Abstract
Pneumothorax is a condition that describes the presence of air between the visceral and parietal pleura sheets and the consequent collapse of the lungs. The collapse of the lungs can be partial or total and can present in different clinical stages, such as a high-pressure pneumothorax that can cause a mediastinal shift. Pneumomediastinum is the presence of free air between the mediastinal tissues due to various causes. It can manifest spontaneously and be minimally symptomatic but can also develop due to severe complications. Its etiology includes numerous iatrogenic and traumatic factors. Although spontaneous pneumothorax and pneumomediastinum that develop in childhood are similar to adult patients, it is important to determine the appropriate treatment strategy in addition to the age group, the effectiveness of the treatment, the role of the applied treatment in reducing recurrence, and the etiologyoriented treatments if there is an underlying pathology.
Keywords: Children patients, pneumomediastinum, pneumothorax.
Introduction
Pneumothorax is a condition that describes the presence of air between the visceral and parietal pleura sheets and the consequent collapse of the lungs (Figure 1). This term was first used in 1803 by Jean Marc Gaspard Itard. The term primary spontaneous pneumothorax (PSP) was first used in 1932 by Kjaergard.[1,2]
Figure 1. Primary spontaneous pneumothorax.
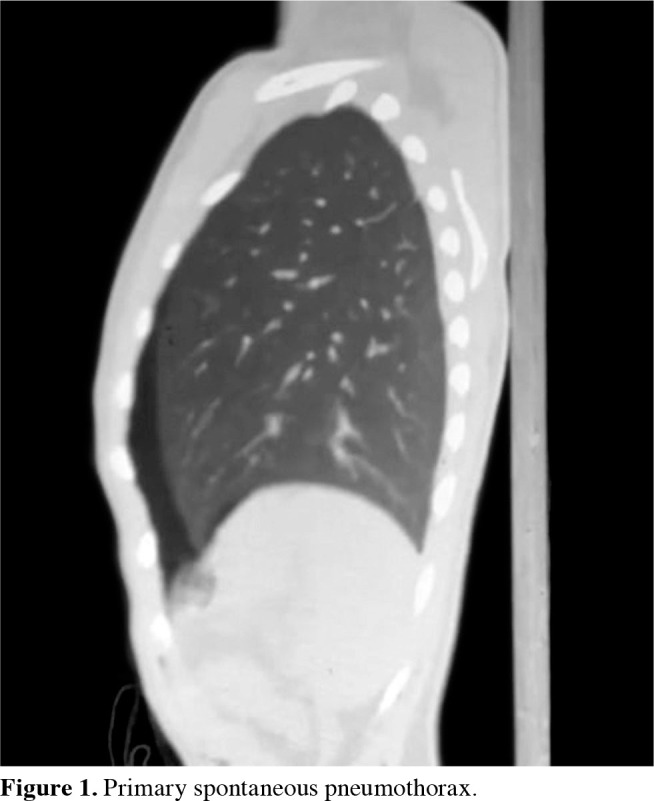
The collapse of the lungs can be partial or total and can present in different clinical stages, such as a tension pneumothorax causing a mediastinal shift. While there are different classifications, pneumothorax can be divided into two main groups based on etiology: spontaneous and acquired. The purpose of the classification is to manage the diagnosis and treatment processes by evaluating them along with the etiology.
Pneumomediastinum (PM) is defined as the presence of free air between the mediastinal tissues due to various causes. It can spontaneously manifest and be minimally symptomatic but can also occur due to severe complications. Its origin includes numerous iatrogenic and traumatic factors (Figure 2).[3] When not occurring secondary to any pathology, it is called primary spontaneous PM. Pneumomediastinum is more frequently observed in the pediatric age group at a rate of about 3%.[4] Patients with asthma are more prone to have spontaneous PM. Although the underlying mechanism is not well preserved, tall and thin athletes are more prone to experience PM. Pneumomediastinum is usually spotted in young patients exposed to sudden pressure changes, particularly during intense physical activity between the ages of 20-40. It also occurs more easily during activities like weight lifting and diving. Clinically, PM typically arises as a result of different precipitating events that initiate the Valsalva maneuver. The incidence of PM rises in age groups with particular diseases. The rate of PM occurrence in emergency department visits is between 1/8,000 and 1/15,000, being more common in males. Another study reported a frequency between 1/7,000 and 1/32,000. Following blunt trauma, the incidence of PM is approximately 10%. Pneumomediastinum can easily occur in mechanical ventilation due to barotrauma. In trauma patients, those undergoing intubation, and those having interventional procedures, the rate of PM is higher. Spontaneous PM, like secondary PM, is more common in males.[4-7]
Figure 2. Traumatic pneumothorax and pneumomediastinum.

