, healthcare personnel; LASA, Linear Analogue Self‐Assessment; LBM, lean body mass; MA, megestrol acetate; MDASI, MD Anderson Symptom Inventory; MPA, medroxyprogesterone acetate; MSAS, Memorial Symptom Assessment Scale; MST, Malnutrition Screening Tool; NA, not applicable; NCCTG, North Central Cancer Treatment Group; NRS 2002, Nutrition Risk Screening 2002; NRS, numerical rating scale; NSCLC, non‐small cell lung cancer; ONS, oral nutritional supplement; OS, overall survival; PG‐SGA (SF), Patient‐Generated Subjective Global Assessment (Short Form); PN, parenteral nutrition; PUFA, polyunsaturated fatty acid; QoL, quality of life; QoL‐ACD, Quality of Life Questionnaire for Cancer Patients Treated with Anticancer Drugs; RCT, randomized controlled trial; REE, resting energy expenditure; SOC, standard of care; SSA, subjective sense of appetite; t.i.d., ter in die (three times a day); THC, tetrahydrocannabinol; TKI, tyrosine kinase inhibitor; VAS, visual analogue scale; WHO PS, World Health Organization Performance Status; WL, weight loss.
Number randomized.
Trials had an intervention period lasting from 2 weeks to 2 years. Of the eligible trials, 65 (81%) measured appetite endpoints, and 26 (40%) showed statistically significant improvements. In 15 studies (23%), appetite was a primary or co‐primary endpoint, whereof 6 (40%) presented statistically significant results in favour of the intervention. Dietary intake endpoints were assessed in 21 (26%) trials, and 12 (57%) showed statistically significant improvements. Seven (33%) had dietary intake as the primary or co‐primary endpoint(s), whereof 4 (57%) were statistically significant in favour of the intervention. Body weight was the most common co‐primary outcome alongside appetite or dietary intake. Sample size calculations were conducted in 12 (54%) of the 22 trials, which had appetite or dietary intake as a primary endpoint (11 [73%] on appetite and 1 [14%] on dietary intake). Six (8%) trials used both appetite and dietary intake endpoints. Five (8%) and 2 (10%) trials used two methods to assess appetite and dietary intake, respectively.
Appetite was primarily investigated in pharmacological interventions (41 studies), while dietary intake was mainly assessed in nutritional intervention trials (14 studies). Figures 2 and 3 illustrate the relationship between the type of study intervention utilized, sample size, and the presence of significant findings related to appetite or dietary intake endpoints.
Figure 2.
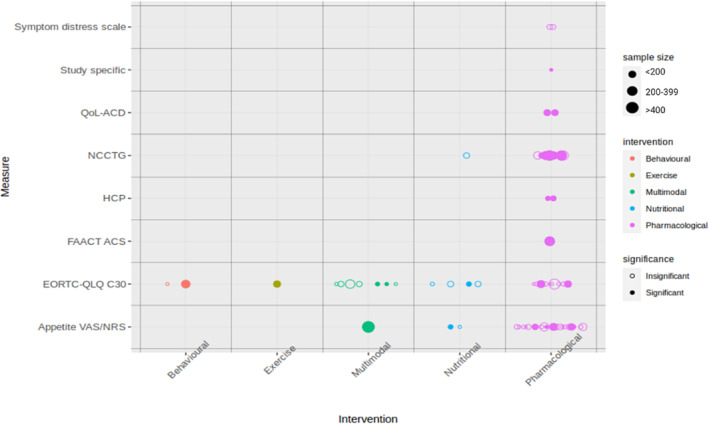
The relationship between study interventions, sample size, statistical significance and appetite measures across eligible trials. EORTC‐QLQ C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30/C15 PAL; FAACT A/CS, Functional Assessment of Anorexia/Cachexia Therapy Anorexia/Cachexia Subscale; HCP, healthcare personnel score; NCCTG, North Central Cancer Treatment Group; QoL‐ACD, Quality of Life Questionnaire for Cancer Patients Treated with Anticancer Drugs; VAS/NRS, visual analogue scale/numerical rating scale.
Figure 3.
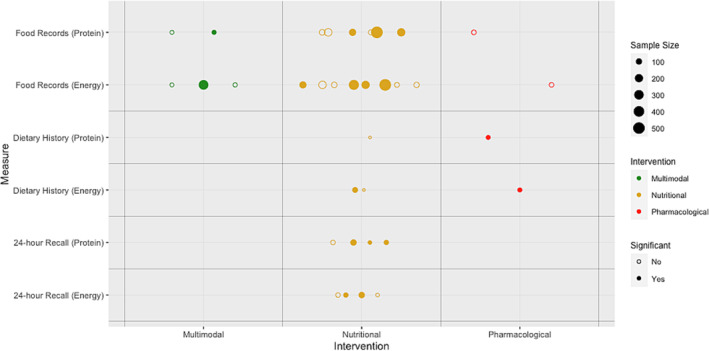
The relationship between study interventions, sample size, statistical significance and dietary intake measures across eligible trials.
Appetite endpoints
Table 2 details the frequency of appetite and dietary intake endpoints used in eligible trials. The most frequent endpoint for assessment of appetite was visual analogue scale (VAS) or numerical rating scale (NRS) (26/65 [40%]). The appetite questions from the EORTC QLQ C30/C15 PAL (25 studies [38%]), as well as the appetite question from North Central Cancer Treatment Group (NCCTG) anorexia questionnaire (11 studies [17%]), were often applied.
Table 2.
