Abstract
Initiation of herpesvirus infection requires attachment of virions to the host cell followed by fusion of virion envelope and cellular cytoplasmic membrane during penetration. In several alphaherpesviruses, glycoprotein C (gC) is the primary attachment protein, interacting with cell-surface heparan sulfate proteoglycans. Secondary binding is mediated by gD, which, normally, is also required for penetration. Recently, we described the isolation of a gD-negative infectious pseudorabies virus (PrV) mutant, PrV gD− Pass (J. Schmidt, B. G. Klupp, A. Karger, and T. C. Mettenleiter, J. Virol. 71:17–24, 1997). In PrV gD− Pass, attachment and penetration occur in the absence of gD. To assess the importance of specific attachment for infectivity of PrV gD− Pass, the gene encoding gC was deleted, resulting in mutant PrV gCD− Pass. Deletion of both known attachment proteins reduced specific infectivity compared to wild-type PrV by more than 10,000-fold. Surprisingly, the virus mutant still retained significant infectivity and could be propagated on normal noncomplementing cells, indicating the presence of another receptor-binding virion protein. Selection of bovine kidney (MDBK) cells resistant to infection by PrV gCD− Pass resulted in the isolation of a cell clone, designated NB, which was susceptible to infection by wild-type PrV but refractory to infection by either PrV gCD− Pass or PrV gD− Pass, a defect which could partially be overcome by polyethylene glycol (PEG)-induced membrane fusion. However, even after PEG-induced infection plaque formation of PrV gCD− Pass or PrV gD− Pass did not ensue in NB cells. Also, phenotypic gD complementation of PrV gCD− Pass or PrV gD− Pass rescued the defect in infection of NB cells but did not restore plaque formation. Glycosaminoglycan analyses of MDBK and NB cells yielded identical results, and NB cells were normally susceptible to infection by other alphaherpesviruses as well as vesicular stomatitis virus. Infectious center assays after PEG-induced infection of NB cells with PrV gD− Pass on MDBK cells indicated efficient exit of virions from infected NB cells. Together, our data suggest the presence of another receptor and receptor-binding virion protein which can mediate PrV entry and cell-to-cell spread in MDBK cells.
Attachment of herpesviruses to target cells is mediated by viral glycoproteins which are embedded in the virion envelope and interact with cellular surface components acting as virus receptors. The best-characterized herpesvirus-cell interaction is the binding of glycoprotein gp350/220 of the gammaherpesvirus Epstein-Barr virus to the B-lymphocyte surface protein CD21, also designated complement receptor 2 (28). Among the alphaherpesviruses, initial interaction between the virion and the target cell involves binding of glycoprotein C (gC) to cell-surface glycosaminoglycans, in particular, heparan sulfate, as components of proteoglycans. This heparan sulfate interaction has been observed for herpes simplex virus type 1 (HSV-1) and HSV-2 (7, 41, 48), pseudorabies virus (PrV) (23, 36), varicella-zoster virus (50), and bovine herpesvirus 1 (BHV-1) (20, 29). In addition, the gammaherpesvirus bovine herpesvirus 4 (BHV-4) (45) and the betaherpesviruses human cytomegalovirus (HCMV) (2) and human herpesvirus 7 (40) have been reported to interact with heparan sulfate proteoglycans during attachment.
Receptor-binding activity has also been shown for gD of HSV-1, PrV, and BHV-1. Soluble HSV-1 gD binds to a saturable number of receptors on the surface of target cells (9), and gD of HSV-1, PrV, and BHV-1 is required for a secondary, stable binding which is no longer sensitive to competition by exogenous heparin (11, 22). Recently, a member of the tumor necrosis factor receptor family, designated herpesvirus entry mediator (HVEM), has been identified which functions in entry of HSV-1 into partially resistant chinese hamster ovary (CHO) cells (27). The viral ligand for HVEM is gD (47). Whereas gC is not required for productive replication of HSV-1, PrV, or BHV-1 and is, therefore, regarded as nonessential, the presence of gD is necessary for replication of wild-type strains of these viruses (24, 42).
Thus, in the absence of gC, attachment could be mediated by gD. Moreover, for HSV-1 it has been shown that heparan sulfate-binding of gC− virions is mediated by the essential gB (8). This indicates that gC-negative HSV-1 is still able to infect cells via a heparan sulfate-dependent pathway. In contrast, gC represents the only PrV virion glycoprotein capable of interacting productively with cell surface heparan sulfate for mediating infection (12). Neither PrV gB nor heterologous BHV-1 gB exhibits heparan sulfate-binding activity in gC-negative PrV virions (14). Therefore, for PrV only two virion proteins have been reported to play a role in attachment, gC and gD.
Evidence for the capability to bind to cellular surface proteins has also been reported for gH of HCMV (13) and gB of BHV-1 (19, 46). However, neither of the postulated receptors has been characterized.
In wild-type PrV, gD is required for penetration but not for direct cell-to-cell spread (30, 32) which allowed copassaging of gD− PrV-infected cells with noninfected cells. Using this approach, we recently isolated an infectious gD-negative PrV mutant, PrV gD− Pass (37). Similar results have also been obtained for BHV-1 (39). Infectivity of PrV gD− Pass is not dependent on the presence of gD, and viral titers of PrV gD− Pass reach up to 107 PFU/ml. We were interested in analyzing the importance of the other attachment protein, gC, for infectivity of PrV gD− Pass. We report here the construction of a mutant of PrV gD− Pass with a deletion of gC and its characterization in cell culture. We also isolated an MDBK cell clone which is specifically refractory to infection by the infectious gD− PrV mutants due to a defect in entry.