Primary spontaneous pneumothorax is a condition where pneumothorax develops without an evident underlying cause. It has a bimodal distribution by age with two peaks, one in the neonatal stage and the other during late adolescence.[8] The incidence of PSP ranges between 4.7 and 28/100,000 per year in males and 1.2 and 6/100,000 per year in females, with a higher prevalence in young, tall, and thin males. The incidence of PSP in pediatric cases is low, and it has been reported to be around 3.4/100,000, with a maleto-female ratio of four-to-one and a peak of incidence during adolescence. However, a higher recurrence rate has been reported in the pediatric cases compared to adults (50-60% vs. 30-50%). Air-containing lesions (blebs or bullae) are thought to play an important role in the etiopathogenesis of PSP.[9-12]
As etiological factors, formations called blebs or bullae, which are pleural air sacs, are held responsible. A bleb is a collection of air surrounded by visceral pleura that forms following the rupture of alveoli. Bullae are intrapulmonary/parenchymal air cysts formed by alveolar destruction, and they have thick fibrous walls. In computed tomography scans of patients with pneumothorax, bullae and blebs are often detected primarily in the upper lobe apical region, less so in the lower lobe's superior segment and other parenchymal areas. Being tall and slim predisposes the formation of apical subpleural bullae and blebs. Secondary spontaneous pneumothorax occurs due to pulmonary pathologies, such as chronic obstructive pulmonary disease or bullous emphysema, in elderly patients. Aside from pulmonary pathologies, many diseases, including connective tissue diseases, metastases, and thoracic endometriosis, are responsible for causing pneumothorax in children.[13,14] Neonatal pneumothorax occurs in approximately 1-2% of newborns within 0-10 days.[5] It is mainly spotted in males. Most cases are attributed to the inhalation of meconium during difficult childbirth (Figure 3). Respiratory distress syndrome is also among the causes. In most cases, pneumothorax resolves spontaneously.[14]
Figure 3. Newborn pneumothorax.

Another type of pneumothorax is acquired pneumothorax. Thoracic traumas are divided into penetrating and blunt traumas. Posttraumatic pneumothorax can develop due to damage to the chest wall, lungs, tracheobronchial tree, and esophagus. The aim of pneumothorax treatment is to achieve complete reexpansion of the lung, remove air from the pleural space, eliminate symptoms, and prevent complications and recurrences.[15,16] Options include observation with oxygen therapy, simple needle aspiration, percutaneous drainage, tube thoracostomy, and surgery (thoracoscopy or thoracotomy).[17]
Observation is a treatment option but does not prevent recurrence. The preferred patient group is clinically stable individuals with small pneumothorax. During observation, patients should receive oxygen support to contribute to the reabsorption of air in the pleural space. Patients with a pneumothorax area less than 10-15% and without dyspnea can be treated with oxygen. Oxygen administration through a face mask can accelerate the rate of air absorption by three to four times. Oxygen also corrects existing hypoxia in patients. These patients should be hospitalized for two to four days until the pneumothorax area is completely absorbed.[17] Advantages of needle aspiration include being less invasive and having lower costs. Disadvantages are that kinking of the catheter is commonly encountered during the procedure.[18]
According to related literature, it has been presented that recurrence develops in approximately half of the patients after needle aspiration in children and that the need for tube thoracostomy occurs. Therefore, tube thoracostomy has been recommended for patients planned to undergo needle aspiration.[16,19] Bruschettini et al.[20] showed no differences in mortality between needle aspiration and intercostal tube drainage in the management of neonatal pneumothorax. Needle aspiration reduces the need for intercostal tube drainage placement.[20]