Frequency of use of appetite and dietary intake endpoints in eligible trials
| Endpoint | Description and time frame | N (trials) | Year |
Total sample Median (min–max) |
Type of intervention | Trials with significant results for appetite or dietary intake endpoints | References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| N (trials) | Total sample (min–max) | Type of intervention | Sign | Ns | ||||||
| Appetite endpoints | ||||||||||
| VAS/NRS |
VAS (0–100 mm) and NRS (0–10) Appetite now–last week–lately–these days |
26 | 1992–2021 |
3510 77 (40–628) |
Pharmacological: 22 Nutritional: 3 Multimodal: 1 |
8 |
1468 (42–628) |
Pharmacological: 6 Nutritional: 1 Multimodal: 1 |
17 , 18 , 25 , 26 , 28 , 57 , 71 , 83 | 27 , 31 , 37 , 45 , 49 , 50 , 53 , 54 , 55 , 56 , 58 , 73 , 75 , 79 , 89 , 90 , 91 , 92 |
| EORTC QLQ C30/C15 PAL (appetite item) |
During the past week: ‘Have you lacked appetite?’ 0–4 Likert scale ‘not at all’ to ‘very much’ |
25 | 1996–2022 |
3396 93 (43–518) |
Pharmacological: 7 Exercise: 1 Nutritional: 8 Multimodal: 7 Behavioural: 2 |
8 |
1267 (61–307) |
Pharmacological: 2 Exercise: 1 Nutritional: 2 Multimodal: 2 Behavioural: 1 |
25 , 32 , 43 , 61 , 68 , 77 , 84 , 86 | 31 , 35 , 44 , 51 , 52 , 60 , 62 , 66 , 70 , 73 , 74 , 75 , 76 , 81 , 82 , 87 , 94 |
| NCCTG (appetite questions) |
Appetite now compared to before illness (‘increased’ to ‘markedly reduced’) Appetite now compared to before study medication (‘reduced’ to ‘increased very much’) How would you rate/describe your appetite? ‘very good’ to ‘very poor’ |
11 | 1990–2017 |
2752 263 (61–496) |
Pharmacological: 9 Nutritional: 2 |
5 |
1733 (133–496) |
Pharmacological: 5 | 15 , 16 , 19 , 30 , 34 | 22 , 39 , 46 , 48 , 64 , 67 |
| QoL‐ACD (Item 8) |
In the past few days: ‘Did you have good appetite?’ 1–5 (‘not at all’ to ‘very much’) |
2 | 2016–2018 |
354 178 (174–181) |
Pharmacological: 2 | 2 |
354 (174–181) |
Pharmacological: 2 | 65 , 72 | ‐ |
| Symptom Distress Scale (Item 3) | Appetite lately 1–5 Likert statements from ‘I have my normal appetite and enjoy good food’ to ‘I cannot stand the thought of food’ | 2 | 1996–2002 |
241 (119–122) |
Pharmacological: 2 | ‐ | ‐ | ‐ | ‐ | 24 , 36 |
| Scored by HCP | Scored 1–5 based on interview | 2 | 1996–2000 |
229 (100–129) |
Pharmacological: 2 | 2 |
229 (100–129) |
Pharmacological: 2 | 23 , 33 | ‐ |
| FAACT (Item C6) | In the past 7 days: ‘I have good appetite’ 0–4 (‘not at all’ to ‘very much’) | 1 | 2004 | 421 | Nutritional: 1 | 1 | 421 | Nutritional: 1 | 39 | ‐ |
| Study specific | Appetite 1–5 ‘none’, ‘poor’, ‘fair’, ‘good’, ‘excellent’ | 1 | 1994 | 52 | Pharmacological: 1 | 1 | 52 | Pharmacological: 1 | 21 | ‐ |
| Dietary intake endpoints | ||||||||||
| Food records | Prospective recording (usually 3–4 days) of all foods, beverages and dietary supplements consumed within a designated period | 13 | 1993–2021 |
2269 183 (52–506) |
Pharmacological: 2 Nutritional: 8 Multimodal: 3 |
Energy: 5 |
1493 (105–506) |
Nutritional: 4 Multimodal: 1 |
Energy: 20 , 40 , 41 , 59 , 88 | 37 , 38 , 47 , 66 , 78 , 87 , 93 |
| Protein: 4 |
901 (58–506) |
Nutritional: 3 Multimodal: 1 |
Protein: 20 , 41 , 76 , 88 | |||||||
| 24‐h recall | Retrospective assessment of intake in the previous 24 h | 6 | 2005–2020 |
515 64 (47–166) |
Nutritional: 6 | Energy: 3 |
238 (63–100) |
Nutritional: 3 | Energy: 43 , 68 , 85 | 42 , 78 |
| Protein: 4 |
285 (47–100) |
Nutritional: 4 | Protein: 43 , 68 , 80 , 85 | |||||||
| Dietary history | Retrospective interview about usual intake of foods and drinks over a specified period | 4 | 1998–2017 |
248 65 (40–79) |
Nutritional: 3 Pharmacological: 1 |
Energy: 2 | 133 (54–79) |
Pharmacological: 1 Nutritional: 1 |
Energy: 29 , 63 | 43 , 69 |
| Protein: 1 | 54 | Pharmacological: 1 | Protein: 29 | |||||||
Abbreviations: EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; FAACT, Functional Assessment of Anorexia/Cachexia Therapy; HCP, healthcare personnel; NCCTG, North Central Cancer Treatment Group; NRS, numerical rating scale; Ns, non‐significant; QoL‐ACD, Quality of Life Questionnaire for Cancer Patients Treated with Anticancer Drugs; Sign, statistically significant; VAS, visual analogue scale.
The most frequent intervention was progestin treatment with either megestrol acetate (MA) or medroxyprogesterone acetate (MPA) (10 studies with a placebo control arm). These studies measured appetite with four different assessment tools, and comparison of effect sizes to assess whether one of these tools was more sensitive to changes was not possible.
Visual analogue scale and numerical rating scale
VAS and NRS were categorized together in this review as it was challenging to interpret which of the two had been applied. Twenty‐two of the 26 studies were pharmacological, as were six of the eight studies presenting statistically significant results. VAS/NRS was the primary endpoint in 9 of the 26 studies, and 3 of these studies reported statistically significant results (Table 2 ). Twenty of the 26 studies also examined body weight. In 14 of these trials, results for body weight and appetite were aligned in that both were either statistically significantly different in favour of the intervention or statistically non‐significant. In the remaining six studies, there were statistically significant differences in only one of the endpoints (appetite [three studies] or weight [three studies]). Only 1 of the 26 studies reported both dietary intake and appetite, and results were statistically non‐significant for both endpoints.
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30/C15 PAL
The use of EORTC QLQ C30/C15 PAL was more equally distributed between trials exploring a wide variety of different interventions (Figure 2 ). Eight of the 25 studies assessing appetite using EORTC QLQ C30/C15 PAL showed statistically significant differences in appetite between the study arms in favour of the intervention. In two studies, appetite was defined as the primary outcome, and one of these studies reported a statistically significant improvement in appetite.
Sixteen of the 25 studies measuring appetite with the EORTC QLQ C30/C15 PAL also reported results for weight; 11 studies reported either changes between arms in both endpoints and in the same direction or no change in both endpoints. Five studies described statistically significant changes between the arms, in either appetite or weight, but not for both endpoints. Five of the 25 studies assessed both dietary intake and appetite. In one study, there were significant changes between the arms for both endpoints, and in three studies, there were non‐significant differences. In one study, appetite was non‐significant, while dietary intake was significant.
North Central Cancer Treatment Group anorexia questionnaire
NCCTG anorexia questionnaire was used to assess appetite in large, predominantly pharmacological studies. Some of the studies used slightly modified versions of the questionnaire. Five of the 11 studies presented statistically significant differences between the treatment arms. Three studies used NCCTG anorexia questionnaire as primary outcome measure for appetite, whereof two studies presented statistically significant results.
Dietary intake endpoints
As presented in Table 2 , food records were the method most often used to assess dietary intake (13 out of 21 trials [62%]). Six trials (29%) used 24‐h recall, and four trials (19%) used dietary history method. One trial combined food record with 24‐h recall, and one trial combined 24‐h recall with dietary history.
Fifteen of the 21 trials (71%) investigated effects of various nutritional interventions. Three pharmacological intervention trials and three multimodal intervention trials assessed dietary intake to determine whether improved appetite or other symptoms affected dietary intake.
