Abstract
Rationale
A recent randomized controlled trial revealed that a multicomponent sepsis transition and recovery (STAR) program delivered through specialized nurse navigators was effective in reducing a composite of 30-day readmission and mortality. Better understanding of patterns of care provided by the STAR program is needed to promote implementation and dissemination of this effective program.
Objectives
This study characterizes individual care activities and distinct “packages” of care delivered by the STAR program.
Methods
We performed a secondary analysis of data from the intervention arm of the IMPACTS (Improving Morbidity during Post–Acute Care Transitions for Sepsis) randomized controlled trial, conducted at three urban hospitals in the southeastern United States from January 2019 to March 2020. We used a structured data collection process to identify STAR nurse navigator care activities from electronic health record documentation. We then used latent class analysis to identify groups of patients receiving distinct combinations of intervention components. We evaluated differences in patient characteristics and outcomes between groups receiving distinct intervention packages.
Results
The 317 sepsis survivors enrolled into the intervention arm of the IMPACTS trial received one or more of nine unique care activities delivered by STAR nurse navigators (care coordination, health promotion counseling, emotional listening, symptom management, medication management, chronic disease management, addressing social determinants of health, care setting advice and guidance, and primary palliative care). Patients received a median of three individual care activities (interquartile range, 2–5). Latent class analysis revealed four distinct packages of care activities delivered to patients with different observable characteristics and different frequency of 30-day readmission and mortality.
Conclusions
We identified nine care activities delivered by an effective STAR program and four distinct latent classes or packages of intervention delivery. These results can be leveraged to increase widespread implementation and provide targets to augment future program delivery.
Keywords: sepsis, septic shock, readmission, mortality, recovery
Sepsis is the leading cause of hospital mortality, unplanned hospital readmission, and readmission-related costs in the United States (1–5). Management of sepsis survivors is complex, as patients frequently face diverse combinations of complications from incomplete resolution of the primary infection, worsening control of existing chronic conditions, and persistent organ dysfunction (6, 7). These and other preventable conditions result in worsening patient morbidity, mortality, and hospital readmission, including a 90-day hospital readmission rate of 40% and more than $3 billion in potentially avoidable costs (3).
Multicomponent interventions are the most promising strategies for reducing hospital readmissions and other adverse outcomes after sepsis (8–10). Previously, we demonstrated that a novel sepsis transition and recovery (STAR) program delivered through specialized nurse navigators was effective in reducing a composite of 30-day readmission and mortality (11) and sustained improvement in readmission at 12 months (12). The program was intentionally designed as a multicomponent, complex healthcare intervention to provide personalized support to a broad range of patients with heterogeneous needs. However, better understanding of patterns of care provided by the STAR program is needed to promote implementation and dissemination of this effective program. In this study, we systematically characterized individual care activities delivered by the STAR program and used latent class analysis (LCA) to identify groups of patients receiving distinct combinations of intervention components. We hypothesized that STAR program care activities were delivered in distinct “packages” for patients with different characteristics and recovery needs.
Methods
We conducted a secondary analysis of IMPACTS (Improving Morbidity during Post–Acute Care Transitions for Sepsis), a pragmatic randomized controlled trial that enrolled 691 patients hospitalized for sepsis with high risk of postdischarge readmission or mortality between January 2019 and March 2020 (NCT 03865602). The protocol and primary trial findings have been reported elsewhere (11–13). Briefly, the STAR intervention leverages nurse navigators to promote care planning and self-management; proactive follow-up; and patient, provider, and community engagement during care transitions after sepsis. The STAR navigator provided sepsis education and recovery planning before hospital discharge and support at close, regular intervals through 30 days after discharge. Sepsis survivors randomly allocated to receive support from the STAR program had a statistically significant 5% lower frequency of 30-day readmission or mortality. For the present study, we examined care delivered to the subpopulation of patients who were randomized to receive STAR and were discharged alive without referral to hospice care.
Data Collection
The primary new data sources were unstructured data from electronic health records (EHRs) collected for patients randomized to the STAR program who met the inclusion criteria. We reviewed STAR nurse navigator EHR documentation to identify care activities delivered by the intervention. We developed and iteratively refined a coding system using the intervention standard operating practice documents and a preliminary review of navigator EHR notes. Trained physician chart abstractors used a structured data collection tool to characterize types of care delivered for each patient during the intervention. All records were double reviewed with discrepancies, which were minimal (<5% of cases), resolved by a formal reconciliation process leveraging team discussions to foster shared meaning and interpretation. Member checking with STAR nurse navigators was performed to discuss findings and to validate representativeness for credibility.
Indicators of STAR Program Activities
On the basis of STAR program design and initial document review, we defined nine care activities to be included in LCA model development. The individual-level care activity indicators were care coordination, health promotion counseling, emotional listening, symptom management, medication management, chronic disease management, addressing social determinants of health, care setting advice and guidance, and primary palliative care. These care indicators align with recent best-practice care delivery recommendations to enhance sepsis recovery (6). Each care activity was categorized as being present if delivered at any time during a patient’s STAR program participation, identified from the nurse navigator EHR documents.
