Abstract
Objective:
To prospectively analyze the feasibility of an algorithm for patient preparation, treatment planning, and selection for deep inspiration breath-hold (DIBH) treatment of left-sided breast cancer.
Methods:
From February 2017 to July 2019, 135 patients with left-sided breast cancer were selected and prepared for radiotherapy in DIBH. 99 received radiotherapy for the breast alone and 36 for the breast including the lymphatic drainage (RNI). Treatment plans DIBH and free breathing (FB) were calculated. Dosimetrical analyses were performed, and criteria were defined to assess whether a patient would dosimetrically profit from DIBH.
Results:
Of the 135 patients, 97 received a DIBH planning CT and 72 were selected for treatment in DIBH according to predefined criteria. When using DIBH, there was a mean reduction of the DmeanHeart of 2.8 Gy and DmeanLAD of 4.2 Gy. seven patients did not benefit from DIBH regarding DmeanHeart, 23 regarding DmeanLAD. For the left lung, the V20Gy was reduced by 4.9%, the V30Gy by 2.7% with 15 and 29 patients not benefiting from DIBH, respectively. In the 25 patients treated in FB, the benefit of DIBH would have been lower than for patients treated with DIBH (ΔDmeanHeart0.7 Gy vs 3.4 Gy).
Conclusion:
Dosimetrically, DIBH is no “one-fits-all” approach. However, there is a statistically significant benefit when looking at a larger patient population. DIBH should be used for treatment of left-sided breast cancer in patients fit for DIBH.
Advances in knowledge:
This analysis offers a well-designed dosimetrical analysis in patients treated with DIBH radiotherapy in an “every day” cohort.
Background
Postoperative radiotherapy after breast conserving surgery in patients with breast cancer is associated with reduced local recurrence and increased overall survival rates. 1–3 Depending on tumor stage, the local and regional lymphatic drainage should be irradiated as well. 4,5 As a downside of the treatment, postoperative irradiation of left-sided breast cancer has shown to be associated with a higher cardiac morbidity and mortality. 6–10 This was also proven for low heart doses. This cardiac mortality can compromise the benefit of an increased disease-free and overall survival gained by radiotherapy.
Deep inspiration breath-hold (DIBH) is a method to reduce the cardiac dose during breast cancer treatment. This was shown in small patient populations, although almost all patients were treated without regional lymph node irradiation (RNI). 11–22 On the assumption that the significant reduction of cardiac dose leads to a reduction in cardiac mortality, DIBH has been widely implemented in the radiotherapy of left-sided breast cancer. The question remains however, if this DIBH is a one-fits-all approach that will benefit all patients with left-sided breast cancer equally. To further elaborate on this question, we developed a comprehensive algorithm for patient preparation, treatment planning, and selection for DIBH treatment. The goal was to perform dosimetrical comparisons of DIBH and FB plans and to evaluate the feasibility of a patient selection algorithm under the assumption that not all patients are fit for treatment and equally benefit from treatment in breath-hold technique.
Methods
Patient characteristics
From February 2017 to July 2019, 135 patients were included in this analysis. The inclusion criteria were <60 years or >60 years of age for patients with a very good performance status and an expected overall survival of >10 years, no severe obesity and the consent of the patient. Until 10/2017, only patients treated without RNI were included. After that date, patients with RNI and matching inclusion criteria were included as well.
All patients had a good performance status (ECOG 0–1) at the time of first consultation. The median age was 54 years (range 30–71 years). 99 patients received breast irradiation only, 16 received additional irradiation of the supraclavicular nodes and 20 of the supraclavicular and internal mammary nodes (IMN).
