Abstract
Background
Stereotactic radiosurgery (SRS) is used to treat recurrent or residual nonfunctioning pituitary neuroendocrine tumors (NFPA). The objective of the study was to assess imaging and development of new pituitary hormone deficiency.
Methods
Patients treated with single-session SRS for a NFPA were included in this retrospective, multicenter study. Tumor control and new pituitary dysfunction were evaluated using Cox analysis and Kaplan–Meier curves.
Results
A total of 869 patients (male 476 [54.8%], median age at SRS 52.5 years [Interquartile range (IQR): 18.9]) were treated using a median margin dose of 14Gy (IQR: 4) for a median tumor volume of 3.4 cc (IQR: 4.3). With a median radiological follow-up of 3.7 years (IQR: 4.8), volumetric tumor reduction occurred in 451 patients (51.9%), stability in 364 (41.9%) and 54 patients (6.2%) showed tumor progression.
The probability of tumor control was 95.5% (95% Confidence Interval [CI]: 93.8–97.3) and 88.8% (95%CI: 85.2–92.5) at 5 and 10 years, respectively. A margin dose >14 Gy was associated with tumor control (Hazard Ratio [HR]:0.33, 95% CI: 0.18–0.60, P < 0.001). The probability of new hypopituitarism was 9.9% (95% CI: 7.3–12.5) and 15.3% (95% CI: 11–19.4) at 5 and 10 years, respectively. A maximum point dose >10 Gy in the pituitary stalk was associated with new pituitary hormone deficiency (HR: 3.47, 95% CI: 1.95–6.19). The cumulative probability of new cortisol, thyroid, gonadotroph, and growth hormone deficiency was 8% (95% CI: 3.9–11.9), 8.3% (95% CI: 3.9–12.5), 3.5% (95% CI: 1.7–5.2), and 4.7% (95% CI: 1.9–7.4), respectively at 10 years.
Conclusions
SRS provides long-term tumor control with a 15.3% risk of hypopituitarism at 10 years.
Keywords: hypopituitarism, nonfunctioning, pituitary adenoma, pituitary neuroendocrine tumors, stereotactic radiosurgery
Key Points.
Tumor control for NFPA was 95.5% at 5 years and 88.8% at 10 years.
The probability of new hypopituitarism was 9.9% at 5 years and 15.3% and 10 years.
Dose >14Gy leads to better local control. Maximum dose <10Gy to the pituitary stalk reduces the risk of new hypopituitarism.
Importance of the Study.
Recurrent or residual nonfunctioning pituitary neuroendocrine tumors are a difficult entity to manage. Stereotactic radiosurgery seems a reasonable alternative to new open surgery. However, long-term outcomes evaluation and refinement in techniques are required to improve the quality of care. This study presented, to the best of our knowledge, is the largest multicentric cohort (869 patients) evaluating outcomes after radiosurgery for nonfunctioning pituitary neuroendocrine tumors. Our finding demonstrates a good probability of tumor control at 10 years with a reasonable risk of new pituitary hormone deficiency. It is the first time that new cortisol, thyroid, gonadotroph, and growth hormone deficiencies were individually described to provide specific advice to patients. Risk factors of tumor control and new hormonal deficiency were studied. Treatment with a dose of more than 14Gy to the tumor and a dose to the stalk of less than 10 Gy demonstrated improved outcomes.
Pituitary neuroendocrine tumors, also known as pituitary adenomas, occur with an incidence between 3.9 and 7.4 cases per 100 000 per year,1 nonfunctioning pituitary adenomas (NFPA) represent 15% to 30% of them.1 Stereotactic radiosurgery (SRS) is used to treat residual and recurrent tumors or as primary treatment in carefully selected patients.2,3
Hypopituitarism is the most common complication of SRS, ranging between 23% and 50%4–10 and with increasing rates over time: 7.8%, 16.2%, 22.4%, 27.5%, and 31.3% at 1, 3, 5, 7, and 10 years, respectively.4 Probability rates of individual pituitary axis deficiencies are either not reported or their predicted rates are limited by the small number of patients included in the studies.11–13 To our knowledge, this is the largest series of NFPA treated with radiosurgery.3
The aim of this study is to evaluate both tumor control and complications of new pituitary hormone deficiency after radiosurgery for NFPA.
Material and Methods
Twelve centers participated in this retrospective study and contributed clinical and radiological data on 869 patients treated with SRS, between 1992 and 2022, for a residual NFPA. Each participating center was responsible for data collection and IRB approval. Patient consent was not required because this is a retrospective study. A deidentified database was shared and checked for inconsistencies. Each center was asked to re-evaluate and correct its database if missing or inconsistent values were found. This study follows the STROBE (Strengthening the reporting of observational studies in epidemiology) criteria.
Study Inclusion Criteria
Each institutional radiosurgical database was queried for patients treated for a pituitary adenoma. Patients were included in the study if they fulfilled all of the following criteria: (1) had histologic confirmation of a pituitary adenoma, (2) had no evidence of a secreting tumor, (3) were treated in a single session, (4) had no prior irradiation to the sellar region, and (5) had at least 1 clinical and radiographic follow-up study.