MATERIALS AND METHODS
Viruses and cells.
Virus mutants were derived from the wild-type PrV strain Kaplan (PrV-Ka) (10). The gG− gD− PrV mutants 133 (PrV-gD−) (32) and PrV gD− Pass (37) have been described previously. Both mutants carry a gG–β-galactosidase expression cassette at the gG locus (25) as does PrV-1112 (25), which replicates like wild-type PrV. PrV-gC−, a deletion mutant lacking most of the gC gene and the 3′ end of the upstream UL43 gene, has been described previously (12). BHV-1 was obtained from G. Keil, vesicular stomatitis virus (VSV) was obtained from H. Schirrmeier, and equine herpesvirus 1 (EHV-1) was obtained from N. Osterrieder (all from the Federal Research Centre for Virus Diseases of Animals, Insel Riems, Germany). The HSV-1 strain KOS was obtained from P. Spear, Northwestern University, Chicago, Ill. Viruses were propagated on porcine (PSEK), bovine (MDBK), or African green monkey kidney (Vero) cells. For detection of β-galactosidase activity, monolayers were fixed and overlaid with staining solution containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (34). Phenotypic gD complementation of gD-negative viruses was achieved by propagation of the respective virus mutant on PrV gD-expressing cells (32).
Southern blot hybridization.
Southern blotting was performed by standard procedures (33) using 32P-labelled hybridization probes.
Virus purification and immunoblot.
Monoclonal antibodies (MAbs) against gB, gC, gD, and gH (15) were used. Proteins of sucrose gradient-purified virions (16) were analyzed by Western blotting (43) after electrophoresis in sodium dodecyl sulfate–10% polyacrylamide gels (17) under reducing conditions. Specific infectivities of virion preparations were determined by calculating particle numbers in preparations of gradient-purified virions obtained from supernatants of infected cells, based on their DNA content (37). Virion preparations were routinely assayed by electron microscopy for purity and presence of enveloped virions.
Glycosaminoglycan differentiation.
Analysis of glycosaminoglycan composition was performed according to a protocol by Yamagata et al. (49) as modified by Gressner et al. (5). Briefly, Na2 35SO4-labelled proteoglycans were extracted from cell cultures, and glycosaminoglycans were purified by ion-exchange chromatography after digestion of the protein moiety. Distribution of heparan sulfate and chondroitin sulfate was determined by digestion with heparinase-heparitinase or chondroitinase ABC and quantitated by measuring radioactivity in reaction products.
Titration and infectious-center assay.
To determine virus titers, MDBK cells were infected with serial dilutions of virus suspensions and incubated at 37°C for 1 h. Thereafter, the inoculum was removed and cells were overlaid with semisolid methylcellulose medium. Cells were stained with crystal violet, by immunostaining (for HSV-1), or by X-Gal overlay after 2 or 3 days of incubation at 37°C. Plaques or infected single cells were then quantitated. For infectious-center assays, two wells of a six-well tissue culture dish were inoculated with an appropriate virus dilution containing between 200 and 500 PFU. Since NB cells are normally not susceptible to PrV gD− Pass and PrV gCD− Pass, they were infected with undiluted stock solutions of these viruses. After incubation at 37°C for 1 h, polyethylene glycol (PEG) fusion was performed in one well, as described below, and control cells were treated with cell culture medium instead of PEG. Extracellular virus was then inactivated by treatment with citrate buffer (CBS) (40 mM citric acid–sodium citrate [pH 3.0], 10 mM KCl, 135 mM NaCl) for 1 min. Cells were washed with phosphate-buffered saline, trypsinized, resuspended in medium, and coseeded with MDBK cells in a 10-cm-diameter culture dish. Cells were stained with X-Gal after 2 days, and plaques were counted.
PEG-induced fusion.
For PEG fusion experiments (35) cells were inoculated with the respective virus suspension and incubated at 37°C for 1 h. The inoculum was then removed, and cells were washed with phosphate-buffered saline and overlaid for 30 s with PEG50 (50% PEG 6000 in modified Eagle medium [MEM]). PEG was removed by consecutive washes with a 1:2 and 1:4 dilution in MEM of PEG50, followed by three washes in MEM supplemented with 5% fetal calf serum. Cells were further incubated for 2 days at 37°C prior to X-Gal staining.
RESULTS
Isolation and characterization of PrV gCD− Pass.
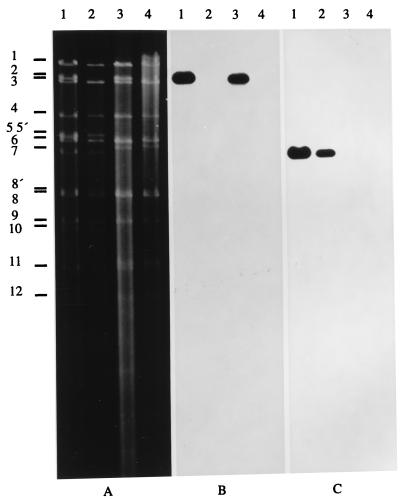
To isolate a gC− variant of PrV gD− Pass, DNA of purified PrV gD− Pass virions was cotransfected into Vero cells with plasmid TN90/3 (38) in which most of the gC gene and the 3′ end of the UL43 gene has been deleted. Virus progeny was enriched for gC-negative viruses by complement-mediated neutralization with an anti-gC MAb. Surviving viruses were plated onto Vero cells, and five plaques were randomly picked. Southern blot analysis showed that all five plaques contained the desired deletion in the gC gene. One isolate, designated PrV gCD− Pass, was further tested. As shown in Fig. 1, after agarose gel electrophoresis (Fig. 1A) of BamHI-digested DNA of PrV-Ka (Fig. 1, lanes 1), PrV-gC− (Fig. 1, lanes 2), PrV gD− Pass (Fig. 1, lanes 3), and PrV gCD− Pass (Fig. 1, lanes 4), hybridization with a gC gene-specific probe (Fig. 1B) yielded the expected signals in the gC+ viruses whereas it failed to hybridize to DNA of the gC− viruses. Moreover, BamHI fragment 2 containing the gC gene shifted to the size of BamHI fragment 3 (Fig. 1A, lanes 2 and 4) due to the introduction of the ca. 1.4-kbp deletion. Hybridization with a gD-specific probe was also performed (Fig. 1C). All gD+ viruses showed the expected signals, whereas the gD− viruses did not exhibit specific reactivity.