Food records
Of the 13 trials using food records, eight trials were nutritional interventions assessing energy and/or protein intake mainly to evaluate compliance with food‐based advice (Table 2 ). Four (50%) of these eight trials reported a statistically significantly higher energy intake in the intervention group than in the control group, while three trials reported statistically significantly higher protein intake. In two trials the comparators were standard of care, in one trial isocaloric oral nutritional supplements (ONS) without n‐3 polyunsaturated fatty acids (PUFAs) and in the last one the comparator was a daily multivitamin‐mineral tablet. Of the remaining five trials using food records (three multimodal and two pharmacological), one multimodal trial (parenteral nutrition [PN], cyclooxygenase [COX] and erythropoietin [EPO]) showed statistically significantly higher energy intake in the intervention group. In the four trials showing no statistically significant difference in energy and protein intake between arms, two used isocaloric comparators (ONS with or without n‐3 PUFAs and capsules with or without n‐3 PUFAs). The other two trials presented no statistically significant effect of either family‐centred psychosocial nutritional intervention or multimodal intervention (exercise and whey protein supplement) compared to standard of care.
Twelve of the 13 trials assessed dietary intake and body weight. Seven trials consistently showed a pattern where dietary intake corresponded to changes in body weight, and in two of these trials, both energy and protein intake increased significantly and corresponded to body weight gain, favouring the treatment arm. In the remaining five trials, no significant changes were observed in either dietary intake or body weight between the study arms. However, in the other five trials, a diversity pattern was observed. In one trial, a significant increase in energy intake coincided with a decline in body weight, while in two other trials, it corresponded to no change in body weight. Moreover, significantly increased protein intake did not correspond to changes in body weight. Finally, one study that used both food records and 24‐h dietary recall reported no increase in energy or protein intake, while body weight increased. Four trials assessed both dietary intake and appetite. In one trial, there was a statistically significant improvement in protein intake but no change in appetite. The three remaining studies showed no changes in either endpoint.
24‐h dietary recall
Six nutritional trials used 24‐h recall to assess intake. Three trials that compared dietary counselling with or without ONS showed higher energy and protein intake in favour of the intervention. The comparators in these trials were standard of care or dietary advice. Further, in one trial, a higher protein intake was shown by supplemental PN compared to dietary advice. In the remaining two trials, no differences in energy or protein intake were found, and comparators were dietary counselling, enriched oral diet or enteral nutrition (EN).
Five trials using 24‐h recall assessed dietary intake and body weight. In two trials, both energy and protein and body weight increased significantly. Among the remaining three trials, one reported a significant increase in energy and protein intake but did not observe a significant change in body weight. Further, one trial showed no change in dietary intake or body weight. One trial using both 24‐h recall and food records showed no significant increase in energy or protein intake, although body weight increased. Two trials assessed both dietary intake and appetite, and a significant increase in energy and protein intake, as well as in appetite, was observed in both. Additionally, this trial also reported a significant increase in body weight.
Dietary history
Four trials used retrospective interviews to assess the usual consumption of foods and drinks over a specified period. In one pharmacological trial, significantly higher energy and protein intake in favour of the intervention were reported, and in one EN trial, significantly higher energy intake was reported. Another trial reported significantly higher energy and protein when using 24‐h recall, but not the dietary history method.
In three trials, body weight was also reported. In the trial using EN, the significant increase in energy intake corresponded to a significant increase in body weight. Additionally, one trial reported no increase in energy or protein intake or body weight. The trial using both dietary history and 24‐h recall showed no statistically significant increase in energy or protein intake, although body weight increased. None of the trials assessing dietary history also assessed appetite.
Effect size/sample size estimation
Available raw scores (baseline, post‐treatment and changes) for all studies are shown in Tables S1 – S4 . Eight studies on appetite (4 VAS/NRS, 3 EORTC and 1 QoL‐ACD) reported sufficient data (mean change and SD) to calculate effect size (Hedge's g) (Tables S1 and S2 ). Assuming that Hedge's g of 0.2, 0.5 and 0.8 corresponds to a small, medium or large effect size, one can use the pooled standard deviations for the control groups for VAS/NRS and EORTC to estimate the corresponding changes on these two scales (estimated needed sample size per arm for 80% power and alpha of 0.05 in parentheses): For VAS/NRS (0–10), a mean change in score of 0.5 (n = 362), 1.3 (n = 58) and 2.2 (n = 23) corresponds to a small, medium or large effect size, respectively. For EORTC (0–100), a mean change in score of 7 (n = 331), 18 (n = 53) and 29 (n = 21) corresponds to a small, medium or large effect size, respectively.
Five studies assessing energy intake (four with food records and one with 24‐h recall) and four studies assessing protein intake (three with food records and one with 24‐h recall) reported sufficient data to calculate effect sizes (Tables S3 and S4 ). Based on three studies using the same unit of kcal/day assessed with food records, a mean change of 93 kcal/day (n = 427), 231 kcal/day (n = 68) and 370 kcal/day (n = 27) represents a small, medium and large effect size, respectively. Based on the same three studies reporting protein intake in g/day, a mean change of 5 g/day (n = 370), 11 g/day (n = 59) and 18 g/day (n = 23) represents a small, medium and large effect size, respectively.
Discussion
This review identified a multitude of measures that have been used to assess nutritional aspects in cancer cachexia clinical trials. VAS/NRS and the EORTC QLQ C30/C15 PAL were the most commonly used endpoints for assessment of appetite, but measures of appetite were rarely the primary endpoint (23%). About 40% of studies reported a statistically significant improvement in appetite in favour of the intervention. In 70% of the studies, changes in appetite and weight coincided. Dietary intake tended to be used more for monitoring compliance with dietary interventions than as a primary endpoint per se. Prospective food records were the most frequently used method to assess dietary intake of energy and/or protein. Statistically significantly higher energy and/or protein intakes in favour of the intervention group were reported in ~60% of trials, and increased dietary intake coincided with weight gain or stabilization in approximately half of these. Very few studies reported both dietary intake and appetite.
Appetite is generally defined as a person's desire to eat and is a subjective feeling that is best assessed with a PROM, 95 which was the case in nearly all trials included in this review. Two main groups of PROMS were identified: VAS/NRS and categorical response scales (CRSs), such as EORTC QLQ C30/C15 PAL and NCCTG anorexia questionnaire. VAS/NRS asks the respondent to grade their appetite loss somewhere between two extremities (no appetite loss vs. worst appetite loss), while CRS asks the respondent to pick the most fitting response out of a set of (usually) four or five available responses that are ordered in terms of intensity of appetite loss. For the purpose of this review, VAS and NRS results were grouped together. Many prior studies have shown a high degree of correlation between VAS and NRS, 96 and it is not clear which is optimal. When assessing appetite, NRS is a well‐known, user friendly and validated assessment, and both its prognostic abilities and minimally clinically important difference have been evaluated. 96 A relatively small study from a palliative care unit reported that VAS was the least preferred appetite scale by patients, while CRS was favoured, slightly more than NRS. 97 NRS or EORTC could thus preferably be recommended when assessing appetite in clinical trials.