DIBH treatment preparation
Patient selection, preparation, and the decision for treating in DIBH were done according to the following algorithm:
Patients included in this analysis received information about DIBH and instructions for breathing exercises upon first consultation. This included information about the differences of thoracic and abdominal breathing and the recommendations on training thoracic breathing and breath-hold in supine position. Before the planning CT scan, there was an additional DIBH training of about 30 min. If patients could hold her breath for at least 15 s and reach a breath amplitude of at least 1.2 cm, they received both, a DIBH CT scan and a free-breathing (FB) CT scan. Else both the planning CT and radiation treatment were done in free breathing. Both CT scans were contoured by the same physician. Contouring was done equally regarding organs-at-risk (OAR) and target volumes. Treatment plans were calculated for both scans by a medical physicist using Aria Eclipse (Varian Medical Systems). Both treatment plans were evaluated by the treating physician. The decision to treat in DIBH or FB was done based on pre-defined dosimetric criteria. Patients were treated in DIBH if the following criteria were fulfilled: DmeanHeart-DIBH < 5 Gy and Δ (DmeanHeart-DIBH-DmeanHeart-FB)>2 Gy, Dmean left lung <Increase 2 Gy and V20Gy/30 Gy left lung <Increase 5%. Patients were treated in FB if DmeanHeart < 5 Gy or Δ (DmeanHeart-DIBH-DmeanHeart-FB)<2 Gy.
Radiation therapy
Depending on histology (DCIS vs invasive carcinoma) and boost application, patients were treated with three different treatment concepts:
Patients treated without boost received 15 fractions with a single dose of 2.67 Gy up to a total dose of 40.05 Gy.
Patients treated with boost received 28 fractions with a single dose of 1.8 Gy up to a total dose of 50.4 Gy for the breast tissue and RNI (if applicable) and a simultaneous integrated boost to the tumor bed with a single dose of 2.25 Gy up to a total dose of 63.0 Gy
Patients treated for DCIS received 25 fractions with a single dose of 2 Gy up to a total dose of 50 Gy.
The breast tissue/thoracic wall was contoured as clinical target volume (CTV). The dorsal border included the thoracodorsal artery and the ventromedial Sternum. RNI was contoured if applicable. The boost CTV included all surgical clips (six clips) plus a 1.5 cm margin. Parts extending the breast CTV were excluded. An additional PTV margin of 8 mm in all directions was added to account for motion and setup errors both in patients treated in DIBH and FB.
Patients were treated either with 3D-tangential fields, 3D-tangential fields with additional volumetric intensity modulated (VMAT) fields or VMAT fields. Gating was done using the Varian Real-time Position Management™ (RPM) System. The marker was placed centrally caudal of the right breast, outside of the treatment fields.
Statistical analysis
For all treatment plans, the breath amplitude as well as several dose parameters were analyzed. For the heart as the main organ at risk the mean heart dose (Dmean (Gy)), the maximum heart dose (Dmax (Gy)) and the Dmean (Gy) and Dmax (Gy) of the left anterior descending coronary artery (LAD) were evaluated. As for the lung dose volume parameters (V5Gy (%), V20Gy (%), V30Gy (%)) and the lung volume (cc) were analyzed. Additionally, the Dmean (Gy) of the contralateral breast was examined. For those dose parameters, descriptive statistics in terms of mean values and standard deviation (SD) were calculated as well as the dosimetric differences between the DIBH and FB plans. Additionally, the number of patients that did not benefit from DIBH treatment (defined as difference FB – DIBH ≤0) with regard to a given dosimetric factor was analyzed.
For the statistical comparison of the DIBH and FB plans, a paired t-test was used. Because of the low total patient count, patients treated with RNI were grouped together. For statistical analysis, SPSS v.25 was used.
Results
Practical aspects on the feasibility of the preparation and patient selection algorithm
The training program was well understood by all but one patient. The compliance for the home training program was high.
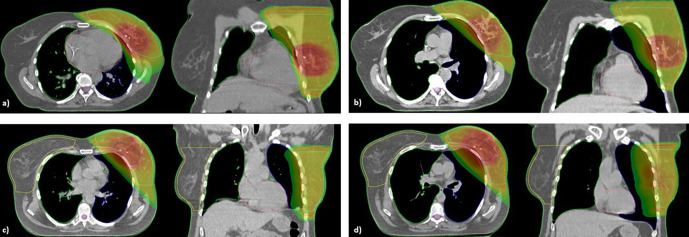
Of the 135 patients initially included, a DIBH CT scan could be acquired in 97 patients (71.1 %). The most common reasons for failure were obesity (n = 14), lack of physical stamina (n = 8), pre-existing lung disease (n = 5), a breath-hold time <15 sec (n = 4) or a breath amplitude of <1.2 cm (n = 4) and unwillingness to do DIBH (n = 4). 72 of the 97 patients receiving both a FB and DIBH CT scan (74.2 %), received radiation treatment in DIBH after evaluation of the pre-defined dosimetric criteria. In two patients, the treatment in DIBH had to be changed to FB later on due to physical exhaustion and lack of reproducibility. An example of the FB and DIBH plans two patients is shown in Figure 1a) – d). Patient 1 (Figure 1a) – b) was considered to profit from treatment in DIBH according to the dosimetric criteria while patient 2 (Figure 1c) – d)) did not.
