Abstract
Lung transplantation is a well-established treatment for children facing advanced lung disease and pulmonary vascular disorders. However, organ shortage remains highest in children. For fitting the small chest of children, transplantation of downsized adult lungs, lobes, or even segments were successfully established. The worldwide median survival after pediatric lung transplantation is currently 5.7 years, while under consideration of age, underlying disease, and peri- and posttransplant center experience, median survival of more than 10 years is reported. Timing of referral for transplantation, ischemia-reperfusion injury, primary graft dysfunction, and acute and chronic rejection after transplantation remain the main challenges.
Keywords: Children, lung donor, lung transplantation, pediatrics.
Introduction
Lung transplantation (LTx) is a well-established treatment for children facing advanced lung disease and pulmonary vascular disorders. Children differ from adults in various aspects, including their smaller anatomy, which necessitates modified surgical approaches. Additionally, the developing immune system in pediatric patients, the impact of pharmacokinetics on immunosuppressant medications, and the unique psychological implications during childhood and adolescence all demand special consideration in the context of LTx.
HISTORY AND DEMOGRAPHIC DEVELOPMENT
The inaugural clinical LTx in adults traces back to 1963, but it was not until 1983 that successful long-term outcomes were firmly established.[1] The first recorded pediatric LTx took place in 1987, involving a 16-year-old boy.[1] Notably, the first instance of living donor lobar transplantation was reported in 1990, featuring a 12-year-old girl.[1] Additionally, the first heart-lung transplantation occurred in 1968, involving a 2.5-year-old girl.[1]
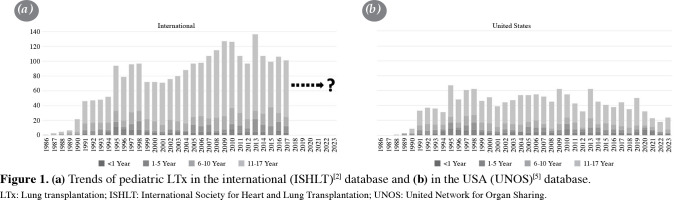
As per recent reports from the International Society for Heart and Lung Transplantation (ISHLT),[2,3] the global count of LTx and heart-lung transplants performed in individuals under the age of 18 between 1992 and 2018 reached 2,514 and 733, respectively. In comparison, adults underwent a significantly higher number of LTx procedures, 67,493 in total during the same period.[4] The incidence of heart-lung transplantation in pediatric patients peaked at 59 cases in 1989 but has since experienced a substantial decline worldwide over the last two decades, with only three reported cases in 2017.[2] Recent data from the USA, obtained from the United Network for Organ Sharing (UNOS) database,[5] suggests that this downward trend likely persists at a low level and is primarily attributed to various factors that make replacement by singular heart or lung transplants more viable.[6] The number of pediatric LTx, which reached a peak of 136 in 2013 (Table 1a),[2] also appear to be on a downward trend, as indicated by recent data from the USA (Table 1b).[5,7] This decline is considered multifactorial, influenced in part by the recent COVID-19 pandemic but predominantly associated with the efficacy of new medications and surgical alternatives.[6]
Table 1. Disease specific referral criteria for pediatric lung transplantation, based on the ISHLT consensus document,[47] including guidelines from the European Pediatric Pulmonary Vascular Disease Network[53].
| Cystic fibrosis |
• A forced expiratory volume in one second (FEV1) a. Between 40% and 30% in general, or b. <50% and rapidly declining or c. <50%, accompanied by a low 6-minute walk (<400 m), hypoxemia (PaO2 <8 kPa or <60 mmHg), hypercarbia (PaCO2 >6.6 kPa or >50 mmHg) or pulmonary hypertension (mPAP >25 mmHg) |
| Pulmonary hypertension |
• Worsening nutritional status and growth despite intervention • Respiratory failure, long-term non-invasive ventilation • Clinical evidence of right heart failure, impaired growth • WHO functional class III-IV • Signs of disease-related secondary liver or kidney dysfunction, recurrent hemoptysis or syncope • Need for prostacyclin therapy • Significantly elevated levels of B-type natriuretic peptide (BNP) or N-terminal (NT)-pro hormone BNP (NT-proBNP) • In echocardiography: severe enlargement of right atrium and ventricle, as well as systolic dysfunction of the left and right ventricle • Invasive hemodynamic measures: cardiac index (CI) <2.5l/min/m2, mean pulmonary artery pressure over mean systemic arterial pressure (mPAP/mSAP) >0.75, mean right atrial pressure (mRAP) >14 mmHg, and pulmonary vascular resistance index (RVRi) >15WUm2 |
| Interstitial lung disease |
• Histopathological proven or radiographic probable usual interstitial pneumonia (UIP) • Any form of pulmonary fibrosis with a. Forced vital capacity (FVC) <80% or b. Diffusion capacity of carbon monoxide (DLCO) <40%, or c. A relative decline one of the following in the past 2 years: FVC ≥10%, DLCO ≥15%, FVC ≥5% in combination with worsening of respiratory symptoms or radiographic progression • Supplemental oxygen requirement • Inflammatory progression despite treatment |
| ISHLT: International Society for Heart and Lung Transplantation; WHO: World Health Organization. | |
Currently, pediatric LTx is exclusively performed in 37 centers across 31 countries.[2,8] Among these centers, only five perform more than four transplant annually.[2] In many countries outside of North America, the majority of LTx is conducted in adult centers with high case volumes.[9]
TYPES OF LUNG TRANSPLANTATION IN CHILDREN
The surgical procedure for pediatric LTx is essentially the same as in adults, aside from the smaller anatomical dimensions. The approach typically involves a clamshell incision (bilateral thoracosternotomy) or two separate anterolateral thoracotomies.[10] However, in very small patients weighing less than 15 kg, certain centers may opt for a sternotomy.[11] The diminutive airways in children can also present a challenge to insert isolated lung ventilation through a double-lumen endotracheal tube. Consequently, cardiopulmonary bypass or extracorporeal membr e ECMO is utilized in as much as 90% of procedures.[13]
The size of the lung allograft significantly influences posttransplant outcomes, a fact supported by evidence from large animal models[14,15] and extensive adult databases.[16] Oversized lung grafts can potentially lead to complications such as atelectasis or distortion of the segmental or subsegmental bronchial anatomy, hindering airway clearance and predisposing to recurrent pulmonary infections.[17-19] Conversely, an undersized lung graft is associated with lower expiratory airflow, higher pulmonary vascular resistance, persistent pleural space, and an increased likelihood of developing PGD and chronic lung allograft dysfunction (CLAD).[17,20] The different approaches to pediatric LTx are depicted in Figure 2.
Figure 1. (a) Trends of pediatric LTx in the international (ISHLT)[2] database and (b) in the USA (UNOS)[5] database. LTx: Lung transplantation; ISHLT: International Society for Heart and Lung Transplantation; UNOS: United Network for Organ Sharing.
Figure 2. Types of lung transplantation in children. LTx: Lung transplantation.

Bilateral lung transplantation
Similar to procedures in adults, bilateral sequential LTx is now the predominant method for most pediatric cases. The procedure involves removing the native lung with the least perfusion, followed by implantation of the donor lung. During bronchial anastomosis, an end-to-end technique is recommended over a telescoping approach to minimize the risk of stenosis.[21] Subsequently, pulmonary arterial anastomosis is performed, and finally, the venous anastomosis is done, combining the donor and recipient’s left atrium. The same process is then repeated on the contralateral side.