Other Patient Characteristics
In addition to STAR program care activities abstracted from the EHR, we used data collected for the original clinical trial: routinely collected patient and clinical characteristics obtained directly from the health system’s enterprise data warehouse (EDW). Specifically, we included patient age, sex, race, and insurance variables, together with census tract–level area deprivation index values based on geocoding of the patient’s residential address in the EHR. In addition, we included medical history information (e.g., Charlson comorbidity index and its individual component conditions on the basis of International Classification of Diseases, 10th Revision, diagnosis codes present at the time of admission with a 12-month lookback, prior hospitalizations in the past 12 mo) and clinical data from the index sepsis hospitalization (e.g., site of infection on the basis of International Classification of Diseases, 10th Revision, diagnosis codes, medication administration, organ dysfunction on the basis of physiologic measurements [e.g., mean arterial pressure] and laboratory values [e.g., complete blood count, basic or comprehensive metabolic panel, lactate], surgical or other hospital procedure data), hospital length of stay, and discharge disposition. Finally, we used navigator documentation of time spent per encounter to calculate total navigator time spent providing support to each patient.
Outcomes
The primary clinical outcome of interest was the dichotomous composite endpoint of mortality and hospital readmission within 30 days of hospital discharge. We obtained deaths documented within 30 days of index hospital discharge, captured in the EDW and including events from national death record data uploaded monthly into the EDW via our institutional subscription. Hospital readmissions within 30 days of index hospital discharge were captured from healthcare use data in the EDW. All inpatient or observation status readmissions to any Atrium Health hospital were counted toward the readmission event definition. For analysis, patients who experienced either death or hospital readmission outcomes within 30 days of index hospital discharge were categorized as event positive.
Analysis
We used descriptive statistics to characterize the STAR program care activities overall and quantify patients receiving each care activity. We then applied LCA, a type of mixture modeling (14), to identify unobserved (latent) classes representing care activity packages. Applying an exploratory approach without prespecifying the hypothesized number of expected classes, we sequentially derived LCA models containing an increasing number of classes. Selection of the most appropriate latent class model was determined using a combination of the Akaike information criterion (AIC) and Bayesian information criterion (BIC) and their adjusted versions (consistent AIC and sample size–adjusted BIC [SABIC], respectively), probability of class assignment (class separation), class prevalence (favoring models with classes >10% of the sample population), and clinical interpretability (15). Lower values for the AIC, BIC, and their adjusted versions indicate a better balance of model fit and parsimony. Although there are commonly discrepancies between these values used to inform model selection decisions, several recent simulations studies suggest the SABIC has superior performance over other relative fit indices and may be prioritized when the number of parameters is large or the sample size is small (16). After identifying class membership using LCA, we evaluated characteristics of patients who received each package of care activities and fit multivariable logistic regression models to explore pairwise comparisons of the associations between class membership (i.e., STAR program packages) and clinical outcomes after controlling for age, comorbidity burden, and organ failure, as we have done in prior analyses of the primary trial. Pairwise comparisons were presented as odds ratios and 95% confidence intervals. LCA models were estimated using Latent GOLD 6.0 software (Statistical Innovations Inc.). Regression models were performed using SAS Enterprise Guide version 7.1 (SAS Institute). Ethical approval was granted by the Wake Forest University institutional review board (study title 12-19-22E, IRB00082615 approved July 2022). Study procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki of 1975.
Results
This secondary analysis included 317 patients from the intervention arm of the IMPACTS trial, with a median age of 67 years; 54% were women, 27% were Black, and 69% were White (Table 1). Patients had a median Charlson comorbidity index score of 5 (interquartile range [IQR], 3–7) and experienced a median of two organ failures (IQR, 1–3) during sepsis hospitalization. Thirty-nine percent required intensive care unit admission, and the average length of hospital stay was 7 days (IQR, 4–11 d).
Table 1.
Characteristics of sepsis survivors randomized to receive the sepsis transition and recovery intervention in the Improving Morbidity during Post–Acute Care Transitions for Sepsis trial
| Age at admission, yr, median (IQR) | 67 (57–75) |
| Sex, n (%) | |
| Female | 171 (54) |
| Male | 146 (46) |
| Race, n (%) | |
| Black | 84 (27) |
| White | 220 (69) |
| Other | 13 (4) |
| Charlson comorbidity index, median (IQR) | 5 (3–7) |
| Number of failed organs, median (IQR) | 2 (1–3) |
| Required ICU admission, n (%) | 123 (39) |
| Length of hospital stay, d, median (IQR) | 7 (4–11) |
| Index discharge disposition, n (%) | |
| Home with self-care | 138 (44) |
| Home with health services | 86 (27) |
| Skilled nursing facility | 71 (22) |
| Long-term acute care facility | 17 (5) |
| Other acute care hospital | 5 (2) |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range.
Characterization of STAR Program Care Activities
We identified nine unique care activities delivered by STAR nurse navigators (Table 2). Patients received a median of three distinct care activities (IQR, 2–5). Care coordination was the most frequently delivered activity (57%), followed by health promotion counseling (37%), emotional listening (32%), medication management (29%), and chronic disease management (29%).
Table 2.
