Abstract
To test the potential of a multigene DNA vaccine against lentivirus infection, we generated a defective mutant provirus of feline immunodeficiency virus (FIV) with an in-frame deletion in pol (FIVΔRT). In a first experiment, FIVΔRT DNA was administered intramuscularly to 10 animals, half of which also received feline gamma interferon (IFN-γ) DNA. The DNA was administered in four 100-μg doses at 0, 10, and 23 weeks. Immunization with FIVΔRT elicited cytotoxic T-cell (CTL) responses to FIV Gag and Env in the absence of a serological response. After challenge with homologous virus at week 26, all 10 of the control animals became seropositive and viremic but 4 of the 10 vaccinates remained seronegative and virus free. Furthermore, quantitative virus isolation and quantitative PCR analysis of viral DNA in peripheral blood mononuclear cells revealed significantly lower virus loads in the FIVΔRT vaccinates than in the controls. Immunization with FIVΔRT in conjunction with IFN-γ gave the highest proportion of protected cats, with only two of five vaccinates showing evidence of infection following challenge. In a second experiment involving two groups (FIVΔRT plus IFN-γ and IFN-γ alone), the immunization schedule was reduced to 0, 4, and 8 weeks. Once again, CTL responses were seen prior to challenge in the absence of detectable antibodies. Two of five cats receiving the proviral DNA vaccine were protected against infection, with an overall reduction in virus load compared to the five infected controls. These findings demonstrate that DNA vaccination can elicit protection against lentivirus infection in the absence of a serological response and suggest the need to reconsider efficacy criteria for lentivirus vaccines.
The continued spread of human immunodeficiency virus (HIV) worldwide makes the development of an effective vaccine an urgent priority for world health. However, HIV and its animal lentivirus counterparts present formidable challenges for vaccine development because they cause persistent infection and induce disease despite a vigorous host immune response (8). Among the available animal analogs of HIV, feline immunodeficiency virus (FIV) has unique value as a widespread naturally occurring infectious agent in its natural host (45). Conventional approaches to vaccine development have been pursued extensively for FIV and simian immunodeficiency virus (SIV) but have yielded only qualified successes. Whole inactivated virus vaccines have proved effective in the FIV model, and protection is virus specific (48) but limited to the homologous virus and may depend on a fortuitous vaccine strain of virus which elicits strong neutralizing-antibody responses (16). Subunit vaccines based on purified or recombinant Env glycoproteins have given some evidence of protection against HIV in a small number of chimpanzees, but similar SIV or FIV vaccines have led at best to reduced virus loads after challenge and in some instances to enhancement of infection (17, 22, 40). More robust resistance has been observed to SIV after infection with attenuated strains lacking regulatory genes such as nef (6), but safety fears make the use of this approach in humans controversial (3, 46).
DNA-mediated immunization has emerged recently as a promising alternative approach to the development of viral vaccines, with protective immunity against viral infections such as avian influenza (33), Newcastle disease (36), and Aujeszky’s disease (13), as well as a wide range of nonviral pathogens (reviewed in reference 42), being generated following in vivo administration of naked DNA to muscle or skin. Given that antiretroviral therapy is unlikely to be economically viable in developing countries, a further attraction of DNA-mediated immunization is the prospect of stable and affordable vaccines.
Several HIV-1 DNA constructs induce immune responses in mice and primates (25, 38, 43), and forthcoming phase I trials will assess the safety and immunogenicity of HIV-1 env DNA in human subjects. Encouraging evidence for the use of DNA vaccines was described recently when an HIV-1 DNA vaccine containing env, rev, and gag/pol induced protective immunity in the chimpanzee model (4). However, since HIV-1 is apathogenic in the chimpanzee model system, the question arises whether similar approaches will be effective in inducing protection against lentivirus infection in the natural host species that are susceptible to virus-induced disease, such as FIV in the domestic cat.
In this study, we generated an FIV DNA vaccine construct based on a replication-defective but essentially full-length proviral genome which should express both structural and regulatory proteins in vivo. We investigated the efficacy of this FIV DNA vaccine and demonstrated that a significant proportion of the cats vaccinated with this DNA were protected from subsequent challenge with the homologous virus. Furthermore, the protective effect was retained in a second trial involving a much shorter immunization schedule. This study provides the first evidence that DNA-mediated immunization can prevent lentivirus infection in its natural host species.
MATERIALS AND METHODS
Cells and viruses.
Cell culture media and supplements were obtained from Life Technologies Inc., Paisley, United Kingdom. Crandell feline kidney (CrFK) cells (5) were maintained in Dulbecco’s modification of minimal essential medium Eagle supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 0.11 mg of sodium pyruvate per ml, 100 μg of streptomycin per ml, and 100 IU of penicillin per ml. MYA-1 cells (27) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 5 × 10−5 M 2-mercaptoethanol (complete RPMI 1640 medium), and 2% culture supernatant from the Ltk−IL2.23 cell line, a murine cell line stably transfected with the human interleukin-2 (IL-2) gene and which produces high levels of human IL-2 (addition of 2% supernatant was found to be equivalent to addition of approximately 100 IU of recombinant human IL-2). The Ltk−IL2.23 cell line was a kind gift of M. Hattori, University of Tokyo. The FIV-PET challenge virus was prepared as described previously (32) by transfection of the murine fibroblast cell line NIH 3T3 with the F14 molecular clone of FIV-PET (28) and recovery into the IL-2-dependent feline T-cell line Q201 (44).
Preparation of DNA immunogens.
The reverse transcriptase (RT) deletion mutant FIVΔRT was prepared from the F-14 molecular clone of FIV-PET (28). Cloning and characterization of feline gamma interferon (IFN-γ) have been described previously (1). For use as an adjuvant gene, the IFN-γ cDNA was subcloned into the pRC-RSV vector (Invitrogen B.V., De Schelp, The Netherlands) as a HindIII-NotI fragment.
Plasmid DNAs were purified by cesium chloride-ethidium bromide gradient centrifugation followed by butanol extraction and ethanol precipitation. Following resuspension in 10 mM Tris plus 1 mM EDTA, the plasmids were dialyzed extensively against phosphate-buffered saline (PBS). Endotoxin levels were quantified commercially by Q1 Biotech, Glasgow, United Kingdom, by the Limulus amebocyte lysate technique and were found to be <5 EU/ml.
DNA immunization and virus challenge.