Downsizing lung transplantation
This technical variation involves downsizing the large donor lung using a linear stapling device, either in a nonanatomical manner or through segmentectomy or lobectomy, to tailor it to fit into the smaller chest cavity of the child.[17,22-24] This approach constitutes more than 40% of procedures in certain centers.[25,26]
Lobar lung transplantation
In this procedure, instead of transplanting whole lungs, only lobes are transplanted into the right and left chest cavities. This option becomes particularly beneficial when utilizing a large adult donor lung for a child with a smaller chest. In some centers, lobar transplantations account for up to 15% of pediatric transplants.[27] There are even reports of performing two concomitant pediatric LTx from one large adult lung.[22] The technique known as pulmonary bipartitioning or split LTx is a highly efficient application of lobar LTx. With this method, either the left or right donor lung of an adult is divided into an upper and lower lobe, which are then used for bilateral transplantation in a smaller recipient.[24] Lobar LTx demands advanced skills in preparing the anatomy. In contrast to regular LTx, only a single pulmonary vein of the donor lobe is anastomosed to a recipient pulmonary vein, rather than the usual connection to the large surface of an entire left atrium.
Living donor lobar lung transplantation
Living-donor lobar LTx, involving a right lower lobe from one donor and a left lower lobe from a second donor, was frequently employed in the USA until 2005 when allocation methods improved.[28,29] Presently, this approach is predominantly utilized in pediatric programs in Japan due to their ongoing shortage of suitable organs.[30,31] Recently, there has even been a reported series of six children who underwent successful living-donor segmental LTx.[32] In this segmental approach, the basal segments of the lower lobes, or segment 6, were used. Importantly, a downside of living-donor LTx is that both the recipient and the donor face risks during the procedure and lose a portion of their lung volume.[28]
Single lung transplantation
While largely abandoned, this procedure may still be infrequently considered for a patient who has undergone a previous pneumonectomy to replace the remaining single lung. The presence of a remaining recipient lung carries the risk of mucus, bacterial and fungal infections spreading from the remaining lung into the graft, or the persistence of PH potentially damaging the graft.
RECIPIENT INDICATIONS
In the past decade, there has been a significant change in referral diagnoses, reflecting shifts in treatment options. Figure 3a provides an international overview of the period between 2010 and 2018,[3] while Figure 3b illustrates the trend shift in the USA from 2016 to 2021.[7]
Figure 3. Pediatric recipient diagnoses (a) in the era between 2010 and 2018 in the international (ISHLT)[3] database and (b) in the years 2016 and 2021 the USA (UNOS)[7] database. IPAH: Idiopatic pulmonary hypertension; ReTx: Re-lung transplantation; CF: Cystic fibrosis; ILD: Interstitial lung disease; IIP: Idiopatic interstitial pneumonias; OB: Obliterative bronchiolitis; PH: Pulmonary hypertension; NA: Not available; ISHLT: International Society for Heart and Lung Transplantation; UNOS: United Network for Organ Sharing.
Cystic fibrosis
In earlier periods, cystic fibrosis (CF) was the predominant diagnosis among children undergoing LTx.[3] Cystic fibrosis results from loss-of-function mutations in the CF transmembrane conductance regular (CFTR) gene.[33] Since 2012, CFTR modulators targeting the underlying cellular mechanisms have been available, even for younger children, positively impacting approximately 90% of CF patients based on their genotype.[34-36] These CFTR modulators have clinically stabilized even patients with advanced stages of CF, leading to a significant postponement of listing or even removal of CF patients from the transplant waitlist.[37-39] After 2013, data from UNOS demonstrated a consistent decline of pediatric CF recipients undergoing LTx in the US, reaching a low of only 16% in 2021 (Figure 3b).[7]
Pulmonary hypertension
In the past decade, idiopathic pulmonary arterial hypertension (IPAH) has emerged as the leading indication for pediatric LTx, alongside CF,[3,6,7] constituting approximately 16% of cases in the USA.[7] The incidence of IPAH continues to rise despite the availability of novel pulmonary vasodilators and interventions, such as atrial septostomy and the reversed Potts shunt (left pulmonary artery to descending aorta).[40-42] This trend may be partly explained by a shift from combined heart-lung transplantation in earlier years to successful LTx alone.[6] Severe right-sided ventricular dysfunction due to IPAH generally improves when the right ventricle is unloaded after LTx.
While IPAH accounts for about 70% of PH cases, approximately 30% of pediatric LTx are performed for secondary PH (SPH).[3] Children requiring LTx for IPAH are typically between one and five years of age, whereas those with SPH tend to be older.[2] A significant subgroup of SPH cases referred for pediatric LTx is those with Eisenmenger-associated severe PH related to congenital heart disease.[6]
Interstitial lung disease
Pediatric interstitial lung diseases (ILDs), or, more broadly, pulmonary fibrosis, are a frequent indication for LTx.[3,7] These diseases encompass a heterogenous group of syndromes[43] and include various conditions, such as surfactant protein deficiencies, disorders leading to alveolar proteinosis, growth abnormalities, such as bronchopulmonary dysplasia, alveolar-capillary dysplasia, obliterative bronchiolitis, and entities such as pleuroparenchymal fibroelastosis.
Retransplantation
The improved outcomes after pediatric LTx have resulted in more long-term survivors, and some of these individuals, who are still children, may become potential candidates for retransplants. International data report a retransplant rate of about 5%,[3] with some centers having rates approaching almost 13%.[44] The most common diagnosis requiring retransplantation is CLAD.[13,45]
Recipient selection criteria
The selection of candidates necessitates a collaborative effort involving multiple disciplines, including pediatrics, medicine, surgery, and anesthesia. Specific pediatric recommendations were introduced in the “consensus document on the selection of lung transplant candidates” by ISHLT in 2014,[46] which were subsequently updated in 2021.[47] The pediatric section in this document relies on the limited available literature, primarily drawing from expert opinions and extrapolations from adult literature.
In the earlier guideline,[46] the timing was determined by factors such as high risk of death from lung disease within two years if LTx is not performed, progressive lung disease despite maximal medical therapy, and poor quality of life. However, in the recent guideline,[47] the timing of referral is kept more open. It is specifically emphasized that children should be referred early and undergo detailed review due to the often longer wait time in this population and the challenge of acquiring suitable-sized organs.
Absolute contraindications specifically relevant for children include multisystem organ failure (unless considered for multiorgan transplant), malignancy with high risk of recurrence, active systemic infections, and expected limited survival even with a transplant. Chest wall deformities or a remodeled or adhesive situs due to previous thoracic surgeries increase the complexity of the transplantation and are therefore considered a relative contraindication and a risk factor, respectively.
Pretransplant mechanical ventilation is a known risk factor for survival in children, except in the subpopulation of infants, which needs to be considered.[48] Nonadherence is viewed as an absolute contraindication, with mental disorders in the child or their caregiver being considered risk factors. In earlier eras of pediatric LTx (1996-2013) adolescents were at a higher risk of developing CLAD due to nonadherence with medical therapy, but this has been successfully addressed with a stronger focus in recent years.[48] No risk association was found in previous studies regarding the severity of pretransplant mean pulmonary artery pressure.[49,50] Being severely underweight, measured by body mass index percentiles, was identified as an independent predictor for poor survival in a current UNOS data analysis.[51] Consequently, addressing this modifiable target is important for improving survival.
