Abstract
Background:
The BREAST-Q is an important tool for evaluating patient satisfaction and quality of life in breast-conserving therapy (BCT) patients, but its clinical utility is limited by the lack of guidance on score interpretation. This study determines reference values and the minimal important difference (MID) for the BREAST-Q BCT module.
Methods:
A retrospective review of BCT patients at Memorial Sloan Kettering Cancer Center from 01/2011–12/2021 was performed. Descriptive statistics were used to summarize median BREAST-Q scores. Distribution-based analyses estimated MIDs based on 0.2 standard deviation of baseline BREAST-Q scores and 0.2 standardized response mean of the difference between baseline and 1-year postoperative BREAST-Q scores. MIDs for different clinical groupings based on BMI, radiation, and re-excision were also estimated.
Results:
Overall, 8060 patients were included for determining reference values, and 5673 patients were included for estimating MIDs. Median BREAST-Q scores trended upwards and stabilized by 2 years after surgery for all domains except Physical Well-Being of the Chest, which decreased and stabilized by 2 years. A score interpretation tool, the Real-Time Engagement and Communication Tool (REACT), was created based on 25th percentile, median, and 75th percentile scores trajectories. All MID estimates ranged from 3 to 5 points; 4 points was determined to be appropriate for use in clinical practice and research.
Conclusions:
Reference values and MIDs are crucial to BREAST-Q score interpretation, which can lead to improved clinical evaluation and decision making, and improved research methodology. Future research should validate this study’s findings in different patient cohorts.
Keywords: BREAST-Q, patient-reported outcomes, breast-conserving therapy, minimal important difference, breast cancer, breast surgery, quality of life
INTRODUCTION
Patient satisfaction and quality of life are increasingly important considerations for how clinicians and researchers evaluate the outcome of cancer treatment.1–3 This is especially true for women with breast cancer, many of whom enter survivorship as a result of improved detection and treatment. Breast cancer patients often experience multiple physical and psychosocial issues, such as adverse effects from cancer treatment, anxiety or depression, or dissatisfaction with appearance, that can adversely affect quality of life years into survivorship.4–9 Therefore, it is crucial to recognize and act on these concerns. The BREAST-Q, a patient-reported outcome measure (PROM) designed specifically to measure patient satisfaction and quality of life in breast cancer patients, was developed by our group in 2009 and has been widely adopted worldwide as the gold-standard PROM following breast surgery.10–13 As such, it is uniquely positioned to be used as a tool for longitudinal follow-up of these patients from the postoperative period to survivorship. However, there exists a paucity of research examining how BREAST-Q scores should be interpreted in the clinical and research context, thus limiting the clinical utility of the BREAST-Q.
One crucial component for meaningful interpretation of PROMs is the minimal important difference (MID). The MID is the smallest difference in the scale’s score that patients feel is beneficial; therefore, knowing the MID can inform clinicians whether an intervention had a meaningful impact on the patient.14 The MID for the BREAST-Q Reconstruction module in cases of postmastectomy breast reconstruction has been studied in a multi-center cohort of 3052 breast reconstruction patients, and a MID of 3–4 points was found to be appropriate for this patient population.15 However, nationwide trends indicate that less than half of breast cancer patients receive mastectomy with or without reconstruction, with the remaining patients undergoing breast-conserving therapy (BCT).16,17 While a BCT-specific BREAST-Q module has been validated for use within this patient population, no efforts have been made to determine an appropriate MID.
Another component important for BREAST-Q score interpretation is its clinical reference values. Reference values can inform patients and surgeons what scores are expected for a typical patient at specific timepoints. Patients who have scores that are unexpectedly low can then be identified and potentially targeted for intervention. Previously, reference values have been determined for the BREAST-Q Reconstruction module using a large cohort of postmastectomy breast reconstruction patients.18 These values were used to create a score interpretation tool that provided 25th, 50th, and 75th percentile score trajectories from baseline to 2 years after surgery. A similar score interpretation tool currently does not exist for BCT patients. The purpose of this study was to determine appropriate MIDs and clinical reference values for the BREAST-Q Breast-Conserving Therapy (BREAST-Q BCT) module.
METHODS
Study Design
Upon institutional review board approval, the authors performed a retrospective analysis of breast-conserving therapy (BCT) patients at Memorial Sloan Kettering Cancer Center (New York, NY, USA) from January 2011 to December 2021. To determine clinical reference values, patients were included if they underwent at least one BCT procedure and completed the BREAST-Q BCT module at least once from before their initial BCT procedure to 2 years after surgery. To determine MID, patients must also have completed either the preoperative or 1-year BREAST-Q.