Description and frequency of sepsis transition and recovery program intervention components delivered to sepsis survivors, obtained from electronic health record review
| Care Component | Description | n (%) |
|---|---|---|
| Care coordination | Organize follow-up testing and appointments with primary care and other healthcare providers to best meet patient’s needs and preferences. | 181 (57.1) |
| Health promotion counseling | Provide support and guidance to improve patient’s understanding and control over their health by making positive changes (e.g., attitudes, behaviors [diet, smoking cessation, exercise], environment). | 116 (36.6) |
| Emotional listening | Listen and offer emotional support for patients to promote shared understanding of the patient’s recovery experiences, build rapport, trust, and social connection during recovery, and enhance care. | 100 (31.6) |
| Symptom management | Screen for new or increasing limitations in activities of daily living, physical functioning, cognitive changes, and mental health symptoms. Provide resources or referrals to address identified needs. | 81 (25.6) |
| Medication management | Review medications with the patient and/or caregiver. Reassess medications changed during hospitalization and adjust for physiologic changes secondary to acute illness. | 93 (29.3) |
| Chronic disease management | Assess status of chronic illnesses (e.g., CHF, COPD, diabetes), address disease information needs to promote self-management, and confirm optimal therapy to enhance control. | 91 (28.7) |
| Addressing social determinants of health | Screen for social support needs related to housing, food, medications, and transportation. Refer to social worker or other community assistance programs as needed or available. | 78 (24.6) |
| Care setting advice and guidance | Support patient’s recovery with information and advice on when and where to seek care. | 70 (22.1) |
| Primary palliative care | Provide basic, palliative-focused supportive care and management to patients with serious illness. Refer to palliative care specialists, if appropriate. | 10 (3.2) |
Definition of abbreviations: CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease.
Identification of STAR Program Packages
We considered models with one to five latent classes (Table 3). We observed that different model fit criteria favored either the three- or four-class models. The SABIC, shown previously to perform well in situations in which classes have fewer than 50 subjects (17), indicated a four-class model. Increasing classes above four led to an increase in class assignment uncertainty and the emergence of classes comprising <5% of patients. The four-class model included class proportions between 14% and 35%, with high mean class probabilities (i.e., class 1, 92.0%; class 2, 92.8%; class 3, 89.1%; and class 4, 92.0%) and had the strongest clinical interpretability.
Table 3.
Description of model fit for one-, two-, three-, four-, and five-class models
| Number of Classes | Number of Parameters | Smallest Class Size [n (%)] | Log Likelihood | BIC | SABIC | AIC | CAIC |
|---|---|---|---|---|---|---|---|
| 1 | 9 | 317 (100) | −1,573.0 | 3,197.8 | 3,169.3 | 3,164.0 | 3,206.8 |
| 2 | 19 | 92 (29) | −1,315.5 | 2,740.3 | 2,680.1 | 2,668.9 | 2,759.3 |
| 3 | 29 | 73 (23) | −1,255.7 | 2,678.4 | 2,586.4 | 2,569.4 | 2,707.4 |
| 4* | 39 | 43 (14) | −1,240.9 | 2,706.5 | 2,582.8 | 2,559.9 | 2,745.5 |
| 5 | 49 | 13 (4) | −1,229.8 | 2,741.9 | 2,586.4 | 2,557.7 | 2,790.9 |
Definition of abbreviations: AIC = Akaike information criterion; BIC = Bayesian information criterion; CAIC = consistent Akaike information criterion; SABIC = sample size–adjusted Bayesian information criterion.
Chosen model.
Figure 1 shows the relative proportion of each care activity across the different packages. On the basis of distribution of these features, we named the four classes as follows: 1) barriers to engagement, 2) health promotion and behavior, 3) disease and medication management, and 4) comprehensive care. The barriers to engagement package made up 35% of the cohort and was characterized by a low frequency of all care activities. The health promotion and behavior package made up 33% of the cohort, characterized by a high frequency of care coordination, health promotion counseling, and emotional listening. The disease and medication management package constituted 14% of the cohort and had high frequencies of medication management, chronic disease management, and primary palliative care. The comprehensive care package made up 18% of the cohort, characterized by high frequencies of all care activities except for primary palliative care. For these latent classes, total STAR program engagement per patient ranged from a mean of 9.8 contacts and mean of 98.6 minutes of support (barriers to engagement class) to a mean of 17.8 contacts and mean of 212.6 minutes of support (disease and medication management class).
Figure 1.
