Abstract

The present review summarizes important aspects of the crystal chemistry of ytterbium-based intermetallic compounds along with a selection of their outstanding physical properties. These originate in many cases from the ytterbium valence. Different valence states are possible here, divalent (4f14), intermediate-valent, or trivalent (4f13) ytterbium, resulting in simple diamagnetic, Pauli or Curie–Weiss paramagnetic, or valence fluctuating behavior. Especially, some of the Yb3+ intermetallics have gained deep interest due to their Kondo or heavy Fermion ground states. We have summarized their property investigations using magnetic and transport measurements, specific heat data, NMR, ESR, and Mössbauer spectroscopy, elastic and inelastic neutron scattering, and XAS data as well as detailed thermoelectric measurements.
Keywords: ytterbium, intermediate valence, Kondo effect, heavy Fermion, 170Yb Mössbauer spectroscopy, 171Yb NMR, ESR, XAS, XPS, valence fluctuations
Ytterbium is one of the few rare earth elements that is prone to valence instabilities in intermetallic compounds. In the rare earth series, many examples are known for cerium, where the interplay between the 4f1 configuration for Ce3+ and the empty f shell for tetravalent cerium led to a huge number of compounds with broadly varying magnetic and electrical properties, such as long-range magnetic ordering (antiferro-, ferro-, or ferrimagnetism), Kondo-type behavior, heavy Fermion behavior as well as static or dynamic intermediate cerium valence. These many facets of structure–property relationships of cerium intermetallics have been summarized in review articles.1−6 While only a few examples are known for divalent samarium (the trivalent state is usually observed),7 many europium compounds have been studied with respect to their valence behavior.8−10 Most of these compounds exhibit the stable half-filled f shell (4f7 configuration), and consequently, divalent europium is the usual case for intermetallics along with paramagnetism and long-range magnetic ordering, sometimes at comparatively high ordering temperatures. The scarce case of trivalent europium in intermetallic phases has recently been reviewed.10
The present review focuses on intermetallic ytterbium compounds. Here, the configurations 4f14 (filled f shell; divalent ytterbium; diamagnetic) and 4f13 (paramagnetic trivalent ytterbium) are the edge states, and static and dynamic ytterbium valence states are observed in-between. Especially, the trivalent ytterbium compounds have attracted high attention in solid-state chemistry and physics, since the 4f13 configuration is the 4f hole analogue to Ce3+. Thus, the ground state properties of these ytterbium-based phases are highly sensitive to the amount of 4f electron (hole)–conduction electron hybridization (Kondo effect).11−14 This influences the Ruderman–Kittel–Kasuya–Yosida (RKKY) exchange interactions and consequently the magnetic ordering. The broad analogy to cerium intermetallics is especially interesting with respect to intermediate-valence and potential heavy Fermion materials.15−17
Materials science can only work with an excellent interplay of solid-state physics and solid-state chemistry, whereby detailed phase analyses and high-quality sample preparation along with precise structure determination are the indispensable prerequisites for reliable property measurements. Herein we review these features for the field of intermetallic ytterbium compounds with a strong emphasis on the ytterbium valence behavior.
The review is written from a solid-state chemist’s point of view. Bearing the enormous number of compounds18 and property measurements in mind, it is simply not possible to include all known intermetallic ytterbium compounds and all references into such an overview (e.g., > 350 references for YbRh2Si2 in SciFinder19). Herein we mainly concentrate on the role of the ytterbium valence in intermetallics and underpin these peculiarities with selected examples. Any missing compound or reference is unintentional.
SYNTHESES AND CRYSTAL GROWTH
The peculiar electronic structure of ytterbium has drastic effects on its physical properties, when compared with the neighboring rare earth elements. Its divalent nature already in the elemental state leads to drastically reduced melting (1097 K) and boiling (1466 K) points.20 Arc-melting is a broadly used synthesis technique for intermetallic samples;21 however, drastic evaporation losses can occur in the cases of ytterbium-based synthesis, when reacting ytterbium with the high-melting noble metals, e.g., platinum (mp 2025 K) or iridium (mp 2683 K). To give an example, during an arc-melting synthesis of YbRh2Si2,22 the ytterbium would largely evaporate before the rhodium melts. Thus, only a few ytterbium intermetallics were prepared by arc melting. Representative examples are YbNiAl423 or Yb3Rh9Si2Sn3.24 For the precise reaction conditions, we refer to the individual publications. In the present chapter, we summarize the different techniques that find application.
Broader phase analytical studies were conducted by simple, direct reaction of the elements in sealed evacuated fused silica ampules. This is possible for almost all YbxTyXz samples with X = elements of the group 13, 14, or 15. The inner surface of the silica ampules can be graphitized (thermal decomposition of acetone) for passivation, or alumina crucibles can be used as inert container material. The reactions and annealing sequences are usually carried out in tube or muffle furnaces. These reactions are not that far from the classical ceramic method25 for the preparation of solids. Often the samples are still multiphase after the first annealing cycle. Thus, repeated grinding, pressing of pellets, and reannealing is necessary in order to obtain samples that are at least phase-pure on the level of powder X-ray diffraction. A critical feature is the repeated grinding, since the increasing surface area might lead to surface oxidation and thus Yb2O3 contamination (vide infra). This ceramic technique was frequently used for the synthesis of ytterbium-based ternary phosphides and arsenides.26 The best way to avoid these Yb2O3 contaminations is the use of distilled ytterbium and working under purified inert gas.
For physical property measurements, high sample purity is required. Nowadays, the intermetallic ytterbium phases are prepared in sealed (arc-welded27 under argon or helium atmosphere) high-melting metal tubes, usually niobium or tantalum, but molybdenum can also find application. This ensures largely inert crucible conditions and prevents evaporation losses of ytterbium. For the reaction, the metal tubes can be sealed in silica tubes (oxidation protection), and the annealing sequence can be run in a tube or muffle furnace. Much better results are obtained through induction melting and subsequent annealing.28,29 Good quantities of X-ray pure polycrystalline samples are available by this technique.
Direction-dependent property measurements can only be performed on high-quality single crystals. This deserves sophisticated crystal growth conditions. The flux growth technique30−33 is meanwhile well established in the field of crystal growth of intermetallic phases. Usually the low-melting p block elements aluminum, gallium, indium, tin, and bismuth find application as flux medium. Two strategies for crystal growth are possible. The easy way is the self-flux technique (the so-called reactive flux), where the low-melting p element is a component of the product and flux medium at the same time. Otherwise, one can use an inert flux (the so-called nonreactive flux), which does not enter the product crystals. There are some prerequisites for targeted crystal growth: (i) the flux medium should be inert toward the crucible material and (ii) the excess flux should easily be separable from the product crystals.
Typical examples for flux-grown crystals are YbRh2Si2 and YbIr2Si2,34 or Yb2CuGe6 and Yb3Cu4Ge4,35 which form in liquid indium. Also, Yb2AuGe3 crystals can be grown from liquid indium with up to 2 mm edge size.36 The excess flux can be dissolved in diluted hydrochloric acid. Similar flux growth experiments are feasible with aluminum and gallium as the flux medium.37 A interesting example for a nonreactive flux is the growth of YbRh6P4 crystals in liquid bismuth.38 Besides dissolution of the flux after successful crystal growth, the liquid flux can also be separated by centrifugation at elevated temperatures. Here are two possible ways: (i) a second crucible filled with quartz wool or a quartz wool plug can be placed (reversed) on top of the one containing the starting materials30 or (ii) a frit-disc crucible set (“Canfield crucible set”) can be utilized.39
Finally, we turn back to the classical textbook examples for crystal growth. Due to the high vapor pressure of molten ytterbium, the Czochralski technique cannot be used. The Bridgman technique (where again sealed tubes are used) was successfully applied for crystal growth of the copper-rich ytterbium compounds YbCu4.25 and YbCu4.4.40 Tantalum was used as container materials, and the crucible lowering speed at the high-frequency coil was 3 mm h–1. Crystals for diffraction experiments were finally separated from different slices that were prepared from the bulk crystal via spark erosion. Further examples for the Bridgman crystal growth are the germanides Yb2Ru3Ge4,41 YbPdGe, and YbPtGe.42
CRYSTAL CHEMICAL PRINCIPLES
The present review has a higher focus on the physical properties of ytterbium intermetallics, and we therefore only give a short and concise overview on some of the basic crystal chemical principles that are important for the family of YbxTyXz intermetallics. The structural chemistry is mostly driven by the ytterbium valence. As discussed above, the filled 4f shell of Yb(II) might be considered as a diamagnetic core–electron shell, and consequently, we observe a crystal chemical behavior of an alkaline earth element. This is readily understandable when comparing the ionic radii for coordination number 6 for Ca2+ (100 pm) and Yb2+ (102 pm).43 We thus observe a close structural relationship of the divalent ytterbium intermetallics with their respective calcium analogues. Typical examples are the binary Zintl phases Ca2Ge44 and Yb2Ge45 as well as the ternary Zintl phases CaZnSn46 and YbZnSn47 with many more pairs of isotypic compounds than for Yb2+/Ca2+.48
A similar close relationship is known for Eu2+ (117 pm) and Sr2+ (118 pm).43 Some examples are EuAl2Pt and SrAl2Pt (MgCuAl2 type, space group Cmcm),49 EuAl5Pt350 and SrAl5Pt351 (YNi5Si3 type, space group Pnma), SrAu3Al252 and EuAu3Al253 (RbAu3Ga2 type, space group Pnma), EuAu2Al254 and SrAu2Al254,55 (CaBe2Ge2 type, space group P4/nmm), Eu2T2In56 and Sr2T2In57 (T = Pd, Pt; Ca2Ir2Si type, space group C2/c), EuRh2In858 and SrRh2In859 (CeFe2Al8 type, space group Pbam), EuPtSi and SrPtSi (LaIrSi type, space group Pnma),60 or finally EuPt2Si2 and SrPt2Si2 (ThCr2Si2 type, space group I4/mmm).61 Besides the direct comparisons Sr2+/Eu2+ and Ca2+/Yb2+, it is not always possible to directly compare an Eu(II) example and an Yb(II) example within one series of compounds, since (i) the structure type is not formed with both cations or (ii) the given ytterbium is trivalent. And even if both compounds are known and adopt the same structure type, the physical properties are significantly different due to Yb(II) being a diamagnetic cation while Eu(II) exhibits a half-filled 4f shell (4f7).
In the case of trivalent ytterbium intermetallics, the ytterbium compound usually adopts the crystal structure of the neighboring compounds existing with thulium or lutetium. A first fast hint for the ytterbium valence can be gathered from the plot of the unit cell volumes of a given series of rare earth compounds, a so-called Iandelli plot. As examples, we present the series of REPdZn62−64 and RE2Pt2Pb47,65 phases in Figure 1. Since divalent ytterbium has a distinctly larger radius than trivalent ytterbium, positive deviations in the Iandelli plot (this is the case for the REPdZn series) indicate divalent ytterbium. If the unit cell volume fits smoothly into the plot, then ytterbium is trivalent (this is the case for the RE2Pt2Pb series). Nevertheless, one important observation needs to be mentioned at this point. Although the physical property measurements (vide infra) show stable trivalent ytterbium in Yb2Pt2Pb, the Iandelli plot shows a very small positive deviation. Such an anomaly is frequently observed for rare earth series with trivalent ytterbium. Another striking series with such a weak positive deviation is the one of REAl3C3.66 Many more examples for both cases can be found when checking structural databases like the Pearson database.18
Figure 1.

Plot of the unit cell volumes for the series REPdZn (per formula unit) and RE2Pt2Pb (subcell volumes). Data was taken from the Pearson database.18
Compounds that exhibit temperature- or pressure-dependent valence phase transitions show a discontinuity in their lattice parameters. YbPd2Al3 (YNi2Al3 type, space group P6/mmm), for example, exhibits a mixed valent state (YbII and YbIII) at room temperature which transitions into an almost fully divalent state below Ttrans = 110 K. The temperature dependence of the lattice parameters is shown in Figure 2. Here, a clear increase of the c lattice parameter is visible (+3 pm, 0.7%). Due to the relatively high transition temperature, single crystal studies were possible, that confirmed the thermal behavior of the lattice parameters and clearly indicated that no structural phase transition takes place. However, significant distortions in the respective Yb coordination polyhedra are observed.67
Figure 2.

Lattice parameters of YbPd2Al3 obtained from temperature-dependent powder X-ray diffraction experiments upon cooling from 300 to 10 K and from single-crystal X-ray diffraction experiments. Reprinted with permission from ref (67).
Finally, a very recent study of Yb2Pd2Cd should be mentioned. Here, a structural phase transition at ∼150 K was observed. The high-temperature modification adopts the Mo2B2Fe type (space group P4/mbm) while below the phase transition temperature, LT-Yb2Pd2Cd is found in a new structure type (space group P4/mbm) with a doubled c axis. The striking structural feature concerns the ytterbium–palladium coordination. The average Yb–Pd distances distort, forming a smaller and a larger coordination environment and enabling a charge separation for Yb2+ and Yb3+.68
SELECTED CRYSTAL STRUCTURES
In the present chapter, we want to selectively focus on some structural principles and refer to extended crystal chemical review articles for further reading. Some of the YbxTyXz phases crystallize with their individual structure type which have all been thoroughly described in the original articles.
Many of the intermetallic ytterbium compounds belong to the so-called equiatomic phases YbTX (T = (transition) metal; X = element of group 13, 14, or 15). The most frequent structure types that occur for these phases are TiNiSi (space group Pnma), ZrNiAl (space group P62m), MgAgAs (space group F43m), PbFCl (space group P4/nmm), and other AlB2 (space group P6/mmm) derived superstructures. Their crystal chemistry has been reviewed in the Handbook on the Physics and Chemistry of Rare Earths.13 These structure types allow for a certain structural flexibility. To give an example, for the orthorhombic TiNiSi type, a manifold of ytterbium-based phases YbTX (T = Mg, Ni, Cu, Rh, Pd, Pt, Au; X = Al, Si, P, Ga, Ge, Sn, Sb) have been synthesized.13,18 Apart from the overview on the equiatomic YbTX phases, the crystal chemistry of these versatile structure types has repeatedly been discussed in various other review articles.3−6,8,69−72 We refer to these overviews for further reading.
A huge number of property studies has been conducted for the binary aluminides YbAl2 (MgCu2 type, cubic Laves phase, space group Fd3m)73 and YbAl3 (Cu3Au type; ordered cubic closest packing, space group Pm3m).74,75 Both compounds crystallize with well-known basic structure types. In this context it is interesting to note that ternary ones have also been reported with the structure types of the cubic and hexagonal Laves phases. In the case of the MgCu2 type phases, especially the solid solution members Yb1–xInxCu2 have been studied, where the ytterbium substructure has been magnetically diluted through indium substitution. YbCu4In76 shows 1:1 Yb/In ordering and crystallizes with the noncentrosymmetric MgCu4Sn type (space group F43m). The crystal chemical details of Laves phase superstructures have recently been reviewed.77 Several aluminides were reported as equiatomic compounds, and the MgZn2 type (space group P63/mmc) was assigned.13 Meanwhile, it is known that an equiatomic composition with complete atom ordering is not possible. The superstructure formation leads to a small deviation. The correct model was solved for Yb6Ir5Ga7 (space group P63/mcm) and the series of gallides RE6Ir5Ga7.78,79 Shortly later, also the aluminum containing RE6T5Al7 phases (RE = Sc, Y, Ce–Nd, Sm, Gd–Lu; T = Ru, Ir)80 have been structurally characterized. With respect to the physical properties, one must differentiate between the fully ordered 6–5–7 phases and solid solutions where mixed occupied sites occur. Often, disorder destroys magnetic ordering or drastically reduces the magnetic ordering temperature.
We now discuss the crystal chemistry of some selected examples. One of the striking ternary ytterbium intermetallics is YbRh2Si2 (ThCr2Si2 type, space group I4/mmm) originally reported in 1979 by Rossi et al.22 Due to its outstanding physical properties (vide infra), meanwhile >350 publications are listed in SciFinder.19 The YbRh2Si2 unit cell is presented in Figure 3. The rhodium atoms have a tetrahedral silicon coordination with Rh–Si distances of 235 pm. These [RhSi4] tetrahedra are condensed via edge-sharing, building a dense layer in the ab plane. Adjacent layers are connected via Si–Si bonds (246 pm). The resulting network provides large cages for the ytterbium atoms which have a coordination number of 18, i.e., Yb@Si10Rh8. Due to the large cages, the shortest Yb–Yb distances are 401 pm, corresponding to the a lattice parameter. The ThCr2Si2 family of compounds is one of the largest among all intermetallic compounds with more than 4000 entries in the Pearson database.18 The crystal chemical details for these materials are summarized in diverse overviews.1,69,81,82
Figure 3.

Crystal structures of YbRh2Si222 and YbIrIn5.83 Ytterbium, rhodium (iridium), and silicon (indium) atoms are drawn as medium gray, blue, and magenta circles, respectively. The network of edge-sharing [RhSi4] tetrahedra for YbRh2Si2 and the characteristic polyhedra for YbIrIn5 are emphasized.
Another, smaller series of tetragonal compounds concerns those with HoCoGa5 type structure84 (space group P4/mmm). This type has been observed for YbCoGa5 and the indides YbTIn5 (T = Co, Rh, Ir).83,85 The YbIrIn5 unit cell83 is also presented in Figure 3. The YbIrIn5 structure is composed of two striking building units. The ytterbium atoms have slightly tetragonally distorted cuboctahedral indium coordination (8 × 320 and 4 × 327 pm Yb–In), which can be considered as a cutout of an ordered closest fcc related packing (given the comparable size of ytterbium and indium). These cuboctahedra condense to layers via four common rectangular faces. These layers alternate with layers of condensed Ir@In8 square prisms (275 pm Ir–In). Similar to YbRh2Si2 discussed above, the ytterbium atoms in YbIrIn5 are also well separated from each other. The shortest Yb–Yb distance of 462 pm again corresponds to the lattice parameter a.
Yb2Pt2Pb43 is one of the few ytterbium intermetallics that belong to the family of U3Si2 derived phases. Due to a puckering effect, one observes superstructure formation for Yb2Pt2Pb (space group P42/mnm) with a small distortion of the ytterbium trigonal and square prisms. For better visibility, only one layer is presented in Figure 4. A more detailed crystal chemical discussion is given in references (86 and 87). The striking feature of this plumbide is the Shastry–Sutherland-like substructure of the ytterbium atoms, which gives rise to magnetic frustration (discussed in detail in the magnetic part, vide infra). Several years after the discovery of the compound,47 this has motivated many detailed property studies, which are addressed below.
Figure 4.

Cutout of the Yb2Pt2Pb47 structure. Ytterbium, platinum, and lead atoms are drawn as medium gray, blue, and magenta circles, respectively. The CsCl and AlB2 related slabs and the two crystallographically independent ytterbium sites are emphasized. Only one layer is shown for better visibility.
A complex cubic structure occurs for the aluminum- and zinc-rich compounds YbT2Al20 (T = Ti, V, Cr) and YbT2Zn20 (T = Co, Rh, Ir).18 They crystallize with the CeCr2Al20 type, space group Fd3m with 184 atoms per unit cell. The main interest in these ytterbium compounds concerned their heavy-Fermion type behavior. The many facets of their physical properties have recently been summarized.88 As an example we present the YbCo2Zn20 structure89 in Figure 5. Although the structure seems complex at first sight, it can easily be constructed from two basic building units. The ytterbium and cobalt atoms of YbCo2Zn20 form a substructure that is isopointal with the cubic Laves phase MgCu2, i.e., has the same space group symmetry. The zinc atoms now surround the ytterbium and cobalt atoms, leading to Yb@Zn16 and Co@Zn12 polyhedra which condense in a tetrahedral motif. Again, the ytterbium atoms are well separated from each other and show no bonding contacts. This “highly diluted” rare earth substructure is prone for basic property studies.
Figure 5.

Cutout of the YbCo2Zn2089 structure. Cobalt and zinc atoms are drawn as medium blue and magenta circles, respectively. The connectivity pattern of the Yb@Zn16 and Co@Zn12 polyhedra is emphasized. For details see text.
In Figure 6, we now turn to a series of ytterbium compounds with complex extended polyanionic networks which exhibit multiple ytterbium sites. Exemplarily, we present the structures of Yb3Pd4Ge4,90 Yb2Pt3Sn5,29 and Yb2Au3In5.91 The common structural feature concerns the dense condensation of the transition metal and p element atoms within the polyanionic networks [Pd4Ge4], [Pt3Sn5], and [Au3In5]. The latter are stabilized through Pd–Ge, Pt–Sn, Au–In as well as Ge–Ge, Sn–Sn, and In–In bonding interactions. The networks then leave cavities of different sizes which are filled by the ytterbium atoms. This leads to the coordination polyhedra Yb1@Ge6Pd6 and Yb2@Ge6Pd8 for Yb3Pd4Ge4, Yb1@Sn6Pt2 and Yb2@Pt5Sn8 for Yb2Pt3Sn5 as well as Yb1@Au4In10 and Yb2@Au5In8 for Yb2Au3In5. The three compounds tend to exhibit mixed ytterbium valences. From a crystal chemical point of view, the smaller cavities are filled by trivalent ytterbium (static mixed valency). For Yb2Pt3Sn5, this feature was tested also from a synthetic point of view. Given the comparable size of Yb2+ and Ca2+ it was possible to synthesize the stannides CaErPt3Sn5, CaTmPt3Sn5, CaYbPt3Sn5, and CaLuPt3Sn5 with calcium site preference on the “divalent” place.92
Figure 6.

View of the Yb3Pd4Ge4,90 Yb2Pt3Sn5,29 and Yb2Au3In591 structures along the short unit cell axis. Ytterbium, palladium (platinum, gold), and germanium (tin, indium) atoms are drawn as medium gray, blue, and magenta circles, respectively. The [Pd4Ge4], [Pt3Sn5], and [Au3In5] networks and the crystallographically independent ytterbium sites are emphasized.
The last crystal chemical example concerns ytterbium aluminum borides. Figure 7 shows projections of the α- and β-YbAlB493 and Yb2AlB694 structures along the short unit cell axis. The boron atoms build up planar layers with different tessellations, which are stacked in a ...AA... sequence. Between the layers, pentagonal prisms for the smaller aluminum atoms and hexagonal, respectively, heptagonal ones for the larger ytterbium atoms are formed. The two modifications of YbAlB4 show ytterbium exclusively in the heptagonal prisms, while two prism types occur in Yb2AlB6. The YbAlB4 modifications gained high interest in recent years since they exhibit heavy Fermion ground state and superconductivity at low temperatures (vide infra). Already more than 120 publications appeared for this composition.19
Figure 7.

View of the α-YbAlB4,93 β-YbAlB493 and Yb2AlB694 structures along the short unit cell axis. Ytterbium, aluminum, and boron atoms are drawn as medium gray, blue, and magenta circles, respectively. The boron nets and the crystallographically independent ytterbium sites are emphasized.
PHYSICAL PROPERTIES AND SPECTROSCOPY
Given the large number of known phases and the fact that some of them have only been characterized with respect to their structure, the review focuses on the ytterbium valence and selected physical properties that arise from these. Thus, selected compounds with a divalent or trivalent ytterbium state are discussed to outline these properties.
Most compounds have routinely been studied with respect to their magnetic behavior in order to get a first hint for the ytterbium valence. A significantly smaller section of these studies is additionally accompanied by resistivity and specific heat measurements; however, these measurements are usually not routinely conducted. Therefore, magnetic, resistivity, and specific heat investigations are, where applicable, discussed together in the following chapter. Subsequently, separate chapters summarize the data on more sophisticated investigation techniques such as (i) inelastic neutron scattering, (ii) XANES/XAS, (iii) X-ray photoelectron spectroscopy (XPS), (iv) nuclear magnetic and electron resonance spectroscopy (NMR and ESR), (v) 171Yb Mössbauer spectroscopy, and (vi) thermoelectric measurements.
Within each property chapter, the data has been summarized with respect to composition (binary or ternary) as well as structure types or structural families (e.g., Zintl phases), facilitating the comparability with respect to the valence electron count, etc.
MAGNETISM, RESISTIVITY AND SPECIFIC HEAT
Before starting with a broader discussion on the magnetic properties of intermetallic ytterbium compounds, we need to comment on ytterbium sesquioxide, Yb2O3, the most inconvenient byproduct occurring during the synthesis of Yb intermetallics. Yb2O3 exhibits paramagnetism (4f13 configuration; TN = 2.3 K95) and can dominate the susceptibility in the low-temperature regime,96 especially in the case of intermediate-valent or almost divalent ytterbium intermetallics. In those cases, a clear quantification of the Yb2O3 content is necessary along with a correction procedure.97 If this feature is not taken into account, the interpretation of the magnetic data remains ambiguous. Since many of the ytterbium intermetallics order magnetically at very low temperature, the Yb2O3 impurity might overlay the intrinsic magnetism of the sample.
Divalent Ytterbium Compounds
Purely divalent ytterbium compounds have the valence electron configuration 4f14, which exhibits high stability. This filled 4f shell can be considered as a core-electron shell; therefore, no magnetic moment is observed. Such ytterbium intermetallics behave either as diamagnets or as Pauli paramagnets. Since most of these phases are metallic conductors, the observed susceptibility usually arises from an overcompensation of the intrinsic core diamagnetism by the Pauli susceptibility caused by the conduction electrons.
Exemplarily, the temperature dependences of the magnetic susceptibility of Yb5Cu2Zn and Yb3Ag4Mg12 are given in Figure 8. Above 100 K, the susceptibilities are very weak and almost temperature-independent. The room temperature value of 7.2 × 10–6 emu mol–1 for Yb5Cu2Zn indicates Pauli paramagnetism.98 In contrast, in Yb3Ag4Mg12 the intrinsic diamagnetism is not as strongly overcompensated by the Pauli contribution, leading to a room temperature value of −4.61 × 10–4 emu mol–1.99 The susceptibility increases in the low-temperature regimes (the so-called Curie tails), which originates from traces of paramagnetic impurities. Further examples of room temperature susceptibilities are given in Table 1. In these compounds, the ytterbium atoms are divalent and therefore carry no magnetic moment.
Figure 8.

Temperature dependence of the magnetic susceptibility χ of Yb3Ag4Mg1299 and Yb5Cu2Zn,98 measured at 10 kOe.
Table 1. Magnetic Susceptibilities of Selected Ytterbium Intermetallics at Room Temperaturea.
| Compound | χ/emu mol–1 | Reference |
|---|---|---|
| YbAgMg | +1.2(1) × 10–4 | (100) |
| YbAuMg | +2.4 × 10–4 | (101) |
| Yb3Ag4Mg12 | –4.61 × 10–4 | (99) |
| Yb5Cu2Zn | +7.2 × 10–6 | (98) |
| YbAgCd | +4.8 × 10–5 | (102) |
| YbAuCd | +5.8 × 10–5 | (101) |
| YbPdIn2 | –4.9(2) × 10–4 | (103) |
| YbCuSn | +4.22 × 10–4 | (104) |
| YbAgSn | –1.37 × 10–5 | (104) |
| YbAuSn | –2.62 × 10–5 | (104) |
| YbZnSn | +2.0(1) × 10–4 | (47) |
| YbCuSb | +1.38 × 10–4 | (104) |
| YbAgSb | –7.69 × 10–5 | (104) |
| YbAuSb | –8.47 × 10–5 | (104) |
Standard deviations are given in parentheses, where available.
Trivalent Ytterbium Compounds
Trivalent ytterbium cations
have a 4f13 valence electron configuration. The g value of 8/7 and the total angular momentum of J = 7/2 lead to a magnetic moment of μ = 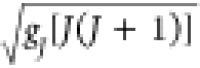 = 4.54 μB per Yb3+.105 The f electrons may play an important
role in bonding/hybridization of intermetallic ytterbium compounds,
leading to interesting properties, such as heavy Fermion behavior,
Kondo behavior, intermediate valence, or complex magnetic structures.
These properties can be influenced by different parameters, like the
chemical environment, the magnetic field, temperature, or pressure.106
= 4.54 μB per Yb3+.105 The f electrons may play an important
role in bonding/hybridization of intermetallic ytterbium compounds,
leading to interesting properties, such as heavy Fermion behavior,
Kondo behavior, intermediate valence, or complex magnetic structures.
These properties can be influenced by different parameters, like the
chemical environment, the magnetic field, temperature, or pressure.106
Characteristic for heavy Fermion behavior is the large electronic specific heat coefficient γ at low temperatures, 2 or 3 orders of magnitude larger (arbitrary value of 400 mJ mol–1 K–2 according to ref (107)) than that of copper (∼1 mJ mol–1 K–2).108 In addition, heavy Fermion compounds typically have large Pauli susceptibilities at low temperatures, due to the large density of states.109 It is very difficult to establish the heavy Fermion behavior of ytterbium compounds, due to the challenging distinction between magnetic, crystal field, and Kondo energy scales. Thus, many compounds show both, magnetic and heavy Fermion character.110
The common way to fit the dependence of the magnetic susceptibility is using the Curie–Weiss law. Within the modified Curie–Weiss law, contributions of the conduction electrons are also taken into account. This makes it possible to adjust even slight curvatures of the reciprocal susceptibility. However, both laws do not consider the temperature dependency of the effective magnetic moment, which marks the only way to explain decrease of the χ–1 value. The ICF model (interconfiguration fluctuation model),111 developed by Sales and Wohlleben, addresses a possible mixed-valent behavior of rare earth atoms (like Yb or Ce) and is represented by
with Tsf as the spin fluctuation temperature and v(T) as the temperature-dependent mean occupation of the ground state given by
where Eex is the energy gap between the ground and the excited state. In most cases, the experimental χ(T) data can be described by
consisting in part of the ICF model (χICF), the Curie–Weiss impurity (χCW), and χ0, the latter being a sum of temperature-independent contributions.111,112
In the following paragraph, some striking examples will be briefly discussed. The magnetic properties and specific heat coefficients of these and further compounds were summarized in Table 2.
Table 2. Magnetic Properties of Selected Ytterbium Compoundsa.
| Compound | TC/K | TN/K | μeff/μB | γ/mJ mol–1 K–2 | Reference |
|---|---|---|---|---|---|
| YbAlB4 | 125 | (204,222) | |||
| YbAl3C3 | 4.66 | (177) | |||
| YbFe2Zn20 | Near 4.53 | 520 | (88,183−186) | ||
| YbCo2Zn20 | Near 4.53 | 7900 | (88,183−186) | ||
| YbNiAl4 | 2.9 | 16 | (23,215,216) | ||
| YbNi3Al9 | – | 3.4 | 4.37 | 110 | (223) |
| YbNi4Si | 4.15 | 25 | (224) | ||
| YbNiGa | 1.7, 1.9 | 4.4 | 450 | (126) | |
| YbNiSn | 5.65 | – | 4.3 | 300 | (121−123,125) |
| YbCuAl | 4.32 | 260 | (146,147,225) | ||
| YbCu2Si2 | 4.19 | 135 | (226) | ||
| YbPdCu4 | – | 0.8 | 4.33 | 200 | (227) |
| YbInCu4 | 4.37 | 50–400 | (228,229) | ||
| YbRh2Si2 | – | 0.07 | 2 × 10–3 | (155,161) | |
| YbRhSn | – | 1.4, 1.85 | 4.3–4.5 | 1200(200) | (110,126−128) |
| YbRhSb | 2.7 | – | 1.4 | 370 | (129−131) |
| Yb4Rh7Ge6 | 4.55–4.65 | 0.184 | (230) | ||
| YbPdBi | 1 | – | 4.11(5) | 1200(100) | (110,127) |
| Yb4Ir7Ge6 | 4.8 | 0.281 | (230) | ||
| YbPtGe | 5.4 | – | 4.48 | 209 | (231) |
| YbPtIn | 1.4, 3.4 | 4.21–4.4 | 400 | (126,232) | |
| YbPtSn | 3.5(4) | – | 4.27(2) | (127) | |
| Yb2Pt2Pb | – | 2.07 | 4.42–4.54 | 30 | (173,233) |
| YbPtBi | – | 0.4 | 8000 | (135−139) |
Magnetic properties include Néel temperature, TN; Curie temperature, TC; effective magnetic moment, μeff; and specific heat capacity (Sommerfeld) coefficient (γ). Standard deviations are given in parentheses, where available.
Binary Compounds
During the last 50 years, YbAl2 (MgCu2 type, space group Fd3m) and YbAl3 (Cu3Au type, space group Pm3m) have been extensively examined regarding their physical properties. Both compounds show Curie–Weiss behavior above 100 K, with a broad maximum of the susceptibility around 850 K for YbAl2. The detected characteristic spin fluctuation temperatures are Tsf = 200 K for YbAl2 and Tsf = 125 K in case of YbAl3.13,114 In a hard X-ray photoemission spectroscopy study for YbAl2, below 300 K, a ytterbium valence of ca. +2.2 was determined.115,116 The Kondo temperature suggested from the magnetic measurements is very high (TK > 2000 K).97,117 The valence of YbAl3 (+2.75) results in a ground state with 4f occupancy of 0.75.118,119 In contrast, photoemission experiments revealed a valence of +2.73, whereas the determined Kondo temperature is in an order of 500–600 K, and the specific heat coefficient is γ = 40 mJ/mol K2. Below the Fermi-liquid temperature Tfl = 50 K, a quadratic temperature dependence of the resistivity was found.118−120
Equiatomic Ternary Compounds
Weak ferromagnetism is reported for the equiatomic compound YbNiSn (TiNiSi type, space group Pnma). The dense Kondo compound has a Curie-temperature of TC = 5.65 K, and the magnetic moment lies along the c axis. A value of μeff = 4.3 μB was determined as an effective magnetic moment close to the value of a free Yb3+ ion (4.54 μB). The maximum detected magnetization is 0.85 μB per Yb atom. Kondo fluctuations with a Kondo temperature TK = 2 K lead to the reduction of the ferromagnetism.121−124 Further investigations by Mössbauer spectroscopy, neutron diffraction, and resistivity measurements confirm these results.121−125
The susceptibility of YbRhSn with ZrNiAl type structure (space group P62m) follows the Curie–Weiss law at high temperatures, with a trivalent state of the Yb ions. The deduced effective magnetic moment was in a range of 4.4–4.5 μB, and a negative Weiss constant was observed.126−128 Two different magnetic transitions at T = 1.85 and 1.4 K were reported via heat capacity data, which shift to lower temperatures with applied magnetic fields as expected for antiferromagnetic ordering. In addition, the heat capacity data indicate a possible heavy Fermion ground state. The resistivity of YbRhSn is Kondo-like and decreases almost linearly down to 20 K, followed by a rapid decrease at 1.8 K. Below 1 K it has a Fermi-liquid-like temperature variation.126−128
YbRhSb was first synthesized by Muro et al. in 2004 and crystallizes with the TiNiSi type structure. It reveals a ferromagnetic transition at TC = 2.7 K. For an applied magnetic field parallel to the crystallographic b axis, a spontaneous moment of only ∼3.0 × 10–3 μB per Yb atom was observed, although the magnetic easy axis is the a axis. At around 2 T a metamagnetic transition occurs, and at 15 T the magnetization reaches 1.4 μB/Yb for the B∥a axis, somewhat reduced when compared with the theoretical saturation magnetization of 4 μB.105 The specific heat coefficient was determined to be γ ∼ 370 mJ mol–1 K–2 in the ferromagnetically ordered state.129 Instead of typical ferromagnets, the Curie temperature furthermore decreases with higher applied magnetic fields. Thus, the weak ferromagnetism of YbRhSb is possibly the result of a canted antiferromagnetic structure. Resistivity measurements and investigations in a p/T phase diagram revealed a minimum for TM(p) at pC = 1.7 GPa. For p ≥ 2 GPa, the canted antiferromagnetic structure changes to a ferromagnetic structure and a moment of 0.4 μB/Yb within the bc plane.130−133
YbBiPt crystallizes in the cubic MgAgAs type structure with space group F43m.134 Antiferromagnetic ordering was detected at TN = 0.4 K, while the determined Weiss constant is ΘP = 0.4 K.135−137 Heat capacity data (Figure 9) revealed an extremely large low-temperature specific heat coefficient of γ = 8 J mol–1 K–2,135−137 and the also determined Kondo temperature was measured to be TK = 1 K.134,136 Further experiments showed that the antiferromagnetism is very sensitive to magnetic field, pressure, and strain.137−140 In addition, below TN very broad magnetic neutron-diffraction peaks were observed.141,142
Figure 9.