Demographic data were collected on patient characteristics, including age, race, body mass index (BMI), and smoking status, as well as on clinical characteristics, including history of radiation therapy, chemotherapy, axillary lymph node dissection, and number of additional re-excision procedures. BREAST-Q scores were collected preoperatively, and at the 6-month, 1-year, and 2-year postoperative timepoints.
The BREAST-Q BCT Module
The BREAST-Q is a PROM in questionnaire format, developed and validated to assess satisfaction and quality of life for breast surgery patients. Separate BREAST-Q modules have been developed and are specific to the type of breast surgery procedure performed, including BCT. The BREAST-Q BCT module has both preoperative and postoperative versions; Psychosocial Well-Being, Sexual Well-Being, Physical Well-Being of the Chest, and Satisfaction with Breasts domains are asked in both preoperative and postoperative versions. Each domain can be completed and scored independently. Raw scores for each domain are converted into a 0–100 score, with higher scores indicating better satisfaction or quality of life. More information on the development, administration, and scoring of the BREAST-Q can be found at https://qportfolio.org/breast-q/breast-cancer/ (accessed November 30, 2022).
At our institution, BREAST-Q scales are administered to breast surgery patients as standard of care. All scales are administered electronically, and can be completed by patients either at home through the patient portal, or in clinic on tablets.
Determining Clinical Reference Values
Patient characteristics were summarized using descriptive statistics (mean and standard deviation [SD] or median and interquartile range [IQR]) for all patients who completed at least 1 BREAST-Q. BREAST-Q scores were summarized using descriptive statistics (median, IQR) for Satisfaction with Breasts, Physical Well-Being of the Chest, Psychosocial Well-Being, and Sexual Well-Being preoperatively, and at the 6-month, 1-year, and 2-year postoperative timepoints. Median, 25th percentile, and 75th percentile scores were used to generate a score interpretation tool.
Determining MID
Patient characteristics and BREAST-Q BCT module scores were summarized using descriptive statistics (mean, SD). This was performed separately for patients who completed 1) the preoperative or 1-year postoperative BREAST-Q, and 2) for patients who completed both the preoperative and 1-year postoperative BREAST-Q.
To determine the MID for each BREAST-Q BCT module domain, a distribution-based analysis was performed. This method has been used previously to determine the MID for the BREAST-Q Reconstruction module. Two analyses were performed. In the first analysis, the MID was determined using 0.2 SD of preoperative BREAST-Q scores. In the second analysis, the MID was determined by calculating the change in score from preoperative to 1 year postoperative for patients who completed BREAST-Q at both time horizons. The 0.2 standardized response mean (SRM) was used to estimate the MID from the change in score
In addition to MID estimates for the overall cohort, the MID was also estimated based on 3 different clinical groupings to evaluate whether a separate MID was necessary based on clinical characteristics. Clinical groupings included: 1) radiation versus no radiation; 2) normal versus overweight versus obese BMI; and 3) re-excision versus no re-excision procedure.
All data analyses were performed using R (version 4.0.3, tidyverse, readxl). Chi-square tests were used for categorical variables, and t-tests were used for continuous variables.
RESULTS
Study Population
A total of 8060 patients completed at least 1 BREAST-Q within 2 years of surgery (Table 1). Average age at time of surgery was 58.1 years (SD 12.0). Average BMI was 28.0 (SD 6.3). The patients were primarily White (75.6%) and never smokers (55.0%). Overall, 28.4% received chemotherapy, 62.0% received radiation, 3.3% had an axillary lymph node dissection, and 13.0% had another excision procedure.
TABLE 1.
Demographics and clinical characteristics of breast-conserving therapy patients, overall
| Overall n = 8060 |
|
|---|---|
| Age, average (SD) | 58.1 (12.0) |
| BMI, average (SD) | 28.0 (6.3) |
| Race, n (%) | |
| White | 6090 (75.6%) |
| Black | 664 (8.2%) |
| Asian | 702 (8.7%) |
| Other | 294 (3.6%) |
| Unknown | 310 (3.8%) |
| Smoking, n (%) | |
| Never smoker | 4435 (55.0%) |
| Previous smoker | 2073 (25.7%) |
| Current smoker | 403 (5.0%) |
| Unknown | 1149 (3.1%) |
| Chemotherapy, n (%) | 2286 (28.4%) |
| Radiation therapy, n (%) | 4994 (62.0%) |
| Axillary lymph node dissection, n (%) | 268 (3.3%) |
| Additional excision procedure, n (%) | 1047 (13.0%) |
SD standard deviation, BMI body mass index
A total of 5673 patients completed either the preoperative or the 1-year postoperative BREAST-Qs (Table 2). Average age at time of surgery was 57.9 years (SD 11.7). Average BMI was 28.0 (SD 6.3). The cohort was primarily White (75.6%) and non-smokers (53.9%). Overall, 63.5% received radiation, 27.3% received chemotherapy, and 3.4% had an axillary lymph node dissection. Most patients did not undergo additional re-excision procedures (88.2%).