Radiosurgical Technique
Radiosurgery was performed with the technology available at each center as described previously.14 SRS was performed in all centers using the Gamma Knife (Elekta AB). The stereotactic frame was placed under local anesthesia, with or without intravenous conscious sedation, and a high-resolution, stereotactic magnetic resonance imaging, or computed tomography scan was acquired. The local multidisciplinary team approved the treatment plan.
Clinical and Radiological Follow-up
Radiological and clinical follow-up was performed according to the local policy.
Tumor size was documented on serial neuroimaging at each treatment center. Tumor progression was defined as a volumetric increase of ≥20% from baseline, and regression as a volumetric decrease of ≥20%. Lesions with volumetric changes of less than <20% were considered stable.9,15 Tumor volumetric analysis was performed and reviewed at each participating center.
Pre- and post-SRS clinical endocrine evaluation was performed at each center to confirm the nonsecreting nature of the tumor and to assess pituitary function. Endocrine assessment included measurement of adrenocorticotropic hormone (ACTH) and serum cortisol, thyroid-stimulating hormone (TSH) and free thyroxine (T4), follicle-stimulating hormone (FSH) and testosterone or estradiol, insulin-like growth factor–1 (IGF-1), growth hormone (GH), prolactin. Clinical evaluation included assessment of symptoms of adrenal insufficiency, hypothyroidism, diabetes insipidus (DI), and hypogonadism.4,14
New-onset hypopituitarism was defined as dysfunction of at least 1 pituitary axis, documented either with a decrease in a hormone level below the limit of normal or a new requirement for hormone replacement by the endocrinologist. The occurrence of a new visual field or cranial nerve deficit was also recorded.
Statistical Analysis
Data were analyzed using R language (R foundation of Statistical computing).16 Missing data were not imputed. Continuous variables are presented as the median and interquartile range (IQR); normality was assessed by graphical representation and the Shapiro test.
A P value <0.05 was considered statistically significant. Cox regression was used to assess predictive factors for tumor control (no growth) and new pituitary hormone deficiency. Continuous variables were dichotomized using the Youden Index. Significant and relevant factors with a P value <0.20 were included in the multivariable analysis. The Cox model assumptions were verified using Schoenfeld residuals for the proportional hazard assumption and Martingale residuals for the linearity assumption.
Kaplan–Meier curves were plotted for the probability of tumor control and new onset hypopituitarism.
Results
Demographics and SRS Characteristics
The study included 869 patients (men: 476 [54.8%]). The median age at SRS was 52.5 years (IQR: 18.9). The median time interval between the last surgical resection and SRS was 1.2 years (IQR: 3.2). Indication for SRS was residual growth in 403 patients (46.5%), adjuvant treatment in 307 patients (35.4%), tumor recurrence in 139 patients (16.0%), and patient preference in 18 (2.1%). In 2 cases, the reason was unknown (Table 1).
Table 1.
Description of the Cohort in Before SRS
| Parameters | Number (%) |
|---|---|
| Median (IQR) age at SRS, y | 52.5 (18.9) |
| Gender | |
| Male | 476 (54.8%) |
| Female | 393 (45.2%) |
| Number of surgical resections pre-SRS, median | |
| One surgical resection | 558 (64.2%) |
| Two surgical resections | 226 (26.0%) |
| Three or more surgical resections | 85 (9.7%) |
| Median (IQR) time between resection and SRS, y | 1.2 (3.2) |
| Pre-SRS visual field deficit | 381 (44.0%) |
| Pre-SRS cranial nerve deficit (III–IV–VI) | 34 (3.9%) |
| Pre-SRS cranial nerve deficit (V) | 5 (0.6%) |
| Pre-SRS hormone deficiency (unknown 6) | |
| None | 464 (53.8%) |
| One hormone | 180 (20.9%) |
| Two hormones | 97 (11.2%) |
| Three hormones | 75 (8.7%) |
| Four hormones | 21 (2.4%) |
| Five hormones | 26 (3.0%) |
| ACTH deficiency | 226 (26.2%) |
| TSH deficiency | 270 (31.3%) |
| Gonadotropin deficiency | 205 (23.8%) |
| IGF-1 deficiency | 56 (6.5%) |
| Diabetes insipidus | 56 (6.5%) |
| Histologya | |
| ACTH | 55/566 (9.7%) |
| GH | 20/565 (3.5%) |
| TSH | 13/555 (2.3%) |
| LH | 79/552 (14.3%) |
| FSH | 104/555 (18.7%) |
| Prolactin | 31/567 (5.5%) |
| PIT 1 | 6/75 (8%) |
| TPIT | 1/69 (1.4%) |
| SF1 | 18/84 (21.4%) |
Notes: ACTH = Adrenocorticotropic Hormone; FSH = follicle-stimulating hormone; GH = Growth hormone; IQR = Interquartile range; LH = Luteinizing hormone; PIT1 = Pituitary-specific positive transcription factor 1; SF-1 = Steroidogenic factor 1; SRS = Stereotactic radiosurgery; TPIT = T-box transcription factor; TSH = Thyroid-stimulating hormone.
aThe histological analysis was not fully standardized among centers and have evolved over the study period. Additionally, in some cases the precise histological report was not available, as patients were referred to tertiary centres for radiosurgery. We provide in the table the number of patients with positive result among the total number for which data were available and the corresponding percentages for more clarity.
