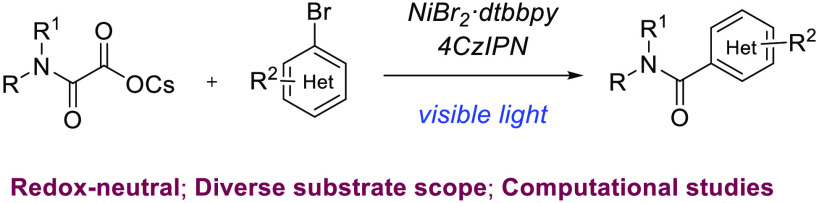
Abstract
Dual nickel-photoredox-enabled direct synthesis of amides through cross-coupling of cesium oxamates with aryl bromides has been developed. This methodology’s key advantages are mild reaction conditions, utilizing organic dye as a photocatalyst, employing readily available starting chemicals as coupling partners, and late-stage carbamoylation of pharmaceutically relevant molecules. DFT studies suggested that the nickel catalytic cycle proceeds via a radical addition pathway prior to the oxidative insertion.
Keywords: Visible light, nickel, carbamoylation, amides, cross-coupling, aryl bromides, oxamates
The amide bond formation is unarguably one of the most important transformations in synthetic organic chemistry.1−5 Molecules with amide bonds are important structural units found throughout nature. This amide linkage serves as the backbone of various biomolecules such as proteins, peptides, natural products, polymers, and pharmaceutical drugs.6−9 Furthermore, many naturally existing plant-based amides have antitumor, antibacterial, antifungal, and insecticidal effects. Considering the widespread utility and importance of amide bonds, various methodologies have already been developed for their synthesis.1−5 The dehydrative condensation in the presence of the stoichiometric amount of coupling reagents is the most commonly employed method for the installation of amide functionality.10 However, the employed coupling reagents are often quite expensive and produce stoichiometric amounts of byproducts. In order to overcome the aforementioned problems, a catalytic method was introduced for the synthesis of amides where metals were used as catalysts.11−14 Among them, the Pd-catalyzed aminocarbonylation process was materialized to some extent.15−19 However, the use of high-pressure toxic CO gas and specialized reaction conditions limits the broad applicability of this method. Therefore, developing a new and benign catalytic protocol for amide bond synthesis from commercially available chemicals is highly enviable.
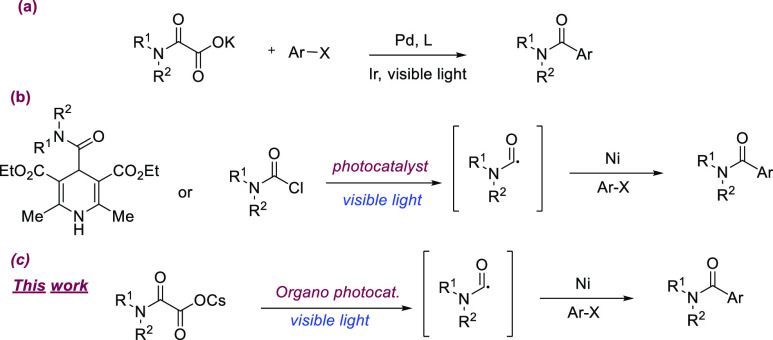
Recently, C–C and C–heteroatom bond formation reactions driven by visible light photocatalysis have emerged as the fastest-growing field in organic synthesis.20−23 Furthermore, the integration of photocatalysis with metal catalysis unlocked new opportunities in the cross-coupling domain.24−29 The generation of carbamoyl radicals and their application for the synthesis of amides through visible light photocatalysis has been well reported.30 Also, few reports have been demonstrated for the synthesis of amides through the dual metallo-photoredox catalysis, in which radical cross-coupling takes place between aryl halides and carbamoyl radical.31−33 In 2015, Shang and Fu jointly reported dual Pd/photoredox-mediated decarboxylative coupling of potassium oxamates with aryl bromides/iodides to produce corresponding amides in good yields (Scheme 1, a).31 In 2020, Melchiorre and co-workers utilized a less expensive combination of photosensitizer and metal catalyst in the presence of visible light, where a variety of bench-stable 4-carbamoyl-1,4-dihydropyridine derivatives act as the source of carbamoyl radical and couples with a range of aryl/heteroaryl bromides lead to the formation of amides in good yields (Scheme 1, b).32 However, the complexity associated with the synthesis of the carbamoyl radical precursors limits its applications. Very recently, Maiti and co-workers demonstrated the direct generation of carbamoyl radical from carbamoyl chloride in the presence of visible light through the halogen-atom transfer (XAT) concept, and further integration with nickel catalysis led to the formation of amides in good yields (Scheme 1, b).33 This methodology tolerates a variety of aryl/hetero aryl bromides and aryl chlorides. Moreover, the authors utilize this methodology for the late-stage amidation of halide-containing drug molecules and pharmacophores. However, carbamoyl chlorides were synthesized from the corresponding amines by the treatment of triphosgene, which is toxicologically unsafe. In the present work, we employed easily accessible and stable oxamates 1 as a source of carbamoyl radical in visible-light photocatalysis, and further integration with nickel cross-coupling events in the presence of aryl/heteroaryl bromides led to the formation of amide products (Scheme 1, c).
