Abstract
Chest wall deformities are congenital disorders characterized by abnormal development and appearance of the thoracic wall. The most common form is the pectus excavatum deformity, known as shoemaker's chest. Pectus carinatum, known as pigeon chest, is the second most common deformity. In general, most patients are asymptomatic, but cardiopulmonary problems may accompany the disease. The indication for treatment is mostly cosmetic. Treating patients before they reach adulthood increases the chance of success. Surgical treatment can be open or minimally invasive.
Keywords: Chest wall, deformity, pectus carinatum, pectus excavatum, surgery.
Introduction
Chest wall deformities (CWD) consist of a group of congenital diseases covering a wide spectrum.[1] Chest wall deformities are characterized by abnormal development and appearance of the chest wall. Congenital CWD may be associated with various anomalies of the musculoskeletal system or may occur in isolation affecting the costae, cartilage, and sternum. These deformities are common deformities, occurring in 1/300-400 live births and more frequently in males.[2] The most common CWD is pectus excavatum (PE), known as shoemaker's chest. It is followed by pectus carinatum (PC), known as pigeon chest.
The first records related with congenital CWD were found in Egyptian civilizations.[3] German physician Schenck von Grafenberg[4] was the first person to collect literature on PE in the early 16th century. Later, Bauhinus,[5] a Swiss anatomist, described the clinical features of PE for the first time in 1594 in a patient with respiratory failure due to severe deformity. The first surgical repair was performed by Meyer[6] in 1911 by removing the cartilage part of the rib. In 1949, subperichondrial resection of the lower costal cartilages and sterna wedge osteotomy popularized by Ravitch[7] was published. This procedure was considered the gold standard treatment for many years. Later, the technique was modified with the addition of the support wire to the operation. In 1956, Judet and Judet[8] described the sternal turnover method. In 1970, Wada[9] reported a series of 270 patients with this procedure. However, sterna turnover was not accepted due to the high morbidity associated with complications such as infection and necrosis.[9] In 1998, Nuss et al.[10] revolutionized the surgical field by describing minimally invasive repair, and the Nuss procedure has taken its place as the most commonly used surgical technique today.
The etiology of PE and PC is not clearly known. Disorder of cartilage development is the basis of the pathology. Today, the most accepted opinion is that the sternum is displaced anterior or posteriorly as a result of overgrowth of the lower costal cartilage and costae, and the diaphragm plays a role in this displacement.[11] In other chest wall deformities, it is thought that the problem may be related to pathologies such as blood supply and malnutrition during pregnancy. It is also known that CWD may be related to genetic syndromes. Although genetic transmission has not been demonstrated, a familial relationship has been reported in most cases.[12]
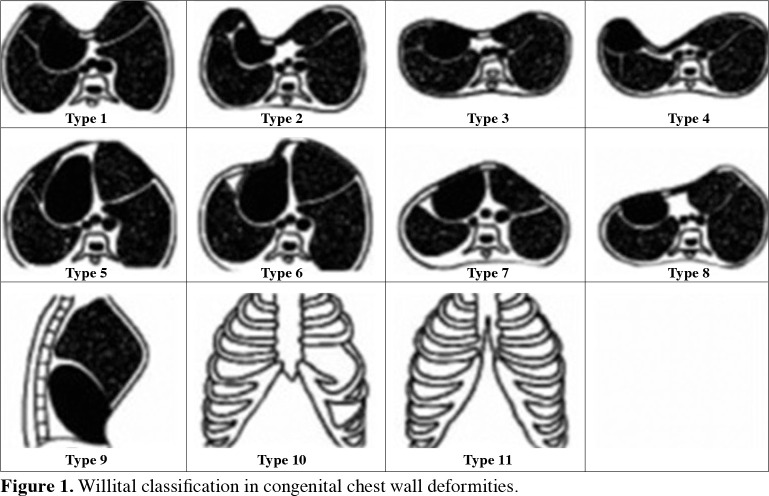
Most patients are asymptomatic. The most common symptoms are exertional dyspnea and chest pain.[13] Although CWD patients usually do not have life-threatening functional disorders in intrathoracic organs, cardiopulmonary pathologies may be encountered rarely. The deformity usually becomes visible in childhood and becomes more prominent over time. In some patients, deformities may show a regressive course with advancing age. Most of the time, physical examination is sufficient for the diagnosis. Patients are evaluated with additional tests according to the accompanying findings. Chest wall deformities may be symmetrical, asymmetrical, or combined. Patients are classified according to Willital et al.’s[14] classification, which divides chest wall deformities into 11 groups based on the morphologic structure of the thorax. Classically, anterior CWD is categorized under five main headings:[15] PE, PC, Poland syndrome (PS), sternal defects, and other deformities (vertebral and costal anomalies, costal dysplasia, Jeune's disease, JarchoLevin syndrome) (Figure 1 and Table 1).