TABLE 2.
Patient characteristics for MID analysis
| Characteristic | Completed Preoperative or 1-Year BREAST-Qs | Completed Preoperative and 1-Year BREAST-Qs |
|---|---|---|
| Number of patients | 5673 | 990 |
| Average age (SD) | 57.9 (11.7) | 58.1 (11.3) |
| Average BMI in kg/m2 (SD) | 28.0 (6.3) | 27.8 (6.1) |
| Race | ||
| White | 4287 (75.6%) | 790 (79.8%) |
| Black | 449 (7.9%) | 58 (5.9%) |
| Asian | 511 (9.0%) | 68 (6.9%) |
| Other | 209 (3.7%) | 35 (3.5%) |
| Unknown | 217 (3.8%) | 39 (3.9%) |
| Smoking status | ||
| Non-smoker | 3056 (53.9%) | 579 (58.5%) |
| Previous smoker | 1456 (25.7%) | 300 (30.3%) |
| Current smoker | 270 (4.8%) | 41 (4.1%) |
| Unknown | 891 (15.7%) | 70 (7.1%) |
| Radiation | 3603 (63.5%) | 679 (68.6%) |
| Chemotherapy | 1549 (27.3%) | 263 (26.6%) |
| Axillary lymph node dissection | 192 (3.4%) | 17 (1.7%) |
| Re-excisions | ||
| None | 5003 (88.2%) | 876 (88.5%) |
| At least 1 | 670 (11.8%) | 114 (11.5%) |
MID minimal important difference, SD standard deviation, BMI body mass index
A total of 990 patients completed both the preoperative and the 1-year postoperative BREAST-Q scales (Table 2). Average age at time of surgery for this cohort was 58.1 years (SD 11.3), and average BMI was 27.8 (6.1). Patients were predominantly White (79.8%) and non-smokers (58.5%). In this cohort, 68.6% received radiation, 26.6% received chemotherapy, and 1.7% had an axillary lymph node dissection. Most patients did not have additional re-excision procedures (88.5%).
