Abstract
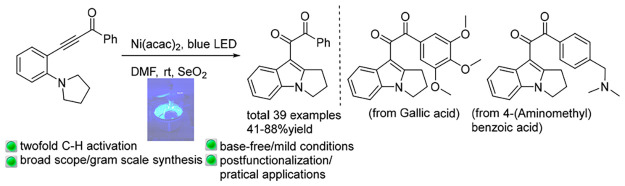
The combination of visible light catalysis and Ni catalysis has enabled the synthesis of indolyl phenyl diketones through the cyclization/oxidation process of ynones. This reaction proceeded under mild and base-free conditions and showed a broad scope and feasibility for gram-scale synthesis. Several natural products and biologically interesting molecules could be readily postfunctionalized by this method.
Keywords: diketones, photocatalysis, Csp3−H bond functionalization, ynones, nickel, cyclization
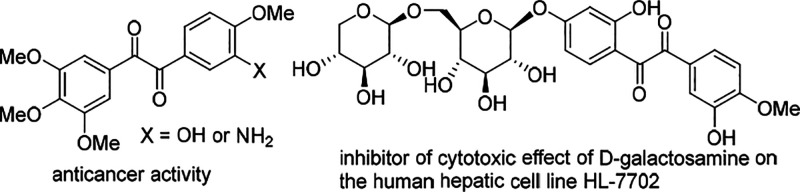
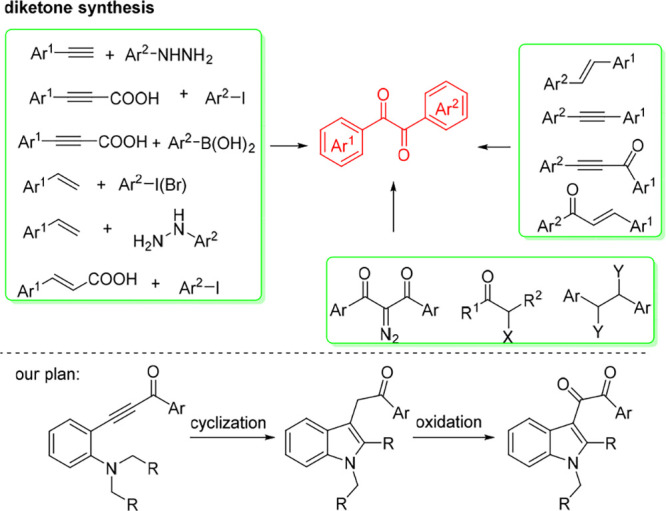
Aryl diketones, also called benzyls, are versatile synthetic intermediates in the synthesis of N-containing heterocycles1 and relevant compounds.2 They are ubiquitous subunits of natural products and biologically active molecules.3 For example, benzyls (Scheme 1, left) exhibited excellent antiproliferative activity on a nanomolar scale on four human tumor cell lines. There are three existing routes (Scheme 2) for the synthesis of diketones: (1) the oxidation of unsaturated motifs, such as alkynes, alkenes, ynones, and enones;4 (2) the coupling of unsaturated components with electrophiles (or nucleophiles);5 and (3) others, which include oxidation of diazo compounds, α-ketones, and alcohols (or halides).6 The main limitation of these methodologies is the requirement of the substituents present on diketones to be preinstalled on the starting material, itself. To increase the molecule complexity, it is highly desirable to realize simultaneous generation of substituents during diketone formation. With our continuous endeavors on ynone conversions,7 we envisioned that the cyclization of tertiary amine-substituted ynone through Csp3-H bond functionalization8,9 and subsequent oxidation would provide indolyl- and phenyl-substituted diketones. Toward this goal, herein, a visible-light-induced Ni-catalyzed strategy10 for the synthesis of indolyl11 phenyl diketones through double Csp3–H bond functionalization under base-free and mild conditions has been disclosed.
Scheme 1. Biologically Active Molecules with Diketone Structure.
Scheme 2. State of the Art on Synthesis of Diketones and Our Plan.
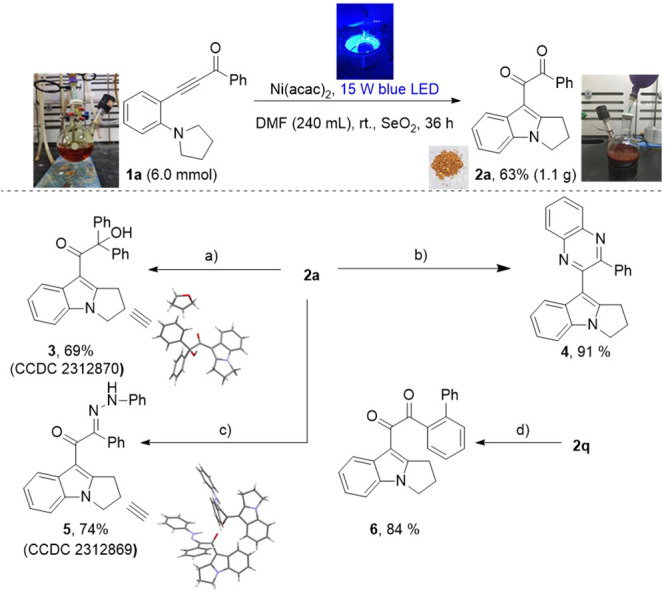
Initially, 1a was used as the model substrate. After extensively studying a series of reaction parameters, phenyl indolyl dione product 2a was obtained in 76% yield when 10 mol % of Ni(acac)2 was used as the catalyst and SeO2 (1.5 equiv) was used as the oxidant in DMF with irradiation of a 15 W blue LED lamp (464 nm) for 5 h (Table 1). The screening of different nickel catalysts showed that the expected product could be formed in decent yields only with Ni(cod)2 or NiCl2[P(CH3)3]2, whereas other Ni catalysts failed (entries 2–7). Irradiation with a 254 nm UV, 365 nm UV, or filament lamp as the light source provided only trace yields of the product (entries 8–10). Next, a range of solvents were screened (entries 11–16). The expected cyclization could proceed in EtOAc, o-xylene, and THF to produce 2a in 16%, 29%, and 50% yields, respectively. Next, a series of commonly used oxidants was tested (entries 17–25). With air, Cu(OAc)2, BPO, or AgOAc as the oxidant, 2a was produced in 15–49% yields, whereas other oxidants failed to give the product.