Low-temperature specific heat Cp(T) of YbBiPt between 0.09 and 0.85 K. Inset: Same data as Cp/T vs T. Reprinted with permission from ref (136).
YbCuAl crystallizes with a ZrNiAl type structure143−145 (space group P62m), was first discovered by Dwight et al. in 1968144 and shows mixed-valent character for Yb in this compound.146,147 Down to T = 30 K, the susceptibility follows the Curie–Weiss law, while at the lowest temperatures it is marked by a saturation at a high level. The observed intermediate valence is close to 3, and YbCuAl can be described as a heavy Fermion compound with a high value of its specific heat coefficient (γ = 260 mJ mol–1 K–2).148,149 For more details, we refer to the review of Bonville in 1988, who summarized the results of the different physical experiments on YbCuAl together with other ytterbium compounds.149
Further Ternary Compounds
One of the most intensively studied ytterbium compounds is YbRh2Si2. Over the last 20 years, more than 350 publications have been published on this silicide. It was first described by Rossi et al. in 1979,22 adopts the tetragonal ThCr2Si2 type structure (space group I4/mmm), and has a (theoretically) three-dimensional Fermi surface.150−154 Antiferromagnetic ordering was detected at very low temperatures, with the Néel temperature being TN = 70 mK. Yet, the nature of this order is still not clear and was further examined with different techniques such as neutron scattering,155 static susceptibility,15629Si NMR,157 or ESR158−160 (vide infra). Ferromagnetic fluctuations cause incommensurate antiferromagnetic correlations, which were detected by these investigations above 70 mK.
YbRh2Si2 exhibits a non-Fermi-liquid behavior. The ordered Yb3+ moment is very small, with only 2 × 10–3 μB/Yb atom (Figure 10). The characteristic Kondo-temperature is TK = 25 K.161 A quantum critical point is suggested for the suppression of TN at ambient pressure by a magnetic field of only Bc = 60 mT.162 The substitution of Si by Ge shifts the quantum critical point to lower fields,156,163 whereas Co substitution leads to ferromagnetism.164,165 In addition, superconductivity was reported for YbRh2Si2, thus leading to the assumption of a strong coupling of electronic and nuclear magnetism.166,167 Reasons for this superconductivity as well as the mentioned nature of ordering and the quantum critical point itself remain unclear and are the subject of further research.168
Figure 10.
Temperature dependence of the magnetic and inverse magnetic susceptibility of YbRh2Si2 measured parallel to the a and c axis with different applied fields. Reprinted with permission from ref (169).
One of the remarkable trivalent ytterbium compounds is the Shastry–Sutherland170−172 phase Yb2Pt2Pb, originally reported in 1999.47 Yb2Pt2Pb adopts the Er2Au2Sn type structure (space group P42/mnm). Later property studies were performed on single crystals which were grown from lead flux (Yb:Pt:Pb = 5:4:40 starting composition).173 Orientation-dependent magnetic susceptibility measurements showed a high anisotropy with an about 30 times larger value for χ(100) as compared to χ(001) (Figure 11).174 The Néel temperature of Yb2Pt2Pb is TN = 2.07 K.173
Figure 11.
Temperature dependence of the magnetic and inverse magnetic susceptibility of Yb2Pt2Pb measured parallel (100) and (001). Reprinted with permission from ref (174).
Besides, Yb2Pt2Pb was studied by specific heat and resistivity measurements and with respect to its crystal field parameters obtained by inelastic neutron scattering data. All these property studies were supported by quantum chemical calculations for a better understanding of the structure–property relationships. The complete property data of Yb2Pt2Pb has recently been reviewed,87 and we refer to this broad overview for more details.
Magnetization data support the anisotropic behavior observed in the orientation-dependent susceptibility measurements. Yb2Pt2Pb shows multiple metamagnetic steps in both the (100) and (110) directions.175 The maximum magnetization observed in (100) at 5 T is around 2.4 μB per ytterbium atom, significantly reduced when compared with the theoretical value of 4 μB (g × J). The magnetic structure of Yb2Pt2Pb was studied from powder and single crystal neutron diffraction data.176 The ordered ytterbium magnetic moments were described in a 5 × 5 × 1 magnetic supercell.
YbAl3C3 is another example of isotropic exchange interaction and crystallizes with the hexagonal ScAl3C3 type structure (space group P63/mmc). The ytterbium atoms form a triangular lattice, then a structural phase transition to an orthorhombic phase (own structure type) occurs at TS = 80 K.177−179 Above this temperature, Curie–Weiss behavior with an effective magnetic moment of μeff = 4.66 μB was observed, underlining the trivalent state of the ytterbium atoms. The calculated Weiss constant of ΘP = −120 K indicates antiferromagnetic interactions in the paramagnetic regime.177,178 Below TS, no long-range magnetic order was visible by measurements of the magnetization or specific heat. It has been proposed that the anisotropic Kramers doublet of YbAl3C3 forms an antiferromagnetic dimer ground state with a single-triplet energy gap of about 15 K in a relatively narrow field and the 4f electrons are expected to behave as a S = 1/2 spin system at low temperatures, thus suggesting only weaker interdimer interaction in the ytterbium triangles.177,178,180−182
YbCo2Zn20 is a member of the CeCr2Al20 type family (space group Fd3m). The high temperature susceptibility follows the Curie–Weiss law with an effective magnetic moment near the value of a free Yb3+ ion (μeff = 4.54 μB). No magnetic ordering down to 20 mK was observed, while the recorded magnetization isotherms at low temperatures indicate a metamagnetic transition. At lowest temperatures, the magnetic ground state is enhanced by the Pauli susceptibility. By heat capacity measurements, a large Sommerfeld coefficient of γ = 7.9 J–1 mol–1K–2 was observed. Kondo interactions were confirmed via the T2 resistivity, resulting from the A coefficient (A = 165 μΩ K–2). This classifies YbCo2Zn20 as a superheavy electron metal. For further detail, we refer to the literature and reviews.88,183−186
Stoichiometric YbInCu4 adopts the MgCuSn4 type structure187,188 (cubic C15B, space group F43m);189 however, the disordered structure (solid solution) Yb1–xInxCu2 (C15, MgCu2 type, space group Fd3m) has also been observed.190,191 It is one of the few Yb compounds (YbPd2Al3 is another one67) that exhibits a first-order isostructural valence transition (VT) with the transition temperature Ttrans = 42 K.189−191 In the high-temperature regime, the Yb valence has been reported to be +2.9 and suddenly drops to +2.74 when cooling below Ttrans.192 At the same time, the magnetic ground state undergoes a transition from a local moment susceptibility of 4.5 μB to temperature-independent Pauli paramagnetism below Ttrans.193 No change in the structure is observed during the transition, although the unit cell volume increases by ∼0.5%.194,195
Furthermore, the transition was confirmed by synchrotron radiation X-ray absorption, X-ray emission spectroscopy, inelastic neutron scattering (vide infra),196−200 and abrupt changes in other physical properties like electrical resistivity, specific heat capacity, and nuclear spin-relaxation rate (vide infra).198,201,202 The transition is also accompanied by a drastic change of the Kondo temperature (TK) from 20 to ∼400 K in the low-temperature phase.199,203
In Yb-based heavy Fermion systems, β-YbAlB4 was the first discovered superconductor with the transition temperature TC = 80 mK.204,205 The results of transport and thermodynamic properties suggest non-Fermi liquid behavior in the normal β-YbAlB4 state.205,206 It adopts the orthorhombic ThMoB4 type structure (space group Cmmm) with the Yb and Al atoms being in the ab plane between two B layers of hepta- and hexagonal boron rings (vide ultra).93 This structural environment and the strong observed valence fluctuations207 are essential for understanding the properties of β-YbAlB4. Within the ab plane, the Kondo hybridization was discussed to be isotropic, in contrast to the anisotropy between the crystal c axis and the ab plane.208 This may explain the observed T/B scaling of the magnetic susceptibility and the intrinsic quantum criticality.209−214
Another example of an intermediate valence ytterbium compound is YbNiAl4, which adopts the YNiAl4 type structure (space group Cmcm). The temperature dependence of the magnetic susceptibility is compatible with the ICF model. At T = 4.2 K the determined valence of the Yb atoms is +2.1, in contrast to +3 at T = 1000 K. Resistivity measurements reveled a Fermi-liquid behavior, due to the observed AT2 variation below 60 K. At room temperature, it shows a metallic-like behavior. The heat capacity measurements gave no hints for any magnetic ordering down to 1.9 K. For the electronic specific heat capacity, a value of γ = 16 mJ mol–1 K–2 was determined, so that a heavy Fermion state can be excluded.23,215 In addition, various transport measurements (thermal and electrical) were analyzed.216 In-situ high-pressure studies show an increase of the ytterbium valence with increasing pressure for YbNiGa4 and YbNiIn4.217
Also, the complex structure of Yb4Ga24Pt9, a platinide, shows intermediate ytterbium valence. Hard X-ray photoelectron spectra and magnetic susceptibility measurements point to an ytterbium valence of ∼2.5.218 The stannide YbRhSn2219 and the solid solution YbIn1–xAu1+x (x = 0–0.3)220 show comparable behavior.
An intermediate ytterbium valence can also be induced by isovalent substitution. The phosphide Yb2Ni12P7 exhibits a heavy-Fermion paramagnetic ground state and switches to intermediate valence in Yb2Ni12As7.221
INELASTIC NEUTRON SCATTERING
Inelastic neutron scattering is often used to probe crystal field excitations which allow drawbacks on the crystal electric field (CEF) splitting for a certain atom and magnetic phase transitions. In contrast to elastic scattering, in this case, the energy difference of the scattered neutrons is detected. Therefore, it is a useful tool to obtain information about the ground state and the magnetic exchange interactions of, in this case, the Yb atoms.234
Binary Compounds
One of the first compounds that has been studied by inelastic neutron scattering was YbBe13 (NaZn13 type, space group Fm3c).235 The spectra were recorded on a polycrystalline sample between 1.2 and 300 K. Since YbBe13 undergoes antiferromagnetic ordering at TN = 1.28 K,236 the single line observed at 1.2 K was interpreted as a magnon. The spin fluctuations associated with the magnetic ordering, however, persist for quite a large temperature range and are still observable at 10 K. At 39 K, the CEF splitting can be observed with Γ7 being the ground state and Γ8 (3.20 meV) and Γ6 (4.39 meV) being the next excited states. These observations contradict the results from the literature where a different sequence was reported; however, all agree on the Γ7 ground state.236,237
For YbPd (CsCl type, space group Pm3m) and Yb3Pd4 (Pu3Pd4 type, space group R3), inelastic neutron scattering has been used to probe the proposed magnetic transitions in these intermediate valent compounds and to determine the CEF splitting to gain information about the ground state. For YbPd, Γ8 was determined to be the ground state with Γ7 being 4.75 meV and Γ6 being 12.3 meV higher in energy. This scheme is in line with trivalent Yb in a cubic environment. At 1.3 K, an additional contribution with magnetic origin is observed for YbPd. For Yb3Pd4, the first excitation was shifted to lower energy (4.08 meV) and is more distinct compared to the one in YbPd. It is interesting to note that despite Yb3Pd4 being trigonal (26 independent CF parameters are expected–note, the referenced paper states that Yb3Pd4 is triclinic; this statement has been corrected) the observed transitions still can be interpreted by a cubic site symmetry. Finally, measurements between 1.3 and 6 K revealed a strong inelastic line at low temperatures that softens toward the proposed magnetic phase transition near 3 K. An abrupt change of the line width at T = 3.1 K indicates that this excitation can be attributed to a magnon in the magnetically ordered state.238
Ternary Compounds
To understand the valence fluctuating behavior of YbInAu2 (MnCu2Al type, space group Fm3m), neutron scattering experiments were conducted and compared to the results of YbAl3 (Cu3Au type, space group Pm3m) which exhibits similar physical properties. The data obtained at temperatures near the susceptibility maxima (∼80 K for YbInAu2 and ∼120 K for YbAl3) could be fitted with a single spectral function. At 5 K, however, the data of YbAl3 needs more components while the data of YbInAu2 still can be fitted with a single function. The differences are also visible in the phonon scattering (high angle data). Here, a well-defined phonon mode is visible for YbAl3 in contrast to YbInAu2.239
As mentioned before, Yb1–xInxCu2 (MgCu2 type, space group Fd3m) shows a temperature-dependent valence phase transition. It changes from a nearly trivalent state at high temperatures to a mixed-valent Pauli paramagnetic state at low temperatures. A sample with x = 0.5 was investigated at T = 30 and 60 K, above and below the transition temperature of T = 40 K. The spin dynamics in the mixed-valent phase (T = 30 K) can be modeled with a Lorentzian power spectrum with the center at E = 40 meV.240
Also, different YbT2X2 compounds were investigated by inelastic neutron scattering. Among the first investigations was the work on YbT2Si2 for T = Fe, Co, and Ni (ThCr2Si2 type, space group I4/mmm).241 The performed experiments showed that YbCo2Si2 exhibits three excited states at ∼4, 12.5, and 30.5 meV above the ground state, while for YbNi2Si2, two clear transitions at ∼7.5 and 13.5 meV are visible alongside a shoulder at lower energy of the 7.5 meV peak. For YbFe2Si2, no clear inelastic signals are observed.241 Taking the experimentally determined energies for YbCo2Si2 into account, the CF level scheme could be derived, indicating Γ7 to be the ground state.242 YbMn2Si2 (ThCr2Si2 type, space group I4/mmm) has the peculiarity that it shows two antiferromagnetic transitions at TN,1 = 526 and TN,2 = 30 K originating from the Mn atoms.243 Determination of the magnetic structure showed that, below TN,1, the Mn atoms show a + – + – sequence, while below TN,2, a + – – + magnetic substructure arises, which leads to a doubling of c in the magnetic unit cell. At 1.5 K also the Yb atoms are antiferromagnetically ordered, however, with their spins orthogonal to the Mn spins.244 Above 30 K, only one Yb site is present which exhibits a doublet ground state as well as three excited states at 4.65, 11.89, and 15.05 meV. Below 30 K, however, a second Yb site emerges due to the antiferromagnetically ordered Yb atoms. This leads to two additional signals in the inelastic neutron spectrum of YbMn2Si2 at 2.5 K. These transitions can be associated with a different CEF scheme for the Yb2 site with energy levels at 7.24, 8.76, and 16.4 meV.245,246 For the heavy Fermion compound YbRh2Si2 (ThCr2Si2 type, space group I4/mmm), inelastic neutron scattering experiments conducted at 1.5 K are also dominated by the CEF excitations of the Yb3+ ion. Besides a ground state doublet, three excited state doublets were observed at higher energies of 17, 25, and 43 meV, in line with the CF theory for a tetragonal system.247 Subsequent calculations showed that the ground state is a Γ6 Kramers doublet.248 For YbIr2Si2, both modifications (CaBe2Ge2 type, P4/nmm, and ThCr2Si2 type, I4/mmm) were investigated by inelastic neutron scattering experiments at 1.5 K. As for YbRh2Si2, a doublet ground state and three excited doublet states are expected. For the body-centered polymorph, energies of 0, 18, 25, and 36 meV were observed, while for the primitive polymorph they are slightly lower in energy at 0, 15, 22, and 33 meV.249 These values are in good agreement with the calculated values for both YbRh2Si2 and YbIr2Si2.250
XANES/XAS
X-ray absorption spectroscopy (XAS) describes the overall measurement of the absorption spectrum of electrons, which interact with polychromatic X-ray photons and absorbs characteristic wavelengths. The XA spectrum can be separated into two parts. First, the area at higher photon energy than the absorption, called extended X-ray absorption fine structure (EXAFS) which contains information about the local environment of the absorbing atom. The second area is at the absorption energy, the X-ray absorption near edge structure (XANES) or near edge X-ray absorption fine structure (NEXAFS), which gives insight into the electronic properties, e.g., the oxidation state of the absorbing atom. In the following chapter, examples of classical intermetallic Yb-compounds as well as Zintl phases will be addressed with regards to their respective valence in the XAS and XANES measurements.
Binary Compounds
The binary compounds YbAl2 (MgCu2 type, space group Fd3m) and YbAl3 (Cu3Au type, space group Pm3m) are among the most investigated intermetallics. Both have one crystallographic Yb position in the crystal structure. One of the earliest X-ray absorption measurements of YbAl2 was done by Beaurepaire, Kappler, and Krill in 1986.251 In their work, YbAl2 was measured at room temperature under pressures of 10 and 30 kbar. At room temperature, YbAl2 has a valence of +2.3, whereas under pressure the valence rises to +2.6 at 30 kbar (pressure-induced oxidation). Due to the width of the 2p core hole of 4.6 eV, the Yb2+ and Yb3+LIII edges could not be resolved, but the two contributions are separated by about 6 eV.251 Dallera et al. measured the pressure dependence of the Yb LIII edge in YbAl2 up to 385 kbar using the partial fluorescence yield mode of the X-ray absorption (PFY-XAS), giving a higher resolution of the spectra compared to the conventional measurement mode. The absorption spectra show the valence shift from a valence in YbAl2 of +2.25 at ambient pressure to a valence of +2.9 at 385 kbar. At pressures of 170 and 385 kbar, the spectra of YbAl2 become similar to Yb1–xInxCu2, which has a stated valence of +2.96, indicating a valence close to +3.252
XA spectra of YbAl3 were measured at 10 and 300 K, showing the Yb 3d → 4f transition and indicating an open 4f shell in the ground state. A decrease in the intensity of 11% at 10 K compared to 300 K is additionally reported by Tjeng et al., suggesting a decrease in the valence of the Yb atom, which is also shown in the photoemission spectroscopy.253 The pressure-induced valence change of YbAl3 was measured by PFY-XAS starting at ambient pressure and the valence of +2.75 for YbAl3, which subsequently rises alongside the increasing pressure. At 9 GPa, the valence is +2.82, then +2.86 at 20 GPa, and finally +2.93 at an applied pressure of 38 GPa. The incident energy for Yb2+ is 8.942 keV, Yb3+ resonates at a slightly higher energy of 8.950 keV.254 By changing from bulk materials to nanoparticles, the valence of YbAl3 changes from +2.86 to +2.70 after mechanical milling in a planetary high-energy ball milling system for 120 h. The reference Yb2O3 indicates the Yb3+ peak at 8946.5 eV, and the spectra of the milled YbAl3 shows an increase of the Yb2+ contribution at 8941 eV. The valences for 20 and 70 h milling time are +2.74 and +2.70, respectively. A cause of the valence change is the size effect stated. The milled YbAl3 particles possess two types of Yb atoms, the trivalent in the bulk of the sample and the ones near the surface having a variation of their coordination number, leading to divalent Yb. Another reason is the disorder introduced in the ball milling process, which is particularly visible in the X-ray diffraction data.255
YbGa2 crystallizes at ambient pressure in the hexagonal CaIn2 type structure (P63/mmc), at higher pressures above 22 GPa a phase transition into the UHg2 type (P6/mmm) occurs. XANES measurements of the Yb LIII-edge show a valence increase from Yb2+ at ambient pressure to Yb3+ in the high-pressure structure. At low pressures, two peaks are visible, wherein the peak at higher energy represents the Yb3+ and is within 1 eV to the reference Yb2O3, and the second peak is 7 eV lower, corresponding to Yb2+. Measurements were taken at 0.6, 4.7, and 28.8 GPa, showing a continuous increase in the intensity of Yb3+ and decrease of the Yb2+ white line until the phase transition occurs and only Yb3+ is present (Figure 12). In the high-pressure modification, YbGa2 possesses a two-dimensional polyanion, compared to the Ga network found in the normal pressure phase. The two-dimensional arrangement can then be supported with the additional electrons from the Yb atoms.256
Figure 12.
Yb LIII XANES spectra of YbGa2 at different pressures. The white lines at low energies are attributed to Yb2+, and the lines at higher energies are attributed to Yb3+. Figure reproduced with permission from ref (256). Copyright 2001 John Wiley & Sons, Inc.
The hexagonal phase Yb5Si3 crystallizes in the Mn5Si3 type structure (P63/mcm) and possesses two distinct crystallographic Yb positions (4d and 6g). Alongside XANES, Mössbauer spectroscopic investigations and magnetic measurements were also conducted. The magnetic measurements indicated that only trivalent Yb is present, in contrast to the Mössbauer spectrum proposing 60% of Yb3+. XANES measurements were performed at 10 and 300 K; these show two white lines at 8943 and 8935 eV corresponding to Yb3+ and Yb2+, respectively. The calculated valence from the spectra is +2.88, which is higher than the expected value of +2.6 if the Yb atoms on the 6d site were purely trivalent and the ones on the 4g site divalent. The authors suggested that a hybridization of the 4f electrons is occurring, whose strength is different for the two different crystallographic sites. The Yb atoms on the 4d site are more strongly influenced than the Yb atoms on 6g.257
Yb5Ge4 (Sm5Ge4 type, space group Pnma) is reported to be a mixed valence compound with a valence of +2.42, showing the white band at 8948 eV and a big shoulder at 8941 eV, indicating Yb3+ and Yb2+, respectively.258
Equiatomic Ternary Compounds
The heavy Fermion compound YbCuAl (ZrNiAl type, space group P62m) is described with a Yb valence of nearly +3. With increasing pressure, the valence of Yb can be increased, and with lowering the temperature, the Yb becomes more divalent as shown by XES measurements.225 YbAuIn also crystallizes in the hexagonal ZrNiAl type structure with one crystallographic Yb position. X-ray absorption measurements of YbAuIn were taken at 15–18 and 300 K at ambient pressure, resulting in an intensive white line at 8941 eV corresponding to a divalent Yb, but also a weak shoulder at 8949 eV showing a small amount of trivalent Yb. By fitting the spectra and excluding Yb2O3 impurities, a valence of +2.17 was determined.259 YbGaGe possesses two crystallographically different Yb positions and crystallizes in the hexagonal YPtAs type structure (P63/mmc). The compound shows a white band at around 8940 eV and a small shoulder around 8950 eV, indicating a divalent system with oxidic impurities, which is in line with the magnetic measurements.260
Further Ternary Compounds
YbRh2Si2 (ThCr2Si2 type, space group I4/mmm) has a temperature- and magnetic-field-dependent Yb valence, which is decreasing with lower temperature and increasing alongside higher magnetic fields. To measure the influence of the two parameters, XAS measurements were conducted by Nakai et al. The Yb LIII absorption spectra show a shoulder at 8940 eV appearing at decreasing temperature and an intensive white line at 8946 eV, indicating the trivalent Yb, whose intensity decreases with lower temperatures. Above 200 K, the valence was determined to be +2.96, while below 25 K, a valence of +2.92 was reported. In contrast, the valence change of Yb was measured by increasing magnetic field at 2 K. At 0 T, the valence is +2.92, and it rises to +2.93 monotonically with stronger magnetic fields up to 33 T.261
X-ray absorption spectra of YbCu2Si2, YbCu2Ge2 (ThCr2Si2 type, space group I4/mmm), and YbPt2 (MgCu2 type, space group Fd3m) were measured at 300 K, showing a pure trivalent Yb in YbPt2, divalent Yb in YbCu2Ge2, and a mixed valence occurring in YbCu2Si2 with a higher amount of Yb3+ than Yb2+. It was also stated that the Yb valence in YbCu2Si2 does not show any signs of a temperature dependency.262 Similar to YbCu2Ge2, YbAl2Ga2 possesses a white line at 8936 eV and a resulting valence of Yb2+.263 YbMn2Ge2 (ThCr2Si2 type, space group I4/mmm) is also described as a mixed valent compound with a Yb valence of +2.40 at 300 K. This compound shows not only a negative thermal expansion, but also a temperature dependence of the Yb valence. With the increase of the temperature, the white line at 8943 eV decreases while the one at 8949 eV increases, corresponding to the Yb2+ and Yb3+ respectively, leading to a valence of +2.82 at 700 K. Also, the valence of Yb can be influenced by applying pressure; e.g., at a pressure of 1.4 GPa, the Yb valence is +2.82.264
The YbT2X20 compounds with T = Cr, Ti, V, Co, Fe, Ir, Rh and X = Al, Zn all crystallize in the cubic CeCr2Al20 type structure (space group Fd3m). These structures are heavy Fermion compounds and can possess a particularly high specific heat coefficient. XAS measurements were taken of YbRh2Zn20 and YbIr2Al20 at 2, 250, and 290 K, respectively, with additional measurements at 2 K with an applied external field of 10 T. The absorption spectra of YbRh2Zn20 have two peaks, the white line at 8950 eV indicating Yb3+ and a shoulder at 8940 eV originating from Yb2+. The valence at 250 K is stated as +2.89 and is decreasing with decreasing temperature, especially below 100 K, reaching a valence of +2.87 at 20 K. The same trend is visible for YbIr2Al20, also having a decreasing Yb valence with decreasing temperatures below 90 K; above 100 K, the valence is nearly constant. As for the measurements under an applied field, only changes in the valence are present below 80 K.265 In contrast, YbCo2Zn20 was investigated by XAS measurements, showing no indication of temperature or magnetic field dependency.266 YbFe2Zn20 and Cd-substituted YbFe2Zn20–xCdx with x = 0–1.4 were analyzed by Fahl et al. Their XANES studies of the Yb LIII absorption show only the white line at 8946 eV corresponding to Yb3+ and no additional shoulder, making all compounds formally trivalent.267
For the valence transition compounds Yb1–xInxCu2 (x = 0.5), the valence transition temperature is stated to be Ttrans = 42 K where the valence changes from +2.98 to +2.85, as calculated from the XANES measurements at 300 and 7 K, respectively.268 Another study on Yb0.5In0.5Cu2 by Yamaoka et al. focused on the pressure-induced valence transition. In their work, X-ray absorption spectra of Yb0.5In0.5Cu2 were taken at 300 and 13 K under pressure. At 300 K, Yb0.5In0.5Cu2 shows no pressure dependent valence; at 13 K on the other hand, it presents an increase of valence at pressures of 3.1 GPa and above.269 The XAS spectra of YbAgCu4 (MgCu4Sn type, space group F43m) measured at 10 and 300 K by Lawrence et al. show the white line at 8947 eV and a shoulder at 8940 eV, corresponding to Yb3+ and Yb2+, respectively. Also, they are stating that the valence of YbAgCu4 is nearly +3 and changes continuously with temperature.270 Sarrao et al. investigated the Yb valence in the YbTCu4 series (MgCu4Sn type, space group F43m) with T = Mg, Ag, Au, Zn, Cd, Tl by XAS of the Yb LIII line between 20 and 300 K, showing the Mg compound with the lowest Yb valence of +2.64 at 20 K and +2.69 at 300 K, followed by YbTlCu4 with +2.75 to +2.82, YbCdCu4 +2.76 to +2.82, YbZnCu4 +2.84 to +2.88, YbAgCu4 +2.86 to +2.93, and finally YbAuCu4 with a valence of +2.95 at 20 K and +2.96 at 300 K.240 XANES studies of YbPtIn4 and YbPdIn4 (YNiAl4 type, space group Cmcm) were reported, stating both compounds containing mixed valent Yb.271
Zintl Phases
The Zintl compounds Yb14TSb11 (T = Zn, Mn, Mg; Ca14AlSb11 type, space group I41/acd) exhibit besides intriguing magnetic also highly important thermoelectric properties (see thermoelectric properties). Using the Zintl counting rules, the Mn-containing compounds only have Yb2+ cations, while the Zn and Mg compound should also contain one Yb3+. To verify the valence of the Yb atoms in these compounds, XANES measurements were taken by He et al. The absorption spectra of all three compounds indicate the presence of both Yb2+ and Yb3+, but the overall valence is close to Yb2+. Additionally, it is visible that the Mg and Mn compounds have a constant amount of Yb3+, but in the Zn compound, the valence is highly temperature-dependent. The formation of Yb3+ is explained by introducing disorder in the structure of Yb14TSb11.272
X-RAY PHOTOELECTRON SPECTROSCOPY (XPS)
X-ray photoelectron spectroscopy (XPS) describes the analysis of a (solid) material, using the photoelectric effect, meaning the emission of an electron due to absorption of X-ray radiation. Typically, Al Kα is used as an X-ray source with a photon energy around 1486 eV. Due to the nature of the interaction between condensed matter and electrons, especially the short inelastic mean free path of, e.g., 1–2 nm for metallic systems, makes this analysis a rather surface-sensitive method.
Binary Compounds
YbAl2 (MgCu2 type, space group Fd3m) was measured by XPS in 1985 by Oh et al. at 120, 460, and 750 K. The spectra at the two lower temperatures show the peaks of both Yb2+ and Yb3+, with the 4f14 → 4f13 and 4f13 → 4f12 bands, respectively. At higher temperatures, the intensity of the peak originating from the divalent Yb atoms is reduced, while the trivalent one increases, leading to valences of +2.32 at 120 K, + 2.40 at 460 K, and finally +2.53 at 750 K.273 In 2012, the valence of Yb in YbAl2 was analyzed by HAXPES using hard X-ray radiation of 7.94 keV for excitation at 20 K, allowing the Yb 3d core levels to be shown. In the spectrum recorded at 20 K, the Yb 3d core-levels can be seen at 1515–1540 eV (3d5/2) and 1560–1585 eV (3d3/2). The spectra at 20 and 300 K both show Yb2+ and Yb3+ species, leading to a mean valence of +2.20, staying nearly unchanged below 300 K.116
The valence of YbAl3 (Cu3Au type, space group Pm3m) was analyzed using XPS by Buschow et al. in 1977. The Yb 4f spectra show a peak multiplet extending from 5 to 10 eV, corresponding to Yb3+. Also, a spin doublet is visible from 0 to 3 eV, indicating divalent Yb, but no mean valence of the compound could be calculated from the measured spectra.274 Later, in 1996, YbAl3 was measured by XPS, with a well-resolved doublet of divalent Yb and the multiplet of trivalent Yb. The mean valence of YbAl3 was determined to be +2.63 based on the recorded XPS spectra.275 The nearly same results were reported by Joyce et al. in 2001, measuring YbAl3 around 20 K showing both Yb valence species in the spectra with a mean valence around +2.6.276
YbCu2 and YbAg2 (both KHg2 type, space group Imma), YbAu2 (MoSi2 type, space group I4/mmm), and YbNi5 (CaCu5 type, space group P6/mmm) were measured by XPS to determine the valence states of ytterbium in these compounds. The measured spectra show the 4f and the 5p levels. As expected, the trivalent Yb compound YbNi5 has peaks only in the region of Yb3+ while YbCu2, which is purely divalent, shows the two peaks corresponding to Yb2+. YbAg2 on the other hand, exhibits a small trivalent signal due to oxidic impurities. According to the unit cell volume, YbAu2 should possess a trivalent Yb state, but the XPS measurements showed not only the Yb3+ peaks, but also the more intensive peaks of divalent Yb in the spectra, indicating YbAu2 to rather be an intermediate valence system, compared to YbCu2.277
The valence fluctuating compound YbB12 (UB12 type, space group Fm3m) was investigated at room temperature, showing the Yb2+ doublet at 0–3 eV and the Yb3+ multiplet between 5 and 12 eV. The mean valence of Yb was determined to be +2.9.278
Finally, Yb4As3, Yb4Sb3, and Yb4SbBi3 (all Th3P4 type, space group I43d) are also known to be valence fluctuating compounds. The XPS spectra, measured at room temperature, all show the peaks for divalent and trivalent Yb, although the Yb3+ peak in Yb4Bi3 is of low intensity. After analyzing the Yb 4f spectra, the mean Yb valences were calculated to be +2.32, +2.34, and +2.10 for Yb4As3, Yb4Sb3, and Yb4Bi3, respectively.279
Equiatomic Compounds
YbAuMg crystallizes in the orthorhombic TiNiSi type structure (space group Pnma), and its electronic structure was investigated by XPS measurements. The spectrum was recorded at room temperature with Al Kα excitation radiation (1486.6 eV), showing the two peaks for divalent Yb 4f7/2 and 4f5/2 at 0.5 and 1.7 eV binding energy, rendering the system purely divalent.280
Further Ternary Compounds
The tetrelides YbNi2Ge2, YbPd2Si2, and YbCu2Si2 all crystallize in the ThCr2Si2 type structure (space group I4/mmm). Their spectra show the 4f level and 4d core levels; in all of them, Yb3+ and Yb2+ are visible, and the divalent peaks are in the range of 0–2 eV for the 4f level and around 185 eV in the 4d spectra. The signal from the trivalent atoms forms a multiplet ranging from 5 to 12 eV and centered around 195 eV for the 4f and 4d spectra, respectively. Due to the presence of the two Yb valence species, these compounds are mixed valent.281
The valence of Yb in YbInCu4 was investigated by Maeda et al. in 2019 using HAXPES with an excitation energy of 8915–8965 eV at 20 and 70 K. The Yb 3d5/2 spectra show a Yb2+ single peak at 1520 eV and the Yb3+ multiplet at 1525–1540 eV. With lower temperature, the intensity of the divalent Yb increases, while the trivalent Yb decreases. The mean valence at 70 K is +2.91 and +2.67 at 20 K.282 Before this, investigation on Yb0.5In0.5Cu2 via XPS measurements at 20 K and excitation energies of 90 to 500 eV also showed both species present in the spectra. A valence of +2.6 was calculated from the data.276 The series of YbInCu4, YbCdCu4, and YbMgCu4 was investigated between 10 to 300 K. The Yb 4d XPS spectra show both Yb species in all samples. The valence of the Cd- and In-containing compounds gradually decreases with decreasing temperature, while the valence in YbMgCu4 does not change to lower temperatures.283 Upon replacing In with a precious metal like Ag, Au, and Pd, YbAgCu4, YbAuCu4 and YbPdCu4 can be obtained. The electronic structure of these three compounds was investigated by Kang et al. The XPS spectra were recorded with Al Kα radiation and an excitation energy of 1486.6 eV at a temperature around 110 K. The spectra show the 4f line of Yb and the Cu 3d, Pd 4d, Pt 5d, and Ag 4d lines. Due to the overlap of the transition metal signals with the emission of divalent Yb between 0 and 4 eV, no valence can be reliably determined. However, based on the high intensity of the trivalent Yb multiplet, it can be assumed that the three compounds have a valence close to Yb3+ and also that the Pd containing compound is closer to the trivalent state than the Au- and Ag-containing ones.284
YbPd2Al3 and the solid solution Yb1–xCaxPd2Al3 crystallize in the hexagonal YNi2Al3 type structure (P6/mmm), containing two crystallographically different Yb positions. The solid solution has a mixing of Yb and Ca on both Yb positions. The compounds were analyzed by XPS with Al Kα radiation (1486 eV). The spectra depict the Yb 4d lines and possess five peaks for the Yb3+ and two rather small peaks forming a doublet for the Yb2+, in which a partial overlap of the trivalent multiplet with the divalent Yb peaks occurs. The calcium-substituted samples Yb0.33Ca0.67Pd2Al3 and Yb0.67Ca0.33Pd2Al3 became oxidized due to the handling in air or due the X-ray radiation, leading to an increased Yb3+ content, which cannot be used to determine the real amount of Yb2+ in the samples.67
The Kondo lattice compounds Yb2Al15Pt6 and Yb2Ga15Pt6 were investigated via XPS at an excitation energy of 5.95 keV at 20 to 300 K. The Yb 3d levels indicate the presence of both di- and trivalent Yb. Also, in the Al-containing compound, with decreasing temperature, the intensity of the Yb3+ multiplet (1524–1536 eV) decreases and the Yb2+ single peak (1520 eV) intensity is observable, shifting the valence from +2.89 at 250 K to +2.83 at 20 K. In comparison, the Ga compound possesses a lower Yb valence of +2.34. However, this valence changes only minimally toward lower temperatures, meaning the Yb valence in Yb2Ga15Al6 has nearly no temperature dependence. The peak of divalent Yb is at 1568 eV, and the multiplet of trivalent Yb at 1574–1582 eV.285
Zintl Phases
The Zintl compounds Yb14MnSb11 and Yb14ZnSb11 (both Ca14AlSb11 type, space group I41/acd) were measured by Holm et al. to analyze the Yb valence by XPS analysis with an excitation energy of 1250 eV. The spectrum for the Mn-containing sample shows the Yb 4s, 4p1/2, 4p3/2, 4d3/2, and 4d5/2 core levels corresponding to the binding energies of 486, 395, 345, 197, and 188 eV. For the Zn sample, the Yb levels are slightly shifted and found at 484, 394, 345, 195, and 187 eV. Additional valence band scans with an excitation energy of 120 eV were taken, showing the Yb 4f multiplet for Yb3+ and the doublet of Yb2+, respectively. Also, it is stated that these compounds are prone to oxidation, leading to a higher amount of Yb3+ on the surface. The amount of trivalent Yb for Yb14MnSb11 is small compared to the divalent Yb and originates from the surface oxidation, in comparison to Yb14ZnSb11, which has a higher amount of Yb3+, leading to the assumption that trivalent Yb is intrinsically present in the sample, making Yb14ZnSb11 an intermediate Yb valence system.286
The Yb-filled skutterudites Yb0.075CoSb3, Yb0.5CoSb3, and YbFe4Sb12 have been studied by XPS measurements, showing the presence of both Yb2+ and Yb3+ in these compounds. The binding energies are in good agreement with Yb metal and Yb2O3, corresponding to divalent and trivalent Yb, respectively. The doublet for Yb2+ is located at 1–2.2 eV, while the multiplet corresponding to Yb3+ is between 6 and 14 eV.287
MAGNETIC RESONANCE SPECTROSCOPY
This chapter is supposed to give an overview about studies in which magnetic resonance spectroscopy was applied to intermetallic Yb compounds. Nuclear magnetic resonance (NMR) in these systems can on one hand be used directly on the ytterbium nucleus to investigate the electronic situation on the Yb atom itself, while on the other hand hetero nuclear NMR is important for the measurement of effects concerning the influence of Yb on its surrounding structure.288,289
In addition, electron paramagnetic resonance (EPR) spectroscopy is a valuable option for paramagnetic species. Here most of the studies were conducted to investigate the interesting physical properties such as the Kondo effect or heavy Fermion behavior.290−292 These methods are, so to say, complementary to many other methods like, e.g., structure determination, since NMR spectroscopy is mainly used to confirm the results of the obtained structural models since it is a site-specific technique. Temperature-dependent measurements of both methods can give information about the temperature of possible phase transitions as well as the electronic situations. Here, important information about the interactions of the 4f electrons and the conduction electrons in the intermetallic compounds are obtained.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Starting with Yb NMR spectroscopy itself, it must be mentioned that in theory the application of NMR spectroscopy to two different Yb isotopes could be possible: 171Yb (S = 1/2, 14.3% natural abundance, 7.4 MHz T–1) and 173Yb (S = 5/2, 16.4% natural abundance, 1.3 MHz T–1).288 In literature, to the best of our knowledge, except for the very first study on Yb metal, no 173Yb study has been conducted, which is obviously due to the higher resonance frequency and the absence of a quadrupole moment in 171Yb.288
As for all compounds with ytterbium, the successful application of magnetic resonance is limited to the diamagnetic closed-shell systems. Besides Yb, only Sc, Y, La, and Lu can play a role here. Therefore, in principle one must exclude the trivalent Yb which has a paramagnetic ground state (4f13). The magnetic moment of Yb3+ leads to extremely short relaxation times and therefore to substantial line broadening and makes the detection of a NMR signal impossible.288 However, examples making use of these significant facts have been reported.