Figure 1. Willital classification in congenital chest wall deformities.
Table 1. Willital classification.
| Type | Description |
| 1 | Symmetrical pectus excavatum, thorax in normal configuration |
| 2 | Asymmetric pectus excavatum, thorax in normal configuration |
| 3 | Symmetrical pectus excavatum, with platythorax |
| 4 | Asymmetric pectus excavatum, with platythorax |
| 5 | Symmetrical pectus carinatum, thorax in normal configuration |
| 6 | Asymmetric pectus carinatum, thorax in normal configuration |
| 7 | Symmetrical pectus carinatum, with platythorax |
| 8 | Asymmetric pectus carinatum, with platythorax |
| 9 | Combination of pectus excavatum and pectus carinatum |
| 10 | Chest wall aplasia/hypoplasia |
| 11 | Sternal cleft defects |
Chest wall deformities can be treated conservatively or surgically. Conservative methods include vacuum, orthosis, and various brace applications and have gained popularity in recent years. For conservative treatments, early ages give better results compared to surgery. Surgical treatments are either closed or open surgery and should be planned in the most appropriate way for the patient. Although there are different opinions on the age of treatment, it is advantageous to start at an early age. The age range of 10-16 years is accepted as the most appropriate age for surgery. The most preferred method in PE surgery today is the minimally invasive repair of PE, developed by the American pediatric surgeon Nuss[16] and modified by other surgeons.[17] In PC, the minimally invasive PC surgery developed by the Argentinian thoracic surgeon Abramson et al.[18] is ideal today. In some PE or PC deformities, where the deformity is asymmetric, open surgical technique known as the Ravicth method may be required.
PECTUS EXCAVATUM
Pectus excavatum is the most common CWD and accounts for 90% of all congenital chest wall deformities.[19] Pectus excavatum is an inward collapse of the sternum and costochondral cartilage structures. It is popularly known as shoemaker's chest. Its incidence is higher in males than in females. The approximate ratio is four-to-one.[20] It usually occurs at birth or in childhood and becomes more prominent in adolescence. The depression in the chest wall is usually asymmetrical and localized on the right side. The manubrium sterni and the first and second costae are usually in normal position. In some cases, the sternum is rotated. It may be associated with PC, and in this case, the disease is called mixed type.
The pathogenesis of PE is not clear. Genetic transmission has not been demonstrated, but familial predisposition is known. It is thought that pathologic development in costal cartilage may be related to collagen metabolism disorder. Pectus excavatum, which usually occurs in isolation, may be a part of genetic syndromes. Marfan syndrome, Ehler-Danlos syndrome, and Noonan syndrome are the most commonly associated syndromes. These syndromes are usually autosomal dominant and affect connective tissue.
Pectus excavatum is often associated with psychosocial and rarely organic problems. The majority of children, particularly in adolescence, become withdrawn, shy, and avoid social activities as they complain about their appearance. The deformity may cause airway obstruction in newborns. Later in life, they may cause exercise intolerance, chest pain, and palpitations. Pectus excavatum may be associated with mitral valve prolapse, arrhythmia, and cardiac murmur. In 2% of patients, PE is associated with congenital heart anomalies. Cardiopulmonary problems are directly proportional to the severity of the disease. Generally, PE patients do not have significant impairment of pulmonary function. Scoliotic deformity accompanies 21% of cases. The rate of scoliosis in the families of PE patients has been reported to be 11%.[21] Aortic root dilatation may be observed in isolated PE (Figure 2 and Table 2).
Figure 2. Patient with pectus excavatum deformity.