Table 1. Reaction Conditions Optimizationa.
| entry | change from the standard conditions | yield (%)b |
|---|---|---|
| 1 | none | 76 |
| 2 | NiCl2(PPh3)2 instead of Ni(acac)2 | trace |
| 3 | Ni(cod)2 instead of Ni(acac)2 | 40 |
| 4 | NiCl2[P(CH3)3]2 instead of Ni(acac)2 | 28 |
| 5 | Ni(dppf)Cl2 instead of Ni(acac)2 | trace |
| 6 | Ni(PPh3)4 instead of Ni(acac)2 | trace |
| 7 | NiCl2 instead of Ni(acac)2 | trace |
| 8 | 254 nm UV instead of blue LED | trace |
| 9 | 365 nm UV instead of blue LED | trace |
| 10 | filament lamp instead of blue LED | trace |
| 11 | DMSO instead of DMF | no reaction |
| 12 | o-xylene instead of DMF | 29 |
| 13 | THF instead of DMF | 50 |
| 14 | dioxane instead of DMF | trace |
| 15 | EtOAc instead of DMF | 16 |
| 16 | DCE instead of DMF | trace |
| 17 | air instead of SeO2 | 49 |
| 18 | CuCl2 instead of SeO2 | |
| 19 | Cu(OAc)2 instead of SeO2 | 20 |
| 20 | BQ instead of SeO2 | |
| 21 | MnO2 instead of SeO2 | |
| 22 | m-CPBA instead of SeO2 | |
| 23 | BPO instead of SeO2 | 15 |
| 24 | t-BuOOH instead of SeO2 | |
| 25 | AgOAc instead of SeO2 | 30 |
Ynone 1a (0.2 mmol, 1.0 equiv) and catalyst (0.02 mmol, 10 mol %) in solvent (2 mL) under irradiation using light source with oxidant (0.3 mmol, 1.5 equiv) at room temperature for 5 h.
Isolated yield.
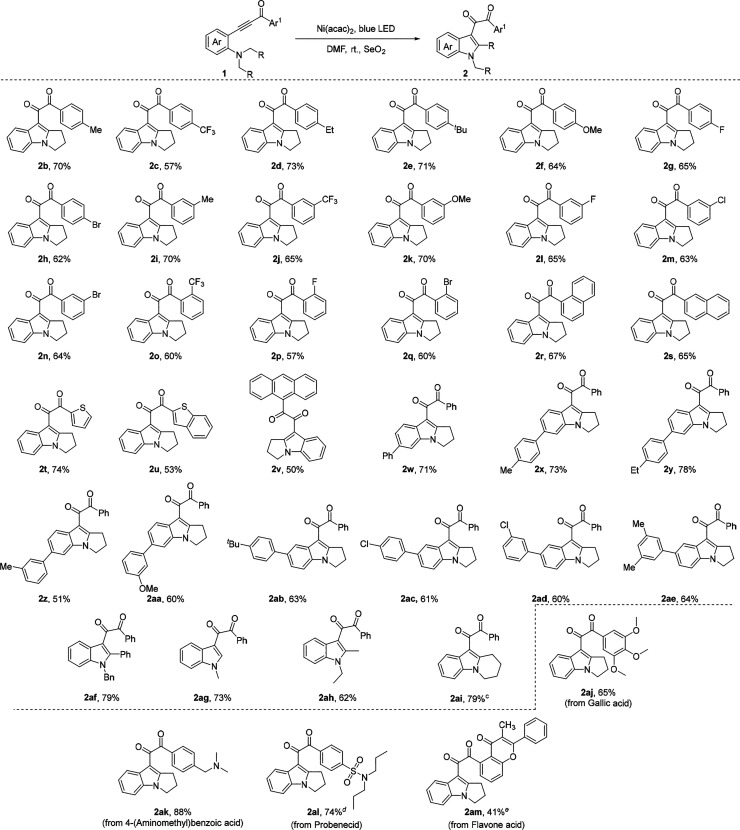
After the establishment of optimal reaction conditions, the scope of various substituted ynones with a pyrrolidinyl group for the cyclization reaction was tested (Scheme 3). Ynones with substituents on both the benzoyl group and aniline smoothly underwent the expected diketone formation reaction and assembled into the corresponding products in 51–78% yields. Functional groups, such alkyl (2b–e, 2i), highly useful halides (2g,h; 2l–n; 2p,q), methoxyl (2f, 2k), and CF3 (2c, 2j, 2o) on the benzoyl group, were well tolerated and afforded the desired products in moderate to good yields. In general, the cyclization–oxidation reaction was not sensitive to the electronic nature of the substituent. In addition, ynones bearing naphthyl group provided the desired products (2r, 2s) in 65–67% yields. Moreover, ynones containing heterocycles, such as thiophenyl and benzothiophenyl rings, furnished the corresponding diketones (2t, 2u) in 53–74% yields. To our delight, a ynone bearing an anthracene group produced the corresponding diketone (2v) in 50% yield. In addition, ynones derived from aryl-substituted anilines delivered the cyclized products in 51–78% yields (2w–ae). This diketone formation reaction could be extended to benzyl, methyl, ethyl, and piperidinyl-substituted ynones, which generate corresponding products in 62–79% yields (2af–i). Importantly, natural products and biologically interesting molecules-derived ynones smoothly undertook the cyclization/oxidation sequence to provide envisioned products in 41–88% yields (2aj–m), thereby demonstrating the postfunctionalization capability of this approach.
