Abstract
We previously reported that human cytomegalovirus (CMV) glycoprotein B (gB) is vectorially transported to apical membranes of CMV-infected polarized human retinal pigment epithelial cells propagated on permeable filter supports and that virions egress predominantly from the apical membrane domain. In the present study, we investigated whether gB itself contains autonomous information for apical transport by expressing the molecule in stably transfected Madine-Darby canine kidney (MDCK) cells grown on permeable filter supports. Laser scanning confocal immunofluorescence microscopy and domain-selective biotinylation of surface membrane domains showed that CMV gB was transported to apical membranes independently of other envelope glycoproteins and that it colocalized with proteins in transport vesicles of the biosynthetic and endocytic pathways. Determinants for trafficking to apical membranes were located by evaluating the targeting of gB derivatives with deletions in the lumen, transmembrane (TM) anchor, and carboxyl terminus. Derivative gB(Δ717-747), with an internal deletion in the luminal juxtamembrane sequence that preserved the N- and O-glycosylation sites, retained vectorial transport to apical membranes. In contrast, derivatives that lacked the TM anchor and cytosolic domain (gBΔ646-906) or the TM anchor alone (gBΔ751-771) underwent considerable basolateral targeting. Likewise, derivatives lacking the entire cytosolic domain (gBΔ772-906) or the last 73 amino acids (gBΔ834-906) showed disrupted apical transport. Site-specific mutations that deleted or altered the cluster of acidic residues with a casein kinase II phosphorylation site at the extreme carboxyl terminus, which can serve as an internalization signal, caused partial missorting of gB to basolateral membranes. Our studies indicate that CMV gB contains autonomous information for apical targeting in luminal, TM anchor, and cytosolic domain sequences, forming distinct structural elements that cooperate in vectorial transport in polarized epithelial cells.
Human cytomegalovirus (CMV) glycoprotein B (gB) is highly conserved among the human herpesviruses (reviewed in reference 61). It functions in virus entry by promoting fusion of the virion envelope with the plasma membrane, cell-cell spread in nonpolarized human fibroblasts, and syncytium formation (55, 89). gB is a major component of the virion envelope and elicits a strong neutralizing antibody and cytotoxic T-cell immune response in infected individuals (33, 40, 54; reviewed in reference 67). CMV(AD169) gB is a type I glycoprotein, 906 amino acids (aa) in length (15), which is glycosylated at N- and O-linked sites (31, 62, 68), cleaved by the endoprotease furin (81, 82, 93), folded in the endoplasmic reticulum with the aid of protein chaperones (96, 98), and assembled into dimers (10).
CMV replication in epithelial cells is a crucial step in invasion of the body and the dissemination of infection. In newborns, organ transplant recipients, and immunocompromised patients, human CMV causes disease in tissues composed of epithelial cells, including the salivary gland, lung, kidney, colon, and, in patients with AIDS, the retina (reviewed in references 9, 18, and 28). Epithelial cells that carry out secretory functions have evolved distinct domains divided by tight junctions into apical (AP) and basolateral (BL) membranes, which have different compositions of protein and lipids that are maintained through vectorial secretory pathways (reviewed in references 19, 77, and 78). How CMV enters and egresses from epithelial cells in body tissues is poorly understood, since most of our knowledge of CMV replication was acquired by studies carried out with human fibroblasts. Cultures of primary human retinal pigment epithelial (RPE) cells support CMV replication (17, 50), and we used a human RPE cell line, ARPE-19, to examine the process of entry and cell-cell spread in epithelial cells. When cultured on permeable filter supports, ARPE-19 cells differentiate, forming distinct AP and BL membrane domains (20). We reported that CMV enters ARPE-19 cells asymmetrically through the AP membrane domain, facilitated by gB (88). In contrast, an accessory glycoprotein, gpUS9, promotes the transmission of infection from cell to cell (44, 45), suggesting that different glycoproteins may function in vectorial trafficking of progeny virions. Examination of CMV-infected ARPE-19 cells by immunofluorescence laser scanning confocal microscopy showed that gB is sorted to AP membranes; infectivity studies indicate that virions are released predominantly from this membrane domain (88). AP release would promote CMV infection of other epithelial cells, whereas spread of virus across BL membranes would transmit infection to adjacent cells and other types of susceptible cells in contact with the epithelial surface.
In polarized epithelial cells, selective delivery of membrane-anchored glycoproteins is regulated by the formation and targeting of transport vesicles along the secretory route (reviewed in reference 48). Vectorial transport to AP or BL membranes occurs in vesicles that bud from the trans Golgi network. Studies with polarized Madine-Darby canine kidney (MDCK) cells reported that membrane-anchored proteins contain signals in the cytosolic domain that recruit them into clathrin-coated pits that are internalized from the cell surface (reviewed in reference 11). Certain proteins are delivered first to one (BL) membrane domain and then endocytosed and transcytosed to the opposite (AP) membrane (51, 80). Asymmetrical release of influenza virus, vesicular stomatitis virus (VSV), human immunodeficiency virus (HIV), and others from MDCK cells is regulated by vectorial sorting of the virion envelope glycoproteins by means of specific determinants (22, 34, 42, 71, 84). Our observation that gB was sorted to the AP membrane domain of CMV-infected epithelial cells suggested that it may contain autonomous targeting information that directs virion-containing vesicles to AP membranes.
Since ARPE-19 cells lose their polarized properties following transfection and vectorial sorting cannot be studied in transiently transfected cells because polarity is lost, in the present study we used stably transfected MDCK cells to evaluate the transport of gB independently of other viral glycoproteins. Examination of wild-type (WT) gB transport in MDCK cells showed that it was targeted apically as in CMV-infected human ARPE-19 cells. This indicated that gB specifies autonomous determinants for vectorial trafficking in the secretory pathway of polarized epithelial cells. We found that deletions in the transmembrane (TM) anchor (aa 751 to 771) and cytosolic domain (aa 834 to 906) disrupt the AP membrane targeting, as do site-specific mutations that delete or modify the charge of an acidic cluster with a casein kinase II (CKII) phosphorylation site (aa 899 to 904). Targeting determinants in the luminal domain and TM anchor of gB resemble those of other apically sorted glycoproteins. In contrast, the cytosolic domain contains potential determinants for internalization from the cell surface, which may direct gB to endocytic vesicles and the recycling pathway and function to missort derivatives with partial deletions in the carboxyl terminus.
MATERIALS AND METHODS
Cells and culture medium.
MDCK type II cells were purchased from the American Type Culture Collection. Cells were grown in T-75-cm2 flasks (Costar) at 37°C in minimal essential medium containing 5% fetal bovine serum, 200 mM l-glutamine, 0.1 mg of streptomycin per ml, and 100 U of penicillin per ml. To form polarized monolayers, MDCK cells expressing gB and the mutated derivatives were grown on microporous filters (Transwell; Costar) with a 0.4-μm pore size. At 4 days after plating, the cells were judged to be polarized when the transepithelial resistance ranged between 200 and 250 Ω/cm2, as determined with a Millicell electrical resistance system (Millipore).
Construction of mutated derivatives in CMV gB.
The construction of deletion derivatives in CMV gB with internal deletions of the luminal juxtamembrane hydrophobic sequence (aa 717 to 747), TM anchor (aa 751 to 771), and both hydrophobic sequences (aa 717 to 772) and truncation mutations in part of the lumen, TM anchor, and cytosolic domains (gBΔ646-906), the TM anchor and cytoplasmic domain (gBΔ761-906), and part of the cytosolic domain (gBΔ834-906) has been published previously (64, 90, 98). Derivative gB(Δ772-906) was constructed by inserting a stop codon at position 772 that precludes synthesis of the cytosolic domain. Mutations in the CKII phosphorylation site in the cytosolic domain of gB (56) were constructed by substituting valine or glutamic acid for serine at position 900. To construct gB(ser900val), the carboxyl coding sequence and 3′-untranslated sequence of the gB gene were subcloned as a 463-bp NsiI-XbaI fragment in the modified pBluescript KS (−). Uracil-containing single-stranded DNA of this subclone was isolated from Escherichia coli CJ236 and was used for site-directed mutagenesis (36). Oligonucleotide 5′-CTTGAAAGACgtCGACG AAGAAG-3′ (the mutated sequence [lowercase]) was used to change the codon 900 from Ser to Val and concurrently to introduce a SalI restriction site. After mutagenesis, the selected SalI-positive clone was sequenced by the dideoxy chain termination method, using double-stranded DNA as a template (74). Other mutations in the extreme carboxyl terminus of the gB molecule were constructed by site-directed mutagenesis by a PCR-uracyl DNA glycosylase method (66). For mutagenesis, a template plasmid, psp/gB, was constructed by inserting the EcoRI-XbaI fragment of gB (aa 685 to 906) into plasmid psp72 (Promega), which was then used as the template for mutagenesis. Primers used for mutagenesis were as follows (substitutions and stop codon indicated by lowercase letters): gB(s899-903) primer 1, 5′-AggaggagcaggugcuGAGAACGTCTGAACCAGGAGG-3′; gB(s899-903) primer 2, 5′-agcaccugctcctccUTTCAAGTGTCTGTAGCCG-3′; gB(ser900glu) primer 1, 5′-aGACGAAGAAGAGAACGUCTGAAC-3′; gB(ser900glu) primer 2, 5′-ACGTTCTCTTCUTCGTCutcGTCTTTCAAGTGTCTGTAGCCGTTTTTGCG-3′; gB(Δ900-906) primer 1, 5′-AGACACTUGAAAGACugaAC CAGGAGGAAAAAAAAACTAGAC-3′; and gB(Δ900-906) primer 2, 5′-AGTCTTUCAAGUGTCUGTAGAC-3′. After mutagenesis, fragments containing the mutations were subcloned into pRC/CMVgB with the EcoRI and XbaI sites to substitute the mutated sequence for the corresponding region of WT gB. Sequence changes were confirmed by both manual and automated sequencing before and after subcloning the mutated fragments.