BREAST-Q Reference Values
Median, 25th percentile, and 75th percentile BREAST-Q scores for each domain from baseline to 2 years after surgery are shown in Fig. 1 as well as in Supplementary Table 1. Psychosocial Well-Being, Sexual Well-Being, and Satisfaction with Breasts scores improved and then stabilized after surgery; however, Physical Well-Being of the Chest scores decreased after surgery and remained lower than baseline at 2 years after surgery.
Fig. 1.
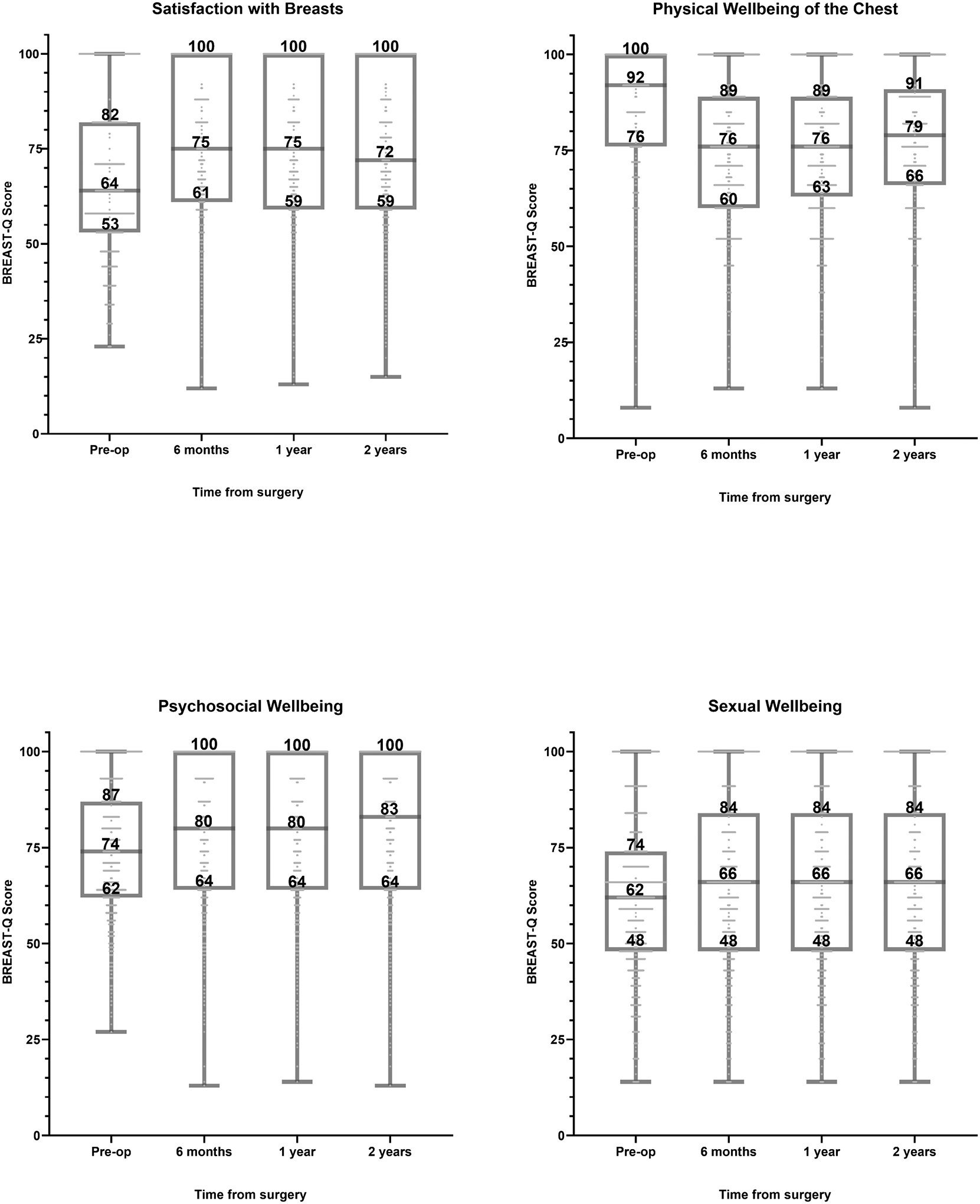
Breast-conserving therapy BREAST-Q scores from baseline to 2 years after surgery.
A score interpretation tool, the BREAST-Q Real-time Engagement and Communication Tool (BREAST-Q REACT), was created based on the score trajectories of this cohort (Fig. 2). This tool provides patients and surgeons with expected scores over time and allows for quick comparison of a patient’s score or scores with these reference values.
Fig. 2.
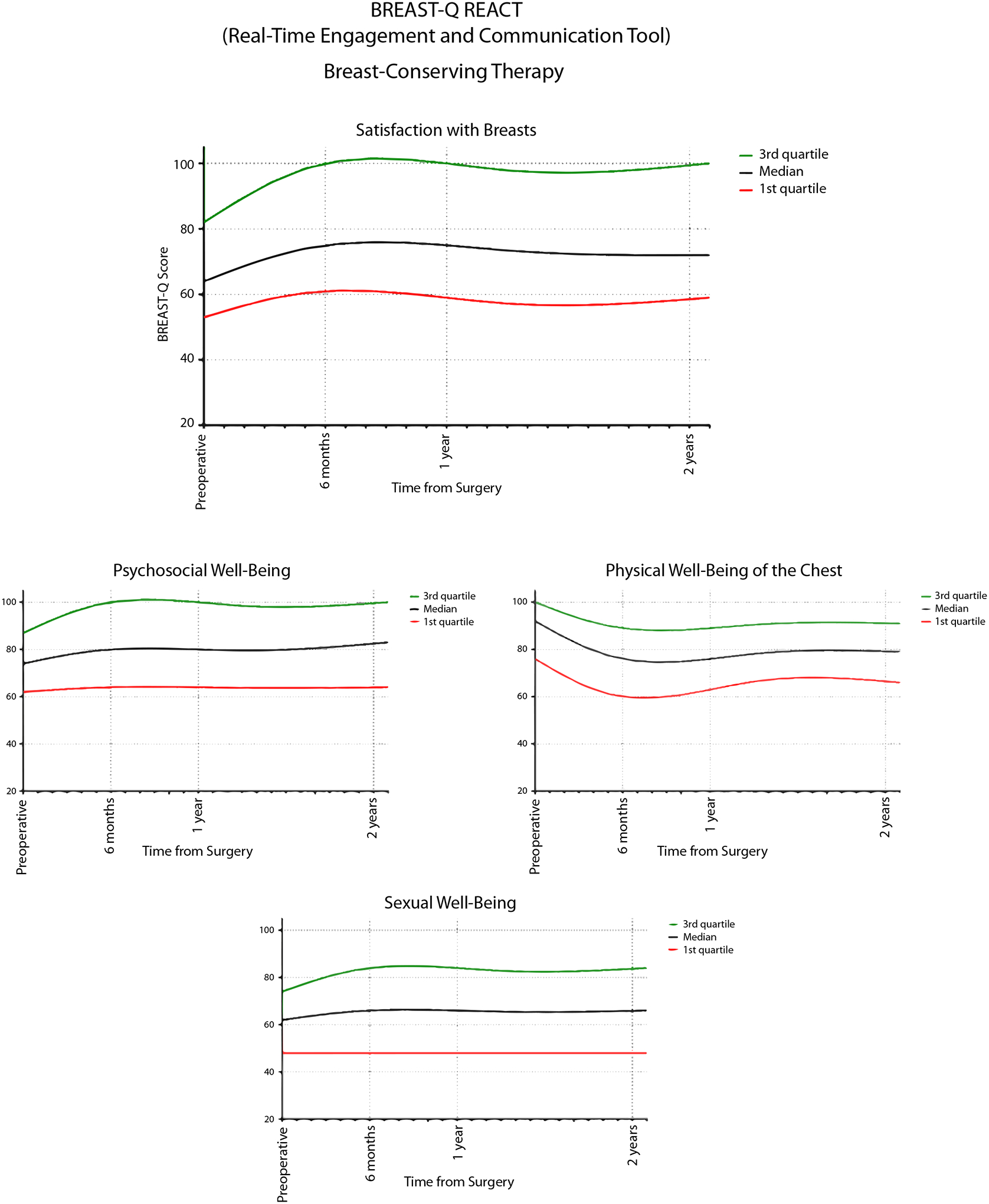
Breast-conserving therapy Real-time Engagement and Communication Tool (REACT).
BREAST-Q MID
Mean BREAST-Q scores at the preoperative time horizon were 67.3 (SD 21.0) for Satisfaction with Breasts, 74.8 (SD: 17.2) for Psychosocial Well-Being, 85.7 (SD 15.4) for Physical Well-Being of the Chest, and 62.2 (SD 20.1) for Sexual Well-Being (Table 3). MID estimates based on 0.2 SD were 4 for Satisfaction with Breasts, 3 for Psychosocial Well-Being, 