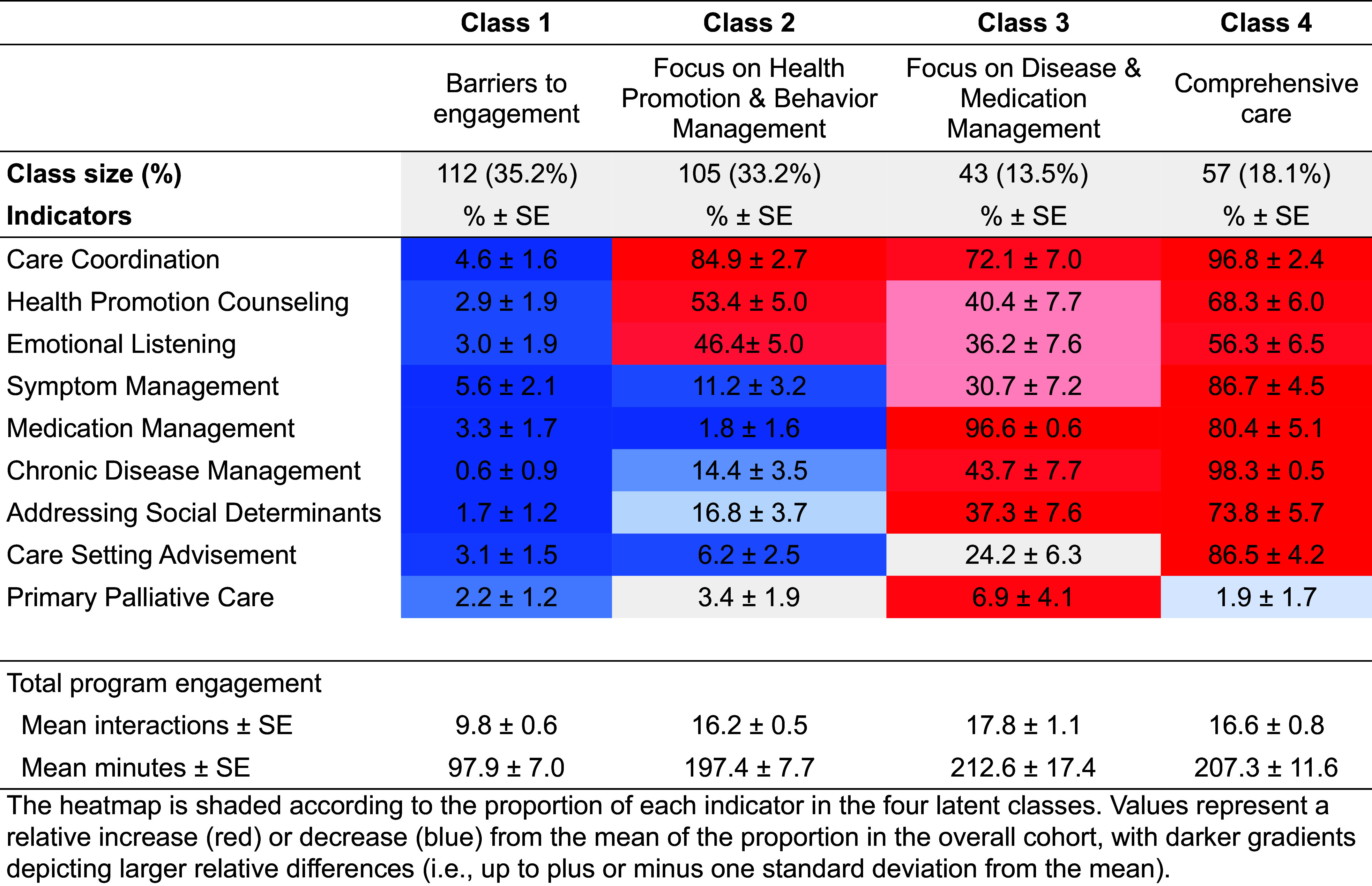
Heatmap displaying the standardized mean values for receipt of each intervention component indicator across four classes, with description of total program engagement. The heatmap is shaded according to the proportion of each indicator in the four latent classes. Values represent a relative increase (red) or decrease (blue) from the mean of the proportion in the overall cohort, with darker gradients depicting larger relative differences (i.e., up to ±1 standard deviation from the mean). SE = standard error.
Characteristics of Patients Receiving Different STAR Program Packages
Table 4 shows clinical characteristics of patients receiving the four different program packages. The barriers to engagement group was similar to other groups except that it had the highest proportion of patients discharged to post–acute care facilities. The health promotion and behavior group had the oldest mean age and the lowest proportion of patients who required organ support during their sepsis hospitalization. The disease and medication management group had the highest proportion of Black patients, Medicaid or uninsured status, heart failure, diabetes, immunosuppressive comorbidities, frequent prior hospitalizations, severe sepsis course requiring organ support, acquisition of new device or wound, and polypharmacy at discharge. The comprehensive care group had the highest proportion of White patients, patients from disadvantaged neighborhoods, and chronic obstructive pulmonary disease and renal comorbidities.
Table 4.
