Abstract
There is considerable interest in the potential for cell-based therapies, particularly mesenchymal stromal cells (MSCs) and their products, as a therapy for acute respiratory distress syndrome (ARDS). MSCs exert effects via diverse mechanisms including reducing excessive inflammation by modulating neutrophil, macrophage and T-cell function, decreasing pulmonary permeability and lung edema, and promoting tissue repair. Clinical studies indicate that MSCs are safe and well tolerated, with promising therapeutic benefits in specific clinical settings, leading to regulatory approvals of MSCs for specific indications in some countries.
This perspective reassesses the therapeutic potential of MSC-based therapies for ARDS given insights from recent cell therapy trials in both COVID-19 and in ‘classic’ ARDS, and discusses studies in graft-vs.-host disease, one of the few licensed indications for MSC therapies. We identify important unknowns in the current literature, address challenges to clinical translation, and propose an approach to facilitate assessment of the therapeutic promise of MSC-based therapies for ARDS.
Keywords: cell therapy, mesenchymal stem cells, mesenchymal stromal cells, ARDS therapies
Cell-based therapies, particularly mesenchymal stromal cells (MSCs) and their products, have considerable therapeutic potential for patients with acute respiratory distress syndrome (ARDS). It was initially believed that cell therapies would be composed of “stem” cells that would differentiate into and replace damaged pulmonary epithelial cells. Interestingly, MSCs are not stem cells but are one step differentiated (hence the term “stromal” cells) and exert their effects via diverse mechanisms, including reducing excessive inflammation by modulating neutrophil, macrophage, and T-cell function (1–3), decreasing pulmonary permeability and lung edema (4, 5), and promoting tissue repair (6, 7) (Figure 1). They can be isolated from different tissue sources (e.g., bone marrow, umbilical cord, adipose tissue), which has implications for their ease of harvest and potentially their profile of mechanistic effects.
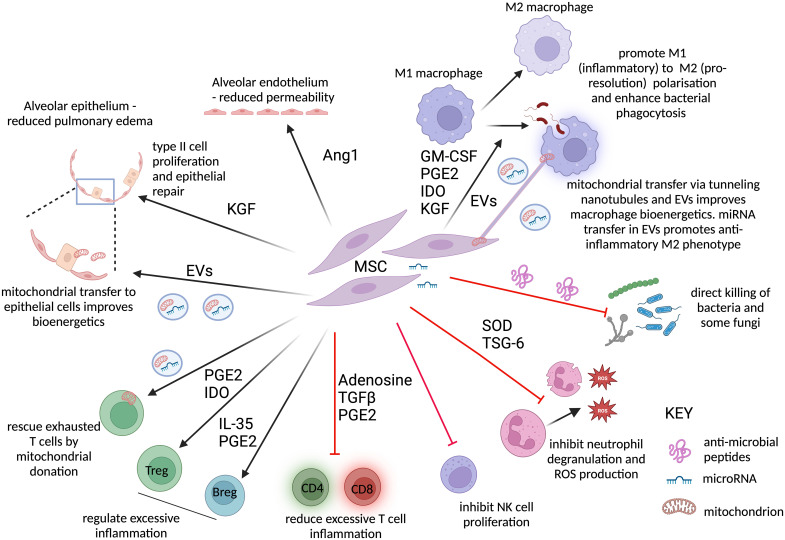
Figure 1.
Potential mechanisms by which mesenchymal stromal cells (MSCs) may improve outcomes in patients with ARDS. In preclinical models, MSCs have been shown to promote repair, reduce inflammation, and improve microbial clearance. Secretion of Ang1 promotes repair of the alveolar endothelium, reducing vascular leak. MSC-derived mitochondria, transported via extracellular vesicles (EVs) to the epithelium, and KGF (keratinocyte growth factor) secretion by MSCs drive type II alveolar epithelial cell proliferation, replacing the denuded epithelial layer, and promote epithelial sodium channel–mediated alveolar fluid clearance, with subsequent reduction in pulmonary edema. MSCs drive an antiinflammatory M2-like proresolution macrophage phenotype, characterized by low TNFalpha but high IL-10 production. This is mediated by MSC-secreted proteins and lipid mediators, including GM-CSF, KGF, indolamine 2,3-dioxygenase (IDO), and PGE2 (prostaglandin E2), but also by mitochondria transferred directly by MSCs to the macrophages by tunneling nanotubules or via EVs. EVs also contain microRNAs that activate antiinflammatory signaling pathways in the macrophages. These macrophages show increased bacterial phagocytosis and efferocytosis, clearing infection and dead cells more effectively. Antimicrobial peptide secretion by MSCs can lead to direct lysis of common bacteria and fungi such as Candida. TSG-6 inhibits neutrophil transepithelial migration and degranulation, reducing the release of tissue-damaging proteases, while SOD inhibits the oxidative stress caused by neutrophil-derived ROS. Natural killer (NK) cells contribute to the excessive inflammatory alveolar response to SARS-CoV-2 and contribute to neutrophil recruitment in preclinical models of ARDS: MSCs reduce NK proliferation. T-cell exhaustion is a feature of severe infection in ARDS and sepsis. Mitochondrial transfer from EVs can rescue exhausted T cells. Where T cells are excessively activated, MSC-derived adenosine, transforming growth factor-β, PGE2, and IL-10 inhibit both CD4 (cluster of differentiation 4) and CD8 T-cell proliferation and inflammatory responses. Treg and Breg expansion is promoted by PGE2/IDO and PGE2/IL-35, respectively, and both limit inflammation. This figure was created using BioRender.com. Ang1 = angiopoietin-1; ARDS = acute respiratory distress syndrome; Breg = regulatory B cell; GM-CSF = granulocyte–monocyte colony-stimulating factor; miRNA = microRNA; ROS = reactive oxygen species; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SOD = superoxide dismutase; TNF = tumor necrosis factor; Treg = regulatory T cell; TSG-6 = tumor necrosis factor–stimulated gene 6 protein.