Selection of MDCK cells expressing derivatives of gB.
Approximately 106 MDCK cells were transfected with 10 μg of DNA from plasmid pcDNA3 containing the gB derivatives by published procedures (24). After 4 to 6 h, fresh medium containing 10% fetal calf serum was added. On the following day, cells were trypsinized and 5 × 104 cells/ml were plated into 24-well culture dishes in medium containing G418 (1.2 mg/ml; Gibco). G418-resistant colonies were selected as previously described (89).
Serological reagents.
The following serological reagents were used: a rat monoclonal antibody (MAb) to ZO-1 in tight junctions (Chemicon International); a rat MAb to E-cadherin in adherens junctions (Sigma); antibodies to VIP-21, β-COP, and furin (Affinity Bioreagents); antibodies to Rab11 (Zymed), Rab4, and Rab5 (Quality Controlled Biochemicals); antibody to cathepsin B (Calbiochem); and fluorescein isothiocyanate (FITC)- and Texas red-conjugated anti-mouse and anti-rat reagents (Jackson ImmunoResearch). Antibodies to adaptor protein complexes 1 and 2 (Ap-1 and Ap-2) in clathrin-coated vesicles were a gift of Frances Brodsky (University of California, San Francisco). The pool of MAbs to CMV gB reacted with epitopes spanning different domains in the gB molecule (5, 64). MAbs to continuous and assembled epitopes in the luminal domain of gB were CH408-1 (domain DC1v), CH177-3 and CH253-1 (domain D1), CH130-9 (domain D2a), and CH442-1 (domain D2b). MAbs to continuous epitopes in the cytosolic domain were CH409-2 (domain D3) and CH28-2 (domain DC3).
Immunofluorescence analyses.
Surface immunofluorescence analyses with laser scanning confocal microscopy were performed as follows. MDCK cells expressing the gB derivatives were grown on permeable filters for 4 days. Then, they were washed with cold phosphate-buffered saline (PBS; pH 7.2), a pool of MAbs to gB was added, and the cells were kept on ice for 30 min. The cells were again washed and fixed with fresh 3% paraformaldehyde in the cold for 5 min. Fixed cells were incubated on ice with secondary antibodies applied from the AP or BL surface. In experiments to stain for gB and cellular proteins, cells were reacted with antibody to gB, fixed with 3% paraformaldehyde, and then incubated with FITC-labeled secondary antibody applied from both surfaces. The cells were then permeabilized with 0.5% Triton X-100 (5 min), reacted with primary antibodies to cellular proteins, and incubated with Texas red-conjugated secondary antibodies applied to both surfaces. Filters were cut and mounted on glass slides in Mowiol solution (Calbiochem-Behring). The cells were analyzed with a krypton-argon laser coupled with a Bio-Rad MRC 600 confocal head, which was attached to a Nikon Optiphot II microscope with a Plane Apo 60 ×1.4 objective lens. The cells were scanned simultaneously for FITC and Texas red emission with the K1 and K2 filter blocks. For Z section analysis, cells were scanned from AP to BL membranes with an increment of 0.5 to 1.0 μm between sections. The data were analyzed with Comos software.
Domain-selective labeling and Western blot assays.
Domain-selective labeling was done as previously reported (26). MDCK cells were grown on filters with a 75-mm diameter and 0.4-μm pore size (Transwell; Costar) for 4 days and then washed with cold Ringer’s buffer (10 mM HEPES [pH 7.4], 154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2). Cells were incubated with Ringer’s buffer containing 20 μg of sulfo-NHS-biotin (Pierce) per ml, which was applied from AP (1.5 ml) or BL (5 ml) membrane domains, for 30 min at 4°C on a rocker platform. Then, they were washed five times with Tris saline (10 mM Tris-HCl [pH 7.4], 120 mM NaCl), harvested with a rubber policeman, and extracted in radioimmunoprecipitation (RIP) buffer containing 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride (PMSF), and aprotinin (1 μg/ml). Cell extracts were clarified in a microcentrifuge at 4°C for 15 min, adsorbed to streptavidin-Sepharose (Pierce) for 2 h at 4°C, and then washed six times with RIP buffer. The samples were subjected to SDS-polyacrylamide gel electrophoresis and were electrotransferred to nitrocellulose. Secreted derivatives of gB were detected as follows. After 4 days’ growth on filters, the medium was aspirated and replaced by medium without serum. At 24 h, the media from AP and BL compartments were collected separately, concentrated 20-fold, and extracted with RIP buffer. Samples were electrophoresed in 12% polyacrylamide gels, electrotransferred to nitrocellulose, and reacted with MAbs to CMV gB. The bands were visualized by exposure to Hyperfilm by the enhanced chemiluminescence procedure (Amersham).
Analysis of CMV gB solubility.
Polarized MDCK cells grown on filters for 4 days were solubilized as published previously (27, 79). Cells were washed with PBS and solubilized in Triton X-100 (1%) or octylglucoside (65 mM) in MBS-buffered saline (25 mM 2-N-morpholinoethanesulfonic acid [MES; pH 6.5], 15 M NaCl, 1 mM PMSF) for 20 min at 4°C on a rocking platform. Cells were scraped from the filter with a rubber policeman and centrifuged (14,000 rpm for 15 min). The soluble supernatant was collected into clean tubes, the insoluble pellet was extracted in SDS immunoprecipitation buffer (15 mM Tris [pH 7.5], 5 mM EDTA, 2.5 EGTA, 1% SDS), and the denatured samples were electrophoresed in denaturing polyacrylamide gels.
RESULTS
Construction of stably transfected MDCK cells that express WT gB and mutated derivatives.
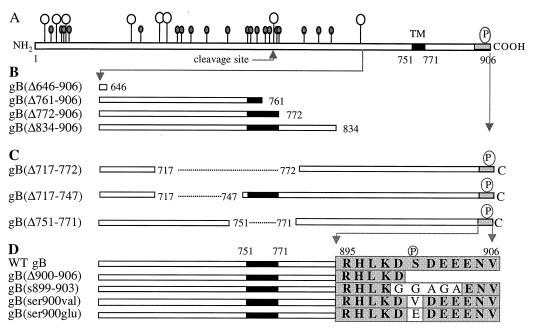
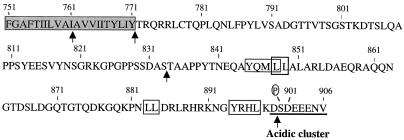
In this, the first study to determine whether sequences in CMV gB encode information for AP transport, we took advantage of previously characterized deletion derivatives that retain many of the antigenic and functional properties of the molecule, fold correctly, assemble into dimers, and undergo transport to the cell surface. Figure 1 shows the sequences of the previously published deletion derivatives of CMV gB and new constructs used in this study. Figure 1A shows sites for N and O glycosylation and endoproteolytic cleavage in the luminal domain, the hydrophobic TM anchor, and the CKII phosphorylation site in the cytosolic domain. Figure 1B shows derivatives with large deletions that span the molecule. gB(Δ646-906), which has been previously reported, has a deletion of 104 aa in the luminal domain and is missing the entire TM anchor and the cytosolic domain (64). This glycoprotein folded correctly, as indicated by a panel of MAbs to conformational epitopes in the lumen, and formed oligomers (64). gB(Δ761-906), which lacks part of the TM anchor and the entire carboxyl terminus, and gB(Δ834-906), which lacks the extreme carboxyl terminus, also retained all of the conformational epitopes and formed oligomers but were impaired in syncytium formation in U373 glioblastoma cells (90). gB(Δ772-906), which was constructed with a stop codon that precluded synthesis of the carboxyl terminus, folded properly but lacked epitopes mapping in the cytosolic domain (5). Figure 1C shows deletion mutations in hydrophobic sequences, which were published previously (98). We and others reported that aa 751 to 771 function as the TM anchor sequence of gB and that derivatives lacking this sequence, gB(Δ751-771) and gB(Δ717-772), were secreted from U373 glioblastoma cells (69, 98). gB(Δ717-747), which lacks a hydrophobic juxtamembrane sequence in the lumen, folds correctly as indicated by MAbs to conformational epitopes but has impaired transport from the endoplasmic reticulum of U373 cells and consequently is not cleaved (98). Figure 1D shows site-specific mutations in the cluster of charged amino acids, which includes a CKII phosphorylation site, in the cytosolic domain (56). They were constructed for the present study and are shown relative to the WT gB sequence in this region. gB(Δ900-906) lacked most of the cluster of acidic residues, as did gB(s899-903), which had five glycine substitutions. The substitution mutation in gB(ser900val) precluded phosphorylation (91), and that in gB(ser900glu) mimicked a constitutively charged residue.
FIG. 1.