The residual treated involved the sella in 547 (62.9%) patients, the cavernous sinus in 560 (64.4%), the suprasellar region in 206 (23.7%), the clivus in 9 (1.0%), the sphenoid sinus in 4 (0.5%), and the middle cranial fossa in 1 (0.1%) patient. The median tumor volume was 3.4 cm3 (IQR: 4.3) administering a median margin dose of 14Gy (IQR: 4, Table 2).
Table 2.
SRS procedure Characteristics
| Parameters | Number (%) |
|---|---|
| Location of treated lesion | |
| Sella | 547 (62.9%) |
| Cavernous sinus | 560 (64.4%) |
| Suprasellar | 201 (23.1%) |
| Other | 26 (3.0%) |
| Median (IQR) margin dose, Gy | 14.0 (4.0) |
| Median (IQR) number of isocenters | 11.0 (8.0) |
| Median (IQR) prescription isodose line, % | 50.0 (0.0) |
| Median (IQR) tumor volume, cm3 | 3.4 (4.3) |
| Median (IQR) optic apparatus maximum point dose, Gy | 7.5 (2.7) |
| Median (IQR) pituitary stalk maximum point dose, Gy | 9.0 (6.5) |
Note: IQR = Interquartile range; SRS = Stereotactic radiosurgery.
Tumor Control
At the last median radiological follow-up of 3.7 years (IQR: 4.8), the tumor decreased in size in 451 patients (51.9%), was stable in 364 (41.9%), and increased in size in 54 (6.2%, Table 3).
Table 3.
Post-SRS Characteristics
| Parameters | Number (%) |
|---|---|
| Median (IQR) clinical follow-up, y | 3.6 (5.0) |
| Median (IQR) radiological follow-up, y | 3.7 (4.8) |
| Median (IQR) time from SRS to first hormone deficiency, y | 2.2 (3.5) |
| New hormone deficiency post-SRS | 73 (8.7%) |
| At 5 y | 9.9% (95% CI: 7.3–12.5) |
| At 10 y | 15.3% (95% CI: 11–19.4) |
| ACTH deficiency | |
| At 5 y | 5.5% (95% CI: 3.3–7.7) |
| At 10 y | 8% (95% CI:3.9–11.9) |
| TSH deficiency | |
| At 5 y | 4.6% (95% CI: 2.5–6.7) |
| At 10 y | 8.3% (95% CI: 3.9–12.5) |
| Gonadotropin deficiency | |
| At 5 y | 2.9% (95% CI: 1.5–4.4) |
| At 10 y | 3.5% (95% CI: 1.7–5.2) |
| Growth hormone (IGF-1) deficiency | |
| At 5 y | 2.8% (95% CI: 1.2–4.4) |
| At 10 y | 4.7% (95% CI: 1.9–7.4) |
| New visual field defect | 12 (1.4%) |
| Other cranial nerve deficit | 6 (0.7%) |
| Tumor response post-SRS | |
| Stable | 364 (41.9%) |
| Decreased | 451 (51.9%) |
| Increased | 54 (6.2%) |
Note: ACTH = Adrenocorticotropic hormone; IGF-1 = Insulin growth factor 1; SRS = Stereotactic radiosurgery; TSH = Thyroid stimulating hormone.
The probability of tumor control was 97.7% (95% Confidence Interval [CI]: 96.6–98.9), 95.5% (95% CI: 93.8–97.3), and 88.8% (95% CI: 85.2–92.5) at 3, 5, and 10 years, respectively (Figure 1A).
Figure 1.
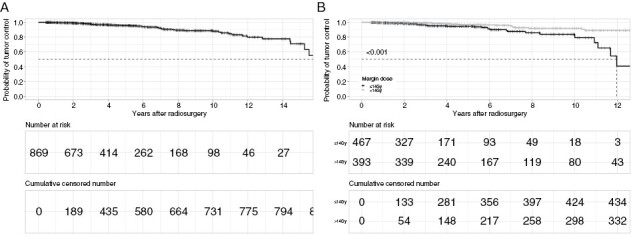
Kaplan–Meier curves demonstrating tumor control following SRS treatment in the entire cohort (A) and in function of margin dose (B). The probability of tumor control was 95.5% and 88.8% at 5 and 10 y, respectively. Tumors treated with prescription doses >14Gy exhibited higher long-term tumor control (P < 0.001).