Disease-specific selection criteria
Table 1 gives an overview of the current referral criteria in pediatric patients with CF, PH, and ILD. Children with CF suffering from pulmonary infection with Burkholderia cenocepacia and nontuberculous mycobacteria, once considered contraindications for LTx in both adults and children, are no longer regarded as such in recent guidelines when well-managed prior to transplant.[47]
In the context of PH, the timing of listing for transplantation poses a challenge. Children with PH are known to exhibit much more preserved cardiac indices and exercise tolerance than adults, even in advanced disease. However, they may experience sudden and rapid deterioration, leading to fatal outcomes.[52] The current guidelines[47] for candidate selection refer to a pediatric-specific consensus statement from 2019.[53]
The timing of retransplant is a complex issue. Immediate retransplantation due to PGD in children,[2] as in adults,[54] is considered inferior to primary operations. Studies have shown that retransplantation after a period of 12 months and without requiring invasive ventilator support at the time of retransplant tends to be more successful.[55]
Extracorporeal membrane oxygenation
In children with respiratory failure, using ECMO as a bridge to the transplant is currently considered a superior alternative with fewer risks compared to long-time mechanical ventilation. Various singlecenter studies[13,56-59] and a recent propensitymatched UNOS data study[60] show that there is no negative impact on the postoperative survival. Based on data from the USA, 16% of pediatric recipients are currently bridged by mechanical ventilation and ECMO, 8% by ECMO only, and 16% by mechanical ventilation only.[7] Most candidates are hemodynamically stable, and veno-venous ECMO via a single bicaval dual-lumen catheter in the internal jugular vein is often sufficient.[12,60] The consensus document by the ISHLT,[47] based on knowledge from adults, lists contraindications for ECMO as a bridge to transplantation, such as septic shock and multiorgan failure, which are also applicable for pediatric candidates. A recent UNOS data study identified age >12 years and compromised kidney function at the time of listing as risk factors.[60] If possible, candidates should be kept awake and spontaneously breathing to allow regular physiotherapy and avoid rapid physical deconditioning.[58,61,62] Lung transplantation outcomes in patients receiving transplant from ECMO were significantly better in patients who were able to ambulate than in those who were not.[59,63]
In some centers, pumpless, low-resistance membrane oxygenator devices are employed for bridging purposes.[64,65] These oxygenators are positioned between the pulmonary artery and the left atrium and have been successfully used in a child as young as two years of age.[65]
DONOR SELECTION AND ALLOCATION
Selection
Donor selection criteria are primarily the same as those for adults.[66] However, the chronic shortage of organs and the challenge of finding organs that fit the size of the child are significant limiting factors in the success of pediatric LTx. Historically, children have experienced higher rates of waitlist mortality compared to adults,[8,67] with small children facing particularly elevated risks. For instance, in the USA, children under six years of age have a 42% chance of dying while on the wait list.[67]
The majority of transplants are conducted using lungs from brain-dead donors.[2] To address the limited donor pool, various strategies are being explored, including size reduction or lobar transplantation,[23] living donation,[30] donation after cardiocirculatory death,[68,69] graft improvement through ex vivo lung perfusion,[70] and the utilization of extended criteria donor organs.[71]
An analysis of international data on deceased donor characteristics revealed no significant association between donor age and one-year survival, as well as freedom from CLAD.[72] Additionally, there was no observed association between short- and long-term survival concerning donor smoking, donor substance abuse, and donor cause of death.[72] However, an ischemic time of 4 h or more was linked to inferior short- and long-term survival, except in a subgroup of very young children.[72]
Allocation
Currently, allocation policies for pediatric LTx vary across different countries, encompassing differences in both prioritization and distribution.[8] In response to longer wait times and higher rates of waitlist mortality, several countries have modified their lung allocation strategies for children. The USA, the UK, Italy, France, and several European countries served by Eurotransplant, as well as Australia and New Zealand, now prioritize children, employing various donor and recipient age algorithms or considering medical urgency over accumulated waitlist time.[8] In the USA, for instance, the implementation of these changes in the lung allocation score has significantly reduced waitlist mortality.[73]
OUTCOMES
Table 2 provides an overview of the most recent reported short- and long-term survival in children, drawing from the extensive ISHLT and UNOS databases, as well as from some high-volume centers. According to ISHLT data, worldwide median survival in children is reported as 5.7 years compared to 6.2 years in adults.[2] However, a noteworthy fact is that all children who survive the first year after LTx have an expected median survival of 9.1 years, surpassing that of adults with 8.3 years from that point onwards.[2] Comparisons of different eras by various registries and single centers generally indicate improved survival in the present day.
Table 2. Short- and long-term survival after pediatric LTx.
| n | Era | 1 year (%) |
3 years (%) |
5 years (%) |
10 years (%) |
Median (years) |
|||
| Overall | |||||||||
| ISHLT (2019)[2] | Pediatric | All | 2,223 | 1992-2017 | 81* | 64* | 53* | 39* | 5.7* |
| Adult | All | 63,410 | 1992-2017 | 81* | 67* | 56* | 34* | 6.2* | |
| All | 124 | 2014-2016 | 84 | 62 | 57 | NA | NA | ||
| UNOS (2023)[7] | Pediatric | ||||||||
| All | 43 | 2011 | 77* | 56* | 48* | 36 | NA | ||
| Vienna, Austria (2018)[13] | Pediatric | All, median age 12.9 All, median age 13.0 |
86 65 |
1990-2015 2003-2015 |
79 86 |
72* 77* |
68 74 |
57 74 |
10.4* NA |
| Hannover, Germany (2009)[25] | Pediatric | All double lung | 31 | 1987-2007 | 73* | 69* | 47* | 37* | 4.7* |
| Italy (9 centers) (2023)[44] | Pediatric | All, median age 14.5 | 100 | 1992-2019 | 72 | 55 | 52 | 33 | 4.3* |
| By age | |||||||||
| Age <1 | 106 | 1992-2017 | 75* | 63* | 56* | 41* | 7.3 | ||
| Age 1-5 | 171 | 1992-2017 | 78* | 59* | 52* | 44* | 5.8 | ||
| ISHLT (2019)[2] | Pediatric | Age 6-10 | 336 | 1992-2017 | 84* | 71* | 61* | 44* | 8.4 |
| Age 11-17 | 1610 | 1992-2017 | 81* | 63* | 51* | 37* | 5.4 | ||
| By diagnosis | |||||||||
| ISHLT (2019)[2] | Pediatric | CF | 1220 | 1992-2017 | 84* | 65* | 52* | 36* | 5.6 |
| ILD | 92 | 1992-2017 | 76* | 61* | 48* | 36* | 4.5 | ||
| ILD other | 103 | 1992-2017 | 81* | 69* | 58* | 34* | 7.3 | ||
| OB (non-ReTx) | 103 | 1992-2017 | 87* | 71* | 64* | 57* | 12.4 | ||
| Secondary PH | 117 | 1992-2017 | 71* | 51* | 43* | 34* | 3.2 | ||
| IPAH | 219 | 1992-2017 | 81* | 69* | 64* | 44* | 7.4 | ||
| St. Louis, USA (2011)[49] | Pediatric | IPAH | 19 | 1991-2009 | 95 | 72* | 61 | 27* | 5.8 |
| By type | |||||||||
| ISHLT (2019)[2] | Pediatric | Single Ltx | 72 | 1992-2017 | 53* | 38* | 28* | NA | 1.9 |
| ISHLT (2019)[2] | Pediatric | ReLTx | 103 | 2000-2017 | 66* | 53* | 44* | 33* | 3.8* |
| UNOS (2011)[55] | Pediatric | ReLTx all | 81 | 1988-2008 | 48 | 38 | 28 | NA | 0.9 |
| ReLTx after <1 year | 40 | 1988-2008 | 40 | 36 | 30 | NA | 0.3 | ||
| ReLTx after >1 year | 41 | 1988-2008 | 56 | 49 | 34 | NA | 2.8 | ||
| Vienna, Austria (2018)[13] | Pediatric | ReLTx | 17 (15 late ReLTx) | 1990-2015 | 92 | 92 | 80 | NA | 7.3* |
| Hannover (2011)[45] | Pediatric | ReLTx, mean age 14.1 | 7 (6 late ReLTx) | 1994-2009 | 71 | 71 | NA | NA | 4.7* |
| ISHLT (2019)[2] | Pediatric | Living donor | 84 | 1992-2017 | 71* | 57* | 39* | 27* | 3.8 |
