Abstract
Following intracerebral infection with Theiler’s murine encephalomyelitis virus (TMEV), susceptible strains of mice (SJL and PLJ) develop virus persistence and demyelination similar to that found in human multiple sclerosis. Resistant strains of mice (C57BL/6) clear virus and do not develop demyelination. To resolve the controversy about the role of CD4+ and CD8+ T cells in the development of demyelination and neurologic deficits in diseases of the central nervous system, we analyzed TMEV infection in CD4- and CD8-deficient B6, PLJ, and SJL mice. Genetic deletion of either CD4 or CD8 from resistant B6 mice resulted in viral persistence and demyelination during the chronic stage of disease. Viral persistence and demyelination were detected in all strains of susceptible background. Although genetic deletion of CD8 had no effect on the extent of demyelination in susceptible strains, deletion of CD4 dramatically increased the degree of demyelination observed. Whereas strains with deletions of CD4 showed severe neurologic deficits, mice with deletions of CD8 showed minimal or no deficits despite demyelination. In all strains, deletion of CD4 but not CD8 resulted in a decreased delayed-type hypersensitivity response to viral antigen. We conclude that each T-cell subset makes a discrete and nonredundant contribution to protection from viral persistence and demyelination in resistant strains. In contrast, in susceptible strains, CD8+ T cells do not provide protection against chronic demyelinating disease. Furthermore, in persistent TMEV infection of the central nervous system, neurologic deficits appear to result either from the absence of a protective class II-restricted immune response or from the presence of a pathogenic class I-restricted response.
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system (CNS) in humans. MS lesions are characterized by foci of inflammation, myelin destruction, and formation of astrocytic scars known as plaques. The presence of CD4+ T cells, CD8+ T cells (11), and macrophages in lesions suggests that pathogenesis is immunologically mediated; however, the specific contribution of specific cell types remains unknown (12, 44, 45). Although the etiology of MS is unknown, virus infection is the only epidemiological factor consistently associated with clinical exacerbation (43), and beta interferon, a cytokine with multiple known antiviral properties (46), is the only therapeutic agent definitively shown to decrease exacerbation and limit disability in MS (46). Therefore, the study of viral models of demyelination is extremely relevant.
Theiler’s murine encephalomyelitis virus (TMEV), a picornavirus, induces a pathological and clinical disease similar to MS (24). Intracerebral infection with the Daniel strain (DA) of TMEV induces transient, acute neuronal polioencephalitis followed by chronic white matter demyelination and neurologic deficits in mice with susceptible (H-2f,p,q,r,s,v) major histocompatibility complex (MHC) haplotypes (15, 32). Mice with resistant (H-2b,d,k) MHC haplotypes recover from the acute disease with no obvious long-term sequelae or demyelination. Although TMEV infection of severely immunodeficient SCID mice results in severe neuronal encephalitis and death within approximately 2 weeks, these mice do not develop demyelination in the spinal cord white matter (38). However, when the immune systems of SCID mice are reconstituted by the adoptive transfer of splenocytes from immunocompetent mice or splenocytes treated with antibodies to CD4 or CD8, infection with TMEV results in chronic demyelination (38). These data indicate that similar to human MS, myelin destruction in chronic TMEV infection is immunologically mediated and requires contributions from both CD4+ and CD8+ T cells.
Various reports have implicated both MHC class I- and class II-restricted cells in the pathogenesis of TMEV infection. CD4+ T cells have been implicated by studies demonstrating that demyelination is decreased following treatment with antibodies to CD4 (47) or I-A (34), is increased by adoptive transfer of a CD4+ T-cell line specific for VP2 capsid protein (9), and, in some studies, correlates with the development of a CD4-mediated delayed-type hypersensitivity (DTH) response against virus antigen (5). Furthermore, β2-microglobulin-deficient mice, which are deficient in MHC class I, CD8+ T cells, and natural killer cells, develop demyelinating disease (6, 16, 28). In contrast, a role for CD8+ T cells has been suggested by studies demonstrating that susceptibility to demyelination maps genetically to MHC class I (H-2D) (1, 35), differential expression of MHC class I in the CNS correlates with disease susceptibility (1), and depletion of CD8+ T cells diminishes demyelination (41). Myelin destruction and neurologic deficits develop in TMEV-infected Aβ0 mice which are deficient in functional MHC class II and CD4+ T cells (20). Of interest, both class I and class II-deficient mice share the resistant (H-2b) haplotype. This suggests that although multiple studies have implicated CD4+ and CD8+ T cells in the pathogenesis of TMEV infection, each of these components of the immune response is independently required for maintenance of resistance to demyelination.
In order to definitively establish the contribution of CD4+ and CD8+ T cells to demyelination and neurologic deficits, mice lacking surface expression of CD4 or CD8 were backcrossed onto genetically resistant C57BL/6 (H-2b) and susceptible SJL (H-2s) and PLJ (H-2u) strains. In this report, we confirm that both CD4+ and CD8+ T cells are required for protection from viral persistence and demyelination in resistant strains of mice. We also demonstrate that genetic deletion of CD8 does not significantly affect the degree of demyelination or survival in susceptible strains; however, genetic deletion of CD4 greatly increases the degree of demyelination and worsens clinical disease. Of interest, genetic deletion of CD8 greatly reduces neurologic deficits in animals with demyelination.
MATERIALS AND METHODS
Virus.
TMEV DA was used in all experiments. The passage history of this virus has been described previously (36). Animals were infected by intracerebral injection of 2 × 105 PFU of TMEV DA in 10 μl.
Mice.
Mice lacking surface expression of CD4 or CD8 were generated at the Ontario Cancer Institute (8, 26, 50). By using homologous recombination in ES cells, CD4 (−/−) mice were generated by interrupting exon 5 of the L3T4 gene by the insertion of neomycin resistance gene sequences in the coding sequence (26). Similarly, CD8 (−/−) mice were generated by disrupting the first exon of the LYT-2 gene (8). Mice deficient in CD4 and CD8 were generated on a haplotype normally susceptible to TMEV-induced demyelination, by crossing to SJL and PLJ strains for 8 to 10 generations. Littermate B6 CD4 (+/−), B6 CD8 (+/−), SJL CD4 (+/−), and SJL CD8 (+/−) mice or wild-type PLJ/J (+/+) mice originally obtained from Jackson Laboratory (Bar Harbor, Maine) were used as controls. This study describes pathologic and virologic data on a total of 245 mice. Handling of all animals conformed to the National Institutes of Health and Mayo Clinic institutional guidelines.
Analysis of clinical deficits in mice.
Mice were monitored weekly for clinical deficits in the categories of general appearance, activity level, and paralysis. For the purpose of evaluation, mice considered symptomatic showed changes in coat or fur, were unkempt or incontinent, demonstrated decreased spontaneous movement as observed in the cage, or demonstrated stiffness or paralysis in one or more extremities. Mice which died during the chronic stage of infection were included in the assessment.
Quantitation of pathologic findings in the brain.