3 for Physical Well-Being of the Chest, and 4 for Sexual Well-Being. MID estimates based on 0.2 SRM for patients who completed both the preoperative and 1-year postoperative BREAST-Q scales were 5 for Satisfaction with Breasts, 4 for Psychosocial Well-Being, 4 for Physical Well-Being of the Chest, and 4 for Sexual Well-Being.
TABLE 3.
BREAST-Q mean scores, standard deviations, and minimal important difference estimates
| Preoperative | 1 Year Postoperative | Change: Preoperative to 1 Year Postoperative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Number | Mean | SD | 0.2 SD | MID Estimate | Number | Mean | SD | Mean Change | SD | 0.2 SRM | MID Estimate |
| Satisfaction with Breasts | 2849 | 67.3 | 21.0 | 4.2 | 4 | 3814 | 75.3 | 20.3 | +8.4 | 24.5 | 4.9 | 5 |
| Psychosocial Well-Being | 2888 | 74.8 | 17.2 | 3.4 | 3 | 3813 | 79.3 | 18.5 | +4.0 | 18.0 | 3.6 | 4 |
| Physical Well-Being of the Chest | 2898 | 85.7 | 15.4 | 3.1 | 3 | 3842 | 74.9 | 18.4 | −8.8 | 19.3 | 3.9 | 4 |
| Sexual Well-Being | 2614 | 62.2 | 20.1 | 4.0 | 4 | 3340 | 65.3 | 22.5 | +2.4 | 20.6 | 4.1 | 4 |
SD standard deviation, MID minimal important difference, SRM standardized response mean
MID estimates based on 0.2 SD and 0.2 SRM for each clinical grouping can be found in Table 4. MID estimates using 0.2 SD all ranged from 3 to 4 points, and MID estimates using 0.2 SRM all ranged from 4–5 points regardless of radiotherapy receipt, BMI, or re-excision.
TABLE 4.