Figure 1.
(a) – d) Example of FB and DIBH plans of 2 patients. Figure 1a) and b) show an example of a patient treated in DIBH (a) FB plan, (b) DIBH plan, Dmean heart 6.6 vs 2.1 Gy). Figure 1c) and d) show a patient treated in FB (c) FB plan, (d) DIBH plan, Dmean heart 4 vs 3.5 Gy)
Dosimetric comparison of all DIBH and FB treatment plans for each patient
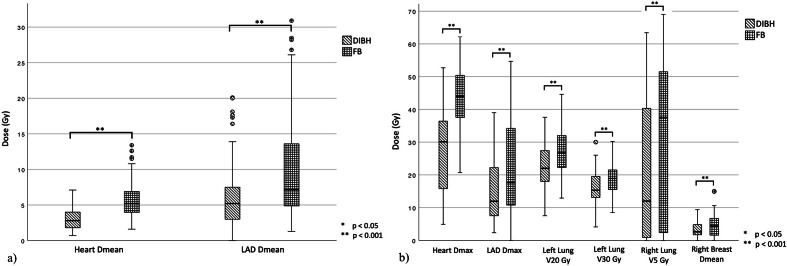
When comparing all FB (n = 97) and DIBH (n = 97) treatment plans that were calculated for each patient, statistically significant differences for all analyzed dose and dose-volume parameters were found (p < 0.001). By using DIBH, the mean heart dose could be reduced by a mean of 2.8 Gy (5.8 vs 3.0 Gy), the maximum heart dose by a mean of 16.6 Gy (43.9 Gy vs 27.3 Gy). For the LAD, those values were 4.2 Gy for Dmean and 8.1 Gy for Dmax. The number of patients who did not benefit from DIBH treatment (Difference FB – DIBH ≤ 0) differed substantially for the analyzed dose parameters. All patients benefitted in terms on lung volume (n = 97) and the majority in terms of Dmax (n = 93) and Dmean (n = 90) of the heart as well as the V20Gy of the left lung (n = 82). The parameters for which a large number of patients did not benefit were V5Gy of the right lung and Dmean of the contralateral breast with 41 and 38 patients not benefitting from DIBH. Further information can be found in Table 1 and corresponding Figure 2a and b.
Table 1.
Comparison of dose parameters for the FB and DIBH plans of all patients (n = 97)
| FB plan | DIBH plan | Mean difference FB - DIBH | sign. | non-profita | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | n (%) | ||||
| Heart | Dmean (Gy) | 5.8 | 2.6 | 3.0 | 1.4 | 2.8 | <0.001 | 7 (7.2) |
| Dmax (Gy) | 43.9 | 8.7 | 27.3 | 12.6 | 16.6 | <0.001 | 4 (4.1) | |
| Left lung | V20Gy (%) | 27.3 | 6.8 | 22.5 | 5.9 | 4.9 | <0.001 | 15 (15.5) |
| V30Gy (%) | 18.6 | 4.8 | 15.9 | 4.3 | 2.7 | <0.001 | 29 (29.9) | |
| Right lung | V5Gy (%) | 29.6 | 23.8 | 21.1 | 21.2 | 8.5 | <0.001 | 41 (42.3) |
| Contralateral breast | Dmean (Gy) | 4.3 | 3.4 | 3.2 | 2.2 | 1.1 | <0.001 | 38 (39.2) |
| LAD | Dmean (Gy) | 10.5 | 8.7 | 6.2 | 4.3 | 4.2 | <0.001 | 23 (23.7) |
| Dmax (Gy) | 23.5 | 13.9 | 15.4 | 10.3 | 8.1 | <0.001 | 20 (20.6) | |
Number of patients that did not statistically benefit from DIBH treatment with regards to the given dosimetric factor
Figure 2.