Scheme 1. Known Strategies for the Photocatalytic Synthesis of Amides from Haloarenes.
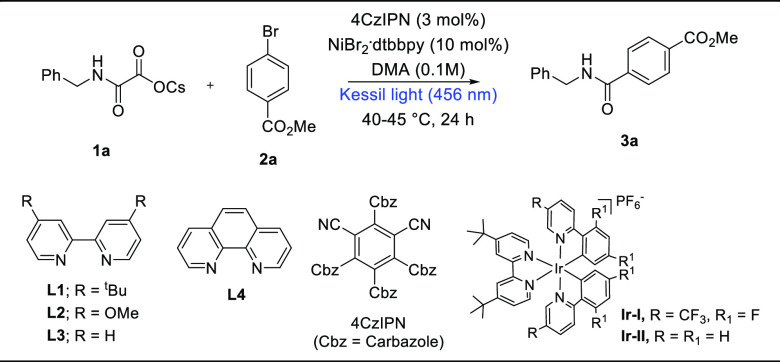
This synergistic dual Ni/photoredox cross-coupling event was conducted using benzyl oxamate 1a and methyl 4-bromobenzoate 2a as a model substrate. After screening several reaction parameters, product 3a was isolated in 71% isolated yield in the presence of 10 mol % of NiBr2·dtbbpy and 3 mol % of photocatalyst (4CzIPN), with DMA as a solvent, and upon irradiation with kessil blue light (456 nm, 40 W) at 40–45 °C (Table 1, entry 1). Oxamates containing different counter cations such as Li+, Na+, and K+ produce slightly lower yields (Table 1, entry 2). Employing NiBr2·L2 (L2 = 4,4′-dimethoxy-2,2′-bipyridine) works well (Table 1, entry 3), while employing NiBr2·L3 and NiBr2·L4 afford low yields of the product (Table 1, entries 4–5). In the presence of Ir-based photocatalysts (Ir–I and Ir–II), the yields are sluggish (Table 1, entries 6–7). Using DMF as a solvent produced a good yield of 3a (Table 1, entry 8), while using DMSO resulted in a low yield of product 3a (Table 1, entry 9). Controlled reactions reveal the role of the Ni, ligand, light, and photocatalyst (Table 1, entries 10–13).
Table 1. Optimization of the Reaction Conditionsa.
| Entry | Deviation from optimized conditions | 3a (%)b |
|---|---|---|
| 1 | None | 75 (71c) |
| 2 | Using Li, Na, and K oxamates | 27, 33, and 52 |
| 3 | Using NiBr2·L2 as ligand | 62 |
| 4 | Using NiBr2·L3 as ligand | 40 |
| 5 | Using NiBr2·L4 as ligand | 36 |
| 6 | Using Ir–I as photocatalyst | 46 |
| 7 | Using Ir–II as photocatalyst | 38 |
| 8 | DMF as solvent | 67 |
| 9 | DMSO as solvent | 47 |
| 10 | In the absence of photocatalyst | 0 |
| 11 | In the absence of light | 0 |
| 12 | In the absence of ligand | 12 |
| 13 | In the absence of nickel | 0 |
Optimization of the reaction conditions: 1a (0.15 mmol), 2a (0.1 mmol), at 40–45 °C, for 24 h.
NMR yields using benzyl benzoate as an internal standard.
Isolated yield.