| LTx (11-17) | |||||||||
| Kyoto, Japan (2022)[31] |
Pediatric | Living donor LTx, median age 11.0 |
25 | 2008-2019 | 88* | 88* | 88 | 75 | NA |
| UNOS (2006)[28] |
Pediatric | Living donor LTx, age 6-10 |
NA | 1994-2003 | 100* | 68* | 50* | NA | NA |
| Living donor LTx, age 11-17 |
NA | 1994-2003 | 66* | 54* | 37* | NA | NA | ||
| St Louis, USA | Pediatric | Living donor LTx | 38 | 1994-2002 | 60 | 48 | NA | NA | NA |
| ISHLT: International Society for Heart and Lung Transplantation; UNOS: United Network for Organ Sharing; CF: Cystic fibrosis; ILD: Interstitial lung diseases; OB: Obliterative bronchiolitis; ReTx: Re-lung transplantation; PH: Pulmonary hypertension; IPAH: Idiopathic pulmonary arterial hypertension; NA: Not available. * Numbers read out of graphs. | |||||||||
Regarding the underlying disease, no significant differences are known, and considerably good outcomes are achieved in children with IPAH, showing an international survival of 7.4 years.[2,3] Recipients with IPAH who survived a year had an expected median survival of 12.4 years.[2] In large contrast to LTx for IPAH, the underlying diagnosis of SPH appears to have the poorest outcomes across all indications for LTx, with a median survival of 3.2 years.[2]
The age of children also plays a certain role in the outcome of LTx.[2] In earlier periods (2000-2005), adolescent recipients had a poorer overall survival compared to younger children.[48] However, this significant trend disappeared with more closely controlled compliance in adolescents in the most recent period (2012-2017).[48]
In terms of the type of LTx, there is a clear survival advantage for bilateral versus single LTx, as the median survival for single LTx is reported to be only 1.9 years.[2]
Earlier reports from the USA presented relatively pessimistic outcomes for living lobar LTx in children,[28,74] likely due to the cohort consisting of severely ill recipients in urgent need of transplantation. In contrast, recent outstanding results have been achieved with living donors in centers of Japan,[31] surpassing the average survival rates of deceased donors.
Historically, retransplantation has shown poor overall survival.[55,75] However, it appears that early retransplantation due to PGD or acute rejection within the first year is associated with poor survival, while at a later state, CLAD and nonrequirement of ventilation are linked to better survival. This is indicated by nonsignificant trends in a study based on UNOS data.[55] Single centers that predominantly retransplanted well-selected children at a later stage have demonstrated favorable results, with medians ranging from 4.7 to 7.3 years.[13,45]
PERI- AND POSTTRANSPLANT CARE AND CHALLENGES
The perioperative period requires a multidisciplinary team approach involving close monitoring and treatment to overcome various challenges, which can lead to short- or long-term morbidity and mortality, as graphically displayed on ISHLT data in Figure 4. Mortality is highest in the first year, with approximately 15% of all recipients succumbing to infection and graft failure.[2]
Figure 4. Relative incidences of leading causes of death after pediatric LTx, based on ISHLT data.[2] CLAD: Chronic lung allograft dysfunction; LTx: Lung transplantation; ISHLT: International Society for Heart and Lung Transplantation.

Immunosuppression
The majority of children undergoing LTx typically receive induction therapy.[7,13] This is usually basiliximab, an interleukin-2 receptor antagonist.[76] Alemtuzumab, a CD52-depleting monoclonal antibody has also shown a significant positive association with median posttransplant survival based on UNOS data.[77] Recently, a multicenter study using the monoclonal antibody rituximab plus the polyclonal antibody rabbit antithymocyte globulin (thymoglobulin) for induction found promising tendencies of reduced rejection.[78]
Posttransplant long-term immunosuppression strategies typically involve a triple-drug maintenance regimen, consisting of a calcineurin inhibitor (usually tacrolimus), a T-cell antiproliferative (mycophenolate mofetil/mycophenolic acid), and corticosteroids (prednisone). A retrospective single-center study found tacrolimus to be positively associated with posttransplant survival in children.[79] However, it is important to note and monitor that calcineurin inhibitors carry the risks of nephrotoxicity, neurological symptoms, the onset of diabetes mellitus, and, along with corticosteroids, the risk of osteoporosis and systemic hypertension.[80-82]
Primary graft dysfunction
Within the first 72 h after LTx, PGD has an incidence of 8-30% in both adults and children.[83] Currently, it accounts for almost 16% of deaths within the first 30 days[2] despite improved surgical techniques and optimized organ preservation. The underlying mechanism predominantly involves damage caused by ischemia-reperfusion injury, leading to severe inflammatory and immunological reactions.[84,85] Clinically, pulmonary edema with diffuse alveolar damage leads to progressive hypoxemia.[83] For graft recovery, along with judicious ventilator management, fluid management to maintain normovolemia and pressor support is employed. In many cases, a transient installation of ECMO support is needed to overcome PGD and ensure sufficient gas exchange. In cases of known previous pulmonary arterial pressure, ECMO is not only used intraoperatively, but its use is extended postoperatively to prevent hyperperfusion and consequent PGD of the new graft until the anatomy and physiology of the heart has readapted.[86]
Acute rejection
Acute cellular rejection (ACR) is the most common form of allograft rejection, affecting almost half of children who undergo LTx. It is most commonly observed during the first three months but remains frequent up to three years later.[87] Acute cellular rejection is a complex activation of innate and adaptive immune responses, resulting in the recruitment of alloreactive T lymphocytes to the lung allograft. This triggers a cascade involving neutrophils, eosinophils, B lymphocytes, macrophages, and natural killer cells, causing lung injury.[88,89] Primary graft dysfunction may trigger ACR.[90] Clinical manifestations of ACR include fever, dyspnea, and hypoxia. Interestingly, the risk of developing ACR appears to increase with the age of the child,[2] with children younger than three years of age appearing to be more protected against ACR.[91,92]
Chronic lung allograft dysfunction
Chronic lung allograft dysfunction is characterized by progressive lung function decline and is subcategorized into obstructive, restrictive, and mixed phenotypes. Among these, the obstructive phenotype of bronchiolitis obliterans syndrome remains a major challenge after pediatric LTx and LTx in general.[93,94] Chronic lung allograft dysfunction is the leading cause of death beyond the first year following pediatric LTx.[3] In the ISHLT database, more than 54% of recipients suffer from CLAD within five years.[2] Chronic lung allograft dysfunction is considered multifactorial, associated with both acute and chronic rejection.[93,94] While a prospective study found no association between episodes of ACR, PGD, or community-acquired respiratory virus infections and the development of CLAD in pediatric lung transplants,[95] a recent retrospective single-center study found an association between CLAD and PGD grade 3 at 48-72 h after transplantation.[96] Children under five years of age appear to have increased freedom from CLAD compared to older children.[48] Immunosuppression nonadherence, particularly in adolescents, is a significant risk factor for CLAD.[97] Currently, there is no well-proven therapeutic approach for managing CLAD, and over the decades, no significant improvement has been achieved.[48] Nonpediatric specific guidelines recommend change/augmentation of immunosuppression, use of macrolides, extracorporeal photopheresis, and total lymphoid irradiation as potential interventions.[98] Retransplantation remains the ultimate treatment option for end-stage CLAD.