Scheme 3. Scope of Diketone Formation,
Ynone 1 (0.2 mmol, 1 equiv), Ni(acac)2 (0.02 mmol, 10 mol %), and SeO2 (0.3 mmol, 1.5 equiv) in DMF (2 mL) under irradiation using 15 W blue LED at room temperature for 5 h.
Isolated yield.
Ni(acac)2 (20 mol %), SeO2 (6.0 equiv), 24 h.
Reacted at 40 °C for 12 h.
Twenty-four hours.
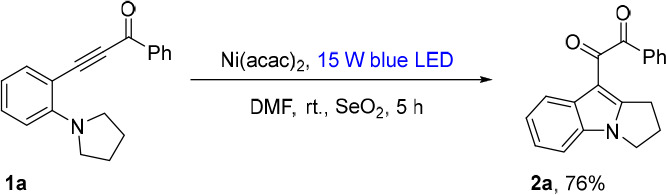
The synthetic application of this methodology was demonstrated by the gram-scale synthesis and selective transformations of adducts (Scheme 4). When the model reaction was scaled up to 6.0 mmol with a diluted concentration and prolonged reaction time, 2a was produced in 63% yield (1.1 g). The selective 1,2-addition between 2a and PhMgBr proceeded to give 3 in a 69% yield. After condensation between 2a and 1,2-diaminobenzene in MeOH at 80 °C for 12 h, quinoxaline product 4 was afforded in excellent yield. The hydrazone formation reaction between 2a and phenylhydrazine readily gave 5 in a 74% yield. By the employment of a classic Pd(0) catalytic system, the coupling between 2q and phenylboronic acid afforded product 6 in an 84% yield. The structures of 3 and 5 were established by X-ray crystallography.
Scheme 4. Synthetic Applications.
Reaction conditions: (a) 2a (0.2 mmol, 1.0 equiv) and PhMgBr (0.6 mmol, 3.0 equiv) in THF (2 mL) under Ar at −78 °C to room temperature for 12 h; (b) 2a (0.2 mmol, 1.0 equiv) and 1,2-diaminobenzene (0.4 mmol, 2.0 equiv) in MeOH (2 mL) under Ar at 80 °C for 12 h; (c) 2a (0.2 mmol, 1.0 equiv), phenylhydrazine (0.3 mmol, 1.5 equiv), and HOAc (0.1 mmol, 50 mol %) in EtOH (2 mL) under Ar at 90 °C for 18 h; (d) 2q (0.2 mmol, 1.0 equiv), phenylboronic acid (0.2 mmol, 1.0 equiv), Pd(PPh3)4 (0.004 mmol, 2 mol %), and Na2CO3 (0.62 mmol, 3.1 equiv) in toluene/MeOH (3/2, 3 mL) under Ar at 90 °C for 12 h.
Next, some preliminary investigations were conducted to study the reaction mechanism (Scheme 5). First, the UV–vis absorbance spectra of 1a, Ni(acac)2, and SeO2 in MeOH were recorded, wherein 1a showed strong absorption in the visible light region. When the reaction was conducted in dark, the starting material was recovered with no formation of the desired product. This demonstrated the key role of light irradiation in this reaction. In the presence of radical scavengers (4.0 equiv), the efficiency of reactions dropped dramatically, which suggests that a radical process should be involved in this reaction. Intermediate G was isolated from the reaction mixture, which was treated with standard conditions to give 2a in 83% yield. In addition, the ON/OFF experiment showed that continuous irradiation was essential for this transformation, and a radical chain process was unlikely to be involved. Furthermore, Stern–Volmer luminescence quenching experiments (see the Supporting Information) indicated that ynone and Ni(acac)2 might form a metal complex through coordination. Next, irradiation of a mixture of 1a, Ni(acac)2, and SeO2 in DMF with blue LEDs for 5 min with 5,5-dimethyl-pyrroline N-oxide (DMPO) as radical spin-trapping agent displayed EPR signals, which could be assigned to carbon radical adduct C-DMPO (see the Supporting Information). We then envisioned a possible reaction mechanism on the basis of these results and previous reports.9,10 Compound 1a and Ni(acac)2 form intermediate A through coordination. Under photo irradiation, A is converted into diradical intermediate B by releasing Ni(acac)2.12 The intramolecular 1,8-HAT13,14 of B(15) gives intermediate C, which next coordinates with Ni(acac)2 to offer intermediate D. The intramolecular radical addition to triple bond on D produces adjacent diradicals intermediate E. Then, E tautomerizes to allene intermediate F, which further undertakes [1,3]-hydride transfer and isomerization to access intermediate G. After tautomerization, G is transformed into enol intermediate H, which is then oxidized by SeO2 to give diketone product.16
Scheme 5. Investigation of the Reaction Mechanism.