171Yb NMR
In one of the already mentioned early studies289 of 171Yb NMR spectroscopy, the resonance of metallic Yb and the resonance in the intermetallic aluminide YbAl2 (MgCu2 type, space group Fd3m) were compared to other divalent ionic species, namely YbCl2 (SrI2 type, space group Pbca) and YbS (NaCl type, space group Fm3m). Knight shifts were determined to be around 0 ppm in the metal and about 7.76% in the intermetallic compound YbAl2. No temperature dependence of the measured Knight shift was found.289
In a following study, the two aluminides YbAl2 (MgCu2 type, space group Fd3m), and YbAl3 (Cu3Au type, space group Pm3m) as prototypes for temperature dependent valence fluctuating systems were investigated with NMR spectroscopy. Here it was possible to detect a signal for the magnetic Yb3+ ion at low temperatures (1.4 to 4.2 for YbAl3 and 4.2–80 K in the case of YbAl2), which is hardly the case in other studies. A determination of the hyperfine field and the relaxation rates has been carried out as well. An extremely large Knight shift of ∼100% was identified in YbAl3.293 In a recent 27Al-NMR and Raman spectroscopic study, the partially divalent character of Yb in the cubic Laves phase YbAl2 at room temperature was investigated by comparing the s electron densities with the observed isotropic resonance shifts δ(27Al). Here, Yb is not comparable to the results obtained for the formally trivalent diamagnetic members of the series.294
The Kondo semiconductor YbB12 (UB12 type, space group Fm3m) has been characterized at low temperatures using 11B as well as 171Yb NMR spectroscopy.295,296 It was possible to observe a spin–echo signal at temperatures below 15 K for 171Yb. In this case, the results of the 1/T1 relaxation of 171Yb is in contrast with the previous 11B experiments. It is suggested that the large susceptibility arises from magnetic moments of the Yb3+ ion, which stands in contrast to results obtained via 11B NMR spectroscopy. The effect of such a behavior is still not fully understood.295 The studies discussed above show the rare case of the occurrence of a detectable NMR signal for trivalent or valence fluctuating, magnetic ytterbium. Here, the NMR signal is found at drastic shifts compared to divalent ionic Yb standards.
In agreement with the structural analysis of Yb3Ag4Mg12 (Gd3Ru4Al12 type, space group P63/mmc) via X-ray diffraction (one Yb site), one single resonance for the 171Yb NMR spectra was observed. The temperature-dependent magnetic susceptibility measurements for this compound show the characteristics of a diamagnetic material, underlining the divalent character of Yb. The resonance of the strongly broadened spectrum recorded under static conditions applying the WURST-CPMG pulse sequence is found to be between 6800 and 7000 ppm, indicating a strong positive Knight shift. Between 200 and 300 K, the temperature dependence of the resonance shift was negligible, suggesting a strong orbital contribution to the Knight shift.99
Recently, the influence on the 171Yb NMR signal, when substituting ternary Yb compounds with Ca, was shown. The divalent, ternary compounds YbZnSn (NdPtSb type, space group P63mc), YbPdSn2, and YbAuIn2 (both MgCuAl2 type, space group Cmcm) as well as the solid solutions (Yb0.5Ca0.5)ZnSn, (Yb0.5Ca0.5)PdSn2, and (Yb0.5Ca0.5)AuIn2 were investigated with 171Yb NMR spectroscopy applying static WURST-CPMG pulse sequences.297 For all three ternary samples, the obtained results are in excellent agreement with the structural investigations and the magnetic susceptibility data for the compounds. In all cases, a single broadened 171Yb spectrum could be recorded, indicating one crystallographic Yb site and underlining the diamagnetic character of Yb2+ in this compound. For YbAuIn2, the magnetic susceptibility data suggest paramagnetic impurities (resulting from intermediate-valent YbAuIn) in higher amounts than for the other compounds, which is given as the reason why here only a poor signal-to-noise-ratio was achieved in the NMR spectroscopic data. After Ca incorporation, a shift for the center of gravity as well as a line broadening is observed. This is due to the different local surrounding caused by the statistic mixing in a solid solution. As an example, the obtained NMR spectra are shown in Figure 13.
Figure 13.

NMR spectra of YbZnSn and YbPdSn2 and the respective Ca-substituted variants Yb0.5Ca0.5ZnSn and Yb0.5Ca0.5PdSn2.297
The germanide YbPtGe2 crystallizes in the orthorhombic YIrGe2 type structure (space group Immm) with two distinct Yb sites. Via 171Yb NMR spectroscopy, it was possible to prove that indeed two distinct Yb sites are present. Moreover, it becomes evident that one site is nearly divalent, while the Yb atoms on the other site are nearly trivalent. The authors explain this by the rather small shift of the observed signal. As mentioned above, in the case of paramagnetic ytterbium ions, larger Knight shifts are expected. Therefore, YbPtGe2 is named a multivalent charge-ordered compound having two different Yb valences in one compound, with no dynamic behavior.298
Finally, in the compound YbGaGe (YPtAs type, P63/mmc) which has two Yb sites in an equivalent coordination, it is quite remarkable that, in a combined 171Yb and 69,71Ga NMR spectroscopic study, the two different Yb sites could be identified separately from each other in NMR spectra recorded at 4.2 K, confirming the proposed crystal structure. Here, no hints for trivalent or valence fluctuating Yb atoms could be found. The Knight shifts are reported with 0.41% (4100 ppm) and 1.1% (11000 ppm), respectively, being in the typical range of divalent nonmagnetic ytterbium compounds.299
EPR
In contrast to the already discussed NMR spectroscopic studies on intermetallic Yb compounds, in which Yb should preferably be in the divalent state, an EPR signal should be detectable, when paramagnetic species such as the open-shell Yb3+ are present. Furthermore, it is possible to obtain electron spin resonance spectra which occur due to conduction electrons. Therefore, EPR spectroscopy is a method that is able to probe the local moments of unpaired 4f electrons of a lanthanide atom as well as the interaction with the surrounding delocalized conduction electrons.211 By temperature-dependent measurements of the g tensor and the line width of the signal, information about the ground state of f electron systems responsible for some important phenomena can be obtained.300 For the determination of the valence state of the Yb atom, the results of EPR spectroscopy can be combined with magnetic measurements. In many recent studies, EPR spectroscopy has been applied to investigate Yb compounds with exceptional low temperature physical properties, such as the Kondo effect and heavy Fermion behavior.
In Kondo systems, the EPR signal should be affected by the Kondo effect in two ways: a temperature-dependent g shift due to the local susceptibility and an additional contribution to the EPR line width.301 At this point, it is interesting to note that it seems to be necessary to have ferromagnetic fluctuations in samples to observe any EPR signal, which could be shown by a study of antiferromagnetic (CeOsPO) and ferromagnetic (CeRuPO) samples, since only the latter give rise to an EPR signal.302
In this chapter, only some selected EPR spectroscopic studies on the most prominent examples of intermetallic Yb compounds shall be summarized. As already mentioned, this quite often goes hand-in-hand with exceptional physical properties of these compounds.
In the first part, some examples will be discussed in which the rare earth atoms were introduced as a substituent/dopant/impurity. Later, it was shown that it is possible to receive experimental EPR spectroscopic data on phase-pure unsubstituted/undoped Yb samples, so to say in a dense lattice.
In many systems, it was not possible to obtain EPR spectra and thereby information about the electronic structure of rare earth atoms by applying EPR spectroscopy on dense lattice systems. Due to ultrafast relaxation times, a doping or substitution of paramagnetic ions into diamagnetic compounds is necessary.290 One example for this is the EPR spectrum of ytterbium recorded in elemental gold (800 and 1600 ppm). Here, temperature-dependent measurements between 0.1 and 1 K resulted in the observation of a nonlinear behavior of the line width when lowering the temperature. This could be attributed to the Kondo effect.301 In a study for the Yb-doped samples (100 up to 1000 ppm) with the composition MPd3 (M = Sc, Y, Lu, La, and Ce), crystallizing in the cubic Cu3Au structure (Pm3m), for all tested metals (Er3+, Gd3+, and Yb3+), a Γ7 ground state indicating trivalent character of the ytterbium was found. The EPR spectrum of YPd3 (1000 ppm Yb) given in reference300 is affected by the hyperfine interaction of the localized moment with the two isotopes 171Yb and 173Yb resulting in additional satellites besides the central transition.
Some examples of EPR spectra of Yb in undoped nonmetallic phases such as hydride (YbHx)303 or oxide phases (YbxY1–xBa2Cu3Oy)304 are reported. In these systems, a narrowing of the observed EPR line is described because of the spin-glass ordering and the absence of heavy Fermion behavior as well as exchange narrowing. In an early example, it is reported that the temperature-dependent EPR spectra of pure YbBe13 and substituted diamagnetic Y1–xYbxBe13 (x = 0.01 and 0.09) are identical, which is linked to the rather large separation of ∼500 pm of the rare earth atoms in the NaZn13 type structure (Fm3c), leading to a small crystal field splitting (Γ7 → Γ6 → Γ8) and next to no interaction between the magnetic moments except for some RKKY exchange. For the exact CEF, the differences in the literature were already discussed above. In this EPR spectroscopic study, the valence state of Yb was determined to be trivalent with a Γ7 ground state, equivalent to Eu and Sm, and in contrast to Ce which is in an intermediate valence state in this structure type.236
When looking in the literature, the application of EPR spectroscopy to intermetallic Yb compounds showing heavy Fermion behavior is the most prominent one. Especially, the compound YbRh2Si2 was the first compound in which the direct investigation of the EPR signal of the rare earth ion responsible for the Kondo effect, up to 25 K, so below and above the Kondo temperature, without any doping, substitution, or impurities, was possible. The detection of the signal was thought to be impossible until this proof. In previous studies of doped samples (e.g., with Gd3+), one cannot exclude the influence of the dopant on the Kondo lattice. The exact origin of the EPR signal in this dense Kondo lattice below the relatively high Kondo temperature of TK = 20 K in this compound is yet unclear.158,305,306 A similar EPR signal could be detected a little later in the isostructural silicide YbIr2Si2. The results are in good agreement with those of the Rh compound. Way below the Kondo temperature of 40 K, it was possible to detect a strong EPR signal down to 1 K. Therefore, it is the second example of strong, intense EPR lines in a dense Kondo heavy Fermion system.307 In the past years, more examples of direct EPR detection in heavy Fermion compounds were reported. For YbNiAl2, the trivalent state of the Yb atom was confirmed by measuring an intense EPR spectrum below 20 K. However, here the authors mention that the intensity of the signal obtained at temperatures below 20 K was about an order of magnitude lower than for YbRh2Si2. By increasing the temperature, the EPR signal disappeared. The reason for the appearance of the EPR signal at low temperatures in this dense heavy Fermion compound is not only the already mentioned ferromagnetic fluctuations but also magnetic anisotropy as well as cumulative effects of the surrounding crystal field and spin–orbit interactions.308
For the case of the above-mentioned heavy Fermion superconductor β-YbAlB4, an interesting dual nature behavior of the EPR signal was observed. At high temperatures, an EPR signal was found for the Yb as well as for the Pauli-paramagnetic Lu compound. The Lu compound shows the characteristic temperature dependence of a conduction electron spin resonance over the whole temperature range (4.2 to 295 K), while in comparison, at 4.2 K, the Yb compound shows a hyperfine splitting caused by the 171Yb isotope, confirming the characteristics of an Yb3+-caused spin resonance. This is a unique behavior of the dual nature observed in EPR spectroscopy.211
For the compounds YbBiPt and YbRh2Pb, EPR measurements could be performed at temperatures of up to 110 K, although for discussion of the nature of the compounds, measurements at 4.4 K were used. The results give hints about the crystal electric field (CEF) for the Yb3+ atom in these ternary compounds. For the plumbide, an extremely small hybridization of the 4f electrons and the conduction electrons is suggested together with weak RKKY interactions. In contrast, the bismuthide shows a strongly broadened CEF due to large hybridization effects.290 In this context, one must mention one important class of isostructural compounds. All Yb compounds with the general formula YbT2Zn20 (T = Fe, Co, Ru, Rh, Os, Ir)309 exhibit heavy Fermion behavior. Therefore, a comparison of the spectroscopic properties such as for the above-mentioned silicides Yb(Ir,Rh)2Si2 was possible.309 Here, the Co and Fe compounds have been investigated with EPR spectroscopy. In an analogous way to the already mentioned studies, it was possible to detect a spectrum without any dilution or paramagnetic impurity. A direct investigation of the dense Yb lattice was possible.290,305,309 A comparison of the EPR absorption of the Co and Fe compounds (Figure 14) gave hints about the involvement of the d electrons of the magnetic atoms. With the Co compound EPR absorption being higher by a factor of 10, it was proposed that the increasing number of available d electrons also plays a crucial role in the intensity of the EPR signal and not only the localized magnetic moment of the f electrons on the rare earth atom.
Figure 14.
EPR spectra of YbCo2Zn20 and YbFe2Zn20. Reprinted from ref (290) with permission from Elsevier.
170Yb MÖSSBAUER SPECTROSCOPY
170Yb is a Mössbauer-active isotope and thus allows studies of the ytterbium valence state, the coordination geometry, and magnetic hyperfine field splitting. The energy Eγ amounts to 84.25 keV. The decisive point for 170Yb Mössbauer spectroscopic studies is the very low natural abundance of 170Yb of only 3%, forcing long data collection times. The basic nuclear moment data for the different ytterbium isotopes (170Yb, 171Yb, 172Yb, 174Yb, 176Yb) have been summarized by Stevens and Dunlap;310 however, 170Yb is the usually studied isotope. Its basic Mössbauer spectroscopic characteristics are: Ie = 2 (nuclear spin operator in the excited state); Ig = 0 (nuclear spin quantum number in the ground state); transition energy of 84.25 keV; TmB12 as the usual source (typically 20 mCi) with a half-life of 130 d; and natural line width of 2.03 mm s–1.288,311−313
The 170Yb isomer shift values for divalent and trivalent ytterbium vary only over a small range. For purely ionic compounds, representative values are δ = −0.49 mm s–1 for YbIISO4 and +0.09 mm s–1 for YbIIICl3.311 It is clear that this marginal separation of the YbII and YbIII isomer shifts hampers the studies of valence changes.314 However, the 170Yb data is very sensitive to the hyperfine splitting of the nuclear energy levels and thus allows detailed studies of the magnetic ordering phenomena. The basics for the 170Yb Mössbauer spectroscopic data with respect to magnetic hyperfine splitting are summarized in a concise resource letter by Cadogan and Ryan.312 In the large field of ytterbium intermetallics, the magnetic ordering of the trivalent compounds often sets in only at very low temperatures. Consequently, 170Yb Mössbauer spectra have frequently been measured down to the mK range. Determination of the quadrupole splitting parameter is the important feature for discriminating divalent and trivalent ytterbium. YbII has a filled 4f shell and thus no 4f contribution to the electric field gradient at the 170Yb nucleus. The YbIII compounds, in contrast, show paramagnetic quadrupole triplets.315 Typical examples for divalent ytterbium intermetallics are the binary ytterbium–cadmium phases YbCd, YbCd2, Yb14Cd51, YbCd5.7, and YbCd6316 or the ternary stannide Yb3Pd2Sn2.317,318
The magnetically ordered YbIII compounds show more or less well-resolved hyperfine field splitting. From the hyperfine field value Hhf, one can derive the ordered moment μ through the relation Hhf = Cμ with a value of C = 102 T/μB for Yb3+ (derived from accurate measurements of the paramagnetic hyperfine constant of 170Yb3+ in the cubic Γ7 state).319−321 Due to the meaninglessness of the isomer shift value, the 170Yb Mössbauer spectroscopic studies on intermetallic compounds only report on the quadrupole splitting parameters and the magnetic hyperfine fields.
Temperature-dependent measurements in the magnetically ordered regimes allow for monitoring the evolution of the magnetic hyperfine field with decreasing temperature. These data can be fitted with an S = 1/2 law, and the magnetic ordering temperature can be derived as complementary experiment to the magnetic characterization. Data of binary Yb3Pd4322 is presented in Figure 15. Further related examples are YbNiBC,323 or samples from the solid solution Yb5SixGe4–x.324 In several cases, the crystal field properties have also been derived from the 170Yb Mössbauer spectroscopic data and compared to LIII-edge X-ray absorption spectra and magnetic data. An exemplary investigation is the 170Yb Mössbauer spectroscopic characterization of YbCu3Al2.325
Figure 15.
Temperature dependence of the hyperfine field at the 170Yb nuclei in Yb3Pd4 (TN = 3.2 K). The thermal variation follows an S = 1/2 mean field law (dashed line). Reprinted from ref (322) with permission from Elsevier.
Early work on 170Yb Mössbauer spectroscopic data focused on the rocksalt type monopnictides YbP, YbAs, and YbSb (space group Fm3m),326−328 which are Kondo-frustrated antiferromagnets with very low Néel temperatures <1 K (Table 3). Although the three phases have cubic symmetry, inhomogeneous hyperfine fields/hyperfine field distributions have been observed. Thus, 170Yb Mössbauer spectroscopy is an excellent complementary tool for monitoring structural distortions. In the following, many other binary, ternary, and quaternary intermetallic phases have been characterized through their 170Yb Mössbauer spectra. Some selected phases, which all have their own peculiarity, are shortly discussed in the following.
Table 3. 170Yb Mössbauer Spectroscopy Derived Parameters for Selected Intermetallic Ytterbium Compoundsa.
| Compound | Structure Type | TM/K | TMb/K | BHf/T | μ/μB | Reference |
|---|---|---|---|---|---|---|
| YbBe13 | NaZn13 | TN = 1.28 | 0.05 | 172 | 1.73 | (336) |
| YbB12 | UB12 | – | 0.04 | <10 | <0.1 | (337) |
| YbP | NaCl | TN = 0.41 | 0.045 | 81 | 0.79 | (326) |
| YbAs | NaCl | TN = 0.58 | 0.045 | 81 | 0.82 | (326) |
| YbSb | NaCl | TN = 0.32 | 0.045 | 64 | 0.63 | (326) |
| YbNi5 | CaCu5 | TC = 0.55 | 0.05 | 399(2) | 3.9 | (338) |
| Yb3Pd4 | Pu3Pd4 | TN = 3.2(1) | 0.05 | 63(5) | 0.62(5) | (322) |
| YbCu3Al2 | PrNi2Al3 | TN = 1.95 | 0.12 | 290(10) | 2.84(10) | (325) |
| YbPtAl | TiNiSi | TN = 5.8 | 4.2 | 159 | 1.56 | (329) |
| Yb3Cu4Ge4 | Gd3Cu4Ge4 | TN = 7.5 | 1.45 | 370 | 3.6 | (332) |
| 148 | 1.45 | |||||
| Yb2Co3Al9 | Y2Co3Al9 | TN = 1.2 | 0.06 | 95 | 0.93 | (333) |
| YbNiSb | MgAgAs | TN = 0.85 | 0.13 | 108(10) | 1.06(10) | (334) |
| YbNiSn | TiNiSi | TN = 5.65 | 1.48 | 870(20) | 0.85(2) | (125) |
Parameters include: magnetic ordering temperature, Tm; measurement temperature for the Mössbauer spectrum, TMB; magnetic hyperfine field, Bhf; and magnetic moment at the 170Yb nuclei, μ. Note that Yb3Cu4Ge4 shows two sub-spectra with different hyperfine fields. Standard deviations are given in parentheses, when available.
The incommensurate modulated structure of YbPtAl (TiNiSi type, space group Pnma, wave-vector k = 0.30, 0, 0) was studied by a combination of neutron powder diffraction and 170Yb Mössbauer spectroscopic data.329 The low-temperature spectroscopic data (0.025 K) clearly shows that the modulated structure is not an antiphase one. The maximum moment of 2.5 μB was derived from the 1.6 K spectrum.
The 170Yb Mössbauer spectroscopic data of YbNiAl4 (YNiAl4 type, space group Cmcm) is an important complementary result to specific heat data. While the latter points to the onset of magnetic ordering, no hyperfine field splitting is evident from the 170Yb Mössbauer spectra down to 1.5 K.23 This is consistent with an intermediate ytterbium valence, since the temperature dependence of the magnetic susceptibility also revealed a substantially reduced experimental magnetic moment.
The ytterbium valence change induced by substitution was studied for the solid solutions Yb5Ge4–x(Sb,Ga)x with x ≤ 1 (Sm5Ge4 type, space group Pnma).330 For influencing the ytterbium valence, the germanium atoms are partially substituted by gallium with lower and antinomy with higher electron count. The YbII/YbIII ratio could be derived from well-resolved 170Yb Mössbauer spectra at 5 K through the subsignals with and without quadrupole splitting (vide infra). The Mössbauer spectroscopic data show an almost gradual increase of the YbII/YbIII ratio from ∼0.95 for Yb5Ge3Ga to ∼1.55 for Yb5Ge3Sb.
The quadrupolar phase transition at 80 K in YbAl3C3 (ScAl3C3 type, space group P63/mmc) was monitored by 170Yb Mössbauer spectroscopy.331 Spectra were collected in the entire range from 0.04 to 150 K. No magnetic ordering of the Yb3+ moments was evident down to the lowest measurement temperature. The symmetry reduction (loss of the mirror plane perpendicular to the 63 screw axis) is well expressed in the course of the quadrupole splitting in the 170Yb spectra in the sequence 85 K → 80 K → 77 K → 75 K with a nonaxial quadrupolar hyperfine interaction in the low-temperature phase. Also in this case, 170Yb Mössbauer spectroscopy served as a valuable complementary probe for studying a structural phase transition.
Yb3Cu4Ge4 with Gd3Cu4Ge4 type structure (space group Immm) is one of the remarkable ytterbium intermetallics, since it exhibits two crystallographically independent ytterbium sites 4e and 2d.332 Although the magnetic data indicate a single transition temperature into the magnetically ordered state, these two ytterbium sites show distinctly different magnetic hyperfine spectra (Figure 16) with ordered moments of 3.6 μB for the 4e and 1.45 μB for the 2d site. Integration of the subspectra is consistent with the 2:1 multiplicity of the individual Wyckoff positions.
Figure 16.
170Yb Mössbauer spectra of Yb3Cu4Ge4. The spectrum at 15 K was taken in the paramagnetic range, while the 1.45 and 6.5 K spectra are derived from the magnetically ordered phase. The subspectra are marked by different solid lines. Reprinted from ref (332) with permission from Elsevier.
170Yb Mössbauer spectroscopy was employed to differentiate the magnetic behavior of the Yb atoms in the isotypic compounds Yb2Co3Al9 and Yb2Co3Ga9 (Y2Co3Ga9 type, space group Cmcm).333 The aluminum compound shows a stable 2F7/2 ground state and well-resolved hyperfine field splitting (95 T) at 0.06 K, while Yb2Co3Ga9 is a paramagnetic heavy-Fermion material showing no hyperfine field splitting over the whole temperature range.
The solid solution Yb1–xInxCu2 is structurally derived from the cubic Laves phase (space group Fd3m, vide ultra). The sample with the composition Yb0.4In0.6Cu2 shows a sharp valence transition around 50 K. 170Yb Mössbauer spectra at 4.2 K indicate that the Yb3+ ions exhibit no magnetic hyperfine field splitting in the cubic environment; however, it shows a strong 4f–5d hybridization.191
The final example shows the necessity of complementary techniques for the precise understanding of structure–property relations. The half-Heusler phase YbNiSb (MgAgAs type, space group F43m) has been characterized through 170Yb Mössbauer spectra at 0.13 and 1.4 K.334 Spectra in the magnetically ordered regime revealed enlarged and inhomogeneous line widths. Based on these results, it was argued that the quadrupolar hyperfine interaction of the paramagnetic phase has no cubic site symmetry. Later, precise single crystal X-ray diffraction data showed that YbNiSb samples are a mixture of equiatomic YbNiSb as the main component along with a distribution of YbNixSb domains with different nickel content.335 Thus, the magnetically split spectrum is a superposition of the magnetically split subspectra of the distribution of YbNixSb domains.
Many other interesting examples for 170Yb Mössbauer spectroscopic studies on ytterbium intermetallics have been published. In such a review, one can only present selected examples. Besides the data discussed above, we have added some further examples in Table 3 with respect to their basic Mössbauer spectroscopic data.
Besides the many 170Yb Mössbauer spectroscopic studies under ambient conditions, some investigations were carried out under application of external magnetic fields or high pressure. The heavy Fermion silicide YbRh2Si2 was additionally studied by 170Yb Mössbauer spectroscopy as a complementary tool to ESR spectroscopy.339 The ESR data for this silicide gave hint for a slow relaxation rate. This issue could be clarified by 170Yb Mössbauer spectroscopy. The 170Yb spectrum only shows a single signal, which does not match with a simulated spectrum for slow relaxation at an applied field of 0.4 T. The 170Yb data thus indicate that the Yb3+ ions relax more rapidly than expected from the ESR data. YbRh2Si2 was furthermore studied at 1.3 K and pressures ranging from 6.4 to 16.5 GPa.340 The experiments show a continuous line broadening with increasing pressure. The pressure-induced first-order magnetic phase transition sets in around 10 GPa. The line broadening indicates increasing magnetic hyperfine field splitting and thus an increase of the ordered moment at the ytterbium nuclei with a value of 1.9 μB (≡191 T) at 16.5 GPa, still reduced when compared with the free ion value of 4 μB.105
The technical details for 170Yb Mössbauer spectroscopy under high-pressure conditions are well documented in an article by Schöppner et al.,341 which focuses on the pressure-induced ytterbium valence change in YbCuAl (ZrNiAl type, space group P62m). The compound was studied at 4.2 K from 0 to 130 kbar pressure. The 170Yb spectra show a continuous increase of the quadrupole splitting parameter with increasing pressure, manifesting the valence transition toward trivalent ytterbium with 4f13 configuration. This ground state is achieved already at around 50 kbar.
170Yb Mössbauer spectroscopic data was collected for the TiNiSi type stannide YbNiSn (space group Pnma) up to 7 GPa.342 A remarkable feature is the increase of the Curie temperature with increasing pressure with a maximum of TC = 7.6 K at 1.7 GPa followed by a rapid decrease. In parallel, the quadrupole splitting parameter and the magnetic hyperfine field increase from ambient pressure to 7 GPa. The maximum field of 175 T corresponds to an ordered moment of 1.72 μB.
Also, the heavy Fermion compound Yb2Ni2Al (W2CoB2 type, space group Immm) shows a pressure-driven first-order magnetic phase transition.343 A crossover from a nonmagnetic heavy Fermion state to a magnetically ordered state occurs at pressures >8 GPa and below 2 K. The 170Yb Mössbauer spectrum (1.8 K data) at the highest pressure of 9 GPa showed an ordered moment of ≈1 μB at the ytterbium nuclei.
Finally, we turn toward an interesting extension of 174Yb Mössbauer spectroscopy which was realized recently using synchrotron radiation as the Mössbauer spectroscopy source. The classical experiments all used the 170Yb isotope. The main disadvantage is its low natural abundance of only 3% as compared to 31.8% for 174Yb. The synchrotron radiation-based technique is described in a resource article by Masuda et al.,344 and exemplified for an YbB12 (UB12 type, space group Fm3m) sample. Further advantages of this new technique are the higher brilliance of the synchrotron radiation as compared to X-rays deriving from a radioactive isotope, and there is no need for isotope enrichment. Along with the use of a windowless avalanche photodiode detector, fast data collections are possible, and this technique will also be helpful for future high-pressure studies. Two further examples for the application of 174Yb Mössbauer spectroscopy are Yb2Fe14B (Nd2Fe14B type, space group P42/mnm)345 and α-YbAlB4 (YCrB4 type, space group Pbam).346
THERMOELECTRICS
Thermoelectric materials are usually heavily doped semiconductors which can produce a charge flow when exposed to a temperature gradient. Depending on the charge carrier type in the respective material, these are denoted as p- or n-type semiconductors. The quality of a thermoelectric material is usually given by the dimensionless figure-of-merit zT which basically incorporates the Seebeck coefficient as well as the thermal and electrical conductivity. Details regarding these values and their interplay can be found in a number of reviews.347−352 However, the determination of the Seebeck coefficient can also be used for identifying magnetic transitions and other physical phenomena as it is very susceptible to changes in the electronic states. In the following chapter, examples of classical intermetallic Yb-compounds as well as examples of Zintl phases, being the more typical thermoelectric materials, will be addressed.
Binary Compounds
We will start this chapter with selected examples of classical intermetallic materials. For this, the binaries YbAl3, YbSi2, YbB6, YbCd6, and Yb3Si5 as well as the solid solutions Yb1–xScxAl2, Yb1–xInxCu2, and YbCu5–xAgx have been chosen. From the ternary systems, equiatomic YbPtBi, YbNiSn, and YbPtGe, as well as YbAlB4, YbNiAl4, YbRh2Al20, and YbT2Zn20 (T = Fe, Co, Ru, Rh, Os, Ir), Yb2Pt3Sn5, Yb2T3X9 (T = Rh, Ir; X = Al, Ga), Yb2Pt6Al15, and Yb2Ni12P7 were selected.