Infections
Infections account for more than a third of deaths during the first year after transplantation in children.[2] Similar to adults, the potent immunosuppression coupled with challenges in mobilizing secretions in the initial weeks after the extensive surgery predisposes recipients to infection.
Cytomegalovirus infection is linked to an increased incidence of both ACR and CLAD. Many transplant centers, including those catering to children, have adopted prophylactic treatment with ganciclovir.[99,100]
Aspergillosis poses a significant challenge in the pediatric lung transplant population,[101] particularly in children with CF who are often colonized by Aspergillus.[101] Therefore, it is widely recommended to initiate antifungal therapy prior to transplantation to reduce the infectious burden and decrease the risk of dissemination during the perioperative period.[102] Some centers perform preventative sinus surgery for CF patients before transplantation, aiming to stabilize respiratory function, improve quality of life, and reduce the incidence of tracheobronchitis and pneumonia.[103-105] However, no direct survival benefit has been established.[104,105]
Posttransplant lymphoproliferative disease
The incidence of malignancy after pediatric LTx is 4.8% at one year, increasing to 9.3% at five years.[2] The majority among these malignancies are posttransplant lymphoproliferative disease (PTLD).[2] Posttransplant lymphoproliferative disease is asignificant and sometimes fatal complication. Primary Epstein-Barr virus (EBV) infection, typically acquired from the donor, is a major risk factor for PTLD development. The incidence of PTLD in EBV-negative recipients is 8.8% at five years after transplant, compared to 1.2% among EBV-positive recipients.[7] During the first year, PTLD typically presents with nodules in the allograft, along with malaise and fever. Later onset of the disease may involve the gastrointestinal tract, skin, and lymphatic tissue, including the nasopharynx. [106,107]Data support the use of prophylactic antiviral therapy in EBV-negative recipients who received a positive donor organ.[108]
CHALLENGES
Children continue to have the highest proportion of deaths on the waitlist across all age groups, with shorter height being associated with increased mortality while awaiting the transplant.[67] Systems prioritizing children worldwide might help overcome this problem.[8] Additionally, as center volume plays a role in the incidence of rejection and survival, more detailed guidelines and experience exchange between centers might improve outcomes.[109] Moreover, further research on subgroups with specific underlying diseases for optimizing candidate selection and defining optimal timing for referral would be needed. This is also true for the increasing number of potential retransplantation candidates, as organ shortage and their often compromised outcomes fuel an ethical dilemma.
Recruitment and management of organ allocation for young pediatric donors may not be as consistent or efficient in some countries.[67] Repeated education and training throughout the allocation process, along with increased knowledge about suitable organs, could potentially enhance the utilization of donors in this age group.
Moreover, similar to adults, additional research into the viability of extended donor lungs and enhancement techniques like ex vivo lung perfusion procedures to expand the limited donor pool would be highly valuable. Given the significance of allograft size in pediatric recipients, exploring the use of threedimensional computed tomography volumetry to assess recipient chest cavity volume could be a promising tool to assist surgeons in accurately downsizing oversized lung grafts before transplantation.[110]
Finally, the persistent challenges of ischemiareperfusion injury, PGD, and, particularly, CLAD require increased focus. Rapid advancements in the development of next-generation technologies may provide insight into the pathophysiology of the dysregulated immune environment associated with ACR and CLAD. This could aid in the early detection and treatment, potentially leading to improved morbidity and mortality.[111]
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept; I.I.; Design, literature review, materials: I.I., J.P.E.; Control/supervision, critical review: I.I., O.M.S.; Data collection and/or processing, references and fundings: I.I., J.P.E., O.M.S.; Analysis and/or interpretation: I.I., J.P.E., O.M.S., M.P.; Writing the article: I.I., J.P.E., M.P.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Mendeloff EN. The history of pediatric heart and lung transplantation. Pediatr Transplant. 2002;6:270–279. doi: 10.1034/j.1399-3046.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayes D Jr, Cherikh WS, Chambers DC, Harhay MO, Khush KK, Lehman RR, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twentysecond pediatric lung and heart-lung transplantation report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1015–1027. doi: 10.1016/j.healun.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes D Jr, Cherikh WS, Harhay MO, Perch M, Hsich E, Potena L, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-fifth pediatric lung transplantation report - 2022; focus on pulmonary vascular diseases. J Heart Lung Transplant. 2022;41:1348–1356. doi: 10.1016/j.healun.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perch M, Hayes D Jr, Cherikh WS, Zuckermann A, Harhay MO, Hsich E, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-ninth adult lung transplantation report-2022; focus on lung transplant recipients with chronic obstructive pulmonary disease. J Heart Lung Transplant. 2022;41:1335–1347. doi: 10.1016/j.healun.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Network for Organ Sharing (UNOS) U.S. Transplants Performed January 1 -O, 2023. OPTN Database. United Network for Organ Sharing (UNOS), 2023. National data; category: transplant; Organ: Lung. Transplants by recipient age. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ .
- 6.Avdimiretz N, Benden C. The changing landscape of pediatric lung transplantation. e14634Clin Transplant. 2022;36 doi: 10.1111/ctr.14634. [DOI] [PubMed] [Google Scholar]
- 7.Valapour M, Lehr CJ, Schladt DP, Smith JM, Goff R, Mupfudze TG, et al. OPTN/SRTR 2021 annual data report: Lung. S379-442Am J Transplant. 2023;23(2 Suppl 1) doi: 10.1016/j.ajt.2023.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avdimiretz N, Benden C. Worldwide organ allocation systems for pediatric lung transplantation. e15018Clin Transplant. 2023;37 doi: 10.1111/ctr.15018. [DOI] [PubMed] [Google Scholar]
- 9.Schmid FA, Inci I, Bürgi U, Hillinger S, Schneiter D, Opitz I, et al. Favorable outcome of children and adolescents undergoing lung transplantation at a European adult center in the new era. Pediatr Pulmonol. 2016;51:1222–1228. doi: 10.1002/ppul.23383. [DOI] [PubMed] [Google Scholar]
- 10.Inci I, Schuurmans MM, Boehler A, Weder W. Zurich University Hospital lung transplantation programme: Update 2012. w13836Swiss Med Wkly. 2013;143 doi: 10.4414/smw.2013.13836. [DOI] [PubMed] [Google Scholar]
- 11.Bryant R 3rd, Morales D, Schecter M. Pediatric lung transplantation. Semin Pediatr Surg. 2017;26:213–216. doi: 10.1053/j.sempedsurg.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Fallon BP, Gadepalli SK, Hirschl RB. Pediatric and neonatal extracorporeal life support: Current state and continuing evolution. Pediatr Surg Int. 2021;37:17–35. doi: 10.1007/s00383-020-04800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waseda R, Benazzo A, Hoetzenecker K, Jaksch P, Muraközy G, Gruber S, et al. The influence of retransplantation on survival for pediatric lung transplant recipients. J Thorac Cardiovasc Surg. 2018;156:2025–2034. doi: 10.1016/j.jtcvs.2018.05.080. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Date H, Ueda K, Nagahiro I, Aoe M, Andou A, et al. Experimental study on size matching in a canine living-donor lobar lung transplant model. J Thorac Cardiovasc Surg. 2002;123:104–109. doi: 10.1067/mtc.2002.117280. [DOI] [PubMed] [Google Scholar]