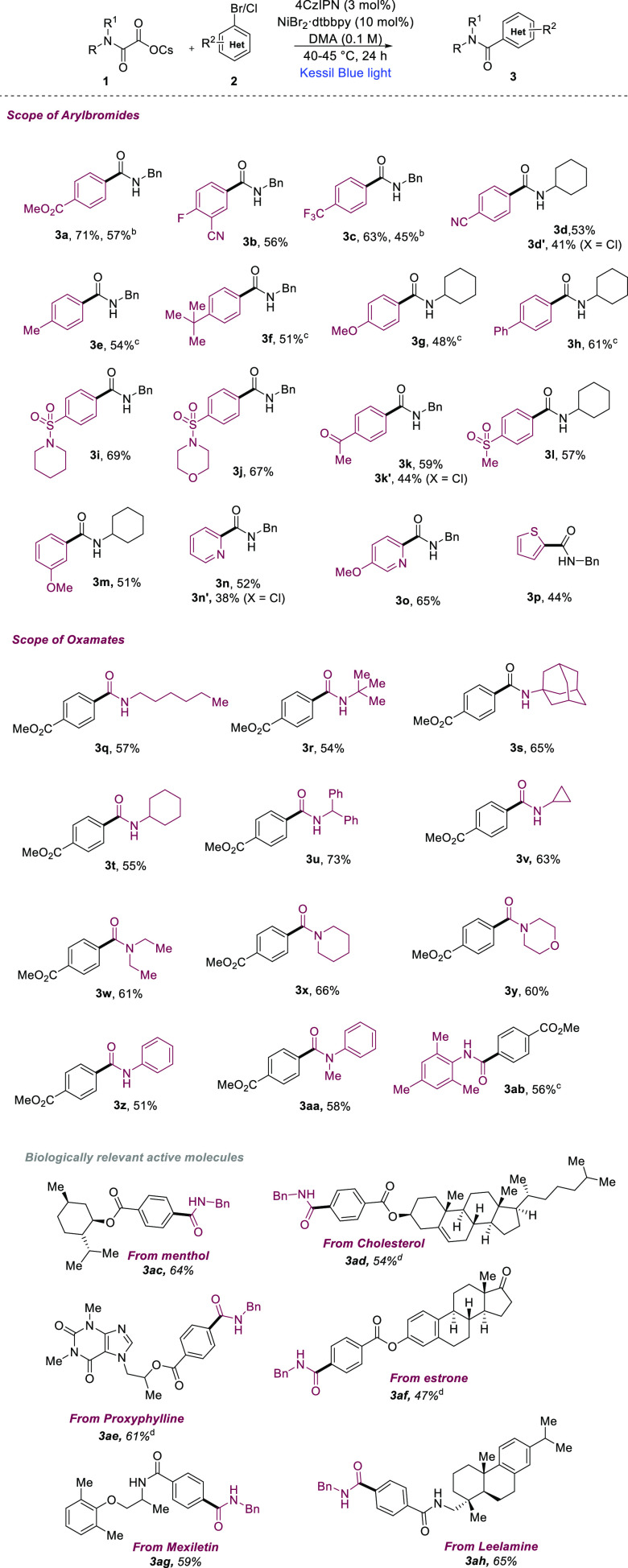
With the optimized reaction conditions as described in Table 1, entry 1, we tested the generality of the synergistic Ni/photoredox catalysis for amide synthesis (Scheme 2). First, the scope of aryl bromides was evaluated. A wide range of aryl bromides, bearing both electron-deficient and electron-rich substituents, undergo cross-coupling reactions with oxamates 1a and 1b to give moderate to good yields of the amide products (3a–3h; 48–71%). Aryl bromides containing different functional groups such as ester, ketone, cyano, ether, and sulfonamide are well reacted with the oxamate (1a or 1b) and afford corresponding amides (3i–3m) in good yields (51–69%). Heteroaryl bromides are well received in our dual catalysis and couple with oxamate 1a to afford corresponding amides (3n–3p) in good yields (44–65%). Electron-deficient aryl chlorides are also suitable candidates in our reaction and afforded amide products (3d′, 3k′, and 3n′) in moderate yields.
Scheme 2. Substrate Scope.
Decarboxylative cross-coupling of oxamates with aryl bromides. Reaction conditions as given in Table 1 (entry 1).
Reaction was carried using 1.5 mmol of 1a and 1 mmol of 2a and 2c for 72 h.
Reaction time 30 h.
Reaction time 35 h. Isolated yields, an average of at least two independent runs.
Next, we focused on the scope of the oxamates, which are easily prepared by treating methyl chlorooxoacetate with amines followed by the addition of CsOH. We first evaluated the sterically different primary alkylamine-derived oxamates. N-Hexylamine-, tert-butyl amine-, 1-adamantylamine-, cyclohexyl amine-, benzhydrylamine-, and cyclopropyl amine-derived oxamates were coupled with methyl 4-bromobenzoate (2a) in our reaction conditions and afford corresponding secondary amide products (3q–3v) in good yields (54–73%). Cyclic and acyclic secondary amines such as morpholine, piperidine, and diethylamine-derived oxamates generate corresponding carbamoyl radicals in our dual catalysis and, coupled with methyl 4-bromobenzoate (2a), led to the formation of tertiary amides (3w–3y) in good yields (60–66%). Finally, oxamates derived from aniline, N-methylaniline, and mesityl amine were successfully converted to their corresponding N-aryl benzamide derivatives (3z–3ab) in our dual Ni/photoredox catalysis. To further investigate the amenability of our methodology toward pharmaceutically relevant molecules, we have employed a few aryl bromides derived from alcohols (menthol, cholesterol, and Proxyphylline), phenol (estrone), and amines (Mexiletin and Leelamine) that were efficiently coupled with benzyl oxamate 1a to afford corresponding amides (3ac–3ah) in moderate to good yields (47–65%).34