Across the spectrum of clinical disorders, there are more than 1,000 clinical trials of MSCs registered at ClinicalTrials.gov, with just over 300 clinical trials published to date. Overall, MSCs have exhibited an acceptable safety profile and demonstrated promising therapeutic benefits in specific clinical settings, which has led to regulatory approvals of MSCs for specific indications (e.g., graft-vs.-host disease [GvHD], Crohn’s fistulae), in a small number of countries worldwide.
In ARDS, a twin-track approach of ongoing preclinical and mechanistic studies, together with phase I and II clinical translational studies, has led to the rapid generation of important insights into the therapeutic potential of MSCs for ARDS. The need for therapies for patients with severe coronavirus infection (COVID-19)–induced ARDS constituted a further catalyst for the larger scale testing of MSC-based therapies. Consequently, it seems timely to reassess the therapeutic potential of MSC-based therapies for ARDS. In this perspective we discuss results from recent cell therapy trials in both COVID-19 and “classical” ARDS and examine insights from studies in GvHD, one of the few licensed indications for MSC therapies. We identify key unknowns and gaps in the current literature, address challenges to clinical translation, and propose an approach to facilitate assessment of the therapeutic promise of MSC-based therapies for ARDS.
Insights from ARDS Clinical Trials
The findings of randomized clinical trials of MSCs in ARDS are summarized in Table 1. The first reported trial was conducted in 12 invasively ventilated patients with ARDS with 1:1 randomization to MSC or placebo (8), with the treatment group receiving a single relatively low intravenous dose of 1 × 106 MSCs/kg adipose-derived MSCs (8). There were no adverse events, and the efficacy endpoints (oxygenation, ventilator-free days [VFDs], ICU-free days, and hospital mortality) were similar between the two groups. Plasma concentrations of surfactant protein D were lower on Day 5 compared with Day 0 in the MSC-treated patients, with no difference in the placebo group. The value of this trial was limited by the small number of patients.
Table 1.
Randomized Clinical Trials of Mesenchymal Stromal Cell Therapies in Patients with Classical Acute Respiratory Distress Syndrome
| Number of Patients |
Patient Population | MSC Therapy Regimen |
Design | Biological Signal | Adverse Events (n) |
Mortality [n (%)]* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Cell Source | Dose Size | Number of Doses | Treatment | Control | Treatment | Control | ||||
| Zheng et al. (2014) (8) | 6 | 6 | Early ARDS; invasive MV (PFR ⩽ 300 mm Hg) | Adipose-derived MSCs | 1 × 106 cells/kg | 1 | Placebo control | No (cytokines) | 1† | 0† | 1 | 2 |
| Matthay et al. (2019) (9) | 40 | 20 | Early ARDS; invasive MV (PFR ⩽ 200 mm Hg) | Bone marrow cord MSCs | 10 × 106 cells/kg | 1 | Placebo control | Yes (biomarkers) | 0† | 0† | 12/40 (30) | 3/20 (15) |
| Bellingan et al. (2022) (11) | 20 | 10 | Early ARDS; invasive MV (PFR ⩽ 200 mm Hg) | Bone marrow cord MAPCs | 9 × 108 cells | 1 | Placebo control | Yes (biomarkers) | 1†/18‡ | 0†/6‡ | 2/20 (10) | 4/10 (40) |
| Ichikado et al. (2023) (12) | 20 | 10 | Pneumonia ARDS with early fibroproliferation | Bone marrow cord MAPCs | 9 × 108 cells | 1 | Placebo control | No (cytokines) | 5†/20‡ | 0†/10‡ | 5/19 (26) | 3/7 (43) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; MAPC = multipotent adult progenitor cell; MSC = mesenchymal stromal cell; MV = mechanical ventilation; PFR = PaO2:FiO2 ratio.
Mortality at the latest time point reported in the study.
Adverse events possibly related to therapy.