Tube thoracostomy is the chosen method for treating moderate and large pneumothoraxes. As catheters, a 14-F (French) thin catheter has been reported as sufficient in children. However, depending on the underlying condition, 20- to 28-F catheters are generally used in pleural drainage. The catheter can be attached to a tube thoracostomy and has a success rate of 90% for the first pneumothorax, 52% for the first recurrence, and 15% for the second recurrence.[21] It has been stated that there is no significant difference in terms of hospitalization between patients who received aspiration, tube thoracostomy, and surgical treatment in the management of first-episode pneumothorax in childhood.[16,22]
SURGICAL TREATMENT
Most European pediatric surgeons prefer chest tube insertion in the management of the first episode of PSP and perform surgical treatment in the second episode in case of underlying bullae >2 cm and recurrent pneumothorax.[23] The aim of surgical treatment is to prevent recurrences by resecting bullae and blebs and performing pleurodesis. During surgery, even in patients with no bullae and bleb formation, apical wedge resection and pleurectomy are recommended. In PSP cases, surgery to prevent potential recurrences should be considered after the second pneumothorax attack. It is debatable to apply surgical treatment immediately at the first attack of spontaneous pneumothorax. Moreover, younger patients and children are the most susceptible to recurrence in PSP, thus surgery can be considered in the first instance of pneumothorax.[17,18,21] Surgical treatment indications for pneumothorax are as follows: prolonged air leakage (five to seven days or longer), recurrent pneumothorax, bilateral pneumothorax, first pneumothorax with history in the other lung, first attack in patients exposed to pressure changes professionally in adult patients (pilots, flight attendants, and divers).
During surgical treatment, video-assisted thoracoscopic surgery (VATS) is the golden standard and should be the preferred operative method. Less invasive and more cosmetic axillar thoracotomy can also be considered. In patients detected with bilateral bullae, median sternotomy can be utilized to operate on both lungs; however, this is not the preferred method. Alternatively, one side can be operated on, and the other side can be electively managed later via VATS.[24]
There are proposals to use a single-port instead of the standard three-port VATS for the treatment of spontaneous pneumothorax to reduce postoperative pain and paresthesia.[25] With VATS, the entire lung parenchyma is generally examined. If the presence of a bleb or bulla is observed, wedge resection is performed with an endolinear stapler. Adding apical pleurectomy to the procedure is controversial. In our clinic, we perform apical parietal pleurectomy on patients who receive surgical treatment with VATS. In our study, the recurrence rate in adult patients was 4.4%.[26]
Thoracotomy should not be applied unless necessary for pneumothorax treatment. Axillary thoracotomy, muscle-sparing thoracotomy, and classic posterolateral thoracotomy are used in appropriate patient groups. It may be necessary to proceed with an emergency thoracotomy in a portion of cases treated with VATS. These include adhesions, bleeding, failure of lung collapse, and giant bullae.[27]
Compared to thoracotomy, the surgical trauma is less in VATS, postoperative pain is minimal, and lung functions are preserved. Hospital stay is shorter, and small skin incisions provide a cosmetic advantage. However, its superiority over axillary thoracotomy has not been demonstrated. In fact, the recurrence rate has been found to be twice that of axillary thoracotomy.[28]