Brains from perfused animals were cut into three coronal sections, embedded in paraffin, and stained with hematoxylin and eosin. The cerebellum, brain stem, hippocampus, striatum, cerebral cortex, and corpus callosum were graded independently, without knowledge of experimental groups, on a four-point scale for the presence of inflammation, demyelination, and necrosis: 0, no pathologic abnormalities; 1, minimal inflammation; 2, moderate inflammation without parenchymal injury; 3, intense inflammation with definite tissue destruction (demyelination, parenchymal damage, cell death, neuronophagia, or neuronal vacuolation); 4, necrosis (complete loss of all tissue elements with associated cellular debris). Meningeal inflammation was assessed as follows: 0, no inflammation; 1, one cell layer of inflammation; 2, two cell layers of inflammation; 3, three cell layers of inflammation; 4, four or more cell layers of inflammation.
Preparation of spinal cords for pathologic analysis.
On days 7 and 45 after infection, mice were anesthetized intraperitoneally with 10 mg of sodium pentobarbital and perfused by intracardiac puncture with Trump’s fixative (phosphate-buffered 4% formaldehyde with 1.5% glutaraldehyde [pH 7.2]), and spinal cords were processed to provide 2-μm-thick glycolmethacrylate-embedded sections. The 7-day time represents the point of maximal inflammation in the brain, and the 45-day time distinguishes between resistance and susceptibility to TMEV-induced demyelination (1, 31). Detailed, nonbiased morphological analyses were performed on 12 to 15 spinal cord sections from each mouse without knowledge of experimental groups. Every quadrant from each coronal section was scored for the presence or absence of neuronal inflammation, meningeal inflammation, and demyelination and was expressed as the percentage of quadrants showing the pathologic abnormality in a given mouse. The maximum score of 100 indicated the presence of the pathologic abnormality in every quadrant of all spinal cord sections of an individual mouse. A total of 8,265 spinal cord quadrants were examined in this study. Statistical significance is reported at P < 0.05 by Student’s t test and is specified in the text.
Virus plaque assays.
Viral titers in clarified CNS homogenates were determined by plaque assay as described previously (36). On days 7 and 45 after infection, CNS homogenates were prepared from brains and spinal cords that had been removed aseptically. A 10% (wt/vol) homogenate was prepared in Dulbecco modified Eagle medium, sonicated three times for 20 s each, and clarified by centrifugation. Virus preparations were stored at −70°C before use. Each assay was performed at least in duplicate, and in most cases in triplicate, on coded samples without knowledge of experimental groups. Data were expressed as log10 PFU per gram of CNS tissue. Statistical significance is reported at P < 0.05 by the Mann-Whitney rank sum test and is specified in the text.
Immunocytochemistry for virus antigen.
For immunoperoxidase studies, coronal spinal cord sections (five or six per mouse) from perfused animals were stored in 0.1 M phosphate buffer, rinsed in 0.1 M Tris buffer with 25 mM hydroxylamine (pH 7.4), treated with 10% dimethyl sulfoxide for 1 h, and quick-frozen in liquid nitrogen-chilled isopentane. Cryostat sections were incubated with a polyclonal rabbit antiserum to purified TMEV DA virions (36), which specifically reacts to all structural proteins of TMEV (36). Slides were developed by using the avidin-biotin immunoperoxidase system (Vector Laboratories, Burlingame, Calif.). For quantitative analysis, a Zeiss microscope attached to a camera lucida was used to project the spinal cord images onto a ZIDAS (Carl Zeiss Inc., Oberkochen, Germany) digitizing tablet. Spinal cord areas were traced to determine the total area (expressed in square millimeters). We analyzed a minimum of 5.89 mm2 to a maximum of 28.07 mm2 of spinal cord for each mouse. The total area of spinal cord examined by combining all animals from the experimental groups was 931 mm2. The number of virus antigen-positive cells for each mouse was counted and expressed per area of spinal cord.
In situ hybridization.
In situ hybridization for TMEV RNA was carried out as described previously (20). Briefly, fixed sections were treated with 1 μg of proteinase K per ml, acetylated, and prehybridized for 4 h at room temperature with buffer containing deionized formamide, Denhardt’s solution, sodium chloride, salmon sperm DNA, and yeast tRNA. Slides were hybridized with 35S-labeled 253-bp (nucleotides 3053 to 3305) and 363-bp (nucleotides 3306 to 3668) cDNA probes corresponding to VP1 of TMEV DA (22). The cDNA probes were obtained by double digestion of the VP1 plasmid with KpnI and SalI and radiolabeling of the probes with 0.5 × 108 to 0.8 × 108 cpm of [α-35S]dATP per μg of DNA by nick translation. TMEV RNA-positive cells were detected by autoradiography in photographic emulsion.
Virus-specific ELISA.
Anti-TMEV antibodies were measured by enzyme-linked immunosorbent assay (ELISA) with purified TMEV antigen. Sera were diluted from 1/400 to 1/102,400 in phosphate-buffered saline. Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) and IgM (heavy and light chains) were used as the detecting antibodies. Known hyperimmune sera and sera from uninfected mice were included as positive and negative controls, respectively.
Virus neutralization assay.
Samples of TMEV DA, diluted to contain 50 PFU/0.2 ml, were mixed with an equal volume of twofold dilutions of heat-inactivated (45 min at 56°C) serum from TMEV DA-infected mice or noninfected mice or with medium alone. After incubation at 25°C for 1 h, the virus-serum mixtures were assayed for residual infectious virus by plaque assay. Neutralization titers were expressed as the log2 dilution of serum which resulted in a 95% reduction in virus titer.
DTH responses to virus antigen.
TMEV-specific DTH responses were evaluated by intradermal injection of 10 μl of UV-inactivated purified virus (2 × 108 PFU/ml before inactivation). The increase in ear thickness over the prechallenge measurement was recorded with a Peacock dial gauge G-50 micrometer (Ozaki Manufacturing Co.) 24 and 48 h after intradermal challenge. Units are expressed as 10−2 millimeters.
RESULTS
Both CD4+ and CD8+ T cells independently protect resistant mice from demyelination.
T- and B-cell deficient SCID mice do not develop demyelination unless they receive an exogenous source of functional lymphocytes (38). To determine the specific T-cell subsets required for immunologically mediated myelin destruction, spinal cords obtained from CD4- and CD8-deficient mice on a resistant background were analyzed for the presence or absence of demyelination 45 days after TMEV infection (Table 1). As expected, demyelination was not detected in spinal cords from B6 CD4 (+/−) or B6 CD8 (+/−) mice (Fig. 1). Although B6 CD8 (−/−) mice did not develop clinical disease, foci of demyelination with meningeal inflammation (Fig. 1) were present in 10 of 12 of these mice. As indicated in Table 1, demyelination (in 6.8 ± 2.2% of quadrants) and inflammation (in 3.3 ± 1.5% of quadrants) was significantly greater than in littermate controls (P < 0.01 by Student’s t test). In contrast, demyelination was detected in only one of six B6 CD4 (−/−) mice at this time point. By 90 days, however, foci of demyelination were detected in all of the B6 CD4 (−/−) mice (n = 17) (Fig. 2). At this time point, lesions were detected in 19.4 ± 4.0% of the 645 quadrants examined. These experiments indicate that neither CD4+ or CD8+ T cells are absolutely required for the development of demyelination and that each subset makes an independent contribution to protection from the development of pathologic changes.