Total number of adverse events (some patients had more than one adverse event).
The START (Human Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome) trial was a phase IIa safety trial of bone marrow–derived performed in 60 patients, with 40 randomized to receive a single dose of MSCs (10 × 106 MSCs/kg) and 20 to receive placebo intravenously within 72 hours of the onset of ARDS (9). There were no safety issues. Post hoc analyses demonstrated that in patients receiving MSCs with moderate or high cell viability (viability varied from 30% to 80%), there was a signal for improved oxygenation and a significant reduction in IL-6 concentrations. A follow-up analysis reported that in 28 patients who underwent BAL at 48 hours after MSC or placebo treatment, the MSC-treated patients had lower total protein (suggesting decreased lung permeability) and lower Ang-2, IL-6, and TNFR1 concentrations in the BAL (10).
The MUST-ARDS (A Phase 1/2 Study to Assess MultiStem® Therapy in Acute Respiratory Distress Syndrome) trial was a randomized, double-blind, placebo-controlled phase II trial of MultiStem (Athersys, Inc.) cells, which are bone marrow–derived multipotent adult progenitor cells that are MSC precursors, in patients with ARDS. Cohort 1 (n = 3) received 300 million cells, while cohort 2 (n = 3) received 900 million cells. In the third cohort, 20 patients received 900 million cells and 10 patients received placebo (11). The patients’ characteristics were well balanced at baseline. Although not powered for clinical outcomes, there were numerical trends that favored MSC treatment, including a lower 28-day mortality rate of 25% in the MSC group versus 40% in the placebo group and more VFDs in the MSC-treated patients. These favorable trends were maintained in a prespecified group with more hypoxemia at baseline (PaO2:FiO2 < 150 mm Hg). A second phase II open-label trial (ONE-BRIDGE [Efficacy and Safety Study of HLCM051(MultiStem®) for Pneumonic Acute Respiratory Distress Syndrome]) of multipotent adult progenitor cells (MAPCs) was performed in 30 Japanese patients with ARDS and pneumonia (20 randomized to MAPCs and 10 to standard of care) within 72 hours of the onset of ARDS (12). Baseline characteristics were well balanced in the two groups. There was no difference in the primary outcome of VFDs, although there was a trend that favored the MAPC group. Mortality was numerically lower, but not statistically different, at 28 and 60 days in the MSC group. Biological markers showed no difference in the two groups. There was significant improvement in functional health the MAPC-treated patients.
The ongoing REALIST (Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration) trial (NCT 03042143) will provide additional data on the use of MSCs in classical ARDS (13)
Insights from COVID-19 Clinical Trials
The findings of randomized clinical trials of MSCs in COVID-19 ARDS are summarized in Table 2. In a meta-analysis investigating MSCs in patients with COVID-19 (of whom 207 received MSCs compared with 196 who received control treatment), MSCs were found to reduce the relative and absolute risk of death. Secondary outcomes were generally consistent with a beneficial effect with MSCs (14). However, several of the studies included were conducted before the routine use of immunomodulation as standard of care and had a high control group mortality of up to 80%, which may limit the generalizability of these findings to current practice.
Table 2.
Randomized Clinical Trials of Mesenchymal Stromal Cell Therapies in Patients with Coronavirus Disease Acute Respiratory Failure/Acute Respiratory Distress Syndrome
| Number of Patients |
Patient Population | MSC Therapy Regimen |
Design | Steroid Use | Biological Signal | Adverse Events (n) |
Mortality [n (%)]* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Therapy | Control | Cell Source | Dose Size | Number of Doses | Treatment | Control | Treatment | Control | |||||
| Cell therapy trials | |||||||||||||
| Shu et al. (2020) (40) | 12 | 29 | Early COVID-19 ARF Not invasive MV (PFR ⩽ 300 mm Hg) |
Umbilical-cord MSCs | 2 × 106 cells/kg | 1 | Placebo control | Yes | Yes (cytokines) | 0† | 0† | 0/12 (0) | 3/29 (10) |
| Adas et al. (2021) (41) | 10 | 10 | Early “severe” COVID-19; invasive MV | Umbilical-cord MSCs | 3 × 106 cells/kg | 3 (D0, D3, D6) | Placebo control | Yes (“per needed”) | Yes (cytokines) | 0† | 0† | 3/10 (30) | 6/10 (60) |
| Dilogo et al. (2021) (42) | 20 | 20 | Early COVID-19 ARF Invasive MV (PFR < 300 mm Hg) Multiorgan failure |