In a bid to assess the multifactorial aspects of appetite loss, some studies used multidimensional assessment tools such as the FAACT questionnaire. However, studies often only reported the summary score, which is influenced by several factors, making it difficult to assess the effect on appetite per se and therefore were not included in the present review Of note, the FAACT questionnaire has been refined to a five‐item anorexia symptoms subscale (5‐IASS), and this is being assessed in ongoing clinical trials of anamorelin, mirtazapine and MA. 12 The EORTC QLQ CAX24, assessing aspects of appetite and QoL in patients with cachexia, has been validated, and a report is awaited (https://qol.eortc.org/questionnaire/qlq‐cax24/); no studies in this review used this tool. Whether 5‐IASS or EORTC QLQ CAX24 will better capture treatment effect is so far unclear. By all accounts, scoring of patients' symptoms by healthcare professionals, like in two studies in this review, should be discouraged.
There is no agreement on how to best measure food intake in patients with cancer, 98 and currently, assessments of dietary intake have not been validated in the cachexia population. Extrapolation from validations in healthy populations is not adequate because dietary intake can vary during the disease and the anti‐cancer treatment trajectory, and there is no agreement when assessment should be performed. Further, due to the catabolic phenotype and muscle wasting in cachexia, sensitive and specific methods validated for estimation of energy and protein intake are needed for trials in this specific patient population.
Weight remains a clinical and relevant measure of energy balance; however, the required energy and protein needed in cancer cachexia to maintain or increase body weight remain unknown. In the current review, only half of the trials showed that intake and body weight changed in the same direction, and when two different methods were applied in the same trial, inconsistencies in results were reported. This highlights a variability in the agreement between dietary intake methods and body weight. Another important aspect of dietary intake methods is that we lack prognostic and predictive value of reduced intake, including relevant prognostic thresholds. This limits dietary intake from serving as a clinically meaningful primary endpoint in cancer cachexia trials, as a statistically significant increase in energy and protein alone may not reflect clinically relevant changes due to the underlying catabolic pathophysiology. Nevertheless, dietary intake assessment is important to monitor and guide nutritional intervention as well as compliance. The superiority of any dietary intake method cannot be concluded based on this review. Additionally, simplified assessments that did not provide detailed reports on calorie and protein intake were not included in this review, as they cannot quantify individual calorie or protein intake in kcal/day or g/day. More simplified methods could be considered when appetite and/or body weight are the main endpoints, but also, these need to be validated with concern to energy and protein intake in the cancer cachexia population.
One of the challenges in assessing appetite and dietary intake in cachexia is the complex interplay between these and confounding factors. Nutritional impact symptoms such as altered sense of smell or taste, early satiety and nausea/vomiting may undermine the desire to eat, yet these were rarely assessed in the reviewed trials. Furthermore, energy expenditure and/or unmitigated catabolism relative to dietary intake of energy/protein determines whether weight can be gained. All these factors are important and need to be collectively understood. This seemingly motivates the inclusion of multiple endpoints in studies of cachexia, which was the case for many of the studies in this review. However, well‐defined primary endpoints are essential, and inclusion of secondary endpoints should be balanced against the risk of spurious findings and preferably be restricted to endpoints of relevance to understand changes in the primary endpoint.
The primary endpoint for about half of the studies in this review was body weight or composition. This is not surprising as weight loss and body composition are key factors defining cachexia. 1 Appetite or dietary intake was only used as primary endpoint in ~25% of trials, including studies using appetite and dietary intake as co‐primary outcomes with weight/body composition. However, although the 2011 international definition on cancer cachexia emphasized that nutritional intervention alone cannot fully reverse cachexia, 99 stakeholders recognized the importance of dietary intake and appetite improvement as part of any cachexia management. 100 Appetite and dietary intake could thus be considered important co‐primary or secondary endpoints in the context of cachexia treatment.
Contrary to body weight and composition, appetite and dietary intake are relevant endpoints throughout the entire cancer trajectory. Interventions against cachexia late in the disease trajectory will have low probability of improving muscle mass or weight due to minimal anabolic potential in patients with a short remaining life expectancy. 0099 In contrast, improving appetite and increasing dietary intake is shown to improve QoL and reduce emotional distress among patients and relatives alike, through the whole disease trajectory. 100 Consequently, appetite and dietary intake are key in each phase of the disease, and intervention trials aiming to improve either of the two should measure appetite and/or dietary intake. Albeit, at the very end of life, preservation of appetite probably becomes more important than amount of energy and protein intake, as the participation in meals and enjoyment of food still are of great value.
When selecting endpoints for a study, the intervention's mechanism of action and the comparators should be considered. For instance, if the effect of an appetite stimulant is evaluated, body weight should not be the only endpoint, as poor appetite alone rarely is the only cause of weight loss in patients with cancer. 100 In such a case, it would be natural to choose appetite as the primary endpoint and weight or dietary intake as a secondary or exploratory endpoint. Dietary intake can serve multiple purposes depending on the specific goals of the trial and the research questions being addressed. Because of lack of validation in cachexia populations, dietary intake has limited value as primary efficacy endpoint. However, in exploratory studies, measurements of energy intake can be used to better understand energy balance, helping researchers addressing cancer cachexia pathophysiology beyond trial endpoints. Dietary intake also could have value as a measure of patient compliance.
The relationship between dietary intake and appetite has been sparsely investigated in patients with cancer, and in the present review, only 8% of the trials measured both, and there may be several reasons for this. Dietary intake is often more time and resource demanding to assess than appetite, and it is sometimes assumed that appetite and dietary intake are two sides to the same story. However, it is important to recognize that although appetite and intake are correlated, some manage to keep a stable dietary intake despite loss of appetite, 101 and thus, endpoints of appetite and dietary intake cannot be used interchangeably or as substitutes for one another. This is also seen in the present review where in studies where weight and dietary intake were assessed together, direction of change in both endpoints coincided in only half of the studies, indicating no real association between the endpoints used to evaluate these two outcomes. On the other hand, concordance between appetite and weight loss was more apparent as changes in weight and appetite coincided in 70% of studies. This could indicate that instruments used to measure appetite have reasonable construct validity; however, above all, this underscores the complexity of cachexia, indicating that dietary intake is not dependent on appetite alone, and weight is not solely dependent on appetite or dietary intake. It is not possible, based on data available, to state whether any of the different measurements of appetite or dietary intake corresponds closer to change in body weight than the others.
Equally important to choosing and prioritizing endpoints is to ensure an adequately sized study sample. Only half of the trials with appetite or dietary intake as primary endpoint reported sample size calculations. This may have led to inconclusive or underpowered trials that fail to provide reliable conclusions. In this review, we provide guidance to what might be appropriate sample sizes in relation to what is perceived to be small, moderate or large effect sizes. According to this, a sample size of <100, akin to approximately half of trials (37 of 80) in this review, is likely too small to detect moderate effect sizes concerning appetite or dietary intake.
The correct sample size depends on what is a clinically meaningful difference in the chosen endpoint. Approximately 40% of the trials reported statistically significant differences between the arms on appetite or dietary intake endpoints. Some of the studies, especially those where appetite and dietary intake were primary endpoints, discussed whether these differences also constituted a clinically important difference. However, there is no clear universal agreement on what a clinically important difference should be. Using VAS/NRS as an example, both a 15% difference between arms and a 25% increase from baseline have been used, as has a numerical difference of 1 and 3 on the 0–10 NRSs. 89 Moderate to high effect sizes for energy intake as seen in this review are sufficient for weight gain in healthy persons, 102 but did not lead to increased body weight in the retrieved studies. Consequently, clinically meaningful changes in the cachexia population seem to be different from the healthy population, and are, as of yet, unknown.