(a) and b) Comparison of dose and dose-volume parameters of the OAR of the DIBH and FB plans of all patients (n = 97) a) Dmean of the heart and the LAD b) Dose parameters of other OAR
Dosimetrical comparison of all DIBH and FB treatment plans for patients treated with or without RNI
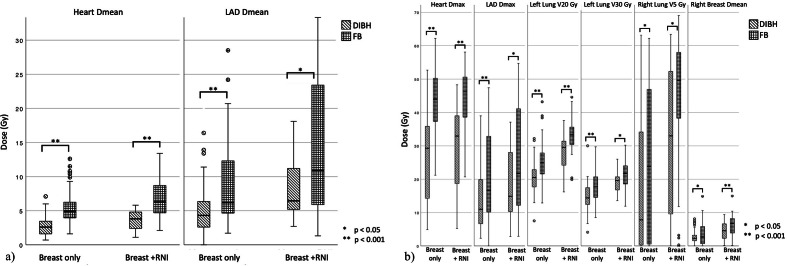
When looking at the differences in dosimetric parameters for the DIBH and FB plans for patients treated with or without RNI separately, there was a statistically significant difference for all analyzed parameters both in patients with and without RNI treatment. As an example, the average difference in mean heart dose between FB and DIBH plans was 2.6 Gy (5.4 Gy vs 2.8 Gy) in patients treated without RNI and 3.1 Gy (6.7 vs 3.6 Gy) for patients treated with RNI. Further details can be found in Table 2 and corresponding Figure 3 a and b.
Table 2.
Dose of the OAR for the FB and DIBH plans for patients treated in FB or DIBH according to the decision criteria
| Treated in FB (n = 25) | Treated in DIBH (n = 72) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FB plan | DIBH plan | sign. | FB plan | DIBH plan | sign. | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Heart | Dmean (Gy) | 3.6 | 1.4 | 2.9 | 1.6 | 0.001 | 6.5 | 2.6 | 3.1 | 1.4 | <0.001 |
| Dmax (Gy) | 39.7 | 8.4 | 21.7 | 12.0 | <0.001 | 45.4 | 8.3 | 29.3 | 12.3 | <0.001 | |
| left lung | V20Gy (%) | 23.6 | 6.1 | 21.4 | 6.7 | 0.004 | 28.6 | 6.6 | 22.8 | 5.6 | <0.001 |
| V30Gy (%) | 16.4 | 4.1 | 14.3 | 4.3 | 0.002 | 19.4 | 4.8 | 16.5 | 4.2 | <0.001 | |
| right lung | V5Gy (%) | 18.6 | 22.6 | 21.0 | 21.2 | 0.448 | 33.4 | 23.1 | 21.1 | 21.4 | <0.001 |
| right breast | Dmean (Gy) | 2.9 | 2.7 | 3.2 | 2.4 | 0.497 | 4.8 | 3.5 | 3.2 | 2.2 | <0.001 |
| LAD | Dmean (Gy) | 8.5 | 6.9 | 6.6 | 4.6 | 0.203 | 11.1 | 9.2 | 6.1 | 4.2 | <0.001 |
| Dmax (Gy) | 21.1 | 13.9 | 14.4 | 8.8 | 0.004 | 24.3 | 13.9 | 15.8 | 10.8 | <0.001 | |
Figure 3.
(a) and b) Comparison of dose and dose-volume parameters of the OAR of the DIBH and FB plans in the patients treated with (n = 26) or without RNI (n = 71) a) Dmean of the heart and the LAD b) Dose parameters of other OAR
Dosimetrical comparison of DIBH and FB plans of patients treated according to the dosimetric decision criteria
According to the predefined dosimetric decision criteria, 72 patients (74.2 %) were treated in DIBH and 25 patients were treated in FB. The percentage of patients treated in DIBH was similar for patients treated with or without RNI (74.6% breast only, 73.1% breast plus RNI). When looking at the 15 patients treated with IMN separately, 80% received treatment in DIBH.
