Abstract
Pulmonary tumors in childhood are rare, but the majority are malignant. The histopathologic spectrum is quite diverse, including inflammatory myofibroblastic tumor, hamartoma, primary pulmonary paraganglioma, carcinoid tumor, mucoepidermoid carcinoma, pleuropulmonary blastoma, adenocarcinoma, squamous cell carcinoma, and sarcomas. Nonspecific clinical and radiological findings result in late and incorrect diagnoses. Although surgical resection is the initial and proper treatment method, additional adjuvant therapy is dependent on both tumor stage and histopathologic type.
Keywords: Childhood, lung, pulmonary tumor.
Introduction
Pulmonary tumors are rare in childhood. Primary malignant tumors and secondary tumors that metastasize to the lung account for about 80% of all cases, while benign tumors are less common.[1]
Due to their rarity and nonspecific clinical and radiological findings, they are often misdiagnosed as foreign body aspiration, infectious conditions, and reactive airway diseases; consequently, they are diagnosed late. However, they still have a better prognosis than those encountered in adulthood.[2]
The presence and variety of symptoms vary depending on the size of the tumor, its benign or malignant nature, vascularity, and peripheral or central location. Nevertheless, they remain nonspecific. Small lesions with a peripheral location and those with central localization can easily overlooked radiologically, which is an obstacle for early diagnosis.
Benign pulmonary tumors in childhood include inflammatory myofibroblastic tumors, hamartoma, and primary pulmonary paraganglioma (PPP). Carcinoid tumors, mucoepidermoid carcinoma, pleuropulmonary blastoma, adenocarcinoma, and squamous cell carcinoma constitute the major primary malignant tumors. Osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, germ cell tumors, and Wilms tumors are secondary malignant tumors that often metastasize to the lung.[1,2]
BENIGN TUMORS
Inflammatory myofibroblastic tumors account for more than 50% of benign pulmonary tumors in childhood.[3] Although they are mostly wellcircumscribed, slow-growing, and peripherally located tumors, they have the potential for relapse and metastasis. Deaths due to metastasis and local invasion have been reported, with a local recurrence rate of 3 to 24%.[4] Although there are reports about spontaneously disappearing tumors or good response to steroid therapy, the main method of treatment is surgery.[5] In the goal of complete resection, wedge resection is sufficient in small and peripheral tumors or lobectomy for larger and aggressive ones. Since inflammatory myofibroblastic tumors may mimic inflammatory reactions and hematolymphoid proliferations, a pre- or intraoperative definitive diagnosis is mandatory to determine the extent of resection. Extended resection or pneumonectomy should not be attempted until the diagnosis is confirmed. Radiotherapy can be considered for incomplete resections or inoperable cases.[6]
Pulmonary hamartomas are benign malformations of the lung and are defined as an abnormal mixture of tissue elements of cartilage, connective, adipose tissue, and epithelium. It is the most frequent benign neoplasm of the lung in adults. However, they are much rarer in children.[7] They grow slowly, tend to be asymptomatic, and usually are found incidentally. On chest imaging, they can appear as a sharply demarcated and round or lobulated structure, commonly <3 cm in diameter, and they tend to occur in the peripheral parenchyma.[8]
Enucleation and wedge resections are the most common surgical choices. Lobectomy, sleeve resection, and pneumonectomy will be required for multiple, large, and hilar lesions, which are making lesser resections impossible. In the case of malignant transformation or accompaniment by primary lung cancer, anatomic resections should be carried out. Endobronchial hamartomas can be removed successfully through bronchoscopy.[9]
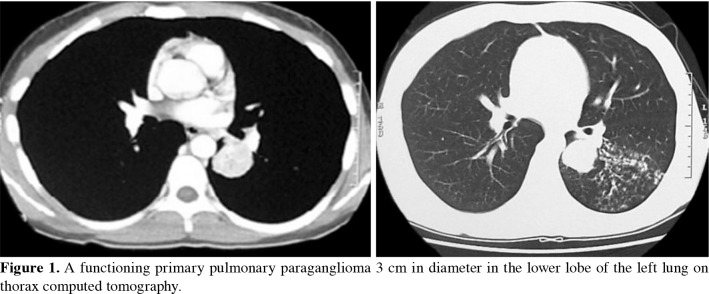
Paraganglioma is a rare tumor originating from the extra-adrenal chromaffin cells. Primary pulmonary paraganglioma is extremely rare and comprises only 1% of all paragangliomas. Metastatic paragangliomas in the lung are more common. Paragangliomas are usually observed as asymptomatic subpleural solitary nodules, and the majority are detected incidentally. They can mimic carcinoid tumors or primary lung cancer when they present as an endobronchial lesion. The proper treatment for paragangliomas is surgery as in other benign lesions of the lung, and parenchyma-sparing resection should be performed when possible.[10] The neuroendocrine immunohistochemical behavior of secreting excessive catecholamines is an exceptional situation in PPP. In our past case report, the clinical presentation of hypertension, tachycardia, and high urine and blood noradrenaline levels is the second pediatric functioning PPP in the literature (Figure 1).[11]
Figure 1. A functioning primary pulmonary paraganglioma 3 cm in diameter in the lower lobe of the left lung on thorax computed tomography.