YbAl3 (Cu3Au type, space group Pm3m) is, as mentioned before, a well studied compound due to the ambivalent nature of the Yb atoms leading to a change of the Yb valence with temperature.113 Due to this phenomenon, the Seebeck coefficient of intermediate valent/valence fluctuating Yb compounds is often significantly higher than expected. This is also the case for YbAl3 where the Seebeck coefficient reaches values over −80 μV K–1 near 120 K,353 and YbSi2 (AlB2 type, space group P6/mmm),354 also a mixed valent compound, shows values between −50 and −35 μV K–1 between 300 and 525 K. The electrical resistivity, however, possesses abnormally large values due to spin-disorder scattering. YbSi2 shows an almost temperature-independent electrical resistivity and a total thermal conductivity between 8 and 11 W m–1 K–1, leading to a zT between 0.08 at 300 K and 0.04 at 525 K. YbB6 adopts the cubic CaB6 type structure (space group Pm3m) and exhibits a certain phase width between x = 5.7 and 6.3.355 Therefore, this compound can be used to study and tune the thermoelectric properties. The compounds with x = 5.7, 5.9, and 6.0 exhibit a negative Seebeck coefficient of approximately −100 μV K–1 between 300 and 1050 K. In contrast, the samples with x = 6.1 and 6.3 start with a positive Seebeck coefficient of +100 μV K–1, which gradually changes to negative values at higher temperatures. YbCd6, despite the same sum formula, crystallizes in a totally different crystal structure (YCd6 type, space group Im3). Instead of octahedral B6 clusters, here a quasi-crystal (QC) approximant with a shell-like structure is present. Both the QC YbCd5.7 and the approximant YbCd6 were investigated with respect to their thermoelectric properties. Both show small positive Seebeck coefficients between +10 and +20 μV K–1. The striking feature is a maximum in the electrical resistivity around 70 K for YbCd5.7. A similar trend can be seen for the different compositions of the quasi-crystals, leading to zT values of 5 × 10–3 near RT.356,357 Finally, Yb5Si3 (Mn5Si3 type, space group P63/mcm) should be discussed as the last binary representative. Again, this compound is valence instable and shows fluctuations between di- and trivalent Yb, leading to two sign changes in the Seebeck coefficient. Below ∼40 K, it is negative with a rather sharp minimum at 10 K, followed by a broad maximum with positive values (∼13 μV K–1) around 100 K, and again a change to a negative Seebeck coefficient close to 300 K. The resistivity is, compared to the close-shell system Lu5Si3, enhanced by a factor of close to two, caused again by the valence fluctuation.358
The solid solution Yb1–xScxAl2 (MgCu2 type, space group Fd3m) was studied to probe the influence of “chemical pressure” on the properties of the valence fluctuating system YbAl2. Different compositions with x = 0.6–1 were synthesized and investigated. Magnetic susceptibility measurements clearly indicate that the intermediate valence (IV) state gets more pronounced with increasing Sc content and a shift toward more Yb3+ is visible. Samples with a pronounced IV state at the same time exhibit a drastically negative Seebeck coefficient up to −50 μV K–1 below 50 K for x = 0.9. This leads to a maximum in the power factor (S2/ρ) of 160 μW cm–1 K–2 for x = 0.9 around 50 K.359
Since Yb1–xInxCu2 (MgCu2 type, space group Fd3m) shows a temperature-induced first-order valence phase transition, its Seebeck coefficient was investigated for x = 0.6 to probe the fine structure of the conduction electron band closely associated with the magnetic state. The temperature dependence of the Seebeck coefficient starts with a positive sign at high temperatures and exhibits a change of sign near 200 K. Around 40 K, a minimum with S = −16 μV K–1 is visible, coinciding with the temperature of the valence phase transition.360
Equiatomic Ternary Compounds
For the ternary compounds, we will start with selected equiatomic representatives, i.e., YbNiSn, YbPdGe, YbPtGe, and YbPtBi. For YbNiSn (TiNiSi type, space group Pnma), the thermoelectric properties were studied to obtain further information about the crystal field splitting. Inelastic neutron scattering should yield three crystal field excitations based on the crystal structure. However, only two excitations were visible; therefore, the thermoelectric properties were determined to gain a deeper insight. The temperature dependence of the Seebeck coefficient shows a broad minimum around 100 K along with a peak at 7.5 K and a change of sign below 5 K. From these investigations, the degeneracy of two crystal field excitations was deduced.361 YbPdGe (YbAuSn type, space group Imm2) and YbPtGe (TiNiSi type, space group Pnma) show a very similar behavior. The Seebeck coefficient shows a broad maximum around 120 K followed by a maximum at 30 K. Three contributions were deduced: a Mott’s diffusion term as well as a Kondo and a resonance term. While the first one is small and temperature-independent, the Kondo term originates from Kondo scattering of conduction electrons by the 4f spins. The inelastic contribution to the resonance term originates from inelastic scattering of the conduction electrons by magnetic interactive spins, respectively.362 Finally, YbPtBi (MgAgAs type, space group F43m) was investigated. This compound is of particular interest since it exhibits one of the largest Sommerfeld coefficients known (γ = 7.4 J mol–1 K–2).137 Thin films of YbPtBi exhibit also a negative Seebeck coefficient with −54 μV K–1.363
Further Ternary Compounds
While the silicides and germanides adopt the tetragonal body centered ThCr2Si2 type structure (space group I4/mmm), the pnictide-based Zintl phases of the same chemical composition crystallize in the trigonal space group P3m1 and adopt the Ce2O2S type structure.
YbRh2Si2 is an antiferromagnetic heavy Fermion compound;162,163,364,365 therefore, investigations of the thermoelectric properties (TEP) can give important information on the behavior of the conduction electrons. The TEP contain information about the relaxation time and the DOS of the quasiparticles at the Fermi level EF. It is interesting to note that at very low temperatures, the TEP is commonly positive for Ce heavy Fermion compounds, in agreement with the Kondo picture, while it is negative for Yb heavy Fermion compounds.366 Field-dependent measurements of the Seebeck coefficient S(H) have been conducted down to 120 mK and up to 16 T.367 Above 3.5 T, a cascade of sharp anomalies can be observed, pointing toward topological transitions of the Fermi surface. Upon substitution of rhodium by cobalt according to Yb(Rh1–xCox)2Si2,368,369 the Kondo temperature decreases significantly, and the sole large minimum in the thermopower that is observed for YbRh2Si2 splits into two minima. In addition, the absolute thermopower is strongly reduced with increasing x, in line with a weaker exchange coupling of the 4f and the conduction electron states making pure YbCo2Si2 a stable trivalent system.241,242,369 However, still two minima are observed in the Seebeck coefficient, indicating weak residual Kondo scattering in line with a tiny residual hybridization between 4f and conduction electron states. A comparative study of the thermoelectric response under magnetic field of YbRh2Si2 and YbAlB4 showed a significant difference in the Seebeck coefficient near the quantum critical points of both compounds. When approaching the critical field, S/T is drastically reduced in YbRh2Si2; however, an enhancement in β-YbAlB4 is observed.370
YbPd2Si2 is another heavy Fermion material within the group of Yb intermetallics and has been studied broadly.371−373 In the range between 1.5 and 300 K, the Seebeck coefficient is negative with a minimum around 50 K, which was attributed to the intermediate valence of this compound.374 Investigations of YbNi2Si2 and YbCu2Si2 revealed a similar behavior with minima between 50 and 100 K; however, the Seebeck coefficient gets positive for YbNi2Si2 below ∼20 K, which was assigned to the antiferromagnetic ordering of this compound.375,376 High-pressure studies of YbCu2Si2 revealed that a magnetic phase transition emerges at Tm = 1.9 K around TC = 10.2 GPa which shifts to higher temperatures with increasing pressure.377 This was later confirmed by high-pressure measurements of the Seebeck coefficient.375 YbPd2Ge1.7 also exhibits a nonmonotonous behavior and shows a negative Seebeck coefficient down to low temperatures, where again the sign changes.378 The solid solutions YbCu2Si2–xGex379 and Yb1–xLaxCu2Si2380 finally were studied with respect to their thermoelectric properties. Materials with large Seebeck coefficients at low temperatures can be utilized for cryogenic Peltier elements for cooling infrared sensors on satellites. With this respect, the solid solutions were investigated to maximize the zT at cryogenic temperatures. In both cases, an enhancement of the power factor as well as a shift of the maximum to higher temperatures was observed. Alongside this, a reduction of the thermal conductivity was noted, leading to an overall higher zT. The observed changes were attributed to the changes in the unit cell dimensions that can be envisioned as “chemical pressure”.
YbAlB4 and YbNiAl4, although having the same chemical composition, adopt two different structure types. YbAlB4 is furthermore dimorphic and crystallizes either in the orthorhombic YCrB4 (α-YbAlB4; space group Pbam) or ThMoB4 (β-YbAlB4; space group Cmmm) type structure (vide ultra). Due to the superconductivity observed in β-YbAlB4204 and its heavy Fermion characteristics, the quantum-critical point (QCP) is of special interest. During these investigations, it became clear that the Seebeck coefficient for β-YbAlB4 behaves significantly different compared to YbRh2Si2. −S(T)/T becomes large for β-YbAlB4 near the zero-field QCP, while for YbRh2Si2, −S(T)/T is strongly suppressed.370,381
Also, in the CeCr2Al20 type structure (space group Fd3m), several Yb-containing compounds are known that exhibit Kondo or heavy Fermion properties. Similar to the examples discussed before, the members of the YbT2Zn20 (T = Fe, Ru, Os, Co, Rh, Ir) series also exhibit heavy Fermion Kondo lattices. In their thermoelectric properties, all members show large, negative minima (between −75 and −45 μV K–1); however, variations with respect to the transition metal T can be observed. The local minimum in S(T) and the local maximum in ρ(T) can be correlated to the transition metals and the Kondo temperatures of the respective compounds as well as with the influence of the crystalline electric field splitting.382 Wei and co-workers have shown, that the compounds of the YbT2Zn20 (T = Co, Rh, Ir) series exhibit increasing figures of merit with increasing mass of the transition metals at low temperatures, peaking at zT = 0.07 at 35 K for YbIr2Zn20.383
Finally, the thermoelectric investigations on Yb2Pt6Al15 (Sc0.6Fe2Si4.9 type, space group P63/mmc),384 Yb2T3Al9 (T = Rh, Ir; Y2Co3Ga9 type, space group Cmcm),385 Yb2Pt3Sn5 (own type, space group Pnma),386 and Yb2Ni12P7 (Zr2Fe12P7 type, space group P6)387 should be addressed. While the first two representatives have been characterized as heavy Fermion systems, Yb2Pt3Sn5 is a rare example of a heterogeneous mixed-valence system. Yb2Ni12P7 finally exhibits a crossover between Fermi-liquid and non-Fermi-liquid behavior and seems to be in close proximity to a quantum critical point. For both Yb2Pt6Al15 and the Yb2T3Al9 (T = Rh, Ir) series, the temperature dependence of the Seebeck coefficient shows a minimum at low temperatures, as expected for Yb-based Kondo lattice systems. While the platinum compound reaches −70 μV K–1 around 35 K, Yb2Rh3Al9 and Yb2Ir3Al9 show a change of sign near 250 K and minima of −15 μV K–1 and −25 μV K–1 at 30 and 40 K, respectively. For the latter, an additional anomaly is observed near their Néel temperature.385 A single anomaly in S(T) is observed for Yb2Pt6Al15 indicating that the Kondo energy scale is close to the CEF splitting. The temperature observed is compatible with a Kondo scale of the order of 60 K. The accordance of these temperatures supports the picture of a Kondo lattice with a large characteristic energy in which the whole multiplet is involved in the low-temperature physics.384 Yb2Pt3Sn5 also shows a minimum in the Seebeck coefficient (−38 μV K–1 at 60 K),386 which is typical for intermediate valent Yb compounds such as YbAgCu4 and YbCu2Si2.374
Zintl Phases
Zintl phases are usually the class of materials that come to mind when speaking about thermoelectric properties. Due to their semiconducting properties, they can be utilized to produce energy based on a temperature gradient. In many cases, the materials have to be optimized with respect to their electronic properties like charge carrier concentration.
One compound with outstanding thermoelectric properties is probably Yb14MnSb11 (Ca14AlSb11 type, space group I41/acd). In 2006, Brown et al. reported that Yb14MnSb11 “achieves quadrupled efficiency and virtually doubled figure of merit over the current state-of-the-art, SiGe”.388 The material reaches a figure of merit of ∼1.0 at 1223 K (Figure 17). The Seebeck coefficient is positive in the whole temperature range and increases linearly with increasing temperature, reaching +185 μV K–1 at 1275 K. Since the resistivity also shows a linear behavior with temperature and Hall measurements indicate a constant carrier concentration over the investigated temperature range, the compound can be considered a heavily doped semiconductor. The high figure of merit finally arises from the low thermal conductivity of Yb14MnSb11, which is on the scale of a glass (7–9 mW cm–1 K–1).
Figure 17.
Figure of merit zT of Yb14MnSb11. Maximum zT value of ∼1.0 at 1200 K. Reprinted with permission from ref (388). Copyright 2006 American Chemical Society.
Since the initial report of Yb14MnSb11 being a high-zT material, numerous reports have been published that modified the original compound via the formation of solid solutions as well as investigations on isostructural compounds. In the following part, selected publications have been chosen to illustrate the overall ideas.
Yb14MnSb11 can be modified on the Yb, the Mn, and the Sb position, either by isovalent atoms, often leading to structural distortions and therefore changes in the electronic structure, or by elements which exhibit different formal oxidation states, leading to changes in the overall electron count. Isovalent substitutions on the Yb position can, for example, be realized by Ca. The solid solution Yb14–xCaxMnSb11 shows that the substitution of Yb by Ca reduces the carrier concentration leading to an increased Seebeck coefficient and at the same time to an increased electrical resistivity. In addition, the mass difference between Ca and Yb leads to a significantly reduced density and mass-disorder scattering is observed, resulting in a lowered lattice thermal conductivity. However, these changes do not lead to a significant improvement of zT. In addition, compositions with values of x > 4 get highly air-sensitive, making the characterization of the thermoelectric properties impossible.389,390
As for a substitution of Yb via a trivalent rare earth element, Sc and Y,391 La,392 Lu,393,394 as well as the full RE3+ (La–Lu) series395 have been reported. While for the latter only the thermochemistry and thermal stability was analyzed, for the other substitutions the influence on the thermoelectric properties was also investigated since the trivalent rare earth cations change the carrier concentration. The Sc- and Y-substituted specimens show higher resistivities and thus higher Seebeck coefficients; however, an overall reduction of the zT is observed.391 Also for the Lu-substituted compounds, a reduced carrier concentration was reported as well as a reduced peak zT.394 Another study, however, reported an increased carrier concentration upon Lu substitution leading to a ∼30% improvement in zT at 670 K.393 For La-substituted Yb14–xLaxMnSb11, finally an increased zT was reported for x ∼ 0.4 with a peak zT of 1.15 at 1150 K.392
Also for the Mn position, an isovalent substitution is possible, e.g., in Yb14Mn1–xZnxSb11.396 The substitution shows a slight improvement up to x ≤ 0.4 by reducing the lattice thermal conductivity at low temperatures; however, only a negligible impact at high temperatures is observed. This is based on the reduction of spin disorder scattering leading to an overall improvement of about 10% with a maximum thermoelectric figure of merit ∼1.1 at 1275 K for x = 0.4. Substituting Mn2+ for Al3+, however, again leads to a reduction of the carrier concentration,397,398 similar to the substitution of Yb2+ by RE3+. For x = 0.6 and 0.8, an improvement of 30% was observed, equal to a zT = 1.3 at 1223 K.
Finally, modifications on the Sb positions should be mentioned. In the solid solution Yb14MnSb11–xBix, overall lower zT values are observed,399 while Yb14MnSb11–xTex shows an improved zT of 1.11 for x = 0.07 at 1240 K.400 This, again, can be attributed to a reduced carrier concentration.
Furthermore, the low-temperature behavior of the thermoelectric properties has been studied at ambient and increased pressure. Also in the range between 4.2 and 300 K, the Seebeck coefficient for Yb14MnSb11 is positive and increases upon heating.401 Increasing the pressure up to 2.3 GPa leads to a further increase of the Seebeck coefficient in the same temperature range.402
Besides Yb14MnSb11, other Yb-containing compounds with the same composition and crystal structure were also investigated. As examples, Yb14MgSb11, Yb14MnBi11, and Yb14MgBi11 will be discussed. Yb14MgSb11 has, compared to its Mn-containing analogue, an increased Seebeck coefficient which leads to an increase of 45% in the figure of merit (zT = 1.02 at 1075 K) at the same temperature.403 The addition of iron leads to the formation of nanosized inclusions, which lead to an even further increase of zT.404,405 Yb14MnBi11 and Yb14MgBi11 are both metallic in nature, and Yb14MgBi11 has an increased carrier concentration in comparison to the isostructural antimonide which compensates potential improvements of the thermoelectric properties. Overall, the zT values of both compounds reach ∼0.2 at 875 K.406
The MT2X2 compounds that adopt the trigonal Ce2O2S type structure (space group P3m1) with the general composition MIITII2Pn2 can also be considered as valence precise Zintl phases. M is a divalent alkaline earth or rare earth metal (Ca, Sr, Eu, Yb), while T is a divalent transition metal (Mn, Zn, Cd) or Mg, and Pn finally a pnictogen (As, Sb, Bi). These compounds are considered to be “electron precise” according to the Zintl formalism.
In the following paragraph, just a small number of studies will be mentioned as there is a plethora of studies related to the optimization of the thermoelectric properties by different strategies. One of the first reports is on the solid solution CaxYb1–xZn2Sb2 from Gascoin and co-workers.407 They showed that YbZn2Sb2 has the smallest Seebeck coefficient but at the same time the smallest electrical resistivity. CaZn2Sb2 in contrast exhibits an over two times larger Seebeck coefficient but also a 7-fold larger resistivity. The solid solution shows an intermediate behavior, depending on the composition. Subsequently, numerous solid solutions were investigated with respect to improving the TEP; for example, substitution of Yb by Eu according to EuxYb1–xZn2Sb2408,409 or by La as in Yb1–xLaxZn2Sb2.410 But also high-quality single crystals of YbZn2Sb2 were grown and investigated.411 Despite modifications by chemical substitution, nanoporous412 or Yb-deficient materials (Yb1−δZn2Sb2)413 were also synthesized and investigated. But not only the substitution of the Yb is possible. Also, the formation of solid solutions with respect to the Zn or Sb position can influence the thermoelectric properties. For example, the solid solutions YbZn2–xMgxSb2,414 YbZn2–xMnxSb2,415,416 YbZn2–xCdxSb2,417 and YbZn2Sb2–xBix418 should be named. Finally, it has been proven that Mg can substitute on both the Yb and Zn sites.419 Besides YbZn2Sb2, also YbMg2Sb2,420 YbMg2Bi2,421−423 YbMn2Sb2,424 YbCd2Sb2, and the solid solutions YbCd2–xMnxSb2425 have been investigated in great detail.
Finally, two other classes of important thermoelectric materials should be addressed: clathrates and skutterudites. Although clathrates are structurally very versatile materials, hardly any ytterbium-containing ones have been reported. Detailed information on the different clathrate structures and their applications can be found in a review.426 The key features that lead to their intriguing thermoelectric properties are the “rattling” atoms within the structure. These are located in the large polyhedra that form the framework and can effectively scatter the heat-carrying phonons, therefore reducing the thermal conductivity. The reports on Yb-substituted clathrates are mainly examples of type-I clathrates, which crystallize in space group Pm3n and exhibit the general formula G8[F46], with F being the framework and G being the guest atoms. In the type-I clathrates, two different polyhedra can be found, a 20-vertex pentagonal dodecahedron and a 24-vertex tetrakaidecahedron. For the framework, different elements and elemental combinations are known as well as a large number of guest atoms occupying these polyhedra.426 The number of Yb-based type-I clathrates (Na4Si23 type, space group Pm3n), however, is extremely low. To the best of our knowledge, no pure Yb clathrates have been reported, but the solid solutions Ba8–xYbxGa16Si30,427 Ba8–xYbxGa16Ge30,428 Ba8–xYbxNi0.1Zn0.54Ga13.8Ge31.56,429 Ba8–xYbxNi3.8Si42.2,430 and Ba8–xYbxCu4.9Si41.1430 have been investigated experimentally. In addition, first principle studies on Ba6Yb2Au6Ge40431 have been conducted. For the silicide, it was shown that the substitution of Ba by Yb is possible, but only to a certain extent of x = 0.25 to 0.38.427 The substitution led to a slight decrease of the Seebeck coefficient, however, also to a significant decrease of the lattice thermal conductivity which resulted in a slight overall increase of zT for Ba7.75Yb0.25Si31.9Ga14.1.427 For the germanide series Ba8–xYbxGa16Ge30, in principle the same trend is observed. Here, samples with x = 0 to 1.3 could be synthesized and investigated, and again a decrease in the Seebeck coefficient is visible for x ≤ 0.7 alongside a decrease of the lattice thermal conductivity leading to a zT of 1.1 (950 K) for Ba7.5Yb0.5Ga16Ge30. This is an increase of 90% compared to the Yb-free sample.428 Also in Ba8–xYbxNi0.1Zn0.54Ga13.8Ge31.56,429 a slightly increased zT (15%, zT = 0.91 at 900 K) was reported for x = 0.3. In this case, the electrical resistivity and lattice thermal conductivity decrease with increasing x, the Seebeck coefficient, however, stays constant. Yb substitution in Ba8–xYbxNi3.8Si42.2 and Ba8–xYbxCu4.9Si41.1 revealed that the majority of the Yb precipitated as secondary phases. The solubility limit is only about 1% on the Ba site. The multiphase samples showed both a lower electrical resistivity and a lower Seebeck coefficient with respect to the unsubstituted compounds; however, the thermal conductivity increases. Overall, the thermoelectric figure of merit of the Ni-based system is not improved, while a slight enhancement above 800 K is observed for Ba8–xYbxCu4.9Si41.1.430 For Ba6Yb2Au6Ge40, finally, the thermoelectric properties were calculated using the full-potential linearized augmented plane wave method based on density functional theory. It was shown that the substitution enhances the density of states at the bottom of the conduction band, leading to an increased Seebeck coefficient due to an increased effective mass of the conduction band.431
Finally, one report on a type-VIII clathrate (G8[F46]; own type, space group I43m) has been published, which investigated the substitution in Ba8–xYbxGa16Sn30.432 Here, an improvement of the Seebeck coefficient up to x = 1.0 was observed. Overall, the situation is similar to the type-I clathrates: a substitution adjusts the Seebeck coefficient as well as the electrical and thermal conductivity, leading to an improvement of the figure of merit zT. In Ba6.5Yb1.5Ga16Sn30, this leads to a maximum zT of 1.35 at 489 K.
The last class of materials that should be discussed with respect to their thermoelectric properties are Yb-containing skutterudites. The name of this class of materials derives from the mineral skutterudite CoAs3 with the general formula TX3 (CoAs3 type, space group Im3). In the crystal structure of the skutterudites, two empty cavities can be found that can accompany a number of different atoms. In the late 1970s, Jeitschko and co-workers coined the term “filled skutterudites” for compounds with the general composition MT4X12 (LaFe4Sb12 type, space group Im3).433−436 In 1996, Sales437 and Fleurial438 showed that filled skutterudites exhibit excellent thermoelectric properties at elevated temperatures. The reviews by Uher from 2001 and Sales from 2003 summarize the important aspects of skutterudites.439,440 One of the first Yb-containing filled skutterudites investigated for its physical properties was YbFe4Sb12. Magnetic measurements indicate an intermediate valence state and a slightly enhanced Sommerfeld coefficient.441,442 In these reports, the potential for YbFe4Sb12 to exhibit good thermoelectric properties was outlined. Low-temperature thermoelectric property measurements showed that YbFe4Sb12 is a p-doped metal with a low carrier density. The high electrical resistivity leads to a zT < 0.02 below 300 K.443 The investigations of the high-temperature properties lead to a zT = 0.4 at 670 K.444 Within the solid solution YbyFe4–xNixSb12, even zT values near 1 (750–850 K) could be obtained for x = 0.6–0.8.445
Due to the chemical versatility of the filled skutterudites, numerous compositions were investigated with respect to their thermoelectric properties. Within the field of Yb-based compounds, YbCo4Sb12 showed an excellent performance, leading to a large number of studies of tuned and improved materials. Therefore, only some examples will be summarized in the next paragraph. Studies on different compounds of the YbxT4Sb12 series (x = 0.1–0.8, T = Fe, Co, Rh, Ir) showed that the physical properties seem to correlate with the filling x and the electronic situation modified by changing T.446 At the same time, studies on YbyCo4SnxSb12–x indicated a maximum zT of 0.1 at 300 K for Yb0.44Co4Sb12.447 More detailed studies including Hall effect, electrical resistivity, Seebeck coefficient, and thermal conductivity measurements were conducted on the solid solution YbyCo4Sb12–xSnx for y = 0.19 and 0.5. It was shown that substituting Sn on the Sb site does not alter the Fermi level with respect to the conduction band; instead, a p-type band for a sufficiently large Sn content x was observed. Therefore, this is not a valid method to optimize the thermoelectric properties of Yb-filled skutterudites.448 Double substitution according to Ba0.08Yb0.09Co4Sb12, however, led to a broad range of resonant phonon scattering and which resulted in a strong suppression in the lattice thermal conductivity. Therefore, high zT values of 1.36 at 800 K could be observed.449 A similar value in zT (1.36 at 850 K) could be observed for Al0.3Yb0.25Co4Sb12.450 By the help of ball-milling in combination with hot-pressing, the value of x in YbxCo4Sb12 could be improved to almost 0.5, leading to a zT of 1.2 at 823 K.451 In recent years, studies addressing the band conversion in YbxCo4Sb12,452 the achievement of ultralow thermal conductivities in YbxCo4–yFeySb12 with x = 0.25–0.5 and y = 0.1–0.5,453 the synthetic approach on YbxCo4Sb12,454 and introducing nanoinclusions455 have led to a deeper understanding of the possibilities to tailor the thermoelectric properties in skutterudite materials.
SUMMARY AND OUTLOOK
The present review impressively underlines that the family of ytterbium-based intermetallic compounds covers extremely broad facets of crystal structures along with interesting and, in some cases, even outstanding physical properties. This interplay is mostly driven by the ytterbium valence: divalent (4f14), intermediate-valent, or trivalent (4f13) ytterbium. Thus, one observes simple diamagnets, Pauli or Curie–Weiss paramagnets as well as valence fluctuating compounds, Kondo behavior, heavy Fermion ground states, or sometimes even superconductivity.
Some of the trivalent ytterbium intermetallics have gained deep interest due to these physical properties. The phases have been meticulously studied with respect to their properties using magnetic and transport measurements, specific heat data, NMR, ESR, and Mössbauer spectroscopy, elastic and inelastic neutron scattering, XAS and XPS data, as well as detailed thermoelectric measurements, then allowing the development of structure–property relations.
Among the binary Yb intermetallics, YbAl2 and YbAl3 have received the most attention due to their Kondo behavior. Overall, however, the number of binary compounds studied in detail is rather low compared to the ternary systems, given the significantly larger combinatory manifold in the ternary systems. Here, YbRh2Si2 is the most outstanding phase with >350 publications on this compound. It exhibits a non-Fermi-liquid behavior along with antiferromagnetic ordering at TN = 70 mK. A quantum critical point at ambient pressure is suggested, at which the magnetic ordering can be suppressed by a magnetic field of only Bc = 60 mT. Yb2Pt2Pb exhibits a Shastry–Sutherland lattice that causes a high anisotropy in the magnetic susceptibility with an about 30 times larger value for χ(100) as compared to χ(001). Finally, among the series of YbT2Zn20 compounds, the cobalt-containing representative YbCo2Zn20 exhibits Kondo interactions, and from specific heat measurements, a large Sommerfeld coefficient of γ = 7.9 J–1 mol–1 K–2 was deduced. Finally, Yb14MnSb11 is one of the most promising p-type thermoelectric materials with a high zT > 1, making this compound suitable for different applications.
Although most of these studies are located in basic research, they help with a deeper understanding of complex intermetallic compounds and motivate for future studies. Especially, the ytterbium-based thermoelectric materials might find application. Even though the family of ytterbium intermetallics is large, a weak point concerns the overall systemization of the property data, since only few larger isotypic series of compounds exist. Already, the equiatomic YbTX phases crystallize with more than 10 different structure types; many of them are superstructures of the aristotype AlB2. The probably best investigated, and thus comparable, series of isotypic compounds concerns the CeCr2Al20 type zinc compounds YbT2Zn20 with T = Fe, Co, Ru, Rh, Os, and Ir.
The study of ytterbium intermetallics is still a vivid field in solid state chemistry and physics. Besides the discovery and characterization of new phases, the understanding and systemization of the structure–property relations is an important future task.
Data Availability Statement
The data underlying this study are available in the published article.
Author Contributions
$ These authors contributed equally. CRediT: Stefan Engel conceptualization, formal analysis, writing-original draft, writing-review & editing; Elias C. J. Gießelmann conceptualization, formal analysis, writing-original draft, writing-review & editing; Maximilian Kai Reimann conceptualization, formal analysis, writing-original draft, writing-review & editing; Rainer Pöttgen conceptualization, formal analysis, project administration, supervision, validation, visualization, writing-original draft, writing-review & editing; Oliver Janka conceptualization, formal analysis, funding acquisition, investigation, supervision, validation, visualization, writing-original draft, writing-review & editing.
Funding is provided by the Deutsche Forschungsgemeinschaft DFG (JA 1891-10-1).
The authors declare no competing financial interest.
References
- Szytuła A.; Leciejewicz J.. Handbook of Crystal Structures and Magnetic Properties of Rare Earth Intermetallics. CRC Press: Boca Raton, 1994. [Google Scholar]
- Matar S. F. Review on cerium intermetallic compounds: A bird’s eye outlook through DFT. Prog. Solid State Chem. 2013, 41, 55–85. 10.1016/j.progsolidstchem.2013.03.001. [DOI] [Google Scholar]
- Pöttgen R.; Chevalier B. Cerium intermetallics with ZrNiAl-type structure – a review. Z. Naturforsch. 2015, 70b, 289–304. 10.1515/znb-2015-0018. [DOI] [Google Scholar]
- Pöttgen R.; Chevalier B. Equiatomic cerium intermetallics CeXX′ with two p elements. Z. Naturforsch. 2015, 70b, 695–704. 10.1515/znb-2015-0109. [DOI] [Google Scholar]
- Pöttgen R.; Janka O.; Chevalier B. Cerium intermetallics CeTX – review III. Z. Naturforsch. 2016, 71b, 165–191. 10.1515/znb-2016-0013. [DOI] [Google Scholar]
- Janka O.; Niehaus O.; Pöttgen R.; Chevalier B. Cerium intermetallics with TiNiSi-type structure. Z. Naturforsch. 2016, 71b, 737–764. 10.1515/znb-2016-0101. [DOI] [Google Scholar]
- Ramirez D.; Gallagher A.; Baumbach R.; Siegrist T. Synthesis and characterization of the divalent samarium Zintl-phases SmMg2Bi2 and SmMg2Sb2. J. Solid State Chem. 2015, 231, 217–222. 10.1016/j.jssc.2015.08.039. [DOI] [Google Scholar]
- Pöttgen R.; Johrendt D. Equiatomic intermetallic europium compounds: syntheses, crystal chemistry, chemical bonding, and physical properties. Chem. Mater. 2000, 12, 875–897. 10.1021/cm991183v. [DOI] [Google Scholar]
- Ramarao S. D.; Singh A. K.; Subbarao U.; Peter S. C. An overview on the structural diversity of europium based ternary intermetallics. J. Solid State Chem. 2020, 281, 121048. 10.1016/j.jssc.2019.121048. [DOI] [Google Scholar]
- Engel S.; Gießelmann E. C. J.; Pöttgen R.; Janka O. Trivalent europium – a scarce case in intermetallics. Rev. Inorg. Chem. 2023, 43 (4), 571. 10.1515/revic-2023-0003. [DOI] [Google Scholar]
- Klaasse J. C. P.; Mattens W. C. M.; van Ommen A. H.; de Boer F. R.; de Châtel P. F. Valency and magnetic behaviour of ytterbium in intermetallic compounds. AIP Conf. Proc. 1976, 34, 184–186. 10.1063/1.2946060. [DOI] [Google Scholar]
- Klaasse J. C. P.; de Boer F. R.; de Châtel P. F. Systematics in intermetallic compounds containing intermediate-valent ytterbium. Physica B 1981, 106, 178–194. 10.1016/0378-4363(81)90078-4. [DOI] [Google Scholar]
- Pöttgen R.; Johrendt D.; Kußmann D., Structure property relations of ternary equiatomic YbTX intermetallics. In Handbook on the Physics and Chemistry of Rare Earths, Gschneidner K. A. Jr; Bünzli J.-C., Eds.; North-Holland/Elsevier: Amsterdam, 2001; Vol. 32, pp 453–513. [Google Scholar]
- Lai Y.; Chan J. Y.; Baumbach R. E. Electronic landscape of the f-electron intermetallics with the ThCr2Si2 structure. Sci. Adv. 2022, 8, eabp8264 10.1126/sciadv.abp8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniach S. The Kondo lattice and weak antiferromagnetism. Physica B 1977, 91, 231–234. 10.1016/0378-4363(77)90190-5. [DOI] [Google Scholar]
- Stewart G. R. Heavy-fermion systems. Rev. Mod. Phys. 1984, 56, 755–787. 10.1103/RevModPhys.56.755. [DOI] [Google Scholar]
- Boursier R.; Villaume A.; Lapertot G.; Aoki D.; Knebel G.; Flouquet J. Comparison between Ce and Yb heavy fermion compounds: CeRh2Si2 versus YbRh2Si2. Physica B 2008, 403, 726–730. 10.1016/j.physb.2007.10.225. [DOI] [Google Scholar]
- Villars P.; Cenzual K.. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds; ASM International: Materials Park, Ohio, USA, release 2022/2023.
- CAS. SciFindern. https://scifinder-n.cas.org/. Last accessed 6th October 2023.