Pleurodesis can be performed via pleurectomy, pleural abrasion, or using chemical agents. The purpose of pleurodesis is to adhere parietal and visceral pleural layers to prevent recurrences. In recent years, with the widespread use of VATS, this procedure has been increasingly performed with thoracoscopy.[29] Since the number of procedures performed in the pediatric group who receive direct surgical treatment decreases, a decrease in hospitalization can also be predicted. In their meta-analysis, Miscia et al.[16] spotted no difference between children who underwent surgery and those managed with other procedures in terms of risk. Therefore, they stated that in case of recurrence or persistent air leak (three to five days), an early surgery following conservative treatment may be recommended in children. Lieu et al.[30] in their review published in 2022, suggested that surgery may be required in children under 12 years old with pneumothorax in which complete expansion cannot be achieved within four days. Miscia et al.,[16] in their study comparing adult and pediatric patients with pneumothorax, reported that observational treatment could be a safe and an effective option in clinically stable children. Tube thoracostomy should be performed in pneumothoraxes that persist despite observation, and surgical treatment may be a treatment alternative in recurrent pneumothoraxes.[31]
The clinical presentation of children with PM is variable, with the most common being pain, painful swallowing, voice changes, subcutaneous emphysema crackling, mediastinal pressure resulting in shortness of breath, cyanosis, and heart failure. The diagnosis can be readily made with history and physical examination findings confirmed with radiological tests. The most common symptoms include pain and dyspnea. Additionally, there may be cyanosis, neck vein distension, neck pain, dysphagia, back pain, feeling of something stuck in the throat, dysphonia, fever, and hypotension. Since clinical findings can easily be confused, differential diagnoses should be considered against urgent clinical conditions such as cardiac tamponade, angina pectoris, aortic aneurysm, mediastinitis, and pulmonary embolism. Morbidity and mortality are dependent on associated diseases. Mortality is high with esophageal rupture accompanying PM following forceful vomiting. Predisposing factors that increase mortality include blunt or penetrating injuries, high-velocity injuries, asthma attacks, and tracheobronchial tears. Air embolism is a less frequent complication. Chest radiography can show associated diseases as well as PM. Radiolucent lines and strips that envelope normal anatomical structures, the heart, retrosternal area, and trachea are easily visible.[30,31]
Observation with oxygen is usually sufficient for treatment of children with SPM. In cases accompanied by pneumothorax, tube thoracostomy and, if necessary, cervical mediastinotomy or mediastinoscopy are performed.[14,17] Fitzwater et al.,[33] according to the study conducted with the data of 96 pediatric patients with a median age 14.1 (IQR: 8.7-16.4) years, reported that if the patients who applied to the emergency department with SPM were clinically stable within 4 h of observation, it would be convenient to plan discharge under adult supervision. In the same study, it was reported that in case of uncontrolled asthma attacks and similar conditions and if the symptoms progress, hospitalization should be extended for further examination and treatment.
In conclusion, although spontaneous pneumothorax and PM that develop in childhood are similar to adult patients, it is important to determine the appropriate treatment strategy according to the age group, the effectiveness of the treatment, the role of the applied treatment in reducing recurrence, and the etiology-oriented treatments if there is an underlying pathology. Tube thoracostomy and VATS are the standard treatment methods in adult patients. On the other hand, there are various treatment options in children. However, studies suggest, as with adult patients, that surgical management is the most effective method in reducing the risk of recurrence. Large series and prospective randomized controlled studies with a large number of patients are needed to create a specific algorithm on this subject.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept: L.C., C.A.; Design: C.A., S.O.M.; Control/supervision, critical review: L.C.; Literature review: C.A.; Writing article: C.A., S.O.M.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Henry M, Arnold T, Harvey J, Pleural Diseases Group. Standards of Care Committee. British Thoracic Society BTS guidelines for the management of spontaneous pneumothorax. ii39-52Thorax. 2003;58 Suppl 2 doi: 10.1136/thorax.58.suppl_2.ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isitmangil T, Balkanli K. In: Gögüs Cerrahisi. Yüksel M, Kalayci G, editors. İstanbul: Bilmedya Grup; 2001. Pnömotoraks ve cerrahisi; pp. 411–446. [Google Scholar]