TABLE 1.
Quantitation of spinal cord pathologic findingsa
| Strain | No. of mice | % of quadrants (mean ± SE) expressing:
|
|
|---|---|---|---|
| Meningeal inflammation | Demyelination | ||
| B6 CD4 +/− | 6 | 0.8 ± 0.0 | 0.6 ± 0.6 |
| B6 CD4 −/− | 6 | 0.9 ± 0.6 | 0.4 ± 0.4 |
| B6 CD8 +/− | 6 | 0.9 ± 0.9 | 0.9 ± 0.9 |
| B6 CD8 −/− | 12 | 3.3 ± 1.5 | 6.8 ± 2.2b |
| PLJ +/+ | 7 | 23.3 ± 2.0 | 39.5 ± 4.9 |
| PLJ CD4 −/− | 7 | 45.3 ± 7.2 | 72.5 ± 6.2b |
| PLJ CD8 −/− | 7 | 23.3 ± 4.9 | 39.2 ± 3.1 |
| SJL CD4 +/− | 4 | 16.5 ± 7.1 | 16.0 ± 6.7 |
| SJL CD4 −/− | 7 | 20.0 ± 3.5 | 70.2 ± 4.6b |
| SJL CD8 +/− | 5 | 13.4 ± 5.6 | 10.8 ± 4.5 |
| SJL CD8 −/− | 11 | 22.4 ± 4.8 | 25.9 ± 4.3 |
Quantitative analysis of meningeal inflammation and demyelination in spinal cords 45 days following TMEV infection. No or minimal gray matter (neuronal) disease was observed in any of these strains.
Statistically significant (P < 0.05) compared to heterozygous (+/−) or wild-type (+/+) controls by Student’s t test.
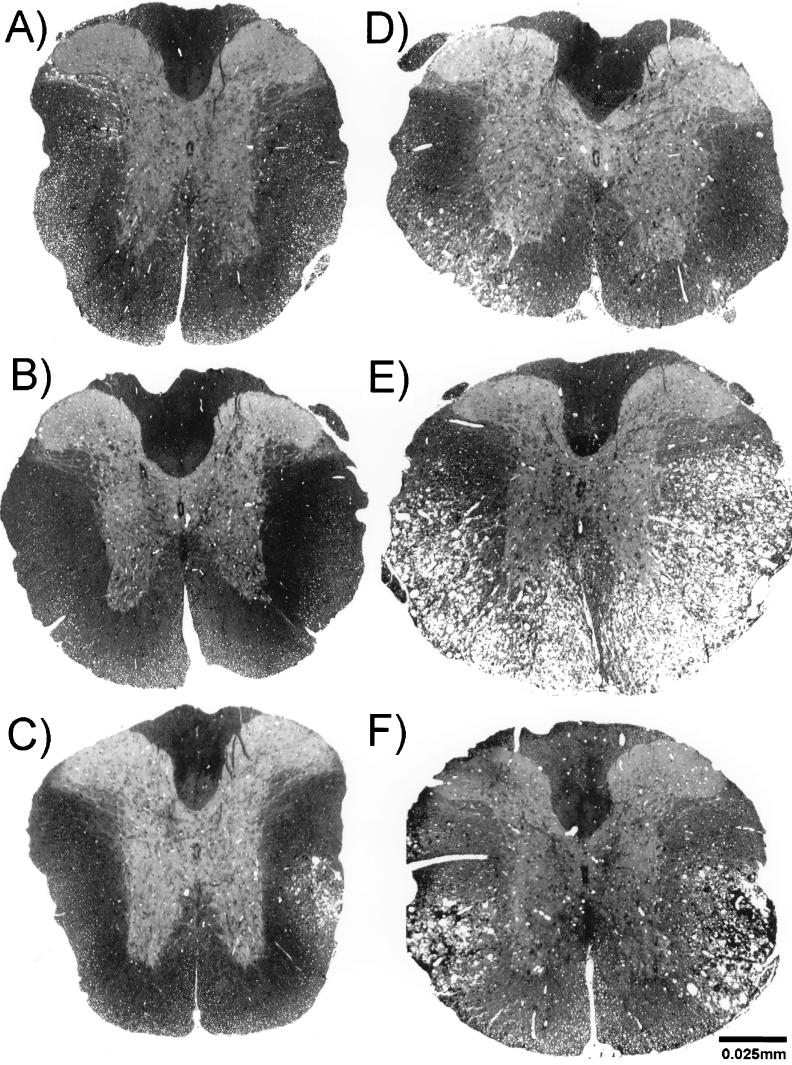
FIG. 1.
Spinal cord cross sections from 45-day-infected B6 (A through C) and PLJ (D through F), wild-type (A and D) CD4-deficient (B and E), or CD8-deficient (C and F) mice. Glycolmethacrylate-embedded sections were stained with erichrome/cresyl violet stain. White matter appeared normal in B6 (+/+) (A) and B6 CD4 (−/−) (B) mice. Focal demyelinating lesions were present in B6 CD8 (−/−) (C), PLJ (+/+) (D), and PLJ CD8 (−/−) (F) mice, and massive demyelination was found in PLJ CD4 (−/−) mice (E).
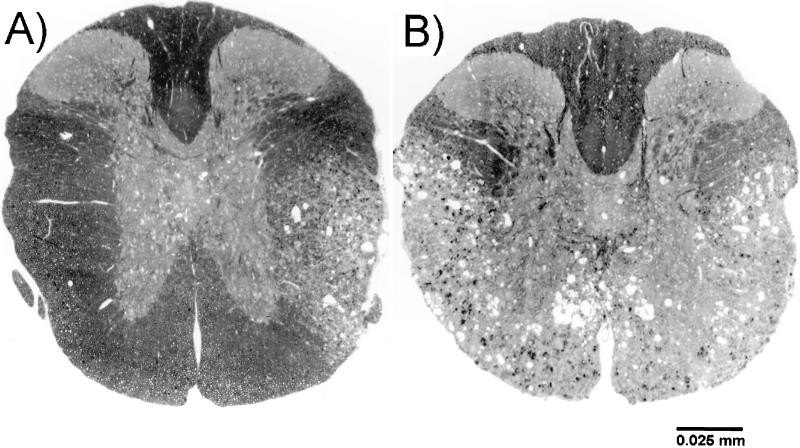
FIG. 2.
Spinal cord cross sections from mice infected for 90 days demonstrates demyelination in B6 CD4 (−/−) mice (A) and more severe demyelination in PLJ CD4 (−/−) mice (B).
CD4+ T cells protect against severe demyelination in mice of susceptible genotype.
