Abstract
Obstructive sleep apnea (OSA) is a multifactorial sleep disorder with a high prevalence in the general population. OSA is associated with an increased risk of developing cardiovascular diseases (CVDs), particularly hypertension, and is linked to worse outcomes. Although the correlation between OSA and CVDs is firmly established, the mechanisms are poorly understood. Continuous positive airway pressure is primary treatment for OSA reducing cardiovascular risk effectively, while is limited by inadequate compliance. Moreover, alternative treatments for cardiovascular complications in OSA are currently not available. Recently, there has been considerable attention on the significant correlation between gut microbiome and pathophysiological changes in OSA. Furthermore, gut microbiome has a significant impact on the cardiovascular complications that arise from OSA. Nevertheless, a detailed understanding of this association is lacking. This review examines recent advancements to clarify the link between the gut microbiome, OSA, and OSA‐related CVDs, with a specific focus on hypertension, and also explores potential health advantages of adjuvant therapy that targets the gut microbiome in OSA.
Keywords: cardiovascular diseases, gut microbiome, intermittent hypoxia, obstructive sleep apnea, prebiotics, probiotics
OSA can lead to dysbiosis and impaired intestinal barrier, resulting in a “leaky gut.”. This allows LPS from intestinal microorganisms to enter the bloodstream, causing endotoxemia. Circulating LPS activates the TLR4/NF‐κB pathway of vascular endothelial cells. This promotes the expression of pro‐inflammatory factors such as IL‐6 and TNF‐α, leading to vascular inflammation. It also promotes ROS generation and decreases eNOS expression, leading to reduced NO bioavailability and endothelial dysfunction. Vascular inflammation and endothelial dysfunction collaborate to promote CVDs, particularly HTN.

1. INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic prevalent condition with a high‐cost burden. An estimated 936 million people between the ages of 30 and 69 have OSA. 1 OSA is characterized by recurrent episodes of partial or complete upper airway obstruction that frequently caused intermittent hypoxia (IH) and sleep fragmentation (SF). 2 These pathophysiological processes can result in a series of complications, including cardiovascular diseases (CVDs), metabolic syndrome, and cognitive function. 3 , 4 Among these sequelae, CVDs, namely hypertension (HTN), are the most common complication of OSA. Several mechanisms have been postulated for explaining the pro‐hypertensive effects of OSA, including endothelial dysfunction, sympathetic nervous system activation, systemic inflammation, and oxidative stress reaction. However, the underlying mechanisms of how OSA leads to HTN remain largely unknown.
Recently, there has been increasing interest in the gut microbiome. Substantial evidence indicates a robust correlation between the gut microbiome and both OSA and OSA‐related CVDs. While intriguing evidence suggests that the gut microbiome plays a significant part in CVDs induced by OSA, specifically HTN, there still remains a limited understanding of this relationship. OSA affects the gut microbial environment and that altered the composition and metabolism of gut microbiome, namely gut dysbiosis. It has been reported that gut dysbiosis interacts with the fundamental mechanisms of OSA, including IH and SF, resulting in elevated inflammation, reduced insulin sensitivity in adipose tissue, and impaired gut barrier function. These pathological changes collectively contribute to the development of OSA. Furthermore, gut microbiome mediates cardiovascular disease‐related pathological changes in patients with OSA. In a murine model of OSA, gut dysbiosis contributed to impairment in artery function and increased arterial blood pressure. 5 In another animal model of OSA, it was observed that OSA rats exhibited a decrease in bacterial‐produced short‐chain fatty acids (SCFAs), which might trigger symptoms of HTN, including an elevation in blood pressure. 6
Consequently, to elucidate the correlation between the gut microbiome, OSA, and OSA‐related CVDs, in particular HTN, this review assesses contemporary research. Additionally, we explore the appliance of therapeutic approaches targeted the gut microbiome in OSA.
2. INTRODUCTION OF GUT MICROBIOME
Bacteria, archaea, fungi, protozoa, viruses, and their collective genomes comprise a microbiome inside and outside the host. It interacts with the host, forms a symbiosis, and maintains a relative balance despite fluctuations. 7 , 8 The most significant component of the human microbiome is the gut microbiome. It is extremely vulnerable to changes in the internal and external environment and a variety of internal and external environmental factors, including the host's immune response, various drugs, infections, and changes in lifestyle (diet, exercise, smoking, etc.), can all trigger their reactions. 9 , 10 The gut microbiome has an initial part in maintaining normal gut physiology. It promotes food digestion, helps maturation of the host immune system, and keeps integrality of gut epithelial barrier.
Dysbiosis, a change in the composition of the gut microbiome, is frequently characterized by the following symptoms: decreased microbial diversity, an increase in the Firmicutes to Bacteroidetes ratio (F: B), and a decrease in Bifidobacterium. 11 , 12 Dysbiosis has been associated with a variety of disorders, including obesity, insulin resistance (IR), 13 CVDs, 14 , 15 autoimmune diseases, 16 and neurodegeneration. 17 Chronic diseases previously believed to be associated with a certain lifestyle are now claimed to be related to microbiome. 18 Additionally, it has been reported that the same Western lifestyle might produce an opposing phenotype, which is directly related to the gut microbiome. 19 Although ample research is lacking, there is evidence that the alterations in the gut microbiome of OSA patients are strongly linked to crucial assessment indicators, such as the severity of OSA.
3. GUT MICROBIOME AND OSA
OSA is a common form of sleep‐disordered breathing that is associated with various detrimental health outcomes. Several studies have identified dysbiosis in individuals with OSA and have analyzed the gut microbiome of OSA patients. First, Bikov et al 20 divided the participants into OSA group (n = 19) and non‐OSA group (n = 20). They discovered no significant change in the gut microbiome composition between the non‐OSA group and OSA in α‐ and β‐diversity analyses. However, a separate study has shown that in OSA patients with varying levels of severity, there is a distinct difference in microbiome characteristics between severe OSA subjects and those with no OSA. 21 Notably, a β diversity analysis based on principal coordinate analysis has revealed significant variations in the OSA groups' composition. 21 Secondly, individuals diagnosed with OSA demonstrate an enhancement in beneficial gut bacteria and a reduction in harmful gut bacteria. Patients with OSA showed elevated levels of Proteobacteria, which synthesizes lipopolysaccharide (LPS), associated with systemic inflammation and obesity. 22 Meanwhile, Actinobacteria were significantly downregulated in OSA. Actinobacteria are renowned for producing the sleep‐inducing neurotransmitter gamma‐aminobutyric acid. 23 Additionally, a negative correlation was observed between Actinobacteria and the number of awakenings in healthy individuals. 24 Therefore, reduced levels of Actinobacteria may exacerbate arousals in OSA.
The researchers investigated the correlations between the microbiome of OSA and the risk factors for OSA, as well as the basic parameters for assessing OSA. Age and body mass index are main risk factors of OSA. Bikov et al 20 have identified a correlation between the abundance of particular bacteria, such as Proteobacteria or Lactobacillae, and age, as well as body mass index. To evaluate OSA comprehensively, three metrics are utilized: the apnea‐hypopnea index, time below 90% oxygen saturation (T90), and the oxygen desaturation index (ODI). These metrics assess OSA individually across three aspects: the overall count of apneas and hypopneas throughout the sleep cycle, the duration of hypoxia, and the total count of oxygen desaturation events. Baldanzi et al 25 carried out a cross‐sectional study examining the associations between gut microbiome and three indicators in a sample of 3570 participants aged 50–64 years. The results revealed a decrease in both richness and evenness of gut microbiome among individuals with OSA, which was linked to the three metrics. However, this is inconsistent with earlier research, 20 , 21 and this difference could be ascribed to the size of the sample. Additionally, 128 species were found to be associated with T90 and/or ODI. Notably, certain species associated with T90/ODI had either a positive or negative correlation with blood pressure.