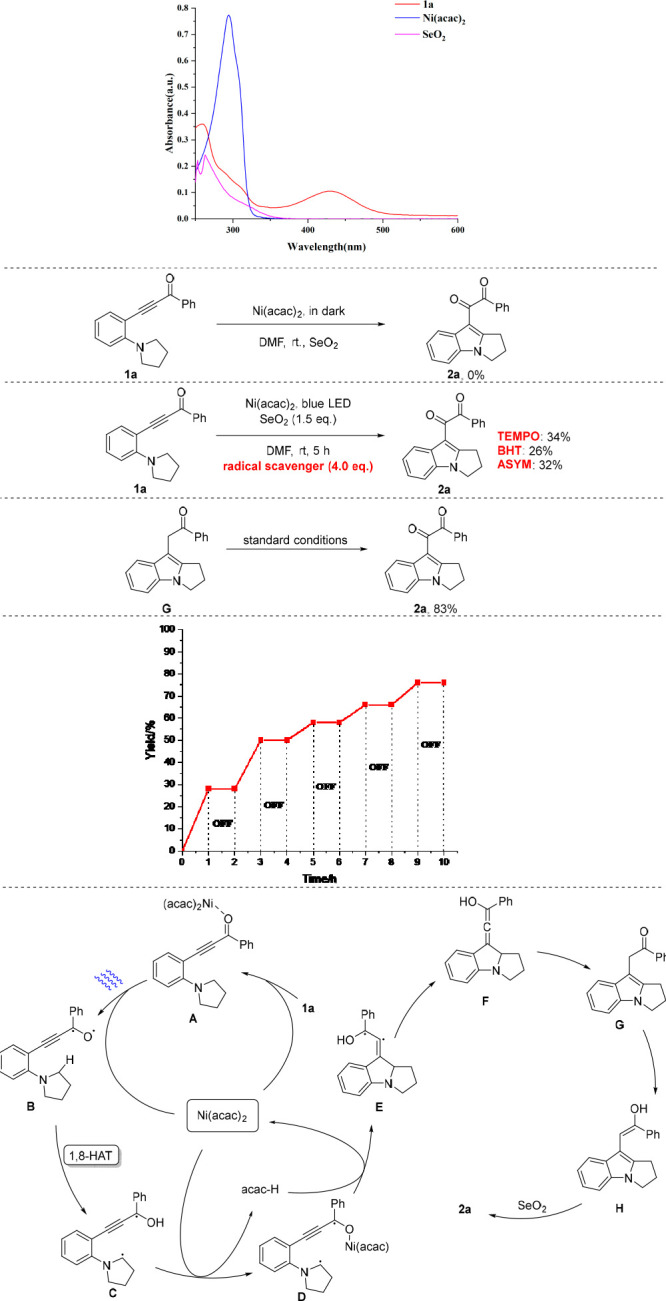
This is the first report on photoinduced Ni-catalyzed synthesis of indolyl phenyl diketones from ynones via twin Csp3–H bond functionalizations. This transformation proceeded smoothly under mild and base-free conditions to produce a wide range of indolyl phenyl diketones in moderate to good yields. Preliminary mechanistic studies indicated that a radical process could be involved in this reaction and that photoirradiation was an indispensable factor for the success of this conversion. Various derivatizations of the coupled product were conducted, which demonstrated the potential synthetic applications. Detailed mechanistic studies are ongoing in our laboratory.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (21801061), Henan University.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.3c00060.
General information; materials; general procedure for the synthesis of derivatives 1a–1am; purification and characterization of derivatives 1a–1am; procedure for the synthesis of derivatives 2a–2am; procedure for the gram synthesis of 2a; procedures for the synthesis of compounds 3–6, as well as their purification and characterization; procedures for control experiments; NMR spectra of 1a–1am; NMR spectra of 2a–2am; and NMR spectra of derivatives 3–6 and G (PDF)
Author Contributions
‡ Y.Z. and Y.W. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Wolkenberg S. E.; Wisnoski D. D.; Leister W. H.; Wang Y.; Zhao Z.; Lindsley C. W. Efficient Synthesis of Imidazoles from Aldehydes and 1, 2-Diketones using Microwave Irradiation. Org. Lett. 2004, 6, 1453–1456. 10.1021/ol049682b. [DOI] [PubMed] [Google Scholar]; b Deng X.; Mani N. S. An efficient route to 4-aryl-5-pyrimidinylimidazoles via sequential functionalization of 2, 4-dichloropyrimidine. Org. Lett. 2006, 8, 269–272. 10.1021/ol052663x. [DOI] [PubMed] [Google Scholar]; c Kong L.; Meng J.; Tian W.; Liu J.; Hu X.; Jiang Z. H.; Zhang W.; Li Y.; Bai L. P. I2-Catalyzed Carbonylation of α-methylene Ketones to Synthesize 1, 2-diaryl Diketones and Antiviral Quinoxalines in One Pot. ACS Omega. 2022, 7, 1380–1394. 10.1021/acsomega.1c06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnáček J.; Weiss R. G.; Lukáč I. Preparation of 4-vinylbenzil and photochemical properties of its homopolymer and copolymer with styrene. Macromolecules 2004, 37, 1304–1311. 10.1021/ma030213j. [DOI] [Google Scholar]
- a Nicolaou K. C.; Gray D. L. F.; Tae J. Total synthesis of hamigerans and analogues thereof photochemical generation and diels-alder trapping of hydroxy-o-quinodimethanes. J. Am. Chem. Soc. 2004, 126, 613–627. 10.1021/ja030498f. [DOI] [PubMed] [Google Scholar]; b Shen Y.; Feng Z.-M.; Jiang J.-S.; Yang Y.-N.; Zhang P.-C. Dibenzoyl and Isoflavonoid Glycosides from Sophora flavescens: Inhibition of the Cytotoxic Effect of D-Galactosamine on Human Hepatocyte HL-7702. J. Nat. Prod. 2013, 76, 2337–2345. 10.1021/np400784v. [DOI] [PubMed] [Google Scholar]; c Mousset C.; Giraud A.; Provot O.; Hamze A.; Bignon J.; Liu J.-M.; Thoret S.; Dubois J.; Brion J.-D.; Alami M. Synthesis and Antitumor Activity of Benzils Related to Combretastatin A-4. Bioorg. Med. Chem. Lett. 2008, 18, 3266–3271. 10.1016/j.bmcl.2008.04.053. [DOI] [PubMed] [Google Scholar]; d Anders M. W. Diacetyl and Related Flavorant α-Diketones: Biotransformation, Cellular Interactions, and Respiratory-Tract Toxicity. Toxicology 2017, 388, 21–29. 10.1016/j.tox.2017.02.002. [DOI] [PubMed] [Google Scholar]; e Wadkins R. M.; Hyatt J. L.; Wei X.; Yoon K. J. P.; Wierdl M.; Edwards C. C.; Morton C. L.; Obenauer J. C.; Damodaran K.; Beroza P.; Danks M. K.; Potter P. M. Identification and Characterization of Novel Benzil (Diphenylethane-1,2-dione) Analogues as Inhibitors of Mammalian Carboxylesterases. J. Med. Chem. 2005, 48, 2906–2915. 10.1021/jm049011j. [DOI] [PubMed] [Google Scholar]; f Mahabusarakam W.; Deachathai S.; Phongpaichit S.; Jansakul C.; Taylor W. C. A benzil and isoflavone derivatives from Derris scandens Benth. Phytochemistry 2004, 65, 1185–1191. 10.1016/j.phytochem.2004.03.006. [DOI] [PubMed] [Google Scholar]; g Maurya R.; Singh R.; Deepak M.; Handa S. S.; Yadav P. P.; Mishra P. K. Constituents of Pterocarpus Marsupium: an Ayurvedic Crude Drug. Phytochemistry 2004, 65, 915–920. 10.1016/j.phytochem.2004.01.021. [DOI] [PubMed] [Google Scholar]; h McKenna J. M.; Halley F.; Souness J. E.; McLay I. M.; Pickett S. D.; Collis A. J.; Page K.; Ahmed I. An Algorithm-Directed Two-Component Library Synthesized via Solid-Phase Methodology Yielding Potent and Orally Bioavailable p38 MAP Kinase Inhibitors. J. Med. Chem. 2002, 45, 2173–2184. 10.1021/jm011132l. [DOI] [PubMed] [Google Scholar]