BREAST-Q minimal important difference estimates based on clinical groupings
| Preoperative | Change: Preoperative to 1 Year Postoperative | |||||
|---|---|---|---|---|---|---|
| Clinical grouping | Number | 0.2 SD | MID Estimate | Number | 0.2 SRM | MID Estimate |
| Radiation therapy | ||||||
| Satisfaction with Breasts | 1731 | 4.2 | 4 | 679 | 4.9 | 5 |
| Psychosocial Well-Being | 1758 | 3.5 | 4 | 677 | 3.5 | 4 |
| Physical Well-Being of the Chest | 1758 | 3.1 | 3 | 675 | 3.9 | 4 |
| Sexual Well-Being | 1598 | 4.0 | 4 | 578 | 4.0 | 4 |
| No radiation therapy | ||||||
| Satisfaction with Breasts | 1118 | 4.2 | 4 | 311 | 5.0 | 5 |
| Psychosocial Well-Being | 1130 | 3.4 | 3 | 306 | 3.8 | 4 |
| Physical Well-Being of the Chest | 1140 | 3.0 | 3 | 309 | 3.7 | 4 |
| Sexual Well-Being | 1016 | 4.0 | 4 | 256 | 4.4 | 4 |
| Normal weight (BMI 18.5–24.9) | ||||||
| Satisfaction with Breasts | 941 | 4.0 | 4 | 332 | 4.9 | 5 |
| Psychosocial Well-Being | 952 | 3.3 | 3 | 331 | 3.6 | 4 |
| Physical Well-Being of the Chest | 955 | 2.9 | 3 | 328 | 3.6 | 4 |
| Sexual Well-Being | 874 | 3.8 | 4 | 287 | 3.9 | 4 |
| Overweight (BMI 25–29.9) | ||||||
| Satisfaction with Breasts | 884 | 4.1 | 4 | 317 | 4.5 | 5 |
| Psychosocial Well-Being | 900 | 3.4 | 3 | 316 | 3.6 | 4 |
| Physical Well-Being of the Chest | 904 | 3.1 | 3 | 317 | 3.9 | 4 |
| Sexual Well-Being | 826 | 4.0 | 4 | 274 | 4.2 | 4 |
| Obese (BMI 30–39.9) | ||||||
| Satisfaction with Breasts | 748 | 4.2 | 4 | 233 | 5.2 | 5 |
| Psychosocial Well-Being | 756 | 3.5 | 4 | 230 | 3.5 | 4 |
| Physical Well-Being of the Chest | 760 | 3.2 | 3 | 232 | 4.1 | 4 |
| Sexual Well-Being | 664 | 4.0 | 4 | 184 | 4.2 | 4 |
| No re-excision | ||||||
| Satisfaction with Breasts | 2580 | 4.2 | 4 | 876 | 4.9 | 5 |
| Psychosocial Well-Being | 2606 | 3.4 | 3 | 869 | 3.6 | 4 |
| Physical Well-Being of the Chest | 2616 | 3.1 | 3 | 872 | 3.9 | 4 |
| Sexual Well-Being | 2365 | 4.0 | 4 | 737 | 4.1 | 4 |
| At least one re-excision | ||||||
| Satisfaction with Breasts | 269 | 4.1 | 4 | 114 | 5.0 | 5 |
| Psychosocial Well-Being | 282 | 3.5 | 4 | 114 | 3.7 | 4 |
| Physical Well-Being of the Chest | 282 | 3.1 | 3 | 112 | 3.7 | 4 |
| Sexual Well-Being | 249 | 4.0 | 4 | 97 | 4.5 | 5 |
SD standard deviation, MID minimal important difference, SRM standardized response mean, BMI body mass index
DISCUSSION
The BREAST-Q is a widely used, well-validated and reliable tool for evaluating satisfaction and quality of life in BCT patients, but its clinical utility may be challenging, as there are no guidelines available for score interpretation. In this study, we used a large cohort of over 8000 patients undergoing breast-conservation surgery to determine appropriate reference values and MIDs. The BREAST-Q REACT was then created for each domain of the BREAST-Q to allow both patients and physicians to compare their results over time. In addition, scores can be compared to reference values to identify patients who may benefit from potential interventions. We currently recommend that a cut-off at the 25th percentile should be used as the threshold for beginning a discussion with a medical provider regarding low BREAST-Q scores.
Additionally, if BREAST-Q scores are collected longitudinally, patients who have a score trajectory that trends unexpectedly can also be identified. For example, a patient who has a Psychosocial Well-Being score of 90 at 6 months (above 50th percentile) and then has a score of 80 at 1 year (50th percentile) may still warrant discussion with a medical provider, as that would constitute an unexpected score trajectory for that BREAST-Q domain. Knowing the MID can help when evaluating whether a change in score over time is clinically meaningful. For example, a patient who experiences a greater than a 4-point drop in BREAST-Q scores between 2 timepoints for Physical Well-Being of the Chest should be identified for a potential discussion about pain or other physical symptoms and potentially referred to physical therapy. The effectiveness of physical therapy can also be evaluated by patient completion of an additional BREAST-Q, looking for score improvements beyond 4 points. During the survivorship period, patients and physicians can use the BREAST-Q for longitudinal monitoring of quality-of-life concerns. For example, a patient with a greater than 4-point decrease in Psychosocial Well-Being should potentially have a discussion with their physician about any new or worsening psychiatric symptoms, and may be referred to appropriate mental health resources. Our recommendations for MIDs in clinical practice can be found in Table 5.
