Abstract
Histology is considered the gold standard for diagnosing the pathological progress of cervical cancer development, while cervical intraepithelial neoplasia of grade 2 or worse (CIN2+) is the cutoff for intervention in clinical practice. The diagnostic value of human papillomavirus (HPV) E6/E7 mRNA in screening for CIN2+ has not been systematically summarized. A meta-analysis was conducted as part of the present study conducted to explore the diagnostic value of HPV E6/E7 mRNA in screening for CIN2+, aiming to provide a new marker for earlier clinical diagnosis of cervical cancer. The PubMed, Embase and Cochrane Library databases were searched from inception to May 2023. Studies reporting the true positive, false positive, true negative and false negative values in differentiating between CIN2+ and CIN2- were included, while duplicate publications, studies without full text, incomplete information or inability to conduct data extraction, animal experiments, reviews and systematic reviews were excluded. STATA software was used to analyze the data. A total of 2,224 patients were included of whom there were 1,274 patients with CIN2+ and 950 patients with CIN2-. The pooled sensitivity and specificity of the studies overall were 0.89 (95% CI, 0.84–0.92) and 0.59 (95% CI, 0.46–0.71), respectively; the positive likelihood ratio (LR) and the negative LR of the studies overall were 2.31 (95% CI, 1.61–3.32) and 0.21 (95% CI, 0.14–0.30), respectively. The pooled diagnostic odds ratio of the studies overall was 11.53 (95% CI, 6.85–19.36). Additionally, the area under the curve was 0.88. The analysis indicated that HPV E6/E7 mRNA has high diagnostic efficacy for CIN2+. HPV E6/E7 mRNA is highly sensitive in the diagnosis of CIN2+, which helps to reduce the rate of missed diagnoses. However, lower specificity may lead to a higher number of misdiagnoses in healthy patients.
Keywords: HPV E6/E7 mRNA, cervical intraepithelial neoplasia, CIN2+, diagnostic value, systematic review, meta-analysis
Introduction
Cervical cancer is one of the most common malignant tumors in the world that can affect the physical and mental health of women (1). Globally, there are ~530,000 new cervical cancer cases and 275,000 cervical cancer-related deaths each year, and incidence has indicated steadily/gradually declining patient age at the time of diagnosis (2). Cervical intraepithelial neoplasia (CIN) is a precancerous lesion of cervical invasive carcinoma, which can be divided into three grades, namely CIN1, 2 and 3; the higher the grade of CIN, the greater the probability of cervical invasive carcinoma development (3). Histology is considered the gold standard for diagnosing the pathological progress of cervical cancer development, while CIN2 or worse (CIN2+) is the cutoff for intervention in clinical practice (4). Additionally, international expert consensus recommendations require demonstration of high intra- and inter-laboratory reproducibility, and non-inferior sensitivity and specificity for the outcome of CIN2+ compared with pathological testing (5).
Persistent or repeated infection with high-risk human papillomavirus (HPV) is the main cause of cervical cancer, which can be prevented and treated (6,7). The progress from precancerous cervical lesions to cancer diagnosis requires 5–12 years. Therefore, early screening and treatment of precancerous cervical lesions are of great significance (8). The E6 and E7 proteins are oncoproteins produced by high-risk HPV types such as HPV-16 and HPV-18. HPV types are classified as low-risk or high-risk based on their association with the development of certain health conditions, particularly cervical cancer. The classification is primarily determined by the potential of the virus to cause malignant transformation in cells. Low-Risk HPV types are less likely to lead to the development of cancer. High-risk HPV types are more likely to cause persistent infections that can lead to the development of cancer (9). These proteins play a pivotal role in the initiation and progression of cervical cancer. E6 and E7 are known for their ability to interact with cellular proteins, disrupting normal regulatory pathways in infected cells. Understanding the significance of E6 and E7 proteins is crucial in the context of cervical cancer diagnosis (9). HPV E6/E7 mRNA plays a role in the transcription and expression of oncogenes and can be used as an early marker for the development of cervical cancer lesions (10,11). The detection of HPV E6/E7 mRNA can serve as a biomarker for identifying infections with high-risk HPV types and assessing the risk of cervical cancer development. Although a previous systematic review investigated HPV E6/E7 mRNA for the detection of CIN2+, it is noteworthy that systematic reviews are only qualitative analyses of the literature. Therefore, the diagnostic value of HPV E6/E7 mRNA in screening for CIN2+ still lacks a more objective meta-quantitative analysis (12). A meta-analysis was conducted as part of the present study to explore the diagnostic value including sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR-), diagnostic odds ratio (DOR) and area under curve (AUC) of HPV E6/E7 mRNA in screening for CIN2+, aiming to provide a new marker for the clinical diagnosis of cervical cancer.