- a Zeng X.; Miao C.; Wang S.; Xia C.; Sun W. Facile and Highly Chemoselective Synthesis of Benzil Derivatives Viaoxidation of Stilbenes in an I2-H2O System. RSC Adv. 2013, 3, 9666–9669. 10.1039/c3ra41489b. [DOI] [Google Scholar]; b Shen D.; Wang H.; Zheng Y.; Zhu X.; Gong P.; Wang B.; You J.; Zhao Y.; Chao M. Catalyst-free and transition-metal-free approach to 1,2-diketones via aerobic alkyne oxidation. J. Org. Chem. 2021, 86, 5354–5361. 10.1021/acs.joc.0c03010. [DOI] [PubMed] [Google Scholar]; c Liu X.; Cong T.; Liu P.; Sun P. Synthesis of 1,2-Diketones via a Metal-Free, Visible-Light-Induced Aerobic Photooxidation of Alkynes. J. Org. Chem. 2016, 81, 7256–7261. 10.1021/acs.joc.6b00097. [DOI] [PubMed] [Google Scholar]; d Kim S.- W.; Um T.-W.; Shin S. Metal-Free Iodine-Catalyzed Oxidation of Ynamides and Diaryl Acetylenes into 1,2-Diketo Compounds. J. Org. Chem. 2018, 83, 4703–4711. 10.1021/acs.joc.8b00484. [DOI] [PubMed] [Google Scholar]; e Yang W.; Chen Y.; Yao Y.; Yang X.; Lin Q.; Yang D. ICl/AgNO3 Co-Catalyzed Radical Oxidation of Diaryl and Alkylaryl alkynes into 1,2-Diketones. J. Org. Chem. 2019, 84, 11080–11090. 10.1021/acs.joc.9b01667. [DOI] [PubMed] [Google Scholar]; f Bansode A. H.; Suryavanshi G. Iodine-Mediated Oxidative Rearrangement of α,β-Unsaturated Diaryl Ketones: A Facile Access to 1,2-Diaryl Diketones. ACS Omega 2019, 4, 9636–9644. 10.1021/acsomega.9b00833. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Ren W.; Liu J.; Chen L.; Wan X. Ruthenium-Catalyzed Alkyne Oxidation with Part-Per-Million Catalyst Loadings. Adv. Synth. Catal. 2010, 352, 1424–1428. 10.1002/adsc.201000250. [DOI] [Google Scholar]; h Zhou J.; Tao X.; Dai J.; Li C.; Xu J.; Xu H.; Xu H. Electrochemical Synthesis of 1,2-Diketones from Alkynes under Transition-Metal-Catalyst-Free Conditions. Chem. Commun. 2019, 55, 9208–9211. 10.1039/C9CC03996A. [DOI] [PubMed] [Google Scholar]; i Cao S.; Zhong S.; Xin L.; Wan J.-P.; Wen C. Visible-light-induced C=C bond cleavage of enaminones for the synthesis of 1,2-diketones and quinoxalines in sustainable medium. ChemCatChem. 2015, 7, 1478–1482. 10.1002/cctc.201500139. [DOI] [Google Scholar]; j Xu F. C.; Xu M.; Jia X. Y.; Li Y. C. Gold-Catalyzed Synthesis of Benzil Derivatives and α-Keto Imides via Oxidation of Alkynes. Org. Lett. 2011, 13, 1556–1559. 10.1021/ol200270t. [DOI] [PubMed] [Google Scholar]; k Chen S.; Liu Z.; Shi E.; Chen L.; Wei W.; Li H.; Cheng Y.; Wan X. Ruthenium-Catalyzed Oxidation of Alkenes at Room Temperature: A Practical and Concise Approach to α-Diketones. Org. Lett. 2011, 13, 2274–2277. 10.1021/ol200716d. [DOI] [PubMed] [Google Scholar]; l Lu Y.; Luo M. J.; Hu M.; Li Y.; Li J. H. Dimethyl Sulfoxide as an Oxygen Atom Source Enabled Tandem Conversion of 2-Alkynyl Carbonyls to 1,2-Dicarbonyls. Adv. Synth. Catal. 2020, 362, 1846–1850. 10.1002/adsc.202000066. [DOI] [Google Scholar]; m Wang X.; Cheng G.; Shen J.; Yang X.; Wei M.; Feng Y.; Cui X. A Metal-Free Synthesis of Diaryl-1,2-diketones by C-C Triple Bond Cleavage of Alkynones. Org. Chem. Front. 2014, 1, 1001–1004. 10.1039/C4QO00174E. [DOI] [Google Scholar]
- a Chand S.; Pandey A. K.; Singh R.; Singh K. N. Visible-Light-Induced Photocatalytic Oxidative Decarboxylation of Cinnamic Acids to 1,2-Diketones. J. Org. Chem. 2021, 86, 6486–6493. 10.1021/acs.joc.1c00322. [DOI] [PubMed] [Google Scholar]; b Lv W.; Zeng Y.; Zhang S.; Li Q.; Wang H. Mild Mn(OAc)3-mediated aerobic oxidative decarboxylative coupling of arylboronic acids and arylpropiolic acids: Direct access to diaryl 1,2-diketones. Org. Lett. 2015, 17, 2972–2975. 10.1021/acs.orglett.5b01265. [DOI] [PubMed] [Google Scholar]; c Saberi D.; Hashemi H.; Niknam K. One-Pot Solvent-Free Synthesis of Diaryl 1,2-Diketones by the Sequential Heck Oxidation Reaction of Aryl Halides with Styrenes. Asian J. Org. Chem. 2017, 6, 169–173. 