Kittens (12 weeks old) were randomized into groups of five for immunization. DNA was administered at four sites in the gastrocnemius and quadriceps muscles (100 μg of each DNA in a total of 200 μl of PBS at each site). The cats were challenged with the homologous F-14 molecular clone of the FIV-PET isolate that had been subjected to titer determination by intraperitoneal inoculation of age-matched cats to calculate the 50% infectious dose.
Detection of FIV-specific CTL.
Lymphocytes were collected from peripheral blood as described previously (10) and assayed directly for cytotoxic T-lymphocyte (CTL) activity. Target cells were autologous or allogeneic skin fibroblasts derived from biopsy material collected prior to vaccination (9). The target cells were labelled with 51Cr and infected with recombinant vaccinia viruses expressing FIV Gag (10) or Env (41) or wild-type vaccinia virus as a control. Effector cells (>99% viable) were added at an effector-to-target-cell (E/T) ratio of 50:1, and lytic activity was measured by monitoring isotope release as described previously (10).
Serological tests.
Plasma samples were tested for the presence of anti-FIV antibodies by immunoblot analysis as described previously (19). Peptide-based enzyme-linked immunosorbent assays (ELISA) were used to determine titers of antibodies recognizing an immunodominant epitope in the transmembrane glycoprotein (TM; CNQNQFFCK) (12, 30) by methods described previously (2, 39). Assays for virus-neutralizing antibodies were performed, by a method described previously (29), on plasma samples taken on the day of challenge.
Immunoblotting.
CrFK cells were transfected with the F-14 molecular clone, the FIVΔRT construct, or no DNA (mock transfected) by using LipofectAMINE (Life Technologies) as specified by the manufacturer. The medium was changed 24 h posttransfection, and supernatants from the transfected cells were harvested 48 h later. These supernatants were clarified by centrifugation at 10,000 × g for 10 min before virions were pelleted by ultracentrifugation at 200,000 × g for 1 h. The virions thus purified from 1 ml of culture fluid were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting as described elsewhere (19). Monoclonal antibody 43/1B9, recognizing FIV p24 (kindly provided by N. Pedersen), was used for immunoblotting.
Isolation of FIV.
On the day of challenge and at intervals of 3 weeks thereafter, peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous peripheral blood by centrifugation over Ficoll-Hypaque (Pharmacia LKB Biotechnology Inc., Piscataway, N.J.) and then either cultured directly or cocultured with the IL-2-dependent feline T-cell line MYA-1 (27) in complete RPMI 1640 medium supplemented with 2% culture supernatant from the Ltk−IL2.23 cell line. Cultures were tested for the production of FIV p24 by ELISA (FIV antigen test kit; IDEXX Laboratories, Portland, Maine) and were maintained for 21 days before being scored as negative.
Quantitative virus isolation.
The infectious virus burden was measured 7 and 12 weeks after challenge. PBMC were isolated as described above, and decreasing numbers of cells (2 × 106, 2 × 105, 2 × 104, 2 × 103, 2 × 102, 20, 2, or 0.2 cells) were cocultivated, in duplicate, in 48-well plates with 106 MYA-1 cells in a total volume of 1.5 ml of complete RPMI medium. Samples of culture supernatant were tested on day 14 for the presence of FIV p24 by ELISA.
Statistical analyses.
The proportions of infected cats in the four groups of cats were compared by Fisher’s exact test. The statistical modelling of the initial number of infected cells per 2 × 106 PBMC for each cat followed the classic dilution assay analysis (26). For the first trial, the maximum-likelihood estimates of the initial concentrations of infected cells were compared among the four groups by a one-way analysis of variance. These estimates were log transformed, after addition of a constant of 1, to better meet the “normality with equal variance” assumptions. Pairwise contrasts between groups were analyzed by two sample t tests. Groups 1 (FIVΔRT) and 2 (FIVΔRT plus IFN-γ) were then aggregated and compared with the two control groups via a two-sample t test. These analyses were performed for each of the two periods separately, ignoring the repeated-measures aspect. The P values cited for the two-sample t tests are adjusted for multiple comparisons via permutation and should be regarded as descriptive, reflecting the exploratory nature of the analyses. For the second trial, similar methods were applied to compare estimates of the initial concentrations of infected cells in the two groups of cats.
Proviral load measurement.
DNA was prepared from PBMC and tested for the presence of FIV sequences by quantitative nested PCR with primers from the env gene as described previously (32). Duplicate assays were performed with pol primers (outer, GAAGATAAATTACAGGAAGAACC and CTCATTTCCTGGAATACCTTTA; inner, GATGGGTTATGAATTACATCCA and GGACCCAATCTATAAATTGC) under identical conditions. Each PCR was validated with a set of dilutions of DNA derived from the FL4 cell line (47). Assays were performed on aliquots of DNA prepared from PBMC at inputs of 250, 500, and 1,000 ng. Positive scores were assigned to denote the amplification in one or more aliquots.
RESULTS
Generation of a replication-defective FIV vaccine construct (FIVΔRT).
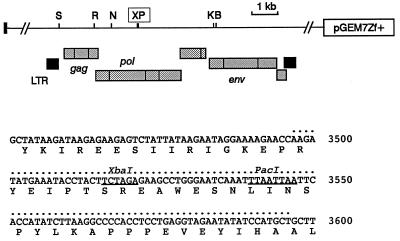
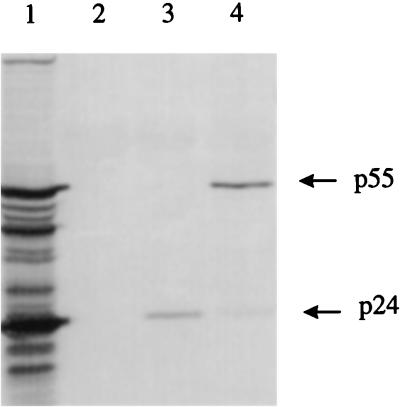
The F-14 molecular clone of FIV-PET has previously been shown to be infectious for cats following intramuscular injection of plasmid DNA (32). To render the FIV provirus noninfectious while leaving as much as possible of the coding potential intact, deletions were generated around a PacI restriction site located within the RT coding sequence at the hinge between the RT and RNase H domains (28). From a library of clones generated by sequential PacI and Bal 31 digestion and religation, we selected a recombinant which had sustained a 33-codon deletion. The precise boundaries of this deletion are shown in Fig. 1 below a partial restriction map of the F-14 proviral clone (28). Characterization of this deletion mutant has shown that it is completely defective with respect to virion infectivity and RT activity. However, transfection studies showed that FIVΔRT is essentially normal in Env-mediated cell fusion and viral antigen release (data not shown). As shown in Fig. 2, CrFK cells transfected with FIVΔRT and the parental F-14 clone release similar levels of viral proteins. The FIVΔRT-derived virions showed a consistent reduction in the extent of Gag protein processing, suggesting some impairment of Gag-Pol precursor transport or function, but since authentic processing was clearly occurring, this proviral construct was adopted as the basis of the FIVΔRT DNA vaccine.