- Emsley J.The Elements. Clarendon Press, Oxford University Press: Oxford, NY, 1998. [Google Scholar]
- Pöttgen R.; Johrendt D.. Intermetallics. 2nd ed.; De Gruyter: Berlin, 2019. [Google Scholar]
- Rossi D.; Marazza R.; Ferro R. Ternary RMe2X2 alloys of the rare earths with the precious metals and silicon (or germanium). J. Less-Common Met. 1979, 66, P17–P25. 10.1016/0022-5088(79)90233-9. [DOI] [Google Scholar]
- Saensunon B.; Nishimura K.; Voyer C. J.; Ryan D. H.; Hutchison W. D.; Stewart G. A. Magnetic ground state at the ytterbium site in YbNiAl4. J. Appl. Phys. 2009, 105, 07E123. 10.1063/1.3067526. [DOI] [Google Scholar]
- Voßwinkel D.; Niehaus O.; Gerke B.; Benndorf C.; Eckert H.; Pöttgen R. Silicon-tin-ordering in RE3Rh9Si2Sn3 (RE = Y, Sm, Gd – Lu). Z. Anorg. Allg. Chem. 2015, 641, 238–246. 10.1002/zaac.201400458. [DOI] [Google Scholar]
- West A. R.Solid State Chemistry and its Applications. 2nd ed.; John Wiley & Sons Inc.: Hoboken, USA, 2022. [Google Scholar]
- Kuz’ma Y.; Chykhrij S.. Phosphides. In Handbook on the Physics and Chemistry of Rare Earths, Gschneidner K. A. Jr; Eyring L., Eds.; Elsevier Science: Amsterdam, 1996; Vol. 23, pp 285–433. [Google Scholar]
- Pöttgen R.; Gulden T.; Simon A. Miniaturisierte Lichtbogenapperatur für den Laborbedarf. GIT Labor-Fachz. 1999, 43, 133–136. [Google Scholar]
- Kußmann D.; Hoffmann R.-D.; Pöttgen R. Syntheses and crystal structures of CaCuGe, CaAuIn, and CaAuSn – three different superstructures of the KHg2 type. Z. Anorg. Allg. Chem. 1998, 624, 1727–1735. . [DOI] [Google Scholar]
- Pöttgen R.; Lang A.; Hoffmann R.-D.; Künnen B.; Kotzyba G.; Müllmann R.; Mosel B. D.; Rosenhahn C. The stannides YbPtSn and Yb2Pt3Sn5. Z. Kristallogr. 1999, 214, 143–150. 10.1524/zkri.1999.214.3.143. [DOI] [Google Scholar]
- Canfield P. C.; Fisk Z. Growth of single crystals from metallic fluxes. Philos. Mag. 1992, 65, 1117–1123. 10.1080/13642819208215073. [DOI] [Google Scholar]
- Kanatzidis M. G.; Pöttgen R.; Jeitschko W. The metal flux: A preparative tool for the exploration of intermetallic compounds. Angew. Chem., Int. Ed. 2005, 44, 6996–7023. 10.1002/anie.200462170. [DOI] [PubMed] [Google Scholar]
- Canfield P. C. Design, discovery and growth of novel materials. Philos. Mag. 2012, 92, 2398–2400. (and review articles within this special issue) 10.1080/14786435.2012.694675. [DOI] [Google Scholar]
- Gille P.; Grin Y.. Crystal Growth of Intermetallics. De Gruyter: Berlin, Boston, 2019. [Google Scholar]
- Krellner C.; Taube S.; Westerkamp T.; Hossain Z.; Geibel C. Single-crystal growth of YbRh2Si2 and YbIr2Si2. Philos. Mag. 2012, 92, 2508–2523. 10.1080/14786435.2012.669066. [DOI] [Google Scholar]
- Peter S. C.; Subbarao U.; Sarkar S.; Vaitheeswaran G.; Svane A.; Kanatzidis M. G. Crystal structure of Yb2CuGe6 and Yb3Cu4Ge4 and the valency of ytterbium. J. Alloys Compd. 2014, 589, 405–411. 10.1016/j.jallcom.2013.11.224. [DOI] [Google Scholar]
- Sarkar S.; Peter S. C. Single crystal growth of europium and ytterbium based intermetallic compounds using metal flux technique. J. Chem. Sci. 2012, 124, 1385–1390. 10.1007/s12039-012-0335-0. [DOI] [Google Scholar]
- Phelan W. A.; Menard M. C.; Kangas M. J.; McCandless G. T.; Drake B. L.; Chan J. Y. Adventures in crystal growth: synthesis and characterization of single crystals of complex intermetallic compounds. Chem. Mater. 2012, 24, 409–420. 10.1021/cm2019873. [DOI] [Google Scholar]
- Pfannenschmidt U.; Rodewald U. Ch.; Pöttgen R. Bismuth flux crystal growth of RERh6P4 (RE = Sc, Yb, Lu): new phosphides with a superstructure of the LiCo6P4 type. Monatsh. Chem. 2011, 142, 219–224. 10.1007/s00706-011-0450-5. [DOI] [Google Scholar]
- Canfield P. C.; Kong T.; Kaluarachchi U. S.; Jo N. H. Use of frit-disc crucibles for routine and exploratory solution growth of single crystalline samples. Philos. Mag. 2016, 96, 84–92. 10.1080/14786435.2015.1122248. [DOI] [Google Scholar]
- Gottlieb-Schönmeyer S.; Brühne S.; Ritter F.; Assmus W.; Balanetskyy S.; Feuerbacher M.; Weber T.; Steurer W. Crystal growth of copper-rich ytterbium compounds: The predicted giant unit cell structures YbCu4.4 and YbCu4.25. Intermetallics 2009, 17, 6–10. 10.1016/j.intermet.2008.08.009. [DOI] [Google Scholar]
- Schappacher F. M.; Katoh K.; Pöttgen R. Bridgman crystal growth of Yb2Ru3Ge4 – A ternary germanide with a three-dimensional network of condensed distorted RuGe5 and RuGe6 units. J. Solid State Chem. 2007, 180, 186–190. 10.1016/j.jssc.2006.10.008. [DOI] [Google Scholar]
- Katoh K.; Heying B.; Hoffmann R.-D.; Rodewald U. Ch.; Pöttgen R. Bridgman crystal growth and structure refinement of YbPdGe and YbPtGe – Two different superstructures of the KHg2 type. Z. Anorg. Allg. Chem. 2008, 634, 1296–1300. 10.1002/zaac.200800036. [DOI] [Google Scholar]
- Shannon R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Eckerlin P.; Wölfel E. Die Kristallstruktur von Ca2Si und Ca2Ge. Z. Anorg. Allg. Chem. 1955, 280, 321–331. 10.1002/zaac.19552800509. [DOI] [Google Scholar]
- Merlo F.; Palenzona A.; Pani M. Yb2Ge, Eu2Ge and Eu2Si: new PbCl2-type compounds. J. Alloys Compd. 2003, 348, 173–175. 10.1016/S0925-8388(02)00851-4. [DOI] [Google Scholar]
- Ganguli A. K.; Corbett J. D. Structure, bonding, and properties of CaZn1–xCdxSn and CaSn0.5Ge1.5. J. Solid State Chem. 1993, 107, 480–488. 10.1006/jssc.1993.1372. [DOI] [Google Scholar]
- Pöttgen R.; Arpe P. E.; Felser C.; Kußmann D.; Müllmann R.; Mosel B. D.; Künnen B.; Kotzyba G. Structure and properties of YbZnSn, YbAgSn, and Yb2Pt2Pb. J. Solid State Chem. 1999, 145, 668–677. 10.1006/jssc.1998.8280. [DOI] [Google Scholar]
- Wiethölter J.; Koldemir A.; Reimann M. K.; Block T.; Kösters J.; Janka O.; Pöttgen R. Mössbauer-spectroscopic characterization of the stannides Sr2Pd2Sn and Eu2Pd2Sn. Z. Naturforsch. 2023, 78b, 301–306. 10.1515/znb-2023-0015. [DOI] [Google Scholar]
- Stegemann F.; Block T.; Klenner S.; Zhang Y.; Fokwa B. P. T.; Timmer A.; Mönig H.; Doerenkamp C.; Eckert H.; Janka O. From 3D to 2D: structural, spectroscopical and theoretical investigations of the dimensionality reduction in the [PtAl2]δ− polyanions of the isotypic MPtAl2 series (M = Ca–Ba, Eu). Chem.—Eur. J. 2019, 25, 10735–10747. 10.1002/chem.201901867. [DOI] [PubMed] [Google Scholar]
- Koizumi T.; Honda F.; Sato Y. J.; Li D.; Aoki D.; Haga Y.; Gouchi J.; Nagasaki S.; Uwatoko Y.; Kaneko Y.; O̅nuki Y. Abrupt change in electronic states under pressure in new compound EuPt3Al5. J. Phys. Soc. Jpn. 2022, 91, 043704. 10.7566/JPSJ.91.043704. [DOI] [Google Scholar]
- Engel S.; Bönnighausen J.; Stegemann F.; Touzani R. S.; Janka O. SrAl5Pt3 and Sr2Al16Pt9 – two new strontium aluminum platinides. Z. Naturforsch. 2022, 77, 367–379. 10.1515/znb-2022-0012. [DOI] [Google Scholar]
- Gerke B.; Pöttgen R. Alkaline earth-gold-aluminides: synthesis and structure of SrAu3Al2, SrAu2.83Al2.17, BaAu2.89Al2.11 and BaAu7.09Al5.91. Z. Naturforsch. 2015, 70b, 903–909. 10.1515/znb-2015-0119. [DOI] [Google Scholar]
- Schmiegel J.-P.; Block T.; Gerke B.; Fickenscher T.; Touzani R. S.; Fokwa B. P. T.; Janka O. EuAu3Al2: Crystal and electronic structures and spectroscopic, magnetic, and magnetocaloric properties. Inorg. Chem. 2016, 55, 9057–9064. 10.1021/acs.inorgchem.6b01530. [DOI] [PubMed] [Google Scholar]
- Hulliger F.; Nissen H. U.; Wessicken R. On new CaBe2Ge2-type representatives MAu2Al2. J. Alloys Compd. 1994, 206, 263–266. 10.1016/0925-8388(94)90047-7. [DOI] [Google Scholar]
- Stegemann F.; Benndorf C.; Zhang Y.; Bartsch M.; Zacharias H.; Fokwa B. P. T.; Eckert H.; Janka O. On ternary intermetallic aurides: CaAu2Al2, SrAu2–xAl2+x and Ba3Au5+xAl6–x. Z. Anorg. Allg. Chem. 2017, 643, 1379–1390. 10.1002/zaac.201700103. [DOI] [Google Scholar]
- Muts I.; Zaremba V. I.; Pöttgen R. Ternary indides Eu2Pd2In and Eu2Pt2In. Z. Anorg. Allg. Chem. 2012, 638, 64–67. 10.1002/zaac.201100417. [DOI] [Google Scholar]
- Muts I.; Nilges T.; Rodewald U. C.; Zaremba V.’ I.; Pöttgen R. Synthesis and structure of Sr2Pd2In and Sr2Pt2In. Z. Naturforsch. 2007, 62b, 1563–1566. 10.1515/znb-2007-1214. [DOI] [Google Scholar]
- Pöttgen R.; Kußmann D. New indium-rich compounds EuRhIn2 and EuRh2In8. Z. Anorg. Allg. Chem. 2001, 627, 55–60. . [DOI] [Google Scholar]
- Muts I. R.; Zaremba V. I.; Pöttgen R. Centered indium cubes as structural motifs in SrRh2In8 and SrIrIn4. Z. Anorg. Allg. Chem. 2007, 633, 2234–2237. 10.1002/zaac.200700116. [DOI] [Google Scholar]
- Evers J.; Oehlinger G. Ternary phases MPtSi (M = Ca, Eu, Sr, Ba) with the LalrSi-type structure. J. Solid State Chem. 1986, 62, 133–137. 10.1016/0022-4596(86)90223-9. [DOI] [Google Scholar]
- Mayer I.; Yetor P. D. MPt2Si2 compounds of the ThCr2Si2 type. J. Less-Common Met. 1977, 55, 171–176. 10.1016/0022-5088(77)90189-8. [DOI] [Google Scholar]
- Iandelli A. Equiatomic ternary compounds of rare earths with the Fe2P or ZrNiAl structure type. J. Alloys Compd. 1992, 182, 87–90. 10.1016/0925-8388(92)90577-V. [DOI] [Google Scholar]
- Dhar S. K.; Kulkarni R.; Manfrinetti P.; Pani M.; Yonezawa Y.; Aoki Y. Synthesis, crystal structure, and physical properties of YbTZn (T = Pd, Pt, and Au) and LuPtZn. Phys. Rev. B 2007, 76, 054411. 10.1103/PhysRevB.76.054411. [DOI] [Google Scholar]
- Mishra T.; Heymann G.; Huppertz H.; Pöttgen R. Dimorphism in the REPdZn series. Intermetallics 2012, 20, 110–114. 10.1016/j.intermet.2011.08.012. [DOI] [Google Scholar]
- Melnyk G.; Gulay L. D.; Tremel W. Crystal structures of new ternary compounds in RE–Pt–Pb and RE–Au–Pb systems (RE = rare earth metal). J. Alloys Compd. 2012, 528, 70–73. 10.1016/j.jallcom.2012.01.063. [DOI] [Google Scholar]
- Gesing T.-M.; Pöttgen R.; Jeitschko W.; Wortmann U. Crystal structure and physical properties of the carbides UAl3C3 and YbAl3C3. J. Alloys Compd. 1992, 186, 321–331. 10.1016/0925-8388(92)90019-6. [DOI] [Google Scholar]
- Stegemann F.; Stahl J.; Bartsch M.; Zacharias H.; Johrendt D.; Janka O. Temperature induced valence phase transition in intermediate-valent YbPd2Al3. Chem. Sci. 2019, 10, 11086–11094. 10.1039/C9SC04437J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kösters J.; Pöttgen R.. Ytterbium valence ordering in the low-temperature superstructure of Yb2Pd2Cd. Z. Kristallogr. 2024, 239, 10.1515/ZKRI-2023-0043. In Press. [DOI] [Google Scholar]
- Parthé E.; Gelato L. M.; Chabot B.; Penzo M.; Cenzual K.; Gladyshevskii E. I.. TYPIX–Standardized data and crystal chemical characterization of inorganic structure types. In Gmelin Handbook of Inorganic and Organometallic Chemistry; Springer, Germany: Berlin, 1993. [Google Scholar]
- Zumdick M. F.; Pöttgen R. Determination of the superstructures for the stannides ZrIrSn, HfCoSn, and HfRhSn. Z. Kristallogr. 1999, 214, 90–97. 10.1524/zkri.1999.214.2.90. [DOI] [Google Scholar]
- Bojin M. D.; Hoffmann R. The REME Phases I. An overview of their structural variety. Helv. Chim. Acta 2003, 86, 1653–1682. 10.1002/hlca.200390140. [DOI] [Google Scholar]
- Bojin M. D.; Hoffmann R. The REME phases II. What’s possible?. Helv. Chim. Acta 2003, 86, 1683–1708. 10.1002/hlca.200390141. [DOI] [Google Scholar]
- Haszko S. E. Intermediate phases with the MgCu2 structure. Trans. Metall. Soc. AIME 1960, 218, 958. [Google Scholar]
- Van Vucht J. H. N.; Buschow K. H. J. On the binary aluminium-rich compounds of the rare-earth elements. Philips Res. Rep. 1964, 19, 319–322. [Google Scholar]
- Moriarty J. L. Jr.; Humphreys J. E.; Gordon R. O.; Baenziger N. C. X-ray examination of some rare-earth-containing binary alloy systems. Acta Crystallogr. 1966, 21, 840–841. 10.1107/S0365110X6600402X. [DOI] [Google Scholar]
- Sysa L. V.; Zaremba V. I.; Kalychak Y. M.; Baranyak V. M. New ternary compounds with indium, rare-earth and 3d metals with MgCu4Sn and ZrNiAl type structure. Visn. Lviv. Derzh. Univ., Ser. Khim. 1988, 29, 32–34. [Google Scholar]
- Gießelmann E. C. J.; Pöttgen R.; Janka O. Laves phases: superstructures induced by coloring and distortions. Z. Anorg. Allg. Chem. 2023, 649, e202300109 10.1002/zaac.202300109. [DOI] [Google Scholar]
- Seidel S.; Pöttgen R. Yb6Ir5Ga7 – A MgZn2 superstructure. Z. Anorg. Allg. Chem. 2017, 643, 261–265. 10.1002/zaac.201600422. [DOI] [Google Scholar]
- Eustermann F.; Pominov A.; Pöttgen R. Rare earth (RE) gallides with closely related compositions: REIrGa and RE6Ir5Ga7. Z. Anorg. Allg. Chem. 2018, 644, 1297–1303. 10.1002/zaac.201800287. [DOI] [Google Scholar]
- Eustermann F.; Stegemann F.; Radzieowski M.; Janka O. Intermetallic RE6T5Al7 phases (RE = Sc, Y, Ce–Nd, Sm, Gd–Lu, T = Ru, Ir): Diversity in their magnetic, magnetocaloric and critical properties. Inorg. Chem. 2019, 58, 16211–16226. 10.1021/acs.inorgchem.9b02732. [DOI] [PubMed] [Google Scholar]
- Just G.; Paufler P. On the coordination of ThCr2Si2 (BaAl4-type compounds within the field of free parameters. J. Alloys Compd. 1996, 232, 1–25. 10.1016/0925-8388(95)01939-1. [DOI] [Google Scholar]
- Shatruk M. ThCr2Si2 structure type: The “perovskite” of intermetallics. J. Solid State Chem. 2019, 272, 198–209. 10.1016/j.jssc.2019.02.012. [DOI] [Google Scholar]
- Zaremba V. I.; Rodewald U. Ch.; Hoffmann R.-D.; Kalychak Y. M.; Pöttgen R. The indium-rich intermetallics YbCoIn5, YbRhIn5, and YbPtIn4. Z. Anorg. Allg. Chem. 2003, 629, 1157–1161. 10.1002/zaac.200300024. [DOI] [Google Scholar]
- Gladyshevskii E. I.; Yarmolyuk Y. P.; Grin Y. Structures of ternary gallides of rare-earth metals-members of the homologous series RmR’3m+2nXn and Rm+nR’nXm+n. Acta Crystallogr. 1978, A34, S148b. [Google Scholar]
- Zaremba V. I.; Rodewald U. Ch.; Pöttgen R. Synthesis and structure of YbIrIn5. Z. Naturforsch. 2003, 58b, 805–808. 10.1515/znb-2003-0814. [DOI] [Google Scholar]
- Lukachuk M.; Pöttgen R. Intermetallic compounds with ordered U3Si2 or Zr3Al2 type structure – crystal chemistry, chemical bonding and physical properties. Z. Kristallogr. 2003, 218, 767–787. 10.1524/zkri.218.12.767.20545. [DOI] [Google Scholar]
- Klenner S.; Pöttgen R., Rare earth transition metal plumbides – An update. In Handbook on the Physics and Chemistry of Rare Earths, Bünzli J.-C. G.; Pecharsky V. K., Eds.; Elsevier: 2020; Vol. 57, pp 1–44. [Google Scholar]
- Pöttgen R.; Janka O. CeCr2Al20-type intermetallics – structure-property relationships. Rev. Inorg. Chem. 2023, 43, 357–383. 10.1515/revic-2023-0012. [DOI] [Google Scholar]
- Jia S.; Ni N.; Bud’ko S. L.; Canfield P. C. Magnetic properties of RFe2Zn20 and RCo2Zn20 (R = Y, Nd, Sm, Gd-Lu). Phys. Rev. B 2009, 80, 104403. 10.1103/PhysRevB.80.104403. [DOI] [Google Scholar]
- Niepmann D.; Prots Y. M.; Pöttgen R.; Jeitschko W. The order of the palladium and germanium atoms in the germanides LnPdGe (Ln = La–Nd, Sm, Gd, Tb) and the new compound Yb3Pd4Ge4. J. Solid State Chem. 2000, 154, 329–337. 10.1006/jssc.2000.8789. [DOI] [Google Scholar]
- Sebastian C. P.; Salvador J.; Martin J. B.; Kanatzidis M. G. New intermetallics YbAu2In4 and Yb2Au3In5. Inorg. Chem. 2010, 49, 10468–10474. 10.1021/ic101502e. [DOI] [PubMed] [Google Scholar]
- Kußmann D.; Pöttgen R.; Kotzyba G. New Yb2Pt3Sn5 type stannides. J. Solid State Chem. 2000, 150, 112–120. 10.1006/jssc.1999.8555. [DOI] [Google Scholar]
- Macaluso R. T.; Nakatsuji S.; Kuga K.; Thomas E. L.; Machida Y.; Maeno Y.; Fisk Z.; Chan J. Y. Crystal structure and physical properties of polymorphs of LnAlB4 (Ln = Yb, Lu). Chem. Mater. 2007, 19, 1918–1922. 10.1021/cm062244+. [DOI] [Google Scholar]
- Mikhalenko S. I.; Kuz’ma Y. B.; Korsukova M. M.; Gurin V. N. Ternary borides containing a rare earth and aluminum, with YCrB4 and Y2ReB6 structures. Inorg. Mater. 1980, 16, 1325–1328. [Google Scholar]
- Bonrath H.; Hellwege K. H.; Nicolay K.; Weber G. Antiferromagnetische Umwandlung von Dy2O3, Er2O3 und Yb2O3 im Temperaturbereich von 1,1 bis 4,2 K. Phys. Konden. Mater. 1966, 4, 382–390. 10.1007/BF02422756. [DOI] [Google Scholar]
- Zell W.; Pott R.; Roden B.; Wohlleben D. Pressure and temperature dependence of the magnetic susceptibility of some ytterbium compounds with intermediate valence. Solid State Commun. 1981, 40, 751–754. 10.1016/0038-1098(81)90822-X. [DOI] [Google Scholar]
- Klaasse J. C. P.; Sterkenburg J. W. E.; Bleyendaal A. H. M.; de Boer F. R. Ambiguous magnetic behaviour of some Yb-compounds. Solid State Commun. 1973, 12, 561–564. 10.1016/0038-1098(73)90658-3. [DOI] [Google Scholar]
- Tappe F.; Schwickert C.; Eul M.; Pöttgen R. Intermetallic cadmium compounds M5T2Cd (M = Ca, Yb, Eu; T = Cu, Ag, Au) with Mo5B2Si-type structure. Z. Naturforsch. 2011, 66b, 1219–1224. 10.1515/znb-2011-1204. [DOI] [Google Scholar]
- Reimann M. K.; Klenner S.; Gerdes J. M.; Hansen M. R.; Pöttgen R. Magnesium-rich intermetallic compounds RE3Ag4Mg12 (RE = Y, La–Nd, Sm–Dy, Yb) and AE3Ag4Mg12 (AE = Ca, Sr). Z. Kristallogr. 2022, 237, 417–427. 10.1515/zkri-2022-0048. [DOI] [Google Scholar]
- Johrendt D.; Kotzyba G.; Trill H.; Mosel B. D.; Eckert H.; Fickenscher T.; Pöttgen R. Magnetic and electrical properties, 151Eu Mössbauer spectroscopy, and chemical bonding of REAgMg (RE = La, Ce, Eu, Yb) and EuAuMg. J. Solid State Chem. 2002, 164, 201–209. 10.1006/jssc.2001.9460. [DOI] [Google Scholar]
- Mishra R.; Pöttgen R.; Hoffmann R.-D.; Kaczorowski D.; Piotrowski H.; Mayer P.; Rosenhahn C.; Mosel B. D. Ternary rare earth (RE) gold compounds REAuCd and RE2Au2Cd. Z. Anorg. Allg. Chem. 2001, 627, 1283–1291. . [DOI] [Google Scholar]
- Fickenscher; Kotzyba G.; Hoffmann R.-D.; Pöttgen R. Synthesis, structure, and magnetic properties of EuAgCd and YbAgCd. Z. Naturforsch. 2001, 56b, 598–603. 10.1515/znb-2001-0706. [DOI] [Google Scholar]
- Galadzhun Y. V.; Hoffmann R.-D.; Kotzyba G.; Künnen B.; Pöttgen R. EuPdIn2, YbPdIn2, and YbAuIn2: Syntheses, structures, and properties of new intermetallic compounds with ordered Re3B-type structure. Eur. J. Inorg. Chem. 1999, 1999, 975–979. . [DOI] [Google Scholar]
- Katoh K.; Takabatake T.; Minami A.; Oguro I.; Sawa H. Crystal growth of YbTX (T = Cu, Ag, Pt, Au; X = Sn, Sb) and the magnetic and transport properties. J. Alloys Compd. 1997, 261, 32–36. 10.1016/S0925-8388(97)00194-1. [DOI] [Google Scholar]
- Lueken H.Magnetochemie. B. G. Teubner: Stuttgart, Leipzig, 1999. [Google Scholar]
- Szytuła A.; Jezierski A.; Penc B.; Winiarski A.; Leithe-Jasper A.; Kaczorowski D. Electronic structure of YbTX compounds. J. Alloys Compd. 2003, 360, 41–46. 10.1016/S0925-8388(03)00344-X. [DOI] [Google Scholar]
- Fritsch G. ″Heavy Fermionen″-Systeme. Phys. Unserer Zeit 1987, 18, 17–20. 10.1002/piuz.19870180103. [DOI] [Google Scholar]
- Fisk Z.; Maple M. B. On the existence of heavy fermion ytterbium compounds. J. Alloys Compd. 1992, 183, 303–311. 10.1016/0925-8388(92)90754-W. [DOI] [Google Scholar]
- Jeong T. Electronic structure and magnetism of YbRhSn. Eur. Phys. J. B 2006, 53, 213–217. 10.1140/epjb/e2006-00371-x. [DOI] [Google Scholar]
- Pietri R.; Andraka B.; Kaczorowski D.; Leithe-Jasper A.; Rogl P. Magnetoresistance and low-temperature specific heat of the Yb compounds YbRhSn, YbPdBi, and YbPtSn. Phys. Rev. B 2000, 61, 12169–12173. 10.1103/PhysRevB.61.12169. [DOI] [Google Scholar]
- Sales B. C.; Wohlleben D. K. Susceptibility of interconfiguration-fluctuation compounds. Phys. Rev. Lett. 1975, 35, 1240–1244. 10.1103/PhysRevLett.35.1240. [DOI] [Google Scholar]
- Grytsiv A.; Kaczorowski D.; Leithe-Jasper A.; Rogl P.; Potel M.; Noël H.; Pikul A. P.; Velikanova T. Structure and properties of Yb3Ge5. J. Solid State Chem. 2002, 165, 178–181. 10.1006/jssc.2002.9531. [DOI] [Google Scholar]
- Havinga E. E.; Buschow K. H. J.; van Daal H. J. The ambivalence of Yb in YbAl2 and YbAl3. Solid State Commun. 1973, 13, 621–627. 10.1016/S0038-1098(73)80026-2. [DOI] [Google Scholar]
- Klaasse J. C. P.; Mattens W. C. M.; de Boer F. R.; de Châtel P. F. Magnetic properties of ytterbium intermetallic compounds with intermediate valency. Physica B 1977, 86–88, 234–236. 10.1016/0378-4363(77)90298-4. [DOI] [Google Scholar]
- Kaindl G.; Reihl B.; Eastman D. E.; Pollak R. A.; Mårtensson N.; Barbara B.; Penney T.; Plaskett T. S. Surface core-level shifts and surface valence change in mixed-valent YbAl2. Solid State Commun. 1982, 41, 157–160. 10.1016/0038-1098(82)91057-2. [DOI] [Google Scholar]
- Matsunami M.; Chainani A.; Taguchi M.; Eguchi R.; Takata Y.; Oura M.; Yabashi M.; Tamasaku K.; Nishino Y.; Ishikawa T.; Kosaka M.; Shin S. Photoemission evidence for valence fluctuations and Kondo resonance in YbAl2. J. Phys. Soc. Jpn. 2012, 81, 073702. 10.1143/JPSJ.81.073702. [DOI] [Google Scholar]
- Matsunami M.; Hajiri T.; Miyazaki H.; Kosaka M.; Kimura S. Strongly hybridized electronic structure of YbAl2: An angle-resolved photoemission study. Phys. Rev. B 2013, 87, 165141. 10.1103/PhysRevB.87.165141. [DOI] [Google Scholar]
- Cornelius A. L.; Lawrence J. M.; Ebihara T.; Riseborough P. S.; Booth C. H.; Hundley M. F.; Pagliuso P. G.; Sarrao J. L.; Thompson J. D.; Jung M. H.; Lacerda A. H.; Kwei G. H. Two energy scales and slow crossover in YbAl3. Phys. Rev. Lett. 2002, 88, 117201. 10.1103/PhysRevLett.88.117201. [DOI] [PubMed] [Google Scholar]
- Okamura H.; Michizawa T.; Nanba T.; Ebihara T. Pseudogap formation and heavy-carrier dynamics in intermediate-valence YbAl3. J. Phys. Soc. Jpn. 2004, 73, 2045–2048. 10.1143/JPSJ.73.2045. [DOI] [Google Scholar]
- Christianson A. D.; Fanelli V. R.; Lindsay L.; Mu S.; Rahn M. C.; Mazzone D. G.; Said A. H.; Ronning F.; Bauer E. D.; Lawrence J. M. Phonons, Q-dependent Kondo spin fluctuations, and 4f phonon resonance in YbAl3. Phys. Rev. B 2020, 102, 205135. 10.1103/PhysRevB.102.205135. [DOI] [Google Scholar]
- Kasaya M.; Tani T.; Iga F.; Kasuya T. Electrical resistivity, hall constant and magnetic susceptibility in the Kondo states Ce(Ni1–xPdx)Sn and YbNiSn. J. Magn. Magn. Mater. 1988, 76–77, 278–280. 10.1016/0304-8853(88)90395-2. [DOI] [Google Scholar]
- Kasaya M.; Tani T.; Kawate K.; Mizushima T.; Isikawa Y.; Sato K. Magnetic properties of the dense Kondo compound YbNiSn. J. Phys. Soc. Jpn. 1991, 60, 3145–3149. 10.1143/JPSJ.60.3145. [DOI] [Google Scholar]
- Kasaya M.; Tani T.; Ohoyama K.; Kohgi M.; Isikawa Y. Magnetic properties of the dense Kondo compounds CePdSn and YbNiSn. J. Magn. Magn. Mater. 1992, 104–107, 665–666. 10.1016/0304-8853(92)90974-S. [DOI] [Google Scholar]
- Bellot P.; Bonville P.; Hammann J.; Hodges J. A.; Imbert P.; Jéhanno G.; Leylekian L.; D’Onofrio L. Ferromagnetic ordering in the Kondo lattice YbNiSn. J. Magn. Magn. Mater. 1992, 108, 141–142. 10.1016/0304-8853(92)91385-7. [DOI] [Google Scholar]
- Bonville P.; Bellot P.; Hodges J. A.; Imbert P.; Jéhanno G.; Le Bras G.; Hammann J.; Leylekian L.; Chevrier G.; Thuéry P.; D’Onofrio L.; Hamzic A.; Barthélémy A. YbNiSn, a ferromagnetic Kondo lattice. Physica B 1992, 182, 105–117. 10.1016/0921-4526(92)90555-7. [DOI] [Google Scholar]
- Trovarelli O.; Geibel C.; Cardoso R.; Mederle S.; Borth R.; Buschinger B.; Grosche F. M.; Grin Y.; Sparn G.; Steglich F. Low-temperature properties of the Yb-based heavy-fermion antiferromagnets YbPtIn, YbRhSn, and YbNiGa. Phys. Rev. B 2000, 61, 9467–9474. 10.1103/PhysRevB.61.9467. [DOI] [Google Scholar]
- Kaczorowski D.; Leithe-Jasper A.; Rogl P.; Flandorfer H.; Cichorek T.; Pietri R.; Andraka B. Magnetic, thermodynamic, and electrical transport properties of ternary equiatomic ytterbium compounds YbTM (T = transition metal, M = Sn and Bi). Phys. Rev. B 1999, 60, 422–433. 10.1103/PhysRevB.60.422. [DOI] [Google Scholar]
- Katoh K.; Terui G.; Niide Y.; Aoki H.; Ochiai A. Magnetic properties and electrical resistivity of YbRhSn and YbIrSn. Physica B 1999, 259–261, 161–162. 10.1016/S0921-4526(98)00829-1. [DOI] [Google Scholar]
- Muro Y.; Haizaki Y.; Kim M. S.; Umeo K.; Tou H.; Sera M.; Takabatake T. Heavy-fermion weak-ferromagnet YbRhSb. Phys. Rev. B 2004, 69, 020401. 10.1103/PhysRevB.69.020401. [DOI] [Google Scholar]
- Muro Y.; Haizaki Y.; Umeo K.; Tou H.; Sera M.; Sakakibara T.; Takabatake T. Effects of magnetic field and pressure on the weak ferromagnetism of YbRhSb. Physica B 2005, 359–361, 124–126. 10.1016/j.physb.2005.01.010. [DOI] [Google Scholar]
- Umeo K.; Kubo H.; Muro Y.; Nakamura F.; Suzuki T.; Takabatake T. Pressure-induced ferromagnetic order in the weak ferromagnet YbRhSb. J. Phys.: Conf. Series 2009, 150, 042223. 10.1088/1742-6596/150/4/042223. [DOI] [Google Scholar]
- Umeo K.; Yamane H.; Kubo H.; Muro Y.; Takabatake T. Pressure-induced transition from a canted antiferromagnetic state to a ferromagnetic state in YbRhSb. J. Phys.: Conf. Series 2010, 200, 012215. 10.1088/1742-6596/200/1/012215. [DOI] [Google Scholar]
- Umeo K.; Yamane H.; Kubo H.; Muro Y.; Nakamura F.; Suzuki T.; Takabatake T.; Sengupta K.; Forthaus M. K.; Abd-Elmeguid M. M. Interplay between crystal electric field and magnetic exchange anisotropies in the heavy-fermion antiferromagnet YbRhSb under pressure. Phys. Rev. B 2012, 85, 024412. 10.1103/PhysRevB.85.024412. [DOI] [Google Scholar]
- Marazza R.; Rossi D.; Ferro R. Magnesium silver arsenide-type phases in the ternary systems of rare earths with palladium and bismuth. Gazz. Chim. Ital. 1980, 11 (44), 357–359. 10.1002/chin.198044016. [DOI] [Google Scholar]
- Canfield P. C.; Thompson J. D.; Beyermann W. P.; Lacerda A.; Hundley M. F.; Peterson E.; Fisk Z.; Ott H. R. Magnetism and heavy fermion-like behavior in the RBiPt series. J. Appl. Phys. 1991, 70, 5800–5802. 10.1063/1.350141. [DOI] [Google Scholar]
- Fisk Z.; Canfield P. C.; Beyermann W. P.; Thompson J. D.; Hundley M. F.; Ott H. R.; Felder E.; Maple M. B.; Lopez de la Torre M. A.; Visani P.; Seaman C. L. Massive electron state in YbBiPt. Phys. Rev. Lett. 1991, 67, 3310–3313. 10.1103/PhysRevLett.67.3310. [DOI] [PubMed] [Google Scholar]
- Mun E.; Bud’ko S. L.; Lee Y.; Martin C.; Tanatar M. A.; Prozorov R.; Canfield P. C. Quantum oscillations in the heavy-fermion compound YbPtBi. Phys. Rev. B 2015, 92, 085135. 10.1103/PhysRevB.92.085135. [DOI] [Google Scholar]
- Movshovich R.; Lacerda A.; Canfield P. C.; Thompson J. D.; Fisk Z. Fermi surface instability and symmetry breaking in heavy-fermion compound YbBiPt. Phys. Rev. Lett. 1994, 73, 492–495. 10.1103/PhysRevLett.73.492. [DOI] [PubMed] [Google Scholar]