TABLE 5.
Recommended minimal important difference estimates in clinical practice and clinical research
| Recommended MID Estimate | |
|---|---|
| Clinical practice | |
| Satisfaction with Breasts | 4 |
| Psychosocial Well-Being | 4 |
| Physical Well-Being of the Chest | 4 |
| Sexual Well-Being | 4 |
| Outcomes research | |
| Satisfaction with Breasts | 4 |
| Psychosocial Well-Being | 4 |
| Physical Well-Being of the Chest | 4 |
| Sexual Well-Being | 4 |
MID minimal important difference
In the research context, the availability of reference values and MIDs can strengthen the methodology of clinical studies which use patient-reported outcomes as an endpoint. Investigators are recognizing the importance of PROMs; the FDA now recommends that PROMs be included as an endpoint for cancer clinical trials. Given this, investigators need PROMs that are not only well validated and reliable, but also interpretable in a clinically meaningful way. PROMs that have known reference values and MIDs can guide investigators in selecting an appropriate effect size for sample size calculations. Additionally, investigators can make more useful comparisons of study groups, as differences between groups may be statistically significant but not necessarily clinically meaningful. For BREAST-Q studies, we recommend using a number-needed-to-treat approach for interpreting study findings, as means and medians do not reflect the entire population. For example, for patients who received BCT with or without radiation, there was a mean change in score from preoperative to 1-year postoperative of 9.0 points for irradiated patients and 8.2 for non-irradiated patients. However, 82.5% (n = 679) of irradiated patients and 82.3% (n = 311) of non-irradiated patients actually had a change in score of greater than the MID. Reference values can also add important context to research studies by informing investigators of how representative their study cohort is of the general BCT population, or by allowing for the selection of study participants using specific score criteria (i.e., only individuals with 25th percentile or lower scores).
This is the first study to determine clinical reference values or the MIDs for the BCT module of the BREAST-Q. Previous work on these score interpretation components has only been done for postmastectomy breast reconstruction.18 We recently determined reference values and generated a score interpretation tool for the BREAST-Q Reconstruction module. We created separate reference values based on type of reconstruction (implant versus autologous) because this characteristic has been extensively shown to influence BREAST-Q scores. For BCT, no similar body of work exists, thereby making it challenging to decide whether separate reference values are warranted based on a particular patient or treatment characteristic. Future research should focus on identifying the characteristics that influence BREAST-Q scores for BCT patients, and reference values should be adjusted accordingly.
A previous study determined the MIDs for the Reconstruction module of the BREAST-Q and similarly found the MID for each domain to be 4 points for almost all domains.15 The similarity in MIDs between the BREAST-Q BCT module and BREAST-Q Reconstruction module supports the validity of the MIDs, as the 2 patient populations are similar at baseline and are expected to have similar score distributions. Additionally, the similarity in MIDs for the 2 modules can streamline score comparisons between postmastectomy breast reconstruction and BCT in clinical research. In clinical settings, surgeons can easily track patient recovery and long-term outcomes using the BREAST-Q, even for breast cancer patients who may undergo a combination of BCT and mastectomy over time.
There are several limitations to our study. First, the small sample size of some subgroups may influence the distribution and estimated MIDs. Second, the distribution of BREAST-Q scores (i.e., standard deviations) used to calculate the MID may be variable from study to study, depending on sample heterogeneity. As such, an alternate participant sample may demonstrate a slight difference in the MID estimate. Third, in contrast to anchor-based methods which seek to establish the MID according to an external measure or phenomenon that is clinically relevant to patients (i.e., the anchor), distribution-based MID methods rely solely on statistical variability in participant scores and are generally considered to be less effective, as they fail to incorporate the patient perspective.19 Finally, a common limitation of distribution-based MID methods is that different effect sizes and standardized response mean values are advocated within the literature. For this study, the authors utilized 0.2 SDs to establish the MID estimate as advocated by Voineskos et al.15; however, authors in different clinical contexts have recommended the use of 0.3 SDs and 0.5 SDs as distribution-based criteria.20,21 The authors believe that this study serves as a conservative starting estimate for the MID and acknowledge that future studies (i.e., anchor-based estimates) may demonstrate different MID values.