10.1002/ajoc.201600529. [DOI] [Google Scholar]; d Matsuda T.; Oyama S. Synthesis of Unsymmetrical Benzils via Palladium-Catalysed α-Arylation-Oxidation of 2-Hydroxyacetophenones with Aryl Bromides. Org. Biomol. Chem. 2020, 18, 3679–3683. 10.1039/D0OB00575D. [DOI] [PubMed] [Google Scholar]; e Zhao B.; Yin X.; Li H.; Cheng K.; Wan J.-P. Transition metal-free, photocatalytic arylation and dioxygenation for vicinal diketone synthesis using alkynes and arene diazonium salts. Org. Chem. Front. 2023, 10, 1942–1947. 10.1039/D3QO00064H. [DOI] [Google Scholar]; f Su Y.; Sun X.; Wu G.; Jiao N. Catalyst-Controlled Highly Selective Coupling and Oxygenation of Olefins: A Direct Approach to Alcohols, Ketones and Diketones. Angew. Chem., Int. Ed. 2013, 52, 9808–9812. 10.1002/anie.201303917. [DOI] [PubMed] [Google Scholar]; g Kumar Y.; Jaiswal Y.; Kumar A. Two-Step One-Pot Synthesis of Unsymmetrical (Hetero)Aryl 1,2-Diketones by Addition Oxygenation of Potassium Aryltrifluoroborates to (Hetero)Arylacetonitriles. Eur. J. Org. Chem. 2018, 2018, 494–505. 10.1002/ejoc.201701625. [DOI] [Google Scholar]; h Min H.; Palani T.; Park K.; Hwang J.; Lee S. Copper-Catalyzed Direct Synthesis of Diaryl 1,2-Diketones from Aryl Iodides and Propiolic Acid. J. Org. Chem. 2014, 79, 6279–6285. 10.1021/jo501089k. [DOI] [PubMed] [Google Scholar]; i Bharate J. B.; Abbat S.; Sharma R.; Bharatam P. V.; Vishwakarma R. A.; Bharate S. B. Cobalt(II) Catalyzed C(sp)-H Bond Functionalization of Alkynes with Phenyl Hydrazines: Facile Access to Diaryl 1,2-diketones. Org. Biomol. Chem. 2015, 13, 5235–5242. 10.1039/C5OB00419E. [DOI] [PubMed] [Google Scholar]
- a Kirihara M.; Ochiai Y.; Takizawa S.; Takahata H.; Nemoto H. Aerobic Oxidation of α-Hydroxycarbonyls Catalysed by Trichlorooxyvanadium: Efficient Synthesis of α-Dicarbonyl Compounds. Chem. Commun. 1999, 1387–1388. 10.1039/a903622i. [DOI] [Google Scholar]; b Zhu J. L.; Tsai Y. T. Rhodium-Catalyzed Aerobic Decomposition of 1,3-Diaryl-2-diazo- 1,3-diketones: Mechanistic Investigation and Application to the Synthesis of Benzils. J. Org. Chem. 2021, 86, 813–828. 10.1021/acs.joc.0c02366. [DOI] [PubMed] [Google Scholar]; c Yuan Y.; Zhu H. Iodine-Catalyzed Synthesis of 1,2-Diaryldiketones by Oxidative Cleavage of 1,3-Diaryldiketones with DMSO. Eur. J. Org. Chem. 2012, 2012, 329–333. 10.1002/ejoc.201101028. [DOI] [Google Scholar]; d Huang L.; Cheng K.; Yao B.; Xie Y.; Zhang Y. Iron-promoted C-C bond cleavage of 1,3-diketones: a route to 1,2-diketones under mild reaction conditions. J. Org. Chem. 2011, 76, 5732–5737. 10.1021/jo200840y. [DOI] [PubMed] [Google Scholar]
- a Feng L.; Hu T.; Zhang S.; Xiong H.-Y.; Zhang G. Copper Mediated Deacylative Coupling of Ynones via C-C Bond Activation under Mild Conditions. Org. Lett. 2019, 21, 9487–9492. 10.1021/acs.orglett.9b03684. [DOI] [PubMed] [Google Scholar]; b Chang Z.; Zhang S.; Wang Y.; Xiong H.-Y.; Zhang G. Catalyst-free synthesis of quinoline-enols through coupling between heterocyclic N-oxides and CF3-ynones under mild conditions. Org. Chem. Front. 2022, 9, 6200–6204. 10.1039/D2QO01260J. [DOI] [Google Scholar]; c Xu P.; Han W.; Zhou Y.; Xiong H.-Y.; Ni S.-F.; Zhang G. Visible Light Mediated Cyclization of Ynones for the Synthesis of 3-Alkyl N-Fused Indoles via Csp3−H Bond Functionalization. Adv. Synth. Catal. 2023, 365, 4533–4537. 10.1002/adsc.202300970. [DOI] [Google Scholar]
- a Chen Z.; Rong M.-Y.; Nie J.; Zhu X.-F.; Shi B.-F.; Ma J.-A. Catalytic Alkylation of Unactivated C(sp3)-H Bonds for C(sp3)-C(sp3) Bond Formation. Chem. Soc. Rev. 2019, 48, 4921–4942. 10.1039/C9CS00086K. [DOI] [PubMed] [Google Scholar]; b Xu Y.; Dong G. sp3 C-H Activation via Exo-Type Directing Groups. Chem. Sci. 2018, 9, 1424–1432. 10.1039/C7SC04768A. [DOI] [PMC free article] [PubMed] [Google Scholar]; c He G.; Wang B.; Nack W. A.; Chen G. Syntheses and Transformations of α-Amino Acids via Palladium-Catalyzed Auxiliary-Directed sp3 C-H Functionalization. Acc. Chem. Res. 2016, 49, 635–645. 10.1021/acs.accounts.6b00022. [DOI] [PubMed] [Google Scholar]; d Saint-Denis T. G.; Zhu R.-Y.; Chen G.; Wu Q.-F.; Yu J.-Q. Enantioselective C(sp3)-H Bond Activation by Chiral Transition Metal Catalysts. Science 2018, 359, eaao4798. 10.1126/science.aao4798. [DOI] [PMC free article] [PubMed] [Google Scholar]; e He C.; Whitehurst W. G.; Gaunt M. J. Palladium-Catalyzed C(sp3)-H Bond Functionalization of Aliphatic Amines. Chem. 2019, 5, 1031–1058. 10.1016/j.chempr.2018.12.017. [DOI] [Google Scholar]; f Mishra A. A.; Subhedar D.; Bhanage B. M. Nickel, Cobalt and Palladium Catalysed C-H Functionalization of Unactivated C(sp3)-H Bond. Chem. Rec. 2019, 19, 1829–1857. 10.1002/tcr.201800093. [DOI] [PubMed] [Google Scholar]