Constructs for expression of CMV gB(AD169) and mutated derivatives in polarized MDCK cells. (A) Drawing of gB based on the amino acid sequence (14, 15) showing positions of potential N- and O-glycosylation sites (full and empty lollipops, respectively) in the amino-terminal domain (aa 1 to 750), endoproteolytic cleavage site (arrow) (aa 460 to 461) (82), TM anchor (aa 751 to 771), and carboxyl terminus (aa 772 to 906). (B) Deletion mutations with truncations in the luminal domain, TM anchor, and cytosolic domain. (C) Internal deletion mutations in hydrophobic sequences in the luminal juxtamembrane region and TM anchor (dashes). (D) Sequence of the cluster of charged residues at the extreme carboxyl terminus of WT gB and derivatives with site-specific substitution and deletion mutations. Designations of the mutated derivatives are indicated at the left. Open bars, large sequences in the lumen and carboxyl terminus; filled bars, TM anchor; shaded region, cluster of charged residues at the extreme carboxyl terminus; Δ, deletion; s, substitution; P, CKII phosphorylation site (56). Amino acids in sequence are indicated by single letters. Antigenic and functional properties of the truncated derivatives and internal deletion mutations in gB (C and D) have been published elsewhere (64, 90, 98).
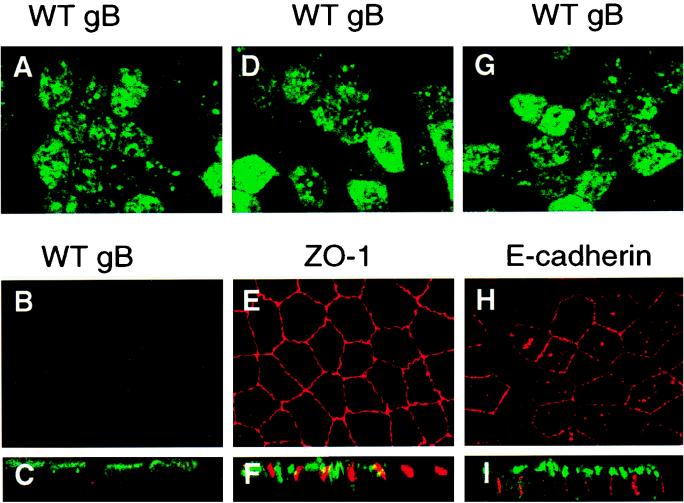
We first sought to examine the vectorial transport of CMV gB expressed autonomously in human ARPE-19 cells and attempted to select stably transfected cells after transfection with plasmid DNA, as described for U373 glioblastoma cells (89). Unfortunately, we found that ARPE-19 cells failed to proliferate following transfection, lost the morphology of epithelial cells, and were impaired in forming tight junctions as determined by staining with antibody to ZO-1 (data not shown). Therefore, we chose to examine the sorting information in autonomously expressed forms of CMV gB by G418 selection of MDCK cells stably transfected with plasmid DNA containing the mutated gB genes regulated by the CMV immediate-early promoter. Several MDCK cell clones expressing the derivatives were isolated, and one or more were examined with the mixture of MAbs for gB transport to the plasma membrane. First, we examined the vectorial transport of WT gB in polarized MDCK cells by surface immunofluorescence of unfixed cells whose AP and BL membranes were stained with FITC- and Texas red-conjugated antisera, respectively (Fig. 2). We found that the glycoprotein was transported preferentially to AP (Fig. 2A) but not BL membranes (Fig. 2B), as shown in horizontal sections (X-Y plane) and vertical sections (X-Z plane) (Fig. 2C). Comparison of the gB staining pattern with that of ZO-1, a marker for tight junctions at the interface between the AP and BL membrane domains (Fig. 2D to F), and E-cadherin, a marker for adherens junctions in BL membranes (Fig. 2G to I), showed that these proteins stained in patterns characteristic of their BL localization. The results of these experiments showed that CMV gB is sorted to the AP membrane domain of stably transfected MDCK cells, as in CMV-infected ARPE-19 cells, which suggested that gB contains autonomous information for vectorial sorting to the AP membrane domain of polarized epithelial cells.
FIG. 2.
Immunofluorescence confocal microscopy showing vectorial transport of CMV gB (A to C, D, and G), ZO-1 (E and F), and E-cadherin (H and I) in polarized MDCK cells. Cells stably expressing WT gB were grown for 4 days on microporous filters. For surface membrane immunofluorescence staining, live cells were incubated with antibodies to gB from AP (A, D, and G) and BL membranes (B, E, and H). Cells were then permeabilized and reacted with antibodies to ZO-1, a protein in tight junctions, and E-cadherin, an adhesion molecule in adherens junctions in BL membranes. Top and middle rows, single 1-μm optical sections in the X-Y horizontal plane; bottom row, X-Z sections showing vertical confocal views through the sample (C, F, and I).
Determinants for AP transport are located in the lumen and TM anchor of gB.
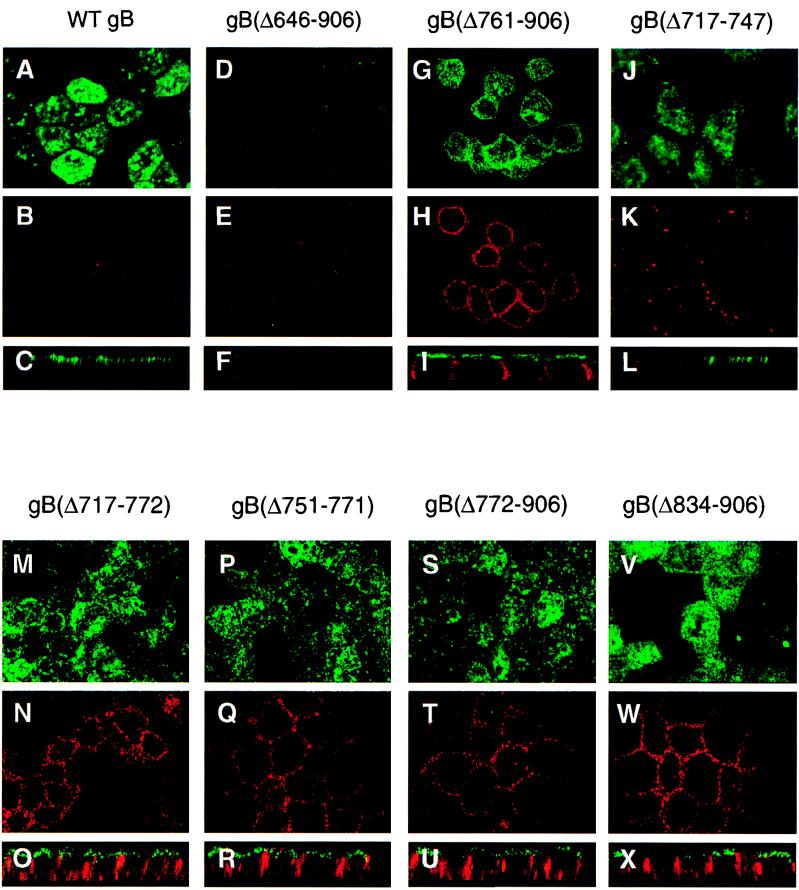
In influenza virus glycoproteins, N- and O-glycosylation sites in the luminal domain and the TM anchor serve as AP sorting information by associating with sphingolipid rafts, microdomains that handle apically sorted proteins (34, 75, 76, 78). To locate sequences that contain vectorial sorting determinants in the CMV gB molecule, we next compared the transport of derivatives with large deletions in the lumen and TM anchor with that of WT gB by immunofluorescence analysis (Fig. 3). Comparison of the staining pattern of WT gB in AP membranes (Fig. 3A to C) with that of gB(Δ646-906), lacking part of the lumen, the entire TM anchor, and the cytosolic domain, showed that the derivative was not detected in either AP or BL surface membranes (Fig. 3D to F). This result suggested that loss of the TM anchor caused this derivative to be secreted from cells. gB(Δ761-906), which lacked the carboxyl-terminal half of the TM anchor and the entire cytosolic domain, retained its membrane anchoring and was detected approximately equally in AP and BL membranes (Fig. 3G to I). These findings indicated that AP targeting information may be lost by deletion of the TM anchor and cytosolic domain of gB.
FIG. 3.
Immunofluorescence confocal microscopy showing the transport of CMV WT gB and deletion derivatives to the apical and basolateral surface membranes of polarized MDCK cells. Cells were incubated with a pool of MAbs to gB, fixed, and then reacted with secondary antibodies conjugated either with fluorescein isothiocyanate (AP membrane) or with Texas red (BL membrane). Derivatives are indicated at the top of each set of panels.
The TM anchor of gB contains AP sorting information.
To determine whether the TM anchor contains AP sorting determinants that function independently of the cytosolic domain, we evaluated the transport of derivatives with deletions in hydrophobic sequences that contained the TM anchor and luminal juxtamembrane proximal sequences. Examination of gB(Δ717-747) showed that it was transported to AP membranes (Fig. 3J to L), whereas derivatives lacking all of the hydrophobic sequence, gB(Δ717-772), or the TM anchor alone, gB(Δ751-771), were transported about equally to both AP and BL membranes (Fig. 3M to R). These results indicated that deletion of the TM anchor alone disrupts AP transport and that a subset of the mutated forms undergo transport to BL membranes.