MALIGN TUMORS
The incidence of primary lung cancer in children and adolescents is rare, and they account for less than 0.1% of all childhood malignancies. They are clinically and histopathologically heterogeneous. Primary malignant pulmonary tumors of epithelial origin in pediatric patients are carcinoid tumors, mucoepidermoid carcinoma, adenocarcinoma, squamous cell carcinoma, small cell carcinoma, and large cell carcinoma.[12]
Shao et al.[13] stated in their study of 301 children that carcinoid tumor was the most common histology (29.6%), followed by pulmonary blastoma (22.3%), mucoepidermoid carcinoma (12.3%), adenocarcinoma (10.3%), neuroendocrine tumor (5.7%), squamous cell carcinoma (5.3%), and atypical carcinoma (2.3%).Rojas et al.[12] reported that the most common histology was carcinoid tumor (63%), followed by mucoepidermoid carcinoma (18%), squamous cell carcinoma (9%), adenocarcinoma (8%), bronchoalveolar carcinoma (2%), and small cell carcinoma (<1%) in their study of 211 children. Abele et al.[2] found that nearly 20% of children had preexisting lung disease, including HPV (human papillomavirus)-associated chronic laryngeal papillomatosis, carcinoma ex-papilloma, or congenital pulmonary airway malformation, indicating that such conditions may increase the relative risk for developing lung tumors, although the absolute risk is likely to remain extremely low in their study of 38 children.
Mucoepidermoid carcinoma was defined as a tumor characterized by a combination of mucus-secreting, squamous, and intermediate cell types. It was reported as the second most common primary malignant pulmonary tumor in children by Dishop et al.[14] Pleuropulmonary blastoma is a rare and aggressive childhood tumor of mesenchymal origin that often affects children under six years of age. Its origin may be pleural, pulmonary, or both. Local recurrence and distant metastasis are frequent clinical behaviors.[15] Adenocarcinoma was more common than squamous cell carcinoma. Fortunately, they appear to have a better prognosis than squamous cell carcinoma in children. Small cell lung carcinoma is exceedingly rare in pediatric patients.[2]
While surgery may be sufficient for localized disease, multimodal treatment is required for advanced conditions. Lobectomy with mediastinal lymph node dissection is the main resection type, followed by sublobar resections and pneumonectomy. As there is no evidence-based treatment method other than surgery, adapting therapeutic regimens from adult practice appears to be the current approach.[12-15]
The five- and 10-year lung cancer specificity survival were 79.4% and 77.7%, respectively. Children with carcinoid tumors and mucoepidermoid carcinoma had a significantly better overall survival compared to patients with other histology. However, squamous cell, large cell, and small cell lung carcinomas have the lowest incidence rate, the lowest overall survival, and the worst prognosis.[13]