Forty-seven patients required a new treatment after SRS. Twenty-nine underwent repeat SRS, 21 repeat surgical resection, 5 conventional or proton beam radiotherapy, and 2 medical therapy. The median time from SRS to a new treatment was 6 years (IQR: 6).
Using Cox analysis, a margin dose >14Gy was associated with a reduction in the risk of tumor progression (Hazard Ratio [HR]: 0.33, 95% CI: 0.18–0.60, P < 0.001; Supplementary Table 1, Figure 1B).
Hypopituitarism
The pre-SRS hormone function was available for 863 patients. The pituitary function was unaffected in 53.8% (464/863) pre-SRS. The most common hormone deficits were: thyroid deficiency in 31.3% (270/863) patients, cortisol deficiency in 26.2% (226/863), gonadotrophin deficiency in 23.8% (205/863), growth hormone deficiency in 6.5% (56/863), and diabetes insipidus in 6.5% (56/863).
After excluding patients with panhypopituitarism (n = 26), the post-SRS pituitary function was evaluated in 837 patients with at least 1 pituitary hormone still at risk (Supplementary Figure 1). At a median follow-up of 3.5 years (IQR: 5.1), 73 patients (8.7%) developed a new hormone deficiency with a median latency from SRS of 2.2 years (IQR: 3.5). Most of the patients developed a deficit of a single pituitary hormone (53/837 [6.3%]), followed by 2 (16/837 [1.9%]), and 3 hormones (4/837 [0.5%]).
The cumulative probability of new hypopituitarism was 6.5% (95% CI: 4.6–8.4), 9.9% (95%CI: 7.3–12.5), and 15.3% (95% CI: 11–19.4) at 3, 5, and 10 years, respectively (Figure 2).
Figure 2.
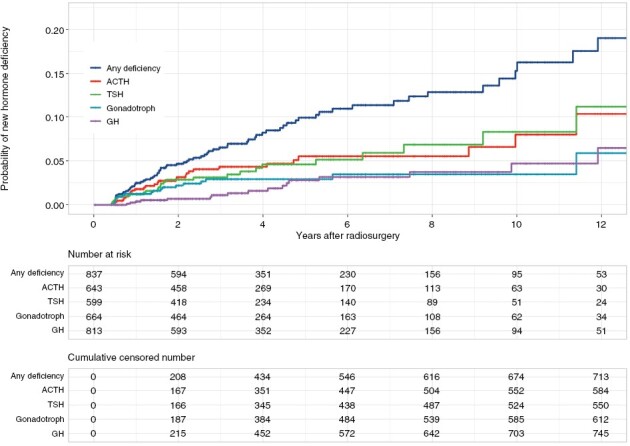
Probability of new hormonal deficit by hormonal subtype. The cumulative probability of gonadotroph (3.5%) and growth hormone (4.7%) deficiency at 10-y was lower than that of ACTH (8%) and TSH (8.3%).
A maximum point dose >10Gy in the pituitary stalk was associated with the occurrence of a new hormone deficiency in multivariate Cox analysis (HR: 3.53, 95% CI: 1.98–6.29, P < 0.001; Supplementary Table 2, Figure 3).
Figure 3.
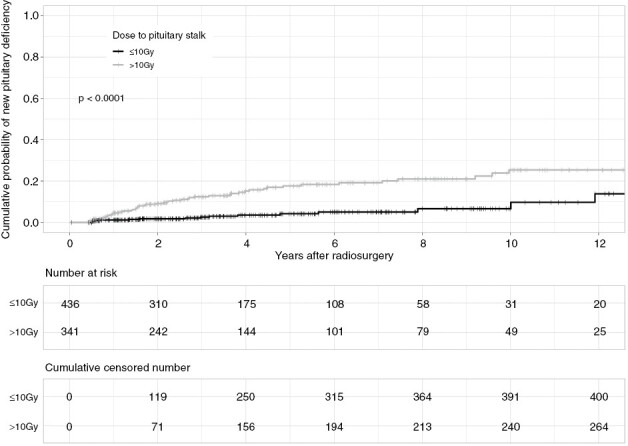
Cumulative probability of new hypopituitarism in function of the median point dose to the stalk. Maximum point doses ≥10Gy on the pituitary stalk correlated with higher probability of new pituitary dysfunction (P < 0.001)
Among patients without dysfunction of the hypothalamic–pituitary–adrenal axis prior to SRS (n = 643), a new deficit was reported in 29 (4.5%) with a median time of 1.5 years (IQR:1.5) after SRS. The cumulative probability was 4.3% (95% CI: 2.6–6.1), 5.5% (95% CI: 3.3–7.7), and 8% (95% CI: 3.9–11.9) at 3, 5, and 10 years, respectively (Supplementary Figure 1).