White matter demyelination was present in all of the spinal cords obtained from both susceptible SJL and PLJ strains; however, the most severe abnormalities occurred in CD4-deficient mice (Table 1). In these mice, extensive meningeal inflammation and severe myelinolysis with vacuolar degeneration were observed (Fig. 1E). Demyelinating lesions were detected in more than 70% of the quadrants examined and could encompass entire spinal cord cross sections. Significantly more demyelination (P < 0.001 by Student’s t test) was detected in CD4 (−/−) (Fig. 1E) mice than in CD8 (−/−) mice (Fig. 1F) or wild-type (+/+) mice (Fig. 1A) for both the PLJ and SJL backgrounds. Although the extent of demyelination in SJL CD8 (−/−) and PLJ CD8 (−/−) mice was greater than observed in B6 CD8 (−/−) mice, it was not significantly different when compared by strain to that in littermate heterozygote SJL CD8 (+/−) or wild-type PLJ (+/+) controls. Therefore, in susceptible strains, genetic deletion of CD4+ T cells dramatically increases the development of demyelination whereas deletion of CD8+ T cells appears to have minimal effect.
Deletion of CD4+ T cells predisposes susceptible strains to severe parenchymal brain disease following TMEV infection.
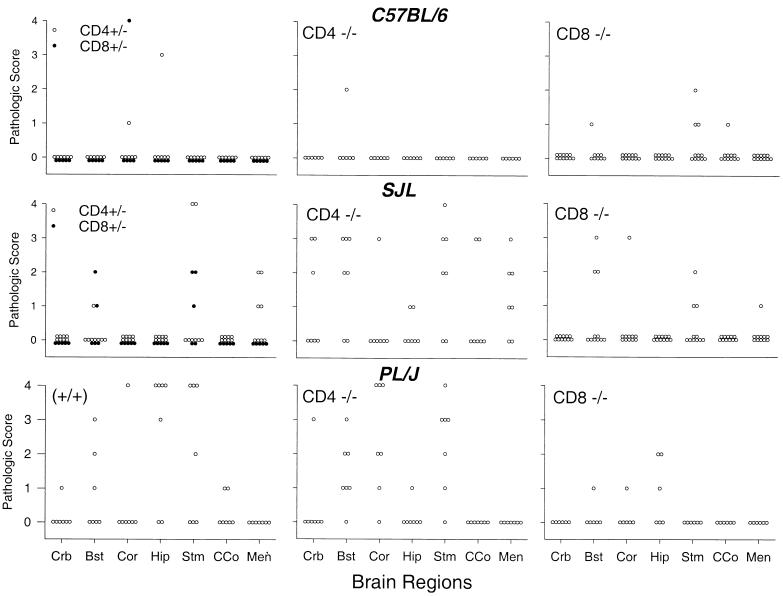
Intracerebral infection with TMEV results in acute inflammation in all strains; however, mice with resistant genotypes completely clear TMEV without evidence of chronic pathologic change (15). In susceptible strains, the majority of brain inflammation is also cleared; however, persistent virus and inflammation can be seen in the brain stem and cerebellum of SJL mice. We assessed the contribution of CD4+ and CD8+ T cells to parenchymal disease of the cerebellum, brain stem, cortex, hippocampus, striatum, and corpus callosum and to infiltration of inflammatory cells in the meninges. At 7 days postinfection, inflammation was widespread in both resistant and susceptible strains of mice, with the most severe disease being localized to the cortex, hippocampus, and striatum (data not shown). At this acute time point, B6 CD4 (−/−) mice had significantly increased inflammation in the brain stem, hippocampus, and corpus callosum compared to B6 CD4 (+/−) controls (P < 0.05, Mann-Whitney rank sum test). No significant differences in the distribution or degree of parenchymal brain disease were detected in the remaining strains irrespective of deletion of CD4 or CD8. By 45 days, however, the majority of brain inflammation had resolved in B6 mice even in the absence of CD4 or CD8 (Fig. 3). In contrast, in SJL and PLJ mice, severe brain disease (scores of ≥2) persisted in the cerebellum, brain stem, cortex, hippocampus, striatum, and corpus callosum. The most severe and extensive disease occurred in SJL CD4 (−/−) and PLJ CD4 (−/−) mice. Increased disease in CD4 (−/−) mice compared to CD8 (−/−) mice of the susceptible haplotype was observed primarily in the cerebellum, brain stem, and striatum.
FIG. 3.
Pathology scores in brain (Crb, cerebellum; Bst, brain stem; Cor, cortex; Hip, hippocampus; Stm, striatum; CCo, corpus callosum; Men, meninges) in C57BL/6, SJL, and PLJ mice infected with TMEV for 45 days. Mice with genetic deletion of CD4 (CD4 −/−) or CD8 (CD8 −/−) were compared to heterozygote CD4 +/− (open circles) or CD8 +/− (solid circles) or wild-type (+/+) mice of each strain.
Both CD4+ and CD8+ T cells protect against persistent virus infection in resistant mice, but deletion of CD8 does not affect infectious virus in susceptible strains.
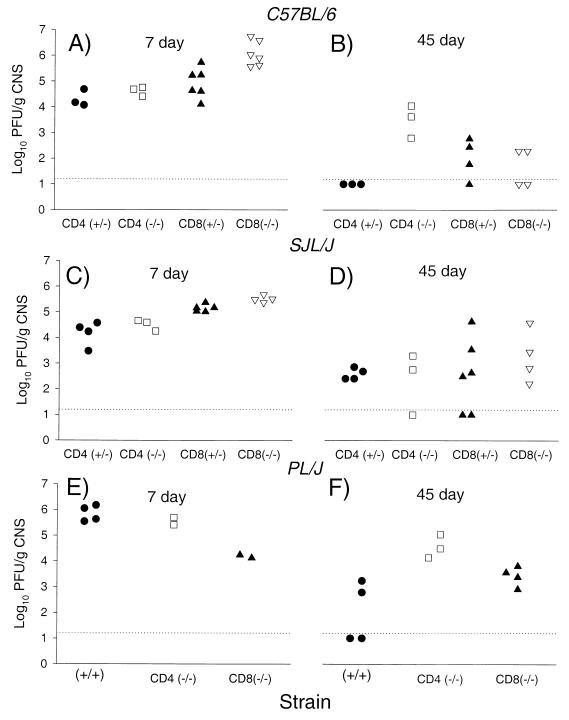
Intracerebral infection with TMEV results in acute encephalitis that is cleared by mice with resistant but not susceptible haplotypes (15). To determine the relative contributions of CD4+ and CD8+ T cells in viral clearance, viral plaque assays were performed on resistant B6 (H-2b) and susceptible PLJ (H-2u) and SJL (H-2s) mice genetically deficient in CD4 or CD8 (Fig. 4). Seven days after infection, 4.07 to 6.74 log10 PFU of infectious virus per g of CNS tissue was detected in all mice of both resistant and susceptible haplotypes. There was a statistically significant increase in replicating virus in B6 CD8 (−/−) mice compared with other mice on the resistant (H-2b) background (P < 0.05, Mann-Whitney rank sum test). Similarly, in the SJL (H-2s) strain, there was a statistically significant increase in the amount of replicating virus in SJL CD8 (−/−) mice compared to littermate controls (P < 0.05, Mann-Whitney rank sum test). These data support a role for CD8+ T cells for clearance of infectious virus during the acute stages of TMEV infection. However, this was not confirmed in experiments with PLJ CD8 (−/−) mice, which demonstrated less virus than did PLJ (+/+) and PLJ CD4 (−/−) mice. At 45 days after infection, a time point which distinguishes resistance and susceptibility to TMEV persistence (1), infectious virus above the sensitivity (1.7 log10 PFU/g of CNS tissue) of the plaque assay was detected in the CNS in none of the three B6 CD4 (+/−) mice and in only one of four B6 (CD8 +/−) mice (Fig. 4). Infectious virus persisted in all B6 CD4 (−/−) mice and two of four B6 CD8 (−/−). On average, 100 times as much virus was detected at 45 days in B6 CD4 (−/−) mice as in B6 CD8 (−/−). As expected, infectious virus was detected 45 days after inoculation in most SJL or PLJ mice irrespective of the CD4 or CD8 deletion. Titers of infectious virus appeared higher in PLJ CD4 (−/−) mice than in PLJ CD8 (−/−) mice, but the data were not statistically significant (P = 0.0571, Mann-Whitney rank sum test). No difference was apparent in chronically infected SJL mice.