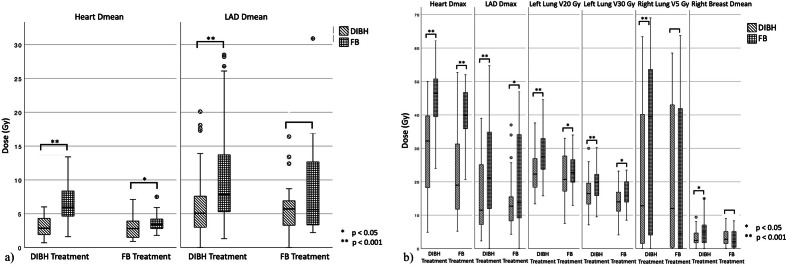
When doing a comprehensive comparison of the calculated DIBH and FB plans for patients receiving DIBH (n = 72) or FB (n = 25) treatment separately, the dose reduction by using DIBH was larger for patients treated in DIBH (acc. to the pre-defined dose criteria) for most dose parameters. The reduction in mean heart dose for the DIBH treatment plans was 0.7 Gy (3.6 Gy vs 2.9 Gy) for patients that were treated in FB and 3.4 Gy (6.5 Gy and 3.1 Gy) for patients that were treated in DIBH. For V20Gy and V30 Gy, those numbers were 2.2% vs 5.8% and 2.1% and 2.9 %, respectively. Further dose parameters regarding the OAR for the FB and DIBH plans for patients treated in FB or DIBH are shown in Figure 4, Table 3 a and b.
Figure 4.
(a and b) Comparison of dose and dose-volume parameters of the OAR of the DIBH and FB plans in the patients treated with DIBH (n = 72) and FB (n = 25) a) Dmean of the heart and the LAD; b) Dose parameters of other OAR
Table 3.
Dose of the OAR for the FB and DIBH plans for patients treated with or without RNI
| Breast without RNI (n = 71) | Breast with RNI (n = 26) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FB plan | DIBH plan | sign. | FB plan | DIBH plan | sign. | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Heart | Dmean (Gy) | 5.4 | 2.4 | 2.8 | 1.4 | <0.001 | 6.7 | 3.0 | 3.6 | 1.4 | <0.001 |
| Dmax (Gy) | 43.8 | 8.6 | 26.7 | 12.6 | <0.001 | 44.1 | 9.1 | 29.0 | 12.7 | <0.001 | |
| left lung | V20Gy (%) | 25.2 | 5.9 | 20.4 | 4.6 | <0.001 | 33.2 | 5.7 | 28.0 | 5.6 | <0.001 |
| V30Gy (%) | 17.6 | 4.4 | 14.8 | 4.0 | <0.001 | 21.6 | 4.4 | 19.1 | 3.5 | 0.008 | |
| right lung | V5Gy (%) | 24.7 | 22.8 | 17.2 | 19.4 | 0.004 | 42.8 | 21.6 | 31.7 | 22.7 | 0.010 |
| right breast | Dmean (Gy) | 3.6 | 3.2 | 2.72 | 2.00 | 0.006 | 6.2 | 3.2 | 4.38 | 2.32 | <0.001 |
| LAD | Dmean (Gy) | 8.7 | 5.7 | 5.5 | 4.0 | <0.001 | 15.1 | 12.7 | 8.1 | 4.5 | 0.001 |
| Dmax (Gy) | 22.6 | 13.1 | 14.3 | 10.1 | <0.001 | 25.9 | 16.1 | 18.4 | 10.5 | 0.001 | |
Discussion
In this analysis, we focused on the question whether DIBH is a one-fits-all approach in the treatment of left-sided breast cancer by developing an algorithm for patient selection. The goal was to dosimetrically compare the DIBH and FB plans and to evaluate the feasibility of this algorithm under the assumption that not all patients are able to tolerate treatment in and/or benefit equally from treatment in breath-hold technique.
With a mean heart dose of 3.0 Gy for the DIBH plans and 5.8 Gy for the FB plans and a max dose of 43.9 Gy and 27.3 Gy, respectively, DIBH proved to be well suited to reduce radiation dose to the heart. The effect of heart and lung sparing has been shown for different treatment techniques (3D, IMRT, VMAT) for patients after breast conserving therapy 11,13–16,18,20,22 as well as post-mastectomy radiotherapy with or without reconstruction. 12,19 With regard to the data by Darby et al, 6 especially the reduction in heart dose is likely clinically relevant. Additionally, the mean dose of the LAD could be reduced from 10.5 to 6.2 Gy by using DIBH. This has to be viewed in the context of the increasing evidence that individual constraints for substructures of the heart might be beneficial compared to the Dmean of the heart. 22–25
Regarding other organs at risk, the analyzed dose-volume parameters of the ipsilateral and contralateral lung were significantly reduced by the use of DIBH. Both effects are mainly the result of the increase in lung volume (2361 cc DIBH vs 1248 cc FB), which leads to an increase in unirradiated lung tissue as well as distance between the target volume and the heart. Also regarding the contralateral breast, the mean dose was reduced by a mean of 1.1 Gy by using DIBH. However, 39.2% of patients did not benefit from DIBH, suggesting that this effect depends on the shape of the thoracic wall and breast tissue.