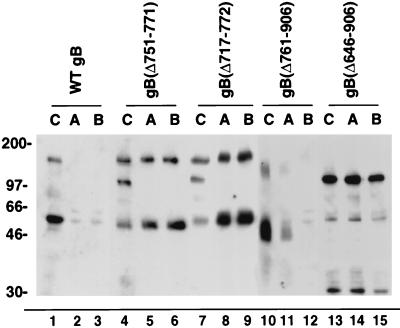
In the ensuing series of experiments, we evaluated directional secretion of gB derivatives lacking all or part of the TM anchor by using Western blotting to analyze the medium collected from the AP and BL membrane domains for the presence of secreted forms (Fig. 4). Comparison of WT gB (lanes 1 to 3) with gB(Δ751-771) and gB(Δ717-772) (lanes 4 to 9) showed that the derivatives were secreted in approximately equal amounts from the AP and BL membrane domains. gB(Δ761-906), missing half of the TM anchor and the entire carboxyl terminus, was released preferentially from AP membranes (lanes 10 to 12). Derivative gB(Δ646-906), with a truncation in part of the ectodomain and all of the TM anchor and cytosolic domain, was secreted in about equal amounts from both membranes (lanes 13 to 15). The results of these experiments suggest that deletion of the TM anchor and cytosolic domain missorts gB either because AP targeting information is lost or because a functional BL targeting signal is exposed. These results also indicate that the luminal domain contains information that promotes secretion from AP membranes. Since all of the potential N- and O-glycosylation sites are retained in gB(Δ646-906), it is possible that the derivative binds to lectins associated with sphingolipid-cholesterol rafts that are apically targeted (75, 78).
FIG. 4.
Secretion from AP and BL membrane domains of polarized MDCK cells of mutated CMV gB derivatives with internal deletions in the TM anchor and carboxyl-terminal truncations. Lanes A and B, medium from AP and BL compartments, respectively; lanes C, total extracts of MDCK cells. Samples were electrophoresed in denaturing gels, electrotransferred to nitrocellulose, and reacted with a pool of MAbs to gB.
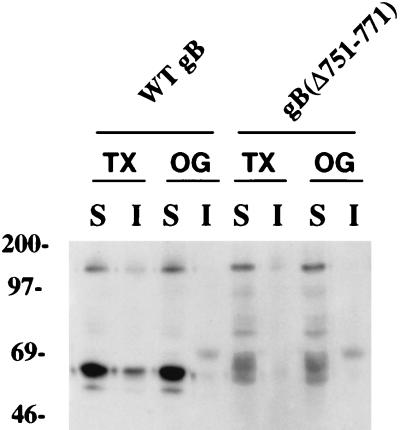
Analysis of derivatives lacking the TM anchor suggested that CMV gB, like the influenza virus glycoproteins hemagglutinin (HA) and neuraminidase (NA), may contain an AP sorting determinant in the TM anchor domain (34, 76, 78). Figure 5 shows the sequence of the TM anchor of gB, which is rich in large hydrophobic residues that might facilitate interaction of the molecule with sphingolipid-cholesterol rafts that are vectorially transported to AP membranes. To determine whether gB is insoluble in Triton X-100, we examined the solubility of WT gB and gB(Δ751-771), lacking the TM anchor, in polarized MDCK cells (Fig. 6). We found that a fraction of WT gB was insoluble in Triton X-100 (lanes 1 to 2). In contrast, WT gB was completely soluble in octylglucoside (lanes 3 to 4), and gB(Δ751-771) was soluble in both Triton X-100 and octylglucoside (lanes 5 to 8). These results suggested that the hydrophobic residues in the TM anchor may promote association of gB with sphingolipid-cholesterol rafts and cooperate in targeting to AP membranes.
FIG. 5.
Hydrophobic sequence of the TM anchor and cytosolic carboxyl terminus of CMV gB. Shaded sequence, TM anchor (aa 751 to 771); aa 772 to 906, cytosolic domain; boxes, Leu-Leu and Tyr-containing signals; underlining, cluster of acidic residues; P, CKII phosphorylation site. Arrows indicate sites of truncation mutations constructed in the TM and cytosolic domain (Fig. 1).
FIG. 6.
Solubility of CMV gB and an internal deletion derivative gB(Δ751-771), lacking the TM anchor, in Triton X-100 and octylglucoside. Western blot analysis of extracted samples electrophoresed in denaturing polyacrylamide gels and transferred to nitrocellulose is shown. TX, Triton X-100; OG, octylglucoside; S, soluble; I, insoluble.
The cytosolic domain of gB facilitates AP sorting.
We then determined whether the cytosolic domain of gB contains AP sorting information by examining two derivatives with large deletions. Immunofluorescence analysis of gB(Δ772-906), lacking all of the carboxyl terminus, and gB(Δ834-906), lacking the last 73 residues, showed that both derivatives had lost AP sorting information and were transported to AP and BL membranes in about equal amounts (Fig. 3S to X). These findings suggested either that information for AP targeting in the carboxyl terminus was lost or that deletion of cytosolic sequences caused a BL sorting determinant to be presented or activated. Since gB internalizes from the plasma membrane (65), vectorial transport in epithelial cells might occur by a combination of AP sorting information (in the lumen and TM anchor) and cytosolic signals for entry into endosomal vesicles, which are then directed to AP membranes and recycled (2). Figure 5 shows the sequence of the cytosolic domain of gB, which contains Tyr-containing sequences, Leu-Leu motifs, and a cluster of charged amino acids with a CKII phosphorylation site that might serve as determinants for endocytosis and targeting to BL membranes (reviewed in reference 48). Relevant to the present study was the report that the endoprotease furin, which cleaves gB in a post-Golgi compartment (93), contains a cluster of charged residues and a CKII site in the cytosolic domain that function as a signal for internalization from plasma membranes into the endocytic pathway (30, 94).
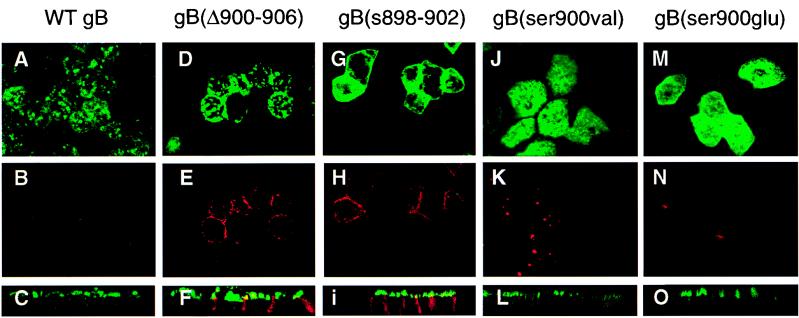
To investigate the role of the cluster of acidic residues in vectorial sorting of gB, we examined the transport of mutated derivatives with deletions or substitutions in this sequence. The sequences of WT gB and the mutated derivatives gB(Δ900-906), gB(s899-903), gB(ser900val), and gB(ser900glu) are shown in Fig. 1D, and their immunofluorescence staining patterns in polarized MDCK cells are shown in Fig. 7. Comparison of WT gB (panels A to C) with gB(Δ900-906) (panels D to F) and gB(s899-903) (panels G to I) indicated that a fraction of the mutated derivatives was missorted to BL membranes. In contrast, derivatives with a single substituted residue, gB(ser900val) and gB(ser900glu), underwent vectorial transport to AP membranes, like WT gB (Fig. 7J to O). These results were confirmed by differences in the staining patterns obtained in vertical sections of the apically sorted derivatives (Fig. 7C, L, and O) and the missorted ones (Fig. 7F and I). It should be mentioned that the distribution of these derivatives with mutations in the acidic cluster differed from that of other forms, in that it was particularly dense (Fig. 7J and M) and was concentrated at the periphery of the AP membrane domain (Fig. 7D and G), suggesting a defect in internalization of gB. That abrogation of the cytosolic charged cluster leads to missorting indicates that this signal participates in vectorial targeting of gB in epithelial cells.
FIG. 7.
Immunofluorescence confocal microscopy showing the transport in polarized MDCK cells of CMV gB derivatives with mutations in the charged cluster of amino acids mapping in the extreme carboxyl terminus. Mutated forms are listed above each set of panels. Top and middle rows, single 1-μm optical section in the X-Y plane for AP and BL, respectively; bottom row, vertical X-Z section through the sample. gB(s899-903), panels G, H, and I.