- 3.Aker C, Sezen CB, Sezen Aİ, Doğru MV, Özbek M, Metin M, et al. Did primary spontaneous pneumomediastinum risk factor alter in the period of COVID-19 pandemia. Interact Cardiovasc Thorac Surg. 2022;34:1031–1037. doi: 10.1093/icvts/ivab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stack AM, Caputo GL. Pneumomediastinum in childhood asthma. Pediatr Emerg Care. 1996;12:98–101. doi: 10.1097/00006565-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Şahin AE, Eren TŞ. In: Mediyasten hastalıkları ve cerrahisi. Balcı AE, editor. İstanbul: TÜSAD; 2015. Primer spontan pnömomediyasten; pp. 145–150. [Google Scholar]
- 6.Campillo-Soto A, Coll-Salinas A, Soria-Aledo V, BlancoBarrio A, Flores-Pastor B, Candel-Arenas M, et al. Spontaneous pneumomediastinum: Descriptive study of our experience with 36 cases. Arch Bronconeumol. 2005;41:528–531. doi: 10.1016/s1579-2129(06)60274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damore DT, Dayan PS. Medical causes of pneumomediastinum in children. Clin Pediatr (Phila) 2001;40:87–91. doi: 10.1177/000992280104000204. [DOI] [PubMed] [Google Scholar]
- 8.O'Lone E, Elphick HE, Robinson PJ. Spontaneous pneumothorax in children: When is invasive treatment indicated. Pediatr Pulmonol. 2008;43:41–46. doi: 10.1002/ppul.20734. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox DT, Glick PL, Karamanoukian HL, Allen JE, Azizkhan RG. Spontaneous pneumothorax: A singleinstitution, 12-year experience in patients under 16 years of age. J Pediatr Surg. 1995;30:1452–1454. doi: 10.1016/0022-3468(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 10.Beg MH, Reyazuddin, Faridi MM, Ahmad SH, Shahab T. Spontaneous pneumothorax in children--a review of 95 cases. Ann Trop Paediatr. 1988;8:18–21. doi: 10.1080/02724936.1988.11748531. [DOI] [PubMed] [Google Scholar]
- 11.Young Choi S, Beom Park C, Wha Song S, Hwan Kim Y, Cheol Jeong S, Soo Kim K, et al. What factors predict recurrence after an initial episode of primary spontaneous pneumothorax in children. Ann Thorac Cardiovasc Surg. 2014;20:961–967. doi: 10.5761/atcs.oa.13-00142. [DOI] [PubMed] [Google Scholar]
- 12.MacDuff A, Arnold A, Harvey J, BTS Pleural Disease Guideline Group Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. ii18-31Thorax. 2010;65 Suppl 2 doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 13.Tokat AO, Karasu S, Özkan M, Kısacık E, Çakmak H. Sekonder spontan pnömotoraks: Etyoloji ve tedavi yöntemleri. Ankara Üniversitesi Tıp Fakültesi Mecmuası. 2010;63:111–113. doi: 10.1501/Tipfak_0000000773. [DOI] [Google Scholar]
- 14.AG A. Plevra hastalıkları ve tedavisi. Ankara: Derman Tıbbi Yayıncılık; 2011. Pnömotoraks; pp. 54–62. [Google Scholar]
- 15.Yousuf S, Cardenas S, Rezaee F. Pediatric pneumothorax: Case studies and review of current literature. Respir Med Case Rep. 2021;34:101548–101548. doi: 10.1016/j.rmcr.2021.101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miscia ME, Lauriti G, Lisi G, Riccio A, Lelli Chiesa P. Management of spontaneous pneumothorax in children: A systematic review and meta-analysis. Eur J Pediatr Surg. 2020;30:2–12. doi: 10.1055/s-0039-3402522. [DOI] [PubMed] [Google Scholar]
- 17.Yıldızhan A. In: Plevra hastalıkları ve cerrahi tedavisi. Yücel O, editor. İstanbul: TÜSAD; 2014. Pnömotoraks ve cerrahi tedavisi; pp. 162–177. [Google Scholar]
- 18.Baysungur V. In: Göğüs cerrahisi. Ökten İ, Kavukçu HŞ, editors. İstanbul: Promat Basım Yayın; 2013. Pnömotoraks; pp. 1493–1518. [Google Scholar]