Distribution of characteristics associated with four-class model of sepsis transition and recovery program receipt
| Class 1 |
Class 2 |
Class 3 |
Class 4 |
|
|---|---|---|---|---|
| Barriers to Engagement | Focus on Health Promotion and Behavior Management | Focus on Disease and Medication Management | Comprehensive Care | |
| Age at admission, yr, mean ± SE | 65.7 ± 1.5 | 66.3 ± 1.4 | 62.2 ± 2.7 | 64.4 ± 2.3 |
| Sex, % ± SE | ||||
| Female | 52.4 ± 5.0 | 55.6 ± 5.2 | 58.4 ± 8.5 | 50.7 ± 7.0 |
| Male | 47.6 ± 5.0 | 44.4 ± 5.2 | 41.6 ± 8.5 | 49.3 ± 7.0 |
| Race, % ± SE | ||||
| Black | 29.4 ± 4.6 | 27.3 ± 4.7 | 46.3 ± 8.6 | 4.8 ± 3.7 |
| White | 67.6 ± 4.7 | 67.3 ± 4.9 | 51.3 ± 8.6 | 90.4 ± 4.6 |
| Other | 3.1 ± 1.6 | 5.5 ± 2.3 | 2.4 ± 2.9 | 4.9 ± 3.0 |
| Medicaid or uninsured status, % ± SE | 18.5 ± 3.9 | 16.6 ± 3.9 | 21.2 ± 7.0 | 11.8 ± 4.7 |
| Census tract ADI > 25th percentile, % ± SE | 24.4 ± 4.3 | 23.9 ± 4.5 | 30.0 ± 7.9 | 38.1 ± 6.8 |
| Coexisting conditions, % ± SE | ||||
| CHF | 37.6 ± 4.8 | 34.2 ± 5.0 | 40.2 ± 8.4 | 34.6 ± 6.7 |
| COPD | 32.0 ± 4.7 | 35.7 ± 5.0 | 27.8 ± 7.9 | 39.7 ± 6.9 |
| Diabetes | 42.4 ± 5.0 | 50.0 ± 5.3 | 63.7 ± 8.4 | 50.2 ± 7.0 |
| Renal disease | 38.5 ± 4.9 | 39.2 ± 5.1 | 30.8 ± 8.0 | 41.1 ± 6.9 |
| Immunosuppressive condition | 25.2 ± 4.4 | 27.2 ± 4.7 | 32.6 ± 8.1 | 17.8 ± 5.5 |
| Two or more hospitalizations in past 12 mo | 25.0 ± 4.4 | 23.3 ± 4.5 | 32.6 ± 8.0 | 23.9 ± 6.0 |
| Site of infection, % ± SE | ||||
| Respiratory | 34.0 ± 4.8 | 33.0 ± 4.9 | 27.3 ± 7.9 | 32.7 ± 6.6 |
| Genitourinary | 23.0 ± 4.2 | 22.9 ± 4.4 | 18.4 ± 6.5 | 26.8 ± 6.2 |
| Gastrointestinal | 4.8 ± 2.2 | 5.2 ± 2.4 | 9.6 ± 5.0 | 3.5 ± 2.7 |
| Wound/soft tissue | 9.2 ± 3.0 | 14.2 ± 3.7 | 11.6 ± 5.4 | 13.7 ± 4.7 |
| Organ dysfunction, % ± SE | ||||
| Required organ support | 35.6 ± 4.8 | 25.5 ± 4.6 | 47.6 ± 8.6 | 29.7 ± 6.5 |
| Bilirubin > 2 mg/dl | 7.8 ± 2.8 | 10.9 ± 3.3 | 13.8 ± 6.0 | 10.2 ± 4.4 |
| Creatinine > 2 mg/dl | 22.6 ± 4.2 | 23.6 ± 4.5 | 22.7 ± 7.2 | 24.9 ± 6.1 |
| Lactate > 2 mmol/L | 59.7 ± 4.9 | 59.4 ± 5.2 | 73.7 ± 7.8 | 61.6 ± 6.8 |
| Mean arterial pressure < 70 mm Hg | 74.4 ± 4.4 | 70.4 ± 4.8 | 91.1 ± 5.6 | 66.1 ± 6.6 |
| Platelet count < 100 cells/μl | 13.6 ± 3.5 | 10.4 ± 3.3 | 3.5 ± 3.6 | 12.8 ± 4.7 |
| New device or surgical procedure, % ± SE | 37.1 ± 4.8 | 34.1 ± 5.0 | 44.0 ± 8.6 | 32.9 ± 6.7 |
| Length of stay > 7 d, % ± SE | 50.9 ± 5.0 | 42.5 ± 5.2 | 55.4 ± 8.6 | 34.5 ± 6.7 |
| Five or more medications at discharge, % ± SE | 49.0 ± 5.0 | 35.4 ± 5.0 | 54.6 ± 8.6 | 24.1 ± 6.2 |
| Discharge to post–acute care facility, % ± SE | 43.3 ± 5.0 | 24.7 ± 4.6 | 21.7 ± 7.0 | 16.3 ± 5.1 |
Definition of abbreviations: ADI = area deprivation index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; SE = standard error.
Associations between STAR Program Packages and Sepsis Recovery Outcomes
Overall, 73 patients (23.0%) experienced the composite outcome of 30-day mortality and hospital readmission, including 8 (2.5%) deaths and 67 (21.1%) hospital readmission events (Figure 2). The composite outcome was most common among patients in the barriers to engagement class (27.6%), and seven of the eight deaths occurred in this group. Although we observed directionally lower event rates in each of the health promotion and behavior, disease and medication management, and comprehensive care classes, compared with the barriers to engagement class, findings from the pairwise comparisons indicated THAT STAR program class membership was not differentially associated with the composite outcome (P > 0.05 for all, Wald test). However, these comparisons are underpowered to obtain definitive conclusions.