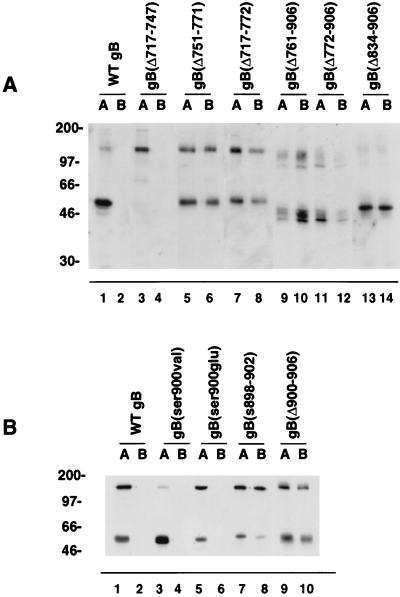
Then, we carried out domain-selective biotinylation of AP and BL membranes of polarized MDCK cells expressing derivatives with deletions in the lumen, the TM anchor, and the cytosolic domain and derivatives with mutations in the cluster of acidic residues to evaluate their transport biochemically (Fig. 8). The results of these experiments showed that WT gB (Fig. 8A, lanes 1 to 2) and gB(Δ717-747) (Fig. 8A, lanes 3 to 4) trafficked to AP membranes. In contrast, gB(Δ751-771) and gB(Δ717-772), lacking the TM anchor (Fig. 8A, lanes 5 to 8), were detected in AP and BL membranes in approximately equal amounts. Likewise, derivatives with deletions in the cytosolic domain, gB(Δ761-906), gB(Δ772-906), and gB(Δ834-906), were present in both AP and BL membranes (Fig. 8A, lanes 9 to 14). Examination of derivatives with mutations in the charged cluster in the carboxyl terminus confirmed that WT gB, gB(ser900val), and gB(ser900glu) were apically targeted (Fig. 8B, lanes 1 to 6), whereas gB(s899-903) and gB(Δ900-906) were partially missorted to BL membranes (Fig. 8B, lanes 7 to 10). The results of domain-selective labeling supported the immunofluorescence studies, which showed that mutations in the TM anchor and cytosolic domain of gB cause missorting to BL membranes and that the cluster of acidic residues, aa 899 to 904, enhances the fidelity of the AP targeting of gB. These findings indicate either that information in the cytosolic domain of gB recognized by the sorting machinery was lost or that functional BL sorting determinants were presented or activated following deletion of the carboxyl terminus, or both.
FIG. 8.
Detection in surface membranes of polarized MDCK cells of CMV gB and derivatives with deletions in the lumen, TM anchor, and cytosolic domain (A) and with site-specific mutations in the cluster of acidic residues in the cytosolic domain (B). Cells were grown on permeable filters and subjected to domain-selective biotinylation from AP (lanes A) or BL (lanes B) membrane domains.
CMV gB colocalizes with proteins associated with endocytic vesicles.
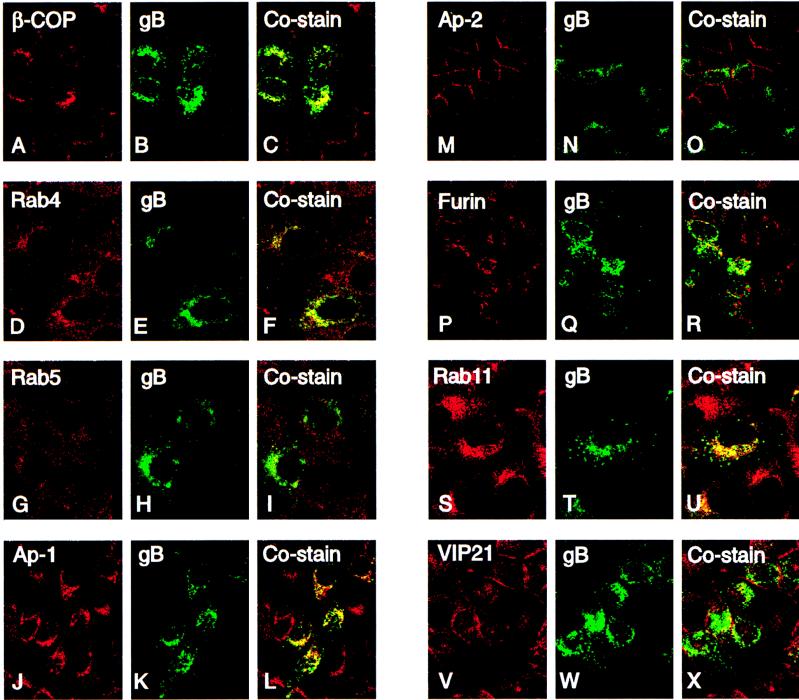
Although the cytosolic domains of membrane-anchored glycoproteins have not been implicated in targeting to AP membranes, our finding that deletions in this domain disrupt AP targeting of gB in epithelial cells suggested that this sequence may participate in targeting. As noted above, the cytosolic sequence contains multiple determinants with the potential for sorting into endocytic vesicles: Tyr-containing and Leu-Leu motifs, which resemble BL sorting signals, and the cluster of acidic residues (Fig. 5) (48, 94). We next determined whether WT gB traffics in the endocytic pathway in MDCK cells by studying gB costaining with cellular proteins that serve as markers for early endosomes and vesicles of the biosynthetic pathway. The results are shown in Fig. 9 and summarized in Table 1. gB stained in a globular pattern and was strongly colocalized with β-COP in transport vesicles trafficking from the endoplasmic reticulum to the Golgi complex (Fig. 9A to C). Some gB costaining was found with Rab4 (Fig. 9D to F) and Rab5 (Fig. 9G to I), which are small GTPases in early endosomes. gB strongly costained with the adaptor protein complex-1 (Ap-1) in clathrin-coated early endosomal vesicles budding from the trans Golgi network (Fig. 9J to L) and weakly costained with AP-2 in clathrin-coated pits internalized from the plasma membrane (Fig. 9M to O). gB costained with furin transported in vesicles from the trans Golgi network and internalized from the cell surface (Fig. 9P to R). gB also colocalized with two proteins that are apically sorted in epithelial cells, Rab11 in recycling vesicles (Fig. 9S to U) and VIP-21, which partitions with sphingolipid-cholesterol rafts (Fig. 9V to X). gB did not costain with cathepsin B, a marker for lysosomes (data not shown). These costaining experiments indicated that gB contains functional sorting determinants, reported to map in the cytosolic domains of membrane-anchored glycoproteins, for trafficking in the biosynthetic pathway, early endosomes, and recycling vesicles from the trans Golgi network and the plasma membrane and also that it sequesters with proteins handled by sphingolipid-cholesterol rafts.
FIG. 9.
Costaining of CMV gB and proteins in secretory vesicles in the biosynthetic and endocytic pathways of MDCK cells, analyzed by immunofluorescence laser scanning confocal microscopy. For each set of three frames, the left, middle, and right rows show cellular protein (red), gB (green), and costaining vesicles (yellow), respectively.
TABLE 1.
Colocalization of CMV gB with vesicles of the biosynthetic and endocytic transport pathways in MDCK cells
| Transport pathway(s) | Vesicle marker | gB costaining | Reference(s) |
|---|---|---|---|
| Coatomers of vesicles trafficking from endoplasmic reticulum to Golgi | β-COP | + | 60 |
| Clathrin-coated vesicles budding from TGNa | Ap-1 | + | 70, 87 |
| Furin | + | 94 | |
| Early endosomes, recycling, and clathrin-coated vesicles | Ap-2 | + | 70, 87 |
| Rab4 | + | 13, 16, 94 | |
| Rab5 | + | ||
| Furin | + | ||
| TGN, apically targeted sphingolipid-cholesterol rafts | VIP-21 | + | 32, 53, 99 |
| Apically targeted vesicles and pericentriolar recycling endosomes | Rab11 | + | 23, 92 |
TGN, trans Golgi network.
DISCUSSION
CMV gB contains information for AP trafficking in polarized epithelial cells.
Our observation that CMV gB is transported to AP membranes in infected ARPE-19 cells prompted the present study to determine whether gB contains autonomous information for AP sorting and, if so, to locate the sequences by expressing derivatives with site-specific mutations in polarized MDCK cells. We found that WT gB expressed in the absence of other viral glycoproteins underwent vectorial transport to AP membranes and that deletions in the TM anchor and the cytosolic domain caused significant missorting to BL membranes, indicating that these regions contain independent sorting determinants. Our results are summarized in Table 2, and the key findings are discussed below.
TABLE 2.
Transport of CMV gB and mutated derivatives to AP and BL membrane domains of polarized MDCK cells
| Derivative | Mutated region | Mol mass (kDa)a | Membrane domainb | Secretion |
|---|---|---|---|---|
| WT gB | None | 150, 55 | AP | None |
| gB(Δ646-906) | Lumen/TM anchor/cytosol | 100, 30 | None | AP/BL |
| gB(Δ717-772) | Lumen/TM anchor | 150, 48 | AP/BLc | AP/BL |
| gB(Δ717-747) | Lumen | 150 | AP | None |
| gB(Δ761-906) | TM anchor/cytosol | 120, 40 | AP/BL | AP |
| gB(Δ751-771) | TM anchor | 150, 52 | AP/BL | AP/BL |
| gB(Δ772-906) | Cytosol | 124, 42 | AP/BL | None |
| gB(Δ834-906) | Cytosol | 150, 50 | AP/BL | None |
| gB(Δ900-906) | Cytosol | 150, 50 | AP/some BLd | NDe |
| gB(s899-903) | Cytosol | 150, 50 | AP/some BLd | ND |
| gB(ser900val) | Cytosol | 150, 50 | AP | ND |
| gB(ser900glu) | Cytosol | 150, 50 | AP | ND |
Mol mass, approximate molecular mass. Products of proteolytic cleavage were detected for all derivatives except gB(Δ717-747).
Membrane domain as determined by immunofluorescence staining of gB in laser scanning confocal microscopy and domain-selective biotinylation.
AP/BP, approximately equal missorting.
AP/some BL, predominantly AP with some BL missorting.
ND, not done.