Bronchial carcinoids account for 42-63% of all primary lung malignancies in the pediatric population. The etiology of bronchial carcinoids in childhood is largely unexplained. They originate from Kulchitsky neuroendocrine cells located in the bronchopulmonary epithelium. In pediatric patients, they are usually diagnosed between a mean age of 11.6 and 13.8 years but may occur as early as at three years of age. Abele et al.[16] confirmed that bronchial carcinoids in children and adolescents are most likely located in the right lung in their study of 32 children. Approximately 75% are located in lobar bronchi, 10-20% in the main bronchi, and a minor proportion in the lung periphery. Bronchial carcinoids are classified as typical low-grade carcinoids and atypical intermediate-grade carcinoids, which can even metastasize. Atypical bronchial carcinoids occur more frequently in children.[16] Metastases were most frequently diagnosed in the liver (75%) and bone (42%).[17] Computed tomography of the thorax is the initial imaging method (Figure 2). Abdominal magnetic resonance imaging is usually preferred in children to avoid radiation exposure for staging. A positron emission tomography with Gallium-68 has the highest sensitivity in detecting pulmonary carcinoids and is therefore recommended in guidelines. Additional imaging by Gallium-68 positron emission tomography/computed tomography should be performed in case of atypical bronchial carcinoid, lymph node, or distant metastases.[16] Bronchoscopic biopsy is recommended prior to definitive surgical resection.[18] Treatment of choice for localized bronchial carcinoids is complete anatomical resection without further adjuvant therapy. The surgical goal is to achieve tumor-free resection margins and preserve as much normal lung tissue as possible. Therefore, bronchial sleeve resection or bronchoplastic techniques should be preferred rather than pneumonectomy.[16-18] In pediatric patients with metastatic spread and recurrence of atypical bronchial carcinoids, treatment options include chemotherapy, somatostatin analogs, and peptide receptor radionucleotide therapy. Five-year overall survivals of approximately 95% have been reported in pediatric patients, including 10-year disease-specific survival of 90.3% with typical histology and 65.0% with atypical histology.[19]
Figure 2. A thorax computed tomography scan of our patient with carcinoid tumor showing (a) a nodule of 7 mm diameter in the inferior lingular segment and (b) a hypodense, well-circumscribed, and homogeneous solid lesion measuring 22×11 mm in the left hilar region.
Most pediatric malignant lung tumors are metastatic from nonpulmonary malignancies. Metastatic tumors from nonpulmonary primary neoplasms comprise approximately 80% of all pediatric lung tumors and more than 95% of malignant tumors of the lung in children. Rhabdomyosarcoma, osteosarcoma, Ewing sarcoma, hepatoblastoma, germ cell tumors, and Wilms’ tumor are major histopathologic types.[19]
In conclusion, surgical resection is the initial treatment method in childhood pulmonary tumors. The main goal is to perform curative resection while preserving as much lung parenchyma as possible. It should be kept in mind that pneumonectomy performed in childhood may cause considerable kyphoscoliosis. Although aggressive oncological treatment methods are applied, innovative methods, such as mutation-guided treatment models, are needed for better prognosis in childhood pulmonary tumors. Establishing a prospective international registry system for new cases will facilitate the management of treatment and will contribute positively to the prognosis of these children.
Footnotes
Conflict of Interest: The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The author received no financial support for the research and/or authorship of this article.