Umbilical-cord MSCs | 1 × 106 cells/kg | 1 | Placebo control | No | Yes (cytokines) | 0† | 0† | 10/20 (50) | 16/20 (80) |
| Shi et al. (2021) (43) | 65 | 35 | Early COVID-19 ARF Not invasive MV (PFR < 300 mm Hg) |
Umbilical-cord MSCs | 4 × 107 cells | 3 (D0, D3, D6) | Placebo control | 20–25% | Yes (lung lesion on CT) | 0†/37‡ | 0†/21‡ | 0/65 (0) | 0/35 (0) |
| Lanzoni et al. (2022) (44) | 12 | 12 | Early COVID-19 ARF Advanced respiratory support including invasive MV (PFR < 300 mm Hg) |
Umbilical-cord MSCs | 1 × 108 cells | 2 (D0, D3) | Placebo control | Yes (80%) | Yes (cytokines) | 1†/35‡ | 1†/53‡ | 1/12 (8) | 7/12 (58) |
| Monsel et al. (2022) (16) | 21 | 24 | Early COVID-19 ARDS Advanced respiratory support including invasive MV (PFR < 300 mm Hg |
Umbilical-cord MSCs | 1 × 106 cells/kg | 3 (D0, D3, D5) | Placebo control | Yes (75%) | Yes (cytokines) | 1†/49‡ | 0†/48‡ | 5/21 (24) | 4/24 (17) |
| Rebelatto et al. (2022) (45) | 11 | 6 | Early ARDS; invasive MV (PFR ⩽ 300 mm Hg) | Umbilical-cord MSCs | 5 × 105 cells/kg | 3 (D0, D3, D5) | Placebo control | Yes (100%) | Yes (cytokines) | 0† | 0† | 5/11 (45) | 1/6 (17) |
| Bowdish et al. (2023) (15) | 112 | 110 | Early ARDS; invasive MV (PFR ⩽ 200 mm Hg) | BM | 2 × 106 cells/kg | 2 (D1, D4) | Placebo control | Yes | Not reported | 0†/167‡ | 0†/153‡ | 42/112 (37.5) | 47/110 (42.7) |
| Gorman et al. (2023) (17) | 30 | 29 | Early ARDS; invasive MV (PFR ⩽ 200 mm Hg) | Umbilical cord | 4 × 108 cells | 1 | Placebo control | Yes | Yes (transcriptome) | 6 | 3 | 7/30 (23) | 8/31 (26) |
| Cell products trials | |||||||||||||
| Fathi-Kazerooni et al. (2002) (46) | 14 | 15 | Early COVID-19 ARF Advanced respiratory support including invasive MV (PFR < 300 mm Hg) |
Menstrual blood MSC | Secretome 5ml | 5 (D0–D4) | Placebo control | 100% | Yes (CRP; D-dimer) | 0† | 0† | 6/14 (43) | 12/15 (80) |
| Lightner et al. (2023) (18) | EF-15: n = 34 EF-10: n = 34 |
34 | Early COVID-19 ARF Advanced respiratory support including invasive MV (PFR < 300 mm Hg) |
BM-MSC–derived EVs | EF-15: 15 ml EF-10: 10 ml |
1 | Placebo control | 100% | Not reported | EF-15: 0†/24‡ EF-10: 0†/26‡ |
1†/23‡ | EF-15: 10 EF-10: 14 |
16/34 (47) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; ARF = acute respiratory failure; BM = bone marrow; CRP = C-reactive protein; CT = computed tomography; D = day; EF-10 = ExoFlo (10 ml); EF-15 = ExoFlo (15 ml); EV = extracellular vesicle; MSC = mesenchymal stromal cell; MV = mechanical ventilation; PFR = PaO2:FiO2 ratio.
Mortality at the latest time point reported in the study.
Only adverse events possible/likely/related to therapy reported.
Total number of adverse events (some patients had more than one adverse event).
These initially promising results have been further tempered by more recent reports from three additional trials. Bowdish and colleagues, in a commercially funded trial, investigated the efficacy of two doses of remestemcel-L (a bone marrow–derived MSC product) at a dose of 2 × 106 cells/kg in 222 patients with COVID-19 ARDS (15). There was no difference in the primary outcome of mortality at 30 days in the overall population. In patients younger than 65 years, there was a signal for benefit, but as this was a subgroup analysis, this finding should be interpreted with caution. Monsel and colleagues investigated the efficacy of three doses of umbilical-cord MSCs (1 × 106 cells/kg) in 45 patients with COVID-19 ARDS (16). There was no difference in the primary outcome of change in the PaO2:FiO2 ratio to Day 7.
Finally, the REALIST-COVID (Repair of Acute Respiratory Distress Syndrome in COVID-19 by Stromal Cells) trial assessed the safety and efficacy of a single dose of ORBCEL-C (Orbsen Therapeutics) (an umbilical-cord MSC product) at a dose of 400 × 106 cells in 60 patients with COVID-19 ARDS (17). ORBCEL-C therapy was safe and well tolerated, with no significant differences between groups in the primary safety outcome. Despite demonstrating biological activity in modulating the peripheral blood transcriptome, there was no signal for efficacy in surrogate pulmonary and nonpulmonary clinical outcomes. Increased duration of ventilation was seen in the ORBCEL-C–treated group, but the trial was not designed to have statistical power to evaluate clinical outcomes, and this should be interpreted cautiously. Long-term follow-up showed similar 2-year mortality rates in both groups, but three patients received cancer diagnoses during the 2-year follow-up period in the MSC-treated group.