Materials and methods
Literature inclusion and exclusion criteria
Inclusion criteria: i) Retrospective or prospective studies evaluating the diagnostic value of E6/E7 mRNA in differentiating between CIN2+ and CIN2-; ii) histopathology as the gold standard; and iii) true positive (TP), true negative (TN), false positive (FP) and false negative (FN) values can be directly or indirectly extracted from the retrieved literature. Exclusion criteria: i) Animal studies, case reports and conference papers; ii) no available data; and iii) duplicate reports or studies based on the same data.
Search strategy
The PubMed, Embase and Cochrane Library databases were searched from inception to May 2023. The search terms included: ‘((((diagnosis[Title/Abstract]) OR (diagnostic[Title/Abstract])) OR (sensitivity[Title/Abstract])) OR (specificity[Title/Abstract])) AND (((((((((((((((Human Papillomavirus Virus[Title/Abstract]) OR (Papillomavirus Virus, Human[Title/Abstract])) OR (Virus, Human Papillomavirus[Title/Abstract])) OR (Human Papillomaviruses[Title/Abstract])) OR (HPV, Human Papillomavirus Viruses[Title/Abstract])) OR (Human Papilloma Virus[Title/Abstract])) OR (Human Papilloma Viruses[Title/Abstract])) OR (Papilloma Virus, Human[Title/Abstract])) OR (Virus, Human Papilloma[Title/Abstract])) OR (HPV Human Papillomavirus[Title/Abstract])) OR (HPV Human Papillomaviruses[Title/Abstract])) OR (Human Papillomavirus, HPV[Title/Abstract])) OR (Human Papillomaviruses, HPV[Title/Abstract])) AND ((Messenger RNA[Title/Abstract]) OR (mRNA[Title/Abstract]))) AND ((E6[Title/Abstract]) OR (E7[Title/Abstract])))’.
Literature screening and data extraction
Literature search, screening and extraction of relevant material was carried out by two researchers. When there were questions or disagreements, a third researcher was consulted before making a decision. The data extraction content included: Author, year of publication, sample size, sex, age and the values of TP, FP, TN and FN. If no TP, FP, TN and FN values were reported, data such as sensitivity, specificity, positive predictive value and negative predictive value were used to reverse the extrapolation.
Literature quality assessment
The QUADAS-2 tool (www.quadas.org) was separately used by two academics for evaluating the quality of published literature (13), and RevMan (version 5.3) (https://training.cochrane.org/online-learning/core-software/revman) was used to draw a quality evaluation map.
Data synthesis and statistical analysis
Bivariate model or hierarchical summary receiver operating characteristic (SROC) model was used to combine sensitivity and specificity. The I2 value was used to evaluate the heterogeneity caused by non-threshold effects. If I2>50%, the random effects model was used, otherwise, the fixed effects model was used. When I2 is 25–50%, heterogeneity is low. When I2 is 50–75%, heterogeneity is at a moderate level, and when I2>75%, there is a high degree of heterogeneity. Subgroup analysis was performed to explore the causes of heterogeneity among the included studies. All analyses were performed with STATA (version 15.1; StataCorp LP). All statistical tests were two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Results of literature search
In the current study, a total of 462 studies were retrieved from the aforementioned databases. After eliminating duplicate studies, 231 studies were obtained. After browsing titles and abstracts, 162 studies were obtained. Finally, 10 articles were included in the present meta-analysis through full-text reading (Fig. 1).
Figure 1.
Flow diagram for selection of studies.
Baseline characteristics and quality assessment of the included studies
Baseline characteristics of the included studies
The present meta-analysis comprised 10 publications. A total of 2,224 patients were included, of whom there were 1,274 patients with CIN2+ and 950 patients with CIN2-. The age range the CIN2+ group was 30.0–48.8 years, while the age range of the CIN2- group was 30.0–45.46 years, which was comparable (Table I) (14–23).
Table I.