Table 2. Syndromes with PE and PC.
| Often | Rarely |
| Aarskog syndrome | Down syndrome |
| Coffin-Lowry syndrome | Hallermann-streiff syndrome Holt-Oram syndrome |
| Homocystinuria | Lowe syndrome |
| Spondylometaphyseal dysplasia Kozlowski type | Mietens syndrome |
| Marfan syndrome | Mohr syndrome |
| Marinesco-Sjögren syndrome | Proteus syndrome |
| Melnick-Needles syndrome | Robinow syndrome |
| Morquio syndrome | Ruvalcaba-Myhre syndrome Tricho-rhinophalangeal syndrome Williams syndrome |
| Mucopolysaccharidosis type 7 | 49,XXXXY syndrome |
| Multiple Lentiginous/Neuroma syndrome Noonan syndrome | |
| Osteogenesis imperfect type 1 | |
| Otopalatodigital syndrome type 1 and 2 | |
| Partial trisomy 10q syndrome | |
| Ruvalcaba syndrome | |
| Schwartz-Jampel syndrome | |
| Spondyloepiphyseal dysplasia congenita | |
| PE: Pectus excavatum; PC: Pectus carinatum. | |
Physical examination is usually sufficient for the diagnosis. Detailed evaluation of symptomatic patients is important. Patients with CWD usually have kyphosis and hook should reappearance, called pectus posture. Posteroanterior and lateral chest radiographs are the first step in the evaluation. Pulmonary function tests should be performed in patients with respiratory complaints. Decreased thoracic volume in patients with advanced deformity may cause a decrease in forced vital capacity and tidal volume. Electrocardiogram and echocardiography should be performed in the presence of cardiac problems, such as chest pain and palpitations. Thoracic tomography is advantageous in terms of providing detailed information about the anatomy. However, although it is the gold standard for diagnosis, physical examination is often sufficient.[22] For the typing and classification of PE, Welch in 1980, Oelsnitz in 1981, Hümmer in 1984, and Haller in 1987 published their studies, including the distance between the sternum and vertebrae.[23] There is still no universally accepted classification. However, the Haller index is the most commonly used and most practical index today. The Haller index is calculated by dividing the transverse width of the chest by the distance between the posterior surface of the sternum and the anterior surface of the spine. A ratio between 2.5-2.7 is considered normal. An index greater than 3.25 is associated with severe deformity (Figure 3).[24]
Figure 3. Calculation of the Haller index.
Treatment of PE consists of follow-up, conservative methods, or surgery. Conservative methods include vacuum correction and the use of various corsets. Surgical treatments consist of open and minimally invasive methods. Apart from these, there are also rare applications such as placing silicone or fat filling in the collapse in the chest. The treatment method should be determined by considering the patient's age, whether the patient wants an operation, the degree of the disease, and the accompanying pathologies. Vacuum therapy may be a good treatment option in patients with early age, nonsevere deformities, and patients who do not want surgery. Minimally invasive repair of PE is the most commonly applied main treatment modality with favorable results. Indications for surgical treatment in PE are psychological, cosmetic, and functional reasons. In clinical practice, most of the patients undergo surgery for psychosocial and cosmetic reasons.[25] The aim of surgical correction is to remove the pressure on the lung and heart, allow the thorax to develop, and provide psychological improvement. In general, the age range of 10-16 years is considered the ideal timing. Some authors recommend surgery at a young age to prevent psychological complications.[26] However, recurrence is more common in operations performed before puberty.
The Nuss method, defined by Nuss[10] in 1998, is the most preferred surgical approach for surgical correction of PE due to its ease of application and successful results. The Nuss method is based on correction of PE with the help of a metal bar. The success rate of the Nuss method is low in asymmetric CWD, and open surgery may be preferred in these patients.
Open surgical methods include external sternal traction, Ravitch and modified Ravitch techniques, placement of supportive tissue under the sternum, sternal turn-over, minimal cartilage resection, resorbable plate/autologous cartilage implantation, and titanium mesh plate applications. Ravitch[7] developed his technique, which he named after himself, inspired by previous methods and published the case series in 1949. Subsequently, the technique was improved by other surgeons and named the modified Ravitch method. In operations performed with the modified Ravitch technique, the chest wall is opened with a median incision after endotracheal intubation. The pectoral muscles are dissected, and the deformed cartilaginous costae are removed subperichondrially, except for the second costae. The xiphosternal joint is separated, and the underside of the sternum is freed. The collapsed sternum is identified, and wedge resection is performed here. After appropriate sternum fixation, the procedure is terminated by supporting the back.