FIG. 1.
Genotypic characterization of PrV gCD− Pass. Virion DNA was isolated from PrV-Ka (lanes 1), PrV-gC− (lanes 2), PrV gD− Pass (lanes 3), and PrV gCD− Pass (lanes 4) and cleaved with BamHI, and resulting fragments were separated in a 0.8% agarose gel by electrophoresis. (A) Ethidium bromide-stained gel. After transfer to nylon filters, hybridization was performed with probes specific for the gC gene (B) or the gD gene (C). Positions of BamHI fragments of PrV-Ka DNA are indicated on the left.
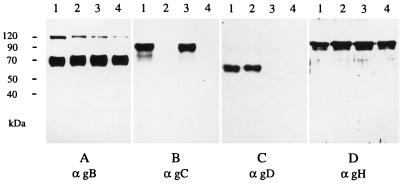
As an additional test, Western blotting was performed on lysates of purified virions of PrV-Ka (Fig. 2, lanes 1), PrV-gC− (Fig. 2, lanes 2), PrV gD− Pass (Fig. 2, lanes 3), and PrV gCD− Pass (Fig. 2, lanes 4). Whereas all virion preparations showed the presence of gB and gH, gC was detected in only the PrV-Ka and PrV gD− Pass virion preparations, and gD was present in only PrV-Ka and PrV-gC−. Specific infectivity of PrV gCD− Pass as determined on MDBK cells was approx. 800-fold lower than that of PrV gD− Pass (1.2 × 107 particles/PFU for PrV gCD− Pass versus 1.5 × 104 particles/PFU for PrV gD− Pass). In contrast, specific infectivity of PrV-gC− was reduced only ca. 50-fold compared to PrV-Ka (1.1 × 104 particles/PFU for PrV-gC− versus 2.3 × 102 particles/PFU for PrV-Ka). This indicates a stronger dependence for infection on the presence of gC in PrV gD− Pass than that in wild-type PrV-Ka, presumably due to the absence of the other known attachment protein, gD, in PrV gD− Pass. In summary, these data show that PrV gCD− Pass simultaneously lacks gC and gD. Deletion of both known attachment proteins strongly impairs virus infectivity. However, since PrV gCD− Pass was isolated and could be propagated on normal cells, these results imply that at least one additional PrV virion protein functions in mediating attachment of this virus mutant.
FIG. 2.
Protein profile of mutant PrV. Virions of wild-type PrV (lanes 1), PrV-gC− (lanes 2), PrV gD− Pass (lanes 3), and PrV gCD− Pass (lanes 4) were lysed, and proteins were separated by acrylamide gel electrophoresis. After transfer to nitrocellulose membranes, the filters were probed with MAbs specific for gB, gC, gD, and gH (α gB, αgC, αgD, and αgH, respectively). Bound antibody was visualized after incubation with peroxidase-conjugated secondary antibody by enhanced chemiluminescence recorded on X-ray film. The anti-gB antibody recognizes the uncleaved precursor as well as one of the two proteolytic cleavage products.
Selection of an MDBK cell clone resistant to infection by PrV gCD− Pass.
MDBK-derived cell clones resistant to infection by PrV gCD− Pass were selected by a procedure adapted from Tufaro et al. (44). Approximately 4 × 107 cells at 80% confluency in a 162-cm2 tissue culture flask were mutagenized by treatment with methylethyl sulfonic acid for 18 h. Cells were cultivated for 24 h at 37°C and split 1:4. Three days later, the resulting four tissue culture flasks were infected with PrV gCD− Pass at a multiplicity of infection of 0.01. Cell cultures were washed three times a day with MEM–10% FCS for the following 7 days. Thereafter, fresh medium was added and cells were incubated at 37°C for 14 days. Colonies of surviving cells were trypsinized and cloned by limiting dilution in 96-well plates. Single-cell clones were grown with conditioned medium for the next 7 to 14 days, followed by further propagation in standard MEM. Individual cell clones were tested for plating efficiencies of PrV-1112, PrV-gC−, PrV gD− Pass, and PrV gCD− Pass in comparison to MDBK cells. Mutant cell clones showing reductions in titer were recloned, and individual clones were judged as free from input PrV gCD− Pass when all of the following tests were negative: (i) indirect immunofluorescence using a polyspecific anti-PrV goat hyperimmune serum; (ii) plating of supernatants from the cell clones on porcine kidney (PK) and MDBK cells and staining with crystal violet and X-Gal after 2 days; (iii) cocultivation of resistant cell clones with PK and MDBK cells for 4 days and staining with crystal violet and X-Gal; and (iv) PCR for a 377-bp fragment of the UL51 gene (18), which reliably allows the detection of a single infected cell in 104 noninfected cells. One cell clone, designated NB, which exhibited the strongest reduction in plating efficiency of PrV gD− Pass and PrV gCD− Pass was selected for further experiments.