- a Zhu S.; Das A.; Bui L.; Zhou H.; Curran D. P.; Rueping M. Oxygen switch in visible-light photoredox catalysis: radical additions and cyclizations and unexpected C-C-bond cleavage reactions. J. Am. Chem. Soc. 2013, 135, 1823–1829. 10.1021/ja309580a. [DOI] [PubMed] [Google Scholar]; b Kohls P.; Jadhav D. D.; Pandey G.; Reiser O. Visible light photoredox catalysis: generation and addition of N-aryl tetrahydroisoquinoline-derived α-amino radicals to Michael acceptors. Org. Lett. 2012, 14, 672–675. 10.1021/ol202857t. [DOI] [PubMed] [Google Scholar]; c Xia X.-F.; Zhang L.-L.; Song X.-R.; Niu Y.-N.; Liu X.-Y.; Liang Y.-M. Palladium-copper-cocatalyzed intramolecular oxidative coupling: an efficient and atom-economical strategy for the synthesis of 3-acylindoles. Chem. Commun. 2013, 49, 1410–1412. 10.1039/c2cc37805a. [DOI] [PubMed] [Google Scholar]; d Gogoi A.; Modi A.; Guin S.; Rout S. K.; Das D.; Patel B. K. A metal free domino synthesis of 3-aroyl indoles via two sp3 C-H activation. Chem. Commun. 2014, 50, 10445–10447. 10.1039/C4CC04407J. [DOI] [PubMed] [Google Scholar]; e Zhang P.; Xiao T.; Xiong S.; Dong X.; Zhou L. Synthesis of 3-Acylindoles by Visible-Light Induced Intramolecular Oxidative Cyclization of o-Alkynylated N,N-Dialkylamines. Org. Lett. 2014, 16, 3264–3267. 10.1021/ol501276j. [DOI] [PubMed] [Google Scholar]
- For selected reviews on photoinduced nickel-catalyzed C–H functionalization, see:; a Mantry L.; Maayuri R.; Kumar V.; Gandeepan P. Photoredox catalysis in nickel-catalyzed C-H functionalization. Beilstein J. Org. Chem. 2021, 17, 2209–2259. 10.3762/bjoc.17.143. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Guillemard L.; Wencel-Delord J. When metal-catalyzed C-H functionalization meets visible-light photocatalysis. Beilstein J. Org. Chem. 2020, 16, 1754–1804. 10.3762/bjoc.16.147. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lee G. S.; Hong S. H. Direct C(sp3)-H Acylation by Mechanistically Controlled Ni/Ir Photoredox Catalysis. Acc. Chem. Res. 2023, 56, 2170–2184. 10.1021/acs.accounts.3c00252. [DOI] [PubMed] [Google Scholar]; d Yue H.; Zhu C.; Huang L.; Dewanji A.; Rueping M. Advances in allylic and benzylic C-H bond functionalization enabled by metallaphotoredox catalysis. Chem. Commun. 2021, 58, 171–184. 10.1039/D1CC06285A. [DOI] [PubMed] [Google Scholar]; e Parasram M.; Gevorgyan V. Visible light-induced transition metal-catalyzed transformations: beyond conventional photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. 10.1039/C7CS00226B. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Cheng W.-M.; Shang R. Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS Catal. 2020, 10, 9170–9196. 10.1021/acscatal.0c01979. [DOI] [Google Scholar]; For selected examples, see:; g Campbell M. W.; Yuan M.; Polites V. C.; Gutierrez O.; Molander G. A. Photochemical C-H Activation Enables Nickel-Catalyzed Olefin Dicarbofunctionalization. J. Am. Chem. Soc. 2021, 143, 3901–3910. 10.1021/jacs.0c13077. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Heitz D. R.; Tellis J. C.; Molander G. A. Photochemical Nickel-Catalyzed C-H Arylation: Synthetic Scope and Mechanistic Investigations. J. Am. Chem. Soc. 2016, 138, 12715–12718. 10.1021/jacs.6b04789. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Xu S.; Ping Y.; Li W.; Guo H.; Su Y.; Li Z.; Wang M.; Kong W. Enantioselective C(sp3)-H Functionalization of Oxacycles via Photo-HAT/Nickel Dual Catalysis. J. Am. Chem. Soc. 2023, 145, 5231–5241. 10.1021/jacs.2c12481. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Kaushik N. K.; Kaushik N.; Attri P.; Kumar N.; Kim C. H.; Verma A. K.; Choi E. Biomedicalimportance of indoles. Molecules 2013, 18, 6620–6662. 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Daly S.; Hayden K.; Malik I.; Porch N.; Tang H.; Rogelj S.; Frolova L. V.; Lepthien K.; Kornienko A.; Magedov I. V. Unprecedented C-2 arylation of indole with diazonium salts: Syntheses of 2,3-disubstituted indoles and their antimicrobial activity. Bioorg. Med. Chem. Lett. 2011, 21, 4720–4723. 