Strengths and limitations
The strengths of this review are its rigorous methodology and also the multinational and multiprofessional collaboration of experts behind it. This has ensured a wider range of inputs when evaluating the multidimensional condition that cancer cachexia is.
The heterogeneity in populations, interventions and usage of the different endpoints included in this review was substantial. Additionally, intervals from baseline to follow‐up assessments varied considerably between studies. Several studies did not describe whether they asked about appetite at worst or on average or which time frame the appetite question concerned (past month, last week or today).
The main inclusion criterion in most studies was unintentional weight loss, and only in relatively few of the analysed trials were loss of appetite and reduced dietary intake required. One could thus discuss if there is potential for improvement when there has been no deterioration in the endpoint prior to inclusion. However, the trials were still included as one could argue that an aim could be to prevent an expected deterioration in appetite and dietary intake in the intervention arm. Indeed, in populations where the prevalence and/or incidence of appetite loss and/or reduced intake is high, screening on these two parameters at study inclusion might not be necessary. However, in future studies aiming to treat appetite or improve dietary intake, this is something that needs to be considered closely.
Another limitation is that data in this review originate from clinical trials and not studies specifically designed to validate endpoints. Non‐significant statistical results do not imply that the trial's outcome measure was unsuitable but could mean that the study was underpowered, the design was wrong or the intervention had no impact on appetite or dietary intake. Also, the variations between interventions, their different targets and assessment methods made it impossible to determine whether any of the endpoints captured changes in appetite to a greater extent than others, also when exclusively comparing studies with the most frequently studied intervention MA/MPA. Nevertheless, when collating all studies published and exploring the endpoints in depth, the totality of studies can give a foundation to improve future study design.
Conclusions
A variety of endpoints have been used to assess appetite and dietary intake in cancer cachexia clinical trials. The optimal nutritional endpoint cannot be determined based on the present review. VAS/NRS and the appetite item from EORTC QLQ C30/C15 PAL were the endpoints most frequently used to assess appetite, while food records were most used to assess dietary intake. From the trials assessed and available literature, NRS and EORTC QLQ C30/C15 PAL can be recommended as measures to assess appetite, either as a primary endpoint when the intervention primarily aims to target appetite or as a secondary measure where other factors that may influence appetite are being assessed. A next step for the academic community might be a formal process to establish an international consensus on which appetite endpoint(s) to use.
Few studies used energy and/or protein intake as a primary endpoint, and when used, it was mainly used to monitor interventions and compliance. This review and previous literature strongly suggest that appetite endpoints should not be used as a surrogate to assess dietary intake. Furthermore, dietary intake methods have not been validated in the present population, and the observed variability in the relationship between intake and body weight underscores the need for studies validating dietary intake in patients suffering from cancer cachexia. At present, dietary intake endpoints cannot be recommended as main efficacy endpoints in clinical trials evaluating treatment of cancer cachexia.
Several studies had multiple endpoints and a relatively small sample size, increasing the risk of spurious findings potentially entailing futile treatment or confirmatory studies with low probability of significant findings. Thus, future studies investigating appetite and dietary intake in patients suffering from, or at risk of developing cancer cachexia, should precisely define primary endpoint and perform sample size calculations.
Conflict of interest statement
MF has received personal fees from Pfizer. MJH has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETS and NIHR. MJH has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board and Steering Committee; has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster and Bristol Myers Squibb; and is a co‐inventor on a European patent application relating to methods to detect lung cancer (PCT/US2017/028013). RJES has received personal fees for consultancy from Artelo, Actimed, Faraday and Helsinn. BJAL has received personal fees for consultancy from Artelo, Actimed, Faraday, Kyowa Kirin and Toray. The remaining authors have not reported any conflicts of interest.
Supporting information
Appendix S1. Documentation of literature search
Appendix S2. Modified Downs and Black Scoring Criteria
Table S1. Raw values of appetite scores pre‐ and posttreatment with delta, significance levels and effect sizes of two‐armed trials
Table S2. Raw values of appetite scores pre‐ and posttreatment with delta, significance levels and effect sizes of multi‐armed trials
Table S3. Raw values of dietary intake of energy and protein pre‐ and posttreatment with delta, significance levels and effect sizes of two‐armed trials
Table S4. Raw values of dietary intake of energy and protein pre‐ and posttreatment with delta, significance levels and effect sizes of multi‐armed trials
Vagnildhaug O. M., Balstad T. R., Ottestad I., Bye A., Greil C., Arends J., et al (2024) Appetite and dietary intake endpoints in cancer cachexia clinical trials: Systematic Review 2 of the cachexia endpoints series, Journal of Cachexia, Sarcopenia and Muscle, doi: 10.1002/jcsm.13434
Richard J. E. Skipworth, Barry J. A. Laird and Tora S. Solheim are joint senior authors.