- Lacerda A.; Movshovich R.; Hundley M. F.; Canfield P. C.; Arms D.; Sparn G.; Thompson J. D.; Fisk Z.; Fisher R. A.; Phillips N. E.; Ott H. R. Doping and pressure studies on YbBiPt. J. Appl. Phys. 1993, 73, 5415–5417. 10.1063/1.353700. [DOI] [Google Scholar]
- Movshovich R.; Lacerda A.; Canfield P. C.; Thompson J. D.; Fisk Z. Low-temperature phase diagram of YbBiPt. J. Appl. Phys. 1994, 76, 6121–6123. 10.1063/1.358326. [DOI] [Google Scholar]
- Ueland B. G.; Kreyssig A.; Prokeš K.; Lynn J. W.; Harriger L. W.; Pratt D. K.; Singh D. K.; Heitmann T. W.; Sauerbrei S.; Saunders S. M.; Mun E. D.; Bud’ko S. L.; McQueeney R. J.; Canfield P. C.; Goldman A. I. Fragile antiferromagnetism in the heavy-fermion compound YbBiPt. Phys. Rev. B 2014, 89, 180403. 10.1103/PhysRevB.89.180403. [DOI] [Google Scholar]
- Ueland B. G.; Kreyssig A.; Mun E. D.; Lynn J. W.; Harriger L. W.; Pratt D. K.; Prokeš K.; Hüsges Z.; Toft-Petersen R.; Sauerbrei S.; Saunders S. M.; Furukawa Y.; Bud’ko S. L.; McQueeney R. J.; Canfield P. C.; Goldman A. I. Magnetic-field effects on the fragile antiferromagnetism in YbBiPt. Phys. Rev. B 2019, 99, 184431. 10.1103/PhysRevB.99.184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krypyakevych P. I.; Markiv V. Y.; Mel’nyk E. V. Crystal structures of the compounds ZrNiAl, ZrCuGa and their analogues. Dopov. Akad. Nauk Ukr. RSR 1967, Ser. A, 750–753. [Google Scholar]
- Dwight A. E.; Mueller M. H.; Conner R. A. Jr.; Downey J. W.; Knott H. W. Ternary compounds with the Fe2P-type structure. Trans. Metall. Soc. AIME 1968, 242, 2075–2080. [Google Scholar]
- Zumdick M. F.; Hoffmann R.-D.; Pöttgen R. The intermetallic zirconium compounds ZrNiAl, ZrRhSn, and ZrPtGa - structural distortions and metal-metal bonding in Fe2P related compounds. Z. Naturforsch. 1999, 54b, 45–53. 10.1515/znb-1999-0111. [DOI] [Google Scholar]
- Mattens W. C. M.; Elenbaas R. A.; de Boer F. R. Mixed-valence behavior in the intermetallic compound ytterbium-copper-aluminum (YbCuAl). Commun. Phys. 1977, 2, 147–150. [Google Scholar]
- Mattens W. C. M.; de Châtel P. F.; Moleman A. C.; de Boer F. R. Intermediate-valence state of Yb in Y- and Gd-substituted YbCuAl. Physica B 1979, 96, 138–143. 10.1016/0378-4363(79)90110-4. [DOI] [Google Scholar]
- Pott R.; Schefzyk R.; Wohlleben D.; Junod A. Thermal expansion and specific heat of intermediate valent YbCuAl. Z. Phys. B: Condens. Matter 1981, 44, 17–24. 10.1007/BF01292647. [DOI] [Google Scholar]
- Bonville P. Kondo effect and heavy fermions in Yb compounds. Hyperfine Interact. 1988, 40, 15–29. 10.1007/BF02049075. [DOI] [Google Scholar]
- Friedemann S.; Wirth S.; Oeschler N.; Krellner C.; Geibel C.; Steglich F.; MaQuilon S.; Fisk Z.; Paschen S.; Zwicknagl G. Hall effect measurements and electronic structure calculations on YbRh2Si2 and its reference compounds LuRh2Si2 and YbIr2Si2. Phys. Rev. B 2010, 82, 035103. 10.1103/PhysRevB.82.035103. [DOI] [Google Scholar]
- Kummer K.; Patil S.; Chikina A.; Güttler M.; Höppner M.; Generalov A.; Danzenbächer S.; Seiro S.; Hannaske A.; Krellner C.; Kucherenko Y.; Shi M.; Radovic M.; Rienks E.; Zwicknagl G.; Matho K.; Allen J. W.; Laubschat C.; Geibel C.; Vyalikh D. V. Temperature-independent Fermi surface in the Kondo lattice YbRh2Si2. Phys. Rev. X 2015, 5, 011028. 10.1103/PhysRevX.5.011028. [DOI] [Google Scholar]
- Zwicknagl G. The utility of band theory in strongly correlated electron systems. Rep. Prog. Phys. 2016, 79, 124501. 10.1088/0034-4885/79/12/124501. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wang Q.; Xu Y.; Xie W.; Yang Y.-F. Nearly degenerate px + ipy and dx2–y2 pairing symmetry in the heavy fermion superconductor YbRh2Si2. Phys. Rev. B 2019, 100, 085132. 10.1103/PhysRevB.100.085132. [DOI] [Google Scholar]
- Güttler M.; Kummer K.; Kliemt K.; Krellner C.; Seiro S.; Geibel C.; Laubschat C.; Kubo Y.; Sakurai Y.; Vyalikh D. V.; Koizumi A. Visualizing the Kondo lattice crossover in YbRh2Si2 with Compton scattering. Phys. Rev. B 2021, 103, 115126. 10.1103/PhysRevB.103.115126. [DOI] [Google Scholar]
- Stock C.; Broholm C.; Demmel F.; Van Duijn J.; Taylor J. W.; Kang H. J.; Hu R.; Petrovic C. From incommensurate correlations to mesoscopic spin resonance in YbRh2Si2. Phys. Rev. Lett. 2012, 109, 127201. 10.1103/PhysRevLett.109.127201. [DOI] [PubMed] [Google Scholar]
- Gegenwart P.; Custers J.; Tokiwa Y.; Geibel C.; Steglich F. Ferromagnetic quantum critical fluctuations in YbRh2(Si0.95Ge0.05)2. Phys. Rev. Lett. 2005, 94, 076402. 10.1103/PhysRevLett.94.076402. [DOI] [PubMed] [Google Scholar]
- Ishida K.; Okamoto K.; Kawasaki Y.; Kitaoka Y.; Trovarelli O.; Geibel C.; Steglich F. YbRh2Si2: Spin fluctuations in the vicinity of a quantum critical point at low magnetic field. Phys. Rev. Lett. 2002, 89, 107202. 10.1103/PhysRevLett.89.107202. [DOI] [PubMed] [Google Scholar]
- Sichelschmidt J.; Ivanshin V. A.; Ferstl J.; Geibel C.; Steglich F. Low temperature electron spin resonance of the Kondo ion in a heavy fermion metal: YbRh2Si2. Phys. Rev. Lett. 2003, 91, 156401. 10.1103/PhysRevLett.91.156401. [DOI] [PubMed] [Google Scholar]
- Abrahams E.; Wölfle P. Electron spin resonance in Kondo systems. Phys. Rev. B 2008, 78, 104423. 10.1103/PhysRevB.78.104423. [DOI] [Google Scholar]
- Wölfle P.; Abrahams E. Phenomenology of ESR in heavy-fermion systems: Fermi-liquid and non-Fermi-liquid regimes. Phys. Rev. B 2009, 80, 235112. 10.1103/PhysRevB.80.235112. [DOI] [Google Scholar]
- Ishida K.; MacLaughlin D. E.; Young B.-L.; Okamoto K.; Kawasaki Y.; Kitaoka Y.; Nieuwenhuys G. J.; Heffner R. H.; Bernal O. O.; Higemoto W.; Koda A.; Kadono R.; Trovarelli O.; Geibel C.; Steglich F. Low-temperature magnetic order and spin dynamics in YbRh2Si2. Phys. Rev. B 2003, 68, 184401. 10.1103/PhysRevB.68.184401. [DOI] [Google Scholar]
- Gegenwart P.; Custers J.; Geibel C.; Neumaier K.; Tayama T.; Tenya K.; Trovarelli O.; Steglich F. Magnetic-field induced quantum critical point in YbRh2Si2. Phys. Rev. Lett. 2002, 89, 056402. 10.1103/PhysRevLett.89.056402. [DOI] [PubMed] [Google Scholar]
- Custers J.; Gegenwart P.; Wilhelm H.; Neumaier K.; Tokiwa Y.; Trovarelli O.; Geibel C.; Steglich F.; Pépin C.; Coleman P. The break-up of heavy electrons at a quantum critical point. Nature 2003, 424, 524–527. 10.1038/nature01774. [DOI] [PubMed] [Google Scholar]
- Hamann S.; Zhang J.; Jang D.; Hannaske A.; Steinke L.; Lausberg S.; Pedrero L.; Klingner C.; Baenitz M.; Steglich F.; Krellner C.; Geibel C.; Brando M. Evolution from ferromagnetism to antiferromagnetism in Yb(Rh1–xCox)2Si2. Phys. Rev. Lett. 2019, 122, 077202. 10.1103/PhysRevLett.122.077202. [DOI] [PubMed] [Google Scholar]
- Lausberg S.; Hannaske A.; Steppke A.; Steinke L.; Gruner T.; Pedrero L.; Krellner C.; Klingner C.; Brando M.; Geibel C.; Steglich F. Doped YbRh2Si2: Not only ferromagnetic correlations but ferromagnetic order. Phys. Rev. Lett. 2013, 110, 256402. 10.1103/PhysRevLett.110.256402. [DOI] [PubMed] [Google Scholar]
- Schuberth E.; Tippmann M.; Steinke L.; Lausberg S.; Steppke A.; Brando M.; Krellner C.; Geibel C.; Yu R.; Si Q.; Steglich F. Emergence of superconductivity in the canonical heavy-electron metal YbRh2Si2. Science 2016, 351, 485–488. 10.1126/science.aaa9733. [DOI] [PubMed] [Google Scholar]
- Nguyen D. H.; Sidorenko A.; Taupin M.; Knebel G.; Lapertot G.; Schuberth E.; Paschen S. Superconductivity in an extreme strange metal. Nature Commun. 2021, 12, 4341. 10.1038/s41467-021-24670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J.; Levitin L. V.; Nyéki J.; Ho A. F.; Cowan B.; Saunders J.; Brando M.; Geibel C.; Kliemt K.; Krellner C. Electronuclear transition into a spatially modulated magnetic state in YbRh2Si2. Phys. Rev. Lett. 2023, 130, 126802. 10.1103/PhysRevLett.130.126802. [DOI] [PubMed] [Google Scholar]
- Trovarelli O.; Geibel C.; Steglich F. Low-temperature properties of YbRh2Si2. Physica B 2000, 284–288, 1507–1508. 10.1016/S0921-4526(99)02716-7. [DOI] [Google Scholar]
- Sriram Shastry B.; Sutherland B. Exact ground state of a quantum mechanical antiferromagnet. Physica B+C 1981, 108, 1069–1070. 10.1016/0378-4363(81)90838-X. [DOI] [Google Scholar]
- Miyahara S.; Ueda K. Exact dimer ground state of the two dimensional Heisenberg spin system SrCu2(BO3)2. Phys. Rev. Lett. 1999, 82, 3701–3704. 10.1103/PhysRevLett.82.3701. [DOI] [Google Scholar]
- Lee J. Y.; You Y.-Z.; Sachdev S.; Vishwanath A. Signatures of a deconfined phase transition on the Shastry-Sutherland lattice: Applications to quantum critical SrCu2(BO3)2. Phys. Rev. X 2019, 9, 041037. 10.1103/PhysRevX.9.041037. [DOI] [Google Scholar]
- Kim M. S.; Bennett M. C.; Aronson M. C. Yb2Pt2Pb: Magnetic frustration in the Shastry-Sutherland lattice. Phys. Rev. B 2008, 77, 144425. 10.1103/PhysRevB.77.144425. [DOI] [Google Scholar]
- Kim M. S.; Bennett M. C.; Aronson M. C. Yb2Pt2Pb: A new quasi-two-dimensional antiferromagnet. Physica B 2008, 403, 1411–1413. 10.1016/j.physb.2007.10.160. [DOI] [Google Scholar]
- Shimura Y.; Sakakibara T.; Iwakawa K.; O̅nuki Y.; Sugiyama K. Magnetization steps in Yb2Pt2Pb with the Shastry-Sutherland lattice. J. Korean Phys. Soc. 2013, 63, 551–554. 10.3938/jkps.63.551. [DOI] [Google Scholar]
- Miiller W.; Wu L. S.; Kim M. S.; Orvis T.; Simonson J. W.; Gamża M.; McNally D. M.; Nelson C. S.; Ehlers G.; Podlesnyak A.; Helton J. S.; Zhao Y.; Qiu Y.; Copley J. R. D.; Lynn J. W.; Zaliznyak I.; Aronson M. C. Magnetic structure of Yb2Pt2Pb: Ising moments on the Shastry-Sutherland lattice. Phys. Rev. B 2016, 93, 104419. 10.1103/PhysRevB.93.104419. [DOI] [Google Scholar]
- Ochiai A.; Inukai T.; Matsumura T.; Oyamada A.; Katoh K. Spin gap state of I = 1/2 Heisenberg antiferromagnet YbAl3C3. J. Phys. Soc. Jpn. 2007, 76, 123703. 10.1143/JPSJ.76.123703. [DOI] [Google Scholar]
- Matsumura T.; Inami T.; Kosaka M.; Kato Y.; Inukai T.; Ochiai A.; Nakao H.; Murakami Y.; Katano S.; S. Suzuki H. Structural phase transition in the spin gap system YbAl3C3. J. Phys. Soc. Jpn. 2008, 77, 103601. 10.1143/JPSJ.77.103601. [DOI] [Google Scholar]
- Mito T.; Tomisawa S.; Wada S.; Harima H.; Hashi K.; Shimizu T.; Goto A.; Ohki S.; Kato Y.; Kosaka M. 27Al NMR/NQR Studies of YbAl3C3. J. Phys. Soc. Jpn. 2009, 78, 014709. 10.1143/JPSJ.78.014709. [DOI] [Google Scholar]
- Hara K.; Matsuda S.; Matsuoka E.; Tanigaki K.; Ochiai A.; Nakamura S.; Nojima T.; Katoh K. Quantum spin state in the rare-earth compound YbAl3C3. Phys. Rev. B 2012, 85, 144416. 10.1103/PhysRevB.85.144416. [DOI] [Google Scholar]
- Kittaka S.; Sugiyama T.; Shimura Y.; Sakakibara T.; Matsuda S.; Ochiai A. Singlet-triplet crossover in the two-dimensional dimer spin system YbAl3C3. J. Korean Phys. Soc. 2013, 62, 2088–2092. 10.3938/jkps.62.2088. [DOI] [Google Scholar]
- Kato Y.; Kosaka M.; Nowatari H.; Saiga Y.; Yamada A.; Kobiyama T.; Katano S.; Ohoyama K.; S. Suzuki H.; Aso N.; Iwasa K. Spin-singlet ground state in the two-dimensional frustrated triangular lattice: YbAl3C3. J. Phys. Soc. Jpn. 2008, 77, 053701. 10.1143/JPSJ.77.053701. [DOI] [Google Scholar]
- Torikachvili M. S.; Jia S.; Mun E. D.; Hannahs S. T.; Black R. C.; Neils W. K.; Martien D.; Bud'ko S. L.; Canfield P. C. YbT2Zn20 (T = Fe, Co, Ru, Rh, Os,Ir): six closely related heavy fermion compounds. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 9960. 10.1073/pnas.0702757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga Y.; Matsubayashi K.; Fujiwara T.; Kosaka M.; Katano S.; Hedo M.; Matsumoto T.; Uwatoko Y. Pressure-induced magnetic transition in a single crystal of YbCo2Zn20. J. Phys. Soc. Jpn. 2008, 77, 053710. 10.1143/JPSJ.77.053710. [DOI] [Google Scholar]
- Matsubayashi K.; Saiga Y.; Matsumoto T.; Uwatoko Y. Fermi-liquid properties of the heavy fermion systems YbT2Zn20 (T = Ir, Rh and Co) under pressure. J. Phys.: Conf. Series 2009, 150, 042117. 10.1088/1742-6596/150/4/042117. [DOI] [Google Scholar]
- Yamanaka R.; Matsubayashi K.; Saiga Y.; Kawae T.; Uwatoko Y. Heat capacity measurement of heavy Fermion YbCo2Zn20 under magnetic field. J. Phys.: Conf. Series 2012, 391, 012078. 10.1088/1742-6596/391/1/012078. [DOI] [Google Scholar]
- Gladyshevskii E. I.; Krypyakevych P. I.; Teslyuk M. Y. Crystal structure of the ternary phase Cu4MgSn. Dokl. Akad. Nauk. SSSR 1952, 85, 81–84. [Google Scholar]
- Osamura K.; Murakami Y. Crystal structures of CuSnMg and Cu4SnMg ternary compounds. J. Less-Common Met. 1978, 60, 311–313. 10.1016/0022-5088(78)90185-6. [DOI] [Google Scholar]
- Kojima K.; Hayashi H.; Minami A.; Kasamatsu Y.; Hihara T. Crystal phases and Yb valence transition in YbxIn1–xCu2. J. Magn. Magn. Mater. 1989, 81, 267–272. 10.1016/0304-8853(89)90004-8. [DOI] [Google Scholar]
- Felner I.; Nowik I. First-order valence phase transition in cubic YbxIn1-xCu2. Phys. Rev. B 1986, 33, 617–619. 10.1103/PhysRevB.33.617. [DOI] [PubMed] [Google Scholar]
- Felner I.; Nowik I.; Vaknin D.; Potzel U.; Moser J.; Kalvius G. M.; Wortmann G.; Schmiester G.; Hilscher G.; Gratz E.; Schmitzer C.; Pillmayr N.; Prasad K. G.; de Waard H.; Pinto H. Ytterbium valence phase transition in YbxIn1-xCu2. Phys. Rev. B 1987, 35, 6956–6963. 10.1103/PhysRevB.35.6956. [DOI] [PubMed] [Google Scholar]
- Sato H.; Shimada K.; Arita M.; Hiraoka K.; Kojima K.; Takeda Y.; Yoshikawa K.; Sawada M.; Nakatake M.; Namatame H.; Taniguchi M.; Takata Y.; Ikenaga E.; Shin S.; Kobayashi K.; Tamasaku K.; Nishino Y.; Miwa D.; Yabashi M.; Ishikawa T. Valence transition of YbInCu4 observed in hard X-ray photoemission spectra. Phys. Rev. Lett. 2004, 93, 246404. 10.1103/PhysRevLett.93.246404. [DOI] [PubMed] [Google Scholar]
- Sarrao J. L. Physics of YbInCu4 and related compounds. Physica B 1999, 259–261, 128–133. 10.1016/S0921-4526(98)00620-6. [DOI] [Google Scholar]
- Lawrence J. M.; Kwei G. H.; Sarrao J. L.; Fisk Z.; Mandrus D.; Thompson J. D. Structure and disorder in YbInCu4. Phys. Rev. B 1996, 54, 6011–6014. 10.1103/PhysRevB.54.6011. [DOI] [PubMed] [Google Scholar]
- Sato H.; Taniguchi M.; Hiraoka K.; Kojima K. Valence transition of YbInCu4 observed by photoemission spectroscopy. e-J. Surf. Sci. Nanotechnol. 2011, 9, 90–94. 10.1380/ejssnt.2011.90. [DOI] [Google Scholar]
- H. Matsuda Y.; Inami T.; Ohwada K.; Murata Y.; Nojiri H.; Murakami Y.; Ohta H.; Zhang W.; Yoshimura K. High-magnetic-field X-ray absorption spectroscopy of field-induced valence transition in YbInCu4. J. Phys. Soc. Jpn. 2007, 76, 034702. 10.1143/JPSJ.76.034702. [DOI] [Google Scholar]
- Dallera C.; Grioni M.; Shukla A.; Vankó G.; Sarrao J. L.; Rueff J. P.; Cox D. L. New spectroscopy solves an old puzzle: The Kondo scale in heavy fermions. Phys. Rev. Lett. 2002, 88, 196403. 10.1103/PhysRevLett.88.196403. [DOI] [PubMed] [Google Scholar]
- Hancock J. N.; McKnew T.; Schlesinger Z.; Sarrao J. L.; Fisk Z. Kondo scaling in the optical response of YbIn1–xAgxCu4. Phys. Rev. Lett. 2004, 92, 186405. 10.1103/PhysRevLett.92.186405. [DOI] [PubMed] [Google Scholar]
- Lawrence J. M.; Shapiro S. M.; Sarrao J. L.; Fisk Z. Inelastic neutron scattering in single-crystal YbInCu4. Phys. Rev. B 1997, 55, 14467–14472. 10.1103/PhysRevB.55.14467. [DOI] [Google Scholar]
- Zhang M. Y.; Chen R. Y.; Dong T.; Wang N. L. Dramatic change of photoexcited quasiparticle relaxation dynamics across Yb valence state transition in YbInCu4. Phys. Rev. B 2017, 95, 165104. 10.1103/PhysRevB.95.165104. [DOI] [Google Scholar]
- Cornelius A. L.; Lawrence J. M.; Sarrao J. L.; Fisk Z.; Hundley M. F.; Kwei G. H.; Thompson J. D.; Booth C. H.; Bridges F. Experimental studies of the phase transition in YbIn1–xAgxCu4. Phys. Rev. B 1997, 56, 7993–8000. 10.1103/PhysRevB.56.7993. [DOI] [Google Scholar]
- Kishimoto Y.; Kawasaki Y.; Ohno T. Mixed valence state in Ce and Yb compounds studied by magnetic susceptibility. Phys. Lett. A 2003, 317, 308–314. 10.1016/j.physleta.2003.08.050. [DOI] [Google Scholar]
- Sarrao J. L.; Immer C. D.; Benton C. L.; Fisk Z.; Lawrence J. M.; Mandrus D.; Thompson J. D. Evolution from first-order valence transition to heavy-fermion behavior in YbIn1–xAgxCu4. Phys. Rev. B 1996, 54, 12207–12211. 10.1103/PhysRevB.54.12207. [DOI] [PubMed] [Google Scholar]
- Nakatsuji S.; Kuga K.; Machida Y.; Tayama T.; Sakakibara T.; Karaki Y.; Ishimoto H.; Yonezawa S.; Maeno Y.; Pearson E.; Lonzarich G. G.; Balicas L.; Lee H.; Fisk Z. Superconductivity and quantum criticality in the heavy-fermion system β-YbAlB4. Nat. Phys. 2008, 4, 603–607. 10.1038/nphys1002. [DOI] [Google Scholar]
- Kuga K.; Karaki Y.; Matsumoto Y.; Machida Y.; Nakatsuji S. Superconducting properties of the non-Fermi-liquid system β-YbAlB4. Phys. Rev. Lett. 2008, 101, 137004. 10.1103/PhysRevLett.101.137004. [DOI] [PubMed] [Google Scholar]
- Nakatsuji S.; Kuga K.; Tomita T.; Matsumoto Y. Pronounced non-Fermi-liquid behavior of the quantum critical heavy fermion superconductor β-YbAlB4. Phys. Status Solidi B 2010, 247, 485–489. 10.1002/pssb.200983080. [DOI] [Google Scholar]
- Okawa M.; Matsunami M.; Ishizaka K.; Eguchi R.; Taguchi M.; Chainani A.; Takata Y.; Yabashi M.; Tamasaku K.; Nishino Y.; Ishikawa T.; Kuga K.; Horie N.; Nakatsuji S.; Shin S. Strong valence fluctuation in the quantum critical heavy fermion superconductor β-YbAlB4: A hard X-ray photoemission study. Phys. Rev. Lett. 2010, 104, 247201. 10.1103/PhysRevLett.104.247201. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y.; Ikeda S.; Kuga K.; Suzuki S.; Nakatsuji S.; Hirao N.; Ohishi Y.; Kobayashi H. Pressure-induced local structural changes in heavy fermion β-YbAlB4. J. Phys. Soc. Jpn. 2016, 85, 023602. 10.7566/JPSJ.85.023602. [DOI] [Google Scholar]
- Matsumoto Y.; Nakatsuji S.; Kuga K.; Karaki Y.; Horie N.; Shimura Y.; Sakakibara T.; Nevidomskyy A. H.; Coleman P. Quantum criticality without tuning in the mixed valence compound β-YbAlB4. Science 2011, 331, 316–319. 10.1126/science.1197531. [DOI] [PubMed] [Google Scholar]
- Ramires A.; Coleman P.; Nevidomskyy A. H.; Tsvelik A. M. β-YbAlB4: A critical nodal metal. Phys. Rev. Lett. 2012, 109, 176404. 10.1103/PhysRevLett.109.176404. [DOI] [PubMed] [Google Scholar]
- Holanda L. M.; Vargas J. M.; Iwamoto W.; Rettori C.; Nakatsuji S.; Kuga K.; Fisk Z.; Oseroff S. B.; Pagliuso P. G. Quantum critical Kondo quasiparticles probed by ESR in β-YbAlB4. Phys. Rev. Lett. 2011, 107, 026402. 10.1103/PhysRevLett.107.026402. [DOI] [PubMed] [Google Scholar]
- Watanabe S.; Miyake K. Charge transfer effect under odd-parity crystalline electric field: Divergence of magnetic toroidal fluctuation in β-YbAlB4. J. Phys. Soc. Jpn. 2019, 88, 033701. 10.7566/JPSJ.88.033701. [DOI] [Google Scholar]
- Ramires A.; Coleman P. Theory of the electron spin resonance in the heavy fermion metal β-YbAlB4. Phys. Rev. Lett. 2014, 112, 116405. 10.1103/PhysRevLett.112.116405. [DOI] [PubMed] [Google Scholar]
- Komijani Y.; Coleman P. Model for a ferromagnetic quantum critical point in a 1D Kondo lattice. Phys. Rev. Lett. 2018, 120, 157206. 10.1103/PhysRevLett.120.157206. [DOI] [PubMed] [Google Scholar]
- Kowalczyk A.; Falkowski M.; Toliński T. Valence fluctuations in YbNiAl4 compound. J. Appl. Phys. 2010, 107, 123917. 10.1063/1.3452388. [DOI] [Google Scholar]
- Falkowski M.; Kowalczyk A. Thermal and electron transport studies on the valence fluctuating compound YbNiAl4. J. Appl. Phys. 2018, 123, 175106. 10.1063/1.5025236. [DOI] [Google Scholar]
- Brubaker Z. E.; Stillwell R. L.; Chow P.; Xiao Y.; Kenney-Benson C.; Ferry R.; Popov D.; Donald S. B.; Söderlind P.; Campbell D. J.; Paglione J.; Huang K.; Baumbach R. E.; Zieve R. J.; Jeffries J. R. Pressure-dependent intermediate valence behavior in YbNiGa4 and YbNiIn4. Phys. Rev. B 2018, 98, 214115. 10.1103/PhysRevB.98.214115. [DOI] [Google Scholar]
- Sichevych O.; Prots Y.; Utsumi Y.; Akselrud L.; Schmidt M.; Burkhardt U.; Coduri M.; Schnelle W.; Bobnar M.; Wang Y.-T.; Wu Y.-H.; Tsuei K.-D.; Tjeng L. H.; Grin Y. Intermediate-calence ytterbium compound Yb4Ga24Pt9: Synthesis, crystal structure, and physical properties. Inorg. Chem. 2017, 56, 9343–9352. 10.1021/acs.inorgchem.7b01530. [DOI] [PubMed] [Google Scholar]
- Schumacher L.; Engelbert S.; Reimann M. K.; Kösters J.; Pöttgen R. Intermediate ytterbium valence in YbRhSn2. Z. Naturforsch. 2022, 77b, 687–692. 10.1515/znb-2022-0099. [DOI] [Google Scholar]
- Grin Y.; Pöttgen R.; Ormeci A.; Kremer R. K.; Wagner F. E. Intermediate-valence intermetallic phase YbIn1–xAu1+x (x = 0–0.3). Cryst. Res. Technol. 2017, 52, 1700101. 10.1002/crat.201700101. [DOI] [Google Scholar]
- Jiang W. B.; Yang L.; Guo C. Y.; Hu Z.; Lee J. M.; Smidman M.; Wang Y. F.; Shang T.; Cheng Z. W.; Gao F.; Ishii H.; Tsuei K. D.; Liao Y. F.; Lu X.; Tjeng L. H.; Chen J. M.; Yuan H. Q. Crossover from a heavy fermion to intermediate valence state in noncentrosymmetric Yb2Ni12(P,As)7. Sci. Rep. 2015, 5, 17608. 10.1038/srep17608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk Z.; Yang N. N.; Maple M. B.; Ott H. R. In Valence fluctuations in solids, Falicov L. M.; Hanke W.; Maple M. B., Eds.; North-Holland: Amsterdam, 1981. [Google Scholar]
- Utsumi Y.; Sato H.; Ohara S.; Yamashita T.; Mimura K.; Motonami S.; Shimada K.; Ueda S.; Kobayashi K.; Yamaoka H.; Tsujii N.; Hiraoka N.; Namatame H.; Taniguchi M. Electronic structure of Kondo lattice compounds YbNi3X9 (X = Al, Ga) studied by hard X-ray spectroscopy. Phys. Rev. B 2012, 86, 115114. 10.1103/PhysRevB.86.115114. [DOI] [Google Scholar]
- Falkowski M.; Kowalczyk A.; Toliński T. Specific heat of YbNi4Si compound. Acta Phys. Polon., A 2008, 113, 641–644. 10.12693/APhysPolA.113.641. [DOI] [Google Scholar]
- Yamaoka H.; Tsujii N.; Utsumi Y.; Sato H.; Jarrige I.; Yamamoto Y.; Lin J.-F.; Hiraoka N.; Ishii H.; Tsuei K.-D.; Mizuki J. I. Valence transitions in the heavy-fermion compound YbCuAl as a function of temperature and pressure. Phys. Rev. B 2013, 87, 205120. 10.1103/PhysRevB.87.205120. [DOI] [Google Scholar]
- Sales B. C.; Viswanathan R. Demagnetization due to interconfiguration fluctuations in the RE-Cu2Si2 compounds. J. Low Temp. Phys. 1976, 23, 449–467. 10.1007/BF00116933. [DOI] [Google Scholar]
- Rossel C.; Yang K. N.; Maple M. B.; Fisk Z.; Zirngiebl E.; Thompson J. D. Strong electronic correlations in a new class of Yb-based compounds: YbXCu4 (X = Ag, Au, Pd). Phys. Rev. B 1987, 35, 1914–1918. 10.1103/PhysRevB.35.1914. [DOI] [PubMed] [Google Scholar]
- Jaime M.; Movshovich R.; Harrison N.; L. Sarrao J. Two energy scales in YbInCu4 from specific heat in high magnetic fields. Physica B 2002, 312–313, 344–345. 10.1016/S0921-4526(01)01304-7. [DOI] [Google Scholar]
- Sarrao J. L.; Ramirez A. P.; Darling T. W.; Freibert F.; Migliori A.; Immer C. D.; Fisk Z.; Uwatoko Y. Thermodynamics of the first-order valence transition in YbCuIn4. Phys. Rev. B 1998, 58, 409–413. 10.1103/PhysRevB.58.409. [DOI] [Google Scholar]
- Katoh K.; Abe H.; Negishi D.; Terui G.; Niide Y.; Ochiai A. Magnetic and transport properties of Yb4Rh7Ge6 and Yb4Ir7Ge6. J. Magn. Magn. Mater. 2004, 279, 118–124. 10.1016/j.jmmm.2004.01.075. [DOI] [Google Scholar]
- Katoh K.; Nakagawa S.; Terui G.; Ochiai A. Magnetic and transport properties of single-crystal YbPtGe. J. Phys. Soc. Jpn. 2009, 78, 104721. 10.1143/JPSJ.78.104721. [DOI] [Google Scholar]
- Kaczorowski D.; Andraka B.; Zaremba V.; Marucha C. YbPtIn – a new ytterbium-based magnetic Kondo lattice. Physica B 2000, 281–282, 44–46. 10.1016/S0921-4526(99)01175-8. [DOI] [Google Scholar]
- Kim M. S.; Aronson M. C. Spin liquids and antiferromagnetic order in the Shastry-Sutherland-lattice compound Yb2Pt2Pb. Phys. Rev. Lett. 2013, 110, 017201. 10.1103/PhysRevLett.110.017201. [DOI] [PubMed] [Google Scholar]
- Rhyne J. J. Neutron scattering in intermetallics. J. Magn. Magn. Mater. 1987, 70, 88–96. 10.1016/0304-8853(87)90369-6. [DOI] [Google Scholar]
- Walter U.; Fisk Z.; Holland-Moritz E. Magnetic excitations and quasielastic linewidths in YbBe13 from neutron scattering. J. Magn. Magn. Mater. 1985, 47–48, 159–162. 10.1016/0304-8853(85)90386-5. [DOI] [Google Scholar]
- Heinrich G.; Kappler J. P.; Meyer A. Magnetic-properties and EPR of YbBe13. Phys. Lett. A 1979, 74, 121–124. 10.1016/0375-9601(79)90603-0. [DOI] [Google Scholar]
- Besnus M. J.; Panissod P.; Kappler J. P.; Heinrich G.; Meyer A. Crystal field parameters in the REBe13 intermetallics. J. Magn. Magn. Mater. 1983, 31–34, 227–228. 10.1016/0304-8853(83)90227-5. [DOI] [Google Scholar]
- Walter U.; Wohlleben D. Unusual magnetic response of intermediate-valent YbPd and Yb3Pd4 as studied by inelastic neutron scattering. Phys. Rev. B 1987, 35, 3576–3584. 10.1103/PhysRevB.35.3576. [DOI] [PubMed] [Google Scholar]
- Murani A. P.; Pierre J. Low temperature paramagnetic scattering from YbInAu2 and YbAl3. Physica B 1995, 206–207, 329–331. 10.1016/0921-4526(94)00449-6. [DOI] [Google Scholar]
- Lawrence J. M.; Osborn R.; Sarrao J. L.; Fisk Z. Time-of-flight neutron-scattering study of YbInCu4 and YbIn0.3Ag0.7Cu4. Phys. Rev. B 1999, 59, 1134–1140. 10.1103/PhysRevB.59.1134. [DOI] [Google Scholar]
- Goremychkin E. A.; Osborn R. Crystal field excitations in YbT2Si2 (T = Fe, Co, Ni). J. Appl. Phys. 2000, 87, 6818–6820. 10.1063/1.372852. [DOI] [Google Scholar]
- Klingner C.; Krellner C.; Brando M.; Geibel C.; Steglich F. Magnetic behaviour of the intermetallic compound YbCo2Si2. New J. Phys. 2011, 13, 083024. 10.1088/1367-2630/13/8/083024. [DOI] [Google Scholar]
- Szytuła A.; Szott I. Magnetic properties of ternary RMn2Si2 and RMn2Ge2 compounds. Solid State Commun. 1981, 40, 199–202. 10.1016/0038-1098(81)90167-8. [DOI] [Google Scholar]
- Hofmann M.; Campbell S. J.; Edge A. V. J.; Studer A. J. The magnetic structures of YbMn2Si2. J. Phys.: Condens. Matter 2001, 13, 9773. 10.1088/0953-8984/13/43/308. [DOI] [Google Scholar]
- Mole R. A.; Hofmann M.; Adroja D. T.; Moze O.; Campbell S. J. Crystal field excitations of YbMn2Si2. J. Magn. Magn. Mater. 2013, 347, 86–94. 10.1016/j.jmmm.2013.07.038. [DOI] [Google Scholar]