Baseline characteristics of the included studies.
| Sample size, n | Age, years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| First author(s), year | CIN 2+ | CIN 2- | CIN 2+ | CIN 2- | TP | FP | FN | TN | Sensitivity, % | Specificity, % | (Refs.) |
| Andersson et al, 2011 | 87 | 68 | 32 (21–79) | 79 | 22 | 8 | 46 | 91.0 | 68.0 | (14) | |
| Waldstrom and | 126 | 67 | 30 (16–65) | 117 | 41 | 9 | 26 | 92.5 | 38.2 | (15) | |
| Ornskov, 2011 | |||||||||||
| Liu et al, 2013 | 57 | 35 | N/A | N/A | 41 | 9 | 16 | 26 | 71.9 | 74.3 | (16) |
| Shi et al, 2017 | 348 | 102 | 39.7±8.9 | 42.2±9.7 | 248 | 33 | 100 | 69 | 71.3 | 67.6 | (17) |
| Camus et al, 2018 | 10 | 10 | N/A | N/A | 9 | 5 | 1 | 5 | 90.0 | 50.0 | (18) |
| Fan and Shen, 2018 | 95 | 97 | N/A | N/A | 87 | 18 | 8 | 79 | 91.5 | 81.4 | (19) |
| Han et al, 2018 | 101 | 96 | 48.8±12.5 | 42.8±10.3 | 86 | 32 | 15 | 64 | 85.1 | 66.7 | (20) |
| Pan et al, 2019 | 92 | 209 | 45.46 (20–89) | 85 | 140 | 7 | 69 | 92.4 | 33.0 | (21) | |
| Zhang et al, 2020 | 328 | 209 | 43.9±11.1 | 308 | 44 | 20 | 165 | 93.8 | 79.0 | (22) | |
| Sun et al, 2021 | 30 | 57 | 35.4±6.5 | 26 | 29 | 4 | 28 | 86.7 | 49.1 | (23) | |
Data are shown as mean ± SD or median (quartiles); N/A, not applicable; TP, true-positive; FP, false-positive; FN, false-negative; TN, true-negative; CIN, cervical intraepithelial neoplasia.
Quality assessment of the included studies
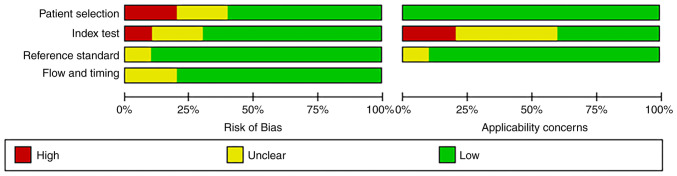
‘Risk of bias’ mainly includes four aspects: ‘Patient selection’, ‘index test’, ‘reference standard’, and ‘flow and timing’ (13). Of the ‘patient selection’ assessment, only two studies were high risk (patients employing selection methods that did not meet the aforementioned criteria, potentially introducing selection bias), and the rest were low risk (patients that adhered to the criteria for random or sequential selection). There was only one study in ‘index test’ showing high risk. Nine studies with regard to the aspect ‘reference standard’ were low-risk and 8 studies with regard to the aspect ‘flow and timing’ were low-risk. Additionally, ‘applicability concerns’ mainly includes three aspects (13): Patient selection, index test and reference standard. For ‘index test’, there was also one study that showed high-risk, and the rest were low risk. Overall, the quality of the literature included in the present review was acceptable (Figs. 2 and 3).
Figure 2.
Methodological quality graph.
Figure 3.