FIG. 4.
Titers of infectious virus isolated by plaque assay from the CNS (brain and spinal cord) of mice infected with TMEV for 7 days (A, C, and E) or 45 days (B, D, and F). Mice with genetic deletion of CD4 [CD4 (−/−)] or CD8 [CD8 (−/−)] are compared to heterozygote [CD4 (+/−) or CD8 (+/−)] littermate or wild-type (+/+) mice. Dashed lines represent the sensitivity of the assay. Data are expressed as log10 PFU per gram of CNS tissue.
To demonstrate conclusively that virus antigen and virus RNA persisted in CNS cells, we performed immunoperoxidase staining and in situ hybridization on a minimum of three mice of the B6 and PLJ strains with and without genetic deletions 7 and 45 days after infection. At 7 days, virus was detected almost exclusively in brain gray matter in all strains. By 45 days, virus antigen and virus RNA were detected in the spinal cord white matter of both B6 CD4 (−/−) and B6 CD8 (−/−) mice but not B6 CD4 (+/−) or B6 CD8 (+/−) mice. There was no statistically significant difference in the number of virus antigen-positive cells in B6 CD4 (−/−) mice (0.2 ± 0.1 cells/mm2) and B6 CD8 (−/−) mice (0.3 ± 0.3 cells/mm2). These data were less striking than those obtained by the viral plaque assay. This may be partially explained by the fact that although immunostaining for viral antigen and in situ hybridization for detection of virus RNA are extremely specific for detecting virus replication in CNS cells, because they do not sample the entire CNS they may be less quantitative methods for detection of virus load than is the viral plaque assay. Similar results were obtained using in situ hybridization to detect virus RNA. Consistent with the plaque assay data, CD4-deficient susceptible PLJ (H-2u) mice demonstrated the greatest number of antigen-positive cells (19.5 ± 16.4 cells/mm2) compared to CD8-deficient mice (2.1 ± 0.8 cells/mm2) or PLJ (+/+) controls (1.8 ± 0.3 cells/mm2).
Genetic deletion of CD4 worsens neurologic deficits in resistant and susceptible strains.
Persistent CNS infection with TMEV results in chronic progressive demyelinating disease in the spinal cord white matter and concomitant development of neurologic deficits. Some experiments argue for a critical role for CD4+ T cells (19) in the induction of neurologic disease; however, other data do not support this hypothesis (20). To resolve this controversy, mice with and without genetic deletion of CD4 or CD8 on a resistant B6 and susceptible PLJ and SJL genetic background were infected with TMEV and monitored weekly for signs of clinical disease, including changes in appearance, spasticity, weakness, paralysis, and death during chronic disease. As expected, resistant B6 (+/+) mice did not develop clinical signs of disease even when observed for 6 months. Of the B6 CD4 (−/−) mice (n = 70), 4.3% showed signs of chronic infection by 45 days postinfection, and by 6 and 10 months 42.5% (17 of 40) and 93.3% (28 of 30), respectively, were symptomatic. In contrast, despite demyelination, clinical disease was absent in resistant B6 CD8 (−/−) mice monitored for a similar period. This is consistent with the hypothesis that CD8+ T cells can mediate neurologic injury (28).
Disease in susceptible SJL (+/+) mice was chronic and progressive and was detected in 11.5% of mice at 3 months (n = 26) and 64% of mice by 6 months (n = 22). Disease was most severe in SJL CD4 (−/−) mice, of which 65.3% showed signs of disease by 2 months (n = 49) and 93% were symptomatic by 6 months (n = 43). In contrast, disease was least severe in SJL CD8 (−/−) mice, of which only 11% (n = 9) showed disease by 6 months. Similarly, in the PLJ strain, susceptibility to clinical disease was dramatically increased in PLJ CD4 (−/−) mice, with 84% being symptomatic by 6 months postinfection (n = 37) compared to 32% of wild-type controls (n = 22). Similar to the observations with resistant strains, PLJ CD8 (−/−) showed minimal signs of disease even at very long time points, with a disease incidence of 7.1% at 6 months (n = 14). Therefore, CD4+ T cells protect or ameliorate neurologic deficits in both susceptible and resistant strains of mice, whereas mice genetically deficient in CD8 develop less significant clinical disease.
TMEV-specific antibody responses are maintained in mice with genetic deletions of CD4 or CD8.