Based on the presented evidence, a noteworthy correlation has been observed between gut microbiome and OSA. It is imperative to examine the complex interrelation between the pathology and physiology of OSA and the gut microbiome.
4. GUT MICROBIOME AND SF
SF is a prevalent sleep disorder with significant impacts on human health. SF significantly impacts the clinical phenotype of OSA, 26 contributing to neuroinflammation, oxidative stress, systemic inflammatory response, cognitive decline, memory impairment, and other common OSA symptoms. SF changes the microbial composition in mice, with more impact observed in the presence of a high‐fat diet (HFD). 27 The modified gut microbiome arising from SF may operate via multiple mechanisms, including disruption of the gut's circadian rhythm, 28 leading to enhanced feeding behavior 18 and inflammation responses. 29 The altered gut microbiome resulting from SF promote the advancement of SF and may even contribute to the pathological changes initiated by SF (Figure 1).
FIGURE 1.
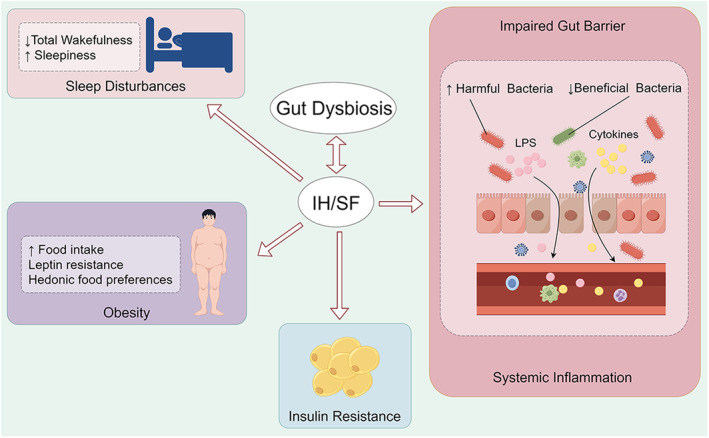
Mechanisms of how the gut microbiome facilitate the development of IH/SF. IH, intermittent hypoxia; SF, sleep fragmentation.
Gut microbiome is associated with SF‐related metabolic syndrome. Prolonged exposure to SF in mice results in an elevated food intake without a corresponding increase in caloric expenditure. 30 Also, leptin resistance develops in response to SF exposure. Consequently, subcutaneous and visceral depots accumulate adipose tissue, yielding weight gain even when consuming a standard diet. Ultimately, these factors contribute to the development of obesity. It is worth noting that SF is linked to alterations in hedonic food preferences, including increased snacking and carbohydrate intake. 31 The gut microbiome is believed to have a significant impact on mediating these changes. Research indicates that Faecalibacterium and Bifidobacterium can boost free fatty acid (FFA) concentrations in males and females, respectively. 32 A significant correlation has been identified between intestinal FFA receptors and appetite. 33 In addition, exposure to OSA leads to an elevation of plasma FFA levels. 34 TUG891, a FFA receptor 4 agonist, displayed significant beneficial effects on body weight, food consumption, and inflammation in the SF model. 35 Moreover, Farré et al 18 discovered that germ‐free (GF) mice received fecal pellets from SF‐exposed animals reproduced several SF behaviors, including increased food intake, significantly decreased adipose tissue insulin sensitivity, and increased leukocyte presence. Together, it is clear that the gut microbiome is strongly linked to metabolic abnormalities associated with SF and may even be a causative factor for such metabolic dysregulation.
The intestinal damage and chronic low‐grade inflammation linked to SF are associated with the gut microbiome. Studies have shown that SF modifies the gut microbiome, leading to an elevation in the Lachnospiraceae family and a decline in the Lactobacillaceae family. 29 The former is associated with intestinal inflammation, whereas the latter acts as a protective barrier. Moreover, SF lowers antioxidant capacity and depletes the number of goblet cells and colonocyte proliferation. 36 It also intensifies LPS‐binding protein circulation levels and stimulates an enhanced inflammatory response. 29 It is reasonable to hypothesize that SF‐related alteration in gut microbiome could risk damage to the gut barrier, thereby causing LPS displacement and provoking chronic low‐grade inflammation. An in vitro study reported that SF mice generated fecal water that resulted in a disruption of the colonic epithelium. 29 Moreover, the transfer of fecal matter from SF mice to GF mice resulted in an increased inflammatory response in the latter. 29 These findings support the abovementioned role of the gut microbiome in SF.
Additionally, studies have reported that probiotic supplements can improve sleep quality, 37 decrease arousal levels, 37 and reduce inflammation and oxidative stress 38 in another sleep disorder, namely sleep deprivation. In the future, there is a need to further investigate the effects of treatments that target gut microbiome on the negative outcomes of SF. In conclusion, the gut microbiome plays a role in mediating certain pathological changes induced by SF.
5. GUT MICROBIOME AND IH
IH affects the arterial partial pressure of oxygen, which could result in a hypoxia/reoxygenation pattern within the gut. 39 , 40 This pattern adversely impacts the environment of the intestinal epithelium and lead to dysbiosis. Systemic low‐grade inflammation caused by IH could also contribute to dysbiosis. In chronic intermittent hypoxia (CIH) mice, beneficial genera like Clostridium, Akkermansia, and Bacteroides reduced in presence while pathogenic genera like Desulfovibrio increased. 41 There is increasing evidence indicating the involvement of gut dysbiosis in promoting and facilitating IH (Figure 1).
The gut microbiome has been associated with disturbed sleep patterns seen in IH. IH exposure may impair sleep architecture by reducing total wakefulness and increasing sleepiness. 42 Badran et al 42 undertook a study that transplanted IH mouse microbiome into naive mice, and for the first time, analyzed the sleep patterns of the recipient mice. The treated mice showed disruptions in their sleep patterns, leading to increased sleep duration during the dark phase when these animals are usually more active and engaged in feeding and play. These findings imply that the gut microbiome changes associated with IH can independently cause sleep disturbances in mice. The sleep disorders observed in mice resemble the excessive daytime sleepiness commonly found in individuals with OSA. Hence, the present study highlights the vital role of the gut microbiome in regulating sleep and suggests a promising therapeutic approach to improving sleep quality and reducing daytime sleepiness in patients with OSA.
The gut microbiome is associated with intestinal damage and systemic inflammatory responses that are linked to IH. CIH damages the colonic mucosa, reducing goblet cells and impairing intestinal tight junctions. 41 These changes may relate with gut microbiome. The gut microbiome and its byproducts have been shown to harm gut barrier function through various mechanisms. 43 IH mice exhibited increased levels of Desulfovibrio, a bacterium that adversely impacts the intestinal barrier, contributing to the disruption of the intestinal barrier. 41 Moreover, researchers transplanted IH mice feces into recipient mice, which also exhibited dysbiosis and elevated intestinal permeability. 44 This indicates that gut barrier injury can be mediated, at least in part, by IH‐induced alterations in gut microbiome. Dysfunctional gut barriers create an atmosphere for bacterial translocation, leading to inflammation. CIH, as expected, increased circulating pro‐inflammatory cytokine 41 and LPS levels. 45 LPS is a byproduct of Gram‐negative gut bacteria and is a biomarker of intestinal barrier dysfunction. 46
There exists a link between gut microbiome changes and IR in IH. Abdelnaby et al 44 found that mice exposed to IH developed IR at a faster rate than those exposed to room air. This observation also applied to GF mice via fecal transplantation. Additionally, plasma exosomes, a kind of extracellular vesicle involved in cell communication, were examined in mice who received IH feces. The effects of these exosomes were comparable to those obtained from IH‐exposed mice. It led to a decrease in insulin sensitivity and a shift in macrophage polarity toward M1 macrophages.