NB cells are not susceptible to infection by gD− passaged virus mutants.
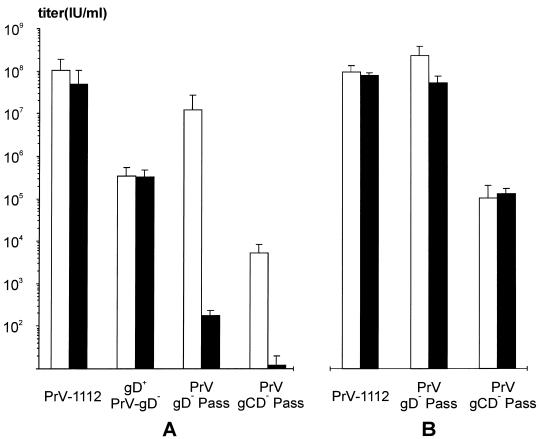
To test susceptibility of NB cells for infection, wild-type-like PrV-1112, phenotypically gD-complemented unpassaged PrV gD−, PrV gD− Pass, and PrV gCD− Pass were titrated on MDBK and NB cells. Results are shown in Fig. 3A. Whereas wild-type PrV and phenotypically gD-complemented PrV-gD− produced plaques on both cell lines with similar efficiencies, PrV gD− Pass and PrV gCD− Pass induced plaque formation only on MDBK cells but not on NB cells. Titers on MDBK cells were ca. 107 PFU/ml for PrV gD− Pass and ca. 104 PFU/ml for PrV gCD− Pass. It is especially noteworthy that even after infection of NB cells with undiluted stocks of either virus, which corresponds to a multiplicity of infection of 10 for PrV gD− Pass and 0.1 for PrV gCD− Pass, no plaques were observed. After careful visual examination only few blue-staining single infected cells could be detected, which amounted to ca. 200 for PrV gD− Pass and <20 for PrV gCD− Pass. Phenotypic gD complementation of PrV gD− Pass and PrV gCD− Pass restored infectivity on NB cells and led to an ∼10-fold increase in infectivity on MDBK cells (Fig. 3B). However, plaque formation still did not ensue in NB cells.
FIG. 3.
Plating efficiency of mutant PrV on MDBK and NB cells. Titers of wild-type-like PrV-1112, phenotypically gD-complemented PrV gD− (gD+ PrV-gD−), PrV gD− Pass, and PrV gCD− Pass were determined on MDBK cells (white bars) or NB cells (black bars) by plaque assay and X-Gal staining (A). (B) Plating efficiencies of PrV-1112, PrV gD− Pass, and PrV gCD− Pass on MDBK and NB cells after propagation on a PrV gD-expressing cell line. Average values and standard variations (error bars) of three independent experiments are shown. This corresponds to PFU on MDBK cells and to infected single cells after infection of NB cells with PrV gD− Pass and PrV gCD− Pass. These virus mutants do not form plaques on NB cells irrespective of phenotypic gD complementation.
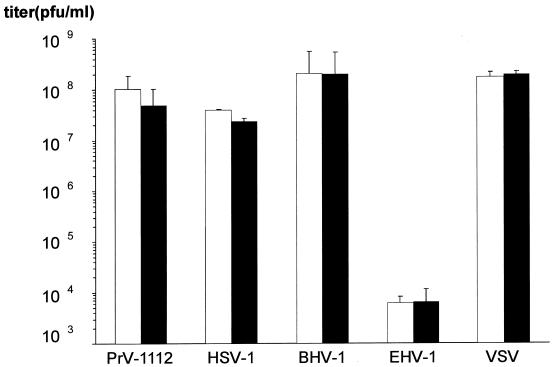
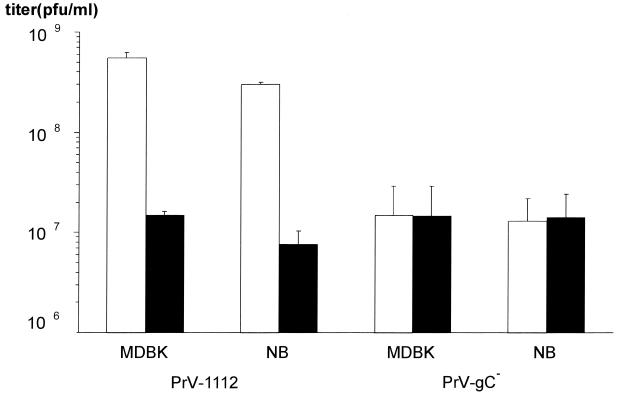
To determine the ability of other viruses to form plaques on NB cells, MDBK and NB cells were infected with PrV, HSV-1, BHV-1, EHV-1, and VSV. As shown in Fig. 4, all viruses induced similar numbers of plaques on either cell line, and no defect in infection of NB cells was observed. Titers for EHV-1 are low on both cells since EHV-1 does not grow well on bovine cells. Together these data indicate that NB cells exhibit a striking restriction in infection which is specific for the infectious gD− mutants.
FIG. 4.
Plating efficiencies of different viruses on MDBK and NB cells. Alphaherpesviruses PrV-1112, HSV-1, BHV-1, and EHV-1 as well as the rhabdovirus VSV were titrated on MDBK and NB cells by plaque assay. Average titers and standard variations (error bars) of three independent experiments are shown.
gD− infectious PrV is unable to enter NB cells.