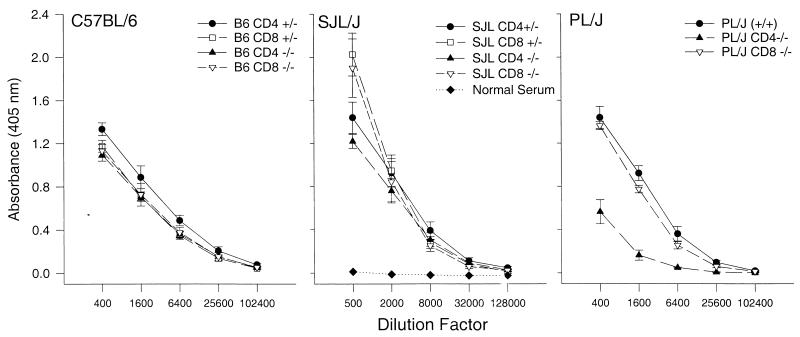
Previous experiments have indicated that CD4-deficient mice maintain the ability to undergo isotype switching from IgM to IgG in vivo (27). To determine if genetic deletion of CD4 or CD8 alters the antibody response to persistent virus, we analyzed TMEV-specific antibody responses in the serum 7 and 45 days after infection. TMEV-specific antibodies were not present 7 days postinfection; however, high titers were detected in all strains 45 days postinfection (Fig. 5). No significant differences were detected in B6, SJL, or PLJ mice. Genetic deletion of CD4 or CD8 did not significantly alter the TMEV antibody response by ELISA in B6 or SJL mice. TMEV-specific antibody responses were reduced in PLJ CD4 (−/−) mice compared to those in PLJ (+/+) or PLJ CD8 (−/−) mice, but these responses were still greater than those observed with sera from uninfected mice. To determine if the antibodies were capable of neutralizing infectious virus, a plaque assay was performed with sera from resistant B6 and susceptible PLJ mice. The minimum log2 serum dilution required to neutralize infectious virus (1,000 PFU/ml) was similar for all groups [B6 CD8 (+/−), 9; B6 CD4 (−/−), 9 to 10; B6 CD8 (−/−), 8 to 10; PLJ (+/+), 8 to 11; PLJ CD4 (−/−), 8 to 11; and PLJ CD8 (−/−), 10 to 11]. Normal mouse serum at a twofold dilution did not neutralize virus. Therefore, in the PLJ strain, although genetic deletion of CD4 reduced the TMEV-specific antibody titers detected by ELISA, levels of neutralizing titers did not appear to be significantly altered. Therefore, alterations in antibody responses are not likely to have been the sole factor in determining disease pathogenesis in CD4- or CD8-deficient mice.
FIG. 5.
Theiler’s virus-specific antibody in the sera of mice infected with virus for 45 days. C57BL6, SJL, and PLJ mice with genetic deletion of CD4 (CD4 −/−) or CD8 (CD8 −/−) were compared to heterozygote (CD4 +/− or CD8 +/−) littermate controls or wild-type (+/+) mice. Results from serum of uninfected wild-type SJL mice are shown for comparison.
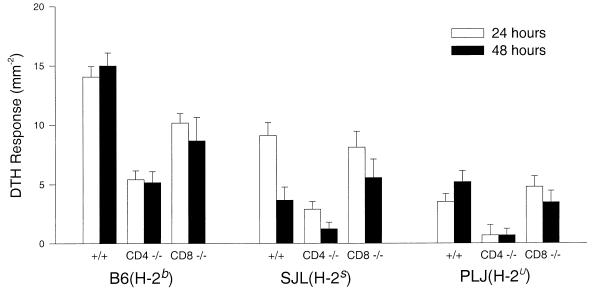
DTH to virus antigen does not correlate with demyelination or the development of neurologic deficits following TMEV infection.
It has been proposed that development of demyelination correlates with DTH responses to virus antigen in the TMEV model (5). However, previous experiments have not supported this hypothesis (33). We therefore investigated the correlation between demyelination and DTH responses in the ears of resistant B6 and susceptible SJL and PLJ mice with and without deletion of CD4 or CD8. Although highest in B6 mice, positive responses were observed consistently in wild-type B6, SJL, or PLJ mice with normal CD4+ or CD8+ T cells (Fig. 6). Lower responses were observed in CD4 (−/−) mice than in CD8 (−/−) mice irrespective of strain. There was no positive correlation between the degree of DTH response and the extent of demyelination or the severity of clinical deficits. As would be expected from an MHC class II-restricted CD4+ T-cell response, the lowest DTH scores were consistently observed in SJL CD4 (−/−) and PLJ CD4 (−/−), the strains with the greatest demyelination and clinical disease.
FIG. 6.
TMEV-specific DTH responses were elicited by intradermal ear injection with 10 μl of UV-inactivated virus (2 × 108 PFU/ml). Immediately before antigen challenge, and 24 and 48 h after antigen challenge, ear thickness was measured and expressed as 10−2 millimeters. Error bars indicate the standard error of the mean.
DISCUSSION
Determining the precise contribution of CD4+ and CD8+ T cells to the pathogenesis of virus-induced demyelination is complex. Experiments with SCID mice demonstrate that T lymphocytes are critical for the development of an effective antiviral immune response but are also required for the development of immunologically mediated tissue damage (38). The present experiments provide important new insights into the contribution of CD4+ and CD8+ T cells in clearance of TMEV from the CNS but also show how the T-cell subsets participate in myelin sheath destruction and induction of neurologic deficits in demyelinating disease.
Mice with resistant haplotypes normally clear TMEV from the CNS within 2 to 3 weeks after infection (23). Here we show that both CD4+ and CD8+ T-cell subsets make independent and nonredundant contributions to protection against persistent TMEV infection and chronic demyelinating disease in the spinal cord white matter of B6 mice. Although B6 CD4 (−/−) mice demonstrated much higher viral titers than did B6 CD8 (−/−) mice, onset of demyelination was delayed compared to that in B6 CD8 (−/−) mice. This may be the result of a protective cytotoxic T-cell response in CD4-deficient mice. It is also possible that CD4-deficient mice have delayed clearance of virus from the spinal cord gray matter or that CD4+ T cells are potent inducers of myelin destruction during early disease but are not necessary during late disease.
In contrast to the resistant strain, demyelination was detected in wild-type SJL and PLJ mice. Although genetic deletion of CD8 had no effect, deletion of CD4 nearly doubled the extent of demyelination in each susceptible strain. Previous experiments in our laboratory have demonstrated that susceptibility and resistance to demyelination map to the H-2D locus and that virus-specific cytotoxicity in CNS-infiltrating lymphocytes is impaired in mice susceptible to demyelination (14). This is consistent with the hypothesis that susceptibility to demyelination is due to an ineffective MHC class I-restricted immune response in SJL (H-2s) and PLJ (H-2u) mice; it is therefore not surprising that deletion of CD8 had no effect. Conversely, because class II-restricted lymphocytes play a critical role in the generation of a protective immune response in these strains, further immunosuppression by genetic deletion of CD4 resulted in a dramatic increase in demyelination.
Neurologic deficits were relatively absent in B6 CD8 (−/−) mice, and fewer clinical deficits were observed in susceptible animals deficient in CD8+ T cells compared to mice deficient in CD4+ T cells. One explanation for the more severe disease phenotype observed in CD4 (−/−) mice is the increased viral burden in the CNS. This was observed in B6 CD4 (−/−) and PLJ CD4 (−/−) mice but not SJL CD4 (−/−) mice. The increased viral burden observed in B6 CD4 (−/−) compared to B6 CD8 (−/−) is consistent with previous experiments demonstrating a 100- to 1,000-fold increase in virus titers in class II-deficient (Aβ0) versus class I-deficient (β2-microglobulin-deficient) mice of identical genotype chronically infected with TMEV (16, 20).