For patients treated with RNI, the dose received by the OAR was generally higher. With, for example, a mean reduction of the Dmean of the heart of 3.1 Gy, they profit equally from treatment in DIBH compared to patients without RNI. This especially applies to the patients that additionally received treatment of the IMN due to the close proximity of the target volume to the heart. Yeung et al could show that patients treated with RNI show a greater decrease of heart dose when using DIBH compared to those treated without RNI, although the patient number was limited. 18
Regarding patient selection despite pre-selection and training there was a relevant amount of patients (28.9 %) that were not able to receive a DIBH CT scan. This emphasizes the point of patient selection, since patients not only have to manage the breath-hold for the planning CT but also for 3–6 weeks of therapy. It is not uncommon that patients have to be changed to FB during the treatment course. Although there was a high dropout at the CT scan, only two patients could not complete the treatment in DIBH. However, reducing the breath-hold time significantly to the point were the treatment of a single field has to be interrupted several times might reduce reproducibility. Notably, the patient cohort of this analysis is from a rural area and might not be comparable to, for example, an urban one.
The algorithm used for patient selection in this analysis is feasible, although the dosimetrical decision criteria were not optimal. Generally, not all patients had a dosimetrical benefit by using DIBH. Regarding the heart sparing as the main benefit of DIBH, the Dmean and Dmax were equal to the DIBH-plan or even lower in the FB plans in 7 (7.2 %) and 4 (4.2 %) of patients. Similarly, the LAD 23 (23.7 %) and 20 (20.6 %) did not benefit at all regarding Dmean and Dmax. Yet when looking at all patients, there is an average benefit, which is statistically significant. For 25 patients treated in FB according to the predefined dosimetrical decision criteria this still applies, although the dosimetrical benefit is smaller. The mean difference of the Dmean of the heart was 0.7 Gy for the patients treated with FB and 3.4 Gy with DIBH and there was no significant difference for the Dmean of the LAD for patients treated with FB (p = 0.2). Still, the criteria of a decrease of the mean heart dose of >2 Gy as well as the mean heart dose of 5 Gy in this analysis were too stern. Also, the initial concern about a possible increase of the lung dose was not justified. For further analyses, the maximum heart dose and the LAD should also be factored in for the decision, as well as the lung sparing.
The limitations of this analysis lie in the limited patient number. A larger cohort is desirable to further specify which patients benefit from DIBH treatment in the context of a personalized treatment. Additionally, a definition of further substructures of the heart might provide further information for radiation tailoring. Another aspect to consider is the heterogeneity in terms of volumes and fractionation schemes. Although we were opting for an analysis of an everyday cohort in a secondary hospital, this might impair the comparability within the cohort.
Conclusion
It can be stated that DIBH is no “one-fits-all” approach for the treatment of left-sided breast cancer. From a practical perspective, nearly a third of patients was unable to receive both the DIBH and FB CT. Of those receiving both CTs, a quarter of patients showed no dosimetrical benefit regarding heart or lung sparing. However, on a practical level, calculating two treatment plans for comparison is not always feasible. Since the majority of patients show a statistical significant benefit, DIBH should be applied when treating left-sided breast cancer with or without RNI. The emphasis is on selecting patients that are physically and mentally capable of a treatment in breath-hold technique.
Contributor Information
Christina Schröder, Email: Christina.schroeder@ksw.ch, Clinic for Radiotherapy and Radiation Oncology, Ruppiner Kliniken GmbH, University Hospital of the Medizinische Hochschule Theodor Fontane Brandenburg, Neuruppin, Germany ; Institute of Radiation Oncology, Cantonal Hospital Winterthur (KSW), Brauerstrasse 15, 8401 Winterthur, Switzerland .