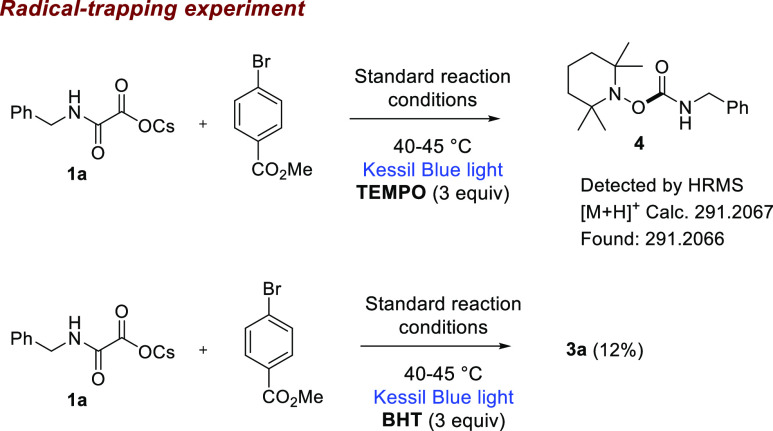
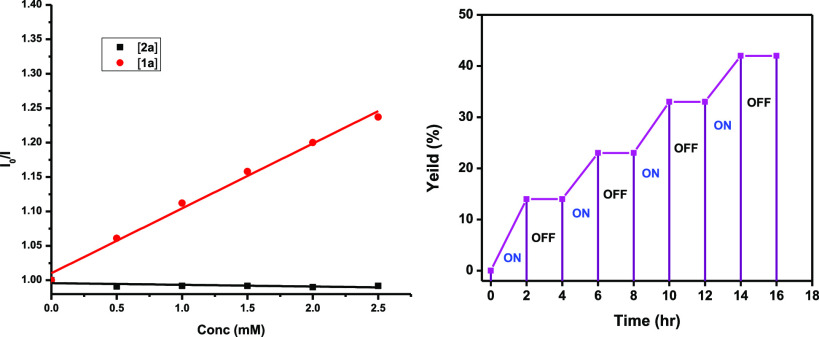
Next, we carried out a series of preliminary mechanistic experiments in order to gain insight into the plausible reaction mechanism (Scheme 3). First, we conducted the reaction in the presence of a radical trapping agent such as TEMPO (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (3.0 equiv), and no desired product 3a was observed. A TEMPO-adduct 4 was confirmed by HRMS analysis (see Supporting Information). When a radical scavenger such as BHT (3.0 equiv) was added in to the standard reaction condition, product 3a was obtained in a low yield (12%). The above experimental observations indicate the involvement of carbamoyl radicals in our reaction conditions. Next, quenching studies clearly indicate the photoexcited photocatalyst undergoes a reductive quenching by benzyl oxamate 1a (Scheme 4, left). Finally, the light ON–OFF experiment reveals that continuous irradiation of light is required throughout the reaction (Scheme 4, right).
Scheme 3. Mechanistic Studies.
Scheme 4. Stern–Volmer Plot (Left), ON–OFF Experiment (Right).
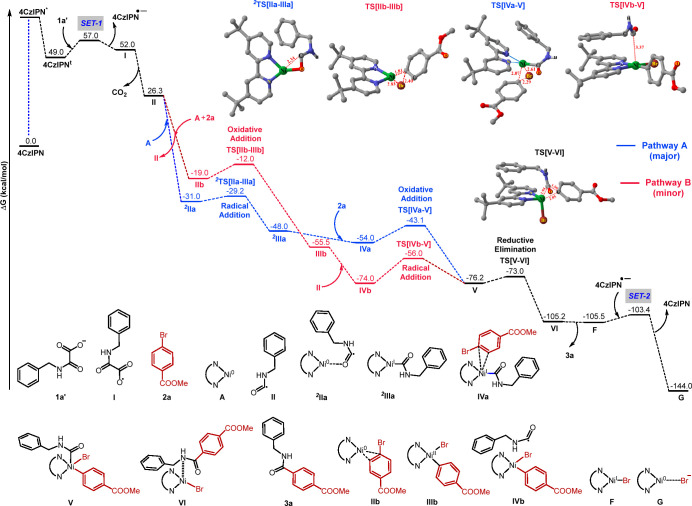
Recently, Jointly Molander–Kozlowski and Terrett–Huestis used density functional theory (DFT) calculations to determine the feasible reaction pathway for the coupling of carbon radicals with aryl bromides in dual Ni-photoredox catalysis.35,36 Their studies revealed that both mechanisms (Ni0–NiI–NiIII and Ni0–NiII–NiIII) are operatively dependent on the type of radical present under the reaction conditions. In order to find the feasible pathway under our reaction conditions, detailed computational studies were conducted using the oxamate anion (1a′) and aryl bromide (2a) as the substrates. The calculations were carried out at the UB3LYP-D3(BJ)/6-31G**, LANL2DZ(Ni)+SMD(DMF)//UB3LYP-D3(BJ)/6-31G*, LANL2DZ(Ni) level of theory. Our study suggests that the most probable mechanism for the reaction follows pathway A as shown in Figure 1. Pathway A in which the initial step is radical addition followed by oxidative insertion is similar to that proposed by Molander and Kozlowski in 2015.35 The other possibility is pathway B, where the initial step is an oxidative addition step followed by a radical addition step proposed by Terrett–Huestis and Doyle–MacMillan, was found to be higher in energy compared to that of pathway A.36,37 The relative Gibbs free energy profile diagram for pathways A and B is given in Figure 1. The important steps involved in the catalytic cycle following pathway A and pathway B are described in the following paragraphs.