References
- 1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Stratton RJ. Elucidating effective ways to identify and treat malnutrition. Proc Nutr Soc 2005;64:305–311. [DOI] [PubMed] [Google Scholar]
- 3. Argiles JM, Lopez‐Soriano FJ, Stemmler B, Busquets S. Cancer‐associated cachexia—understanding the tumour macroenvironment and microenvironment to improve management. Nat Rev Clin Oncol 2023;20:250–264. [DOI] [PubMed] [Google Scholar]
- 4. Barajas Galindo DE, Vidal‐Casariego A, Calleja‐Fernández A, Hernández‐Moreno A, Pintor de la Maza B, Pedraza‐Lorenzo M, et al. Appetite disorders in cancer patients: impact on nutritional status and quality of life. Appetite 2017;114:23–27. [DOI] [PubMed] [Google Scholar]
- 5. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 6. Yeom E, Yu K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp Mol Med 2022;54:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Omlin A, Blum D, Wierecky J, Haile SR, Ottery FD, Strasser F. Nutrition impact symptoms in advanced cancer patients: frequency and specific interventions, a case–control study. J Cachexia Sarcopenia Muscle 2013;4:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laird BJA, Balstad TR, Solheim TS. Endpoints in clinical trials in cancer cachexia: where to start? Curr Opin Support Palliat Care 2018;12:445–452. [DOI] [PubMed] [Google Scholar]
- 9. Delgado A, Guddati AK. Clinical endpoints in oncology—a primer. Am J Cancer Res 2021;11:1121–1131. [PMC free article] [PubMed] [Google Scholar]
- 10. FDA‐NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US); 2016. Co‐published by National Institutes of Health (US), Bethesda (MD). https://www.ncbi.nlm.nih.gov/books/NBK326791/pdf/Bookshelf_NBK326791.pdf. Accessed 19 Jan 2024. [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gelhorn HL, Gries KS, Speck RM, Duus EM, Bourne RK, Aggarwal D, et al. Comprehensive validation of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) Anorexia/Cachexia Subscale (A/CS) in lung cancer patients with involuntary weight loss. Qual Life Res 2019;28:1641–1653. [DOI] [PubMed] [Google Scholar]
- 13. Bauer J, Capra S, Ferguson M. Use of the scored Patient‐Generated Subjective Global Assessment (PG‐SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779–785. [DOI] [PubMed] [Google Scholar]
- 14. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kardinal CG, Loprinzi CL, Schaid DJ, Hass AC, Dose AM, Athmann LM, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer 1990;65:2657–2662. [DOI] [PubMed] [Google Scholar]
- 16. Loprinzi CL, Ellison NM, Schaid DJ, Krook JE, Athmann LM, Dose AM, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127–1132. [DOI] [PubMed] [Google Scholar]
- 17. Feliu J, González‐Barón M, Berrocal A, Artal A, Ordónez A, Garrido P, et al. Usefulness of megestrol acetate in cancer cachexia and anorexia: a placebo‐controlled study. Am J Clin Oncol 1992;15:436–440. [DOI] [PubMed] [Google Scholar]
- 18. Downer S, Joel S, Allbright A, Plant H, Stubbs L, Talbot D, et al. A double blind placebo controlled trial of medroxyprogesterone acetate (MPA) in cancer cachexia. Br J Cancer 1993;67:1102–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loprinzi CL, Michalak JC, Schaid DJ, Mailliard JA, Athmann LM, Goldberg RM, et al. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol 1993;11:762–767. [DOI] [PubMed] [Google Scholar]
- 20. Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol 1993;11:2043–2049. [DOI] [PubMed] [Google Scholar]
- 21. Lai Y‐L, Fang F‐M, Yeh C‐Y. Management of anorexic patients in radiotherapy: a prospective randomized comparison of megestrol and prednisolone. J Pain Symptom Manage 1994;9:265–268. [DOI] [PubMed] [Google Scholar]
- 22. Goldberg RM, Loprinzi CL, Mailliard JA, O'Fallon JR, Krook JE, Ghosh C, et al. Pentoxifylline for treatment of cancer anorexia and cachexia? A randomized, double‐blind, placebo‐controlled trial. J Clin Oncol 1995;13:2856–2859. [DOI] [PubMed] [Google Scholar]
- 23. Chen H‐C, Leunga SW, Wang C‐J, Suna L‐M, Fanga F‐M, Hsu J‐H. Effect of megestrol acetate and prepulsid on nutritional improvement in patients with head and neck cancers undergoing radiotherapy. Radiother Oncol 1997;43:75–79. [DOI] [PubMed] [Google Scholar]
- 24. Gebbia V, Testa A, Gebbia N. Prospective randomised trial of two dose levels of megestrol acetate in the management of anorexia‐cachexia syndrome in patients with metastatic cancer. Br J Cancer 1996;73:1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simons JPFHA, Aaronson NK, Vansteenkiste JF, ten Velde GP, Muller MJ, Drenth BM, et al. Effects of medroxyprogesterone acetate on appetite, weight, and quality of life in advanced‐stage non‐hormone‐sensitive cancer: a placebo‐controlled multicenter study. J Clin Oncol 1996;14:1077–1084. [DOI] [PubMed] [Google Scholar]
- 26. Beller E, Tattersall M, Lumley T, Levi J, Dalley D, Olver I, et al. Improved quality of life with megestrol acetate in patients with endocrine‐insensitive advanced cancer: a randomised placebo‐controlled trial. Ann Oncol 1997;8:277–283. [DOI] [PubMed] [Google Scholar]
- 27. Catalina V, Seguí MA, Giménez‐Arnau JM, Morales S, Cirera L, Bestit I, et al. Anticachectic efficacy of megestrol acetate at different doses and versus placebo in patients with neoplastic cachexia. Am J Clin Oncol 1998;21:347–351. [DOI] [PubMed] [Google Scholar]
- 28. De Conno F, Martini C, Zecca E, Balzarini A, Venturino P, Groff L, et al. Megestrol acetate for anorexia in patients with far‐advanced cancer: a double‐blind controlled clinical trial. Eur J Cancer 1998;34:1705–1709. [DOI] [PubMed] [Google Scholar]
- 29. Simons JP, Schols AM, Hoefnagels JM, Westerterp KR, ten Velde GP, Wouters EF. Effects of medroxyprogesterone acetate on food intake, body composition, and resting energy expenditure in patients with advanced, nonhormone‐sensitive cancer: a randomized, placebo‐controlled trial. Cancer 1998;82:553–560. [PubMed] [Google Scholar]
- 30. Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol 1999;17:3299–3306. [DOI] [PubMed] [Google Scholar]
- 31. McMillan DC, Wigmore SJ, Fearon KC, O'Gorman P, Wright CE, McArdle CS. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer 1999;79:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Westman G, Bergman B, Albertsson M, Kadar L, Gustavsson G, Thaning L, et al. Megestrol acetate in advanced, progressive, hormone‐insensitive cancer. Effects on the quality of life: a placebo‐controlled, randomised, multicentre trial. Eur J Cancer 1999;35:586–595. [DOI] [PubMed] [Google Scholar]
- 33. Erkurt E, Erkisi M, Tunali C. Supportive treatment in weight‐losing cancer patients due to the additive adverse effects of radiation treatment and/or chemotherapy. J Exp Clin Cancer Res 2000;19:431–439. [PubMed] [Google Scholar]
- 34. Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer‐associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567–573. [DOI] [PubMed] [Google Scholar]
- 35. Persson CR, Johansson BB, Sjoden PO, Glimelius BL. A randomized study of nutritional support in patients with colorectal and gastric cancer. Nutr Cancer 2002;42:48–58. [DOI] [PubMed] [Google Scholar]
- 36. Ulutin HC, Arpaci F, Pak Y. Megestrol acetate for cachexia and anorexia in advanced non‐small cell lung cancer: a randomized study comparing two different doses. Tumori 2002;88:277–280. [DOI] [PubMed] [Google Scholar]
- 37. Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double‐blind, placebo‐controlled study. J Clin Oncol 2003;21:129–134. [DOI] [PubMed] [Google Scholar]
- 38. Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense N‐3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer‐associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004;22:2469–2476. [DOI] [PubMed] [Google Scholar]