The severely truncated derivative gB(Δ646-906), missing part of the lumen, the entire TM anchor, and the cytosolic domain, was secreted from AP and BL membrane domains; this indicated that the remaining luminal sequences contain AP sorting information, although considerable missorting occurred. That derivative gB(Δ751-771), lacking only the hydrophobic TM anchor sequence, was also missorted to BL membranes indicated that AP sorting information may have been lost. Missorting of gB(Δ772-906) and gB(Δ834-906) to BL membranes suggests that the cytosolic domain contains independent AP targeting information distinct from determinants contained in the TM anchor. Deletion and substitution mutations that altered the cluster of acidic residues (aa 899 to 904) changed the trafficking of a fraction of the mutated forms expressed in MDCK cells, indicating that the charged sequence at the extreme carboxyl terminus of gB influences vectorial sorting of the glycoprotein in epithelial cells. We showed that determinants in separate regions—the lumen, TM anchor, and cytosolic domain—participate in vectorial transport of gB to AP membranes of epithelial cells and that loss of structural elements in the TM anchor and the cytosolic domain causes missorting to BL membranes.
Sorting determinants in the lumen and TM domain that are recognized by the cellular sorting machinery.
Intense research efforts have focused on understanding the mechanism of protein sorting in epithelial cells, deciphering the sorting determinants, and identifying the cytosolic protein complexes that decode them. Most of these studies have been performed with polarized MDCK cells that are stably transfected with a gene encoding the protein of interest. It is thought that AP sorting is regulated by information in the lumen and TM anchor, whereas the cytosolic domain contains determinants for BL sorting and entry of glycoproteins into the endocytic pathway. N-glycosylation sites and an O-glycosylated stalk domain serve as functional elements for targeting proteins to AP membranes (25, 75, 97). The glycophosphatidylinositol anchor functions as an AP sorting determinant (38), as does the hydrophobic TM anchor of influenza virus envelope glycoproteins HA and NA (34, 76). It has been reported that these glycoproteins traffic vectorially to the AP membrane domain by association with sphingolipid-cholesterol rafts—which are protein-lipid microdomains formed in the Golgi complex (reviewed in references 39 and 78)—by means of residues in the TM anchor and the affinity of the luminal domain for raft-associated lectins (34, 73, 79).
Our findings that a fraction of WT gB molecules is insoluble in Triton X-100, like influenza virus HA and NA (32, 34, 79), and colocalizes with VIP-21, a raft-associated, cholesterol-binding protein (53), suggest that some of the gB molecules may also undergo AP targeting in sphingolipid-cholesterol rafts. Many N- and O-glycosylation sites between aa 1 and 646 in the lumen of gB, shown in Fig. 1, may promote AP targeting of the anchorless derivative, gB(Δ646-906), and the partially anchored derivative, gB(Δ761-906), by associating with lectins in apically sorted lipid rafts (75, 78, 97). Since some of these gB molecules are missorted, affinity of the luminal domain for raft-associated proteins may not be sufficient for clustering into lipid rafts. The missorting of gB that occurs following deletion of the TM anchor could also result from less stringent exclusion of gB from clathrin-coated vesicles and suggests that cooperation between determinants in the lumen and TM anchor enhances AP targeting. Comparison of the TM anchor sequence (Fig. 7) with those of influenza virus HA and NA glycoproteins, which contain AP sorting information, indicates that the gB anchor is rich in large hydrophobic residues that may promote cooperative interactions with cholesterol in sphingolipid rafts (32, 76). The finding that the truncated derivative gB(Δ761-906) undergoes transport to AP and BL membranes but is secreted predominantly from AP membranes suggests that the remaining hydrophobic residues may serve to anchor gB in BL membranes but are not sufficient for its stable distribution in AP membranes. Whether the association of gB with sphingolipid-cholesterol rafts depends on the capacity of the TM anchor to bind cholesterol remains to be investigated.
Determinants for endocytosis and vectorial sorting in the cytosolic domain of gB.
Signals for rapid internalization into the endocytic pathway, which may overlap BL targeting determinants, are found in the cytosolic domain of membrane-anchored glycoproteins (reviewed in references 46 and 48). These include a Tyr (Y) within the sequence YXXØ, where X is any amino acid and Ø is one with a bulky hydrophobic group; Leu-Leu motifs (29, 47); and a cluster of acidic residues (94). Since primary determinants can have diverse targeting activities, other sequence and contextual requirements influence protein sorting within cells (46, 47). Among these are flanking residues that favor sorting to particular compartments, the position of the signal within the cytosolic domain, and secondary determinants that operate together with it. Entry into the endocytic pathway involves the recruiting of membrane-anchored glycoproteins into clathrin-coated regions by the binding of Ap-1 and Ap-2 to cytosolic signals (57, 58, 70). Potential determinants in the cytosolic domain for sorting CMV gB into the endocytic pathway include five Tyr-containing motifs, two Leu-Leu motifs, and a cluster of acidic residues with a CKII site (Fig. 5) (56). Immunofluorescence studies showed that gB colocalized with Ap-1 in the trans Golgi network, with Ap-2 at the plasma membrane, and with proteins in endocytic vesicles. This indicates that gB traffics in clathrin-coated vesicles, early endosomes, and recycling vesicles in MDCK cells. It is notable that varicella-zoster virus gE and gI and pseudorabies virus gE and gB also contain functional cytosolic signals that regulate the trafficking of these alphaherpesvirus glycoproteins in clathrin-coated vesicles of the endocytic pathway (1, 59, 85).
The results of mutagenesis studies indicate that the acidic cluster in the cytosolic domain of gB, which functions as an endocytic sorting signal in furin (8, 94), may influence the targeting of gB in polarized cells. We recently observed that derivatives with site-specific mutations in the cluster of acidic residues fail to internalize from the plasma membrane (91). The finding that these mutations alter the vectorial sorting of gB suggests that trafficking through the endocytic pathway in polarized cells may play a key role in gB targeting. The acidic cluster is immediately preceded by a Tyr-containing signal, which may assume a functional role in the BL sorting of mutated derivatives gB(s899-903) and gB(Δ900-906), in which this signal is intact. Tyr-containing signals, Leu-Leu motifs, and other elements that may function in BL targeting form a type I β-turn configuration (4). Interestingly, several MAbs to gB that recognize the carboxyl terminus fail to react with overlapping peptides constructed from this sequence, indicating that the intact cytosolic domain may possess secondary structure (5). Possibly determinants used for endocytosis have a different context after truncation of the molecule and consequently missort gB to BL membranes. A study of the apically targeted human nerve growth factor receptor supports this idea (37). An internal cytoplasmic deletion, which moved a cytoplasmic Tyr closer to the membrane into a more charged environment, caused missorting of the mutated form basolaterally and more rapid internalization of the ligand. The results suggested that a BL targeting signal related to endocytic signals, dominant over AP targeting information in the TM anchor and luminal domains, was expressed. Given the presence of multiple sorting determinants in CMV gB and the potential of the molecule to interact with cellular proteins in different compartments (63, 96, 98), it will be important to identify specific signals for endocytosis in the cytosolic domain and those that may promote BL targeting following structural changes.
Vectorial trafficking of viral envelope glycoproteins and directional release of virions from polarized epithelial cells.
Viruses infecting epithelial cells coopt the cellular machinery to sort their envelope glycoproteins and thus regulate the direction of progeny virion egress to maximize the spread of infection. In MDCK cells, influenza virus is released from AP membranes, which are enriched in the envelope glycoproteins HA and NA (35, 72). In contrast, VSV (22), HIV, and human T-cell leukemia virus type 1 (41, 42) are released from BL membranes, to which their envelope glycoproteins are vectorially sorted. In human RPE cells, influenza virus and VSV are released with the same polarity as in MDCK cells, which suggests that the sorting information is recognized similarly (6, 79, 84). In the present study, we showed that CMV gB contains autonomous determinants for AP transport in MDCK cells, which is comparable to the sorting in CMV-infected human ARPE-19 cells (88). We have also employed a nonreplicating adenovirus vector to express CMV gB and found that it is sorted apically in polarized MDCK and ARPE-19 cells infected with the vector (43), which supports the similar vectorial targeting of gB in these cells.
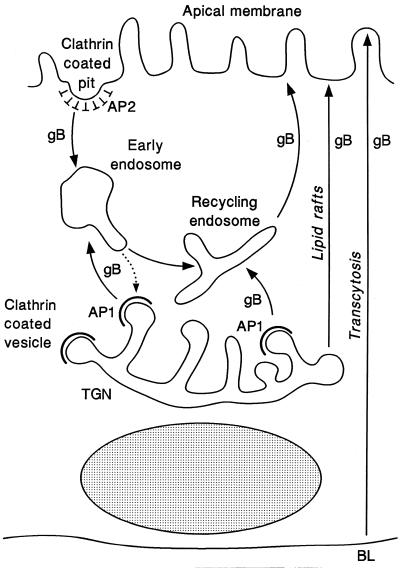
Multiple pathways for trafficking membrane-anchored glycoproteins to the plasma membrane have been demonstrated for polarized epithelial cells, i.e., direct sorting to AP and BL membranes in vesicles of the biosynthetic pathway and indirect transport in vesicles of the endocytic and transcytotic pathway (48, 52, 78, 95). Figure 10 shows a model of the transport pathways for trafficking CMV gB to AP membranes of polarized epithelial cells based on colocalization studies and published work from our laboratory and others (7, 65, 88). Structural determinants in the lumen, TM anchor, and cytosolic domain may influence the pathway used for trafficking gB to AP membranes. N- and O-glycosylation sites in the luminal domain and hydrophobic residues in the TM anchor domain may promote association of gB with glycosphingolipid-cholesterol rafts (75, 76, 78, 97). Cytosolic determinants may bind Ap-1 in clathrin-coated vesicles budding from the TGN, which enter the endocytic pathway by fusing with early endosomes or recycling endosomes, or may bind Ap-2 in clathrin-coated pits at the plasma membrane during internalization. Once in AP membranes, gB may be internalized and enter the recycling endosomes that are apically targeted. It was recently reported that influenza virus HA is slowly transcytosed from BL to AP membranes in rat RPE cells (7). Although only a trace amount of gB was detected in BL membranes of human ARPE-19 cells infected with CMV (88) or with a nonreplicating adenovirus vector expressing gB (43), the possibility that the transcytotic pathway is used for indirect targeting to AP membranes cannot be excluded and remains to be examined.