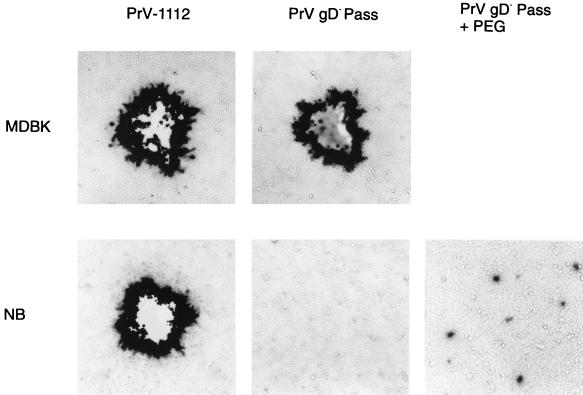
To assay whether the restriction in infectivity of the passaged gD-negative virus mutants on NB cells occurs at the level of entry, MDBK and NB cells were inoculated with PrV gD− Pass and PrV gCD− Pass, and membrane fusion was experimentally induced by PEG. PEG-mediated fusion enhanced the number of infected NB cells by ca. 200-fold and 1,000-fold, respectively (Fig. 5 and 6). In contrast, titers on MDBK cells were not affected by PEG treatment. Thus, at least part of the restriction appears due to a defect in entry of the gD− infectious PrV mutants. However, even after PEG-induced entry of the infectious gD− virus mutants, only single blue-staining infected NB cells were observed (Fig. 5).
FIG. 5.
Infectivity of mutant PrV on MDBK and NB cells. MDBK and NB cells in six-well tissue culture plates were infected with 1 ml of a PrV-1112 stock diluted 1/106 or with 1 ml of a PrV gD− Pass stock diluted 1/106 (MDBK cells) or 1/10 (NB cells). Both stock solutions contained 107 PFU of the respective virus per ml as determined on MDBK cells. Two days after infection cells were stained with X-Gal. In this experiment, no plaques or single infected cells were observed after infection of NB cells with PrV gD− Pass. After PEG-induced fusion (+ PEG) the amount of infected single cells increased but plaque formation did not ensue.
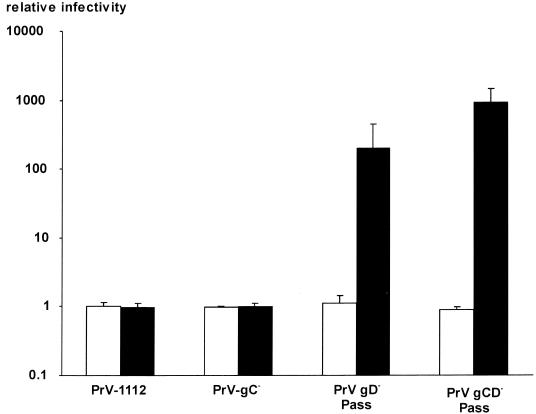
FIG. 6.
PEG-induced infectivity of PrV on MDBK and NB cells. MDBK (white bars) and NB cells (black bars) were infected with serial dilutions of PrV-1112, PrV-gC−, PrV gD− Pass, and PrV gCD− Pass. After X-Gal staining, infectious titers were determined by counting either plaques (for PrV-1112 and PrV-gC− on both cell lines and for PrV gD− Pass and PrV gCD− Pass on MDBK cells) or single infected cells (for PrV gD− Pass and PrV gCD− Pass on NB cells). Relative infectivities and standard variations (error bars) compared to control plates which were not treated with PEG are indicated.
NB cells do not differ from MDBK cells in glycosaminoglycan composition.
Infection of target cells by PrV is initiated by interaction of gC with cell-surface heparan sulfate. To examine whether the entry defect of gD− infectious PrV mutants in NB cells is associated with a difference in glycosaminoglycans, radiolabelled proteoglycans were extracted, protein moieties were digested with papain, and the amount of heparan sulfate and chondroitin sulfate was determined after digestion with heparinase-heparitinase or chondroitinase. No significant differences in the amount of total radiolabelled material were detected between MDBK and NB cells (ca. 106 cpm in 105 cells). In both cell lines, ∼50% of radioactively labelled material was sensitive to digestion with heparinase-heparitinase and ∼30% was digested with chondroitinase (21). Thus, NB cells exhibit no gross defect in glycosaminoglycan synthesis, indicating that the primary receptor for PrV is present in comparable amounts in both cell lines. This is further demonstrated by a comparable inhibition of plaque formation by exogenous heparin of gC+ PrV-1112 and insensitivity of gC− PrV toward heparin inhibition on both cell lines (Fig. 7).
FIG. 7.
Heparin inhibition. PrV-1112 and PrV-gC− were titrated on MDBK and NB cells in the presence (black bars) and absence (white bars) of heparin (50 μg/ml). The addition of heparin reduced titers of gC+ PrV-1112 on both cell lines to a similar extent, whereas PrV-gC− was not affected by the presence of heparin on either cell line. Average values and standard variations (error bars) of three independent experiments are shown.
Egress of gD− infectious PrV from NB cells is not impaired.