Moreno‐Indias et al 45 have shown that plasma concentrations of LPS in mice exposed to IH remained significantly increased even after 6 weeks of normoxic recovery, surpassing the levels measured after 6 weeks of IH exposure by a factor of one. Moreover, gut dysbiosis still persisted after exposure to normoxia. These findings indicate that the harmful consequences of IH may last for an extended period and require intervention, despite the cessation of IH exposure.
6. MOLECULAR MECHANISMS BETWEEN GUT MICROBIOME AND OSA
Leaky gut is characterized by chronic elevation of intestinal permeability. It has been suggested that this phenomenon fosters systemic inflammation. Normally, the intestinal barrier prevents the transmission of harmful agents to the internal environment. However, compromised intestinal integrity can facilitate the entry of harmful substances, such as pathogens, pro‐inflammatory substances, and antigens, leading to the development of diseases or inflammation. 47 One such substance is LPS, also known as endotoxin, is a major cell wall component of Gram‐negative bacteria. It is released during infection or the lysis of Gram‐negative bacteria. The gut microbiome is the biggest reservoir of Gram‐negative bacteria, making it the primary source of circulating LPS. Although serum LPS concentration is typically low, compromised intestinal barrier integrity can cause an increase in LPS levels. 48 Elevated LPS levels can activate the Toll‐like receptor 4 (TLR4)/nuclear factor‐kappaB (NF‐κB) pathway, resulting in a systemic inflammatory response that contributes to the development of various diseases. Numerous studies have shown that OSA is a well‐established cause of tight junction dysfunction and increased circulating LPS levels. 46
Individuals with OSA experience narrowing or collapse of the upper airway during sleep, leading to episodes of apnea or hypopnea. Craniofacial abnormalities, such as excessive or elongated soft palate tissue, are a common contributing factor to upper airway stenosis in OSA. 49 Su et al 50 discovered a noteworthy increase in blood vessels in the lamina propria of the soft palate tissue of OSA patients. The study also revealed significantly higher levels of TLR4, p‐NF‐κB p65, vascular endothelial‐derived growth factor, and matrix metalloproteinase‐9 in the soft palate of OSA patients compared to controls. The TLR4/NF‐κB pathway promotes the production of vascular endothelial‐derived growth factor and matrix metalloproteinase‐9, contributing to the angiogenesis of the soft palate in OSA patients. The experiment confirmed the upregulation of the TLR4/NF‐κB pathway in the soft palate of OSA patients, which may be linked to the activation of the TLR4 receptor by high mobility group protein box 1. However, the impact of dysbiosis and elevated circulating LPS on OSA has not yet been investigated. Furthermore, a separate study found that individuals with moderate‐to‐severe OSA exhibited elevated levels of TLR4 in their carotid plaques when compared to both control groups and those with mild OSA. 51
In previous studies, the role of the TLR4/NF‐κB pathway has also been investigated in animal models of OSA. It has been suggested that IH‐mediated organ damage may be associated with the activation of this pathway. Zhang et al 52 found that the TLR4/NF‐κB pathway plays a vital role in IH‐related renal injury. They conducted a study on TLR4 knockout mice and TLR4 wild‐type (WT) C57BL/6J mice exposed to normal air or CIH. In comparison with TLR4 knockout mice, the WT mice exhibited renal insufficiency and histological damage. Additionally, CIH treatment led to a significant accumulation of macrophages and fibroblasts, release of pro‐inflammatory cytokines, and renal fibrosis in WT mice. The controls also showed a notable increase in the expression of myeloid differentiation primary‐response protein 88 and NF‐κB p65 in the kidney. In a separate study, Zou et al 53 found that TLR4 deficiency effectively prevented lung injury induced by IH. The study discovered that TLR4‐deficient (TLR4−/−) mice did not exhibit any noticeable lung changes, while C57BL/6J mice exposed to IH showed severe alveolar septal thickening, alveolar constriction, structural disruption, leukocyte infiltration, and hemorrhage. Furthermore, C57BL/6J‐IH mice had higher expression of NF‐κB p65 protein than TLR4−/− mice. The TLR4/NF‐κB pathway may also be associated with SF‐induced damage. Liu et al 54 utilized the modified multi‐platform sleep deprivation method to simulate rapid eye movement sleep deprivation, a common method for SF modeling. The rapid eye movement sleep‐deprived rats exhibited prolonged sleep latency and shortened sleep duration compared to the control group. Additionally, the sleep‐deprived rats displayed significant cognitive impairment and pathological injury in the hippocampus and cortex. In the model group, TLR4, p‐NF‐κB p65, and phosphorylated NF‐κB inhibitor(IκB) alpha levels were significantly higher than those in the control group.
These findings suggest that the activation of the pro‐inflammatory TLR4/NF‐κB pathway may be a mechanism that links OSA to its associated target‐organ damage. However, the substances that activate the TLR4 receptor were not investigated. Additionally, the potential link between OSA‐induced dysfunction of the intestinal barrier and increased levels of circulating LPS with TLR4 activation was not explored. Future research should prioritize investigating the relationship between leaky gut and OSA by targeting the TLR4/NF‐κB pathway.
7. OSA, GUT MICROBIOME, AND CVDs
It is widely acknowledged that a correlation exists between OSA and CVDs. Furthermore, these two conditions are known to interact with each other. Furthermore, evidence of interaction between these two conditions exists, with OSA affecting between 40% and 80% of patients with HTN, heart failure, coronary artery disease, pulmonary HTN, atrial fibrillation (AF), and stroke. 55 Individuals diagnosed with OSA are more susceptible to developing CVDs, which can lead to a worse prognosis. 56
In addition to the direct damage that OSA inflicts on the cardiovascular system, the gut microbiome and its metabolites can also exert an impact on it. In fact, there is a causal link between the gut microbiome and CVDs. 57 Gut microbiome is found to promote myocardial damage in ST‐elevation myocardial infarction (STEMI) patients. Chen et al 58 introduced fecal samples from such patients into GF mice. The outcome suggested that STEMI combined with fecal microbiota transplantation (FMT) treatment decreased baseline cardiac function and increased left ventricular stiffness, thereby affecting the contractility of recipient mice. After undergoing surgery for myocardial infarction, mice that received STEMI‐FMT showed a surprisingly high mortality rate. Interestingly, supplementing mice with butyrate led to a decrease in infarct size and an improvement in cardiac mechanical properties, especially in the presence of a healthy gut microbiome. Additionally, butyrate treatment showed a cardioprotective effect that depended on the dosage. Together, these findings imply that during a myocardial infarction, coexisting dysbiosis of the gut microbiome plays a role in exacerbating cardiac damage and that butyrate has a protective impact on the myocardium. Trimethylamine N‐oxide (a microbial metabolic product) and its precursors have been linked to a higher risk of significant adverse cardiovascular incidents and all‐cause mortality, irrespective of traditional risk factors for CVDs. 59 Jin et al 60 report that trimethylamine N‐oxide treatment impaired cardiac function and T‐tubule network in mice. Dysbiosis of the gut microbiome has been shown to have detrimental effects on ischemic stroke through the brain–gut axis. Patients with ischemic stroke have been discovered to have significant gut dysbiosis. In mice used as an ischemic stroke model, proximal middle cerebral artery occlusion (MCAO) increased intestinal permeability and translocation of the gut microbiome and bacterial toxins. Infarct volume and dysfunction following FMT were significantly higher in recipient mice (3 days before distal MCAO) receiving feces from stroke mice (previously had proximal transient MCAO). On the other hand, recipient mice who received feces (beginning on the day of proximal MCAO) had a significantly reduced gut dysbiosis and infarct volume. Additionally, probiotics and prebiotics use can enhance stroke results. 61 Alterations in the gut microbiome composition may be a mediator of aging‐related AF. According to Zhang et al, 62 compared to young mice, aged mice showed changed gut microbiome composition and higher circulating LPS (primarily gut‐metabolized). A significant increase in AF and a higher incidence of burst pacing‐induced AF were observed in young mice that received fecal transplants from older mice. However, the antagonistic potential of R. sphaeroides LPS significantly reduced the occurrence and duration of AF in aged‐FMT rats and significantly alleviated AF.