Figure 2.
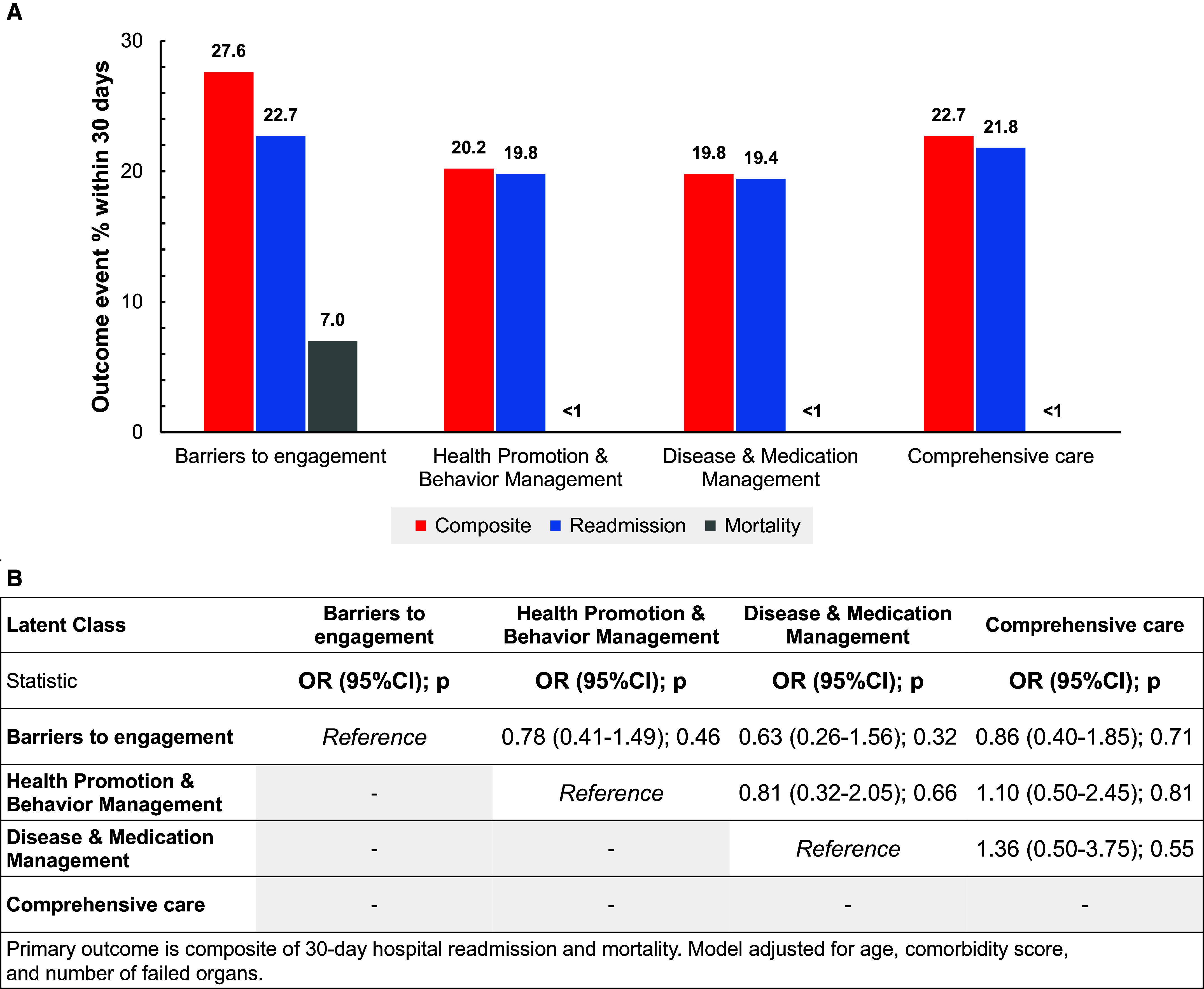
(A and B) Distribution (A) and pairwise comparisons (B) of composite 30-day hospital readmission and mortality outcomes for four-class model of sepsis transition and recovery program receipt. The primary outcome is a composite of 30-day hospital readmission and mortality. The model is adjusted for age, comorbidity score, and number of failed organs. CI = confidence interval; OR = odds ratio.
Discussion
In this secondary analysis, we applied a novel approach using LCA to examine distinct packages of delivering an effective STAR program. We successfully identified four packages for delivery of specific care activities in a population of adult sepsis survivors enrolled in a pragmatic randomized trial. This approach is a unique methodological contribution to process evaluation of complex interventions, especially when effectiveness was demonstrated in a pragmatic trial that allowed flexible intervention delivery to address diverse patient needs. Our method of explicitly characterizing intervention activities promotes opportunity for ongoing learning about optimal intervention delivery (i.e., the science of adaptation), an important step in optimal implementation (18). Our study contributes new, practical knowledge in key areas to improve understanding of the mechanism of the STAR program’s effectiveness and advance implementation of best-practice care for patients after sepsis.