The Nuss method was inspired by the increase in the anteroposterior diameter of the thorax in adult chronic obstructive pulmonary disease patients over time. In operations performed with this method, the patient is intubated with a double-lumen endotracheal tube under general anesthesia in the supine position. After general draping and sterility are ensured, the deepest part of the PE is determined. The metal bar is shaped outside according to the characteristics of the deformity. An incision of a few centimeters is made bilaterally on the lateral chest wall and under the pectoral muscles. A small incision is made in the lower intercostal space, and the right hemithorax is observed by videothoracoscopy. During this procedure, one-lung ventilation is started with the help of selective bronchial intubation. The right lung is not ventilated, and the air inside is aspirated by the anesthesiologist. The intraducer is inserted into the thorax through the appropriate intercostal space in the right-sided incision and passed behind the sternum and in front of the pericardium towards the left hemithorax and then out of the thorax through the left-sided incision. The metal bar, which is given the corrected shape of the chest wall, is tied to the end of the intraducer in the opposite way with nylon tape. The intraducer is removed as it is inserted into the thorax. When the intraducer leaves the thorax and the metal bar takes its place, the nylon tape is cut, and the bar is separated from the intraducer. When the inverted metal bar is turned with the help of surgical instruments, the chest wall takes the desired shape. Unilateral (usually left) or bilateral stabilizers are placed to prevent the bar from moving, according to the surgeon's preference to support the upper and lower costae. After the lung is inflated and the air in the hemithorax is evacuated, the procedure is terminated by closing the subcutaneous and skin incisions (Figure 4).
Figure 4. Preoperative and postoperative images of the patient who underwent Nuss operation.
Preoperative epidural catheter placement is useful for analgesia control. In terms of stabilization of the bar, patients should avoid heavy physical exercises for the first one to two months and contact sports until the third month. The average bar removal time is 2.5-3 years. Early removal may be possible in case of clinical necessity, but recurrence is more likely in these patients (Figure 5).
Figure 5. The appearance of the bar and stabilizer on chest radiograph after Nuss operation.

Various complications may occur during and after PE surgery. The most serious complication during surgery is cardiac injury. Bar rotation or slippage is rare.
The most common complications are pneumothorax, wound infection, and bleeding. Pleural effusion, pneumonia, pericarditis, and bar allergy are other postoperative complications. As the age of the patient increases, the likelihood of complications increases. In general, the use of appropriate instruments and the surgeon's experience are the most important factors in preventing these complications.
PECTUS CARINATUM
Popularly known as pigeon breast, PC is the most common CWD after PE. Pectus carinatum is characterized by outward protrusion of the sternum and related costal cartilage. As in PE, it is observed four times more frequently in males.[27] Musculoskeletal anomalies, most commonly scoliosis and mitral valve problems may accompany the deformity. Marfan syndrome, PS, and Noonan syndrome may rarely be associated with PC.[28] Pectus carinatum may not be observed at the time of birth. It occurs at a more advanced age compared to PE. It consists of three subtypes, including chondrogladiolar, chondromanubrial, and mixed type. Chondrogladiolar type is the most common. It may be symmetrical or asymmetrical. In chondromanubrial type, protrusion is observed in the manubrium sterni and upper costal cartilages. It is a rare subtype and is also known as pectus arcuatum (Figure 6 and 7).
Figure 6. A patient with asymmetric pectus carinatum.

Figure 7. A patient with pectus arcuatum.

The diagnosis is usually made by physical examination. Most patients are asymptomatic. Laboratory findings are usually normal in the majority of patients who describe cardiopulmonary symptoms. In most cases, preoperative posteroanterior and lateral chest radiographs are sufficient before treatment.
Treatment of PC is determined according to the presence of scoliosis, whether the deformity is symmetrical or not, the compliance of the chest wall, whether it is associated with PE or not, and the presence of concomitant congenital heart disease or syndromic disease. The appropriate age is considered to be the age range of 10-16 years in PE.
Conservative or surgical treatment modalities are available. In conservative treatment, orthotic treatment has gained popularity in recent years and has become the first choice in many centers.[29] Visible change in corset use starts within two to three months. Patients are recommended to use the brace for 15-20 h daily and intermittently. The daily duration of corset use has an effect on the total duration of treatment. The average duration of corset use is around 2-2.5 years. It is possible to treat 70-80% of the disease with this application.