Figure 1.
Computed Gibbs free energy profile diagram for the redox-neutral decarboxylative cross-coupling reaction catalyzed by the photocatalyst 4CzIPN and the LnNi0 species A obtained at the UB3LYP-D3(BJ)/6-31G**,LANL2DZ(Ni)+SMD(DMF)//UB3LYP-D3(BJ)/6-31G*,LANL2DZ(Ni) level of theory. The relative Gibbs free energies (in kcal/mol) of all intermediates and transition states are with respect to the oxamate anion 1a′, 4CzIPN in singlet state, LnNi0 species A, and the aryl bromide 2a. Ln = 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbbpy).
Initially, the photocatalyst 4CzIPN goes to an excited singlet state, 4CzIPN*, upon irradiation with Kessil light (456 nm) and then relaxes to the triplet state 4CzIPNt as shown in Figure 1. The single electron transfer (SET-1) from the oxamate anion 1a′ to the 4CzIPNt generates radical I and 4CzIPN•–. The energy barrier for SET-1 is 8.0 kcal/mol. The carbamoyl radical II is formed from I upon decarboxylation. Next, through pathway A, carbamoyl radical II enters the nickel catalytic cycle by coordinating to LnNi0 (Ln = 4,4′-di-tert-butyl-2,2′-dipyridyl) species A through the O atom, forming an intermediate 2IIa of energy −31.0 kcal/mol. Further, the intermediate 2IIa converted to a more stable LnNiI intermediate 2IIIa (−48.0 kcal/mol) in the doublet state and proceeded via a low barrier (1.8 kcal/mol) transition state 2TS[IIa-IIIa].38 The intermediate IVa of energy −54.0 kcal/mol is formed by the coordination of the LnNiI intermediate 2IIIa and aryl bromide 2a. The next step is the transformation of intermediate IVa through the oxidative insertion of nickel into the aryl–Br bond to form LnNiIII intermediate V of energy −76.2 kcal/mol that proceeded via the energy barrier (10.9 kcal/mol) transition state TS[IVa-V]. An alternative possibility is considered in pathway B, where the substrate 2a coordinates to LnNi0 species A to form intermediate IIb which has an energy of −19.0 kcal/mol. Next, intermediate IIb is transformed to more stable LnNiII intermediate IIIb (−55.5 kcal/mol) via a high energy barrier (7.0 kcal/mol) transition state TS[IIb-IIIb]. The intermediate V has been generated via the addition of carbamoyl radical II to the LnNiII intermediate IIIb, which proceeds through a high energy barrier (18 kcal/mol) via TS[IVb-V]. Next, in both pathways, the C–C bond formation between the aryl carbon of 2a and the amide carbon of 1a' via the reductive elimination of transition state TS[V-VI] takes place to form the product–catalyst complex VI. The energies of TS[V-VI] and VI are −73.0 and −105.2 kcal/mol, respectively. When the product 3a decoordinates from the nickel center, Ni(I) species F is formed, which in turn accepts an electron from 4CzIPN•– through single electron transfer (SET-2) process to form LnNi0 species G and 4CzIPN. The SET-2 barrier of 2.1 kcal/mol serves as the catalyst regeneration step in this mechanism. The increase in energy barrier (7.0 kcal/mol) for oxidative addition of 2a with LnNi0 makes pathway A a more favorable pathway.