- 40. Lundholm K, Daneryd P, Bosaeus I, Korner U, Lindholm E. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: effects on survival, metabolism, and function. Cancer 2004;100:1967–1977. [DOI] [PubMed] [Google Scholar]
- 41. Bauer J, Capra S, Battistutta D, Davidson W, Ash S. Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clin Nutr 2005;24:998–1004. [DOI] [PubMed] [Google Scholar]
- 42. Dias MCG, Marucci MFN, Nadalin W, Waitzberg DL. Nutritional intervention improves the caloric and proteic ingestion of head and neck cancer patients under radiotherapy. Nutr Hosp 2005;5:320–325. [PubMed] [Google Scholar]
- 43. Ravasco P, Monteiro‐Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659–668. [DOI] [PubMed] [Google Scholar]
- 44. Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, et al. Double‐blind, placebo‐controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol 2006;24:3401–3407. [DOI] [PubMed] [Google Scholar]
- 45. Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, et al. Comparison of orally administered cannabis extract and delta‐9‐tetrahydrocannabinol in treating patients with cancer‐related anorexia‐cachexia syndrome: a multicenter, phase III, randomized, double‐blind, placebo‐controlled clinical trial from the Cannabis‐In‐Cachexia‐Study‐Group. J Clin Oncol 2006;24:3394–3400. [DOI] [PubMed] [Google Scholar]
- 46. Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, Rowland KM Jr, et al. A placebo‐controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007;110:1396–1403. [DOI] [PubMed] [Google Scholar]
- 47. Beijer S, Hupperets PS, van den Borne BE, Eussen SR, van Henten AM, van den Beuken‐van EM, et al. Effect of adenosine 5′‐triphosphate infusions on the nutritional status and survival of preterminal cancer patients. Anticancer Drugs 2009;20:625–633. [DOI] [PubMed] [Google Scholar]
- 48. Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, et al. A placebo‐controlled, double‐blind trial of infliximab for cancer‐associated weight loss in elderly and/or poor performance non‐small cell lung cancer patients (N01C9). Lung Cancer 2010;68:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Navari RM, Brenner MC. Treatment of cancer‐related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010;18:951–956. [DOI] [PubMed] [Google Scholar]
- 51. Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, Thomas K, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non‐small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 2011;24:431–440. [DOI] [PubMed] [Google Scholar]
- 52. Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer—a randomized study. Head Neck 2012;34:1–9. [DOI] [PubMed] [Google Scholar]
- 53. Wen HS, Li X, Cao YZ, Zhang CC, Yang F, Shi YM, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy 2012;58:461–467. [DOI] [PubMed] [Google Scholar]
- 54. Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol 2012;124:417–425. [DOI] [PubMed] [Google Scholar]
- 55. Madeddu C, Dessi M, Panzone F, Serpe R, Antoni G, Cau MC, et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib ± megestrol acetate for patients with cancer‐related anorexia/cachexia syndrome. Clin Nutr 2012;31:176–182. [DOI] [PubMed] [Google Scholar]
- 56. Del Fabbro E, Dev R, Hui D, Palmer L, Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double‐blind placebo‐controlled trial. J Clin Oncol 2013;31:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dobrila‐Dintinjana R, Tricanovic D, Zelic M, Radíc M, Dintinjana M, Petranovic D, et al. Nutritional support in patients with colorectal cancer during chemotherapy: does it work? Hepatogastroenterology 2013;60:475–480. [DOI] [PubMed] [Google Scholar]
- 58. Kanat O, Cubukcu E, Avci N, Budak F, Ercan I, Canhoroz M, et al. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori 2013;99:229–233. [DOI] [PubMed] [Google Scholar]
- 59. Bourdel‐Marchasson I, Blanc‐Bisson C, Doussau A, Germain C, Blanc JF, Dauba J, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two‐year randomized controlled trial. PLoS ONE 2014;9:e108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pottel L, Lycke M, Boterberg T, Pottel H, Goethals L, Duprez F, et al. Echium oil is not protective against weight loss in head and neck cancer patients undergoing curative radio (chemo)therapy: a randomised‐controlled trial. BMC Complement Altern Med 2014;14:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Poulsen GM, Pedersen LL, Osterlind K, Baeksgaard L, Andersen JR. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clin Nutr 2014;33:749–753. [DOI] [PubMed] [Google Scholar]
- 62. Focan C, Houbiers G, Gilles L, Steeland TV, Georges N, Maniglia A, et al. Dietetic and psychological mindfulness workshops for the management of cachectic cancer patients. A randomized study. Anticancer Res 2015;35:6311–6315. [PubMed] [Google Scholar]
- 63. Gavazzi C, Colatruglio S, Valoriani F, Mazzaferro V, Sabbatini A, Biffi R, et al. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: a multicentre randomised clinical trial. Eur J Cancer 2016;64:107–112. [DOI] [PubMed] [Google Scholar]
- 64. Jatoi A, Qin R, Satele D, Dakhil S, Kumar P, Johnson DB, et al. “Enjoy glass of wine before eating:” a randomized trial to test the orexigenic effects of this advice in advanced cancer patients. Support Care Cancer 2016;24:3739–3746. [DOI] [PubMed] [Google Scholar]
- 65. Takayama K, Katakami N, Yokoyama T, Atagi S, Yoshimori K, Kagamu H, et al. Anamorelin (ONO‐7643) in Japanese patients with non‐small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer 2016;24:3495–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Woo SM, Joo J, Kim SY, Park SJ, Han SS, Kim TH, et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology 2016;16:1099–1105. [DOI] [PubMed] [Google Scholar]
- 67. Jatoi A, Steen PD, Atherton PJ, Moore DF, Rowland KM, Le‐Lindqwister NA, et al. A double‐blind, placebo‐controlled randomized trial of creatine for the cancer anorexia/weight loss syndrome (N02C4): an Alliance trial. Ann Oncol 2017;28:1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kapoor N, Naufahu J, Tewfik S, Bhatnagar S, Garg R, Tewfik I. A prospective randomized controlled trial to study the impact of a nutrition‐sensitive intervention on adult women with cancer cachexia undergoing palliative care in India. Integr Cancer Ther 2017;16:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leedo E, Gade J, Granov S, Mellemgaard A, Klausen TW, Rask K, et al. The effect of a home delivery meal service of energy‐ and protein‐rich meals on quality of life in malnourished outpatients suffering from lung cancer: a randomized controlled trial. Nutr Cancer 2017;69:444–453. [DOI] [PubMed] [Google Scholar]
- 70. Werner K, Kullenberg de Gaudry D, Taylor LA, Keck T, Unger C, Hopt UT, et al. Dietary supplementation with n‐3‐fatty acids in patients with pancreatic cancer and cachexia: marine phospholipids versus fish oil—a randomized controlled double‐blind trial. Lipids Health Dis 2017;16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zietarska M, Krawczyk‐Lipiec J, Kraj L, Zaucha R, Malgorzewicz S. Chemotherapy‐related toxicity, nutritional status and quality of life in precachectic oncologic patients with, or without, high protein nutritional support. A prospective, randomized study. Nutrients 2017;9:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, et al. Anamorelin (ONO‐7643) for the treatment of patients with non‐small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer 2018;124:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kouchaki B, Janbabai G, Alipour A, Ala S, Borhani S, Salehifar E. Randomized double‐blind clinical trial of combined treatment with megestrol acetate plus celecoxib versus megestrol acetate alone in cachexia‐anorexia syndrome induced by GI cancers. Support Care Cancer 2018;26:2479–2489. [DOI] [PubMed] [Google Scholar]