NB cells are refractory to entry of gD− infectious PrV. After PEG-induced fusion the number of infected cells increased but plaque formation did not ensue. To analyze whether this phenotype reflects an additional egress defect, NB cells were infected with PrV gD− Pass and PrV gCD− Pass by PEG fusion. One parallel well was then overlaid with methylcellulose medium and stained with X-Gal after 2 days. Cells from the other well were trypsinized and reseeded with susceptible MDBK cells. Monolayers were then also stained with X-Gal after 2 days. As shown in Table 1, the number of infected single cells in the NB monolayer and the number of plaques formed in the infectious center assay correlated quite well, which indicates that virus is released from infected NB cells and is able to enter neighboring susceptible MDBK cells.
TABLE 1.
Comparison between PEG-induced infectivity and infectious centersa
| Virus | MDBK
|
NB
|
||
|---|---|---|---|---|
| Infectivity | Infect. centers | Infectivity | Infect. centers | |
| PrV-1112 | 1.0 × 108 | 1.0 × 108 | 8.9 × 107 | 4.0 × 107 |
| 9.3 × 107 | 9.9 × 107 | 8.4 × 107 | 3.7 × 107 | |
| PrV gD− Pass | 4.0 × 106 | 2.1 × 106 | 9.2 × 105 | 2.9 × 105 |
| 5.6 × 104 | 9.0 × 104 | 9.3 × 104 | 4.6 × 104 | |
| PrV gCD− Pass | 1.2 × 103 | 1.3 × 103 | 3.9 × 102 | 2.9 × 102 |
| 7.8 × 103 | 3.7 × 103 | 9.8 × 102 | 8.3 × 102 | |
MDBK and NB cells were inoculated with serial dilutions of the indicated virus stock and treated with PEG. Thereafter, one well was stained after 48 h with X-Gal and plaques or infected single cells (for PrV gD− Pass and PrV gCD− Pass on NB cells) were counted. A parallel well was trypsinized and reseeded together with excess MDBK cells. At 48 h after infection, plaques were stained with X-Gal and counted. Indicated are titers determined directly (Infectivity) compared to titers determined in infectious-center assays (Infect. centers). Representative data (in infectious units per milliliter of virus suspension) from two independent experiments each are shown.
DISCUSSION
Initiation of infection by herpesviruses requires interaction between virion glycoproteins and cellular receptors. For alphaherpesviruses, several virion envelope constituents have been implicated in receptor binding. gC of HSV-1, PrV, and BHV-1 binds to cell surface heparan sulfate, resulting in an initial interaction which is sensitive to competition with exogenous heparin (7, 23, 29). This first binding converts into a more stable attachment via gD (11, 22). In addition, HSV-1 virion gB has also been shown to mediate attachment by interaction with heparan sulfate (8). Thus, three receptor binding proteins have been identified in HSV-1. In contrast, in PrV virion gB does not productively interact with heparan sulfate (12), and only gC and gD of PrV are thought to interact with cellular receptors (11, 23). The isolation of a PrV mutant which is infectious even in the absence of gC and gD indicates that another virion component(s) must also have or be able to acquire receptor binding activity. These studies were possible due to the isolation of a PrV mutant, PrV gD− Pass, which by copassaging of infected with noninfected cells, acquired the ability to replicate productively in the absence of gD (37). This was surprising since gD had hitherto been regarded as a glycoprotein which is required for infectious entry of PrV (30, 32). Interestingly, PrV gD is not necessary for direct cell-to-cell spread, a prerequisite for the copassaging experiment (30, 32).
Deletion of gC from wild-type PrV reduced specific infectivity by ∼50-fold (12). In contrast, deletion of gC from PrV gD− Pass reduced specific infectivity by ∼800-fold, demonstrating a higher degree of dependence on gC-heparan sulfate interaction in the gD− virions than in gD+ virions. This further supports our previous data indicating that in the absence of gC, attachment of virions to target cells is mainly mediated by gD (12). Obviously, in the absence of gD, primary gC-dependent attachment becomes of paramount importance for the virus to bind to its target cell. Thus, the presence of either gC or gD is necessary for efficient initial virus-cell contact.
However, since even virions lacking both gC and gD are able to infect target cells, though with a strikingly reduced efficiency, an additional or alternative virion protein-cell receptor interaction has to be postulated, if it is assumed that nonspecific binding of virions to target cells does not occur. gB and the gH/gL complex have been shown to be required for entry of virus into target cells and direct viral cell-to-cell spread (1, 30–32). We hypothesize that in the absence of gC and gD, one of these proteins might provide the relatively inefficient attachment function in PrV gCD− Pass. Both, gB and gH of other herpesviruses, i.e., BHV-1 and HCMV, respectively, have been postulated to bind to proteinaceous cell surface receptors (13, 19, 46).
Recently, expression cloning has been successfully used to identify a cell surface protein, HVEM (27), which belongs to the tumor necrosis factor alpha receptor family and is able to mediate HSV-1 infection of CHO cells by binding to virion gD (47). A basic requirement for this approach is the availability of cells with a restriction of virus infection at the level of entry. Unfortunately, PrV exhibits a very wide host range in vitro. Therefore, we selected for mutant cell clones which are specifically resistant to infection by PrV. Infection with a gC− virus mutant should avoid selection for cells exhibiting defects in proteoglycan biosynthesis, as has been observed before (6, 26). In addition, selection with infectious gD− PrV was used to gain evidence for the presence of novel receptors which do not interact with either gC or gD. The NB cells described here exhibit a block in entry of PrV gD− Pass and PrV gCD− Pass as indicated by PEG fusion experiments. In contrast, these cells are fully permissive for several other PrV glycoprotein mutants (e.g., PrV-gM− [3] and PrV-gE− [25] [data not shown]), as well as for other alphaherpesviruses and VSV. Thus, the defect in NB cells is specific for entry of gD− infectious PrV mutants, which indicates that it affects a cellular component which is critical for infectivity of these particular mutants. The NB cell phenotype also further supports our hypothesis that gD− infectious PrV mutants use an additional, or alternative, receptor for entry (37).