- 15.Oto T, Date H, Ueda K, Hayama M, Nagahiro I, Aoe M, et al. Experimental study of oversized grafts in a canine living-donor lobar lung transplantation model. J Heart Lung Transplant. 2001;20:1325–1330. doi: 10.1016/s1053-2498(01)00362-x. [DOI] [PubMed] [Google Scholar]
- 16.Chambers DC, Cherikh WS, Harhay MO, Hayes D Jr, Hsich E, Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1042–1055. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inci I, Schuurmans MM, Kestenholz P, Schneiter D, Hillinger S, Opitz I, et al. Long-term outcomes of bilateral lobar lung transplantation. Eur J Cardiothorac Surg. 2013;43:1220–1225. doi: 10.1093/ejcts/ezs541. [DOI] [PubMed] [Google Scholar]
- 18.Mueller C, Hansen G, Ballmann M, Schwerk N, Simon AR, Goerler H, et al. Size reduction of donor organs in pediatric lung transplantation. Pediatr Transplant. 2010;14:364–368. doi: 10.1111/j.1399-3046.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 19.Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451–460. doi: 10.1378/chest.11-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberlein M, Reed RM, Bolukbas S, Diamond JM, Wille KM, Orens JB, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. 2015;34:233–240. doi: 10.1016/j.healun.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huddleston CB. Pediatric lung transplantation. Semin Pediatr Surg. 2006;15:199–207. doi: 10.1053/j.sempedsurg.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Inci I, Benden C, Kestenholz P, Hillinger S, Schneiter D, Ganter M, et al. Simultaneous bilateral lobar lung transplantation: one donor serves two recipients. e69-71Ann Thorac Surg. 2013;96 doi: 10.1016/j.athoracsur.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 23.Inci I, Schuurmans MM, Caviezel C, Hillinger S, Opitz I, Schneiter D, et al. Long-term outcomes of cadaveric lobar lung transplantation: An important surgical option. Ann Thorac Cardiovasc Surg. 2021;27:244–250. doi: 10.5761/atcs.oa.20-00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aigner C, Mazhar S, Jaksch P, Seebacher G, Taghavi S, Marta G, et al. Lobar transplantation, split lung transplantation and peripheral segmental resection--reliable procedures for downsizing donor lungs. Eur J Cardiothorac Surg. 2004;25:179–183. doi: 10.1016/j.ejcts.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Görler H, Strüber M, Ballmann M, Müller C, Gottlieb J, Warnecke G, et al. Lung and heart-lung transplantation in children and adolescents: A long-term single-center experience. J Heart Lung Transplant. 2009;28:243–248. doi: 10.1016/j.healun.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Martens T, Kanakis M, Spencer H, Muthialu N. Pediatric lung transplantation: Results of volume reduction in smaller children. e13752Pediatr Transplant. 2020;24 doi: 10.1111/petr.13752. [DOI] [PubMed] [Google Scholar]
- 27.Solomon M, Grasemann H, Keshavjee S. Pediatric lung transplantation. Pediatr Clin North Am. 2010;57:375–391. doi: 10.1016/j.pcl.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Sweet SC. Pediatric living donor lobar lung transplantation. Pediatr Transplant. 2006;10:861–868. doi: 10.1111/j.1399-3046.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 29.Conrad C, Cornfield DN. Pediatric lung transplantation: Promise being realized. Curr Opin Pediatr. 2014;26:334–342. doi: 10.1097/MOP.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Date H. Living-donor lobar lung transplantation. J Heart Lung Transplant. 2024;43:162–168. doi: 10.1016/j.healun.2023.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Nakajima D, Sakamoto R, Oguma T, Kawaguchi A, Ohsumi A, et al. Outcome and growth of lobar graft after pediatric living-donor lobar lung transplantation. J Heart Lung Transplant. 2023;42:660–668. doi: 10.1016/j.healun.2022.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima D, Tanaka S, Ikeda T, Baba S, Hiramatsu H, Suga T, et al. Living-donor segmental lung transplantation for pediatric patients. J Thorac Cardiovasc Surg. 2023;165:2193–2201. doi: 10.1016/j.jtcvs.2022.07.031. [DOI] [PubMed] [Google Scholar]
- 33.Ratjen FA. Cystic fibrosis: Pathogenesis and future treatment strategies. Respir Care. 2009;54:595–605. doi: 10.4187/aarc0427. [DOI] [PubMed] [Google Scholar]
- 34.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benden C, Schwarz C. CFTR modulator therapy and its impact on lung transplantation in cystic fibrosis. Pulm Ther. 2021;7:377–393. doi: 10.1007/s41030-021-00170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Boeck K. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatr. 2020;109:893–899. doi: 10.1111/apa.15155. [DOI] [PubMed] [Google Scholar]
- 37.Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax. 2018;73:731–740. doi: 10.1136/thoraxjnl-2017-210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myerburg M, Pilewski JM. CFTR modulators to the rescue of individuals with cystic fibrosis and advanced lung disease. Am J Respir Crit Care Med. 2021;204:7–9. doi: 10.1164/rccm.202103-0674ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgel PR, Durieu I, Chiron R, Ramel S, Danner-Boucher I, Prevotat A, et al. Rapid improvement after starting elexacaftor-tezacaftor-ivacaftor in patients with cystic fibrosis and advanced pulmonary disease. Am J Respir Crit Care Med. 2021;204:64–73. doi: 10.1164/rccm.202011-4153OC. [DOI] [PubMed] [Google Scholar]
- 40.Baruteau AE, Serraf A, Lévy M, Petit J, Bonnet D, Jais X, et al. Potts shunt in children with idiopathic pulmonary arterial hypertension: Long-term results. Ann Thorac Surg. 2012;94:817–824. doi: 10.1016/j.athoracsur.2012.03.099. [DOI] [PubMed] [Google Scholar]
- 41.Carotti A. Surgical management of Fallot's tetralogy with pulmonary atresia and major aortopulmonary collateral arteries: Multistage versus one-stage repair. World J Pediatr Congenit Heart Surg. 2020;11:34–38. doi: 10.1177/2150135119884914. [DOI] [PubMed] [Google Scholar]
- 42.Lammers AE, Haworth SG, Diller GP. Atrial septostomy in patients with pulmonary hypertension: Should it be recommended. Expert Rev Respir Med. 2011;5:363–376. doi: 10.1586/ers.11.25. [DOI] [PubMed] [Google Scholar]
- 43.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, et al. An official American Thoracic Society clinical practice guideline: Classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiavon M, Camagni S, Venuta F, Rosso L, Boffini M, Parisi F, et al. A multicentric evaluation of pediatric lung transplantation in Italy. J Thorac Cardiovasc Surg. 2023;165:1519–1527. doi: 10.1016/j.jtcvs.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Müller C, Görler H, Ballmann M, Gottlieb J, Simon AR, Strüber M, et al. Pulmonary retransplantation in paediatric patients: A justified therapeutic option. A single-centre experience. Eur J Cardiothorac Surg. 2011;39:201–205. doi: 10.1016/j.ejcts.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes D Jr, Harhay MO, Cherikh WS, Chambers DC, Perch M, Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-fourth pediatric lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant. 2021;40:1023–1034. doi: 10.1016/j.healun.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein BS, Sweet SC, Mao J, Huddleston CB, Grady RM. Lung transplantation in children with idiopathic pulmonary arterial hypertension: An 18-year experience. J Heart Lung Transplant. 2011;30:1148–1152. doi: 10.1016/j.healun.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Hubbard R, Miller R, Tumin D, Tobias JD, Hayes D Jr. Transplant outcomes for idiopathic pulmonary hypertension in children. J Heart Lung Transplant. 2019;38:580–581. doi: 10.1016/j.healun.2019.01.1314. [DOI] [PubMed] [Google Scholar]