First, the program delivered a broad range of care activities to a diverse patient population. This breadth of intervention scope is consistent with other recovery interventions demonstrating promising effects (e.g., Intensive Care Syndrome: Promoting Independence and Return to Employment program [19]) and is distinct from interventions that focus primarily on a single transitional care element (e.g., medication reconciliation). In addition, we found that the program’s care activities could be classified into patterns or packages that were delivered to patients with distinct observed characteristics. Despite different counts and types of care activities delivered, the time spent by the highly trained, pluripotent nurse navigators interacting with these patients was similarly high in three of the four latent classes. These findings, together with the similar clinical outcomes observed across these same three classes, highlight the potential for STAR program delivery to be effectively organized in a manner that prioritizes and tailors care to the individual patient’s needs. These findings also align with prior literature showing that high intensity of support is important to maintain the effectiveness of transitional care delivered to complex patients (20, 21).
Second, our findings not only illustrate how current program delivery was efficiently organized but also suggest potential opportunities to enhance support or augment care in the identified classes. For example, the health promotion and behavior class had fewer sepsis-specific recovery needs (e.g., the majority of patients did not require organ support, who has an established long-term recovery impact). These patients’ care focused largely on activities that may be effectively supported by general transitional care programs or with primary support from a community health worker or health coach to address primary transitional care needs, complemented by planned, but less frequent, sepsis nurse navigator touchpoints at critical time points (e.g., early discharge). Conversely, patients in the disease and medication management group had the most lengthy and severe sepsis course and often left the hospital with five or more medications and other complications influencing their postdischarge needs, requiring more dedicated support of their acute and chronic medical conditions during program participation. These individuals may benefit from the integration of a virtual pharmacist and early connection to primary care or brick-and-mortar recovery clinics (where available) for close clinical management. Furthermore, patients who received the comprehensive care STAR package had the highest proportion of individuals living in areas of high deprivation (i.e., in the top quartile, according to census tract area deprivation index). Although individuals who received this package often had a moderate acute illness course, the potential lack of community resources may be an important marker for the broad range of patient needs or care gaps to be addressed during transitional care after sepsis. These individuals may benefit from added social work or other multidisciplinary services to help bridge these access limitations.
Finally, not all packages were differentiated by increased delivery of care activities; the barriers to engagement class was characterized by the absence of targeted program care components. This group comprised a large proportion of patients who were discharged to post–acute care facilities, a subgroup with established poor outcomes such as high rates of unplanned hospital readmission (22). Prior research, including our own qualitative data, has shown that transitions from hospital to skilled nursing facilities (SNFs) are often challenged by poor information transfer and engagement (23). Indeed, in the present study, this class had the fewest STAR program contacts and received limited STAR-directed care activities during program enrollment. Importantly, STAR and other hospital–SNF collaborations have been shown to improve readmissions (24, 25), indicating that developing implementation strategies that overcome engagement barriers for this population is a top priority. Our findings highlight the need for further research on collaborative care models that enable stronger linkages between hospitals and SNFs to foster improved care coordination, promote increased sepsis recovery competencies among SNF staff members, and facilitate better engagement among staff members, patients, and families in implementing transitional care programs to work more effectively in these settings. In addition, this group had the highest mortality. We were unable to determine whether lower program engagement may have contributed to higher mortality or whether lower program engagement was a result of mortality during the program period.
Strengths and Limitations
Our study has several notable limitations. First, we analyzed EHR data that were extracted retrospectively from an existing cohort of patients enrolled in a pragmatic randomized trial at multiple hospitals in a single large health system.
Second, we abstracted documented care elements via chart review, requiring that elements be captured in the electronic documentation as part of routine care. Although this study design is subject to multiple sources of bias, chart review was necessary to gather the data needed to address our study question, and we took deliberate steps to mitigate the potential for error. For example, we included all eligible patients from the trial cohort to limit selection bias. In addition, STAR navigators entered data in the EHR using a standardized progress-note format to promote accurate documentation of the care actions taken as part of STAR program delivery, and we used standardized data abstraction tools and a structured reconciliation process to avoid misclassification bias.
Third, we deliberately focused our review on the care actions driven by the STAR navigators. However, other providers may have facilitated additional care actions that were outside the scope of our analyses. Our finding that care within each STAR package aligned with the expected needs of the patients in each class suggests that STAR navigators focused program resources on the highest prioritized care needs and add face validity to our distinct latent classes of STAR program delivery.
Conclusions
This study identified four distinct latent classes or packages of delivering an effective STAR program. These results further support our hypotheses that care delivered by the STAR program is appropriately tailored to meet high-priority recovery needs of different patient groups, which can be leveraged to increase widespread implementation. In addition, our data provide targets to facilitate future program delivery and address barriers to program engagement and effectiveness in hard to reach patients such as those discharged to SNFs.
Footnotes
Supported by National Institutes of Health grant R01NR018434 and National Library of Medicine grant R21LM013373.