Recently, cell-free products such as MSC-derived extracellular vesicles (EVs) isolated from bone marrow MSCs have also been investigated as a potential treatment for patients with COVID-19. EVs are small cell-derived particles containing proteins, mRNA, microRNA, and lipids, which arise from the plasma membrane. Lightner and colleagues, in a commercially funded trial of 102 patients, investigated the efficacy of up to two doses of ExoFlo (Direct Biologics), bone marrow MSC–derived EVs, at doses of 15 and 10 ml (providing 1.2 trillion and 0.9 trillion EV particles per dose) up to Day 4 in 102 patients with COVID-19 ARDS (18). ExoFlo therapy was well tolerated. Although not statistically significant, 60-day mortality was reduced in the 15-ml ExoFlo group (P = 0.134). In post hoc subgroup analyses, greater benefits were seen in participants aged 18–65 years with moderate to severe ARDS, including VFDs (P = 0.045), although again, as these were post hoc subgroup analyses, the findings should be interpreted with caution. These investigators have now begun a phase III trial of ExoFlo in the United States for classical ARDS, using the expanded definition of ARDS (19), with the primary endpoint of 60-day all-cause mortality (EXTINGUISH [Extracellular Vesicle Treatment for Acute Respiratory Distress Syndrome] trial [NCT 05354141]).
One further trial investigating the safety and efficacy of a single dose of a bone marrow–derived MSCs, at a dose of 10 × 106 cells/kg, in patients with COVID-19 and non–COVID-19 ARDS, has recently completed (STAT [Mesenchymal Stromal Cells For Acute Respiratory Distress Syndrome] trial [NCT 03818854]), and results are awaited.
Insights from GvHD
GvHD, an unfavorable immune reaction after hematopoietic stem cell transplantation when donor lymphocytes attack the recipient’s healthy tissue, is the clinical condition for which bone marrow–derived MSCs have been most frequently studied. After in vitro studies that demonstrated strong immunomodulatory properties of MSCs (20), and case studies in patients with steroid refractory GvHD (21), the clinical benefit of MSC infusion was more definitively demonstrated in a nonrandomized phase II trial (22) of 55 patients (30 adults and 25 children) with severe steroid-resistant acute GvHD treated with allogeneic HLA-identical, haploidentical, or mismatched MSCs. The study patients were all severely ill, mainly with GvHD of the gastrointestinal tract and liver. Thirty patients achieved complete remission and 9 patients achieved partial clinical improvement, and no side effects were observed. Subsequently, these findings were replicated in multiple studies (23, 24).
In contrast, a large, multicenter phase III clinical trial assessing the use of a commercial MSC product (Prochymal; Mesoblast [remestemcel-L]) failed to meet its primary clinical endpoint, defined as complete resolution of acute GvHD symptoms for at least 28 days after beginning the treatment (25). Remestemcel-L was proved to be safe and well tolerated in this 260-patient study. Post hoc analyses of patients with liver involvement or with high-risk disease revealed both higher complete remission and partial remission rates in the remestemcel-L group. There was also a trend toward a superior clinical response in children compared with adults. Further encouraging studies in children and adults were subsequently published (26, 27).
Interestingly, country regulators have adopted different approaches to licensing MSCs for GvHD. In 2015, the Japanese Pharmaceuticals and Medical Devices Agency granted approval to JR-031 (TEMCELL; JCR Pharmaceuticals Co., Ltd.) for the treatment of acute GvHD in both children and adults on the basis of the findings of these studies. In contrast, a U.S. Food and Drug Administration application to treat children with steroid-resistant acute GvHD with remestemcel-L was declined in 2020, and again declined in 2023 following resubmission (28).
In terms of insights relevant to ARDS, a number of factors appear to be associated with improved responsiveness to MSC therapy in GvHD, including a high cell dose, younger patient age (22, 29, 30), and gut and/or skin involvement (30, 31). The clinical MSC product has varied between different studies; both HLA-matched, haploidentical cells and mismatched cells have been used. Interestingly, clinical responses do not appear to be influenced by cell culture conditions, including the use of fetal bovine serum or human platelet lysate in the culture medium, or by the degree of HLA disparity or ABO matching between MSC donors and recipients (32).
Key Insights and Knowledge Gaps
In classical ARDS, recent phase II trials of MSCs have provided evidence that MSC therapy appears safe, although the total number of patients studied to date is modest (Table 1). In terms of efficacy, therapy with MultiStem cells showed the most favorable signal for efficacy. The START trial suggested potential efficacy in a post hoc analysis of higher viability MSCs and, perhaps more important, provided evidence that MSCs may reduce lung permeability injury, inflammation, and endothelial injury. In patients with COVID-19 ARDS, MSCs are safe, but early indications of a signal for efficacy have largely disappeared in subsequent studies as the standard of care has evolved to include steroid therapy and other immunomodulators. Further trials of MSCs for severe COVID-19 ARDS do not seem warranted at present, given the identification of other effective therapies and of the development of vaccines for this condition.