10.1016/j.bmcl.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wu Y. S.; Coumar M. S.; Chang J. Y.; Sun H. Y.; Kuo F. M.; Kuo C. C.; Chen Y. J.; Chang C. Y.; Hsiao C. L.; Liou J. P.; et al. Synthesis and Evaluation of 3-Aroylindoles as Anticancer Agents: Metabolite Approach. J. Med. Chem. 2009, 52, 4941–4945. 10.1021/jm900060s. [DOI] [PubMed] [Google Scholar]; d La Regina G.; Sarkar T.; Bai R.; Edler M. C.; Saletti R.; Coluccia A.; Piscitelli F.; Minelli L.; Gatti V.; Mazzoccoli C.; et al. New Arylthioindoles and Related Bioisosteres at the Sulfur Bridging Group. 4. Synthesis, Tubulin Polymerization, Cell Growth Inhibition, and Molecular Modeling Studies. J. Med. Chem. 2009, 52, 7512–7527. 10.1021/jm900016t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Xie X.; Pan H.; Zhou T.-P.; Han M.-Y.; Wang L.; Geng X.; Ma Y.; Liao R.-Z.; Wang Z.-M.; Yang J.; Li P. ortho-Ethynyl Group Assisted Regioselective and Diastereoselective [2 + 2] Cross-Photocycloaddition of Alkenes. Org. Chem. Front. 2021, 8, 5872–5887. 10.1039/D1QO01017D. [DOI] [Google Scholar]; b Xie X.; Wang L.; Zhou Q.; Ma Y.; Wang Z.-M.; Li P. Visible-light-induced novel cyclization of 2-(2-(arylethynyl)benzylidene)-malononitrile derivatives with 2,6-di(tert-butyl)-4-methylphenol to bridged spirocyclic compounds. Chin. Chem. Lett. 2022, 33, 5069–5073. 10.1016/j.cclet.2022.03.084. [DOI] [Google Scholar]
- a Huo J.; Geng X.; Li W.; Zhang P.; Wang L. A Traceless Heterocyclic Amine Mediator in Regioselectivity-Switchable Formal [1 + 2 + 2] Cycloaddition Reaction to 1,3,4- and 1,4,5-Trisubstituted Pyrazoles. Org. Lett. 2023, 25, 512–516. 10.1021/acs.orglett.2c04227. [DOI] [PubMed] [Google Scholar]; b Baráth E. Hydrogen Transfer Reactions of Carbonyls, Alkynes, and Alkenes with Noble Metals in the Presence of Alcohols/Ethers and Amines as Hydrogen Donors. Catalysts. 2018, 8, 671. 10.3390/catal8120671. [DOI] [Google Scholar]; c Nechab M.; Mondal S.; Bertrand M. P. 1,n-Hydrogen-Atom Transfer (HAT) Reactions in Which n≠5: An Updated Inventory. Chem. - Eur. J. 2014, 20, 16034–16059. 10.1002/chem.201403951. [DOI] [PubMed] [Google Scholar]; d Capaldo L.; Ravelli D.; Fagnoni M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C-H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. 10.1021/acs.chemrev.1c00263. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Cao H.; Kong D.; Yang L. C.; Chanmungkalakul S.; Liu T.; Piper J. L.; Peng Z.; Gao L.; Liu X.; Hong X.; Wu J. Brønsted Acid-Enhanced Direct Hydrogen Atom Transfer Photocatalysis to Enable Selective Late-Stage Functionalization of Unactivated C(sp3)-H Bonds. Nat. Synth. 2022, 1, 794–803. 10.1038/s44160-022-00125-1. [DOI] [Google Scholar]
- a Guyenne S.; León E. I.; Martín A.; Pérez-Martín I.; Suárez E. Intramolecular 1,8-Hydrogen Atom Transfer Reactions in Disaccharide Systems Containing Furanose Units. J. Org. Chem. 2012, 77, 7371–7391. 10.1021/jo301153u. [DOI] [PubMed] [Google Scholar]; b Martín A.; Pérez-Martín I.; Quintanal L. M.; Suárez E. Intramolecular 1,8-Hydrogen Atom Transfer. Stereoselectivity of the Hexopyranos-5-yl Radical Reactions in Hexp-(1→4)-Hexp Disaccharide Systems. J. Org. Chem. 2008, 73, 7710–7720. 10.1021/jo801499d. [DOI] [PubMed] [Google Scholar]
- a Deb M. L.; Baruah P. K. Strategies Toward the Catalyst-Free α-C-H Functionalizations of Tertiary Amines. Top Curr. Chem. 2023, 381, 14. 10.1007/s41061-023-00424-x. [DOI] [PubMed] [Google Scholar]; b Deb M. L.; Borpatra P. J.; Baruah P. K. A One-Pot Catalyst/External Oxidant/Solvent-Free Cascade Approach to Pyrimidines via A 1,5-Hydride Transfer. Green Chem. 2019, 21, 69–74. 10.1039/C8GC03507E. [DOI] [Google Scholar]; c Borpatra P. J.; Deka B.; Deb M. L.; Baruah P. K. Recent Advances in Intramolecular C-O/C-N/C-S Bond Formation via C-H Functionalization. Org. Chem. Front. 2019, 6, 3445–3489. 10.1039/C9QO00863B. [DOI] [Google Scholar]
- a Mlochowski J.; Wójtowicz-Mlochowska H. Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents. Molecules 2015, 20, 10205–10243. 10.3390/molecules200610205. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rabjohn N. Selenium Dioxide Oxidation. Org. React. 2011, 5, 331–386. 10.1002/0471264180.or005.08. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.