- Campbell S. J.; Hofmann M.; Mole R. A.; Prokes K.; Wallacher D.; Wang J. L. Magnetic order in YbMn2Si2 – Neutron scattering investigation. J. Korean Phys. Soc. 2013, 63, 314–319. 10.3938/jkps.63.314. [DOI] [Google Scholar]
- Stockert O.; Koza M. M.; Ferstl J.; Murani A. P.; Geibel C.; Steglich F. Crystalline electric field excitations of the non-Fermi-liquid YbRh2Si2. Physica B 2006, 378–380, 157–158. 10.1016/j.physb.2006.01.059. [DOI] [Google Scholar]
- Leushin A. M.; Ivanshin V. A.; Kurkin I. N. Crystal field of Yb3+ tetragonal centers in the YbRh2Si2 intermetallic compound. Phys. Solid State 2007, 49, 1417–1421. 10.1134/S1063783407080021. [DOI] [Google Scholar]
- Hiess A.; Stockert O.; Koza M. M.; Hossain Z.; Geibel C. Magnetisation dynamics of YbIr2Si2. Physica B 2006, 378–380, 748–749. 10.1016/j.physb.2006.01.272. [DOI] [Google Scholar]
- Leushin A. M.; Ivanshin V. A. Crystalline electric fields and the ground state of YbRh2Si2 and YbIr2Si2. Physica B 2008, 403, 1265–1267. 10.1016/j.physb.2007.10.122. [DOI] [Google Scholar]
- Beaurepaire E.; Kappler J. P.; Krill G. Xanes study of trivalent and homogeneous mixed valent rare earth systems. Solid State Commun. 1986, 57, 145–149. 10.1016/0038-1098(86)90531-4. [DOI] [Google Scholar]
- Dallera C.; Annese E.; Rueff J. P.; Palenzona A.; Vankó G.; Braicovich L.; Shukla A.; Grioni M. Determination of pressure-induced valence changes in YbAl2 by resonant inelastic x-ray emission. Phys. Rev. B 2003, 68, 245114. 10.1103/PhysRevB.68.245114. [DOI] [Google Scholar]
- Tjeng L. H.; Oh S.; Cho E.; Lin H.; Chen C. T.; Gweon G.; Park J.; Allen J. W.; Suzuki T.; Makivic M. S.; Cox D. L. Temperature dependence of the Kondo resonance in YbAl3. Phys. Rev. Lett. 1993, 71, 1419–1422. 10.1103/PhysRevLett.71.1419. [DOI] [PubMed] [Google Scholar]
- Kumar R. S.; Svane A.; Vaitheeswaran G.; Kanchana V.; Bauer E. D.; Hu M.; Nicol M. F.; Cornelius A. L. Pressure-induced valence change in YbAl3: A combined high-pressure inelastic X-ray scattering and theoretical investigation. Phys. Rev. B 2008, 78, 075117. 10.1103/PhysRevB.78.075117. [DOI] [Google Scholar]
- Rojas D. P.; Fernández Barquín L.; Espeso J. I.; Rodríguez Fernández J.; Chaboy J. Reduction of the Yb valence in YbAl3 nanoparticles. Phys. Rev. B 2008, 78, 094412. 10.1103/PhysRevB.78.094412. [DOI] [Google Scholar]
- Schwarz U.; Giedigkeit R.; Niewa R.; Schmidt M.; Schnelle W.; Cardoso R.; Hanfland M.; Hu Z.; Klementiev K.; Grin Y. Pressure-induced oxidation state change of ytterbium in YbGa2. Z. Anorg. Allg. Chem. 2001, 627, 2249–2256. . [DOI] [Google Scholar]
- Rams M.; Królas K.; Bonville P.; Alleno E.; Godart C.; Kaczorowski D.; Canepa F. Valence states of Yb in Yb5Si3. Phys. Rev. B 1997, 56, 3690–3696. 10.1103/PhysRevB.56.3690. [DOI] [Google Scholar]
- Subbarao U.; Sarkar S.; Joseph B.; Peter S. C. Magnetic and X-ray absorption studies on the RE5X2Sb6 (RE = Eu, Yb; X = Al, Ga, In) compounds. J. Alloys Compd. 2016, 658, 395–401. 10.1016/j.jallcom.2015.10.232. [DOI] [Google Scholar]
- Kanatzidis M. G.Chemistry and Properties of Complex Intermetallics from Metallic Fluxes; Northwestern University: Evanston, IL, USA, 2015. [Google Scholar]
- Booth C. H.; Christianson A. D.; Lawrence J. M.; Pham L. D.; Lashley J. C.; Drymiotis F. R. Ytterbium divalency and lattice disorder in near-zero thermal expansion YbGaGe. Phys. Rev. B 2007, 75, 012301. 10.1103/PhysRevB.75.012301. [DOI] [Google Scholar]
- Nakai H.; Ebihara T.; Tsutsui S.; Mizumaki M.; Kawamura N.; Michimura S.; Inami T.; Nakamura T.; Kondo A.; Kindo K.; Matsuda Y. H. Temperature and magnetic field dependent Yb valence in YbRh2Si2 observed by X-ray absorption spectroscopy. J. Phys. Soc. Jpn. 2013, 82, 124712. 10.7566/JPSJ.82.124712. [DOI] [Google Scholar]
- Hatwar T. K.; Nayak R. M.; Padalia B. D.; Ghatikar M. N.; Sampathkumaran E. V.; Gupta L. C.; Vijayaraghavan R. X-ray absorption spectroscopic study of mixed valence systems EuCu2Si2, YbCu2Si2 and Sm4Bi3. Solid State Commun. 1980, 34, 617–620. 10.1016/0038-1098(80)90939-4. [DOI] [Google Scholar]
- Ghatikar M. N.; Hatwar T. K.; Padalia B. D.; Sampathkumaran E. V.; Gupta L. C.; Vijayaraghavan R. Study of X-ray absorption edges of rare earths and gallium in RAl2Ga2 intermetallics. Phys. Status Solidi B 1981, 106, K89–K93. 10.1002/pssb.2221060241. [DOI] [Google Scholar]
- Qiao Y.; Song Y.; Sanson A.; Fan L.; Sun Q.; Hu S.; He L.; Zhang H.; Xing X.; Chen J. Negative thermal expansion in YbMn2Ge2 induced by the dual effect of magnetism and valence transition. npj Quantum Mater. 2021, 6, 49. 10.1038/s41535-021-00348-z. [DOI] [Google Scholar]
- Honda F.; Hirose Y.; Miyake A.; Mizumaki M.; Kawamura N.; Tsutsui S.; Watanuki T.; Watanabe S.; Takeuchi T.; Settai R.; Aoki D.; O̅nuki Y. X-ray absorption spectroscopy and novel electronic properties in heavy fermion compounds YbT2Zn20 (T: Rh and Ir). J. Phys.: Conf. Series 2015, 592, 012021. 10.1088/1742-6596/592/1/012021. [DOI] [Google Scholar]
- Mito T.; Koyama T.; Nakagawara K.; Ishida T.; Ueda K.; Kohara T.; Matsubayashi K.; Saiga Y.; Munakata K.; Uwatoko Y.; Mizumaki M.; Kawamura N.; Idzikowski B.; Reiffers M. Mechanism of field induced Fermi liquid state in Yb-based heavy-fermion compound: X-ray absorption spectroscopy and nuclear magnetic resonance studies of YbCo2Zn20. J. Phys. Soc. Jpn. 2012, 81, 033706. 10.1143/JPSJ.81.033706. [DOI] [Google Scholar]
- Fahl A.; Grossi R.; Rigitano D.; Cabrera-Baez M.; Avila M. A.; Adriano C.; Granado E. Crystal, local atomic, and local electronic structures of YbFe2Zn20–xCdx (0 ≤ x ≤ 1.4): A multiband system with possible coexistence of light and heavy fermions. Phys. Rev. B 2021, 103, 155116. 10.1103/PhysRevB.103.155116. [DOI] [Google Scholar]
- Hosokawa S.; Happo N.; Hayashi K.; Kimura K.; Matsushita T.; Stellhorn J. R.; Mizumaki M.; Suzuki M.; Sato H.; Hiraoka K. Valence-selective local atomic structures on an YbInCu4 valence transition material by X-ray fluorescence holography. J. Phys. Soc. Jpn. 2020, 89, 034603. 10.7566/JPSJ.89.034603. [DOI] [Google Scholar]
- Yamaoka H.; Ohmura A.; Furue Y.; Tsujii N.; Ishii H.; Hiraoka N. Direct observation of pressure-induced Yb valence transition in YbInCu4. J. Phys. Soc. Jpn. 2021, 90, 124801. 10.7566/JPSJ.90.124801. [DOI] [Google Scholar]
- Lawrence J. M.; Kwei G. H.; Canfield P. C.; DeWitt J. G.; Lawson A. C. LIII X-ray absorption in Yb compounds: Temperature dependence of the valence. Phys. Rev. B 1994, 49, 1627–1631. 10.1103/PhysRevB.49.1627. [DOI] [PubMed] [Google Scholar]
- Subbarao U.; Sarkar S.; Joseph B.; Peter S. C. Magnetic and X-ray absorption studies on the RE5X2Sb6 (RE = Eu, Yb; X = Al, Ga, In) compounds. J. Alloys Compd. 2016, 658, 395–401. 10.1016/j.jallcom.2015.10.232. [DOI] [Google Scholar]
- He A.; Wille E. L. K.; Moreau L. M.; Thomas S. M.; Lawrence J. M.; Bauer E. D.; Booth C. H.; Kauzlarich S. M. Intermediate Yb valence in the Zintl phases Yb14MSb11 (M = Zn,Mn,Mg): XANES, magnetism, and heat capacity. Phys. Rev. Mater. 2020, 4, 114407. 10.1103/PhysRevMaterials.4.114407. [DOI] [Google Scholar]
- Oh S. J.; Allen J. W.; Torikachvili M. S.; Maple M. B. Temperature-induced valence change of YbAl2 studied by XPS and BIS. J. Magn. Magn. Mater. 1985, 52, 183–186. 10.1016/0304-8853(85)90250-1. [DOI] [Google Scholar]
- Buschow K. H. J.; Campagna M.; Wertheim G. K. Intermediate valence in YbAl3 and EuCu2Si2 by X-ray photoemission (XPS). Solid State Commun. 1977, 24, 253–256. 10.1016/0038-1098(77)91208-X. [DOI] [Google Scholar]
- Joyce J. J.; Andrews A. B.; Arko A. J.; Bartlett R. J.; Blyth R. I. R.; Olson C. G.; Benning P. J.; Canfield P. C.; Poirier D. M. Photoelectron spectroscopy of strongly correlated Yb compounds. Phys. Rev. B 1996, 54, 17515–17535. 10.1103/PhysRevB.54.17515. [DOI] [PubMed] [Google Scholar]
- Joyce J. J.; Arko A. J.; Morales L. A.; Sarrao J. L.; Höchst H. Comment on “Photoemission experiments on YbInCu4: Surface effects and temperature dependence. Phys. Rev. B 2001, 63, 197101. 10.1103/PhysRevB.63.197101. [DOI] [Google Scholar]
- Wertheim G. K.; Wernick J. H.; Crecelius G. Surface effects on valence in rare-earth intermetallic compounds. Phys. Rev. B 1978, 18, 875–879. 10.1103/PhysRevB.18.875. [DOI] [Google Scholar]
- Iga F.; Takakuwa Y.; Takahashi T.; Kasaya M.; Kasuya T.; Sagawa T. XPS study of rare earth dodecaborides: TmB12, YbB12 and LuB12. Solid State Commun. 1984, 50, 903–905. 10.1016/0038-1098(84)90745-2. [DOI] [Google Scholar]
- Suga S.; Ogawa S.; Namatame H.; Taniguchi M.; Kakizaki A.; Ishii T.; Fujimori A.; Oh S.-J.; Kato H.; Miyahara T.; Ochiai A.; Suzuki T.; Kasuya T. XPS and UPS studies of valence fluctuation and surface states of Yb4As3, Yb4Sb3 and Yb4Bi3. J. Phys. Soc. Jpn. 1989, 58, 4534–4543. 10.1143/JPSJ.58.4534. [DOI] [Google Scholar]
- Gegner J.; Koethe T. C.; Wu H.; Hu Z.; Hartmann H.; Lorenz T.; Fickenscher T.; Pöttgen R.; Tjeng L. H. Electronic structure of RAuMg and RAgMg (R = Eu, Gd, Yb). Phys. Rev. B 2006, 74, 073102. 10.1103/PhysRevB.74.073102. [DOI] [Google Scholar]
- Rao C. N. R.; Sarma D. D.; Sarode P. R.; Sampathkumaran E. V.; Gupta L. C.; Vijayaraghavan R. Valence fluctuation in some Yb intermetallics by X-ray photoemission and X-ray absorption. Chem. Phys. Lett. 1980, 76, 413–415. 10.1016/0009-2614(80)80637-3. [DOI] [Google Scholar]
- Maeda K.; Sato H.; Akedo Y.; Kawabata T.; Abe K.; Shimokasa R.; Yasui A.; Mizumaki M.; Kawamura N.; Ikenaga E.; Tsutsui S.; Matsumoto K.; Hiraoka K.; Mimura K.. Yb L3 resonant hard X-ray photoemission spectroscopy of valence transition compound YbInCu4. In Proceedings of the International Conference on Strongly Correlated Electron Systems (SCES2019), Okayama, 2020; Vol. 30, p. 011137.
- Sato H.; Hiraoka K.; Taniguchi M.; Nishikawa Y.; Nagasaki F.; Fujino H.; Takeda Y.; Arita M.; Shimada K.; Namatame H.; Kimura A.; Kojima K. X-dependent electronic structure of YbXCu4 (X = In, Cd, Mg) investigated by high-resolution photoemission spectroscopy. J. Phys.: Condens. Matter 2002, 14, 4445–4459. 10.1088/0953-8984/14/17/316. [DOI] [Google Scholar]
- Kang J.; Allen J. W.; Rossel C.; Seaman C. L.; Maple M. B. Electron-spectroscopy study of YbXCu4 (X = Ag, Au, Pd). Phys. Rev. B 1990, 41, 4078–4082. 10.1103/PhysRevB.41.4078. [DOI] [PubMed] [Google Scholar]
- Rousuli A.; Nakamura S.; Sato H.; Ueda T.; Matsumoto Y.; Ohara S.; Schwier E. F.; Nagasaki T.; Mimura K.; Anzai H.; Ichiki K.; Ueda S.; Shimada K.; Namatame H.; Taniguchi M. Photoemission study of the electronic structure of the Kondo lattices Yb2Pt6X15 (X = Al, Ga). Phys. Rev. B 2017, 96, 045117. 10.1103/PhysRevB.96.045117. [DOI] [Google Scholar]
- Holm A. P.; Ozawa T. C.; Kauzlarich S. M.; Morton S. A.; Dan Waddill G.; Tobin J. G. X-ray photoelectron spectroscopy studies of Yb14MnSb11 and Yb14ZnSb11. J. Solid State Chem. 2005, 178, 262–269. 10.1016/j.jssc.2004.07.009. [DOI] [Google Scholar]
- Anno H.; Ashida K.; Matsubara K.; Nolas G. S.; Akai K.; Matsuura M.; Nagao J. Electronic structure and thermoelectric properties of ytterbium-filled skutterudites. MRS Proc. 2001, 691, G2.4.1–G2.4.6. 10.1557/PROC-691-G2.4. [DOI] [Google Scholar]
- Eckert H.; Pöttgen R.. Solid-state NMR and Mößbauer spectroscopy. In Rare Earth Chemistry, Pöttgen R.; Jüstel T.; Strassert C. A., Eds.; De Gruyter: Berlin, 2020; pp 299–322. [Google Scholar]
- Gossard A. C.; Jaccarino V.; Wernick J. H. Ytterbium NMR: 171Yb nuclear moment and Yb metal Knight shift. Phys. Rev. 1964, 133, A881–A884. 10.1103/PhysRev.133.A881. [DOI] [Google Scholar]
- Ivanshin V. A.; Sukhanov A. A.; Sokolov D. A.; Aronson M. C.; Jia S.; Bud’ko S. L.; Canfield P. C. Electron spin resonance of dense Yb-based heavy-fermion compounds: New experimental data. J. Alloys Compd. 2009, 480, 126–127. 10.1016/j.jallcom.2008.09.172. [DOI] [Google Scholar]
- Kochelaev B. I.; Belov S. I.; Skvortsova A. M.; Kutuzov A. S.; Sichelschmidt J.; Wykhoff J.; Geibel C.; Steglich F. Why could electron spin resonance be observed in a heavy fermion Kondo lattice?. Eur. Phys. J. B 2009, 72, 485–489. 10.1140/epjb/e2009-00386-9. [DOI] [Google Scholar]
- Schlottmann P. Electron spin resonance in heavy-fermion systems. Phys. Rev. B 2009, 79, 045104. 10.1103/PhysRevB.79.045104. [DOI] [Google Scholar]
- Shimizu T.; Takigawa M.; Yasuoka H.; Wernick J. H. 171Yb nuclear magnetic resonance in YbAl2 and YbAl3. J. Magn. Magn. Mater. 1985, 52, 187–189. 10.1016/0304-8853(85)90251-3. [DOI] [Google Scholar]
- Giesselmann E. C. J.; Engel S.; Kostusiak W.; Zhang Y.; Herbeck-Engel P.; Kickelbick G.; Janka O. Raman and NMR spectroscopic and theoretical investigations of the cubic laves-phases REAl2 (RE = Sc, Y, La, Yb, Lu). Dalton Trans. 2023, 52, 3391–3402. 10.1039/D3DT00141E. [DOI] [PubMed] [Google Scholar]
- Ikushima K.; Kato Y.; Takigawa M.; Iga F.; Hiura S.; Takabatake T. 171Yb NMR in the Kondo semiconductor YbB12. Physica B 2000, 281–282, 274–275. 10.1016/S0921-4526(99)00815-7. [DOI] [Google Scholar]
- Kasaya M.; Iga F.; Takigawa M.; Kasuya T. Mixed valence properties of YbB12. J. Magn. Magn. Mater. 1985, 47–48, 429–435. 10.1016/0304-8853(85)90458-5. [DOI] [Google Scholar]
- Gerdes J. M.; Schumacher L.; Hansen M. R.; Pöttgen R.. A solid-state 171Yb NMR spectroscopic characterization of selected divalent ytterbium intermetallics. Z. Naturforsch. 2024, 79b, 10.1515/ZNB-2023-0070. In Press. [DOI] [Google Scholar]
- Sarkar R.; Gumeniuk R.; Leithe-Jasper A.; Schnelle W.; Grin Y.; Geibel C.; Baenitz M. Unconventional magnetism in multivalent charge-ordered YbPtGe2 probed by 195Pt- and 171Yb-NMR. Phys. Rev. B 2013, 88, 201101(R) 10.1103/PhysRevB.88.201101. [DOI] [Google Scholar]
- Iwamoto Y.; Akazawa T.; Ueda K., NMR study of YbGaGe. In Proceedings of the International Conference on Strongly Correlated Electron Systems (SCES2013), Tokyo, 2014.
- Gambke T.; Elschner B.; Kremer R.; Schanz M. Electron-spin-resonance of some rare-earths ions in cubic intermetallic compounds (AuCu3-structure). J. Magn. Magn. Mater. 1983, 36, 115–124. 10.1016/0304-8853(83)91053-3. [DOI] [Google Scholar]
- Nagel J.; Baberschke K.; Tsang E. ESR of Er3+ and Yb3+ in gold between 100 mK and 1 K. J. Magn. Magn. Mater. 1980, 15–18, 730–732. 10.1016/0304-8853(80)90738-6. [DOI] [Google Scholar]
- Krellner C.; Forster T.; Jeevan H.; Geibel C.; Sichelschmidt J. Relevance of ferromagnetic correlations for the electron spin resonance in Kondo lattice systems. Phys. Rev. Lett. 2008, 100, 066401. 10.1103/PhysRevLett.100.066401. [DOI] [PubMed] [Google Scholar]
- Kotel’nikova E. E.; Suleimanov N. M.; Khaliollin G. G.; Drulis H.; Iwasieczko W. Spin-glass transition as the cause of a false heavy-fermion behavior of the YbHx system. Zh. Eksp. Teor. Fiz. 1993, 58, 276–279. [Google Scholar]
- Likodimos V.; Guskos N.; Wabia M.; Typek J. Exchange interactions of Yb3+ ions in YbxY1–xBa2Cu3Oy. Phys. Rev. B 1998, 58, 8244–8247. 10.1103/PhysRevB.58.8244. [DOI] [Google Scholar]
- Ivanshin V. A.; Litvinova T. O.; Sukhanov A. A.; Ivanshin N. A.; Jia S.; Bud’ko S. L.; Canfield P. C. Dual nature of electron spin resonance in YbCo2Zn20 intermetallic compound. JETP Lett. 2014, 99, 153–157. 10.1134/S0021364014030096. [DOI] [Google Scholar]
- Ivan’shin V. A.; Aminov L. K.; Kurkin I. N.; Sichelschmidt J.; Stockert O.; Ferstl J.; Geibel C. Electron Paramagnetic Resonance of Yb3+ Ions in a concentrated YbRh2Si2 compound with heavy fermions. JETP Lett. 2003, 77, 526–529. 10.1134/1.1591984. [DOI] [Google Scholar]
- Sichelschmidt J.; Wykhoff J.; Krug von Nidda H.-A.; Fazlishanov I. I.; Hossain Z.; Krellner C.; Geibel C.; Steglich F. Electron spin resonance of YbIr2Si2 below the Kondo temperature. J. Phys.: Condens. Matter 2007, 19, 016211. 10.1088/0953-8984/19/1/016211. [DOI] [Google Scholar]
- Gataullin E. M.; Ivanshin V. A.; Izotov V. V.; Yavkin B. V.; Ivanshin N. A.; Sokolov D. A. Electron paramagnetic resonance in YbNiAl2 single crystals with strong magnetic anisotropy. Phys. Solid State 2017, 59, 434–437. 10.1134/S1063783417030118. [DOI] [Google Scholar]
- Torikachvili M. S.; Jia S.; Mun E. D.; Hannahs S. T.; Black R. C.; Neils W. K.; Martien D.; Bud’ko S. L.; Canfield P. C. Six closely related YbT2Zn20 (T = Fe, Co, Ru, Rh, Os, Ir) heavy fermion compounds with large local moment degeneracy. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 9960–9963. 10.1073/pnas.0702757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G.; Dunlap B. D. Nuclear moments and moment ratios as determined by Mössbauer spectroscopy. J. Phys. Chem. Ref. Data 1976, 5, 1093–1122. 10.1063/1.555541. [DOI] [Google Scholar]
- Shenoy G. K.; Wagner F. E.. Mössbauer Isomer Shifts. North-Holland Publishing: Amsterdam, 1978. [Google Scholar]
- Cadogan J. M.; Ryan D. H. An Overview of 166Er, 169Tm and 170Yb Mössbauer Spectroscopy. Hyperfine Interact. 2004, 153, 25–41. 10.1023/B:HYPE.0000024711.45868.29. [DOI] [Google Scholar]
- Gubbens P. C. M., Rare earth Mössbauer spectroscopy measurements on lanthanide intermetallics: A survey. In Handbook of Magnetic Materials, Buschow K. H. J., Ed.; Elsevier: Amsterdam, 2012; Vol. 20, pp 227–335. [Google Scholar]
- Eckert H.Mössbauer spectroscopy of mixed-valent compounds. In Mössbauer Spectroscopy Applied to Inorganic Chemistry, Long G. J., Ed.; Plenum Press: New York, 1987; Vol. 2. [Google Scholar]
- Cadogan J. M.; Ryan D. H. A study on the magnetic behaviour of polymorphic YbFe6Ge6. J. Phys.: Condens. Matter 2010, 22, 016009. 10.1088/0953-8984/22/1/016009. [DOI] [PubMed] [Google Scholar]
- Ryan D. H.; Saleema N. M.; Gagnon R.; van Lierop J. 170Yb Mössbauer study of the YbCd5.7 binary quasi-crystal and related phases. J. Phys.: Condens. Matter 2001, 13, 10159. 10.1088/0953-8984/13/45/304. [DOI] [Google Scholar]
- Solokha P.; Čurlik I.; Giovannini M.; Lee-Hone N. R.; Reiffers M.; Ryan D. H.; Saccone A. Structural and physical properties of the new intermetallic compound Yb3Pd2Sn2. J. Solid State Chem. 2011, 184, 2498–2505. 10.1016/j.jssc.2011.07.031. [DOI] [Google Scholar]
- Čurlik I.; Reiffers M.; Giovannini M.; Solokha P.; Saccone A.; Il’kovič S.; Ryan D. H. Crystal structure and physical properties of the novel stannide Yb3Pd2Sn2. J. Phys.: Conf. Series 2012, 391, 012008. 10.1088/1742-6596/391/1/012008. [DOI] [Google Scholar]
- Bonville P.; Imbert P.; Jéhanno G.; Gonzalez-Jimenez F.; Hartmann-Boutron F. Emission Mössbauer spectroscopy and relaxation measurements in hyperfine levels out of thermal equilibrium: Very-low-temperature experiments on the Kondo alloy Au170Yb. Phys. Rev. B 1984, 30, 3672–3690. 10.1103/PhysRevB.30.3672. [DOI] [Google Scholar]
- Bonville P.; Conte R. Evidence for random strain at the cubic site of Yb3+ in palladium by Mössbauer spectroscopy on 170Yb. J. Phys. (Paris) 1985, 46, 1553–1563. 10.1051/jphys:019850046090155300. [DOI] [Google Scholar]
- Bonville P.; Hodges J. A.; Imbert P.; Jéhanno G.; Jaccard D.; Sierro J. Magnetic ordering and paramagnetic relaxation of Yb3+ in YbNi2Si2. J. Magn. Magn. Mater. 1991, 97, 178–186. 10.1016/0304-8853(91)90178-D. [DOI] [Google Scholar]
- Bonville P.; Hodges J. A.; Imbert P.; Jéhanno G.; Thuéry P. A neutron diffraction and 170Yb Mössbauer study of Yb3Pd4. J. Magn. Magn. Mater. 1994, 136, 238–244. 10.1016/0304-8853(94)00325-4. [DOI] [Google Scholar]
- Bonville P.; Hodges J. A.; Hossain Z.; Nagarajan R.; Dhar S. K.; Gupta L. C.; Alleno E.; Godart C. Heavy electron YBNi2B2C and giant exchange YbNiBC: 170Yb Mössbauer spectroscopy and magnetization studies. Eur. Phys. J. B 1999, 11, 377–383. 10.1007/s100510050948. [DOI] [Google Scholar]
- Voyer C. J.; Ryan D. H.; Ahn K.; Gschneidner K. A.; Pecharsky V. K. Valence and magnetic ordering in the Yb5SixGe4–x pseudobinary system. Phys. Rev. B 2006, 73, 174422. 10.1103/PhysRevB.73.174422. [DOI] [Google Scholar]
- Bonville P.; Bauer E. A 170Yb Mössbauer spectroscopy and LIII-edge study of the Kondo lattice YbCu3Al2. J. Phys.: Condens. Matter 1996, 8, 7797–7812. 10.1088/0953-8984/8/41/023. [DOI] [Google Scholar]
- Bonville P.; Hodges J. A.; Hulliger F.; Imbert P.; Jéhanno G.; Marimon da Cunha J. B.; Ott H. R. 170Yb Mössbauer study of the heavy-electron pnictides YbP, YbAs and YbSb. J. Magn. Magn. Mater. 1988, 76–77, 473–474. 10.1016/0304-8853(88)90459-3. [DOI] [Google Scholar]
- Le Bras G.; Bonville P.; Hodges J. A.; Imbert P.; Polatsek G.; Fischer P.; Keller L.; Dönni A.; Kohgi M.; Suzuki T. 170Yb Mössbauer and neutron diffraction investigation of the antiferromagnetic phase in the Kondo lattices YbP and YbN. Physica B 1993, 190, 333–346. 10.1016/0921-4526(93)90193-A. [DOI] [Google Scholar]
- Bonville P.; Polatsek G.; Hodges J. A.; Imbert P.; LeBras G. Giant exchange fields and reduced moments in Yb Kondo lattices. Physica B 1993, 186–188, 254–257. 10.1016/0921-4526(93)90545-H. [DOI] [Google Scholar]
- Bonville P.; Abd-Elmeguid M.; Malaman B.; Ressouche E.; Sanchez J. P.; Geibel C.; Trovarelli O. Incommensurate modulated magnetic structure in the heavy electron compound YbPtAl. Physica B 2000, 281–282, 144–146. 10.1016/S0921-4526(99)00864-9. [DOI] [Google Scholar]
- Ryan D. H.; Lee-Hone N. R.; Cadogan J. M.; Ahn K.; Pecharsky V. K.; Gschneidner K. A. Doping-induced valence change in Yb5Ge4–x(Sb, Ga)x: (x ≤ 1). Hyperfine Interact. 2012, 208, 59–63. 10.1007/s10751-011-0415-4. [DOI] [Google Scholar]
- Dhar S. K.; Manfrinetti P.; Fornasini M. L.; Bonville P. Phase transition in YbAl3C3. Eur. Phys. J. B 2008, 63, 187–192. 10.1140/epjb/e2008-00241-7. [DOI] [Google Scholar]
- Dhar S. K.; Singh S.; Bonville P.; Mazumdar C.; Manfrinetti P.; Palenzona A. Magnetic behavior of Yb3Cu4Ge4 and Gd3Cu4Ge4. Physica B 2002, 312–313, 846–847. 10.1016/S0921-4526(01)01269-8. [DOI] [Google Scholar]
- Dhar S. K.; Mitra C.; Bonville P.; Rams M.; Królas K.; Godart C.; Alleno E.; Suzuki N.; Miyake K.; Watanabe N.; Onuki Y.; Manfrinetti P.; Palenzona A. Magnetic and 4f quadrupolar behavior of Yb2Co3Al9 and the Kondo lattice compound Yb2Co3Ga9. Phys. Rev. B 2001, 64, 094423. 10.1103/PhysRevB.64.094423. [DOI] [Google Scholar]
- LeBras G.; Bonville P.; Hodges J. A.; Hammann J.; Besnus M. J.; Schmerber G.; Dhar S. K.; Aliev F. G.; Andre G. Local symmetry lowering in the cubic intermetallics YbPdBi and YbNiSb. J. Phys.: Condens. Matter 1995, 7, 5665. 10.1088/0953-8984/7/28/020. [DOI] [Google Scholar]
- Mishra R.; Pöttgen R.; Hoffmann R.-D.; Fickenscher T.; Eschen M.; Trill H.; Mosel B. D. Ternary antimonides YbTSb (T = Ni, Pd, Pt, Cu, Ag, Au) – synthesis, structure, homogeneity ranges, and 121Sb Mössbauer spectroscopy. Z. Naturforsch. 2002, 57b, 1215–1223. 10.1515/znb-2002-1105. [DOI] [Google Scholar]
- Bonville P.; Imbert P.; Jehanno G. Low-temperature Mössbauer study of 170Yb in YbBe13. J. Phys. F: Metal Phys. 1986, 16, 1873–1883. 10.1088/0305-4608/16/11/022. [DOI] [Google Scholar]
- Yaouanc A.; Dalmas de Réotier P.; Bonville P.; LeBras G.; Gubbens P. C. M.; Mulders A. M.; Kunii S. Dynamical magnetic correlations in the Kondo insulator YbB12. Europhys. Lett. 1999, 47, 247–253. 10.1209/epl/i1999-00379-x. [DOI] [Google Scholar]
- Hodges J. A.; Bonville P.; Ocio M. Magnetic properties of YbNi5 from 170Yb Mössbauer and magnetisation measurements. Eur. Phys. J. B 2007, 57, 365–370. 10.1140/epjb/e2007-00184-5. [DOI] [Google Scholar]
- Knebel G.; Boursier R.; Hassinger E.; Lapertot G.; G. Niklowitz P.; Pourret A.; Salce B.; P. Sanchez J.; Sheikin I.; Bonville P.; Harima H.; Flouquet J. Localization of 4f state in YbRh2Si2 under magnetic field and high pressure: Comparison with CeRh2Si2. J. Phys. Soc. Jpn. 2006, 75, 114709. 10.1143/JPSJ.75.114709. [DOI] [Google Scholar]
- Plessel J.; Abd-Elmeguid M. M.; Sanchez J. P.; Knebel G.; Geibel C.; Trovarelli O.; Steglich F. Unusual behavior of the low-moment magnetic ground state of YbRh2Si2 under high pressure. Phys. Rev. B 2003, 67, 180403. 10.1103/PhysRevB.67.180403. [DOI] [Google Scholar]
- Schöppner M.; Moser J.; Kratzer A.; Potzel U.; Mignot J. M.; Kalvius G. M. Changes of valence state of Yb in YbCuAl at high pressure: A Mössbauer study. Z. Phys. B: Condens. Matter 1986, 63, 25–32. 10.1007/BF01312574. [DOI] [Google Scholar]
- Drescher K.; Abd-Elmeguid M. M.; Micklitz H.; Sanchez J. P. Competing anisotropies in the ferromagnetic Kondo-lattice compound YbNiSn: Observation of a complex magnetic ground state under high pressure. Phys. Rev. Lett. 1996, 77, 3228–3231. 10.1103/PhysRevLett.77.3228. [DOI] [PubMed] [Google Scholar]
- Winkelmann H.; Abd-Elmeguid M. M.; Micklitz H.; Sanchez J. P.; Geibel C.; Steglich F. Pressure-induced local moment magnetism in the nonmagnetic heavy fermion compound Yb2Ni2Al. Phys. Rev. Lett. 1998, 81, 4947–4950. 10.1103/PhysRevLett.81.4947. [DOI] [Google Scholar]
- Masuda R.; Kobayashi Y.; Kitao S.; Kurokuzu M.; Saito M.; Yoda Y.; Mitsui T.; Iga F.; Seto M. Synchrotron radiation-based Mössbauer spectra of 174Yb measured with internal conversion electrons. Appl. Phys. Lett. 2014, 104, 082411. 10.1063/1.4866280. [DOI] [Google Scholar]
- Meyer C.; Gavigan J. P.; Czjzek G.; Bornemann H. J. A study of crystal fields in Yb2Fe14B by 174Yb Mössbauer spectroscopy. Solid State Commun. 1989, 69, 83–86. 10.1016/0038-1098(89)90031-8. [DOI] [Google Scholar]
- Oura M.; Ikeda S.; Masuda R.; Kobayashi Y.; Seto M.; Yoda Y.; Hirao N.; Kawaguchi S. I.; Ohishi Y.; Suzuki S.; Kuga K.; Nakatsuji S.; Kobayashi H. Valence fluctuating compound α-YbAlB4 studied by 174Yb Mössbauer spectroscopy and X-ray diffraction using synchrotron radiation. Physica B 2018, 536, 162–164. 10.1016/j.physb.2017.09.035. [DOI] [Google Scholar]
- Snyder G. J.; Toberer E. S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. 10.1038/nmat2090. [DOI] [PubMed] [Google Scholar]
- Gayner C.; Kar K. K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. 10.1016/j.pmatsci.2016.07.002. [DOI] [Google Scholar]
- Orr B.; Akbarzadeh A.; Mochizuki M.; Singh R. A review of car waste heat recovery systems utilising thermoelectric generators and heat pipes. Appl. Therm. Eng. 2016, 101, 490–495. 10.1016/j.applthermaleng.2015.10.081. [DOI] [Google Scholar]