Open and minimally invasive techniques are used in surgical treatment. The classical method in open surgery is Ravitch, and the technique is similar to that in PE. Surgical treatment is not recommended in children under five years of age unless there are severe symptoms related to the disease since it is thought to disrupt chest wall development.[30] Closed surgery of PC is the Abramson minimally invasive surgical method. This method is similar to the Nuss method in PE. The difference is that bilateral thoracic incisions opened for insertion of a metal bar are combined under the skin above the sternum without entering the thorax. After the bar shaped according to the deformity is passed through this tunnel and the chest wall is shaped, bilateral stabilizers are placed. Since the thoracic cavity is not entered, singlelumen endotracheal intubation is sufficient, and videothoracoscopy is not needed.
Effective analgesia is important for patient comfort and early discharge. Patients should avoid excessive physical exercise for up to three months. The bar removal period is similar to PE and averages three years. Early removal of the bar increases the possibility of recurrence.
Since the procedure is performed outside the thoracic cavity, intraoperative complications are rare. Postoperative complications are similar to those in PE. In closed technique surgery, rupture or loosening of the steel wires used for stabilization may occur. Iatrogenic PE is a possible complication depending on the elasticity of the chest wall and the shape of the bar. Complications in patients with modified Ravitch are similar to those in PE.
POLAND SYNDROME
Alfred Poland found a partial absence of the left pectoralis major and minor muscles and serratus anterior muscle in the dissection of the cadaver of a 27-year-old former prisoner and published the disease in 1841.[31] Poland syndrome is a rare congenital anomaly in which partial or complete absence of the pectoral muscles may be accompanied by many thoracic anomalies. Poland syndrome is right-sided in 60-75% of cases. There are two hypotheses in its etiology. The first one is developmental failure in the first month of pregnancy, and the other and more accepted one is blood flow disturbance in the subclavian artery in the early embryonic period.[32] Absence of the pectoralis major is the major component of the disease. Minor components include muscle agenesis or hypoplasia, costal anomalies, skin anomalies, breast and nipple anomalies, and hand and finger anomalies (Figure 8 and 9).
Figure 8. A 15-year-old patient with pectus excavatum with loss of the left axillary fold due to absence of the pectoralis major muscle.

Figure 9. A patient with poland syndrome and scapula anomaly.

Physical examination and chest radiographs are usually sufficient for diagnosis. It may resemble asymmetric PC cases. Different diagnostic methods may need to be used to reveal other anomalies that may accompany PS. Thorax computed tomography is a frequently used method both for diagnosis and to reveal accompanying anomalies.
The indication for treatment is usually cosmetic. In most patients, achieving body symmetry is satisfactory, and therefore, treatment after puberty is more appropriate. Silicone implant placements in the pectoral muscle area or fat injection are commonly used methods. In cases with CWD and lung herniation, surgical treatment is performed, regardless of age. The defect can be surgically repaired with muscle flaps, costal grafts, plates, or meshes. The most common associated finger anomaly is syndactyly and should be treated surgically.
STERNAL DEFECTS
Sternal cleft is type 11 according to the Willital classification of chest deformities and is a congenital CWD observed in 1/50,000-100,000 births. It is more common in females. It is caused by the interruption of sternal development in the embryologic period. Sternal defects are classified into three groups: sternal cleft, ectopia cordis, and Cantrellʼs pentalogy. The sternal cleft may be total or partial, is most commonly located superiorly, and the heart position is usually normal. Skin integrity is intact. Ectopia cordis is an abnormal position of the heart and may be associated with a sternal defect. It can be cervical, thoracic, or thoracoabdominal. In the cervical form, the heart is displaced superiorly. Concomitant cervicofacial anomalies may also be encountered, and these patients usually have a mortal course. In the thoracic type, the heart is displaced outside the chest wall. The thoracoabdominal type is known as Cantrell's pentalogy. It consists of an omphalocele, anterior diaphragmatic hernia, ectopia cordis, cardiac anomalies, and sternal cleft.[33] Early surgical treatment is necessary in cases with disrupted skin integrity and herniation of the heart with Valsalva (Figure 10).
Figure 10. Cantrell’s pentalogy.

LESS COMMON DEFORMITIES OF THE CHEST WALL JEUNE SYNDROME
It is a syndrome characterized by a narrow and rigid chest and multiple cartilaginous rib anomalies.[34] It may be congenital or acquired. Congenital Jeune syndrome is an autosomal recessive skeletal deformity. It is observed rarely. Pelvic and iliac bone pathologies may accompany them. Retinal, renal, hepatic, and pancreatic anomalies may be observed in some cases. The main goal of surgical treatment is to expand the thoracic volume and reduce hypoventilation. The acquired form is caused by impaired cartilage and costal growth after PE surgery in preadolescence (Figure 11 and 12).