An alternative explanation is that in the context of demyelinating disease, class I-restricted cytotoxic lymphocytes induce neurologic deficits following demyelination (28). This hypothesis is supported by experiments in which MHC class I-deficient mice show demyelination but no clinical deficits (16, 28) whereas class II-deficient (Aβ0) mice of identical H-2b haplotype show demyelination and neurological deficits and die (20). Similarly, experiments on experimental allergic encephalomyelitis (EAE) showed that even though CD8 (−/−) mice had a higher frequency of relapses, acute EAE was less severe and was associated with reduced mortality (13). Also, CD8 (−/−) mice infected with lymphocytic choriomeningitis virus survive acute choriomeningitis without clinical deficits, in contrast to wild-type mice (7). Because CD8+ T cells and CD4 (Th1 subset) T cells have many cytokines in common, it is unlikely that their shared effector molecules are critical for the induction of neurologic disease. Instead, the present data suggests that a factor exclusively generated during a cytotoxic class I-restricted immune response might injure vulnerable denuded axons and slow or block neuronal conduction.
At least one T-cell subset is required for demyelination to occur in SCID mice (38), although the exact mechanism by which demyelination develops during TMEV infection is not known. Major hypotheses include (i) direct cytolytic infection of oligodendrocytes (5, 30, 36), (ii) an autoimmune attack against myelin antigens (17), (iii) “bystander demyelination” from the release of toxic mediators from macrophages recruited by CD4+ T cells (9), and (iv) TMEV-specific, immunologically mediated tissue destruction of persistently infected glial cells (39).
Several studies support direct cytolytic infection of oligodendrocytes as a mechanism of demyelination. Persistent virus is required for the development of demyelination (4), TMEV infects oligodendrocytes in vitro (10, 21, 40, 49) and in vivo (2, 3, 25, 36, 37, 48), and TMEV preferentially kills oligodendrocytes in mixed glial cultures (10, 40). Although small foci of demyelination have been observed in nude mice of the BALB/c genotype (42), the massive viral burden but lack of demyelination in SCID mice argues against this hypothesis. Immune cells reactive against myelin epitopes are found during the chronic phase of disease, supporting the autoimmune hypothesis (19). However, they are not detected until demyelinating lesions are well developed. TMEV-induced demyelination does not induce significant proliferative responses against myelin antigens prior to the onset of demyelination, and the disease is not protected by tolerizing to myelin antigens, which is effective in EAE (17). The bystander hypothesis proposes that activation of macrophages by CD4+ T cells is responsible for myelin destruction (18, 19). However, in the present experiments, CD4 (−/−) mice from susceptible and resistant genotypes developed severe demyelination, clinical disease, and low or absent DTH responses to viral antigen challenge. Therefore, data from these experiments are most consistent with the hypothesis that demyelination results from an immune response against infected glial cells. Although either CD4+ or CD8+ T cells appear to participate independently in the development of white matter pathologic changes, the ultimate effector is not known. Activated macrophages may engulf myelin debris or injured oligodendrocytes, or, alternatively, CD4+ or CD8+ T cells may directly interfere with the myelinating function of oligodendrocytes without actually killing the cell (29). Such a process would be manifested morphologically as dying-back oligodendrogliopathy (29).
ACKNOWLEDGMENTS
These experiments were supported by grants from the National Institutes of Health (R01-NS24180, NS32129, and N01-AI-45197), the National Multiple Sclerosis Society (RG2203B-6), and the Multiple Sclerosis Society of Canada.
We appreciate the excellent technical assistance of Mabel L. Pierce, Roger L. Thiemann, and Laurie Zoecklein for tissue processing and histological sections. We thank Tak Mak for donation of CD4- and CD8-deficient mice, and we thank Alexandra Ho for breeding CD4 (−/−) and CD8 (−/−) mice onto PLJ and SJL backgrounds. We also thank Rafael L. Ufret-Vincenty (University of Puerto Rico School of Medicine, San Juan, Puerto Rico) for assistance in this project as a Minority Medical School Student Summer Research Trainee in the Department of Immunology (Mayo Medical School).
REFERENCES
- 1.Altintas A, Cai Z, Pease L R, Rodriguez M. Differential expression of H-2K and H-2D in the central nervous system of mice infected with Theiler’s virus. J Immunol. 1993;151:2803–2812. [PubMed] [Google Scholar]
- 2.Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore W F, Welsh C J, Tonks P, Nash A A. Observations on demyelinating lesions induced by Theiler’s virus in CBA mice. Acta Neuropathol. 1988;76:581–589. doi: 10.1007/BF00689596. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro M, Aubert C, Brahic M. Demyelinating lesions due to Theiler’s virus are associated with ongoing central nervous system infection. J Virol. 1986;57:992–997. doi: 10.1128/jvi.57.3.992-997.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clatch R J, Melvold R W, Miller S D, Lipton H L. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV-specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–1414. [PubMed] [Google Scholar]
- 6.Fiette L, Aubert C, Brahic M, Rossi C P. Theiler’s virus infection of beta 2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung-Leung W P, Kundig T M, Zinkernagel R M, Mak T W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung-Leung W P, Schilham M W, Rahemtulla A, Kundig T M, Vollenweider M, Potter J, van Ewijk W, Mak T W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 9.Gerety S J, Rundell M K, Dal Canto M C, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 10.Graves M C, Bologa L, Siegel L, Londe H. Theiler’s virus in brain cell cultures: lysis of neurons and oligodendrocytes and persistence in astrocytes and macrophages. J Neurosci Res. 1986;15:491–501. doi: 10.1002/jnr.490150406. [DOI] [PubMed] [Google Scholar]
- 11.Hauser S L, Bhan A K, Gilles F, Kemp M, Kerr C, Weiner H L. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19:578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- 12.Hunter S F, Rodriguez M. Multiple sclerosis: a unique immunopathological syndrome of the central nervous system. Springer Semin Immunopathol. 1995;17:89–105. doi: 10.1007/BF00194102. [DOI] [PubMed] [Google Scholar]
- 13.Koh D R, Fung-Leung W P, Ho A, Gray D, Acha-Orbea H, Mak T W. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Pease L R, Rodriguez M. Differential generation of class I H-2D- versus H-2K-restricted cytotoxicity against a demyelinating virus following central nervous system infection. Eur J Immunol. 1997;27:963–970. doi: 10.1002/eji.1830270424. [DOI] [PubMed] [Google Scholar]