Sebastian Kirschke, Email: s.kirschke@ruppiner-kliniken.de, Clinic for Radiotherapy and Radiation Oncology, Ruppiner Kliniken GmbH, University Hospital of the Medizinische Hochschule Theodor Fontane Brandenburg, Neuruppin, Germany .
Eyck Blank, Email: e.blank@ruppiner-kliniken.de, Clinic for Radiotherapy and Radiation Oncology, Ruppiner Kliniken GmbH, University Hospital of the Medizinische Hochschule Theodor Fontane Brandenburg, Neuruppin, Germany .
Sophia Rohrberg, Email: s.rohrberg@ruppiner-kliniken.de, Clinic for Radiotherapy and Radiation Oncology, Ruppiner Kliniken GmbH, University Hospital of the Medizinische Hochschule Theodor Fontane Brandenburg, Neuruppin, Germany .
Robert Förster, Email: robert.foerster@ksw.ch, Institute of Radiation Oncology, Cantonal Hospital Winterthur (KSW), Brauerstrasse 15, 8401 Winterthur, Switzerland ; Medical Faculty, University of Zurich (UZH), Pestalozzistrasse 3/5, 8091 Zurich, Switzerland .
André Buchali, Email: a.buchali@ruppiner-kliniken.de, Clinic for Radiotherapy and Radiation Oncology, Ruppiner Kliniken GmbH, University Hospital of the Medizinische Hochschule Theodor Fontane Brandenburg, Neuruppin, Germany ; Medizinische Hochschule Theodor Fontane Brandenburg, Neuruppin, Germany .
REFERENCES
- 1. Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML . Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis . J Clin Oncol 2000. ; 18: 1220 – 9 . doi: 10.1200/JCO.2000.18.6.1220 [DOI] [PubMed] [Google Scholar]
- 2. . Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials . Lancet 2005. ; 365: 1687 – 717 . doi: 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 3. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials . Lancet 2011. ; 378: 1707 – 16 . doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. . Internal mammary and medial supraclavicular irradiation in breast cancer . N Engl J Med 2015. ; 373: 317 – 27 . doi: 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 5. Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. . Regional nodal irradiation in early-stage breast cancer . N Engl J Med 2015. ; 373: 307 – 16 . doi: 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. . Risk of ischemic heart disease in women after radiotherapy for breast cancer . N Engl J Med 2013. ; 368: 987 – 98 . doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 7. Sardaro A, Petruzzelli MF, D'Errico MP, Grimaldi L, Pili G, Portaluri M . Radiation-Induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints . Radiother Oncol 2012. ; 103: 133 – 42 . doi: 10.1016/j.radonc.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 8. Schultz-Hector S, Trott K-R . Radiation-Induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys 2007. ; 67: 10 – 18 . doi: 10.1016/j.ijrobp.2006.08.071 [DOI] [PubMed] [Google Scholar]
- 9. Piroth MD, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R, et al. . Heart toxicity from breast cancer radiotherapy : Current findings, assessment, and prevention . Strahlenther Onkol 2019. ; 195: 1 – 12 . doi: 10.1007/s00066-018-1378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corradini S, Ballhausen H, Weingandt H, Freislederer P, Schönecker S, Niyazi M, et al. . Left-sided breast cancer and risks of secondary lung cancer and ischemic heart disease : Effects of modern radiotherapy techniques . Strahlenther Onkol 2018. ; 194: 196 – 205 . doi: 10.1007/s00066-017-1213-y [DOI] [PubMed] [Google Scholar]
- 11. Bruzzaniti V, Abate A, Pinnarò P, D'Andrea M, Infusino E, Landoni V, et al. . Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast cancer . J Exp Clin Cancer Res 2013. ; 32: 88 . doi: 10.1186/1756-9966-32-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darapu A, Balakrishnan R, Sebastian P, Hussain MRK, Ravindran P, John S . Is the deep inspiration Breath-Hold technique superior to the free breathing technique in cardiac and lung sparing while treating both left-sided post-mastectomy chest wall and supraclavicular regions? Case Rep Oncol 2017. ; 10: 37 – 51 . doi: 10.1159/000453607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayden AJ, Rains M, Tiver K . Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer . J Med Imaging Radiat Oncol 2012. ; 56: 464 – 72 . doi: 10.1111/j.1754-9485.2012.02405.x [DOI] [PubMed] [Google Scholar]