Of the 599 patients without hypothalamic–pituitary–thyroid dysfunction prior to SRS, 25 (4.2%) patients demonstrated a new hormone deficiency; the median time to new-onset dysfunction was 1.6 years (IQR: 3.0). The cumulative probability of a new deficit was 3.1% (95% CI: 1.6–4.6), 4.6% (95% CI: 2.5–6.7), and 8.3% (95% CI: 3.9–12.5) at 3, 5, and 10 years, respectively.
Among patients without gonadotroph dysfunction prior to SRS (n = 664), a new deficit was reported in 20 (3.0%) with a median delay of 1.5 years (IQR: 2.0). The cumulative probability was 2.9% (95% CI: 1.5–4.4) at 3 and 5 years and 3.5% (95% CI: 1.7–5.2) at 10 years.
At SRS, 813 patients were at risk for new growth hormone deficiency; new post-SRS deficiency was reported in 17 patients (2.1%) with a delay of 3.7 years (IQR: 2.9). The cumulative probability was 1.1% (95% CI: 0.3–1.9), 2.8% (95% CI: 1.2–4.4), and 4.7% (95% CI: 1.9–7.4) at 3, 5, and 10 years, respectively (Figure 2).
Among patients with intact vasopressin production prior to SRS (n = 813), new diabetes insipidus developed in 5 (0.6%) of the patients with a delay of 3.2 years (IQR: 1.0) from SRS.
Clinical Outcomes
Prior to SRS, 381 (44.0%) patients had a visual field defect, 16 (1.8%) patients had an oculomotor nerve palsy, 2 (0.2%) patients had a trochlear nerve palsy, and 12 (1.4%) abducens nerve palsy alone. One (0.1%) patient had a palsy of the third and fourth cranial nerve, 1 (0.1%) of the third and sixth cranial nerve, and 1 (0.1%) of the third, fourth, and sixth cranial nerves. One (0.1%) patient had diplopia, but the cranial nerves affected were unknown. In 5(0.6%) patients, the fifth cranial nerve was affected.
After SRS, 2 (0.2%) patients developed a third, 2 (0.2%) a sixth, 1 (0.1%) a third and a sixth, and 1 (0.1%) a fifth cranial nerve palsy. In 3 of the cases, the new deficit was associated with tumor progression. A new or worsening of a visual field defect occurred in 12 (1.4%).
Fourteen (1.6%) patients died during follow-up. The reason was unrelated to the NFPA in 8 cases and unknown in the remaining 6.
Discussion
Tumor Control
In this study, 869 patients were treated with single-session SRS for residual or recurrent NFPA. At a median radiological follow-up of 3.7 years, progression occurred in 54 (6.2%) cases. The probability of tumor control was 97.7%, 95.5%, and 88.8% at 3, 5, and 10 years, respectively. Owing to the large number of patients and the long-term follow-up (334 patients with a follow-up of more than 5 years and 97 of more than 10 years), these results are notable for evaluation of the long-term efficacy of SRS. These long-term tumor control rates are in accordance with previous reports.12,13,17–19 However, bias might have been introduced, as the analysis of tumor volume was performed locally and not centrally using a blind evaluation method. Using multivariable analysis, the only factor found to reduce the risk of tumor progression is a higher margin dose (HR: 0.33, 95% CI: 0.18–0.60, P < 0.001). Tumor volume and parasellar invasion were not significantly associated with tumor progression, contrary to prior studies.13,19 The inclusion of pituitary adenomas with a relatively limited volume range (3.4 cm3 [IQR: 4.3]) may be an explanation for these differences.
Hypopituitarism
The probability of developing a new pituitary hormone deficiency was 6.5%, 9.9%, and 15.3% at 3, 5, and 10 years, respectively. This rate is lower than described in the literature ranged between 25.3% and 30% at 5 years for NFPA.13,18,20 In 2 recent meta-analyses by Kotecha et al. and Albano et al., the pooled estimate of post-SRS hypopituitarism was 21% and 18%, respectively.3,21
The 10-year cumulative probability of new specific hormone deficiency was 8% for cortisol, 8.3% for thyroid, 3.5% for gonadotroph, and 4.7% for growth hormone. This is the first time that the risk of new deficiency was evaluated by hormone subtype, providing a better prospect for each patient of the risk for developing another specific deficit. Animal model studies and radiosurgical series of functioning pituitary adenomas have suggested a higher sensitivity to radiation for growth hormone secretion compared with ACTH.10,22 The lower rate of pituitary deficiency found in our study could be due to the higher number of patients and the inclusion of solely nonfunctioning pituitary adenoma patients compared to more heterogenous and smaller series; this should provide a better estimate of long-term hypopituitarism rates. However, it is possible that the new hormonal deficit underreporting could have influenced our results. The lower rate of gonadotroph and growth hormone deficiency observed in the current study could be explained by a lack of systematic testing and replacement for these hormones in the clinical setting, rather than a true difference in radiosensitivity. This is part of the limitations of multicentric studies with local endocrinology evaluation using different laboratory assays with variable reference ranges over time. In the same way, we did not ask for a specific hormone follow-up as the endocrine testing is relatively consensual on which hormone should be tested. So instead of having a specific hormone follow-up, we have a global hormone follow-up which was used for the statistical analysis. This could lead to uncertainty in the rate of new endocrinopathies estimation.