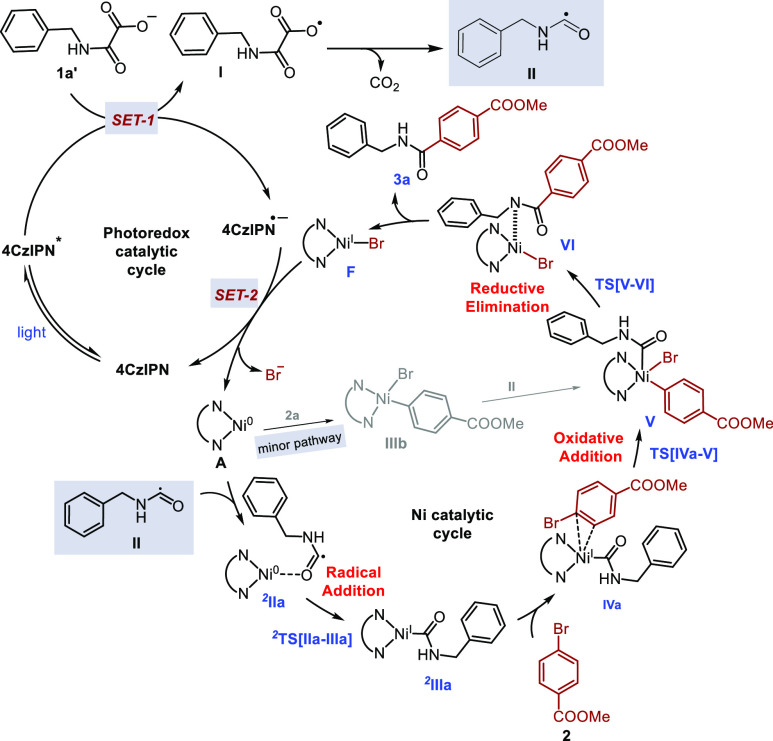
Combining all the above experimental and computational studies and following previous literature precedents,31−33,35,36 we proposed a feasible reaction mechanism in our catalytic cycle as depicted in Scheme 5. Initially, in the photocatalytic cycle, the single electron oxidation of benzylic cesium oxamates 1a (Eox = +0.74 V vs Fc in DMA, see Supporting Information for the CV of 1a) takes place by the photoexcited photocatalyst (4CzIPN*, Ered = +1.35 V vs SCE)39 resulting in the generation of the oxamate radical I and radical anion of the photocatalyst (4CzIPN•–). The radical intermediate I undergoes decarboxylation, leading to carbamoyl radical II. In the nickel cycle, the LnNiIIBr2 (Ln = dtbbpy) complex was transformed to LnNi0 complex (A) in the presence of visible-light photocatalysis utilizing benzylic cesium oxamate as a substoichiometric amount of reductant. Addition of carbamoyl radical to the LnNi0 complex generates LnNiI intermediate (2IIIa) via 2IIa. Next, the addition of aryl bromide to the LnNiI intermediate generates another LnNiIII intermediate V via IVa. Reductive elimination from the LnNiIII intermediate V liberates the product 3a and LnNiIBr intermediate F. Finally, single electron transfer between LnNiIBr and reduced photocatalyst (4CzIPN•–) regenerated ground-state photocatalyst (4CzIPN) and active LnNi0 (A) for the next catalytic cycle. Nevertheless, our catalytic cycle cannot completely ignore the alternative reaction pathway that is the initial oxidative addition of aryl bromide 2a to LnNi0 (A), followed by carbamoyl radical addition to generate the LnNiIII intermediate V (see Supporting Information for the complete cycle).40
Scheme 5. Mechanistic Hypothesis.
In summary, we have developed a new catalytic method for synthesizing amides through cross-coupling cesium oxamates with aryl bromides using dual nickel/photoredox catalysis. This strategy works under mild reaction conditions, and corresponding amides are isolated in good to moderate yields. The detailed analysis of the mechanism using DFT reveals that the favorable pathway of the nickel catalytic cycle begins with the radical addition elementary step prior to the oxidative insertion. Further, a variety of carbamoylation chemistry using the oxamates as the carbamoyl radical source is ongoing in our laboratory.
Acknowledgments
Financial support by the Council of Scientific & Industrial Research-Human Resource Development Group (CSIR-HRDG), Government of India (File Number: 02/0466/23/EMR-II) is greatly acknowledged. A.B. thanks MHRD (for PMRF). V.K.S. thanks CSIR-HRDG for the CSIR-JRF fellowship. The authors thank Alisha Rani Tripathy, Udita Choudhury, and Ashutosh Mishra for the synthesis and purification of some of the products. R.K. and S.K. gratefully acknowledge Science and Engineering Research Board (SERB), Government of India (File Number: SRG/2022/000307), and IIT Palakkad for the financial support, and Chandra high-performance computing cluster at IIT Palakkad for the computational facilities.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.3c00053.
General experimental procedures, mechanism studies, 1H and 13C NMR spectra of all compounds (1a–1m, 2s, 2u, 3a–3ah) (PDF)
Author Contributions
# V.K.S. and S.K. contributed equally. Credit: Akash Bisoyi: conceptualization (lead), data curation (lead), formal analysis (lead), investigation (lead), writing–review and editing (supporting); Vijay Kumar Simhadri: data curation (lead), formal analysis (supporting), investigation (supporting); Surya K: data curation (lead), formal analysis (supporting); Rositha Kuniyil: data curation (lead), formal analysis (lead); funding acquisition (lead), project administration (lead), resources (lead), supervision (lead), writing–original draft (lead); Veera Reddy Yatham: data curation (supporting), formal analysis (supporting), funding acquisition (lead), project administration (lead), resources (lead), supervision (lead), writing–original draft (lead).