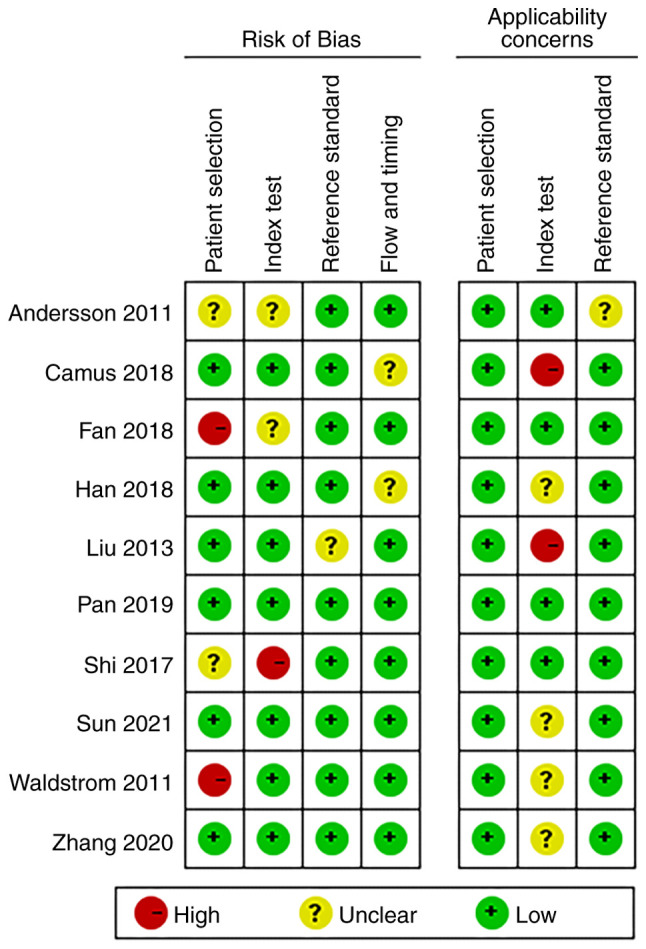
Methodological quality summary.
Results of meta-analysis
Since the I2 for sensitivity (91.71%), specificity (93.95%), LR+ (94.7%), LR- (89.3%) and DOR (84.2%) were >50%, representing a high level of inconsistency among studies, a sensitivity analysis was conducted to find sources of heterogeneity (Fig. S1, Fig. S2, Fig. S3, Fig. S4). The results showed that the two studies by Shi et al (17) and Zhang et al (22) had a greater impact on the results. Both studies were excluded and tested for heterogeneity again. The results of the repeated heterogeneity test showed that the heterogeneity was significantly reduced.
Sensitivity and specificity
Meta-analysis was performed through a random-effect model due to heterogeneity in sensitivity (I2=79.21%) and specificity (I2=93.33%). The pooled sensitivity and specificity of the studies overall were 0.89 (95% CI, 0.83–0.93) and 0.62 (95% CI, 0.46–0.76), respectively (Fig. 4).
Figure 4.
Forest plot of sensitivity and specificity of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; HPV, human papillomavirus.
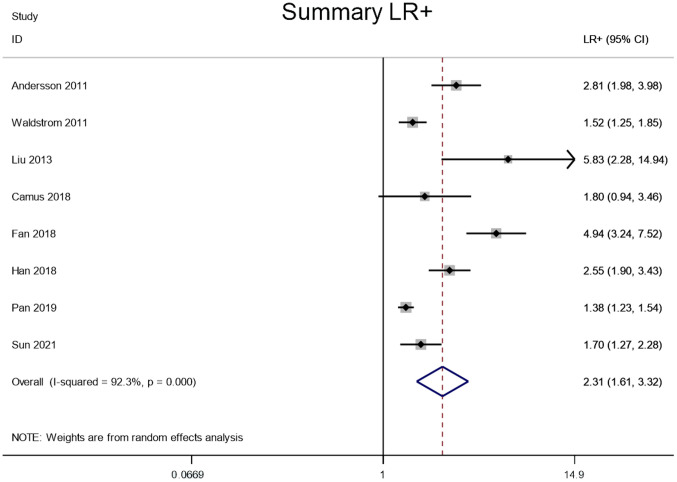
LR+ and LR-
Meta-analysis was performed through a random-effect model due to lower heterogeneity in LR+ (I2=92.3%) and LR- (I2=56.8%). The pooled LR+ and LR- of the studies overall were 2.31 (95% CI, 1.61–3.32) and 0.21 (95% CI, 0.14–0.30), respectively (Figs. 5 and 6).
Figure 5.
Forest plot of the LR+ of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; LR+, positive likelihood ratio; HPV, human papillomavirus.
Figure 6.
Forest plot of the LR- of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; LR-, negative likelihood ratio; HPV, human papillomavirus.
DOR
Meta-analysis was performed through a random-effect model due to lower heterogeneity in DOR (I2=57.5%). The pooled DOR of the studies overall was 11.53 (95% CI, 6.86–19.36; Fig. 7).
Figure 7.
Forest plot of the DOR of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; DOR, diagnostics odd ratio; HPV, human papillomavirus.
ROC analysis
When the AUC value is 0.5–0.6, it is considered that the diagnostic tool is ineffective, 0.6–0.7 is poor, 0.7–0.8 is average, 0.8–0.9 is good and 0.9–1.0 is excellent (24). The SROC curve of the present study shows that AUC was 0.88 (95% CI, 0.84–0.90), indicating that E6/E7 mRNA has good diagnostic value for cervical cancer screening (Fig. 8).