There is also some direct research on the relationship between OSA, the gut microbiome, and CVDs. According to Xue et al, 63 IH and hypercapnia accelerate the development of atherosclerosis, especially in the pulmonary artery. The 3,3‐dimethyl‐1‐butanol (a microbial trimethylamine production inhibitor) may also partially inhibit its growth. The cardiac transcriptome is influenced by the microbiome during normoxia and intermittent hypoxia and hypercapnia (IHH) treatment, as reported by Zhou et al. 64 After administering IHH to both conventional and GF mice for 2 weeks, researchers discovered 192 differentially expressed genes (DEGs) in the hearts of conventional mice and just 161 in GF mice with minimal overlap. This indicates that the presence or absence of gut microbiome leads to heterogeneous cardiac responses during exposure to IHH. Interestingly, in conventional mice, 24% of the DEGs detected had a role in regulating transcription from the DNA template. By contrast, DEGs in the heart of GF mice induced by IHH mostly participated in regulating biological functions or activities, for instance, the circadian rhythm and cell cycle. Furthermore, SF exposure resulted in an increase in mean arterial pressure in mice. This manifestation could potentially be linked to a decrease in the abundance of bacteria that produce SCFAs. 65
8. OSA, GUT MICROBIOME, AND HTN
The gut microbiome of hypertensive patients often has some characteristics. Unlike the healthy populations, both pre‐hypertensive and hypertensive populations usually have markedly decreased microbial richness and diversity, and distinct metagenomic composition. 66 , 67 For example, bacteria associated with healthy status are reduced in HTN, such as Lactobacillus 66 , 68 and Oscillibacter. 66 , 67 Disease‐linked bacteria overgrow in HTN, such as Parabacteroides. 66 , 67 Of note, SCFAs‐producing bacteria like Clostridium butyricum (C. butyricum) are normally less prevalent in HTN. SCFAs are known for regulating the blood pressure by SCFAs receptor.
In the last 5 years, the relation between gut microbiome and blood pressure has moved from association to causation, with researches that have used supplementation with specific strains or microbial metabolites, and FMT. A meta‐analysis of 14 human randomized and controlled trials has shown that probiotic supplementation can notably reduce blood pressure. 69 Another study described a causal relationship with FMT from one normotensive control and two untreated hypertensive patients to GF mice. 67 When compared to control mice, HTN mice had greater systolic blood pressure (SBP), diastolic blood pressure, mean blood pressure, and heart rates after 10 weeks following transplantation.
This causal relationship can also be seen in OSA‐related HTN and gut microbiome. One of the earliest investigations verified the association by FMT. The researchers mimicked OSA in rats by inflating a tracheal balloon in sync with the sleep cycle. 70 Rats developed HTN after being subjected to OSA and HFD. By contrast with control group, OSA combined with HFD led to a significant alteration in composition of gut microbiome. Using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States, the researchers predicted that several steps in the butyrate metabolism pathway would be downregulated. Afterwards, to identify the role of the gut in OSA‐induced HTN, they transplanted cecal contents obtained from OSA + HFD rats into the intestinal tract of recipient rats, who were not anticipated to have HTN. The recipient rats showed an increase in blood pressure. Thus, current evidences suggest that the gut microbiome contributes to the development of OSA‐induced HTN.
9. MOLECULAR MECHANISMS BETWEEN GUT MICROBIOME AND OSA, CVDs, AND HTN
Accumulating evidence suggests an important role of microbiome in blood pressure control. It has been hypothesized that one of the main mechanisms involved is the gut‐derived LPS/TLR4 pathway activation. The activation of the gut‐derived LPS/TLR4 pathway is primarily caused by excessive LPS production, which enters the circulation and activates vascular or heart TLR4. Furthermore, intestinal barrier damage is a critical step in this process. Excessive production of LPS is the primary cause of its activation, which then enters the circulation and activates vascular or heart TLR4. Additionally, damage to the intestinal barrier is a critical step in this process. 71
Gut‐derived LPS/TLR4 pathway is believed to have a strong association with CVDs. Several studies have shown that people with an increased risk of developing the blood major adverse cardiovascular events, such as heart attack and stroke, exhibit the heightened levels of LPS. 72 , 73 Zhang et al 74 discovered that Desulfovibrio desulfuricans (D. desulfuricans) worsened the progression of atherosclerosis in apolipoprotein E‐deficient mice. The study suggests that treatment with D. desulfuricans led to increased gut permeability and elevated serum LPS levels in apolipoprotein E‐deficient mice. Additionally, D. desulfuricans activated the endothelial TLR4/NF‐κB pathway. The researchers used TAK‐242, a specific inhibitor of TLR4 that blocks interactions between TLR4. As anticipated, TAK‐242 treatment significantly reduced the lesion area in the aorta and decreased the mRNA expression levels of TLR4 and NF‐κB, without affecting LPS levels. This study indicates that the gut‐derived LPS/TLR4 pathway activation may have a proatherosclerotic effect.
There is increasing evidence indicating the significant involvement of gut‐derived LPS in HTN. Vascular function is widely regarded as the direct cause of high blood pressure. The primary actors in vascular function are the endothelial and smooth muscle cells. 75 Numerous studies have investigated endothelial dysfunction triggered by LPS. Exposure to LPS, both through ex vivo incubation and in vivo intraperitoneal administration, has been shown to impair the aortic vasodilatory response to acetylcholine. 76 Su et al 71 modeled metabolic hypertension (MH) in rats by feeding them a diet high in sugar and fat, along with excessive alcohol. After 5 weeks of modeling, the MH rats exhibited significant increases in SBP and mean blood pressure. In comparison with rats fed normal chow and water, the model group exhibited a decrease in intestinal diversity and an impaired intestinal barrier. This was demonstrated by the loss of colonic villi and the downregulation of protein expression levels of zonula occludens‐1, occludin, and claudin‐1 when compared to normal rats. Additionally, the model group had higher serum levels of LPS. Vessels from MH rats also exhibited detached endothelial cells, smooth muscle cell hypertrophy and reduced endothelial nitric oxide synthase (eNOS) levels. eNOS produces vascular nitric oxide (NO) by using l‐arginine as a substrate. Nitric oxide is a crucial molecule released by endothelial cells. Endothelial dysfunction, which underlies the pathogenesis of CVDs, is commonly characterized by reduced bioavailability of NO. 77 Thus, reduced levels of eNOS reflected impaired endothelial dysfunction. In this study, it was also found that MH increased the expression of TLR4 and the phosphorylation of IκB kinase‐beta, IκB alpha, and NF‐κB p65 in rat vessels. 71 Therefore, it was concluded that the LPS‐dependent activation of TLR4 is responsible for vascular structural and functional dysfunction in MH. Furthermore, LPS/TLR4 pathway may decrease eNOS levels, impairing endothelial function. Robles‐Vera et al 78 discovered significant gut dysbiosis and endotoxemia in spontaneously hypertensive rats (SHR) compared to Wistar Kyoto rats (WKY). The vasodilatory response to the endothelium‐dependent dilator acetylcholine was weaker in the aorta of the SHR than that of the WKY. In both WKY and SHR, this acetylcholine‐induced vasorelaxation was entirely dependent on NO from the endothelium, as NG‐nitro‐l‐arginine methyl ester, an eNOS inhibitor, completely inhibited acetylcholine‐induced relaxation. The bioavailability of NO is heavily dependent on the reactive oxygen species (ROS) generated by NADPH oxidase in the endothelium, in addition to eNOS. In aortic rings from SHR, NADPH oxidase ROS production increased by approximately 60%, whereas in WKY, it did not. In aortic homogenates, TLR4 mRNA levels were approximately 8 times higher in SHR than in WKY. Notably, supplementing with probiotics and SCFAs can attenuate these changes. Taken together, these studies demonstrate that the gut dysbiosis may mediate the production of ROS and decrease the level of eNOS through the LPS/TLR4 pathway. This can lead to disruption of NO signaling and endothelial dysfunction.