The authors declare no competing financial interest.
Supplementary Material
References
- Sabatini M. T.; Boulton L. T.; Sneddon H. F.; Sheppard T. D. A green chemistry perspective on catalytic amide bond formation. Nature Catal. 2019, 2, 10–17. 10.1038/s41929-018-0211-5. [DOI] [Google Scholar]
- Pattabiraman V. R.; Bode J. W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]
- Shen B.; Makley D. M.; Johnston J. N. Umpolung reactivity in amide and peptide synthesis. Nature 2010, 465, 1027–1033. 10.1038/nature09125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massolo E.; Pirola M.; Benaglia M. Amide Bond Formation Strategies: Latest Advances on a Dateless Transformation. Eur. J. Org. Chem. 2020, 2020, 4641–4651. 10.1002/ejoc.202000080. [DOI] [Google Scholar]
- de Figueiredo R. M.; Suppo J.-S.; Campagne J.-M. Nonclassical Routes for Amide Bond Formation. Chem. Rev. 2016, 116, 12029–12122. 10.1021/acs.chemrev.6b00237. [DOI] [PubMed] [Google Scholar]
- Greenberg A.; Breneman C. M.; Liebman J. F.. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley–Interscience: Chichester, NY, 2000. [Google Scholar]
- Kumar V.; Bhatt V.; Kuma N.. Amides From Plants: Structures and Biological Importance. In Studies in Natural Products Chemistry; Rahman A.-U., Ed., Elsevier B.V.: Amsterdam, 2018, Vol. 56, pp 287–333. [Google Scholar]
- Roughley S. D.; Jordan A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- Bray B. L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discovery 2003, 2, 587–593. 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- Valeur V.; Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. 10.1039/B701677H. [DOI] [PubMed] [Google Scholar]
- Allen C. L.; Williams J. M. J. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. 10.1039/c0cs00196a. [DOI] [PubMed] [Google Scholar]
- Roy S.; Roy S.; Gribble G. W. Metal-catalyzed amidation. Tetrahedron 2012, 68, 9867–9923. 10.1016/j.tet.2012.08.065. [DOI] [Google Scholar]
- Veatch A. M.; Alexanian E. J. Cobalt-catalyzed aminocarbonylation of (hetero)aryl halides promoted by visible light. Chem. Sci. 2020, 11, 7210–7213. 10.1039/D0SC02178D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kancherla R.; Naveen T.; Maiti D. Palladium-Catalyzed [3 + 3] Annulation between Diarylamines and α,β-Unsaturated Acids through C–H Activation: Direct Access to 4-Substituted 2-Quinolinones. Chem.—Eur. J. 2015, 21, 8360–8364. 10.1002/chem.201500774. [DOI] [PubMed] [Google Scholar]
- Schoenberg A.; Heck R. F. Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic halides. J. Org. Chem. 1974, 39, 3327–3331. 10.1021/jo00937a004. [DOI] [Google Scholar]
- Brennfuhrer A.; Neumann H.; Beller M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem., Int. Ed. 2009, 48, 4114–4133. 10.1002/anie.200900013. [DOI] [PubMed] [Google Scholar]
- Martinelli J. R.; Clark T. P.; Watson D. A.; Munday R. H.; Buchwald S. L. Palladium-Catalyzed Aminocarbonylation of Aryl Chlorides at Atmospheric Pressure: The Dual Role of Sodium Phenoxide. Angew. Chem., Int. Ed. 2007, 46, 8460–8463. 10.1002/anie.200702943. [DOI] [PubMed] [Google Scholar]
- Friis S. D.; Skrydstrup T.; Buchwald S. L. Mild Pd-Catalyzed Aminocarbonylation of (Hetero)Aryl Bromides with a Palladacycle Precatalyst. Org. Lett. 2014, 16, 4296–4299. 10.1021/ol502014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang T. T.; Zhu Y.; Ngiam J. S. Y.; Ghosh S. C.; Chen A.; Seayad A. M. Palladium Nanoparticles Supported on ZIF-8 As an Efficient Heterogeneous Catalyst for Aminocarbonylation. ACS Catal. 2013, 3, 1406–1410. 10.1021/cs400232b. [DOI] [Google Scholar]
- Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Kärkäs M. D.; Porco J. A. Jr; Stephenson C. R. J. Photochemical approaches to complex chemotypes: applications in natural product synthesis. Chem. Rev. 2016, 116, 9683–9747. 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover J.; Prakash G.; Teja C.; Lahiri G. K.; Maiti D. Metal-Free Photoinduced Hydrogen Atom Transfer Assisted C(sp3)–H Thioarylation. Green Chem. 2023, 25, 3431–3436. 10.1039/D3GC00359K. [DOI] [Google Scholar]