- 51.Heidel JS, Dani A, Towe C, Schecter M, Zhang Y, Hossain MM, et al. Body mass index percentage and survival in pediatric patients listed for lung transplantation: A modernera multi-institutional analysis. J Heart Lung Transplant. 2023;42:1242–1250. doi: 10.1016/j.healun.2023.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melicoff E, Hayes D Jr, Benden C. Lung transplantation as an intervention for pediatric pulmonary hypertension. Pediatr Pulmonol. 2021;56:587–592. doi: 10.1002/ppul.25154. [DOI] [PubMed] [Google Scholar]
- 53.Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879–901. doi: 10.1016/j.healun.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Inci I, Ehrsam JP, Van Raemdonck D, Ceulemans LJ, Krüger T, Koutsokera A, et al. Extracorporeal life support as a bridge to pulmonary retransplantation: Prognostic factors for survival in a multicentre cohort analysis. Eur J Cardiothorac Surg. 2022;61:405–412. doi: 10.1093/ejcts/ezab514. [DOI] [PubMed] [Google Scholar]
- 55.Scully BB, Zafar F, Schecter MG, Rossano JW, Mallory GB Jr, Heinle JS, et al. Lung retransplantation in children: Appropriate when selectively applied. Ann Thorac Surg. 2011;91:574–579. doi: 10.1016/j.athoracsur.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Toprak D, Midyat L, Freiberger D, Boyer D, Fynn-Thompson F, Visner G. Outcomes of mechanical support in a pediatric lung transplant center. Pediatr Pulmonol. 2017;52:360–366. doi: 10.1002/ppul.23535. [DOI] [PubMed] [Google Scholar]
- 57.Casswell GK, Pilcher DV, Martin RS, Pellegrino VA, Marasco SF, Robertson C, et al. Buying time: The use of extracorporeal membrane oxygenation as a bridge to lung transplantation in pediatric patients. E182-8Pediatr Transplant. 2013;17 doi: 10.1111/petr.12152. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt F, Sasse M, Boehne M, Mueller C, Bertram H, Kuehn C, et al. Concept of “awake venovenous extracorporeal membrane oxygenation” in pediatric patients awaiting lung transplantation. Pediatr Transplant. 2013;17:224–230. doi: 10.1111/petr.12001. [DOI] [PubMed] [Google Scholar]
- 59.Inci I, Klinzing S, Schneiter D, Schuepbach RA, Kestenholz P, Hillinger S, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation: An institutional experience and literature review. Transplantation. 2015;99:1667–1671. doi: 10.1097/TP.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 60.Koh W, Zang H, Ollberding NJ, Ziady A, Hayes D Jr. Extracorporeal membrane oxygenation bridge to pediatric lung transplantation: Modern era analysis. e14570Pediatr Transplant. 2023;27 doi: 10.1111/petr.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kearns SK, Hernandez OO. “Awake” extracorporeal membrane oxygenation as a bridge to lung transplant. AACN Adv Crit Care. 2016;27:293–300. doi: 10.4037/aacnacc2016792. [DOI] [PubMed] [Google Scholar]
- 62.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: A new approach for bridge-to-lung transplantation. e137-9J Thorac Cardiovasc Surg. 2010;139 doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Rehder KJ, Turner DA, Hartwig MG, Williford WL, Bonadonna D, Walczak RJ Jr, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care. 2013;58:1291–1298. doi: 10.4187/respcare.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Perrot M, Granton JT, McRae K, Cypel M, Pierre A, Waddell TK, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30:997–1002. doi: 10.1016/j.healun.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Hoganson DM, Gazit AZ, Boston US, Sweet SC, Grady RM, Huddleston CB, et al. Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. J Thorac Cardiovasc Surg. 2014;147:420–426. doi: 10.1016/j.jtcvs.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 66.Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–1200. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 67.Spielberg DR, Melicoff E, Heinle JS, Hosek K, Mallory GB. Differential donor management of pediatric vs adult organ donors and potential impact on pediatric lung transplantation. J Heart Lung Transplant. 2023;42:522–532. doi: 10.1016/j.healun.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Yoo PS, Olthoff KM, Abt PL. Donation after cardiac death in pediatric organ transplantation. Curr Opin Organ Transplant. 2011;16:483–488. doi: 10.1097/MOT.0b013e32834a8bf5. [DOI] [PubMed] [Google Scholar]
- 69.Ehrsam JP, Benden C, Immer FF, Inci I. Current status and further potential of lung donation after circulatory death. e14335Clin Transplant. 2021;35 doi: 10.1111/ctr.14335. [DOI] [PubMed] [Google Scholar]
- 70.Luc JG, Nagendran J. The evolving potential for pediatric ex vivo lung perfusion. Pediatr Transplant. 2016;20:13–22. doi: 10.1111/petr.12653. [DOI] [PubMed] [Google Scholar]
- 71.Ehrsam JP, Held U, Opitz I, Inci I. A new lung donor score to predict short and long-term survival in lung transplantation. J Thorac Dis. 2020;12:5485–5494. doi: 10.21037/jtd-20-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayes D Jr, Harhay MO, Cherikh WS, Chambers DC, Khush KK, Hsich E, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-third pediatric lung transplantation report - 2020; focus on deceased donor characteristics. J Heart Lung Transplant. 202;39:1038–1049. doi: 10.1016/j.healun.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lancaster TS, Miller JR, Epstein DJ, DuPont NC, Sweet SC, Eghtesady P. Improved waitlist and transplant outcomes for pediatric lung transplantation after implementation of the lung allocation score. J Heart Lung Transplant. 2017;36:520–528. doi: 10.1016/j.healun.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sweet S, de la Morena MT, Schuler P, Patterson A, Meyers B, Schuller D, et al. Single center comparison of pediatric living donor lobar and cadaveric lung transplant. S145-6J Heart Lung Transplant. 2003;22(Suppl 1) doi: 10.1016/S1053-2498(02)00917-8. [DOI] [Google Scholar]
- 75.Goldfarb SB, Hayes D Jr, Levvey BJ, Cherikh WS, Chambers DC, Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric lung and heart-lung transplantation report-2018; Focus theme: Multiorgan transplantation. J Heart Lung Transplant. 2018;37:1196–1206. doi: 10.1016/j.healun.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 76.Hayes D Jr, Kirkby S, Wehr AM, Lehman AM, McConnell PI, Galantowicz M, et al. A contemporary analysis of induction immunosuppression in pediatric lung transplant recipients. Transpl Int. 2014;27:211–218. doi: 10.1111/tri.12240. [DOI] [PubMed] [Google Scholar]
- 77.Whitson BA, Lehman A, Wehr A, Hayes D Jr, Kirkby S, Pope-Harman A, et al. To induce or not to induce: A 21st century evaluation of lung transplant immunosuppression's effect on survival. Clin Transplant. 2014;28:450–461. doi: 10.1111/ctr.12339. [DOI] [PubMed] [Google Scholar]
- 78.Sweet SC, Armstrong B, Blatter J, Chin H, Conrad C, Goldfarb S, et al. CTOTC-08: A multicenter randomized controlled trial of rituximab induction to reduce antibody development and improve outcomes in pediatric lung transplant recipients. Am J Transplant. 2022;22:230–244. doi: 10.1111/ajt.16862. [DOI] [PubMed] [Google Scholar]