Moreover, increased LPS levels triggered vascular inflammation through TLR4 activation. Vascular inflammation is widely acknowledged as a key precursor of endothelial dysfunction and the inciting factor in HTN. Many clinical studies have reported a positive association between levels of inflammatory markers and blood pressure. 79 Yuan et al 80 demonstrated that LPS treatment of EA.hy926 cells resulted in higher levels of IκB and NF‐κB phosphorylation compared to the control group. Additionally, LPS treatment raised the levels of typical inflammatory cytokines, interleukin 6 (IL‐6), and tumor necrosis factor‐alpha (TNF‐α), in EA.hy926 cells. Likewise, LPS treatment in mice led to significant inflammatory cell infiltration in vascular tissues. In the aortae of LPS mice, the mRNA levels of IL‐6, TNF‐α, and TLR4 were elevated. Importantly, in WT mice, the injection of LPS led to a notable rise in NF‐κB activation. However, this effect was not observed in TLR4−/− mice, which further supports the hypothesis that TLR4 activation is necessary for LPS‐induced vascular inflammation. 76
In conclusion, the gut‐derived LPS/TLR4 pathway induces vascular dysfunction and inflammation by disrupting NO signaling and promoting an inflammatory response, ultimately leading to elevated blood pressure. These findings are consistent with the clinically observed phenotype. Patients with OSA exhibit reduced circulating NO and impaired endothelium‐dependent vasodilation. 81 In addition, as a well‐studied pathological process, the LPS/TLR4 pathway has been implicated in the disease phenotype of OSA. The gut‐derived LPS/TLR4 pathway has been shown to play a pro‐cardiovascular role in animal models of HTN. However, there is currently no conclusive evidence to confirm the role of this molecular mechanism in the OSA combined with HTN model. Future work should give priority attention to verifying whether this pathway is involved in the development of OSA‐related HTN. Moreover, manipulating the microbiome and metabolome may be a promising treatment strategy to block the cascade of responses generated by leaky gut in OSA patients.
10. THERAPEUTIC CONSIDERATIONS
The goals of treatment for OSA are to reduce symptoms, improve quality of life, reduce complications, and decrease mortality. As stated above, the gut microbiome plays a role in the pathological processes of both OSA and its cardiovascular complications. Adjuvant therapies targeting the gut microbiome, such as probiotics and prebiotics supplements, SCFAs supplements, and FMT, are currently undergoing preclinical and clinical testing due to their potential impact on OSA. These therapies may soon have applications in clinical settings.
10.1. Probiotics and prebiotics
Probiotics are presently defined as live bacteria that, when consumed in sufficient quantities, provide advantageous health effects to the host. 82 Probiotics can affect various internal pathways or the intestinal barrier directly, resulting in the regulation of metabolism and immunity. 83 , 84 The present prebiotic concept pertains to “a substrate utilized selectively by host microorganisms leading to health benefit.” 85 Prebiotics can combat enterogenous infections due to their anti‐adhesive characteristics against enteropathogens and enterotoxins, 86 while stimulate selectively the growth and/or activity of probiotic bacteria. 87 Certain probiotics and prebiotics have shown to have positive effects in relation to cardiovascular complications in OSA through experimental and clinical research (Figure 2).
FIGURE 2.

The probiotics, prebiotics, and SCFAs supplements may reduce the progression of OSA and the cardiovascular morbidity. CVDs, cardiovascular diseases; IH, intermittent hypoxia; OSA, obstructive sleep apnea; SF, sleep fragmentation; SCFAs, short‐chain fatty acids.
C. butyricum, a commensal bacterium in the human gut, is a member of the Clostridium genus. 88 C. butyricum ferments undigested dietary fibers and synthesizes SCFAs, especially butyrate and acetate. 89 Hylon VII is a resistant corn starch that serves as a substrate for bacterial fermentation and SCFAs generation. Study has demonstrated that its consumption results in elevated concentrations of butyrate in the caecum, as well as acetate and butyrate in fecal matter. 90 Ganesh et al 6 established rat model of OSA by implanting an endotracheal obstruction device into rat and evaluated the impact of C. butyricum and Hylon VII supplementation on HTN in OSA rats with one billion CFU of C. butyricum or 20% high amylose corn starch Hylon VII. OSA rats and sham rats are, respectively, randomized to HFD, HFD + Hylon, or HFD+ C. butyricum group. They found that HFD‐fed rats showed increased SBP after 1 and 2 weeks of OSA, as compared with sham rats. In contrast, there was no substantial difference in SBP between OSA and sham rats in the HFD + Hylon or HFD+ C. butyricum groups. That means the Hylon and C. butyricum can prevent SBP elevation in OSA without affecting SBP in normotensive sham rats. Furthermore, the number of mucus‐producing goblet cells in the cecum decreased, and the mucus barrier thickness that spans the distance from luminal bacteria to the underlying epithelium was reduced by OSA. These changes were countered by C. butyricum and Hylon.
Lactobacillus rhamnosus GG (LGG) is a member of the Lactobacillus genus, which comprises a substantial portion of the human microbiota. LGG is the most common probiotic available. It has the ability to adhere to the intestinal mucosa, improve the epithelial barrier, and concomitantly inhibit pathogen adhesion. Additionally, it modulates type 1 immune responsiveness. 91 To develop the condition of obesity in OSA, Xu et al 92 treat the mice with a high‐fat high‐fructose diet and IH. Based the model, they investigated the effects of LGG (109 CFU bacteria/day) and LGG cell‐free supernatant (dose equivalent to 109 CFU bacteria/day). Transthoracic echocardiography analysis revealed that IH‐exposed obese mice exhibited an increase in left ventricular internal systolic diameter and left ventricular end systolic volume, as well as a decrease in EF%. However, these alterations were absent in high‐fat high‐fructose diet/IH + LGG mice. Furthermore, the myocardium did not exhibit IH‐induced structural disorganization in both LGG and LGG cell‐free supernatant groups. These findings illustrated the anti‐ventricular remodeling and cardio protection ability of LGG in OSA. Additionally, LGG diminished the IH‐elevated cardiac inflammatory level in obese mice. In another investigation, important differences were observed between normal diet (ND), CIH + ND, high salt diet (HSD), and CIH + HSD rats. 93 CIH + HSD rats showed increased blood pressure and altered gut microbiome composition, particularly in Lactobacillus. For 4 weeks of LGG intervention, CIH + HSD rats were sequentially divided into ND, HSD, and ND + LGG groups averagely and randomly. Importantly, LGG therapy resulted in a significant reduction in blood pressure compared to controls and their blood pressure before intervention.
Beyond that, other probiotics like bifidobacterium has been found worked in cardiovascular models. Lu et al 94 reported that the growth of HTN and vascular remodeling in SHR was inhibited by Bifidobacterium longum CCFM752. Nevertheless, these abilities have yet to be confirmed in an OSA‐related HTN model.
In addition to the abovementioned effects, probiotics and prebiotics could aid in managing CVDs by regulating metabolic disorders encompassing obesity and diabetes, 95 reducing cholesterol levels, 96 and curbing oxidative stress. 97 Collectively, it is evident that the data from these experimental studies demonstrate considerable cardiovascular advantages associated with the ingestion of probiotics and prebiotics in OSA. However, further mechanistic studies and more clinical studies in OSA patients are required.