FIG. 1.
Partial restriction map of the FIV-PET F-14 molecular clone which was received as a subclone in plasmid pGEM7Zf+. The location of the major open reading frames (stippled boxes) and long terminal repeats (solid boxes) is shown underneath. The unique PacI site (P) used in mutagenesis and an adjacent XbaI site (X) are boxed in the diagram and underlined in the primary sequence below. The extent of the Bal 31-generated deletion in FIVΔRT is indicated by dots above the sequence. Numbering is relative to the published sequence (GenBank accession no. M25381 [28]). Other restriction sites: S, SacI; R, EcoRI; N, NcoI; K, KpnI; B, BglII.
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of sedimented virus particles released from FL4 cells persistently infected with FIV-PET (lane1), mock-transfected CrFK cells (lane 2), CrFK cells transfected with the F-14 molecular clone (lane 3), and CrFK cells transfected with the FIVΔRT construct (lane 4).
DNA vaccination with FIVΔRT induces a virus-specific CTL response.
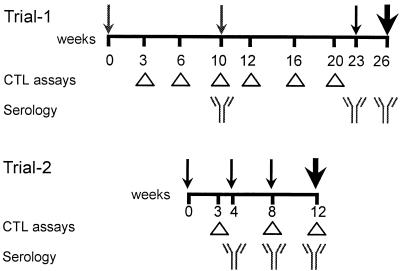
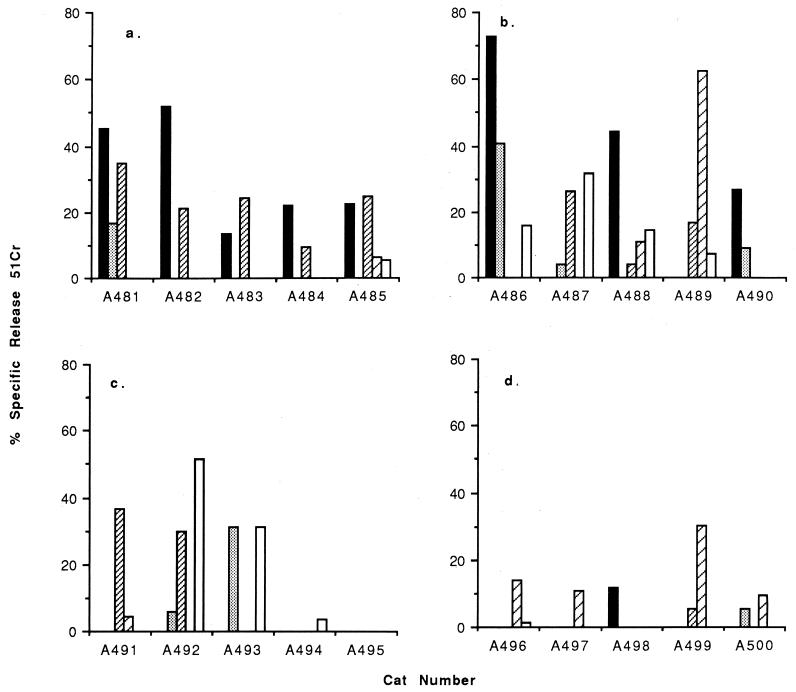
In the first vaccine trial of FIVΔRT, three groups of five cats were immunized with DNA comprising either FIVΔRT, FIVΔRT in conjunction with IFN-γ, or IFN-γ alone. A fourth control group received no DNA. A line diagram depicting the schedule is shown in Fig. 3. Virus-specific CTL responses were first detected in unstimulated peripheral blood three weeks following the first immunization. Gag- and Env-specific CTL responses were observed in all cats immunized with FIVΔRT (Fig. 4a), although the magnitude of the response varied between individual animals, as might be expected given the outbred nature of the study population, with higher levels of 51Cr release observed in cats A481 to A483 than in cats A484 and A485. However, in all cats the observed lysis exceeded 10% at an E/T ratio of 50:1. The CTL activity was not observed when allogeneic target cells were used in the assay, suggesting that the observed activity was major histocompatibility complex restricted. There was also no recognition of autologous target cells infected with wild-type vaccinia virus, confirming the specificity of the response.
FIG. 3.
Design of the two trials. Timings of immunizations and challenges (in weeks) are represented by small and large arrows, respectively. CTL and serological assays were performed at the intervals denoted by the triangles and symbols representing antibodies, respectively.
FIG. 4.
FIV Gag- and Env-specific effector CTL responses were measured directly on the fresh PBMC collected from vaccinated (FIVΔRT [a]; FIVΔRT plus IFN-γ [b]) and control (IFN-γ [c]; PBS alone [d]) cats in trial 1, 3 weeks after the first immunization. Autologous or allogeneic skin fibroblasts infected with recombinant vaccinia viruses expressing either FIV Gag (10) (■ and ░⃞) or FIV Env (41) (▨ and ▨) or wild-type vaccinia virus (□) were labelled with 51Cr and used as targets in the assay. The release of 51Cr into the culture supernatant was detected after 4 h of incubation at 37°C. The results shown represent the mean values for triplicate cultures at an E/T ratio of 50:1.
Coimmunization with FIVΔRT and IFN-γ DNA also elicited FIV Gag- and Env-specific CTL responses in the unstimulated PBMC of all cats, and in some of these animals higher levels of 51Cr release were detected than in cats inoculated with FIVΔRT alone (Fig. 4b). However, these responses were not entirely major histocompatibility complex restricted since lysis of allogeneic target cells was also observed. The nonspecific nature of the cytolytic responses observed following IFN-γ DNA immunization was also indicated by the recognition of autologous target cells infected with wild-type vaccinia virus. Furthermore, immunization with IFN-γ DNA alone resulted in the induction of CTL responses recognizing either autologous or allogeneic target cells or target cells infected with wild-type vaccinia virus (Fig. 4c), suggesting that in vivo delivery of the feline IFN-γ plasmid may have stimulated a nonspecific cellular immune response. No FIV-specific CTL activity was observed in control cats inoculated with PBS alone (Fig. 4d).