- 14. Nemoto K, Oguchi M, Nakajima M, Kozuka T, Nose T, Yamashita T . Cardiac-sparing radiotherapy for the left breast cancer with deep breath-holding . Jpn J Radiol 2009. ; 27: 259 – 63 . doi: 10.1007/s11604-009-0336-1 [DOI] [PubMed] [Google Scholar]
- 15. Pham TT, Ward R, Latty D, Owen C, Gebski V, Chojnowski J, et al. . Left-Sided breast cancer loco-regional radiotherapy with deep inspiration breath-hold: does volumetric-modulated Arc radiotherapy reduce heart dose further compared with tangential intensity-modulated radiotherapy? J Med Imaging Radiat Oncol 2016. ; 60: 545 – 53 . doi: 10.1111/1754-9485.12459 [DOI] [PubMed] [Google Scholar]
- 16. Stranzl H, Zurl B . Postoperative irradiation of left-sided breast cancer patients and cardiac toxicity . Strahlentherapie und Onkologie 2008. ; 184: 354 – 8 . doi: 10.1007/s00066-008-1852-0 [DOI] [PubMed] [Google Scholar]
- 17. Taylor CW, Wang Z, Macaulay E, Jagsi R, Duane F, Darby SC . Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013 . Int J Radiat Oncol Biol Phys 2015. ; 93: 845 – 53 . doi: 10.1016/j.ijrobp.2015.07.2292 [DOI] [PubMed] [Google Scholar]
- 18. Yeung R, Conroy L, Long K, Walrath D, Li H, Smith W, et al. . Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation . Radiat Oncol 2015. ; 10: 200 . doi: 10.1186/s13014-015-0511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dumane VA, Saksornchai K, Zhou Y, Hong L, Powell S, Ho AY . Reduction in low-dose to normal tissue with the addition of deep inspiration breath hold (DIBH) to volumetric modulated Arc therapy (VMAT) in breast cancer patients with implant reconstruction receiving regional nodal irradiation . Radiat Oncol 2018. ; 13: 187 . doi: 10.1186/s13014-018-1132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oechsner M, Düsberg M, Borm KJ, Combs SE, Wilkens JJ, Duma MN . Deep inspiration breath-hold for left-sided breast irradiation: analysis of dose-mass histograms and the impact of lung expansion . Radiat Oncol 2019. ; 14: 109 . doi: 10.1186/s13014-019-1293-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hepp R, Ammerpohl M, Morgenstern C, Nielinger L, Erichsen P, Abdallah A, et al. . Deep inspiration breath-hold (DIBH) radiotherapy in left-sided breast cancer: Dosimetrical comparison and clinical feasibility in 20 patients . Strahlenther Onkol 2015. ; 191: 710 – 6 . doi: 10.1007/s00066-015-0838-y [DOI] [PubMed] [Google Scholar]
- 22. Loap P, Kirova Y . Evaluating cardiac substructure radiation exposure in breast rotational intensity modulated radiation therapy: effects of cancer laterality, fractionation and deep inspiration breath-hold . Cancer Radiother 2021. ; 25: 13 – 20 . doi: 10.1016/j.canrad.2020.05.016 [DOI] [PubMed] [Google Scholar]
- 23. Loap P, Fourquet A, Kirova Y . Should we move beyond mean heart dose? Int J Radiat Oncol Biol Phys 2020. ; 107: 386 – 7 . doi: 10.1016/j.ijrobp.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 24. van den Bogaard VAB, Ta BDP, van der Schaaf A, Bouma AB, Middag AMH, Bantema-Joppe EJ, et al. . Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures . J Clin Oncol 2017. ; 35: 1171 – 8 . doi: 10.1200/JCO.2016.69.8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loap P, Servois V, Dhonneur G, Kirov K, Fourquet A, Kirova Y . A radiation therapy contouring atlas for cardiac conduction node delineation . Pract Radiat Oncol 2021. ; 06 Feb 2021 . doi: 10.1016/j.prro.2021.02.002 [DOI] [PubMed] [Google Scholar]