The multicentric design allows a more generalizable study but outcomes (endocrine and tumoral follow-up) can be affected by differences in local habits, patients’ selection, and length of follow-up. The direction of these differences in the results cannot be defined. A maximum point dose >10 Gy in the pituitary stalk was associated with the occurrence of new hormone deficiency (HR: 3.53, 95% CI: 1.98–6.29, P < 0.001). Some factors that show association with the development of hypopituitarism in other studies such as the total dose to the gland7,23 or distance from the center of the tumor6 were not evaluated in the current study.
Histology
NFPAs encompass a wide spectrum of immunohistological subtypes.24 Specific subtypes, such as silent corticotroph adenomas, exhibit more aggressive natural history25,26 and recur more frequently after SRS.27 ACTH staining was only available in 566 patients in this study, precluding its inclusion as a potential factor in the Cox analysis. In the same way, Ki-67 was only available in 116 patients with a clear bias between centers. As such, this factor could not be included in the multivariable analysis and its impact on prognosis in radiosurgical outcomes could not be evaluated.
The 2021 World Health Organization classification introduced cell transcription factors, such as the pituitary-specific positive transcription factor 1 (PIT1), the T-box transcription factor (TPIT), and the steroidogenic factor 1 (SF-1), to better characterize pituitary adenomas. As our study involved patients treated from 1999 to 2021, these factors were only available for a small number of patients (75 for PIT1, 69 for TPIT, and 84 for SF-1). Silent PIT-1 lineage tumors have been suggested to exhibit more aggressive behavior.28 The exact implication of this tumor subtype in radiosurgery outcomes needs further evaluation.
Conclusions
SRS for NFPA affords long-term tumor control, with the probability exceeding 88% at 10 years. The cumulative probability of new pituitary dysfunction was 15.3% at 10 years. Treatment with a dose of more than 14Gy to the tumor and a dose to the stalk of less than 10 Gy demonstrated improved outcomes.
Supplementary Material
Acknowledgments
Dr. Dumot gratefully acknowledges receipt of a grant for mobility from the “Hospices civils de Lyon,” France, from the “Institut Servier,” France, from the “Societe française of Neurochirurgie (SFNC),” France, from the “Fondation Planiol,” France and from the “Phillip foundation.”
Contributor Information
Chloe Dumot, Department of Neurological Surgery, University of Virginia, Charlottesville, Virginia, USA; Department of Neurological Surgery, Hospices civils de Lyon, Lyon, France.
Georgios Mantziaris, Department of Neurological Surgery, University of Virginia, Charlottesville, Virginia, USA.
Sam Dayawansa, Department of Neurological Surgery, University of Virginia, Charlottesville, Virginia, USA.
Selcuk Peker, Department of Neurosurgery, Koc University School of Medicine, Istanbul, Turkey.
Yavuz Samanci, Department of Neurosurgery, Koc University School of Medicine, Istanbul, Turkey.
Ahmed M Nabeel, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt.
Wael A Reda, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Departments of Neurosurgery, Ain Shams University, Cairo, Egypt.
Sameh R Tawadros, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Departments of Neurosurgery, Ain Shams University, Cairo, Egypt.
Khaled Abdelkarim, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Departments of Clinical Oncology, Ain Shams University, Cairo, Egypt.
Amr M N El-Shehaby, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Departments of Neurosurgery, Ain Shams University, Cairo, Egypt.
Reem M Emad, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Department of Radiation Oncology, National Cancer Institute, Cairo University, Cairo, Egypt.
Ahmed Ragab Abdelsalam, Neurosurgery Department, Military Medical Academy, Cairo, Egypt.
Roman Liscak, Department of Stereotactic and Radiation Neurosurgery, Na Homolce Hospital, Prague, Czech Republic.
Jaromir May, Department of Stereotactic and Radiation Neurosurgery, Na Homolce Hospital, Prague, Czech Republic; Department of Neurosurgery, University of Miami, Miami, Florida, USA; Department of Radiation Oncology, James Cancer Hospital at The Ohio State University, Columbus, Ohio, USA.
Elad Mashiach, Department of Neurosurgery, NYU Langone, New York City, New York, USA.
Fernando De Nigris Vasconcellos, Department of Neurosurgery, NYU Langone, New York City, New York, USA.
Kenneth Bernstein, Department of Radiation Oncology, NYU Langone, New York City, New York, USA.
Douglas Kondziolka, Department of Neurosurgery, NYU Langone, New York City, New York, USA.
Herwin Speckter, Departments of Neurosurgery, Dominican Gamma Knife Center and Radiology Department, CEDIMAT, Santo Domingo, Dominican Republic.
Ruben Mota, Departments of Neurosurgery, Dominican Gamma Knife Center and Radiology Department, CEDIMAT, Santo Domingo, Dominican Republic.