Figure 11. A patient with Jeune syndrome.

Figure 12. Chest radiograph of a patient with Jeune syndrome.

JARCHO-LEVIN SYNDROME
Spondylothoracic dysplasia is an autosomal recessive deformity with multiple vertebrae and rib anomalies described by Jarcho and Levin[35] in 1938. There are multiple hemivertebrae in all or most of the thoracic and lumbar vertebrae. Vertebral ossification centers rarely cross the midline. Due to costal deformities, the chest wall gives a crab-like appearance on chest radiography. Respiratory failure develops due to a small and nonfunctional thorax. Surgical treatment is performed before the age of two years and mostly to increase the thoracic volume (Figure 13).
Figure 13. Chest radiograph of a patient with Jarcho-Levin syndrome.

ISOLATED COSTAL DEFORMITIES
Isolated costal deformities are characterized by fusion of one or more costae. They can be unilateral or bilateral and are usually detected incidentally. They do not require surgical treatment since they do not show functional impairment.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Concept, materials, critical rewiev, control: C.T.; Design, data collection, literature rewiev, writing, references and funding: Z.O.B.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Rea G, Sezen CB. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. Chest Wall Deformities. [Google Scholar]
- 2.Shamberger RC, Welch KJ. Surgical repair of pectus excavatum. J Pediatr Surg. 1988;23:615–622. doi: 10.1016/s0022-3468(88)80629-8. [DOI] [PubMed] [Google Scholar]
- 3.Yavuzer Ş. Chest surgery RedBook. 2. Istanbul: Nobel Medical Book; 2015. Chest wall deformities and surgical treatment; pp. 625–631. [Google Scholar]
- 4.Schenck von Grafenberg J. Observationummedicarum, rararum, novarum, admirabilium, et montrosarum, libersecundus. De partibusvitalibus, thoracecontentis. Observation. 1594;264:516–516. [Google Scholar]
- 5.Bauhinus J. Sterni cum costis ad interna reflexio native, spirandi difficultatis causes. Frankfurt: Johannes Schenckvon Grafenberg; 1594. [Google Scholar]
- 6.Meyer L. Zur chirurgischen Behandlung der angeborenen trichterbrust. Berl Klin Wschr. 1911;48:15–15. [Google Scholar]
- 7.Ravitch MM. The operative treatment of pectus excavatum. Ann Surg. 1949;129:429–444. doi: 10.1097/00000658-194904000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judet J, Judet R. Sternum en entonnoir par resection et retournement. Mem Acad Chir (Paris) 1956;82:250–250. [PubMed] [Google Scholar]
- 9.Wada J, Ikeda K, Ishida T, Hasegawa T. Results of 271 funnel chest operations. Ann Thorac Surg. 1970;10:526–532. doi: 10.1016/s0003-4975(10)65390-8. [DOI] [PubMed] [Google Scholar]
- 10.Nuss D, Kelly RE Jr, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545–552. doi: 10.1016/s0022-3468(98)90314-1. [DOI] [PubMed] [Google Scholar]
- 11.Fonkalsrud EW, Salman T, Guo W, Gregg JP. Repair of pectus deformities with sternal support. J Thorac Cardiovasc Surg. 1994;107:37–42. [PubMed] [Google Scholar]
- 12.Quigley PM, Haller JA Jr, Jelus KL, Loughlin GM, Marcus CL. Cardiorespiratory function before and after corrective surgery in pectus excavatum. J Pediatr. 1996;128:638–643. doi: 10.1016/s0022-3476(96)80128-4. [DOI] [PubMed] [Google Scholar]
- 13.Kelly RE Jr. Pectus excavatum: Historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg. 2008;17:181–193. doi: 10.1053/j.sempedsurg.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Willital GH, Saxena AK, Schütze U, Richter W. Chestdeformities: A proposal for a classification. World J Pediatr. 2011;7:118–123. doi: 10.1007/s12519-011-0263-y. [DOI] [PubMed] [Google Scholar]
- 15.Shamberger RC. General thoracic surgery. 1. Philadelphia: Lippincott Williams and Wilkins; 2005. Chest wall deformities; pp. 653–681. [Google Scholar]