The practical challenges of delivering MSC therapies to critically ill patients are increasingly clear (33) and have also been a feature in other research fields. Challenges with maintaining high viability of MSC therapies during the thawing and administration process are highlighted by the findings of the START study, and these must be addressed (9). A key issue is the need to determine the optimal cell source and the optimal dosage regimens. Studies in COVID-19 have been particularly heterogeneous in this regard, with multiple MSC tissue sources, both fresh and cryopreserved cells, single and multiple cell doses, and substantial variability in dose size all well documented (14). The impact of changes over time in the standard of care, particularly regarding the use of steroids and other immunomodulators in COVID-19, which may alter the potential efficacy of MSCs, might account for the lack of efficacy seen in more recent studies in this setting (34).
The finding that three patients had developed malignancy at 2-year follow-up in the REALIST trial in COVID-19 ARDS raises concerns, despite the demonstrated low risk of malignant transformation of MSCs. Although an increased risk of malignancy was not found in a meta-analysis that investigated the safety of MSC administration in a range of clinical conditions (35), this finding does highlight the importance of undertaking long-term follow-up in MSC trials to identify any potential long-term adverse outcomes.
Insights from GvHD, which remains one of the few conditions for which MSCs are licensed, are worth considering. In particular, the identification of factors that are associated with improved responsiveness to MSC therapy in GvHD suggest that it may be possible to identify subpopulations with ARDS more likely to benefit from MSC therapies. The identification of hyperinflammatory subphenotypes in patients with ARDS may be a relevant target population for MSC-based therapies (36), but this remains unproven. Conversely, preclinical studies demonstrating that MSCs may exert detrimental effects within profibrotic lung microenvironment, suggesting that there may be subgroups of patients with ARDS for whom MSC therapies should be avoided (37).
The limitations of clinical studies of MSC therapies for ARDS conducted to date must be addressed. These studies have been quite small in size, were conducted in heterogeneous ARDS populations, and had significant variability in treatment protocols. In a recent meta-analysis, Kirkham and colleagues reported that studies in COVID-19 have tested diverse MSC tissue sources and used variable dose sizes and both single and multiple dose regimens, with variability in concomitant therapies and different outcome measures (38). Methodologic issues were also identified, with low rates of reporting details of MSC product characterization and concerns regarding risk of bias reported in some studies (38). Consequently, despite the publication of multiple recent trials in the field, it remains difficult to draw clear conclusions on the therapeutic potential of MSCs for ARDS given the current evidence base.
Future Directions: Developing Better Trials
Clinical studies to date of MSC therapies for patients with ARDS have not realized the therapeutic promise demonstrated in preclinical studies (33). MSCs are complex biological therapeutics, and so apparently minor differences across studies in MSC tissue source, in cell isolation and ex vivo processing, in cell cryopreservation and thawing strategies, and in dosing regimens make it difficult to meaningfully pool results to generate generalizable insights. Consequently, we endorse the call to develop a “core protocol” (39) for evaluating MSC therapies for ARDS (38). This protocol would provide guidance regarding MSC product characteristics, dose and dose regimens, target ARDS subpopulations, recommended sample sizes, patient populations, administration strategies, concomitant therapies, outcome measures, and blinding procedures to be followed. This would facilitate the standardization of future studies, thereby permitting meaningful aggregation of results in meta-analyses.
Regarding the specifics of such a core protocol, we offer the following suggestions (Box 1). All studies should provide detailed characterization of the MSC therapeutic studied, including their isolation, laboratory processing, and viability at cell administration. In terms of MSC source, umbilical cord–derived MSCs may be the most promising option, given the ease of isolation of MSCs and the fact that this is a widely available source. In addition, cell-free alternatives, such as MSC-derived EVs, may provide advantages by avoiding any risks of administering the whole MSCs, and these deserve further study.
Box 1. Proposals to Enhance the Likelihood of Effective MSC Trials in ARDS
Develop a core protocol to improve the standardization of future mesenchymal stromal cell (MSC) studies in acute respiratory distress syndrome (ARDS).
Provide detailed characterization for the MSC therapeutic (cell isolation, processing, key markers, cell viability at administration) in study reports.
Focus on umbilical cord–derived MSCs as the most promising current option.
Test MSC doses of at least 2 million MSCs/kg body weight delivered intravenously.
Examine multiple (two or three) dose regimens delivered early in the course of ARDS.
Ensure that all patients enrolled receive the best current care and standardize concomitant therapies.
Enroll sufficiently large patient numbers to assess clinically meaningful outcomes.
Collect biological samples to characterize mechanisms of action of MSC therapeutic.
Identify more treatment-responsive subpopulations for MSCs within the ARDS population.