Anderson Brito, Departments of Neurosurgery, Dominican Gamma Knife Center and Radiology Department, CEDIMAT, Santo Domingo, Dominican Republic.
Shray Kumar Bindal, Departments of Neurosurgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Ajay Niranjan, Departments of Neurosurgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Dade L Lunsford, Departments of Neurosurgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Carolina Gesteira Benjamin, Department of Neurosurgery, University of Miami, Miami, Florida, USA.
Timoteo Abrantes de Lacerda Almeida, Department of Radiation Oncology, University of Miami, Miami, Florida, USA.
Jennifer Mao, Department of Stereotactic and Radiation Neurosurgery, Na Homolce Hospital, Prague, Czech Republic; Department of Neurosurgery, University of Miami, Miami, Florida, USA; Department of Radiation Oncology, James Cancer Hospital at The Ohio State University, Columbus, Ohio, USA.
David Mathieu, Division of Neurosurgery, Université de Sherbrooke, Centre de recherche du CHUS, Sherbrooke, Quebec, Canada.
Jean-Nicolas Tourigny, Division of Neurosurgery, Université de Sherbrooke, Centre de recherche du CHUS, Sherbrooke, Quebec, Canada.
Manjul Tripathi, Departments of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Joshua David Palmer, Department of Radiation Oncology, James Cancer Hospital at The Ohio State University, Columbus, Ohio, USA.
Jennifer Matsui, Department of Stereotactic and Radiation Neurosurgery, Na Homolce Hospital, Prague, Czech Republic; Department of Neurosurgery, University of Miami, Miami, Florida, USA; Department of Radiation Oncology, James Cancer Hospital at The Ohio State University, Columbus, Ohio, USA.
Joe Crooks, College of Medecine, Drexel University, Philadelphia, Pennsylvania, USA.
Rodney E Wegner, Allegheny Health Network Cancer Institute, Allegheny Health Network, Pittsburgh, Pennsylvania, USA.
Matthew J Shepard, Department of Neurosurgery, Allegheny Health Network, Pittsburgh, Pennsylvania, USA.
Mary Lee Vance, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
Jason P Sheehan, Department of Neurological Surgery, University of Virginia, Charlottesville, Virginia, USA.
Conflict of Interest
D.L.L. is a stockholder in AB Elekta, Stockholm, Sweden. J.D.P. is part of Novocure advisory board and receives Varian speaking fees, Kroger trial funding, NIH R01CA269948, NIH R702 award, Biocept clinical trial funding, and Genentech clinical trial funding. The other authors report no conflicts of interest.
Funding
None declared.
Data Availability
Data are available upon reasonable request to the corresponding author.
Author Contributors
Conception and design: Sheehan, Dumot, Mantziaris, Vance. Acquisition of data: Dumot, Mantziaris, Dayawansa Peker, Samanci, Nabeel, Reda, Tawadros, Abdelkarim, El-Shehaby, Emad, Abdelsalam, Liscak, May, Mashiach, De Nigris Vasconcellos, Bernstein, Kondziolka, Speckter, Mota, Brito, Bindal, Niranjan, Lunsford, Benjamin, Abrantes de Lacerda Almeida, Mao, Mathieu, Tourigny, Tripathi, Palmer, Matsui, Crooks, Wegner, Shepard. Analysis and interpretation of data: Dumot, Mantziaris, Dayawansa. Drafting the article: Dumot, Mantziaris. Critically revising the article: Sheehan, Vance, Mantziaris, Dayawansa, Peker, Nabeel, Bernstein, Kondziolka, Speckter, Lunsford, Mathieu, Tourigny, Tripathi, Reviewed-submitted version of manuscript: all authors. Statistical analysis: Dumot, Mantziarias. Study supervision: Sheehan.