- 74. Schink K, Herrmann HJ, Schwappacher R, Meyer J, Orlemann T, Waldmann E, et al. Effects of whole‐body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: a controlled pilot trial. BMC Cancer 2018;18:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Turcott JG, Del Rocío Guillen Núñez M, Flores‐Estrada D, Oñate‐Ocaña LF, Zatarain‐Barrón ZL, Barrón F, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double‐blind clinical trial. Support Care Cancer 2018;26:3029–3038. [DOI] [PubMed] [Google Scholar]
- 76. Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr 2018;37:1202–1209. [DOI] [PubMed] [Google Scholar]
- 77. Britton B, Baker AL, Wolfenden L, Wratten C, Bauer J, Beck AK, et al. Eating As Treatment (EAT): a stepped‐wedge, randomized controlled trial of a health behavior change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiation therapy (TROG 12.03). Int J Radiat Oncol Biol Phys 2019;103:353–362. [DOI] [PubMed] [Google Scholar]
- 78. Cereda E, Turri A, Klersy C, Cappello S, Ferrari A, Filippi AR, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med 2019;8:6923–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laviano A, Calder PC, Schols A, Lonnqvist F, Bech M, Muscaritoli M. Safety and tolerability of targeted medical nutrition for cachexia in non‐small‐cell lung cancer: a randomized, double‐blind, controlled pilot trial. Nutr Cancer 2020;72:439–450. [DOI] [PubMed] [Google Scholar]
- 80. Obling SR, Wilson BV, Pfeiffer P, Kjeldsen J. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr 2019;38:182–190. [DOI] [PubMed] [Google Scholar]
- 81. Bouleuc C, Anota A, Cornet C, Grodard G, Thiery‐Vuillemin A, Dubroeucq O, et al. Impact on health‐related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist 2020;25:e843–e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dehghani M, Mirzaie M, Farhadi P, Rezvani A. The effect of ACE inhibitor on the quality of life amongst patients with cancer cachexia. Asian Pac J Cancer Prev 2020;21:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Famil‐Dardashti A, Hajigholami A, Badri S, Yekdaneh A, Moghaddas A. The role of Trigonella, Cichorium, and Foeniculum herbal combination in the treatment of cancer‐induced anorexia/cachexia: a quasi‐experimental study. Int J Cancer Manag 2020;13:e102515. [Google Scholar]
- 84. Hong Y, Wu C, Wu B. Effects of resistance exercise on symptoms, physical function, and quality of life in gastrointestinal cancer patients undergoing chemotherapy. Integr Cancer Ther 2020;19:1534735420954912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Movahed S, Seilanian Toussi M, Pahlavani N, Motlagh AG, Eslami S, Nematy M, et al. Effects of medical nutrition therapy compared with general nutritional advice on nutritional status and nutrition‐related complications in esophageal cancer patients receiving concurrent chemoradiation: a randomized controlled trial. Mediterranean J Nutr Metab 2020;13:265–276. [Google Scholar]
- 86. Qiu Y, You J, Wang K, Cao Y, Hu Y, Zhang H, et al. Effect of whole‐course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomized control trial. Nutrition 2020;69:110558. [DOI] [PubMed] [Google Scholar]
- 87. Storck LJ, Ruehlin M, Gaeumann S, Gisi D, Schmocker M, Meffert PJ, et al. Effect of a leucine‐rich supplement in combination with nutrition and physical exercise in advanced cancer patients: a randomized controlled intervention trial. Clin Nutr 2020;39:3637–3644. [DOI] [PubMed] [Google Scholar]
- 88. Bargetzi L, Brack C, Herrmann J, Bargetzi A, Hersberger L, Bargetzi M, et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: secondary analysis of a prospective randomized trial. Ann Oncol 2021;32:1025–1033. [DOI] [PubMed] [Google Scholar]
- 89. Currow DC, Glare P, Louw S, Martin P, Clark K, Fazekas B, et al. A randomised, double blind, placebo‐controlled trial of megestrol acetate or dexamethasone in treating symptomatic anorexia in people with advanced cancer. Sci Rep 2021;11:2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hunter CN, Abdel‐Aal HH, Elsherief WA, Farag DE, Riad NM, Alsirafy SA. Mirtazapine in cancer‐associated anorexia and cachexia: a double‐blind placebo‐controlled randomized trial. J Pain Symptom Manage 2021;62:1207–1215. [DOI] [PubMed] [Google Scholar]
- 91. Izumi K, Iwamoto H, Yaegashi H, Nohara T, Shigehara K, Kadono Y, et al. Androgen replacement therapy for cancer‐related symptoms in male: result of prospective randomized trial (ARTFORM study). J Cachexia Sarcopenia Muscle 2021;12:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ko MH, Song SY, Ha SJ, Lee JY, Yoon SW, Park JH, et al. Efficacy and safety of Yukgunja‐Tang for patients with cancer‐related anorexia: a randomized, controlled trial, pilot study. Integr Cancer Therapies 2021;20:15347354211019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Molassiotis A, Brown T, Cheng HL, Byrnes A, Chan RJ, Wyld D, et al. The effects of a family‐centered psychosocial‐based nutrition intervention in patients with advanced cancer: the PiCNIC2 pilot randomised controlled trial. Nutr J 2021;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kutz LM, Abel J, Schweizer D, Tribius S, Krull A, Petersen C, et al. Quality of life, HPV‐status and phase angle predict survival in head and neck cancer patients under (chemo)radiotherapy undergoing nutritional intervention: results from the prospective randomized HEADNUT‐trial. Radiother Oncol 2022;166:145–153. [DOI] [PubMed] [Google Scholar]
- 95. Patient‐reported outcome measures: use in medical product development to support labeling claims. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research; Center for Devices and Radiological Health. 2009. https://www.fda.gov/media/77832/download. Accessed on 19 Jan 2024.
- 96. Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011;41:1073–1093. [DOI] [PubMed] [Google Scholar]
- 97. Jeter K, Blackwell S, Burke L, Joyce D, Moran C, Conway EV, et al. Cancer symptom scale preferences: does one size fit all? BMJ Support Palliat Care 2018;8:198–203. [DOI] [PubMed] [Google Scholar]
- 98. Martin L, Kubrak C. How much does reduced food intake contribute to cancer‐associated weight loss? Curr Opin Support Palliat Care 2018;12:410–419. [DOI] [PubMed] [Google Scholar]
- 99. Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 2013;98:1012–1019. [DOI] [PubMed] [Google Scholar]
- 100. Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021;6:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Solheim TS, Blum D, Fayers PM, Hjermstad MJ, Stene GB, Strasser F, et al. Weight loss, appetite loss and food intake in cancer patients with cancer cachexia: three peas in a pod?—analysis from a multicenter cross sectional study. Acta Oncol 2014;53:539–546. [DOI] [PubMed] [Google Scholar]
- 102. Hall KD. What is the required energy deficit per unit weight loss? Int J Obes (Lond) 2008;32:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Documentation of literature search
Appendix S2. Modified Downs and Black Scoring Criteria
Table S1. Raw values of appetite scores pre‐ and posttreatment with delta, significance levels and effect sizes of two‐armed trials
Table S2. Raw values of appetite scores pre‐ and posttreatment with delta, significance levels and effect sizes of multi‐armed trials
Table S3. Raw values of dietary intake of energy and protein pre‐ and posttreatment with delta, significance levels and effect sizes of two‐armed trials
Table S4. Raw values of dietary intake of energy and protein pre‐ and posttreatment with delta, significance levels and effect sizes of multi‐armed trials