10.2. SCFAs
SCFAs are generated by the gut microbiota during the fermentation of dietary fiber, which includes acetate, butyrate, and propionate. SCFAs have multiple physiological impacts both inside and outside of the colon. Locally, they increase mucosal blood flow and stimulate the growth of colonic epithelial cells. Furthermore, they can regulate intestinal barrier function and maintain intestinal homeostasis. Unlike most other types of cells, which utilize glucose as their primary energy source, colonocytes mainly use SCFAs, especially butyrate, for energy. 98 Butyrate enhances Mucin2 expression, and thereby improving the intestinal physical barrier. 99 Outside of the colon, SCFAs possesses an anti‐inflammatory characteristic. Butyrate suppressed neuroinflammation induced by chronic alcohol exposure by reducing pro‐inflammatory cytokines TNF‐α, interleukin‐1beta, and IL‐6 in the brain. It also regulated the polarization of M1/M2 microglia and downregulated TLR4/NF‐κB activation. 100 SCFAs play a crucial role in regulating immune function. Butyrate increases the expression of aryl hydrocarbon receptor and hypoxia‐inducible factor 1α, which enhances the production of interleukin‐22 in group 3 innate lymphoid cells and CD4+ T cells by binding to G‐protein receptor 41. 101 In addition, SCFAs can affect metabolic state of the host. Insulin secretion in islet β‐cells is regulated by SCFAs through FFA2 and FFA3 receptors. 102 Moreover, SCFAs stimulate leptin release in mouse adipose tissue and adipocytes via the G protein‐coupled receptor41. 103
The role of SCFAs was also validated in the OSA‐induced HTN model (Figure 2). Ganesh et al 6 found the rats developed HTN, after gavaged with cecal and colonic contents of HTN rats which caused by a combination of OSA and HFD. To determine the specific substance responsible for HTN in the gut contents, they analyzed the gut microbiota profile and metabolism. Their investigation revealed a decrease in both acetate‐produced bacteria and acetate level in OSA + HFD rats. Then, they examine the effect of acetate supplementation (cecal infusion of acetate). Acetate infusion reduced the heightened mRNA level of cecal interleukin‐1α and interleukin‐6 mRNA in OSA rats. It is worth noting that there was no significant difference in the interleukin‐1α and interleukin‐6 mRNA levels between the OSA + acetate group and the sham group. Since its known that OSA companied by HFD leads to HTN, they next measured SBP. SBP of OSA + acetate rats were not remarkably different from sham group. It is evidence that acetate supplementation can prevent OSA‐related inflammation and HTN.
10.3. FMT
FMT is a procedure in which fecal material containing gut microbiota from a healthy donor is infused into the gastrointestinal tract of a recipient patient. 104 FMT is a therapy recommended for instances of recurrent or refractory Clostridium difficile infection. 105 FMT also conducted for inflammatory bowel disease, specifically ulcerative colitis, in several clinical trials. 106 , 107 , 108 While multiple variables, like administered dose, varied in the studies mentioned, the overarching results seem to demonstrate that FMT may lead to clinical remission of ulcerative colitis. In type 2 diabetes, FMT effectively improved IR and mended impaired islets. 109
Given the implication of gut microbiome and its metabolites in the progression of OSA and many pathological manifestations of OSA can be transmitted via FMT, it seems plausible to suggest that FMT could present a feasible advantage for OSA. As stated earlier, FMT is currently being utilized in animal models to investigate whether gut microbiota play a part in mediating pathological processes linked to OSA. It has not yet been implemented therapeutically. However, the present clinical indication for FMT in humans remains limited to recurrent or refractory Clostridium difficile infection. To date, FMT is not indicated for human non‐gastrointestinal diseases.
11. SUMMARY
The influence of the gut microbiome on the host has been extensively investigated in the context of OSA. According to recent findings, the gut microbiome plays a crucial role in OSA. IH and SF, the fundamental features of OSA, have been found to induce alterations in the gut microbiome, aggravate gut permeability, modify microbial metabolites, and trigger low‐grade chronic inflammatory, metabolic syndrome, and sleep disturbance. Based on the current evidence, some researchers have established the causal association between the gut microbiome and OSA. Among them, the gut‐derived LPS/TLR4 pathway appears to play a significant role. Furthermore, interventions targeting the gut microbiome, including probiotic and prebiotic supplements, SCFAs supplements, and FMT, have demonstrated the ability to mitigate pathophysiology triggered by OSA. Of note, in animal models, probiotic or prebiotic supplements display promise as a therapy for CVDs arising from OSA. Further randomized and controlled trials are necessary to demonstrate the efficacy of using probiotics or prebiotics as an adjunct therapeutic remedy in preventing, eliminating, or reducing complications associated with OSA.
AUTHOR CONTRIBUTIONS
Xiaotong Zhang: Writing—original draft, Conceptualization; Haifen Zhang: Investigation; Shuai Li: Investigation; Fan Fang: Conceptualization; Yanran Yin: Supervision; Qiang Wang: Writing—review & editing.
FUNDING INFORMATION
This work was supported by foundation of Health Commission of Shanxi Province of China, Grant/Award Number: 2023ZYYDA2035.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
Zhang X, Zhang H, Li S, Fang F, Yin Y, Wang Q. Recent progresses in gut microbiome mediates obstructive sleep apnea‐induced cardiovascular diseases. FASEB BioAdvances. 2024;6:118‐130. doi: 10.1096/fba.2023-00153
DATA AVAILABILITY STATEMENT
Stored in repository.
REFERENCES
- 1. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature‐based analysis. Lancet Respir Med. 2019;7(8):687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ji Y, Liang Y, Mak JCW, Ip MSM. Obstructive sleep apnea, intermittent hypoxia and non‐alcoholic fatty liver disease. Sleep Med. 2022;95:16‐28. [DOI] [PubMed] [Google Scholar]
- 3. Bauters F, Rietzschel ER, Hertegonne KB, Chirinos JA. The Link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep. 2016;18(1):1. [DOI] [PubMed] [Google Scholar]
- 4. Kheirandish‐Gozal L, Gozal D. Pediatric OSA syndrome morbidity biomarkers: the hunt is finally on! Chest. 2017;151(2):500‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badran M, Khalyfa A, Ericsson A, et al. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnea: effect of probiotics. Eur Respir J. 2022;61:2200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganesh BP, Nelson JW, Eskew JR, et al. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72(5):1141‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barko PC, McMichael MA, Swanson KS, Williams DA. The gastrointestinal microbiome: a review. J Vet Intern Med. 2018;32(1):9‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369‐2379. [DOI] [PubMed] [Google Scholar]
- 10. Redondo‐Useros N, Nova E, González‐Zancada N, Díaz LE, Gómez‐Martínez S, Marcos A. Microbiota and lifestyle: a special focus on diet. Nutrients. 2020;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durgan DJ. Obstructive sleep apnea‐induced hypertension: role of the gut microbiota. Curr Hypertens Rep. 2017;19(4):35. [DOI] [PubMed] [Google Scholar]
- 12. Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74(16):2959‐2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55‐71. [DOI] [PubMed] [Google Scholar]
- 14. Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018;214:153‐157. [DOI] [PubMed] [Google Scholar]
- 16. Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quigley EMM. Microbiota‐brain‐gut Axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17(12):94. [DOI] [PubMed] [Google Scholar]
- 18. Farré N, Farré R, Gozal D. Sleep apnea morbidity: a consequence of microbial‐immune cross‐talk? Chest. 2018;154(4):754‐759. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez‐Castaño GP, Caro‐Quintero A, Reyes A, Lizcano F. Advances in gut microbiome research, opening new strategies to Cope with a Western lifestyle. Front Genet. 2016;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bikov A, Szabo H, Piroska M, et al. Gut microbiome in patients with obstructive sleep Apnoea. Appl Sci. 2022;12(4). [Google Scholar]