Author Contributions: S.P.T. and M.A.K.: conception, design, analysis and interpretation of data, drafting the work, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. C.M., M.D., P.S., N.R., A.N., N.S., and A.Z.: acquisition and interpretation of data for the work, reviewing the work critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. B.B.: design of the work, analysis and interpretation of data, reviewing the work critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA . 2015;313:1055–1057. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA . 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 3. Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA . 2017;317:530–531. doi: 10.1001/jama.2016.20468. [DOI] [PubMed] [Google Scholar]
- 4.Torio C, Moore B.2016. https://hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf
- 5. Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med . 2015;43:738–746. doi: 10.1097/CCM.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA . 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor SP, Bray BC, Chou SH, Burns R, Kowalkowski MA. Clinical subtypes of sepsis survivors predict readmission and mortality after hospital discharge. Ann Am Thorac Soc . 2022;19:1355–1363. doi: 10.1513/AnnalsATS.202109-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hope AA, McPeake J. Healthcare delivery and recovery after critical illness. Curr Opin Crit Care . 2022;28:566–571. doi: 10.1097/MCC.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 9. McPeake J, Boehm LM, Hibbert E, Bakhru RN, Bastin AJ, Butcher BW, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor . 2020;2:e0088. doi: 10.1097/CCE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med . 2014;174:1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor SP, Murphy S, Rios A, McWilliams A, McCurdy L, Chou SH, et al. Effect of a multicomponent sepsis transition and recovery program on mortality and readmissions after sepsis: the improving morbidity during post–acute care transitions for sepsis randomized clinical trial. Crit Care Med . 2022;50:469–479. doi: 10.1097/CCM.0000000000005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kowalkowski MA, Rios A, McSweeney J, Murphy S, McWilliams A, Chou SH, et al. Effect of a transitional care intervention on rehospitalization and mortality after sepsis: a 12-month follow-up of a randomized clinical trial. Am J Respir Crit Care Med . 2022;206:783–786. doi: 10.1164/rccm.202203-0590LE. [DOI] [PubMed] [Google Scholar]
- 13. Kowalkowski M, Chou SH, McWilliams A, Lashley C, Murphy S, Rossman W, et al. Atrium Health ACORN Investigators Structured, proactive care coordination versus usual care for Improving Morbidity during Post–Acute Care Transitions for Sepsis (IMPACTS): a pragmatic, randomized controlled trial. Trials . 2019;20:660. doi: 10.1186/s13063-019-3792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanza ST, Bray BC, Collins LM. In: Handbook of psychology. 2nd ed. Schinka JA, Velicer WF, Weiner IB, editors. Vol. 2. Hoboken, NJ: John Wiley; 2013. An introduction to latent class and latent transition analysis; pp. 691–716. [Google Scholar]
- 15. Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling . 2007;14:535–569. [Google Scholar]
- 16.Henson JM, Reise SP, Kim KH. Detecting mixtures from structural model differences using latent variable mixture modeling: a comparison of relative model fit statistics. Struct Equ Modeling. 2007;14:202–226. [Google Scholar]
- 17.Chih-Chien Yang. Evaluating latent class analysis models in qualitative phenotype identification. Comput Stat Data Anal. 2006;50:1090–1104. [Google Scholar]
- 18. Chambers DA, Norton WE. The adaptome: advancing the science of intervention adaptation. Am J Prev Med . 2016;51:S124–S131. doi: 10.1016/j.amepre.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McPeake J, Henderson P, MacTavish P, Devine H, Daniel M, Lucie P, et al. A multicentre evaluation exploring the impact of an integrated health and social care intervention for the caregivers of ICU survivors. Crit Care . 2022;26:152. doi: 10.1186/s13054-022-04014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verhaegh KJ, MacNeil-Vroomen JL, Eslami S, Geerlings SE, de Rooij SE, Buurman BM. Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff (Millwood) . 2014;33:1531–1539. doi: 10.1377/hlthaff.2014.0160. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Fu MR, Fang J, Zheng H, Luo B. The effectiveness of transitional care interventions for adult people with heart failure on patient-centered health outcomes: a systematic review and meta-analysis including dose-response relationship. Int J Nurs Stud . 2021;117:103902. doi: 10.1016/j.ijnurstu.2021.103902. [DOI] [PubMed] [Google Scholar]
- 22. Neuman MD, Wirtalla C, Werner RM. Association between skilled nursing facility quality indicators and hospital readmissions. JAMA . 2014;312:1542–1551. doi: 10.1001/jama.2014.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gadbois EA, Tyler DA, Shield R, McHugh J, Winblad U, Teno JM, et al. Lost in transition: a qualitative study of patients discharged from hospital to skilled nursing facility. J Gen Intern Med . 2019;34:102–109. doi: 10.1007/s11606-018-4695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colucciello NA, Kowalkowski MA, Kooken M, Wardi G, Taylor SP. Passing the SNF test: a secondary analysis of a sepsis transition intervention trial among patients discharged to post-acute care. J Am Med Dir Assoc . 2023;24:742–746.e1. doi: 10.1016/j.jamda.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 25. Rahman M, Gadbois EA, Tyler DA, Mor V, Hospital-Skilled Nursing Facility Collaboration Hospital-skilled nursing facility collaboration: a mixed-methods approach to understanding the effect of linkage strategies. Health Serv Res . 2018;53:4808–4828. doi: 10.1111/1475-6773.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]