Figure 8.

SROC curve of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; SROC, summary receiver operating characteristic; HPV, human papillomavirus; AUC, area under the curve; sens, sensitivity; spec, specificity.
Sensitivity analysis
Sensitivity analysis was carried out by iteratively excluding each included study individually, followed by re-conducting the meta-analysis with the remaining studies. The results of this sensitivity analysis were then compared to the original analysis to evaluate the influence of each study on the meta-analysis outcomes. Notably, after the exclusion of the studies conducted by Shi et al (17) and Zhang et al (22), the subsequent meta-analysis exhibited considerable changes compared with the original analysis. Therefore, it can be inferred that these two studies had a pronounced impact on the overall results (Fig. 9).
Figure 9.

Sensitivity analysis of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; HPV, human papillomavirus.
Publication bias
The P-value of the Deek's funnel plot of HPV E6/E7 mRNA for distinguishing between CIN2+ and CIN2 was 0.96, indicating that there was no obvious publication bias in the current study (Fig. 10).
Figure 10.

Deek's Funnel plot of HPV E6/E7 mRNA to distinguish between CIN2+ and CIN2-. CIN2+, cervical intraepithelial neoplasia grade 2 or worse; HPV, human papillomavirus; ESS, effective sample size.
Discussion
It is now clear that the occurrence and development of cervical cancer and CIN are mainly caused by the continuous infection with high-risk HPV. HPV DNA testing is primarily a way to check if a patient is infected with HPV. Although it has a high sensitivity, its specificity is relatively low, and it cannot evaluate the infection stage of cervical HPV and the activity of viral oncogenes (25). HPV circular DNA is free in the nucleus of the host, and viral nucleic acid is generally integrated in the genome of the host normal cell, which can cause the inactivation or loss of E2 gene fragment, and then lead to the mRNA transcription of viral E6 and E7 oncogenes (19). Basu et al (26) reported that HPV E6/E7 proteins could bind to p53 and pRb, the key tumor suppressor proteins in cervical epithelial cells, respectively, and lead to their inactivity, resulting in abnormal cell cycle regulation and increasing the risk of malignant degeneration of CIN. An increasing number of studies have revealed that the expression level of HPV E6/E7 mRNA is positively associated with the severity of cervical lesions, and the higher the expression level, the greater the risk of high-grade CIN progressing to cervical cancer (27,28). Therefore, the present meta-analysis explored the diagnostic value of HPV E6/E7 mRNA in screening for CIN2+, aiming to provide a new marker for clinical diagnosis of cervical cancer.
Firstly, the pooled sensitivity and specificity of the studies overall were 0.89 (95% CI, 0.84–0.92) and 0.59 (95% CI, 0.46–0.71), respectively. This indicates that HPV E6/E7 mRNA is highly sensitive in the diagnosis of CIN2+, which helps to reduce the rate of missed diagnosis. However, lower specificity may lead to higher misdiagnosis in healthy patients. Additionally, the pooled DOR of the studies overall was 11.53 (95% CI, 6.85–19.36), suggesting that HPV E6/E7 mRNA had high diagnostic efficacy. Notably, the SROC curve of the current study showed that the AUC of 0.88 (95% CI, 0.84–0.90) indicates that E6/E7 mRNA has good diagnostic value for cervical cancer screening. In a study by Camus et al (18), the sensitivity of HPV DNA for CIN2+ diagnosis was 80%, while the AUC was 0.76. In addition, Zhang et al (29) reported an HPV DNA sensitivity of 86.5% and an AUC of 0.865. This suggests that the sensitivity and diagnostic accuracy of HPV E6/E7 mRNA may be higher than that of HPV DNA. When HPV E6/E7 mRNA detection is positive, cervical cancer histopathological examination should be performed for early diagnosis and early intervention.
However, the present study also has certain limitations. First, most of the included studies were retrospective, thus potentially introducing selection bias and limiting the generalizability of the findings to broader populations or screening settings. Further large-scale randomized controlled trials are needed to validate the findings. Second, most of the included studies were single-center, retrospective studies. Third, while the current study reported no obvious publication bias based on Deek's funnel plot, publication bias can be challenging to detect, especially when the number of included studies is limited. Fourth, the study primarily focused on diagnostic accuracy measures. However, it does not directly assess clinical outcomes, such as the impact of HPV E6/E7 mRNA testing on patient management or the reduction in cervical cancer incidence or mortality. Fifth, the study does not directly compare HPV E6/E7 mRNA testing with other screening methods, making it challenging to evaluate whether this biomarker offers advantages over existing diagnostic approaches.