Perform longer term follow-up studies to exclude concerns such as risk of malignancy.
Cell-free alternatives such as MSC-derived extracellular vesicles may provide advantages and deserve further study.
In terms of MSC dose and dosage regimen, we suggest that doses of at least 2 million MSCs/kg body weight should be tested and that multidose regimens should be examined. At present, clinical studies should focus on the intravenous route of administration. As preclinical studies have focused on early ARDS, we suggest that studies should test two or three dose regimens in early ARDS. The new global definition of ARDS (19) opens up the possibility for earlier therapy in patients receiving high-flow nasal oxygen. A research priority should be to identify MSC treatment–responsive populations within the ARDS population. Both the treatment group and standard-care group must receive best current care, with standardized concomitant therapies. Studies should be sufficiently large to permit the measurement of clinically meaningful outcomes. Studies should also collect blood (and ideally BAL) samples to permit post hoc studies to examine mechanisms of effect. This approach would also help identify treatment-responsive subpopulations of patients with ARDS who may be more likely to benefit from MSC-based therapies.
Conclusions
MSC therapies retain considerable therapeutic potential for ARDS, but clinical studies to date have not realized the therapeutic promise demonstrated in preclinical studies. We have ample evidence that MSCs are safe when administered to patients with ARDS from studies to date, in terms of short-term outcomes, although we still need studies with long-term follow-up to definitively exclude any malignancy risk. Further trials of MSCs for patients with severe COVID-19 ARDS are not warranted at present, given the identification of other effective therapies and the development of vaccines for this condition. In classical ARDS, the development of an agreed core protocol is necessary to enable the greater standardization of future clinical trials. This approach should lead to an efficient and robust determination of the efficacy of MSC-based therapies for treatment-responsive subpopulations of patients with ARDS.
Footnotes
Supported by Science Foundation Ireland grant 16-FRL-3845 (J.G.L.).
Author Contributions: All authors contributed to drafting and editing the manuscript and have reviewed and approved the final version.
Originally Published in Press as DOI: 10.1164/rccm.202311-2046CP on February 7, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Rabani R, Volchuk A, Jerkic M, Ormesher L, Garces-Ramirez L, Canton J, et al. Mesenchymal stem cells enhance NOX2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur Respir J . 2018;51:1702021. doi: 10.1183/13993003.02021-2017. [DOI] [PubMed] [Google Scholar]
- 2. O’Kane CM, Matthay MA. Understanding the role of mesenchymal stromal cells in treating COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med . 2023;207:231–233. doi: 10.1164/rccm.202209-1838ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med . 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol . 2014;306:L809–L815. doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayes M, Curley GF, Masterson C, Devaney J, O’Toole D, Laffey JG. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Med Exp . 2015;3:29. doi: 10.1186/s40635-015-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, et al. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol . 2015;195:875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- 7. Hayes M, Masterson C, Devaney J, Barry F, Elliman S, O’Brien T, et al. Therapeutic efficacy of human mesenchymal stromal cells in the repair of established ventilator-induced lung injury in the rat. Anesthesiology . 2015;122:363–373. doi: 10.1097/ALN.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 8. Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res . 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med . 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wick KD, Leligdowicz A, Zhuo H, Ware LB, Matthay MA. Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight . 2021;6:e148983. doi: 10.1172/jci.insight.148983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bellingan G, Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, et al. Safety and efficacy of multipotent adult progenitor cells in acute respiratory distress syndrome (MUST-ARDS): a multicentre, randomised, double-blind, placebo-controlled phase 1/2 trial. Intensive Care Med . 2022;48:36–44. doi: 10.1007/s00134-021-06570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichikado K, Kotani T, Kondoh Y, Imanaka H, Johkoh T, Fujimoto K, et al. Clinical efficacy and safety of multipotent adult progenitor cells (Invimestrocel) for acute respiratory distress syndrome (ARDS) caused by pneumonia: a randomized, open-label, standard therapy-controlled, phase 2 multicenter study (ONE-BRIDGE) Stem Cell Res Ther . 2023;14:217. doi: 10.1186/s13287-023-03451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorman E, Shankar-Hari M, Hopkins P, Tunnicliffe WS, Perkins GD, Silversides J, et al. Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST): a structured study protocol for an open-label dose-escalation phase 1 trial followed by a randomised, triple-blind, allocation concealed, placebo-controlled phase 2 trial. Trials . 2022;23:401. doi: 10.1186/s13063-022-06220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirkham AM, Bailey AJM, Monaghan M, Shorr R, Lalu MM, Fergusson DA, et al. Updated living systematic review and meta-analysis of controlled trials of mesenchymal stromal cells to treat COVID-19: a Framework for Accelerated Synthesis of Trial Evidence for Rapid Approval—FASTER Approval. Stem Cells Transl Med . 2022;11:675–687. doi: 10.1093/stcltm/szac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowdish ME, Barkauskas CE, Overbey JR, Gottlieb RL, Osman K, Duggal A, et al. A randomized trial of mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome from COVID-19. Am J Respir Crit Care Med . 2023;207:261–270. doi: 10.1164/rccm.202201-0157OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, et al. APHP STROMA–CoV-2 Collaborative Research Group Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care . 2022;26:48. doi: 10.1186/s13054-022-03930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorman EA, Rynne J, Gardiner HJ, Rostron AJ, Bannard-Smith J, Bentley AM, et al. Repair of Acute Respiratory Distress Syndrome in COVID-19 by Stromal Cells (REALIST-COVID trial): a multicenter, randomized, controlled clinical trial. Am J Respir Crit Care Med . 2023;208:256–269. doi: 10.1164/rccm.202302-0297OC. [DOI] [PubMed] [Google Scholar]