- 19.Soccorso G, Anbarasan R, Singh M, Lindley RM, Marven SS, Parikh DH. Management of large primary spontaneous pneumothorax in children: Radiological guidance, surgical intervention and proposed guideline. Pediatr Surg Int. 2015;31:1139–1144. doi: 10.1007/s00383-015-3787-8. [DOI] [PubMed] [Google Scholar]
- 20.Bruschettini M, Romantsik O, Zappettini S, O'Donnell CP, Calevo MG. Needle aspiration versus intercostal tube drainage for pneumothorax in the newborn. CD011724Cochrane Database Syst Rev. 2019;2 doi: 10.1002/14651858.CD011724.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med. 2000;342:868–874. doi: 10.1056/NEJM200003233421207. [DOI] [PubMed] [Google Scholar]
- 22.Williams K, Lautz TB, Leon AH, Oyetunji TA. Optimal timing of video-assisted thoracoscopic surgery for primary spontaneous pneumothorax in children. J Pediatr Surg. 2018;53:1858–1861. doi: 10.1016/j.jpedsurg.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 23.Soyer T, Dariel A, Dingemann J, Martinez L, Pini-Prato A, Morini F, et al. European Pediatric Surgeons' Association survey on the management of primary spontaneous pneumothorax in children. Eur J Pediatr Surg. 2022;32:415–421. doi: 10.1055/s-0041-1739420. [DOI] [PubMed] [Google Scholar]
- 24.Karamustafaoğlu YA, Yanık F, Yörük Y. Wedge resection and pleurodesis through single-incision videothoracoscopic transmediastinal approach for bilateral spontaneous pneumothorax. Turk Gogus Kalp Damar Cerrahisi Derg. 2023;31:295–299. doi: 10.5606/tgkdc.dergisi.2023.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akçay O, Acar T, Cantay S, Anar S. Minimally invasive approach to pneumothorax: Single port or two ports. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:347–351. doi: 10.5606/tgkdc.dergisi.2020.18778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocatürk Cİ, Cansever L, Günlüoğlu MZ, Turna A, Özdemir S, Çınar U, et al. Primer spontan pnömotoraksın cerrahi tedavisinde kama rezeksiyon ve parsiyel plevrektominin sonuçları: Videotorakoskopi mi aksiller torakotomi mi. Turk Gogus Kalp Damar Cerrahisi Derg. 2011;19:213–217. doi: 10.5606/tgkdc.dergisi.2011.015. [DOI] [Google Scholar]
- 27.Mouroux J, Elkaïm D, Padovani B, Myx A, Perrin C, Rotomondo C, et al. Video-assisted thoracoscopic treatment of spontaneous pneumothorax: Technique and results of one hundred cases. J Thorac Cardiovasc Surg. 1996;112:385–391. doi: 10.1016/S0022-5223(96)70266-0. [DOI] [PubMed] [Google Scholar]
- 28.Doğusoy I, Yıldırım M, Ustaalioğlu R, Demirbağ H. A comparison of axillary thoracotomy versus videoassisted thoracoscopic surgery in the surgical treatment of primary spontaneous pneumothorax. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26:132–137. doi: 10.5606/tgkdc.dergisi.2018.15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ünal M, Samancılar Ö, Gülmez B, Yazgan S, Yağcı T, Üçvet A, et al. Axillary thoracotomy and VATS for the treatment of primary spontaneous pneumothorax. Curr Thorac Surg. 2017;2:85–90. doi: 10.26663/cts.2017.0025. [DOI] [Google Scholar]
- 30.Lieu N, Ngo P, Chennapragada SM, Fitzgerald DA, Karpelowsky J, Pandit C, Selvadurai H, Robinson PD. Update in management of paediatric primary spontaneous pneumothorax. Paediatr Respir Rev. 2022;41:73–79. doi: 10.1016/j.prrv.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Zylak CM, Standen JR, Barnes GR, Zylak CJ. Pneumomediastinum revisited. Radiographics. 2000;20:1043–1057. doi: 10.1148/radiographics.20.4.g00jl131043. [DOI] [PubMed] [Google Scholar]
- 32.Ho AS, Ahmed A, Huang JS, Menias CO, Bhalla S. Multidetector computed tomography of spontaneous versus secondary pneumomediastinum in 89 patients: Can multidetector computed tomography be used to reliably distinguish between the 2 entities. J Thorac Imaging. 2012;27:85–92. doi: 10.1097/RTI.0b013e3182103876. [DOI] [PubMed] [Google Scholar]
- 33.Fitzwater JW, Silva NN, Knight CG, Malvezzi L, RamosIrizarry C, Burnweit CA. Management of spontaneous pneumomediastinum in children. J Pediatr Surg. 2015;50:983–986. doi: 10.1016/j.jpedsurg.2015.03.024. [DOI] [PubMed] [Google Scholar]