- 21. Li Q, Xu T, Shao C, et al. Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function. Sci Rep. 2023;13(1):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortés‐Martín A, Iglesias‐Aguirre CE, Meoro A, Selma MV, Espín JC. There is no distinctive gut microbiota signature in the metabolic syndrome: contribution of cardiovascular disease risk factors and associated medication. Microorganisms. 2020;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yunes RA, Poluektova EU, Dyachkova MS, et al. GABA production and structure of gadB/gadC genes in lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe. 2016;42:197‐204. [DOI] [PubMed] [Google Scholar]
- 24. Smith RP, Easson C, Lyle SM, et al. Gut microbiome diversity is associated with sleep physiology in humans. PloS One. 2019;14(10):e0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baldanzi G, Sayols‐Baixeras S, Theorell‐Haglöw J, et al. OSA is associated with the human gut microbiota composition and functional potential in the population‐based Swedish CardioPulmonary bioImage study. Chest. 2023;164(2):503‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue R, Wan Y, Sun X, Zhang X, Gao W, Wu W. Nicotinic mitigation of Neuroinflammation and oxidative stress after chronic sleep deprivation. Front Immunol. 2019;10:2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang F, Zou J, Xu H, et al. Effects of chronic intermittent hypoxia and chronic sleep fragmentation on gut microbiome, serum metabolome, liver and adipose tissue morphology. Front Endocrinol (Lausanne). 2022;13:820939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Wang Z, Cao J, Dong Y, Chen Y. Melatonin improves the homeostasis of mice gut microbiota rhythm caused by sleep restriction. Microbes Infect. 2023;25(6):105121. [DOI] [PubMed] [Google Scholar]
- 29. Poroyko VA, Carreras A, Khalyfa A, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Carreras A, Lee S, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity (Silver Spring). 2014;22(3):758‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernández‐Navarro T, Díaz I, Gutiérrez‐Díaz I, et al. Exploring the interactions between serum free fatty acids and fecal microbiota in obesity through a machine learning algorithm. Food Res Int. 2019;121:533‐541. [DOI] [PubMed] [Google Scholar]
- 33. Freitas RDS, Campos MM. Understanding the appetite modulation pathways: the role of the FFA1 and FFA4 receptors. Biochem Pharmacol. 2021;186:114503. [DOI] [PubMed] [Google Scholar]
- 34. Chopra S, Rathore A, Younas H, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102(9):3172‐3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murtaza B, Hichami A, Khan AS, et al. Novel GPR120 agonist TUG891 modulates fat taste perception and preference and activates tongue‐brain‐gut axis in mice. J Lipid Res. 2020;61(2):133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao T, Wang Z, Dong Y, et al. Role of melatonin in sleep deprivation‐induced intestinal barrier dysfunction in mice. J Pineal Res. 2019;67(1):e12574. [DOI] [PubMed] [Google Scholar]
- 37. Freitas SM, Franco B, Saragiotto G, et al. Effect of a probiotic fermented milk supplementation on behavior and sleep. Nutr Neurosci. 2023;26:1‐13. [DOI] [PubMed] [Google Scholar]
- 38. Zheng Y, Zhang L, Bonfili L, de Vivo L, Eleuteri AM, Bellesi M. Probiotics supplementation attenuates inflammation and oxidative stress induced by chronic sleep restriction. Nutrients. 2023;15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almendros I, Montserrat JM, Torres M, González C, Navajas D, Farré R. Changes in oxygen partial pressure of brain tissue in an animal model of obstructive apnea. Respir Res. 2010;11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moreno‐Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45(4):1055‐1065. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Wang F, Gao Z, et al. Melatonin attenuates chronic intermittent hypoxia‐induced intestinal barrier dysfunction in mice. Microbiol Res. 2023;276:127480. [DOI] [PubMed] [Google Scholar]
- 42. Badran M, Khalyfa A, Ericsson A, Gozal D. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp Neurol. 2020;334:113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khalyfa A, Ericsson A, Qiao Z, Almendros I, Farré R, Gozal D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: effects of physical activity. EBioMedicine. 2021;64:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moreno‐Indias I, Torres M, Sanchez‐Alcoholado L, et al. Normoxic recovery mimicking treatment of sleep apnea does not reverse intermittent hypoxia‐induced bacterial Dysbiosis and low‐grade Endotoxemia in mice. Sleep. 2016;39(10):1891‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mashaqi S, Rangan P, Saleh AA, et al. Biomarkers of gut barrier dysfunction in obstructive sleep apnea: a systematic review and meta‐analysis. Sleep Med Rev. 2023;69:101774. [DOI] [PubMed] [Google Scholar]
- 47. Aleman RS, Moncada M, Aryana KJ. Leaky gut and the ingredients that help treat it: a review. Molecules. 2023;28(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang Y, Kuang X, Yan H, et al. A novel Synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota‐liver Axis and inhibiting the hepatic TLR4/NF‐κB/NLRP3 signaling pathway. mSystems. 2023;8(2):e0112722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lv R, Liu X, Zhang Y, et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct Target Ther. 2023;8(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Su T, Li C, Zhang Y, et al. Upregulation of HMGB1 promotes vascular dysfunction in the soft palate of patients with obstructive sleep apnea via the TLR4/NF‐κB/VEGF pathway. FEBS Open Bio. 2023;13(2):246‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Olejarz W, Głuszko A, Cyran A, et al. TLRs and RAGE are elevated in carotid plaques from patients with moderate‐to‐severe obstructive sleep apnea syndrome. Sleep Breath. 2020;24(4):1573‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Su X, Zou F, Xu T, Pan P, Hu C. Toll‐like receptor‐4 deficiency alleviates chronic intermittent hypoxia‐induced renal injury, inflammation, and fibrosis. Sleep Breath. 2019;23(2):503‐513. [DOI] [PubMed] [Google Scholar]
- 53. Zou F, Su X, Pan P. Toll‐like Receptor‐4‐mediated inflammation is involved in intermittent hypoxia‐induced lung injury. Lung. 2020;198(5):855‐862. [DOI] [PubMed] [Google Scholar]
- 54. Liu B, Li F, Xu Y, Wu Q, Shi J. Gastrodin improves cognitive dysfunction in REM sleep‐deprived rats by regulating TLR4/NF‐κB and Wnt/β‐catenin signaling pathways. Brain Sci. 2023;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144(3):e56‐e67. [DOI] [PubMed] [Google Scholar]
- 56. Tietjens JR, Claman D, Kezirian EJ, et al. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc. 2019;8(1):e010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen HC, Liu YW, Chang KC, et al. Gut butyrate‐producers confer post‐infarction cardiac protection. Nat Commun. 2023;14(1):7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies. J Am Heart Assoc. 2017;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin B, Ji F, Zuo A, et al. Destructive role of TMAO in T‐tubule and excitation‐contraction coupling in the adult Cardiomyocytes. Int Heart J. 2020;61(2):355‐363. [DOI] [PubMed] [Google Scholar]
- 61. Chidambaram SB, Rathipriya AG, Mahalakshmi AM, et al. The influence of gut Dysbiosis in the pathogenesis and Management of Ischemic Stroke. Cells. 2022;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Y, Zhang S, Li B, et al. Gut microbiota dysbiosis promotes age‐related atrial fibrillation by lipopolysaccharide and glucose‐induced activation of NLRP3‐inflammasome. Cardiovasc Res. 2022;118(3):785‐797. [DOI] [PubMed] [Google Scholar]