References
- 1.Weldon CB, Shamberger RC. Pediatric pulmonary tumors: Primary and metastatic. Semin Pediatr Surg. 2008;17:17–29. doi: 10.1053/j.sempedsurg.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Abele M, Bajčiová V, Wright F, Behjati S, Voggel S, Schneider DT, et al. Primary lung carcinoma in children and adolescents: An analysis of the European Cooperative Study Group on Paediatric Rare Tumours (EXPeRT) Eur J Cancer. 2022;175:19–30. doi: 10.1016/j.ejca.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Hancock BJ, Di Lorenzo M, Youssef S, Yazbeck S, Marcotte JE, Collin PP. Childhood primary pulmonary neoplasms. J Pediatr Surg. 1993;28:1133–1136. doi: 10.1016/0022-3468(93)90147-d. [DOI] [PubMed] [Google Scholar]
- 4.Fabre D, Fadel E, Singhal S, de Montpreville V, Mussot S, Mercier O, et al. Complete resection of pulmonary inflammatory pseudotumors has excellent long-term prognosis. J Thorac Cardiovasc Surg. 2009;137:435–440. doi: 10.1016/j.jtcvs.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Cho JH, Park MS, Chung JH, Lee JG, Kim YS, et al. Pulmonary inflammatory pseudotumor--a report of 28 cases. Korean J Intern Med. 2002;17:252–258. doi: 10.3904/kjim.2002.17.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yüksel C, Yenigün BM, Kocaman G, Özkıncı H, Kahya Y, Dizbay Sak S, et al. Operated pulmonary inflammatory myofibroblastic tumors: Our experience with 17 cases. Turk Gogus Kalp Damar Cerrahisi Derg. 2022;30:101–108. doi: 10.5606/tgkdc.dergisi.2022.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saadi MM, Barakeh DH, Husain S, Hajjar WM. Large multicystic pulmonary chondroid hamartoma in a child presenting as pneumothorax. Saudi Med J. 2015;36:487–489. doi: 10.15537/smj.2015.4.10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amini B, Huang SY, Tsai J, Benveniste MF, Robledo HH, Lee EY. Primary lung and large airway neoplasms in children: Current imaging evaluation with multidetector computed tomography. Radiol Clin North Am. 2013;51:637–657. doi: 10.1016/j.rcl.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Önen A, Şanlı A, Karapolat S, Karacam V, Kargı A. Pulmonary hamartoma and squamous cell carcinoma: A very rare coexistence. Turk Gogus Kalp Dama. 2007;15:311–331. [Google Scholar]
- 10.Kavakli K, Ozturk M, Ongoru O, Gürkök S, Genc O. Primary pulmonary paraganglioma with Hodgkin's lymphoma. Thorac Cardiovasc Surg. 2009;57:375–377. doi: 10.1055/s-2008-1038878. [DOI] [PubMed] [Google Scholar]
- 11.Yüksel C, Kocaman G, Yenigün BM, Özakıncı H, Dizbay Sak S, Enön S, et al. Primary pulmonary paraganglioma: Two cases. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:394–398. doi: 10.5606/tgkdc.dergisi.2020.18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rojas Y, Shi YX, Zhang W, Beierle EA, Doski JJ, Goldfarb M, et al. Primary malignant pulmonary tumors in children: A review of the national cancer data base. J Pediatr Surg. 2015;50:1004–1008. doi: 10.1016/j.jpedsurg.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Shao W, Liu J, Li B, Guo X, Sun J, Li H, et al. Primary lung cancer in children and adolescents: Analysis of a surveillance, epidemiology, and end results database. Front Oncol. 2023;13:1053248–1053248. doi: 10.3389/fonc.2023.1053248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: A review and 25-year experience at a large children's hospital. Arch Pathol Lab Med. 2008;132:1079–1103. doi: 10.5858/2008-132-1079-PAMLTI. [DOI] [PubMed] [Google Scholar]
- 15.Demir ÖF, Önal Ö, Hasdıraz L, Oğuzkaya F, Kontaş O, Ülgey A. Pleuropulmonary blastoma: A report of two cases. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:209–212. doi: 10.5606/tgkdc.dergisi.2020.18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abele M, Kunstreich M, Lessel L, Seitz G, Vokuhl C, Lapa C, et al. Bronchial carcinoid tumors in children and adolescents - A report and management considerations from the German MET studies. Lung Cancer. 2023;183:107320–107320. doi: 10.1016/j.lungcan.2023.107320. [DOI] [PubMed] [Google Scholar]
- 17.Robelin P, Hadoux J, Forestier J, Planchard D, Hervieu V, Berdelou A, et al. Characterization, prognosis, and treatment of patients with metastatic lung carcinoid tumors. J Thorac Oncol. 2019;14:993–1002. doi: 10.1016/j.jtho.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up✩. Ann Oncol. 2021;32:439–451. doi: 10.1016/j.annonc.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JY, Sigel K, Martin J, Jordan R, Beasley MB, Smith C, et al. Evaluation of the prognostic significance of TNM staging guidelines in lung carcinoid tumors. J Thorac Oncol. 2019;14:184–192. doi: 10.1016/j.jtho.2018.10.166. [DOI] [PubMed] [Google Scholar]