- Liu W.; Hu J.; Zhang S.; Deng M.; Han C.-G.; Liu Y. New trends, strategies and opportunities in thermoelectric materials: A perspective. Mater. Today Phys. 2017, 1, 50–60. 10.1016/j.mtphys.2017.06.001. [DOI] [Google Scholar]
- Kauzlarich S. M.; Zevalkink A.; Toberer E.; Snyder G. J.. Zintl phases: recent developments in thermoelectrics and future outlook. In Thermoelectric Materials and Devices; The Royal Society of Chemistry: Cambridge, England, 2017; pp 1–26. [Google Scholar]
- Shuai J.; Mao J.; Song S.; Zhang Q.; Chen G.; Ren Z. Recent progress and future challenges on thermoelectric Zintl materials. Mater. Today Phys. 2017, 1, 74–95. 10.1016/j.mtphys.2017.06.003. [DOI] [Google Scholar]
- van Daal H. J.; van Aken P. B.; Buschow K. H. J. The Seebeck coefficient of YbAl2 and YbAl3. Phys. Lett. A 1974, 49, 246–248. 10.1016/0375-9601(74)90870-6. [DOI] [Google Scholar]
- Tanusilp S.-A.; Ohishi Y.; Muta H.; Yamanaka S.; Nishide A.; Hayakawa J.; Kurosaki K. Ytterbium silicide (YbSi2): A promising thermoelectric material with a high power factor at room temperature. Phys. Status Solidi RRL 2018, 12, 1700372. 10.1002/pssr.201700372. [DOI] [Google Scholar]
- Massalski T. B.; Okamoto H.; Subramanian P. R.; Kacprzak L.. Binary alloy phase diagrams, 2nd Ed. ASM International: Ohio, U.S.A., 1990. [Google Scholar]
- Pope A. L.; Tritt T. M.; Gagnon R.; Strom-Olsen J. Electronic transport in Cd–Yb and Y–Mg–Zn quasicrystals. Appl. Phys. Lett. 2001, 79, 2345–2347. 10.1063/1.1406555. [DOI] [Google Scholar]
- Kuo Y. K.; Lai J. R.; Huang C. H.; Ku W. C.; Lue C. S.; Lin S. T. Thermoelectric properties of binary Cd-Yb quasicrystals and Cd6Yb. J. Appl. Phys. 2004, 95, 1900–1905. 10.1063/1.1642282. [DOI] [Google Scholar]
- Iizuka T.; Hori T.; Matsunami M.; Takeuchi T. Thermoelectric properties of Yb5Si3. Jpn. J. Appl. Phys. 2020, 59, 010902. 10.7567/1347-4065/ab5b85. [DOI] [Google Scholar]
- Lehr G. J.; Morelli D. T.; Jin H.; Heremans J. P. Enhanced thermoelectric power factor in Yb1–xScxAl2 alloys using chemical pressure tuning of the Yb valence. J. Appl. Phys. 2013, 114, 223712. 10.1063/1.4842795. [DOI] [Google Scholar]
- Itoh Y.; Kadomatsu H.; Sakurai J.; Fujiwara H. Transport properties of YbxIn1–xCu2. Phys. Status Solidi A 1990, 118, 513–517. 10.1002/pssa.2211180223. [DOI] [Google Scholar]
- Adroja D. T.; Rainford B. D.; Takabatake T. Crystal field excitations in the ferromagnetic Kondo lattice: YbNiSn. Physica B 1998, 253, 269–277. 10.1016/S0921-4526(98)00397-4. [DOI] [Google Scholar]
- Itoh Y.; Kadomatsu H. Electrical and magnetic properties of YbPdGe and YbPtGe. J. Alloys Compd. 1998, 280, 39–43. 10.1016/S0925-8388(98)00744-0. [DOI] [Google Scholar]
- Guner S.; Budak S.; Muntele C. I.; Ila D. Thermoelectric properties of YbBiPt and YBiPt thin films. Mater. Res. Soc. Online Proc. Lib. 2008, 1100, 6. 10.1557/PROC-1100-JJ04-22. [DOI] [Google Scholar]
- Gegenwart P.; Tokiwa Y.; Westerkamp T.; Weickert F.; Custers J.; Ferstl J.; Krellner C.; Geibel C.; Kerschl P.; Müller K. H.; Steglich F. High-field phase diagram of the heavy-fermion metal YbRh2Si2. New J. Phys. 2006, 8, 171. 10.1088/1367-2630/8/9/171. [DOI] [Google Scholar]
- Trovarelli O.; Geibel C.; Mederle S.; Langhammer C.; Grosche F. M.; Gegenwart P.; Lang M.; Sparn G.; Steglich F. YbRh2Si2: pronounced non-Fermi-liquid effects above a low-lying magnetic phase transition. Phys. Rev. Lett. 2000, 85, 626–629. 10.1103/PhysRevLett.85.626. [DOI] [PubMed] [Google Scholar]
- Behnia K.; Jaccard D.; Flouquet J. On the thermoelectricity of correlated electrons in the zero-temperature limit. J. Phys.: Condens. Matter 2004, 16, 5187. 10.1088/0953-8984/16/28/037. [DOI] [Google Scholar]
- Pourret A.; Knebel G.; D. Matsuda T.; Lapertot G.; Flouquet J. Magnetic polarization and Fermi surface instability: case of YbRh2Si2. J. Phys. Soc. Jpn. 2013, 82, 053704. 10.7566/JPSJ.82.053704. [DOI] [Google Scholar]
- Stockert U.; Klingner C.; Krellner C.; Zlatić V.; Geibel C.; Steglich F. Thermopower evolution in Yb(Rh1-xCox)2Si2 upon 4f localization. J. Low Temp. Phys. 2019, 196, 364–374. 10.1007/s10909-019-02187-6. [DOI] [Google Scholar]
- Klingner C.; Krellner C.; Brando M.; Geibel C.; Steglich F.; Vyalikh D. V.; Kummer K.; Danzenbächer S.; Molodtsov S. L.; Laubschat C.; Kinoshita T.; Kato Y.; Muro T. Evolution of magnetism in Yb(Rh1–xCox)2Si2. Phys. Rev. B 2011, 83, 144405. 10.1103/PhysRevB.83.144405. [DOI] [Google Scholar]
- Machida Y.; Tomokuni K.; Ogura C.; Izawa K.; Kuga K.; Nakatsuji S.; Lapertot G.; Knebel G.; Brison J. P.; Flouquet J. Thermoelectric response near a quantum critical point of β-YbAlB4 and YbRh2Si2: a comparative study. Phys. Rev. Lett. 2012, 109, 156405. 10.1103/PhysRevLett.109.156405. [DOI] [PubMed] [Google Scholar]
- Polatsek G.; Bonville P. Interpretation of neutron magnetic scattering in the Kondo lattices YbPd2Si2 and YbAgCu4. Z. Phys. B: Condens. Matter 1992, 88, 189–193. 10.1007/BF01323571. [DOI] [Google Scholar]
- Bonville P.; Hammann J.; Hodges J.; Imbert P.; Jéhanno G.; Besnus M.; Meyer A. Hybridization and crystal field in YbPd2Si2. Physica B 1991, 171, 171–174. 10.1016/0921-4526(91)90512-D. [DOI] [Google Scholar]
- Dhar S. K.; Sampathkumaran E. V.; Vijayaraghavan R.; Kuentzler R. YbPd2Si2, A moderate heavy fermion system. Solid State Commun. 1987, 61, 479–481. 10.1016/0038-1098(87)90495-9. [DOI] [Google Scholar]
- Casanova R.; Jaccard D.; Marcenat C.; Hamdaoui N.; Besnus M. J. Thermoelectric power of YbMCu4 (M = Ag, Au and Pd) and YbPd2Si2. J. Magn. Magn. Mater. 1990, 90–91, 587–588. 10.1016/S0304-8853(10)80217-3. [DOI] [Google Scholar]
- Alami-Yadri K.; Jaccard D.; Andreica D. Thermopower of Yb heavy fermion compounds at high pressure. J. Low Temp. Phys. 1999, 114, 135–149. 10.1023/A:1021801903961. [DOI] [Google Scholar]
- Duc Dung N.; D. Matsuda T.; Haga Y.; Ikeda S.; Yamamoto E.; Ishikura T.; Endo T.; Tatsuoka S.; Aoki Y.; Sato H.; Takeuchi T.; Settai R.; Harima H.; Onuki Y. de Haas–van Alphen effect and Fermi surface properties in high-quality single crystals YbCu2Si2 and YbCu2Ge2. J. Phys. Soc. Jpn. 2009, 78, 084711. 10.1143/JPSJ.78.084711. [DOI] [Google Scholar]
- Alami-Yadri K.; Wilhelm H.; Jaccard D. Pressure-induced magnetically ordered Kondo lattice state in YbCu2Si2. Eur. Phys. J. B 1998, 6, 5–11. 10.1007/s100510050520. [DOI] [Google Scholar]
- Nikiforov V. N.; Pryadun V. V.; Gribanov A. V.; Irkhin V. Y. Electronic properties of the ternary system YbPd2Ge2–x. Physica B 2018, 545, 312–314. 10.1016/j.physb.2018.06.034. [DOI] [Google Scholar]
- Lehr G. J.; Morelli D. T. Interplay of chemical expansion, Yb valence, and low temperature thermoelectricity in the YbCu2Si2–xGex solid solution. J. Appl. Phys. 2015, 117, 135101. 10.1063/1.4916786. [DOI] [Google Scholar]
- Lehr G. J.; Morelli D. T.; Jin H.; Heremans J. P. YbCu2Si2–LaCu2Si2 solid solutions with enhanced thermoelectric power factors. J. Electron. Mater. 2015, 44, 1663–1667. 10.1007/s11664-014-3509-3. [DOI] [Google Scholar]
- Machida Y.; Ogura C.; Izawa K.; Kuga K.; Nakatsuji S. Low-temperature thermal transport coefficients of heavy fermion β-YbAlB4. J. Phys.: Conf. Series 2011, 273, 012005. 10.1088/1742-6596/273/1/012005. [DOI] [Google Scholar]
- Mun E. D.; Jia S.; Bud’ko S. L.; Canfield P. C. Thermoelectric power of the YbT2Zn20 (T = Fe, Ru, Os, Ir, Rh, and Co) heavy fermions. Phys. Rev. B 2012, 86, 115110. 10.1103/PhysRevB.86.115110. [DOI] [Google Scholar]
- Wei K.; Neu J. N.; Lai Y.; Chen K.-W.; Hobbis D.; Nolas G. S.; Graf D. E.; Siegrist T.; Baumbach R. E. Enhanced thermoelectric performance of heavy-fermion compounds YbTM2Zn20 (TM = Co, Rh, Ir) at low temperatures. Sci. Adv. 2019, 5, eaaw6183 10.1126/sciadv.aaw6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M.; Hartmann S.; Macovei M. E.; Oeschler N.; Nicklas M.; Geibel C. Investigation of Yb2Pt6Al15 single crystals: heavy fermion system with a large local moment degeneracy. New J. Phys. 2008, 10, 093017. 10.1088/1367-2630/10/9/093017. [DOI] [Google Scholar]
- Trovarelli O.; Geibel C.; Buschinger B.; Borth R.; Mederle S.; Grosche M.; Sparn G.; Steglich F.; Brosch O.; Donnevert L. Magnetic, transport, and thermal properties of Yb2T3X9 compounds (T = Rh, Ir; X = Al, Ga). Phys. Rev. B 1999, 60, 1136–1143. 10.1103/PhysRevB.60.1136. [DOI] [Google Scholar]
- Muro Y.; Yamane K.; Kim M.-S.; Takabatake T.; Godart C.; Rogl P. Magnetic and thermoelectric properties of a heterogeneous mixed-valence system, Yb2Pt3Sn5. J. Phys. Soc. Jpn. 2003, 72, 1745–1750. 10.1143/JPSJ.72.1745. [DOI] [Google Scholar]
- Jang S.; White B. D.; Ho P. C.; Kanchanavatee N.; Janoschek M.; Hamlin J. J.; Maple M. B. Crossover between Fermi liquid and non-Fermi liquid behavior in the non-centrosymmetric compound Yb2Ni12P7. J. Phys.: Condens. Matter 2014, 26, 425601. 10.1088/0953-8984/26/42/425601. [DOI] [PubMed] [Google Scholar]
- Brown S. R.; Kauzlarich S. M.; Gascoin F.; Snyder G. J. Yb14MnSb11: New high efficiency thermoelectric material for power generation. Chem. Mater. 2006, 18, 1873–1877. 10.1021/cm060261t. [DOI] [Google Scholar]
- Cox C. A.; Brown S. R.; Snyder G. J.; Kauzlarich S. M. Effect of Ca doping on the thermoelectric performance of Yb14MnSb11. J. Electron. Mater. 2010, 39, 1373–1375. 10.1007/s11664-010-1149-9. [DOI] [Google Scholar]
- Uvarov C. A.; Ortega-Alvarez F.; Kauzlarich S. M. Enhanced High-Temperature Thermoelectric Performance of Yb14–xCaxMnSb11. Inorg. Chem. 2012, 51, 7617–7624. 10.1021/ic300567c. [DOI] [PubMed] [Google Scholar]
- Grebenkemper J. H.; Klemenz S.; Albert B.; Bux S. K.; Kauzlarich S. M. Effects of Sc and Y substitution on the structure and thermoelectric properties of Yb14MnSb11. J. Solid State Chem. 2016, 242, 55–61. 10.1016/j.jssc.2016.03.015. [DOI] [Google Scholar]
- Toberer E. S.; Brown S. R.; Ikeda T.; Kauzlarich S. M.; Snyder J. G. High thermoelectric efficiency in lanthanum doped Yb14MnSb11. Appl. Phys. Lett. 2008, 93, 123–128. 10.1063/1.2970089. [DOI] [Google Scholar]
- Yu C.; Chen Y.; Xie H. H.; Snyder G. J.; Fu C. G.; Xu J. S.; Zhao X. B.; Zhu T. J. Improved Thermoelectric Properties in Lu-doped Yb14MnSb11 Zintl Compounds. Appl. Phys. Express 2012, 5, 031801. 10.1143/APEX.5.031801. [DOI] [Google Scholar]
- Justl A. P.; Bux S. K.; Kauzlarich S. M. Evolution of Thermoelectric and Oxidation Properties in Lu-Substituted Yb14MnSb11. ACS Appl. Energy Mater. 2023, 6, 471–483. 10.1021/acsaem.2c03464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyeva I. G.; Nikolaev R. E.; Abdusaljamova M. N.; Kauzlarich S. M. Thermochemistry study and improved thermal stability of Yb14MnSb11 alloyed by Ln3+ (La–Lu). J. Mater. Chem. C 2016, 4, 3342–3348. 10.1039/C6TC00178E. [DOI] [Google Scholar]
- Brown S. R.; Toberer E. S.; Ikeda T.; Cox C. A.; Gascoin F.; Kauzlarich S. M.; Snyder G. J. Improved thermoelectric performance in Yb14Mn1–xZnxSb11 by the reduction of spin-disorder scattering. Chem. Mater. 2008, 20, 3412–3419. 10.1021/cm703616q. [DOI] [Google Scholar]
- Toberer E. S.; Cox C. A.; Brown S. R.; Ikeda T.; May A. F.; Kauzlarich S. M.; Snyder G. J. Traversing the metal-insulator transition in a Zintl phase: Rational enhancement of thermoelectric efficiency in Yb14Mn1–xAlxSb11. Adv. Funct. Mater. 2008, 18, 2795–2800. 10.1002/adfm.200800298. [DOI] [Google Scholar]
- Cox C. A.; Toberer E. S.; Levchenko A. A.; Brown S. R.; Snyder G. J.; Navrotsky A.; Kauzlarich S. M. Structure, heat capacity, and high-temperature thermal properties of Yb14Mn1–xAlxSb11. Chem. Mater. 2009, 21, 1354–1360. 10.1021/cm803252r. [DOI] [Google Scholar]
- Star K.; Zevalkink A.; Huang C.-K.; Dunn B.; Fleurial J.-P. Synthesis and thermoelectric properties of doped Yb14MnSb11-xBix Zintls. MRS Online Proceedings Library (OPL) 2010, 1267 (1267), DD1203–1205. 10.1557/PROC-1267-DD03-05. [DOI] [Google Scholar]
- Yi T.; Abdusalyamova M. N.; Makhmudov F.; Kauzlarich S. M. Magnetic and transport properties of Te doped Yb14MnSb11. J. Mater. Chem. 2012, 22, 14378–14384. 10.1039/c2jm32089d. [DOI] [Google Scholar]
- Ribeiro R. A.; Hadano Y.; Narazu S.; Suekuni K.; Avila M. A.; Takabatake T. Low-temperature thermoelectric properties of Yb14MSb11 (M = Mn, Zn). J. Phys.: Condens. Matter 2007, 19, 376211. 10.1088/0953-8984/19/37/376211. [DOI] [Google Scholar]
- Akrap A.; Barišić N.; Forró L.; Mandrus D.; Sales B. C. High-pressure resistivity and thermoelectric power in Yb14MnSb11. Phys. Rev. B 2007, 76, 085203. 10.1103/PhysRevB.76.085203. [DOI] [Google Scholar]
- Hu Y.; Wang J.; Kawamura A.; Kovnir K.; Kauzlarich S. M. Yb14MgSb11 and Ca14MgSb11 – New Mg-containing Zintl compounds and their structures, bonding, and thermoelectric properties. Chem. Mater. 2015, 27, 343–351. 10.1021/cm504059t. [DOI] [Google Scholar]
- Perez C. J.; Qi X.; Chen Z.; Bux S. K.; Chanakain S.; Li B.; Liu K.; Dhall R.; Bustillo K. C.; Kauzlarich S. M. Improved power factor and mechanical properties of composites of Yb14MgSb11 with iron. ACS Appl. Energy Mater. 2020, 3, 2147–2159. 10.1021/acsaem.9b02168. [DOI] [Google Scholar]
- Perez C. J.; Chen Z.; Beeson W. B.; Chanakian S.; Liu K.; Bux S. K.; Kauzlarich S. M. Chemical route to Yb14MgSb11 composites with nanosized iron inclusions for the reduction of thermal conductivity. ACS Appl. Energy Mater. 2021, 4, 3748–3756. 10.1021/acsaem.1c00163. [DOI] [Google Scholar]
- Hu Y.; Kauzlarich S. M. Yb14MgBi11: structure, thermoelectric properties and the effect of the structure on low lattice thermal conductivity. Dalton Trans. 2017, 46, 3996–4003. 10.1039/C7DT00183E. [DOI] [PubMed] [Google Scholar]
- Gascoin F.; Ottensmann S.; Stark D.; Haïle S. M.; Snyder G. J. Zintl phases as thermoelectric materials: tuned transport properties of the compounds CaxYb1–xZn2Sb2. Adv. Funct. Mater. 2005, 15, 1860–1864. 10.1002/adfm.200500043. [DOI] [Google Scholar]
- Shuai J.; Wang Y.; Liu Z.; Kim H. S.; Mao J.; Sui J.; Ren Z. Enhancement of thermoelectric performance of phase pure Zintl compounds Ca1–xYbxZn2Sb2, Ca1–xEuxZn2Sb2, and Eu1–xYbxZn2Sb2 by mechanical alloying and hot pressing. Nano Energy 2016, 25, 136–144. 10.1016/j.nanoen.2016.04.023. [DOI] [Google Scholar]
- Takagiwa Y.; Sato Y.; Zevalkink A.; Kanazawa I.; Kimura K.; Isoda Y.; Shinohara Y. Thermoelectric properties of EuZn2Sb2 Zintl compounds: zT enhancement through Yb substitution for Eu. J. Alloys Compd. 2017, 703, 73–79. 10.1016/j.jallcom.2017.01.350. [DOI] [Google Scholar]
- Zhang X.; Peng K.; Guo L.; Yan Y.; Zhan H.; Lu X.; Gu H.; Zhou X. Effects of lanthanum substitution on thermoelectric properties of YbZn2Sb2. J. Electron. Mater. 2017, 46, 2611–2615. 10.1007/s11664-016-4824-7. [DOI] [Google Scholar]
- May A. F.; McGuire M. A.; Ma J.; Delaire O.; Huq A.; Custelcean R. Properties of single crystalline AZn2Sb2 (A = Ca, Eu, Yb). J. Appl. Phys. 2012, 111 (3), 033708. 10.1063/1.3681817. [DOI] [Google Scholar]
- Zhao W.-Y.; Liang Z.; Wei P.; Yu J.; Zhang Q.-J.; Shao G.-S. Enhanced thermoelectric performance via randomly arranged nanopores: Excellent transport properties of YbZn2Sb2 nanoporous materials. Acta Mater. 2012, 60, 1741–1746. 10.1016/j.actamat.2011.11.056. [DOI] [Google Scholar]
- Zevalkink A.; Zeier W. G.; Cheng E.; Snyder J.; Fleurial J.-P.; Bux S. Nonstoichiometry in the Zintl phase Yb1−δZn2Sb2 as a route to thermoelectric optimization. Chem. Mater. 2014, 26, 5710–5717. 10.1021/cm502588r. [DOI] [Google Scholar]
- Yang X.; Gu Y.; Li Y.; Guo K.; Zhang J.; Zhao J.-T. The equivalent and aliovalent dopants boosting the thermoelectric properties of YbMg2Sb2. Sci. China Mater. 2020, 63, 437–443. 10.1007/s40843-019-1199-4. [DOI] [Google Scholar]
- Yu C.; Zhu T. J.; Zhang S. N.; Zhao X. B.; He J.; Su Z.; Tritt T. M. Improved thermoelectric performance in the Zintl phase compounds YbZn2–xMnxSb2 via isoelectronic substitution in the anionic framework. J. Appl. Phys. 2008, 104 (1), 013705. 10.1063/1.2939372. [DOI] [Google Scholar]
- Zhu T. J.; Yu C.; He J.; Zhang S. N.; Zhao X. B.; Tritt T. M. Thermoelectric properties of Zintl compound YbZn2Sb2 with Mn substitution in anionic framework. J. Electron. Mater. 2009, 38, 1068–1071. 10.1007/s11664-009-0667-9. [DOI] [Google Scholar]
- Wang X.-J.; Tang M.-B.; Chen H.-H.; Yang X.-X.; Zhao J.-T.; Burkhardt U.; Grin Y. Synthesis and high thermoelectric efficiency of Zintl phase YbCd2–xZnxSb2. Appl. Phys. Lett. 2009, 94 (9), 092106. 10.1063/1.3040321. [DOI] [Google Scholar]
- Zhang X.; Gu H.; Zhang Y.; Guo L.; Yang J.; Luo S.; Lu X.; Chen K.; Chai H.; Wang G.; Zhang X.; Zhou X. Enhanced thermoelectric properties of YbZn2Sb2–xBix through a synergistic effect via Bi-doping. Chem. Eng. J. 2019, 374, 589–595. 10.1016/j.cej.2019.05.206. [DOI] [Google Scholar]
- Zhang Z.; Yan Y.; Li X.; Wang X.; Li J.; Chen C.; Cao F.; Sui J.; Lin X.; Liu X.; Xie G.; Zhang Q. A dual role by incorporation of magnesium in YbZn2Sb2 Zintl phase for enhanced thermoelectric performance. Adv. Energy Mater. 2020, 10 (29), 2001229. 10.1002/aenm.202001229. [DOI] [Google Scholar]
- Zhou T.; Feng Z.; Mao J.; Jiang J.; Zhu H.; Singh D. J.; Wang C.; Ren Z. Thermoelectric properties of Zintl phase YbMg2Sb2. Chem. Mater. 2020, 32, 776–784. 10.1021/acs.chemmater.9b04131. [DOI] [Google Scholar]
- Zhou T.; Mao J.; Jiang J.; Song S.; Zhu H.; Zhu Q.; Zhang Q.; Ren W.; Wang Z.; Wang C.; Ren Z. Large reduction of thermal conductivity leading to enhanced thermoelectric performance in p-type Mg3Bi2–YbMg2Bi2 solid solutions. J. Mater. Chem. C 2019, 7, 434–440. 10.1039/C8TC05424J. [DOI] [Google Scholar]
- May A. F.; McGuire M. A.; Singh D. J.; Ma J.; Delaire O.; Huq A.; Cai W.; Wang H. Thermoelectric transport properties of CaMg2Bi2, EuMg2Bi2, and YbMg2Bi2. Phys. Rev. B 2012, 85, 035202. 10.1103/PhysRevB.85.035202. [DOI] [Google Scholar]
- Kundu A. K.; Roy T.; Pakhira S.; Wu Z.-B.; Tsujikawa M.; Shirai M.; Johnston D. C.; Pasupathy A. N.; Valla T. Topological electronic structure of YbMg2Bi2 and CaMg2Bi2. npj Quantum Mater. 2022, 7 (1), 67. 10.1038/s41535-022-00474-2. [DOI] [Google Scholar]
- Nikiforov V. N.; Pryadun V. V.; Morozkin A. V.; Irkhin V. Y. Anomalies of transport properties in antiferromagnetic YbMn2Sb2 compound. Phys. Lett. A 2014, 378, 1425–1427. 10.1016/j.physleta.2014.03.030. [DOI] [Google Scholar]
- Guo K.; Cao Q.-G.; Feng X.-J.; Tang M.-B.; Chen H.-H.; Guo X.; Chen L.; Grin Y.; Zhao J.-T. Enhanced thermoelectric figure of merit of Zintl phase YbCd2–xMnxSb2 by chemical substitution. Eur. J. Inorg. Chem. 2011, 2011, 4043–4048. 10.1002/ejic.201100282. [DOI] [Google Scholar]
- Dolyniuk J.-A.; Owens-Baird B.; Wang J.; Zaikina J. V.; Kovnir K. Clathrate thermoelectrics. Mater. Sci. Eng.: R: Rep. 2016, 108, 1–46. 10.1016/j.mser.2016.08.001. [DOI] [Google Scholar]
- Liu L.; Li F.; Wei Y.; Chen N.; Bi S.; Qiu H.; Cao G.; Li Y. Synthesis and thermoelectric properties of rare earth Yb-doped Ba8–xYbxSi30Ga16 clathrates. J. Alloys Compd. 2014, 588, 271–276. 10.1016/j.jallcom.2013.10.219. [DOI] [Google Scholar]
- Tang X.; Li P.; Deng S.; Zhang Q. High temperature thermoelectric transport properties of double-atom-filled clathrate compounds YbxBa8–xGa16Ge30. J. Appl. Phys. 2008, 104 (1), 013706. 10.1063/1.2951888. [DOI] [Google Scholar]
- Chen C.; Zhang L.; Dong J.; Xu B. Thermoelectric performance of Yb-doped Ba8Ni0.1Zn0.54Ga13.8Ge31.56 type-I clathrate synthesized by high-pressure technique. J. Electron. Mater. 2017, 46, 2860–2866. 10.1007/s11664-016-5013-4. [DOI] [Google Scholar]
- Tsujii N. Preparation and thermoelectric properties of Yb-doped Si clathrates. J. Jpn. Soc. Powder Powder Metall. 2020, 67, 529–535. 10.2497/jjspm.67.529. [DOI] [Google Scholar]
- Koga K.; Anno H.; Akai K.; Matsuura M.; Matsubara K. First-Principles Study of Electronic Structure and Thermoelectric Properties for Guest Substituted Clathrate Compounds Ba6R2Au6Ge40 (R = Eu or Yb). Mater. Trans. 2007, 48, 2108–2113. 10.2320/matertrans.E-MRA2007865. [DOI] [Google Scholar]
- Shen L.; Chen J.; Li D.; Liu W.; Ge W.; Deng S. Preparation and thermoelectric properties of Yb doped type-VIII Ba8–xYbxGa16Sn30 clathrate. Mater. Rep. 2020, 34, 8136–8140. 10.11896/cldb.19030096. [DOI] [Google Scholar]
- Jeitschko W.; Braun D. LaFe4P12 with filled CoAs3-type structure and isotypic lanthanoid-transition metal polyphosphides. Acta Crystallogr. 1977, B33, 3401–3406. 10.1107/S056774087701108X. [DOI] [Google Scholar]
- Braun D. J.; Jeitschko W. Preparation and structural investigations of antimonides with the LaFe4P12 structure. J. Less-Common Met. 1980, 72, 147–156. 10.1016/0022-5088(80)90260-X. [DOI] [Google Scholar]
- Braun D. J.; Jeitschko W. Ternary arsenides with LaFe4P12-type structure. J. Solid State Chem. 1980, 32, 357–363. 10.1016/S0022-4596(80)80031-4. [DOI] [Google Scholar]
- Braun D. J.; Jeitschko W. Thorium-containing pnictides with the LaFe4P12 structure. J. Less-Common Met. 1980, 76, 33–40. 10.1016/0022-5088(80)90007-7. [DOI] [Google Scholar]
- Sales B. C.; Mandrus D.; Williams R. K. Filled Skutterudite Antimonides: A New Class of Thermoelectric Materials. Science 1996, 272, 1325–1328. 10.1126/science.272.5266.1325. [DOI] [PubMed] [Google Scholar]
- Fleurial J.-P.; Borshchevsky A.; Caillat T.. High Figure of Merit in Ce-Filled Skutterudites. In Proceedings of 15th International Conference on Thermoelectrics, Pasadena, CA, USA, 1996; pp 91–95.
- Sales B. C.Filled Skutterudites. In Handbook on the Physics and Chemistry of Rare Earths, Gschneidner K. A.; Bünzli J. C. G.; Pecharsky V. K., Eds.; Elsevier, 2003; Vol. 33, pp 1–34. [Google Scholar]
- Uher C.Skutterudites: Prospective novel thermoelectrics. In Semiconductors and Semimetals, Tritt T. M., Ed.; Elsevier: Amsterdam, 2001; Vol. 69, pp 139–253. [Google Scholar]
- Dilley N. R.; Freeman E. J.; Bauer E. D.; Maple M. B. Intermediate valence in the filled skutterudite compound YbFe4Sb12. Phys. Rev. B 1998, 58, 6287–6290. 10.1103/PhysRevB.58.6287. [DOI] [Google Scholar]
- Leithe-Jasper A.; Kaczorowski D.; Rogl P.; Bogner J.; Reissner M.; Steiner W.; Wiesinger G.; Godart C. Synthesis, crystal-structure determination and physical properties of YbFe4Sb12. Solid State Commun. 1999, 109, 395–400. 10.1016/S0038-1098(98)00556-0. [DOI] [Google Scholar]
- Dilley N. R.; Bauer E. D.; Maple M. B.; Dordevic S.; Basov D. N.; Freibert F.; Darling T. W.; Migliori A.; Chakoumakos B. C.; Sales B. C. Thermoelectric and optical properties of the filled skutterudite YbFe4Sb12. Phys. Rev. B 2000, 61, 4608–4614. 10.1103/PhysRevB.61.4608. [DOI] [Google Scholar]
- Kuznetsov V. L.; Rowe D. M. High temperature electrical transport properties of the EuFe4Sb12 and YbFe4Sb12 filled skutterudites. J. Phys.: Condens. Matter 2000, 12, 7915. 10.1088/0953-8984/12/36/306. [DOI] [Google Scholar]
- Hiroaki A.; Jiro N.; Kakuei M.. Electronic and thermoelectric properties of YbyFe4–xNixSb12 filled skutterudites. Proceeding of the 21th International Conference on the Thermoelectrics, Long Beach, CA, USA, 2002; pp 56–59.
- Bauer E.; Galatanu A.; Michor H.; Hilscher G.; Rogl P.; Boulet P.; Noël H. Physical properties of skutterudites YbxM4Sb12, M = Fe, Co, Rh, Ir. Eur. Phys. J. B 2000, 14, 483–493. 10.1007/s100510051057. [DOI] [Google Scholar]
- Dilley N. R.; Bauer E. D.; Maple M. B.; Sales B. C. Thermoelectric properties of chemically substituted skutterudites YbyCo4SnxSb12–x. J. Appl. Phys. 2000, 88, 1948–1951. 10.1063/1.1305837. [DOI] [Google Scholar]
- Yang J.; Morelli D. T.; Meisner G. P.; Chen W.; Dyck J. S.; Uher C. Effect of Sn substituting for Sb on the low-temperature transport properties of ytterbium-filled skutterudites. Phys. Rev. B 2003, 67, 165207. 10.1103/PhysRevB.67.165207. [DOI] [Google Scholar]
- Shi X.; Kong H.; Li C. P.; Uher C.; Yang J.; Salvador J. R.; Wang H.; Chen L.; Zhang W. Low thermal conductivity and high thermoelectric figure of merit in n-type BaxYbyCo4Sb12 double-filled skutterudites. Appl. Phys. Lett. 2008, 92, 182101. 10.1063/1.2920210. [DOI] [Google Scholar]
- Elsheikh M. H.; Sabri M. F. M.; Said S. M.; Miyazaki Y.; Masjuki H. H.; Shnawah D. A.; Naito S.; Bashir M. B. A. Rapid preparation of bulk AlxYb0.25Co4Sb12 (x = 0, 0.1, 0.2, 0.3) skutterudite thermoelectric materials with high figure of merit ZT = 1.36. J. Mater. Sci. 2017, 52, 5324–5332. 10.1007/s10853-017-0772-8. [DOI] [Google Scholar]
- Yang J.; Hao Q.; Wang H.; Lan Y. C.; He Q. Y.; Minnich A.; Wang D. Z.; Harriman J. A.; Varki V. M.; Dresselhaus M. S.; Chen G.; Ren Z. F. Solubility study of Yb in n-type skutterudites YbxCo4Sb12 and their enhanced thermoelectric properties. Phys. Rev. B 2009, 80, 115329. 10.1103/PhysRevB.80.115329. [DOI] [Google Scholar]
- Hanus R.; Guo X.; Tang Y.; Li G.; Snyder G. J.; Zeier W. G. A chemical understanding of the band convergence in thermoelectric CoSb3 skutterudites: Influence of electron population, local thermal expansion, and bonding interactions. Chem. Mater. 2017, 29, 1156–1164. 10.1021/acs.chemmater.6b04506. [DOI] [Google Scholar]
- Li W.; Wang J.; Xie Y.; Gray J. L.; Heremans J. J.; Kang H. B.; Poudel B.; Huxtable S. T.; Priya S. Enhanced thermoelectric performance of Yb-single-filled skutterudite by ultralow thermal conductivity. Chem. Mater. 2019, 31, 862–872. 10.1021/acs.chemmater.8b03994. [DOI] [Google Scholar]
- Ryll B.; Schmitz A.; de Boor J.; Franz A.; Whitfield P. S.; Reehuis M.; Hoser A.; Müller E.; Habicht K.; Fritsch K. Structure, phase composition, and thermoelectric properties of YbxCo4Sb12 and their dependence on synthesis method. ACS Appl. Energy Mater. 2018, 1, 113–122. 10.1021/acsaem.7b00015. [DOI] [Google Scholar]
- Qin D.; Cui B.; Zhu J.; Shi W.; Qin H.; Guo F.; Cao J.; Cai W.; Sui J. Enhanced thermoelectric and mechanical performance in n-type Yb-filled skutterudites through aluminum alloying. ACS Appl. Mater. Interface 2020, 12, 12930–12937. 10.1021/acsami.0c01798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Villars P.; Cenzual K.. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds; ASM International: Materials Park, Ohio, USA, release 2022/2023.
Data Availability Statement
The data underlying this study are available in the published article.