Analysis of the CTL response 6 weeks after the initial immunization revealed a general decline in activity (Fig. 5). This was most marked in the group coimmunized with FIVΔRT and IFN-γ, in which no CTL activity could be detected. In the FIVΔRT-immunized group, although Gag- and Env-specific CTL responses were still detectable in four of five cats, the levels of lysis were lower than those observed at week 3. In two of five cats (A481 and A482) in the FIVΔRT group and three of five cats (A487, A488, and A489) in the FIVΔRT-plus-IFN-γ-coimmunized group, this activity remained at background levels despite repeat inoculations of DNA. In contrast, a transient boosting of FIV Gag-specific CTL activity was observed in the remaining five cats at week 12, 2 weeks after the repeat DNA inoculation administered at week 10, and in one animal (A490) a transient increase in Env-specific CTL activity was observed before the level declined to background levels (Fig. 6). Further boosting at week 23 had a negligible effect on the FIV-specific effector CTL responses detected in the peripheral blood (data not shown), and all cats were challenged at week 26.
FIG. 5.
Detection of FIV Gag- and Env-specific CTL responses directly in fresh PBMC collected from vaccinated (FIVΔRT [a]; FIVΔRT plus IFN-γ [b]) and control (IFN-γ [c]; PBS alone [d]) cats in trial 1, 6 weeks after the first immunization. Autologous or allogeneic target cell express either FIV Gag (■ and ░⃞) or FIV Env (▨ and ▨) or no FIV proteins (infected with wild-type vaccinia virus [□]). The results shown represent the mean 51Cr release after a 4-h incubation at 37°C for triplicate cultures at an E/T ratio of 50:1.
FIG. 6.
Detection of FIV Gag- and Env-specific CTL responses directly in fresh PBMC collected from vaccinated (FIVΔRT [a]; FIVΔRT plus IFN-γ [b]) and control (IFN-γ [c]; PBS alone [d]) cats in trial 1, 1 week after the second immunization. Autologous or allogeneic target cells express either FIV Gag (■ and ) or FIV Env (▨ and ▨) or no FIV proteins (infected with wild-type vaccinia virus [□]). The results shown represent the mean 51Cr release after a 4-h incubation at 37°C for triplicate cultures at an E/T ratio of 50:1. ND, not done.
DNA immunization does not induce antiviral antibodies.
In contrast to the CTL responses, FIV-specific antibodies were not detected by any available test, including ELISA for the immunodominant TM peptide and immunoblot analysis with a lysate of FIV-PET-infected cells (Table 1). Previous studies by us and others have demonstrated these assays to be reliable and sensitive measures of FIV-specific serological responses in naturally infected cats (19, 39). Similarly, no virus-neutralizing antibodies were detected in plasma samples taken on the day of challenge.
TABLE 1.
Titers of antibody responses to FIV TM peptide, reactivity of plasma samples by immunoblotting, and results of virus isolation on the day of challenge and at intervals following challenge
| DNA inoculum | Cat | Antibody
responsea at time (wk) postchallenge:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
3
|
6
|
9
|
12
|
|||||||||
| IB | VI | TM | IB | VI | IB | VI | IB | VI | IB | VI | TM | ||
| Trial 1 | |||||||||||||
| FIVΔRT | 1 | − | − | 0 | − | − | − | + | + | + | − | 0 | |
| 2 | − | − | 0 | − | + | − | + | + | + | + | − | 0 | |
| 3 | − | − | 0 | − | − | − | + | + | + | + | + | 0 | |
| 4 | − | − | 0 | − | − | − | − | − | − | − | − | 0 | |
| 5 | − | − | 0 | − | + | + | + | + | + | + | − | 25 | |
| FIVΔRT + IFN-γ | 1 | − | − | 0 | − | − | − | − | − | − | − | − | 0 |
| 2 | − | − | 0 | − | − | + | + | + | + | + | − | 0 | |
| 3 | − | − | 0 | − | − | − | − | − | − | − | − | 0 | |
| 4 | − | − | 0 | − | − | + | + | + | + | + | − | 125 | |
| 5 | − | − | 0 | − | − | − | − | − | − | − | − | 0 | |
| IFN-γ | 1 | − | − | 0 | − | + | + | + | + | + | + | + | 125 |
| 2 | − | − | 0 | − | + | + | + | + | + | + | − | 25 | |
| 3 | − | − | 0 | − | − | + | + | + | + | + | + | 25 | |
| 4 | − | − | 0 | − | − | + | + | + | + | + | + | 25 | |
| 5 | − | − | 0 | − | + | + | + | + | + | + | + | 5 | |
| No DNA | 1 | − | − | 0 | − | − | + | + | + | + | + | + | 25 |
| 2 | − | − | 0 | − | + | + | + | + | + | + | + | 5 | |
| 3 | − | − | 0 | − | − | + | + | + | + | + | + | 5 | |
| 4 | − | − | 0 | − | − | + | + | + | + | + | + | 125 | |
| 5 | − | − | 0 | − | + | + | + | + | + | + | + | 5 | |
| Trial 2 | |||||||||||||
| FIVΔRT + IFN-γ | 1 | − | − | 0 | ND | + | + | + | ND | + | + | + | 25 |
| 2 | − | − | 0 | ND | − | − | − | ND | − | − | − | 0 | |
| 3 | − | − | 0 | ND | − | − | + | ND | + | + | − | 0 | |
| 4 | − | − | 0 | ND | + | + | + | ND | + | + | − | 25 | |
| 5 | − | − | 0 | ND | − | − | − | ND | − | − | − | 0 | |
| IFN-γ | 1 | − | − | 0 | ND | + | + | + | ND | + | + | + | 25 |
| 2 | − | − | 0 | ND | + | + | + | ND | + | + | − | 125 | |
| 3 | − | − | 0 | ND | + | − | + | ND | + | + | + | 5 | |
| 4 | − | − | 0 | ND | − | − | + | ND | + | + | + | 25 | |
| 5 | − | − | 0 | ND | − | − | + | ND | + | + | + | 25 | |
IB, immunoblotting; VI, virus isolation; TM, titer of antibodies recognizing TM peptide; ND, not done.
FIVΔRT vaccine affords protection against challenge with infectious FIV.