As regards relevance of our findings for the entry pathway of wild-type PrV, there are several scenarios to consider. First, gD− infectious PrV mutants may have acquired during the passaging process a novel receptor binding activity which is not present in wild-type PrV virions. This would be indicative of an experimentally induced alteration in use of cell surface receptors. Second, gD− infectious PrV mutants may be dependent on the use of a receptor which is not critical for wild-type virus infection due to the presence of other virion-cell interactive proteins. The presence of receptors which act “downstream” from the gC and gD interactions presumably by binding to either gH/L or gB has been postulated (4). It is conceivable that in the absence of gC and gD, any other receptor-binding activity gains importance, especially when involved in mediating penetration. From our data it is evident that gD− infectious PrV mutants are defective in entry into NB cells despite the propensity, at least for PrV gD− Pass, to efficiently bind to these cells via gC (data not shown). Thus, we hypothesize that the defect in NB cells abolishes function of a “fusion receptor” which is essential for penetration of gD− mutant viruses. In this context it is important to note that both passaged virus mutants are still efficiently neutralized by antibodies against gH and gL, indicating that these two proteins are relevant for entry of wild-type and mutant viruses (data not shown).
Infectivity of wild-type PrV and several other PrV glycoprotein mutants is not impaired on NB cells, which could be interpreted as if the phenotype of NB cells is irrelevant for the entry process of these viruses. However, it is conceivable that there is redundancy in receptor binding by wild-type PrV, as already shown by continued attachment of virions lacking gC or gD to target cells. Thus, the entry pathway requiring the function defective in NB cells may be bypassed by wild-type PrV but not by gD− infectious PrV mutants. Phenotypic complementation of passaged gD-negative PrV mutants by propagation on gD-expressing cells quantitatively restored infectivity of these mutants on NB cells, showing that in the presence of gD, these virus mutants enter cells via the normal pathway. However, as expected, phenotypically gD-complemented gD-negative passaged virus mutants were still not able to form plaques in NB cells.
Especially striking is the prominent phenotype of NB cells as regards susceptibility to infection compared to parental MDBK cells. Infectivity of PrV gD− Pass on NB cells is reduced ca. 105-fold, which effectively means that these cells are not permissive for PrV gD− Pass infection. A similarly striking reduction was observed for PrV gCD− Pass. Thus, NB cells represent a cell clone with a specific entry defect for PrV at a magnitude which, presumably, allows expression screening for receptors as used by Montgomery et al. (27). It is important to note that infectivity of other alphaherpesviruses as well as the nonrelated rhabdovirus VSV is not inhibited in NB cells, further providing specificity of the phenotypic alteration.
Our data also indicate that egress from NB cells of gD− infectious PrV mutants occurs as shown by infectious-center assay. However, plaque formation in NB cells does not ensue even after PEG-induced infection. Thus, it appears as if the defect in plaque formation is correlated with the defect in entry and does not reflect a simultaneous impairment of egress. This highlights the relationship between entry and direct cell-to-cell spread and yields evidence that similar cellular functions are involved in both processes. Interestingly, after infection with gD-complemented PrV-gD−, plaques did form on NB cells; i.e., cell-to-cell spread on NB cells can occur in the absence of gD. Presumably, the mutation(s) leading to gD-independent infectivity of PrV gD− Pass at the same time abolished a function which is necessary for gD-independent cell-to-cell spread in NB cells. Whether these two phenotypes are consequences of the same mutational event remains to be determined.
Deletion of both known attachment proteins of PrV, gC and gD, drastically reduces infectivity of PrV, although it does not completely abolish it. Most importantly, PrV gCD− Pass can be propagated on normal cells without the danger of inadvertent rescue of either mutation. The isolation of PrV gCD− Pass now allows new approaches to specifically alter the host range of PrV. Incorporation of heterologous attachment proteins into PrV gCD− Pass virions could favor attachment to alternative target cells, which is especially intriguing in light of the use of herpesviruses for gene therapy. Experiments to analyze the potential of our gCD− infectious PrV mutant in this context are under way.
ACKNOWLEDGMENTS
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Me 854/4-1).
We thank B. Bettin for expert technical assistance.