FIG. 10.
Model of CMV gB trafficking pathways in polarized epithelial cells with distinct apical and basolateral membrane domains. Determinants in the lumen, TM anchor, and cytosolic domain of the gB molecule may participate in vectorial transport to AP membranes. N- and O-glycosylation sites in the luminal domain and hydrophobic residues in the TM anchor domain may promote association of gB with glycosphingolipid-cholesterol rafts (75, 76, 78, 97). Cytosolic signals may bind Ap-1 in clathrin-coated vesicles budding from the trans Golgi network (TGN) and Ap-2 in clathrin-coated pits internalizing from the plasma membrane, which enter the endocytic pathway by fusing with early endosomes and recycling endosomes. In retinal pigment epithelial cells, cytosolic signals may target delivery to BL membranes and transcytosis to AP membranes (3, 7).
Our study of the mutated derivative gB(Δ717-747) provided a striking example of differences in the transport pathways used for trafficking membrane-anchored glycoproteins in different types of cells. We recently reported that this derivative was retained in the endoplasmic reticulum of U373 glioblastoma cells, where it formed complexes with protein chaperones, although the derivative was neither malfolded nor degraded (98). In the present study, we found that gB(Δ717-747) underwent transport to AP membranes of polarized MDCK cells (Table 2), suggesting that structural features may be recognized by components of the secretory machinery operating in polarized epithelial cells, but not in nonpolarized cells. This idea is supported by the finding that proteins associated with sphingolipid-cholesterol rafts may be sorted to different membrane domains depending on the cell type, which suggests that additional cellular factors may be required for accurate sorting (99).
Egress of the herpesviruses from epithelial cells is a complex process due to the presence of multiple glycoproteins, which may participate in vectorial transport of virion-containing vesicles to distinct membrane domains (reviewed in references 61 and 83). It is generally accepted that the first step in virion egress is the acquisition of an envelope by nucleocapsids budding into the inner nuclear membrane, but the subsequent steps are not well understood. Genetic studies showed that herpes simplex virus may undergo de-envelopment from the first envelope acquired during budding into the endoplasmic reticulum and be reenveloped in a different subcellular compartment (12). In CMV-infected human fibroblasts, gB is internalized from plasma membranes and incorporated into the virion envelope (65), and released virus particles are derived from endocytic vesicles (86). It has long been appreciated that virions are poorly released from CMV-infected U373 cells and that plaque formation fails to occur, which may be a consequence of rapid endocytosis of gB from the surface of infected U373 cells (21). Together, these results suggest that CMV may be enveloped in vesicles derived from the endocytic pathway and that virion egress may be regulated in epithelial cells by targeting of virion-containing vesicles to AP membranes via trafficking determinants in the TM anchor and cytosolic domain of gB distributed in the vesicle membranes. A key question is the relationship between release of CMV virions predominantly from the AP membrane domain, trafficking information in gB, and potentially divergent signals in the other envelope glycoproteins. It is thought that transport determinants are hierarchically arranged and that inactivation of signals specifying BL transport causes efficient AP sorting (49). The hierarchy of signals regulating virion egress from CMV-infected epithelial cells, in which multiple glycoproteins are transported in vesicles of the biosynthetic and endocytic pathways, remains to be examined by using viral recombinants with mutations in trafficking determinants in gB and other envelope glycoproteins.
ACKNOWLEDGMENTS
These studies were supported in part by Public Health Service grants EY10138 and EY11223 from the National Institutes of Health (L.P.). J.X. was supported in part by fellowship awards from Fight for Sight (PD97038) and the Universitywide AIDS Research Program (P97-SF-106). Z.Z. was supported by the Universitywide AIDS Research Program (F94-SF-13).
We thank Janet Wellington for performing pilot studies on the vectorial transport of CMV gB in epithelial cells and Zoya Kharitonov for assistance with cell culture.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca G, Katz L A, Mostov K E. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apodaca G, Mostov K E. Transcytosis of placental alkaline phosphatase-polymeric immunoglobulin receptor fusion proteins is regulated by mutations of Ser664. J Biol Chem. 1993;268:23712–23719. [PubMed] [Google Scholar]
- 4.Aroeti B, Kosen P A, Kuntz I D, Cohen F E, Mostov K E. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basgoz N, Qadri I, Navarro D, Sears A, Lennette E, Youngblom J, Pereira L. The amino terminus of human cytomegalovirus glycoprotein B contains epitopes that vary among strains. J Gen Virol. 1992;73:983–988. doi: 10.1099/0022-1317-73-4-983. [DOI] [PubMed] [Google Scholar]
- 6.Bok D, O’Day W, Rodriguez-Boulan E. Polarized budding of vesicular stomatitis and influenza virus from cultured human and bovine retinal pigment epithelium. Exp Eye Res. 1992;55:853–860. doi: 10.1016/0014-4835(92)90011-g. [DOI] [PubMed] [Google Scholar]
- 7.Bonilha V, Marmorstein A, Cohen-Gould L, Rodriguez-Boulan E. Apical sorting of influenza hemagglutinin by transcytosis in retinal pigment epithelium. J Cell Sci. 1997;110:1717–1727. doi: 10.1242/jcs.110.15.1717. [DOI] [PubMed] [Google Scholar]
- 8.Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters P J, Bonifacino J S. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2493–2523. [Google Scholar]
- 10.Britt W J, Vugler L G. Oligomerization of the human cytomegalovirus major envelope glycoprotein complex gB (gp55-116) J Virol. 1992;66:6747–6754. doi: 10.1128/jvi.66.11.6747-6754.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky F. New fashions in vesicle coats. Trends Cell Biol. 1997;7:175–179. doi: 10.1016/S0962-8924(97)01038-6. [DOI] [PubMed] [Google Scholar]
- 12.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucci C, Wandinger-Ness A, Lutcke A, Chiariello M, Bruni C B, Zerial M. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc Natl Acad Sci USA. 1994;91:5061–5065. doi: 10.1073/pnas.91.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–170. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 15.Cranage M P, Kousarides T, Bankier A T, Satchwell S, Weston K, Tomlinson P, Barrell B, Hart H, Bell S E, Minson A C, Smith G L. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986;5:3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detrick B, Rhame J, Wang Y, Nagineni C N, Hooks J J. Cytomegalovirus replication in human retinal pigment epithelial cells. Altered expression of viral early proteins. Invest Ophthalmol Vis Sci. 1996;37:814–825. [PubMed] [Google Scholar]
- 18.Drew L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988;158:449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 19.Drubin D G, Nelson W J. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 20.Dunn K C, Aotaki K A, Putkey F R, Hjelmeland L M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 21.Fish, K., and J. Nelson. Personal communication.
- 22.Fuller S D, von Bonsdorff C-H, Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- 23.Goldenring J R, Smith J, Vaughan H D, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- 24.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–567. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 25.Gut A, Kappeler F, Hyka N, Balda M S, Hauri H P, Matter K. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanzel D, Nabi I R, Surzolo C, Powell S K, Rodriguez-Boulan E. New techniques lead to advances in epithelial cell polarity. Semin Cell Biol. 1991;2:341–353. [PubMed] [Google Scholar]
- 27.Hinck L, Nathke I S, Papkoff P, Nelson W J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland G N. AIDS: retinal and choroidal infections. In: Lewis H, Ryan S J, editors. Medical and surgical retina: advances, controversies, and management. St. Louis, Mo: Mosby; 1994. pp. 415–433. [Google Scholar]
- 29.Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry M D, Walsh K A, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kari B, Radke R, Gehrz R. Processing of human cytomegalovirus envelope glycoproteins in and egress of cytomegalovirus from human astrocytoma cells. J Gen Virol. 1992;73:253–260. doi: 10.1099/0022-1317-73-2-253. [DOI] [PubMed] [Google Scholar]
- 32.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kniess N, Mach M, Fay J, Britt W J. Distribution of linear antigenic sites on glycoprotein gp55 of human cytomegalovirus. J Virol. 1991;65:138–146. doi: 10.1128/jvi.65.1.138-146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kundu A, Avalos R T, Sanderson C M, Nayak D P. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kundu A, Nayak D P. Analysis of the signals for polarized transport of influenza virus (A/WSN/33) neuraminidase and human transferrin receptor, type II transmembrane proteins. J Virol. 1994;68:1812–1818. doi: 10.1128/jvi.68.3.1812-1818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991;115:607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisanti M P, Caras I W, Davitz M A, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisanti M P, Tang Z, Scherer P E, Sargiacomo M. Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 1995;250:655–668. doi: 10.1016/0076-6879(95)50103-7. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y-N C, Klaus A, Kari B, Stinski M F, Eckhardt K, Gehrz R C. The N-terminal 513 amino acids of the envelope glycoprotein gB of human cytomegalovirus stimulates both B- and T-cell immune responses in humans. J Virol. 1991;65:1644–1648. doi: 10.1128/jvi.65.3.1644-1648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodge R, Delamarre L, Lalonde J P, Alvarado J, Sanders D A, Dokhelar M C, Cohen E A, Lemay G. Two distinct oncornaviruses harbor an intracytoplasmic tyrosine-based basolateral targeting signal in their viral envelope glycoprotein. J Virol. 1997;71:5696–5702. doi: 10.1128/jvi.71.7.5696-5702.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodge R, Lalonde J P, Lemay G, Cohen E A. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 1997;16:695–705. doi: 10.1093/emboj/16.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou, Y., J. Xiao, S. Tugizov, and L. Pereira. Unpublished data.