In view of the apparent refractory immune state of the vaccinated cats, we decided to test their ability to resist viral infection. All 20 cats were challenged at week 26 with the homologous FIV-PET isolate by intraperitoneal inoculation of 25 50% infectious doses. The cats were monitored following challenge for the presence of infectious FIV and viral DNA and for evidence of seroconversion.
Virus isolation was attempted at 0, 3, 6, 9, and 12 weeks following challenge. Virus was isolated from 5 of 10 control cats by week 3 postchallenge, as well as from 2 of 5 cats inoculated with FIVΔRT. In contrast, all of the cats inoculated with FIVΔRT plus IFN-γ remained virus free at this time, indicating that either their viral loads were below the detection limit of the assay or infection was delayed in this group (Table 1). At 6, 9, and 12 weeks postchallenge, one of five FIVΔRT vaccinates and three of five FIVΔRT-plus-IFN-γ vaccinates remained virus free. In contrast, virus was isolated consistently from all of the control cats (IFN-γ alone and no-DNA control groups) at 6, 9, and 12 weeks postchallenge.
Since all of the cats were seronegative on the day of challenge, positive immunoblot results and titers of antibodies recognizing the TM peptide indicated infection. The results of serological tests confirmed previous observations that immunoblot analysis is the more sensitive indicator of infection in cats infected with FIV-PET (18, 32), since not all of the infected and immunoblot-positive cats developed detectable titers of anti-FIV TM peptide antibodies by week 12 postchallenge (Table 1). Furthermore, the immunoblot results correlated closely with virus isolation results. Plasma from the four cats which were virus isolation negative remained negative throughout the experiment (Fig. 7). In contrast, the control groups which received IFN-γ alone or PBS all became seropositive by 6 weeks postchallenge (Table 1).
FIG. 7.
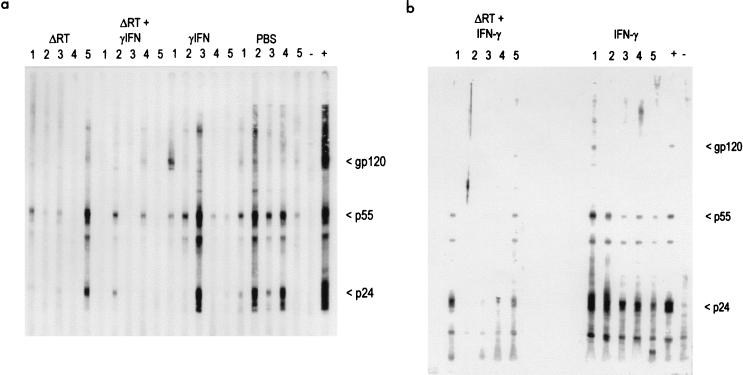
Immunoblot analysis performed as described previously (19) on plasma samples taken from cats 12 weeks after challenge. (a) Trial 1 (numbered lanes) and plasma from an FIV-infected and an unvaccinated, uninfected cat as controls (lanes + and −, respectively). One cat in the group immunized with ΔRT (lane 4) and three cats in the group immunized with ΔRT and IFN-γ (lanes 1, 3, and 5) remained antibody negative after challenge. (b) Trial 2 (numbered lanes) and control lanes as in panel a. Two cats (lanes 2 and 4) immunized with ΔRT and IFN-γ remained antibody negative after challenge.
A total of five FIVΔRT-vaccinated cats from which virus had been isolated at both 6 and 9 weeks postchallenge became negative at 12 weeks postchallenge, including two cats coinoculated with IFN-γ DNA. These cats remained consistently seropositive, confirming their infected status.
Reduced levels of virus and proviral DNA in peripheral blood of FIVΔRT vaccinates.
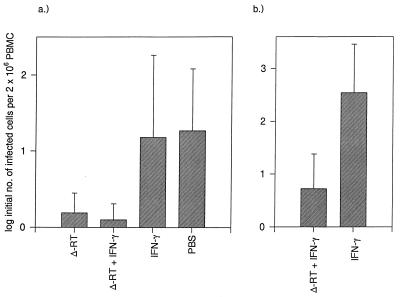
Since there was evidence of lower viral loads in the FIVΔRT vaccinates, with inconsistent virus isolation results and delayed seroconversion in some cats, we measured the infectious-virus burdens in peripheral blood at 7 and 12 weeks postchallenge. Analysis of the results (Fig. 8a) demonstrated that at week 7 there was a statistically significant difference between the groups overall (F test, P = 0.025). However, none of the groups was statistically significantly different pairwise (two-sample t test, adjusted for multiple comparisons by permutation). When aggregated to the two groups immunized with FIVΔRT compared with the two control groups, the FIVΔRT vaccinates had a statistically significantly lower log initial number of infected cells per 2 × 106 PBMC than did the controls (Fig. 8a). At week 12, pairwise contrasts between vaccinated and control groups were all statistically significant, with P < 0.02 (i.e., FIVΔRT compared to IFN-γ, FIVΔRT compared to no DNA, FIVΔRT plus IFN-γ compared to IFN-γ, and FIVΔRT plus IFN-γ compared to no DNA).
FIG. 8.
Viral loads in trial 1 at 7 weeks postchallenge (a) and in trial 2 at 6 weeks postchallenge (b), expressed as the mean ± 2 standard errors of the mean of the log-transformed maximum-likelihood estimates of the initial number of infected cells present in 2 × 106 PBMC.
Similar conclusions emerged from quantitative PCR analysis of proviral DNA in PBMC. The results shown in Table 2 represent the results obtained with the env primers, and these correlated closely with parallel assays involving pol primers (data not shown). Although the levels of proviral DNA were low in the control cats following challenge with the F-14 molecular clone, these findings were broadly consistent with our earlier study of cats infected with a low dose of the FIV-PET isolate (32). Despite the low overall levels of DNA and evidence of declining load from weeks 6 to 12, clear differences among the four vaccine groups were evident. All of the control cats immunized with either IFN-γ or no DNA scored positive in PCRs with 250 ng of DNA at 6 weeks, compared to only 4 of 10 FIVΔRT vaccinates. By 12 weeks postchallenge, only 6 of 10 control cats yielded positive results from 500 ng of DNA compared to 10 of 10 at week 6, indicating that the proviral loads later in infection are decreased. The four cats which remained negative by virus isolation and seronegative by immunoblotting and ELISA gave isolated, transient positive PCR results, three at 6 weeks postinfection and one at 12 weeks. These results indicate that the proviral loads in these cats are close to the detection limit of the assay. Of the remaining six vaccinates, all of which yielded positive results by virus isolation and immunoblotting, a single cat was uniformly negative by PCR, three gave single positive PCR results at week 6 postchallenge, one gave positive results at high DNA concentrations at weeks 6 and 12, and the remaining cat gave positive PCR results at all DNA concentrations tested. It was notable that all of the cats in the group which received FIVΔRT and IFN-γ became uniformly negative by PCR by 12 weeks postchallenge even at the highest input of 1 μg of DNA.