REFERENCES
- 1.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 2.Compton T, Nowlin D L, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 3.Dijkstra J, Gerdts V, Klupp B G, Mettenleiter T C. Deletion of glycoprotein gM of pseudorabies virus results in attenuation for the natural host. J Gen Virol. 1997;78:2147–2151. doi: 10.1099/0022-1317-78-9-2147. [DOI] [PubMed] [Google Scholar]
- 4.Fuller A O, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gressner A M, Pazen H, Greiling H. The biosynthesis of glycosaminoglycans in normal rat liver and in response to experimental hepatic injury. Hoppe-Seyler’s Z Physiol Chem. 1977;358:825–833. doi: 10.1515/bchm2.1977.358.2.825. [DOI] [PubMed] [Google Scholar]
- 6.Gruenheid S, Gatzke L, Meadows H, Tufaro F. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J Virol. 1993;67:93–100. doi: 10.1128/jvi.67.1.93-100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold B C, Visalli R J, Susmarski N, Brandt C, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 9.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan A S, Vatter A. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959;13:78–92. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 11.Karger A, Mettenleiter T C. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology. 1993;194:654–664. doi: 10.1006/viro.1993.1305. [DOI] [PubMed] [Google Scholar]
- 12.Karger A, Saalmüller A, Tufaro F, Banfield B W, Mettenleiter T C. Cell surface proteoglycans are not essential for infection by pseudorabies virus. J Virol. 1995;69:3482–3489. doi: 10.1128/jvi.69.6.3482-3489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keay S, Merigan T C, Rasmussen L. Identification of cell surface receptors for the 86-kilodalton glycoprotein of human cytomegalovirus. Proc Natl Acad Sci USA. 1989;86:10100–10103. doi: 10.1073/pnas.86.24.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klupp B G, Karger A, Mettenleiter T C. Bovine herpesvirus 1 glycoprotein B does not productively interact with cell surface heparan sulfate in a pseudorabies virion background. J Virol. 1997;71:4838–4841. doi: 10.1128/jvi.71.6.4838-4841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klupp B G, Fuchs W, Weiland E, Mettenleiter T C. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J Virol. 1997;71:7687–7695. doi: 10.1128/jvi.71.10.7687-7695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp A, Mettenleiter T C. Stable rescue of a glycoprotein gII deletion mutant of pseudorabies virus by glycoprotein gI of bovine herpesvirus 1. J Virol. 1992;66:2754–2762. doi: 10.1128/jvi.66.5.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lenk M, Visser N, Mettenleiter T C. The pseudorabies virus UL51 gene product is a 30-kilodalton virion component. J Virol. 1997;71:5635–5638. doi: 10.1128/jvi.71.7.5635-5638.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, van Drunen Littel-van den Hurk S, Babiuk L A, Liang X. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 1995;69:4758–4768. doi: 10.1128/jvi.69.8.4758-4768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang X, Babiuk L A, Zamb T. Mapping of heparin-binding structures on bovine herpesvirus 1 and pseudorabies virus gIII glycoproteins. Virology. 1993;194:233–243. doi: 10.1006/viro.1993.1254. [DOI] [PubMed] [Google Scholar]
- 21.Linker A, Hovingh P. Heparinase and heparitinase from flavobacteria. Methods Enzymol. 1972;28:902–911. [Google Scholar]
- 22.McClain D S, Fuller A O. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994;198:690–702. doi: 10.1006/viro.1994.1081. [DOI] [PubMed] [Google Scholar]
- 23.Mettenleiter T C, Zsak L, Zuckermann F, Sugg N, Kern H, Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990;64:278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mettenleiter T C. Initiation and spread of α-herpesvirus infections. Trends Microbiol. 1994;2:2–4. doi: 10.1016/0966-842x(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter T C, Rauh I. A glycoprotein gX-β-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990;30:55–66. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- 26.Mettenleiter, T. C., B. G. Klupp, and A. Karger. Unpublished results.
- 27.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 28.Nemerow G, Wolfert R, McNaughton M, Cooper N R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2) J Virol. 1985;55:347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okazaki K, Matsuzaki T, Sugahara Y, Okadad J, Hasebe M, Iwamura Y, Ohnishi M, Kanno T, Shimizu M, Honda E, Kono Y. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparin-like moiety on the cell surface. Virology. 1991;181:666–670. doi: 10.1016/0042-6822(91)90900-v. [DOI] [PubMed] [Google Scholar]
- 30.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peeters B, deWind N, Broer R, Gielkens A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sanes J R, Rubenstein J L R, Nicolas J F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3313–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarmiento M, Haffey M, Spear P G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7 in virion infectivity. J Virol. 1979;29:1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawitzky D, Hampl H, Habermehl K-O. Comparison of heparin-sensitive attachment of pseudorabies virus (PRV) and herpes simplex virus type 1 and identification of heparin-binding PRV glycoproteins. J Gen Virol. 1990;71:1221–1225. doi: 10.1099/0022-1317-71-5-1221. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt J, Klupp B G, Karger A, Mettenleiter T C. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreurs C, Mettenleiter T C, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988;62:2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schröder C, Linde G, Fehler F, Keil G M. From essential to beneficial: glycoprotein D loses importance for replication of bovine herpesvirus 1 in cell culture. J Virol. 1997;71:25–33. doi: 10.1128/jvi.71.1.25-33.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Secchiero P, Sun D, de Vico A L, Crowley R W, Reitz M S, Hauli G, Lusso P, Gallo R C. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J Virol. 1997;71:4571–4580. doi: 10.1128/jvi.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tufaro F, Snider M D, McKnight S L. Identification and characterization of a mouse cell mutant defective in the intracellular transport of glycoproteins. J Cell Biol. 1987;105:647–657. doi: 10.1083/jcb.105.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderplasschen A, Bublot M, Dubuisson J, Pastoret P-P, Thiry E. Attachment of the gammaherpesvirus bovine herpesvirus 4 is mediated by the interaction of gp8 glycoprotein with heparinlike moieties on the cell surface. Virology. 1993;196:232–240. doi: 10.1006/viro.1993.1471. [DOI] [PubMed] [Google Scholar]
- 46.Varthakavi V, Minocha H C. Identification of a 56kDa putative bovine herpesvirus 1 cellular receptor by anti-idiotype antibodies. J Gen Virol. 1996;77:1875–1882. doi: 10.1099/0022-1317-77-8-1875. [DOI] [PubMed] [Google Scholar]
- 47.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968;243:1523–1535. [PubMed] [Google Scholar]
- 50.Zhu Z, Gershon M D, Ambron R, Gable C, Gershon A A. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose-6-phosphate and heparin. Proc Natl Acad Sci USA. 1995;92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]