- 16.Nuss D. Minimally invasive surgical repair of pectus excavatum. Semin Pediatr Surg. 2008;17:209–217. doi: 10.1053/j.sempedsurg.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Wang F, Ji G, Teng J, Liang X, Liang X, et al. Modified Nuss procedure for the treatment of pectus excavatum: Experience of 259 patients. Asian J Surg. 2023;46:692–697. doi: 10.1016/j.asjsur.2022.06.080. [DOI] [PubMed] [Google Scholar]
- 18.Abramson H, D’Agostino J, Wuscovi S. A 5-year experience with a minimally invasive technique for pectus carinatum repair. J Pediatr Surg. 2009;44:118–123. doi: 10.1016/j.jpedsurg.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Langer E. Zuckerkande l. Untersuchungen über den mißbildeten Brustkorb des. Herrn JW Wiener med Zeit. 1880;49:515–515. [Google Scholar]
- 20.Cartoski MJ, Nuss D, Goretsky MJ, Proud VK, Croitoru DP, Gustin T, et al. Classification of the dysmorphology of pectus excavatum. J Pediatr Surg. 2006;41:1573–1581. doi: 10.1016/j.jpedsurg.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Waters P, Welch K, Micheli LJ, Shamberger R, Hall JE. Scoliosis in children with pectus excavatum and pectus carinatum. J Pediatr Orthop. 1989;9:551–556. doi: 10.1097/01241398-198909010-00009. [DOI] [PubMed] [Google Scholar]
- 22.Folkalsrud EW. Management of pectus chest deformities in female patients. Am J Surg. 2004;187:192–197. doi: 10.1016/j.amjsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira Carvalho PE, da Silva MV, Rodrigues OR, Cataneo AJ. Surgical interventions for treating pectus excavatum. CD008889Cochrane Database Syst Rev. 2014;2014 doi: 10.1002/14651858.CD008889.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JH, Park IK, Kim YT, Kim WS, Kang CH. Classification of pectus excavatum according to objective parameters from chest computed tomography. Ann Thorac Surg. 2016;102:1886–1891. doi: 10.1016/j.athoracsur.2016.05.079. [DOI] [PubMed] [Google Scholar]
- 25.Özalper MH, Yüksel M. Göğüs duvarı deformiteleri. Turkiye Klinikleri J Thor Surg-Special Topics. 2011;4:130–133. [Google Scholar]
- 26.Bilgin M, Fazlıoğlu M, Oral A. Pectus excavatum: Nuss experience in 110 cases. Turk Gogus Kalp Dama. 2014;22:790–794. [Google Scholar]
- 27.Robert C. In: General thoracic surgery. 7th ed. Shields TW, Locicero III, Reed CE, Feins RH, editors. Philadelphia: Lippincott Williams & Wilkins; 2009. Shamberger. Chest wall deformities; pp. 599–628. [Google Scholar]
- 28.Bentz ML, Rowe MI, Wiener ES. Improved sternal fixation in the correction of pediatric pectus excavatum. Ann Plast Surg. 1994;32:638–641. doi: 10.1097/00000637-199406000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Azizkhan RG, Cohen AP. In: Chest wall deformities. Saxena A, editor. Berlin, Heidelberg: Springer; 2017. Nonoperative management of chest wall deformities; pp. 517–522. [Google Scholar]
- 30.Goretsky MJ, Kelly RE Jr, Croitoru D, Nuss D. Chest wall anomalies: Pectus excavatum and pectus carinatum. Adolesc Med Clin. 2004;15:455–471. doi: 10.1016/j.admecli.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Yiyit N. Poland sendromu. Turk Gogus Kalp Dama. 2015;23:413–421. [Google Scholar]
- 32.Yiyit N, Işıtmangil T, Saygın H. Eight patients with multiple bilateral thoracic anomalies: A new syndrome or bilateral Poland's syndrome. Ann Thorac Surg. 2014;97:1758–1763. doi: 10.1016/j.athoracsur.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Ramdial S, Pillay D, Madaree A. Primary closure of a sternal cleft in a neonate. World J Plast Surg. 2016;5:308–312. [PMC free article] [PubMed] [Google Scholar]
- 34.Jeune M, Beraud C, Carron R. Asphyxiating thoracic dystrophy with familial characteristics. Arch Fr Pediatr. 1955;12:886–891. [PubMed] [Google Scholar]
- 35.Jarcho S, Levin P. Hereditary malformation of the vertebral bodies. Bull Johns Hopkins Hosp. 1938;62:216–226. [Google Scholar]