TABLE 2.
Comparison of viral loads in trial 1 at 6 and 12 weeks postchallenge as measured by quantitative nested PCR
| DNA inoculum | Cat | PCR result (wk
6)a
|
PCR result (wk
12)a
|
Serocon- version | |||
|---|---|---|---|---|---|---|---|
| 500 ng | 250 ng | 1,000 ng | 500 ng | 250 ng | |||
| FIVΔRT | 1 | + | − | − | − | − | + |
| 2 | − | − | − | − | − | + | |
| 3 | + | − | + | − | − | + | |
| 4 | − | − | − | + | − | − | |
| 5 | + | + | − | + | + | + | |
| FIVΔRT + IFN-γ | 1 | + | − | − | − | − | − |
| 2 | − | + | − | − | − | + | |
| 3 | + | − | − | − | − | − | |
| 4 | − | + | − | − | − | + | |
| 5 | NDb | + | − | − | − | − | |
| IFN-γ | 1 | + | + | + | + | − | + |
| 2 | + | + | + | − | − | + | |
| 3 | + | + | + | − | + | + | |
| 4 | + | + | − | − | − | + | |
| 5 | + | + | + | − | − | + | |
| No DNA | 1 | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + | |
| 3 | + | + | − | + | + | + | |
| 4 | + | + | − | + | + | + | |
| 5 | + | + | + | + | + | + | |
Positive scores denote a positive amplification in one or more aliquots. Serological responses were determined 12 weeks after challenge by immunoblotting, as shown in Fig. 7.
ND, not done.
The FIVΔRT immunization schedule can be reduced without apparent loss of efficacy.
To investigate whether the lengthy immunization schedule could be reduced without compromising protection, we conducted a second experiment in which two groups of five cats received either FIVΔRT plus IFN-γ or IFN-γ alone at 0, 4, and 8 weeks. As in the first trial, this regimen induced cytolytic activity (data not shown) but no detectable antibody responses by the same series of assays (immunofluorescence, immunoblotting, anti-TM peptide ELISA, and assay for virus-neutralizing antibodies). After challenge at 12 weeks, two of five vaccinates remained seronegative and virus could not be isolated at any of the times tested (Table 1) whereas all of the IFN-γ-alone controls became seropositive and positive by virus isolation, consistent with the results of the first trial. Again, immunoblot analysis corroborated these findings (Fig. 7b). Quantitative measurements of virus in the second trial (Fig. 8b) revealed that at 6 weeks postchallenge, the FIVΔRT-plus-IFN-γ vaccinates developed significantly lower viral loads than did the IFN-γ vaccinates (P = 0.027). By 9 weeks postchallenge, the viral loads of all of the cats were low and there were no differences between the groups (data not shown).
DISCUSSION
This study demonstrates that protection against FIV infection can be achieved by intramuscular administration of nonreplicating FIV DNA. A significant proportion of FIVΔRT vaccinates was protected, and the mean viral loads of the vaccinates were significantly reduced compared to those of the control cats. Similar results were obtained in a second trial in which the immunization schedule was shorter, indicating that the interval between immunizations did not appear to influence the protected status of vaccinated cats.
The mechanism of protection is of great interest, particularly in view of the lack of a detectable humoral immune response in the vaccinates prior to challenge. Our results provide an intriguing parallel with the apparently protected status of individuals exposed to HIV who remain seronegative but have measurable CTL responses (35, 37), suggesting that a potent cellular immune response may be sufficient to confer immunity to infection. The induction of CTL in the absence of a detectable humoral immune response following immunization with the FIVΔRT DNA vaccine is in contrast to the responses observed recently following immunization of chimpanzees with a prospective HIV DNA vaccine (4). Following immunization with a plasmid carrying HIV env and rev under the regulatory control of the cytomegalovirus promoter, antibodies specific for HIV Env were detected in all four immunized animals (4). However, it is notable that even with the protracted immunization regime used to immunize the chimpanzees, there were marked differences in the titers of antibodies induced following immunization. Moreover, three of the four immunized chimpanzees failed to generate a humoral response to the viral Gag protein. In this study, we were unable to detect antibodies specific for either FIV Env or Gag proteins in the cats immunized with either FIVΔRT alone or FIVΔRT in conjunction with IFN-γ. In agreement with our findings, a recent study involving FIV env gene-based DNA vaccines induced either low or undetectable levels of FIV-specific antibodies (31).
The bias toward CTL induction rather than antibody production in response to inoculation with FIVΔRT suggested that intramuscular administration of the FIVΔRT DNA generated primarily a Th1-type immune response. Given that previous studies have demonstrated that it is possible to generate potent cellular and humoral immune responses following immunization of macaques with plasmids expressing the env and gag genes of SIVmac251 (24, 34, 49), there is clearly scope for further optimization of the vaccination schedule. However, the question is then which type of immune response is likely to confer protective immunity to challenge with a lentivirus. The SIVmac251-based DNA vaccine neither protected the immunized macaques from challenge with the homologous virus nor led to a reduction in the viral load of the vaccinates (24). Significantly, the SIVmac251 challenge is a highly pathogenic virus; recent data suggest that the FIV-PET challenge virus used in our study is less pathogenic than the FIV GL-8 strain (21). Similarly, the HIV-1 SF2 challenge virus used in the chimpanzee DNA vaccine trial does not induce disease readily in infected animals (4). Future studies should address whether protective immunity to infection with highly pathogenic strains of FIV (7) can be achieved with DNA vaccines.
CTL activity to FIV Env was shown previously to correlate with long-lived immunity induced by whole inactivated virus vaccines (11). In the present study, there was no clear correlation between the elicitation of Env- or Gag-specific CTL responses in the peripheral blood of vaccinated cats and the protection observed following challenge, since qualitatively similar CTL responses were detected in both protected and unprotected cats within each vaccine group. Future studies will address the contribution of CTL responses with other viral specificities in protective immunity and will investigate the sequestration of virus-specific CTLs to the lymphoid organs, which may explain the rather short-lived CTL responses observed in the peripheral blood. It should now be possible to identify the critical targets for the protective immune response by selective gene inactivation. Given that immunization of cats with FIV env gene-based DNA vaccines resulted in enhancement of infection following challenge (31), it is possible that regulatory gene products expressed by the FIVΔRT DNA vaccine contributed to its greater efficacy.