- 18. Lightner AL, Sengupta V, Qian S, Ransom JT, Suzuki S, Park DJ, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for the treatment of respiratory failure from COVID-19: a randomized, placebo-controlled dosing clinical trial. Chest . 2023;164:1444–1453. doi: 10.1016/j.chest.2023.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med . 2024;209:37–47. doi: 10.1164/rccm.202303-0558WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol . 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 21. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet . 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 22. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet . 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 23. Ball LM, Bernardo ME, Roelofs H, van Tol MJ, Contoli B, Zwaginga JJ, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol . 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 24. von Bonin M, Stölzel F, Goedecke A, Richter K, Wuschek N, Hölig K, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant . 2009;43:245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 25. Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A phase 3 randomized study of remestemcel-L versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant . 2020;26:835–844. doi: 10.1016/j.bbmt.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, et al. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant . 2014;20:229–235. doi: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 27. Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, et al. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant . 2014;20:375–381. doi: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 28. Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol . 2023;20:613–625. doi: 10.1038/s41423-023-01022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Resnick IB, Barkats C, Shapira MY, Stepensky P, Bloom AI, Shimoni A, et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC) Am J Blood Res . 2013;3:225–238. [PMC free article] [PubMed] [Google Scholar]
- 30. Galleu A, Milojkovic D, Deplano S, Szydlo R, Loaiza S, Wynn R, et al. Mesenchymal stromal cells for acute graft-versus-host disease: response at 1 week predicts probability of survival. Br J Haematol . 2019;185:89–92. doi: 10.1111/bjh.15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sánchez-Guijo F, Caballero-Velázquez T, López-Villar O, Redondo A, Parody R, Martínez C, et al. Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol Blood Marrow Transplant . 2014;20:1580–1585. doi: 10.1016/j.bbmt.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 32. Moll G, Hult A, von Bahr L, Alm JJ, Heldring N, Hamad OA, et al. Do ABO blood group antigens hamper the therapeutic efficacy of mesenchymal stromal cells? PLoS One . 2014;9:e85040. doi: 10.1371/journal.pone.0085040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang H, Slutsky AS. Enhancing the efficacy of mesenchymal stromal cells in COVID-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med . 2023;208:222–224. doi: 10.1164/rccm.202306-0969ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li T, Xu Y, Wang Y, Jiang Y. Differential expression profiles of long noncoding RNAs and mRNAs in human bone marrow mesenchymal stem cells after exposure to a high dosage of dexamethasone. Stem Cell Res Ther . 2021;12:9. doi: 10.1186/s13287-020-02040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine . 2020;19:100249. doi: 10.1016/j.eclinm.2019.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med . 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Islam D, Huang Y, Fanelli V, Delsedime L, Wu S, Khang J, et al. Identification and modulation of microenvironment is crucial for effective mesenchymal stromal cell therapy in acute lung injury. Am J Respir Crit Care Med . 2019;199:1214–1224. doi: 10.1164/rccm.201802-0356OC. [DOI] [PubMed] [Google Scholar]
- 38. Kirkham AM, Bailey AJM, Shorr R, Lalu MM, Fergusson DA, Allan DS. Systematic review and meta-analysis of randomized controlled trials of mesenchymal stromal cells to treat coronavirus disease 2019: is it too late? Cytotherapy . 2023;25:341–352. doi: 10.1016/j.jcyt.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bitterman DS, Cagney DN, Singer LL, Nguyen PL, Catalano PJ, Mak RH. Master protocol trial design for efficient and rational evaluation of novel therapeutic oncology devices. J Natl Cancer Inst . 2020;112:229–237. doi: 10.1093/jnci/djz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther . 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant . 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med . 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther . 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med . 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, Stimamiglio MA, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther . 2022;13:122. doi: 10.1186/s13287-022-02796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fathi-Kazerooni M, Fattah-Ghazi S, Darzi M, Makarem J, Nasiri R, Salahshour F, et al. Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID-19 patients: clinical trial phase I & II. Stem Cell Res Ther . 2022;13:96. doi: 10.1186/s13287-022-02771-w. [DOI] [PMC free article] [PubMed] [Google Scholar]