- 63. Xue J, Zhou D, Poulsen O, et al. Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine‐oxide. Am J Respir Cell Mol Biol. 2017;57(5):581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhou D, Xue J, Miyamoto Y, Poulsen O, Eckmann L, Haddad GG. Microbiota modulates cardiac transcriptional responses to intermittent hypoxia and hypercapnia. Front Physiol. 2021;12:680275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maki KA, Burke LA, Calik MW, et al. Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats. Physiol Genomics. 2020;52(7):280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dan X, Mushi Z, Baili W, et al. Differential analysis of hypertension‐associated intestinal microbiota. Int J Med Sci. 2019;16(6):872‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilck N, Matus MG, Kearney SM, et al. Salt‐responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551(7682):585‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chi C, Li C, Wu D, et al. Effects of probiotics on patients with hypertension: a systematic review and meta‐analysis. Curr Hypertens Rep. 2020;22(5):34. [DOI] [PubMed] [Google Scholar]
- 70. Durgan DJ, Ganesh BP, Cope JL, et al. Role of the gut microbiome in obstructive sleep apnea‐induced hypertension. Hypertension. 2016;67(2):469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Su J, Wang Y, Yan M, et al. The beneficial effects of Polygonatum sibiricum red. Superfine powder on metabolic hypertensive rats via gut‐derived LPS/TLR4 pathway inhibition. Phytomedicine. 2022;106:154404. [DOI] [PubMed] [Google Scholar]
- 72. Hakoupian M, Ferino E, Jickling GC, et al. Bacterial lipopolysaccharide is associated with stroke. Sci Rep. 2021;11(1):6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Manolis AA, Manolis TA, Melita H, Manolis AS. Gut microbiota and cardiovascular disease: symbiosis versus Dysbiosis. Curr Med Chem. 2022;29(23):4050‐4077. [DOI] [PubMed] [Google Scholar]
- 74. Zhang K, Qin X, Qiu J, et al. Desulfovibrio desulfuricans aggravates atherosclerosis by enhancing intestinal permeability and endothelial TLR4/NF‐κB pathway in Apoe (−/−) mice. Genes Dis. 2023;10(1):239‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ma J, Li Y, Yang X, et al. Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334. [DOI] [PubMed] [Google Scholar]
- 77. Janaszak‐Jasiecka A, Płoska A, Wierońska JM, Dobrucki LW, Kalinowski L. Endothelial dysfunction due to eNOS uncoupling: molecular mechanisms as potential therapeutic targets. Cell Mol Biol Lett. 2023;28(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Robles‐Vera I, Toral M, de la Visitación N, et al. Probiotics prevent Dysbiosis and the rise in blood pressure in genetic hypertension: role of short‐chain fatty acids. Mol Nutr Food Res. 2020;64(6):e1900616. [DOI] [PubMed] [Google Scholar]
- 79. Angeli F, Reboldi G, Verdecchia P. The Link between inflammation and hypertension: unmasking mediators. Am J Hypertens. 2021;34(7):683‐685. [DOI] [PubMed] [Google Scholar]
- 80. Yuan H, Hou Q, Feng X, et al. 5,2'‐Dibromo‐2,4′,5′‐trihydroxydiphenylmethanone inhibits LPS‐induced vascular inflammation by targeting the Cav1 protein. Molecules. 2022;27(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arnaud C, Billoir E, de Melo Junior AF, Pereira SA, O'Halloran KD, Monteiro EC. Chronic intermittent hypoxia‐induced cardiovascular and renal dysfunction: from adaptation to maladaptation. J Physiol. 2023;601(24):5553‐5577. [DOI] [PubMed] [Google Scholar]
- 82. Salminen S, Collado MC, Endo A, et al. The international scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mishra SP, Jain S, Taraphder S, Yadav H. New horizons in microbiota and metabolic Health Research. J Clin Endocrinol Metab. 2021;106(2):e1052‐e1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hou K, Wu ZX, Chen XY, et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491‐502. [DOI] [PubMed] [Google Scholar]
- 86. Douëllou T, Montel MC, Thevenot SD. Invited review: anti‐adhesive properties of bovine oligosaccharides and bovine milk fat globule membrane‐associated glycoconjugates against bacterial food enteropathogens. J Dairy Sci. 2017;100(5):3348‐3359. [DOI] [PubMed] [Google Scholar]
- 87. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stoeva MK, Garcia‐So J, Justice N, et al. Butyrate‐producing human gut symbiont, clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13(1):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nielsen TS, Bendiks Z, Thomsen B, et al. High‐amylose maize, potato, and Butyrylated starch modulate large intestinal fermentation, microbial composition, and oncogenic miRNA expression in rats fed a high‐protein meat diet. Int J Mol Sci. 2019;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Capurso L. Thirty years of lactobacillus rhamnosus GG: a review. J Clin Gastroenterol. 2019;53(Suppl. 1):S1‐s41. [DOI] [PubMed] [Google Scholar]
- 92. Xu H, Wang J, Cai J, et al. Protective effect of lactobacillus rhamnosus GG and its supernatant against myocardial dysfunction in obese mice exposed to intermittent hypoxia is associated with the activation of Nrf2 pathway. Int J Biol Sci. 2019;15(11):2471‐2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu J, Li T, Wu H, et al. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea‐induced hypertension in a high salt diet via regulating TMAO level and CD4(+) T cell induced‐type I inflammation. Biomed Pharmacother. 2019;112:108580. [DOI] [PubMed] [Google Scholar]
- 94. Lu W, Wang Y, Fang Z, et al. Bifidobacterium longum CCFM752 prevented hypertension and aortic lesion, improved antioxidative ability, and regulated the gut microbiome in spontaneously hypertensive rats. Food Funct. 2022;13(11):6373‐6386. [DOI] [PubMed] [Google Scholar]
- 95. Mishra SP, Wang S, Nagpal R, et al. Probiotics and prebiotics for the amelioration of type 1 diabetes: present and future perspectives. Microorganisms. 2019;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mo R, Zhang X, Yang Y. Effect of probiotics on lipid profiles in hypercholesterolaemic adults: a meta‐analysis of randomized controlled trials. Med Clin (Barc). 2019;152(12):473‐481. [DOI] [PubMed] [Google Scholar]
- 97. Chen Z, Liang W, Liang J, et al. Probiotics: functional food ingredients with the potential to reduce hypertension. Front Cell Infect Microbiol. 2023;13:1220877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hou H, Chen D, Zhang K, et al. Gut microbiota‐derived short‐chain fatty acids and colorectal cancer: ready for clinical translation? Cancer Lett. 2022;526:225‐235. [DOI] [PubMed] [Google Scholar]
- 99. Liang L, Liu L, Zhou W, et al. Gut microbiota‐derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin Sci (Lond). 2022;136(4):291‐307. [DOI] [PubMed] [Google Scholar]
- 100. Wei H, Yu C, Zhang C, et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia‐mediated neuroinflammation and modulating the microbiome‐gut‐brain axis. Biomed Pharmacother. 2023;160:114308. [DOI] [PubMed] [Google Scholar]
- 101. Yang W, Yu T, Huang X, et al. Intestinal microbiota‐derived short‐chain fatty acids regulation of immune cell IL‐22 production and gut immunity. Nat Commun. 2020;11(1):4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Teyani R, Moniri NH. Gut feelings in the islets: the role of the gut microbiome and the FFA2 and FFA3 receptors for short chain fatty acids on β‐cell function and metabolic regulation. Br J Pharmacol. 2023;180(24):3113‐3129. [DOI] [PubMed] [Google Scholar]
- 103. May KS, den Hartigh LJ. Modulation of adipocyte metabolism by microbial short‐chain fatty acids. Nutrients. 2021;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gupta A, Khanna S. Fecal microbiota Transplantation. JAMA. 2017;318(1):102. [DOI] [PubMed] [Google Scholar]
- 105. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fang H, Fu L, Li X, et al. Long‐term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Fact. 2021;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Huang C, Huang Z, Ding L, et al. Fecal microbiota transplantation versus glucocorticoids for the induction of remission in mild to moderate ulcerative colitis. J Transl Med. 2022;20(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kedia S, Virmani S, Vuyyuru SK, et al. Faecal microbiota transplantation with anti‐inflammatory diet (FMT‐AID) followed by anti‐inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022;71(12):2401‐2413. [DOI] [PubMed] [Google Scholar]
- 109. Wang H, Lu Y, Yan Y, et al. Promising treatment for type 2 diabetes: fecal microbiota Transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol. 2019;9:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Stored in repository.