- 79.Gruber S, Eiwegger T, Nachbaur E, Tiringer K, Aigner C, Jaksch P, et al. Lung transplantation in children and young adults: A 20-year single-centre experience. Eur Respir J. 2012;40:462–469. doi: 10.1183/09031936.00092211. [DOI] [PubMed] [Google Scholar]
- 80.Fan Y, Xiao YB, Weng YG. Tacrolimus versus cyclosporine for adult lung transplant recipients: A metaanalysis. Transplant Proc. 2009;41:1821–1824. doi: 10.1016/j.transproceed.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 81.Wong M, Mallory GB Jr, Goldstein J, Goyal M, Yamada KA. Neurologic complications of pediatric lung transplantation. Neurology. 1999;53:1542–1549. doi: 10.1212/wnl.53.7.1542. [DOI] [PubMed] [Google Scholar]
- 82.Sweet SC. Pediatric lung transplantation. Proc Am Thorac Soc. 2009;6:122–127. doi: 10.1513/pats.200808-095GO. [DOI] [PubMed] [Google Scholar]
- 83.Porteous MK, Diamond JM, Christie JD. Primary graft dysfunction: Lessons learned about the first 72h after lung transplantation. Curr Opin Organ Transplant. 2015;20:506–514. doi: 10.1097/MOT.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. H1283-99Am J Physiol Heart Circ Physiol. 2010;299 doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 85.Laubach VE, Sharma AK. Mechanisms of lung ischemiareperfusion injury. Curr Opin Organ Transplant. 2016;21:246–252. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moser B, Jaksch P, Taghavi S, Muraközy G, Lang G, Hager H, et al. Lung transplantation for idiopathic pulmonary arterial hypertension on intraoperative and postoperatively prolonged extracorporeal membrane oxygenation provides optimally controlled reperfusion and excellent outcome. Eur J Cardiothorac Surg. 2018;53:178–185. doi: 10.1093/ejcts/ezx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trulock EP. Management of lung transplant rejection. Chest. 1993;103:1566–1576. doi: 10.1378/chest.103.5.1566. [DOI] [PubMed] [Google Scholar]
- 88.Hsiao HM, Scozzi D, Gauthier JM, Kreisel D. Mechanisms of graft rejection after lung transplantation. Curr Opin Organ Transplant. 2017;22:29–35. doi: 10.1097/MOT.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benzimra M, Calligaro GL, Glanville AR. Acute rejection. J Thorac Dis. 2017;9:5440–5457. doi: 10.21037/jtd.2017.11.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–197. doi: 10.1016/j.athoracsur.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elizur A, Faro A, Huddleston CB, Gandhi SK, White D, Kuklinski CA, et al. Lung transplantation in infants and toddlers from 1990 to 2004 at St. Louis Children's Hospital. Am J Transplant. 2009;9:719–726. doi: 10.1111/j.1600-6143.2009.02552.x. [DOI] [PubMed] [Google Scholar]
- 92.Ibrahim JE, Sweet SC, Flippin M, Dent C, Mendelhoff E, Huddleston CB, et al. Rejection is reduced in thoracic organ recipients when transplanted in the first year of life. J Heart Lung Transplant. 2002;21:311–318. doi: 10.1016/s1053-2498(01)00395-3. [DOI] [PubMed] [Google Scholar]
- 93.Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38:493–503. doi: 10.1016/j.healun.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Venado A, Kukreja J, Greenland JR. Chronic lung allograft dysfunction. Thorac Surg Clin. 2022;32:231–242. doi: 10.1016/j.thorsurg.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Sweet SC, Chin H, Conrad C, Hayes D Jr, Heeger PS, Faro A, et al. Absence of evidence that respiratory viral infections influence pediatric lung transplantation outcomes: Results of the CTOTC-03 study. Am J Transplant. 2019;19:3284–3298. doi: 10.1111/ajt.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong W, Johnson B, Cheng PC, Josephson MB, Maeda K, Berg RA, et al. Primary graft dysfunction grade 3 following pediatric lung transplantation is associated with chronic lung allograft dysfunction. J Heart Lung Transplant. 2023;42:669–678. doi: 10.1016/j.healun.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: A review. Chest. 1998;114:1411–1426. doi: 10.1378/chest.114.5.1411. [DOI] [PubMed] [Google Scholar]
- 98.Benden C, Haughton M, Leonard S, Huber LC. Therapy options for chronic lung allograft dysfunction-bronchiolitis obliterans syndrome following first-line immunosuppressive strategies: A systematic review. J Heart Lung Transplant. 2017;36:921–933. doi: 10.1016/j.healun.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 99.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solidorgan transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 100.Perreas KG, McNeil K, Charman S, Sharples LD, Wreghitt T, Wallwork J. Extended ganciclovir prophylaxis in lung transplantation. J Heart Lung Transplant. 2005;24:583–587. doi: 10.1016/j.healun.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Ammerman E, Sweet SC, Fenchel M, Storch GA, Conrad C, Hayes D Jr, et al. Risk and outcomes of pulmonary fungal infection after pediatric lung transplantation. e13100Clin Transplant. 2017;31 doi: 10.1111/ctr.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: Update 2012. Diagn Microbiol Infect Dis. 2012;73:293–300. doi: 10.1016/j.diagmicrobio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Luparello P, Lazio MS, Voltolini L, Borchi B, Taccetti G, Maggiore G. Outcomes of endoscopic sinus surgery in adult lung transplant patients with cystic fibrosis. Eur Arch Otorhinolaryngol. 2019;276:1341–1347. doi: 10.1007/s00405-019-05308-9. [DOI] [PubMed] [Google Scholar]
- 104.Leung MK, Rachakonda L, Weill D, Hwang PH. Effects of sinus surgery on lung transplantation outcomes in cystic fibrosis. Am J Rhinol. 2008;22:192–196. doi: 10.2500/ajr.2008.22.3146. [DOI] [PubMed] [Google Scholar]
- 105.Meier M, Schuurmans MM, Vital D, Inci I, Holzman D, Soyka MB. Impact of extended sinus surgery on allograft infection, allograft function and overall survival in cystic fibrosis lung transplant recipients. Eur Arch Otorhinolaryngol. 2023;280:4501–4507. doi: 10.1007/s00405-023-08028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bakker NA, van Imhoff GW, Verschuuren EA, van Son WJ, Homan van der Heide JJ, Veeger NJ, et al. Early onset posttransplant lymphoproliferative disease is associated with allograft localization. Clin Transplant. 2005;19:327–334. doi: 10.1111/j.1399-0012.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 107.Herrmann BW, Sweet SC, Hayashi RJ, Canter CE, White FV, Lieu JE. Otolaryngological manifestations of posttransplant lymphoproliferative disorder in pediatric thoracic transplant patients. Int J Pediatr Otorhinolaryngol. 2006;70:303–310. doi: 10.1016/j.ijporl.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 108.AlDabbagh MA, Gitman MR, Kumar D, Humar A, Rotstein C, Husain S. The role of antiviral prophylaxis for the prevention of Epstein-Barr virus-associated posttransplant lymphoproliferative disease in solid organ transplant recipients: A systematic review. Am J Transplant. 2017;17:770–781. doi: 10.1111/ajt.14020. [DOI] [PubMed] [Google Scholar]
- 109.Guzman-Gomez A, Ahmed HF, Dani A, Zafar F, Lehenbauer DG, Potter AS, et al. Center volume effect on acute cellular rejection and outcomes in pediatric lung transplant recipients. J Heart Lung Transplant. 2023;42:1030–1039. doi: 10.1016/j.healun.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujimoto R, Nakajima D, Tanaka S, Yamada Y, Yutaka Y, Ohsumi A, et al. Efficacy of three-dimensional computed tomography volumetry for recipients in downsizing oversized grafts in brain-dead donor lung transplantation. Gen Thorac Cardiovasc Surg. 2021;69:1112–1117. doi: 10.1007/s11748-021-01591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conrad C. Update on pediatric lung transplantation: Mir-ando into the mechanisms of chronic lung allograft dysfunction in children. Curr Opin Organ Transplant. 2020;25:293–298. doi: 10.1097/MOT.0000000000000763. [DOI] [PubMed] [Google Scholar]