HPV E6/E7 mRNA testing has high diagnostic efficacy for CIN2+. HPV E6/E7 mRNA is highly sensitive in the diagnosis of CIN2+, which helps to reduce the rate of missed diagnoses. However, lower specificity may lead to more misdiagnoses in healthy patients.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: Not funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
FSX and TFR made substantial contributions in conceving and drafting the manuscript. QGW and RRP made substantial contributions to acquisition of data. SZC and JL made substantial contributions to analysis and interpretation of data. FSX and JL confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nadile M, Kornel A, Sze NSK, Tsiani E. A comprehensive review of Genistein's effects in preclinical models of cervical cancer. Cancers (Basel) 2023;16:35. doi: 10.3390/cancers16010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervical Cancer Treatment, corp-author. PDQ Cancer Information Summaries. National Cancer Institute; Bethesda, MD: 2002. Patient version. [Google Scholar]
- 3.Ikeda S, Ueda Y, Hara M, Yagi A, Kitamura T, Kitamura Y, Konishi H, Kakizoe T, Sekine M, Enomoto T, Sobue T. Human papillomavirus vaccine to prevent cervical intraepithelial neoplasia in Japan: A nationwide case-control study. Cancer Sci. 2021;112:839–846. doi: 10.1111/cas.14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, Committee EG. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28((Suppl 4)):iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 5.Meijer CJLM, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J, Dillner J, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf JL, Billingsley CC, Kendler A, Jackson AL. Cervical stratified mucin-producing intraepithelial lesion: A systematic review of diagnosis and management. J Low Genit Tract Dis. 2020;24:259–264. doi: 10.1097/LGT.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 7.Fischer M, Uxa S, Stanko C, Magin TM, Engeland K. Human papilloma virus E7 oncoprotein abrogates the p53-p21-DREAM pathway. Sci Rep. 2017;7:2603. doi: 10.1038/s41598-017-02831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebolj M, Rimmer J, Denton K, Tidy J, Mathews C, Ellis K, Smith J, Evans C, Giles T, Frew V, et al. Primary cervical screening with high risk human papillomavirus testing: observational study. BMJ. 2019;364:l240. doi: 10.1136/bmj.l240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyri E, Tsimplaki E, Daskalopoulou D, Stravopodis DJ, Kouikoglou O, Terzakis E, Panotopoulou E. E6/E7 mRNA expression of high-risk HPV types in 849 Greek women. Anticancer Res. 2013;33:4007–4011. [PubMed] [Google Scholar]
- 10.Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: Key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26:158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, Gao C, Ma D, Liao S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020;471:88–102. doi: 10.1016/j.canlet.2019.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Derbie A, Mekonnen D, Woldeamanuel Y, Van Ostade X, Abebe T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): A systematic review. Infect Agent Cancer. 2020;15:9. doi: 10.1186/s13027-020-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan D, Giele H. The scratch collapse test: A QUADAS-2 assessment of a systematic review. J Plast Reconstr Aesthet Surg. 2019;72:1418–1833. doi: 10.1016/j.bjps.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Andersson E, Kärrberg C, Rådberg T, Blomqvist L, Zetterqvist BM, Ryd W, Lindh M, Horal P. Type-specific human papillomavirus E6/E7 mRNA detection by real-time PCR improves identification of cervical neoplasia. J Clin Microbiol. 2011;49:3794–3799. doi: 10.1128/JCM.00549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldstrom M, Ornskov D. Clinical performance of a human papillomavirus messenger RNA test (Aptima HPV Assay) on residual material from archived 3-year-old PreservCyt samples with low-grade squamous intraepithelial lesion. Arch Pathol Lab Med. 2011;135:1052–1056. doi: 10.5858/2010-0411-OAR. [DOI] [PubMed] [Google Scholar]