- Chan A. Y.; Perry I. B.; Bissonnette N. B.; Buksh B. F.; Edwards G. A.; Frye L. I.; Garry O. L.; Lavagnino M. N.; Li B. X.; Liang Y.; Mao E.; Millet A.; Oakley J. V.; Reed N. L.; Sakai H. A.; Seath C. P.; MacMillan D. W. C. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. 10.1021/acs.chemrev.1c00383. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Yue H.; Chu L.; Rueping M. Recent advances in photoredox and nickel dual-catalyzed cascade reactions: pushing the boundaries of complexity. Chem. Sci. 2020, 11, 4051–4064. 10.1039/D0SC00712A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A.; Guin S.; Ali W.; Bhattacharya T.; Sasmal S.; Goswami N.; Prakash G.; Sinha S. K.; Chandrashekar H. B.; Panda S.; Anjana S. S.; Maiti D. Photoinduced Regioselective Olefination of Arenes at Proximal and Distal Sites. J. Am. Chem. Soc. 2022, 144, 1929–1940. 10.1021/jacs.1c12311. [DOI] [PubMed] [Google Scholar]
- Saha A.; Ghosh A.; Guin S.; Panda S.; Mal D. K.; Majumdar A.; Akita M.; Maiti D. Substrate-Rhodium Cooperativity in Photoinduced ortho-Alkynylation of Arenes. Angew. Chem., Int. Ed. 2022, 61, e202210492 10.1002/anie.202210492. [DOI] [PubMed] [Google Scholar]
- Ali W.; Saha A.; Ge H.; Maiti D. Photoinduced meta-Selective C–H Oxygenation of Arenes,. JACS Au 2023, 3, 1790–1799. 10.1021/jacsau.3c00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo B. T.; Oliveira P. H. R.; Pissinati E. F.; Vega K. B.; de Jesus I. S.; Correia J. T. M.; Paixao M. Photoinduced Carbamoylation Reactions: Unlocking New Reactivities towards Amide Synthesis. Chem. Commun. 2022, 58, 8322–8339. 10.1039/D2CC02585J. [DOI] [PubMed] [Google Scholar]
- Cheng W.-M.; Shang R.; Yu H.-Z.; Fu Y. Room-Temperature Decarboxylative Couplings of α-Oxocarboxylates with Aryl Halides by Merging Photoredox with Palladium Catalysis. Chem.—Eur. J. 2015, 21, 13191–13195. 10.1002/chem.201502286. [DOI] [PubMed] [Google Scholar]
- Alandini N.; Buzzetti L.; Favi G.; Schulte T.; Candish L.; Collins K. D.; Melchiorre P. Amide Synthesis by Nickel/Photoredox-Catalyzed Direct Carbamoylation of (Hetero)Aryl Bromides. Angew. Chem., Int. Ed. 2020, 59, 5248–5253. 10.1002/anie.202000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S.; Roy S.; Ghosh P.; Kasera A.; Maiti D. Photo-Excited Nickel-Catalyzed Silyl-Radical-Mediated Direct Activation of Carbamoyl Chlorides To Access (Hetero)aryl Carbamides. Angew. Chem., Int. Ed. 2022, 61, e2022074 10.1002/anie.202207472. [DOI] [PubMed] [Google Scholar]
- The mass balance accounts for the reduction of aryl bromides, conversion of oxamates to formamide, and unreacted aryl bromides.
- Gutierrez O.; Tellis J. C.; Primer D. N.; Molander G. A.; Kozlowski M. C. Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereoconvergence in Nickel-Catalyzed Cross-Couplings. J. Am. Chem. Soc. 2015, 137, 4896–4899. 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahdouzan K.; Khalaf R.; Grandner J. M.; Chen Y.; Terrett J. A.; Huestis M. P. Dual Photoredox/Nickel-Catalyzed Conversion of Aryl Halides to Aryl Aminooxetanes: Computational Evidence for a Substrate-Dependent Switch in Mechanism. ACS Catal. 2020, 10, 405–411. 10.1021/acscatal.9b03596. [DOI] [Google Scholar]
- Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alternative radical addition transition state in quartet spin state 4TS[IIa-IIIa] was found to be 23.2 kcal/mol higher in energy compared to that in doublet spin state. See Figure 60 in the Supporting Information for more details on radical addition transition state in quartet spin state.
- Shang T. Y.; Lu L. H.; Cao Z.; Liu Y.; He W. M.; Yu B. Recent advances of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) in photocatalytic transformations. Chem. Commun. 2019, 55, 5408–5419. 10.1039/C9CC01047E. [DOI] [PubMed] [Google Scholar]
- After submission of this manuscript, a related transformation was reported using oxamic acids as carbamoyl radical source and couples with aryl bromides through Dual Nickel-and Photoredox-Cataysis.; Hutskalova V.; Hamdan F. B.; Sparr C.. Org. Lett., 2023, ASAP. 10.1021/acs.orglett.3c02389. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.