- 15.Lipton H L. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller D J, Riveraquinones C, Njenga M K, Leibowitz J, Rodriguez M. Spontaneous CNS remyelination in Beta(2) microglobulin-deficient mice following virus-induced demyelination. J Neurosci. 1995;15:8345–8352. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller S D, Gerety S J, Kennedy M K, Peterson J D, Trotter J L, Tuohy V K, Waltenbaugh C, Dal Canto M C, Lipton H L. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1990;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, S. D., C. L. Vanderlugt, W. S. Begolka, W. Pao, K. L. Neville, R. L. Yauch, and B. S. Kim. 1997. Epitope spreading leads to myelin-specific autoimmune responses in SJL mice chronically infected with Theiler’s virus. J. Neurovirol. 3(Suppl. 1):S62–S65. [PubMed]
- 19.Miller S D, Vanderlugt C L, Begolka W S, Pao W, Yauch R L, Neville K L, Katz-Levy Y, Carrizosa A, Kim B S. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 20.Njenga M K, Pavelko K D, Baisch J, Lin X, David C, Leibowitz J, Rodriguez M. Theiler’s virus persistence and demyelination in major histocompatibility complex class II-deficient mice. J Virol. 1996;70:1729–1737. doi: 10.1128/jvi.70.3.1729-1737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohara Y, Konno H, Iwasaki Y, Yamamoto T, Terunuma H, Suzuki H. Cytotropism of Theiler’s murine encephalomyelitis viruses in oligodendrocyte-enriched cultures. Arch Virol. 1990;114:293–298. doi: 10.1007/BF01310760. [DOI] [PubMed] [Google Scholar]
- 22.Ohara Y, Stein S, Fu J, Stillman L, Klaman L, Roos R P. Molecular cloning and sequence determination of DA strain of Theiler’s murine encephalomyelitis virus. Virology. 1988;164:245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- 23.Patick A K, Lindsley M D, Rodriguez M. Differential pathogenesis between mouse strains resistant and susceptible to Theiler’s virus-induced demyelination. Semin Virol. 1990;1:281–288. [Google Scholar]
- 24.Patick A K, Oleszak E L, Leibowitz J L, Rodriguez M. Persistent infection of a glioma cell line generates a Theiler’s virus variant which fails to induce demyelinating disease in SJL/J mice. J Gen Virol. 1990;71:2123–2132. doi: 10.1099/0022-1317-71-9-2123. [DOI] [PubMed] [Google Scholar]
- 25.Penney J B, Jr, Wolinsky J S. Neuronal and oligodendroglial infection by the WW strain of Theiler’s virus. Lab Invest. 1979;40:324–330. [PubMed] [Google Scholar]
- 26.Rahemtulla A, Fung-Leung W P, Schilham M W, Kundig T M, Sambhara S R, Narendran A, Arabian A, Wakeham A, Paige C J, Zinkernagel R M, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 27.Rahemtulla A, Kundig T M, Narendran A, Bachmann M F, Julius M, Paige C J, Ohashi P S, Zinkernagel R M, Mak T W. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur J Immunol. 1994;24:2213–2218. doi: 10.1002/eji.1830240942. [DOI] [PubMed] [Google Scholar]
- 28.Rivera-Quinones C, McGavern D B, Schmelzer J D, Hunter S F, Low P A, Rodriguez M. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–193. doi: 10.1038/nm0298-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez M. Virus-induced demyelination in mice: “dying back” of oligodendrocytes. Mayo Clin Proc. 1985;60:433–436. doi: 10.1016/s0025-6196(12)60865-9. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez M. Immunoglobulins stimulate central nervous system remyelination: electron microscopic and morphometric analysis of proliferating cells. Lab Invest. 1991;64:358–370. [PubMed] [Google Scholar]
- 31.Rodriguez M, David C S. Demyelination induced by Theiler’s virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- 32.Rodriguez M, David C S, Pease L R. The contribution of MHC gene products to demyelination by Theiler’s virus. In: David C S, editor. H-2 antigens. New York, N.Y: Plenum Publishing Corp.; 1987. pp. 747–756. [Google Scholar]
- 33.Rodriguez M, Dunkel A J, Thiemann R L, Leibowitz J, Zijlstra M, Jaenisch R. Abrogation of resistance to Theiler’s virus-induced demyelination in H-2b mice deficient in β 2-microglobulin. J Immunol. 1993;151:266–276. [PubMed] [Google Scholar]
- 34.Rodriguez M, Lafuse W, Leibowitz J, David C S. Partial suppression of Theiler’s virus-induced demyelination in vivo by administration of monoclonal antibodies to immune response gene products (Ia antigens) Neurology. 1986;36:964–970. doi: 10.1212/wnl.36.7.964. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez M, Leibowitz J, David C S. Susceptibility to Theiler’s virus-induced demyelination: mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez M, Leibowitz J L, Lampert P W. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez M, Leibowitz J L, Powell H C, Lampert P W. Neonatal infection with the Daniels strain of Theiler’s murine encephalomyelitis virus. Lab Invest. 1983;49:672–679. [PubMed] [Google Scholar]
- 38.Rodriguez M, Pavelko K D, Njenga M K, Logan W C, Wettstein P J. The balance between persistent virus infection and immune cells determines demyelination. J Immunol. 1996;157:5699–5709. [PubMed] [Google Scholar]
- 39.Rodriguez M, Pease L P, David C D. Immune-mediated injury of virus-infected oligodendrocytes: a model of multiple sclerosis. Immunol Today. 1986;7:359–363. doi: 10.1016/0167-5699(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez M, Siegel L M, Hovanec-Burns D, Bologa L, Graves M C. Theiler’s virus-associated antigens on the surface of cultured glial cells. Virology. 1988;166:463–474. doi: 10.1016/0042-6822(88)90517-x. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez M, Sriram S. Successful therapy of TMEV-induced demyelination (DA strain) with monoclonal anti lyt2.2 antibody. J Immunol. 1988;140:2950–2955. [PubMed] [Google Scholar]
- 42.Roos R P, Wollmann R. DA strain of Theiler’s murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol. 1984;15:494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- 43.Sibley W A, Bamford C R, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;i:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traugott U, Reinherz E L, Raine C S. Multiple sclerosis. Distribution of T cells, T cell subsets and Ia-positive macrophages in lesions of different ages. J Neuroimmunol. 1983;4:201–221. doi: 10.1016/0165-5728(83)90036-x. [DOI] [PubMed] [Google Scholar]
- 45.Traugott U, Reinherz E L, Raine C S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock-Guttman B, Ransohoff R M, Kinkel R P, Rudick R A. The interferons: biological effects, mechanisms of action, and use in multiple sclerosis. Ann Neurol. 1995;37:7–15. doi: 10.1002/ana.410370105. [DOI] [PubMed] [Google Scholar]
- 47.Welsh C J, Tonks P, Nash A A, Blakemore W F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- 48.Wroblewska Z, Gilden D H, Wellish M, Rorke L B, Warren K G, Wolinsky J S. Virus-specific intracytoplasmic inclusions in mouse brain produced by a newly isolated strain of Theiler virus. I. Virologic and morphologic studies. Lab Invest. 1977;37:595–602. [PubMed] [Google Scholar]
- 49.Wroblewska Z, Kim S U, Sheffield W D, Gilden D H. Growth of the WW strain of Theiler virus in mouse central nervous system organotypic culture. Acta Neuropathol. 1979;47:13–19. doi: 10.1007/BF00698267. [DOI] [PubMed] [Google Scholar]
- 50.Yeung R S, Penninger J, Mak T W. T-cell development and function in gene-knockout mice. Curr Opin Immunol. 1994;6:298–307. doi: 10.1016/0952-7915(94)90105-8. [DOI] [PubMed] [Google Scholar]