- 16.Liu TY, Xie R, Luo L, Reilly KH, He C, Lin YZ, Chen G, Zheng XW, Zhang LL, Wang HB. Diagnostic validity of human papillomavirus E6/E7 mRNA test in cervical cytological samples. J Virol Methods. 2014;196:120–125. doi: 10.1016/j.jviromet.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Shi WJ, Liu H, Wu D, Tang ZH, Shen YC, Guo L. E6/E7 proteins are potential markers for the screening and diagnosis of cervical pre-cancerous lesions and cervical cancer in a Chinese population. Oncol Lett. 2017;14:6251–6258. doi: 10.3892/ol.2017.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camus C, Vitale S, Loubatier C, Pénaranda G, Khiri H, Plauzolles A, Carcopino X, Halfon P, Giordanengo V. Quantification of HPV16 E6/E7 mRNA spliced isoforms viral load as a novel diagnostic tool for improving cervical cancer screening. J Clin Med. 2018;7:530. doi: 10.3390/jcm7120530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Y, Shen Z. The clinical value of HPV E6/E7 and STAT3 mRNA detection in cervical cancer screening. Pathol Res Pract. 2018;214:767–775. doi: 10.1016/j.prp.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Han L, Husaiyin S, Zhao F, Rezhake R, Niyazi M. Clinical value of human papillomavirus E6/E7 mRNA detection in screening for cervical cancer in women positive for human papillomavirus DNA or. Clin Lab. 2018;64:1363–1371. doi: 10.7754/Clin.Lab.2018.180138. [DOI] [PubMed] [Google Scholar]
- 21.Pan D, Zhang CQ, Liang QL, Hong XC. An efficient method that combines the ThinPrep cytologic test with E6/E7 mRNA testing for cervical cancer screening. Cancer Manag Res. 2019;11:4773–4780. doi: 10.2147/CMAR.S197749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SK, Guo Z, Wang P, Kang LN, Jia MM, Wu ZN, Chen Q, Cao XQ, Zhao DM, Guo PP, et al. The potential benefits of HPV E6/E7 mRNA test in cervical cancer screening in China. Front Oncol. 2020;10:533253. doi: 10.3389/fonc.2020.533253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Yue Y, Li R, Sun Q, Hu C, Ge X, Guan Q. Detection of HPV E6/E7 mRNA in the diagnosis of cervical cancer and precancerous lesions after kidney transplantation. Am J Transl Res. 2021;13:7312–7317. [PMC free article] [PubMed] [Google Scholar]
- 24.Qian S, Zhang S, Lu M, Chen S, Liu L, Liu S, Jiang F, Zhang J. The accuracy of screening tools for sarcopenia in older Chinese adults: A systematic review and meta-analysis. Front Public Health. 2024;12:1310383. doi: 10.3389/fpubh.2024.1310383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molden T, Kraus I, Skomedal H, Nordstrom T, Karlsen F. PreTect HPV-proofer: Real-time detection and typing of E6/E7 mRNA from carcinogenic human papillomaviruses. J Virol Methods. 2007;142:204–212. doi: 10.1016/j.jviromet.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Basu P, Banerjee D, Mittal S, Dutta S, Ghosh I, Chowdhury N, Abraham P, Chandna P, Ratnam S. Sensitivity of APTIMA HPV E6/E7 mRNA test in comparison with hybrid capture 2 HPV DNA test for detection of high risk oncogenic human papillomavirus in 396 biopsy confirmed cervical cancers. J Med Virol. 2016;88:1271–1278. doi: 10.1002/jmv.24453. [DOI] [PubMed] [Google Scholar]
- 27.Song Y, Zhang M, Zhang C, Du S, Zhai F. HPV E6/E7 mRNA combined with thin-prep cytology test for the diagnosis of residual/recurrence after loop electrosurgical excision procedure in patients with cervical intraepithelial neoplasia. Diagn Microbiol Infect Dis. 2024;108:116119. doi: 10.1016/j.diagmicrobio.2023.116119. [DOI] [PubMed] [Google Scholar]
- 28.Duvlis S, Popovska-Jankovic K, Arsova ZS, Memeti S, Popeska Z, Plaseska-Karanfilska D. HPV E6/E7 mRNA versus HPV DNA biomarker in cervical cancer screening of a group of Macedonian women. J Med Virol. 2015;87:1578–1586. doi: 10.1002/jmv.24199. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang J, Zhang R, Lei F, Lai S. Application value of detection of high-risk HPV infection in early cervical cancer patients in disease diagnosis and prognosis evaluation. Clin Lab. 2020;66 doi: 10.7754/Clin.Lab.2020.200245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.