A greater proportion of the cats in the group inoculated with IFN-γ in conjunction with FIVΔRT was protected following challenge compared to the proportion in the group inoculated with FIVΔRT alone, suggesting that IFN-γ may have enhanced the protective effect of FIVΔRT. IFN-γ is a pleiotropic immune regulator which is depressed in progressive HIV infection and has been reported to be inversely correlated with lymph node virus load in primary SIV infection of macaques (23). Although recombinant IFN-γ is being investigated widely as a therapeutic agent (15), its use as a gene adjuvant has been limited so far. Interestingly, replacement of the nef gene in infectious SIVmac with a human IFN-γ gene was recently found to cause further attenuation of the virus and to enhance protection against superinfection with virulent virus (14). Whether these effects are mediated by specific or nonspecific immune mechanisms remains to be determined.
Our results raise wider questions about the role of antibodies in natural and vaccinal immunity to lentiviruses. Hitherto, our assumption has been that vaccines should stimulate the broadest possible range of effector mechanisms, and our previous studies with whole inactivated FIV vaccines demonstrated that neutralizing antibodies correlated with protection (16). However, previous studies have described enhancement of FIV infection following immunization with prospective vaccines, including subunit vaccines and a DNA vaccine expressing a limited range of FIV gene products (20, 31, 40). Further studies are necessary to account for the different outcomes of these vaccine experiments compared to the present study and to allow extrapolation of these findings to other immunosuppressive lentiviruses such as HIV in human beings.
ACKNOWLEDGMENTS
This work was supported by the U.K. Medical Research Council and the EC Concerted Action, FAVEUR. T. Miyazawa received a fellowship from the Japanese Society for the Promotion of Science.
We are grateful to P. Johnson, NIAID, NIH, for kindly providing the F14 molecular clone; N. Pedersen, University of California at Davis, for kindly providing monoclonal antibody 43/1B9; and J. Norrie, Robertson Centre for Biostatistics, University of Glasgow, for the statistical analyses. J. Cole, R. Irvine, and V. Dale are also thanked for their assistance.
REFERENCES
- 1.Argyle D J, Smith K, McBride K, Fulton R, Onions D E. Nucleotide and predicted peptide sequence of feline interferon-gamma (IFN-gamma) DNA Sequence. 1995;5:169–171. doi: 10.3109/10425179509029357. [DOI] [PubMed] [Google Scholar]
- 2.Avrameas A, Strosberg A D, Moraillon A, Sonigo P, Pancino G. Serological diagnosis of feline immunodeficiency virus (FIV) infection based on synthetic peptides from Env glycoproteins. Res Virol. 1993;144:209–218. doi: 10.1016/s0923-2516(06)80031-2. [DOI] [PubMed] [Google Scholar]
- 3.Baba T W, Jeong Y S, Penninck D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 4.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 5.Crandell R A, Fabricant C G, Nelson-Rees W A. Development, characterisation and virus susceptibility of a feline (Felis catus) renal cell line (CRFK) In Vitro. 1973;9:176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- 6.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 7.Diehl L J, Mathiason-DuBard C K, O’Neil L L, Obert L, Hoover E A. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J Virol. 1995;69:6149–6157. doi: 10.1128/jvi.69.10.6149-6157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauci A S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 9.Flynn J N, Beatty J A, Cannon C A, Stephens E B, Hosie M J, Neil J C, Jarrett O. Involvement of gag- and env- specific cytotoxic T lymphocytes in protective immunity to feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1995;11:1107–1113. doi: 10.1089/aid.1995.11.1107. [DOI] [PubMed] [Google Scholar]
- 10.Flynn J N, Cannon C A, Reid G, Rigby M A, Neil J C, Jarrett O. Induction of feline immunodeficiency virus-specific cell-mediated and humoral immune responses following immunization with a multiple antigenic peptide from the envelope V3 domain. Immunology. 1995;85:171–175. [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn J N, Keating P, Hosie M J, Mackett M, Stephens E B, Beatty J A, Neil J C, Jarrett O. Env-specific CTL predominate in cats protected from FIV infection by vaccination. J Immunol. 1996;157:3658–3665. [PubMed] [Google Scholar]
- 12.Fontenot J D, Hoover E A, Elder J H, Montelaro R C. Evaluation of feline immunodeficiency virus and feline leukaemia virus transmembrane peptides for serological diagnosis. J Clin Microbiol. 1992;30:1885–1890. doi: 10.1128/jcm.30.7.1885-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdts V, Jons A, Makoschey B, Visser N, Mettenleiter T C. Protection of pigs against Aujeszky’s disease by DNA vaccination. J Gen Virol. 1997;78:2139–2146. doi: 10.1099/0022-1317-78-9-2139. [DOI] [PubMed] [Google Scholar]
- 14.Giavedoni L, Ahmad S, Jones L, Yilma T. Expression of gamma-interferon by simian immunodeficiency virus increases attenuation and reduces postchallenge virus load in vaccinated rhesus macaques. J Virol. 1997;71:866–872. doi: 10.1128/jvi.71.2.866-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holyoake T L. Cytokines at the research-clinical interface—potential applications. Blood Rev. 1996;10:189–200. doi: 10.1016/s0268-960x(96)90026-0. [DOI] [PubMed] [Google Scholar]
- 16.Hosie M, Osborne R, Yamamoto J K, Neil J C, Jarrett O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J Virol. 1995;69:1253–1255. doi: 10.1128/jvi.69.2.1253-1255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosie M J, Dunsford T H, de Ronde A, Willett B J, Cannon C A, Neil J C, Jarrett O. Suppression of virus burden by immunisation with feline immunodeficiency virus Env protein. Vaccine. 1996;14:405–411. doi: 10.1016/0264-410x(95)00193-5. [DOI] [PubMed] [Google Scholar]
- 18.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosie M J, Jarrett O. Serological responses of cats to feline immunodeficiency virus. AIDS. 1990;4:215–220. doi: 10.1097/00002030-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Hosie M J, Osborne R, Reid G, Neil J C, Jarrett O. Enhancement after feline immunodeficiency virus vaccination. Vet Immunol Immunopathol. 1992;35:191–198. doi: 10.1016/0165-2427(93)90149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosie, M. J., B. J. Willett, T. H. Dunsford, C. A. Robertson, C. A. Cannon, J. C. Neil, and O. Jarrett. Unpublished data.