The strengths of our study include the use of a large patient cohort for determining MID estimates. Additionally, we accounted for the potential of different MIDs based on important clinical characteristics by performing subgroup analyses, and we verified that these characteristics do not impact MID estimates. Future research should confirm the MID estimates in larger cohorts and samples with different characteristics, as the MIDs estimated in our study may differ from those of other patient populations. Additional studies should also examine other methods for determining the MID, such as anchor-based methods.
Conclusions
In this study, we determined clinical reference values and MIDs for the BREAST-Q BCT module using a large cohort of BCT patients at a single institution. Clinical reference values and MIDs can impact the interpretability of the BREAST-Q, which improves the utility of this PROM as a tool for clinical research and patient care. Further studies should be performed in different patient cohorts to confirm the estimates in this study.
Supplementary Material
Synopsis:
Here we determine reference values and the MID for the BREAST-Q BCT module. We find that reference values and MIDs are crucial to BREAST-Q score interpretation, which can lead to improved clinical evaluation and decision making and research methodology.
ACKNOWLEDGEMENTS
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the 44th Annual San Antonio Breast Cancer Symposium, December 7–10, 2021, San Antonio, TX. Dr. Babak Mehrara reports receipt of an investigator-initiated grant from Pfizer, Regeneron, and Puretech, receipt of royalty payments from Puretech, and an advisory position with Mediflix. Dr. Andrea L. Pusic is a co-developer of the BREAST-Q and receives totalities when it is used in for-profit, industry-sponsored clinical trials. All other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. [DOI] [PubMed] [Google Scholar]
- 2.Kuehn BM. Collecting Patient-Reported Outcomes in Cancer Clinical Trials. JAMA. 2021;326(5):379. [DOI] [PubMed] [Google Scholar]
- 3.Minvielle E, di Palma M, Mir O, Scotte F. The use of patient-reported outcomes (PROs) in cancer care: a realistic strategy. Ann Oncol. 2022;33(4):357–359. [DOI] [PubMed] [Google Scholar]
- 4.Boquiren VM, Esplen MJ, Wong J, Toner B, Warner E, Malik N. Sexual functioning in breast cancer survivors experiencing body image disturbance. Psychooncology. 2016;25(1):66–76. [DOI] [PubMed] [Google Scholar]
- 5.Carreira H, Williams R, Muller M, Harewood R, Stanway S, Bhaskaran K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J Natl Cancer Inst. 2018;110(12):1311–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32(16):1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch L, Bertram H, Eberle A, et al. Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship--a multi-regional population-based study. Psychooncology. 2014;23(5):547–554. [DOI] [PubMed] [Google Scholar]
- 8.Park J, Rodriguez JL, O’Brien KM, et al. Health-related quality of life outcomes among breast cancer survivors. Cancer. 2021;127(7):1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Przezdziecki A, Sherman KA, Baillie A, Taylor A, Foley E, Stalgis-Bilinski K. My changed body: breast cancer, body image, distress and self-compassion. Psychooncology. 2013;22(8):1872–1879. [DOI] [PubMed] [Google Scholar]
- 10.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293–302. [DOI] [PubMed] [Google Scholar]
- 11.Fuzesi S, Cano SJ, Klassen AF, Atisha D, Pusic AL. Validation of the electronic version of the BREAST-Q in the army of women study. Breast. 2017;33:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klassen AF, Dominici L, Fuzesi S, et al. Development and Validation of the BREAST-Q Breast-Conserving Therapy Module. Ann Surg Oncol. 2020;27(7):2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. [DOI] [PubMed] [Google Scholar]
- 14.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. [DOI] [PubMed] [Google Scholar]
- 15.Voineskos SH, Klassen AF, Cano SJ, Pusic AL, Gibbons CJ. Giving Meaning to Differences in BREAST-Q Scores: Minimal Important Difference for Breast Reconstruction Patients. Plast Reconstr Surg. 2020;145(1):11e–20e. [DOI] [PubMed] [Google Scholar]
- 16.Jonczyk MM, Jean J, Graham R, Chatterjee A. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat. 2019;173(2):267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JA, Chu JJ, McCarthy CM, et al. BREAST-Q REACT: Clinical Reference Values for the BREAST-Q in Post-mastectomy Breast Reconstruction Patients. Ann Surg Oncol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devji T, Carrasco-Labra A, Qasim A, et al. Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. Bmj. 2020;369:m1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898–910. [DOI] [PubMed] [Google Scholar]
- 21.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581–585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