- 44.Maidji E, Tugizov S, Abenes G, Jones T, Pereira L. A novel human cytomegalovirus glycoprotein, gpUS9, which functions in cell-cell spread in polarized epithelial cells, colocalizes with the cytoskeletal proteins E-cadherin, and F-actin. J Virol. 1998;72:5717–5727. doi: 10.1128/jvi.72.7.5717-5727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maidji E, Tugizov S, Jones T, Zheng Z, Pereira L. Accessory human cytomegalovirus glycoprotein US9 in the unique short component of the viral genome promotes cell-to-cell transmission of virus in polarized epithelial cells. J Virol. 1996;70:8402–8410. doi: 10.1128/jvi.70.12.8402-8410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marks M, Ohno H, Kirchhausen T, Bonifacino J D. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 47.Matter K, Yamamoto E M, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 49.Mellman I, Yamamoto E, Whitney J A, Kim M, Hunziker W, Matter K. Molecular sorting in polarized and non-polarized cells: common problems, common solutions. J Cell Sci Suppl. 1993;17:1–7. doi: 10.1242/jcs.1993.supplement_17.1. [DOI] [PubMed] [Google Scholar]
- 50.Miceli M, Newsome D, Navak L, Beuerman R. Cytomegalovirus replication in cultured retinal pigment epithelial cells. Curr Eye Res. 1989;8:835–839. doi: 10.3109/02713688909000873. [DOI] [PubMed] [Google Scholar]
- 51.Mostov K E. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 52.Mostov K E, Altschuler Y, Chapin S J, Enrich C, Low S H, Luton F, Richman E J, Singer K L, Tang K, Weimbs T. Regulation of protein traffic in polarized epithelial cells: the polymeric immunoglobulin receptor model. Cold Spring Harbor Symp Quant Biol. 1995;60:775–781. doi: 10.1101/sqb.1995.060.01.083. [DOI] [PubMed] [Google Scholar]
- 53.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia T V, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro D, Lennette E, Tugizov S, Pereira L. Humoral immune response to functional regions of human cytomegalovirus glycoprotein B. J Med Virol. 1997;52:451–459. [PubMed] [Google Scholar]
- 55.Navarro D, Paz P, Tugizov S, Topp K, LaVail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, the transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 56.Norais N, Hall J A, Gross L, Tang D, Kaur S, Chamberlain S H, Burke R L, Marcus F. Evidence for a phosphorylation site in cytomegalovirus glycoprotein gB. J Virol. 1996;70:5716–5719. doi: 10.1128/jvi.70.8.5716-5719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohno H, Fournier M C, Poy G, Bonifacino J S. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- 58.Ohno H, Stewart J, Fournier M C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 59.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pepperkok R, Scheel J, Horstmann H, Hauri H P, Griffiths G, Kreis T E. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- 61.Pereira L. Function of glycoprotein B homologues of the family herpesviridae. Infect Agents Dis. 1994;3:9–28. [PubMed] [Google Scholar]
- 62.Pereira L, Hoffman M, Tatsuno M, Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984;139:73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- 63.Pietropaolo R L, Compton T. Direct interaction between human cytomegalovirus glycoprotein B and cellular annexin II. J Virol. 1997;71:9803–9807. doi: 10.1128/jvi.71.12.9803-9807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qadri I, Navarro D, Paz P, Pereira L. Assembly of conformation-dependent neutralizing domains on human cytomegalovirus glycoprotein B. J Gen Virol. 1992;73:2913–2921. doi: 10.1099/0022-1317-73-11-2913. [DOI] [PubMed] [Google Scholar]
- 65.Radsak K, Eickmann M, Mockenhaupt T, Bogner E, Kern H, Eis H A, Reschke M. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch Virol. 1996;141:557–572. doi: 10.1007/BF01718317. [DOI] [PubMed] [Google Scholar]
- 66.Rashtchian A, Thornton C G, Heidecker G. A novel method for site-directed mutagenesis using PCR and uracil DNA glycosylase. PCR Methods Appl. 1992;2:124–130. doi: 10.1101/gr.2.2.124. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen L. Immune response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:221–254. doi: 10.1007/978-3-642-74980-3_9. [DOI] [PubMed] [Google Scholar]
- 68.Rasmussen L, Nelson M, Neff M, Merigan T C. Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology. 1988;163:309–318. doi: 10.1016/0042-6822(88)90271-1. [DOI] [PubMed] [Google Scholar]
- 69.Reschke M, Reis B, Noding K, Rohsiepe D, Richter A, Mockenhaupt T, Garten W, Radsak K. Constitutive expression of human cytomegalovirus glycoprotein B (gpUL55) with mutagenized carboxy-terminal hydrophobic domains. J Gen Virol. 1995;76:113–122. doi: 10.1099/0022-1317-76-1-113. [DOI] [PubMed] [Google Scholar]
- 70.Robinson M S. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Boulan E, Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980;20:45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Boulan E, Sabatini D D. Asymmetric budding of viruses in epithelial cell monolayers: a model system for study of epithelial cell polarity. Proc Natl Acad Sci USA. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanderson C M, Avalos R, Kundu A, Nayak D P. Interaction of Sendai viral F, HN, and M proteins with host cytoskeletal and lipid components in Sendai virus-infected BHK cells. Virology. 1995;209:701–707. doi: 10.1006/viro.1995.1308. [DOI] [PubMed] [Google Scholar]
- 74.Sanger R, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 76.Scheiffele P, Roth M G, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simons K, Fuller S D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- 78.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 79.Skibbens J E, Roth M G, Matlin K S. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989;108:821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song W, Apodaca G, Mostov K. Transcytosis of the polymeric immunoglobulin receptor is regulated in multiple intracellular compartments. J Cell Biol. 1994;269:29474–29480. [PubMed] [Google Scholar]
- 81.Spaete R R, Saxena A, Scott P I, Song G J, Probert W S, Britt W J, Gibson W, Rasmussen L, Pachl C. Sequence requirements for proteolytic processing of glycoprotein B of human cytomegalovirus strain Towne. J Virol. 1990;64:2922–2931. doi: 10.1128/jvi.64.6.2922-2931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spaete R R, Thayer R M, Probert W S, Masiarz F R, Chamberlain S H, Rasmussen L, Merigan T C, Pachl C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology. 1988;167:207–225. doi: 10.1016/0042-6822(88)90071-2. [DOI] [PubMed] [Google Scholar]
- 83.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press; 1993. pp. 201–232. [Google Scholar]
- 84.Thomas D C, Brewer C B, Roth M G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- 85.Tirabassi R S, Enquist L W. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 87.Traub L M. Clathrin-associated adaptor proteins—putting it all together. Trends Cell Biol. 1997;7:43–46. doi: 10.1016/S0962-8924(96)20042-X. [DOI] [PubMed] [Google Scholar]
- 88.Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77:61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 89.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology. 1994;201:263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 90.Tugizov S, Wang Y, Qadri I, Navarro D, Maidji E, Pereira L. Mutated forms of human cytomegalovirus glycoprotein B are impaired in inducing syncytium formation. Virology. 1995;209:580–591. doi: 10.1006/viro.1995.1290. [DOI] [PubMed] [Google Scholar]
- 91.Tugizov, S., J. Xiao, E. Maidji, and L. Pereira. Unpublished data.
- 92.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton R G. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vey M, Schafer W, Reis B, Ohuchi R, Britt W, Garten W, Klenk H D, Radsak K. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology. 1995;206:746–749. doi: 10.1016/s0042-6822(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 94.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weimbs T, Low S-H, Chapin S J, Mostov K E. Apical targeting in polarized epithelial cells: there’s more afloat than rafts. Trends Cell Biol. 1997;7:393–399. doi: 10.1016/S0962-8924(97)01130-6. [DOI] [PubMed] [Google Scholar]
- 96.Yamashita Y, Shimokata K, Mizuno S, Daikoku T, Tsurumi T, Nishiyama Y. Calnexin acts as a molecular chaperone during the folding of glycoprotein B of human cytomegalovirus. J Virol. 1996;70:2237–2246. doi: 10.1128/jvi.70.4.2237-2246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yeaman C, Le Gall A H, Baldwin A N, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng Z, Maidji E, Tugizov S, Pereira L. Mutations in the carboxyl-terminal hydrophobic sequence of human cytomegalovirus glycoprotein B alter transport and protein chaperone binding. J Virol. 1996;70:8029–8040. doi: 10.1128/jvi.70.11.8029-8040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zurzolo C, van’t Hof W, van Meer G, Rodriguez-Boulan E. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphosphatidylinositol-anchored proteins in epithelial cells. EMBO J. 1994;13:42–53. doi: 10.1002/j.1460-2075.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]