- 22.Israel Z R, Edmonson P F, Maul D H, O’Neil S P, Mossman S P, Thiriart C, Fabry L, Van Opstal O, Bruck C, Bex F, Burny A, Fultz P N, Mullins J I, Hoover E A. Incomplete protection, but suppression of virus burden, elicited by subunit simian immunodeficiency virus vaccines. J Virol. 1994;68:1843–1853. doi: 10.1128/jvi.68.3.1843-1853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatissian E, Monceaux V, Cumont M C, Montagnier L, Hurtrel B, Chakrabarti L. Interferon-gamma expression in macaque lymph nodes during primary infection with simian immunodeficiency virus. Cytokine. 1996;8:844–852. doi: 10.1006/cyto.1996.0113. [DOI] [PubMed] [Google Scholar]
- 24.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus-DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu S, Santoro J C, Fuller D H, Haynes J R, Robinson H L. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody-responses in mice. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 26.McCullagh P, Nelder J A. Generalized linear models. London, United Kingdom: Chapman & Hall; 1989. pp. 11–12. [Google Scholar]
- 27.Miyazawa T M, Furuya T, Itagaki S, Tohya Y, Takahashi E, Mikami T. Establishment of a feline T-lymphoblastoid cell line highly sensitive for replication of feline immunodeficiency virus. Arch Virol. 1989;108:131–135. doi: 10.1007/BF01313750. [DOI] [PubMed] [Google Scholar]
- 28.Olmsted R A, Hirsch V M, Purcell R H, Johnson P R. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci USA. 1989;86:8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne R, Rigby M, Siebelink K, Neil J C, Jarrett O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J Gen Virol. 1994;75:3641–3645. doi: 10.1099/0022-1317-75-12-3641. [DOI] [PubMed] [Google Scholar]
- 30.Pancino G, Chappey C, Saurin W, Sonigo P. B epitopes and selection pressures in feline immunodeficiency virus envelope glycoproteins. J Virol. 1993;67:664–672. doi: 10.1128/jvi.67.2.664-672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson J, Moraillon A, Baud S, Cuisinier A-M, Sonigo P, Pancino G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J Virol. 1997;71:9640–9649. doi: 10.1128/jvi.71.12.9640-9649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigby M A, Hosie M J, Willett B J, Mackay N, McDonald M, Cannon C, Dunsford T, Jarrett O, Neil J C. Comparative efficiency of feline immunodeficiency virus infection by DNA inoculation. AIDS Res Hum Retroviruses. 1997;13:405–412. doi: 10.1089/aid.1997.13.405. [DOI] [PubMed] [Google Scholar]
- 33.Robinson H L, Hunt L A, Webster R G. Protection against a lethal influenza virus challenge by immunization with a hemagglutinin-expressing plasmid DNA. Vaccine. 1993;11:957–960. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- 34.Robinson H L, Lu S, Mustafa F, Johnson E, Santoro J C, Arthos J, Winsink J, Mullins J I, Montefiori D, Yasutomi Y, Letvin N L, Haynes J R, Manson K, Wyand M. Simian immunodeficiency virus-DNA vaccine trial in macaques. Ann NY Acad Sci. 1995;772:209–211. doi: 10.1111/j.1749-6632.1995.tb44746.x. [DOI] [PubMed] [Google Scholar]
- 35.Rowland-Jones S L, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schulz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi M, Nakamura H, Sonoda K, Hamamda F, Hirai K. Protection of chickens from Newcastle disease by vaccination with a linear plasmid DNA expressing the F protein of Newcastle disease virus. Vaccine. 1996;14:747–752. doi: 10.1016/0264-410x(95)00254-x. [DOI] [PubMed] [Google Scholar]
- 37.Shearer G M, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 38.Shiver J W, Davies M E, Perry H C, Freed D C, Liu M A. Humoral and cellular immunities elicited by HIV-1 DNA vaccination. J Pharm Sci. 1996;85:1317–1324. doi: 10.1021/js9600991. [DOI] [PubMed] [Google Scholar]
- 39.Sibille P, Avrameas A, Moraillon A, Richardson J, Sonigo P, Pancino G, Strosberg A D. Comparison of serological tests for the diagnosis of feline immunodeficiency virus infection of cats. Vet Microbiol. 1995;45:259–267. doi: 10.1016/0378-1135(94)00128-j. [DOI] [PubMed] [Google Scholar]
- 40.Siebelink K H J, Tijhaar E, Huisman R C, Huisman W, de Ronde A, Darby I H, Francis M J, Rimmelzwaan G F, Osterhaus A D M E. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J Virol. 1995;69:3704–3711. doi: 10.1128/jvi.69.6.3704-3711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens E B, Butfiloski E J, Monck E. Analysis of the amino terminal presequence of the feline immunodeficiency virus glycoprotein: effect of deletions on the intracellular transport of gp95. Virology. 1992;190:569–578. doi: 10.1016/0042-6822(92)90894-u. [DOI] [PubMed] [Google Scholar]
- 42.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Boyer J, Srikantan V, Coney L, Carrano R, Phan C, Merva M, Dang K, Agadjanan M, Gilbert L, Ugen K E, Williams W V, Weiner D B. DNA inoculation induces neutralizing immune responses against human immunodeficiency virus type-1 in mice and non-human primates. DNA Cell Biol. 1993;12:799–805. doi: 10.1089/dna.1993.12.799. [DOI] [PubMed] [Google Scholar]
- 44.Willett B, Hosie M J, Dunsford T, Neil J C, Jarrett O. Productive infection of helper T lymphocytes with FIV is accompanied by reduced expression of CD4. AIDS. 1991;5:1469–1475. doi: 10.1097/00002030-199112000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 46.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto J K, Ackley C D, Zochlinski H, Louie H, Pembroke E, Torten M, Hansen H, Munn R, Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency viruses: importance for diagnostic reagents and vaccines. Intervirology. 1991;32:361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto J K, Okuda T, Ackley C D, Louie H, Pembroke E, Zochlinski H, Munn R J, Gardner M B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991;7:911–921. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- 49.Yasutomi Y, Robinson H L, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins J I, Voss G, Manson K, Wyand M, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]