References
- 1. Daly AF, Beckers A.. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. 2020;49(3):347–355. [DOI] [PubMed] [Google Scholar]
- 2. Sheehan J, Lee CC, Bodach ME, et al. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E539–E540. [DOI] [PubMed] [Google Scholar]
- 3. Kotecha R, Sahgal A, Rubens M, et al. Stereotactic radiosurgery for non-functioning pituitary adenomas: meta-analysis and International Stereotactic Radiosurgery Society practice opinion. Neuro Oncol. 2020;22(3):318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cordeiro D, Xu Z, Mehta G, et al. Hypopituitarism after Gamma Knife radiosurgery for pituitary adenomas: a multicenter, international study. J Neurosurg. 2018;131(4):1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pomeraniec IJ, Xu Z, Lee CC, et al. Dose to neuroanatomical structures surrounding pituitary adenomas and the effect of stereotactic radiosurgery on neuroendocrine function: an international multicenter study. J Neurosurg. 2022;136(3):813–821. [DOI] [PubMed] [Google Scholar]
- 6. Ironside N, Snyder H, Xu Z, et al. Effect of distance from target on hypopituitarism after stereotactic radiosurgery for pituitary adenomas. J Neurooncol. 2022;158(1):41–50. [DOI] [PubMed] [Google Scholar]
- 7. Oh JW, Sung KS, Moon JH, et al. Hypopituitarism after Gamma Knife surgery for postoperative nonfunctioning pituitary adenoma. J Neurosurg. 2018;129(Suppl1):47–54. [DOI] [PubMed] [Google Scholar]
- 8. Leenstra JL, Tanaka S, Kline RW, et al. Factors associated with endocrine deficits after stereotactic radiosurgery of pituitary adenomas. Neurosurgery. 2010;67(1):27–32; discussion 32–33. [DOI] [PubMed] [Google Scholar]
- 9. Sheehan JP, Starke RM, Mathieu D, et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013;119(2):446–456. [DOI] [PubMed] [Google Scholar]
- 10. Cohen-Inbar O, Ramesh A, Xu Z, et al. Gamma knife radiosurgery in patients with persistent acromegaly or Cushing’s disease: long-term risk of hypopituitarism. Clin Endocrinol (Oxf). 2016;84(4):524–531. [DOI] [PubMed] [Google Scholar]
- 11. Lee CC, Kano H, Yang HC, et al. Initial gamma knife radiosurgery for nonfunctioning pituitary adenomas. J Neurosurg. 2014;120(3):647–654. [DOI] [PubMed] [Google Scholar]
- 12. Sun S, Liu A, Zhang Y.. Long-term follow-up studies of gamma knife radiosurgery for postsurgical nonfunctioning pituitary adenomas. World Neurosurg. 2019;124(19):e715–e723. [DOI] [PubMed] [Google Scholar]
- 13. Park KJ, Kano H, Parry PV, et al. Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery. 2011;69(6):1188–1199. [DOI] [PubMed] [Google Scholar]
- 14. Mehta GU, Ding D, Patibandla MR, et al. Stereotactic radiosurgery for Cushing disease: results of an international, multicenter study. J Clin Endocrinol Metab. 2017;102(11):4284–4291. [DOI] [PubMed] [Google Scholar]
- 15. Snell JW, Sheehan J, Stroila M, Steiner L.. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error Technical note. J Neurosurg. 2006;104(1):157–162. [DOI] [PubMed] [Google Scholar]
- 16. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- 17. Losa M, Spatola G, Albano L, et al. Frequency, pattern, and outcome of recurrences after gamma knife radiosurgery for pituitary adenomas. Endocrine. 2017;56(3):595–602. [DOI] [PubMed] [Google Scholar]
- 18. Deng Y, Li Y, Li X, et al. Long-term results of gamma knife radiosurgery for Postsurgical residual or recurrent nonfunctioning pituitary adenomas. Int J Med Sci. 2020;17(11):1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Wu L, Quan T, et al. Characteristic of tumor regrowth after gamma knife radiosurgery and outcomes of repeat gamma knife radiosurgery in nonfunctioning pituitary adenomas. Front Oncol. 2021;11(1):627428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu J, Li Y, Quan T, et al. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas: results from a 26-year experience. Endocrine. 2020;68(2):399–410. [DOI] [PubMed] [Google Scholar]
- 21. Albano L, Losa M, Barzaghi LR, et al. Gamma knife radiosurgery for pituitary tumors: a systematic review and meta-analysis. Cancers. 2021;13(19):4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson IC, Fairhall KM, Hendry JH, Shalet SM.. Differential radiosensitivity of hypothalamo-pituitary function in the young adult rat. J Endocrinol. 2001;169(3):519–526. [DOI] [PubMed] [Google Scholar]
- 23. Graffeo CS, Link MJ, Brown PD, Young WF, Pollock BE.. Hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis based on patients treated using contemporary techniques. Int J Radiat Oncol Biol Phys. 2018;101(3):618–623. [DOI] [PubMed] [Google Scholar]
- 24. Asa SL, Mete O, Perry A, Osamura RY.. Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol. 2022;33(1):6–26. [DOI] [PubMed] [Google Scholar]
- 25. Langlois F, Lim DST, Yedinak CG, et al. Predictors of silent corticotroph adenoma recurrence: a large retrospective single center study and systematic literature review. Pituitary. 2018;21(1):32–40. [DOI] [PubMed] [Google Scholar]
- 26. Strickland BA, Shahrestani S, Briggs RG, et al. Silent corticotroph pituitary adenomas: clinical characteristics, long-term outcomes, and management of disease recurrence. J Neurosurg. 2021;135(6):1706–1713. [DOI] [PubMed] [Google Scholar]
- 27. Cohen-Inbar O, Xu Z, Lee CC, et al. Prognostic significance of corticotroph staining in radiosurgery for non-functioning pituitary adenomas: a multicenter study. J Neurooncol. 2017;135(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mete O, Gomez-Hernandez K, Kucharczyk W, et al. Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod Pathol. 2016;29(2):131–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the corresponding author.


