Abstract
Objective:
The present systematic review and meta-analysis compared the diagnostic performance of F-18 fludeoxyglucose positron emission tomography (18F-FDG PET) and conventional imaging, including MRI, echocardiography, and CT, in characterising cardiac masses.
Methods:
A literature search of the PubMed, Cochrane, and EMBASE databases for studies comparing the diagnostic accuracies of 18F-FDG PET and conventional imaging in characterising cardiac masses, from inception of indexing to 31 July 2020, was performed. The Quality Assessment of Diagnostic Accuracy Studies-2 tool was used to assess study quality. Sensitivity and specificity across the studies were determined, positive and negative likelihood ratios (LR+ and LR-, respectively) were calculated, and summary receiver operating characteristic curves were constructed.
Results:
Of six included studies (n = 212 patients), 18F-FDG PET demonstrated a pooled sensitivity of 0.89 (95% confidence interval [CI] 0.81–0.94) and a pooled specificity of 0.89 (95% CI 0.80–0.94). LR syntheses yielded an overall LR+ of 7.9 (95% CI 4.3–14.6) and LR- of 0.12 (95% CI 0.07–0.22). The calculated pooled diagnostic odds ratio (DOR) was 64 (95% CI 23–181). For conventional imaging, the pooled sensitivity was 0.70 (95% CI 0.57–0.81) and the pooled specificity was 0.96 (95% CI 0.88–0.98). LR syntheses yielded an overall LR+ of 16.1 (95% CI 5.8–44.5) and LR- of 0.31 (95% CI 0.21–0.46). The evaluated pooled DOR was 52 (95% CI 17–155).
Conclusion:
18F-FDG PET and conventional imaging demonstrated comparable diagnostic accuracies for the characterisation of cardiac masses. Further large multicentre studies are, however, required to corroborate the diagnostic performances of 18F-FDG PET and conventional imaging for the characterisation of cardiac masses.
Advances in knowledge:
No previous studies have comprehensively analysed the diagnostic performance of 18F-FDG PET/CT compared with conventional imaging techniques including echocardiography, CT, and MRI. According to the current study, 18F-FDG PET/CT yielded a pooled DOR of 64, whereas other conventional imaging techniques demonstrated a DOR of 52. As such, 18F-FDG PET/CT demonstrated sensitivity and specificity, with a high pooled DOR comparable with other conventional imaging modalities.
Introduction
Cardiac masses are rare but widely heterogeneous entities, and include benign, malignant, and non-tumour masses—both primary and secondary tumours. 1–3 In previous autopsy studies, the incidence of primary cardiac masses ranged from 0.001 to 0.03%, and approximately 25% of primary tumours were malignant. 2–4 Sarcoma is the most common malignant cardiac disease (95%). 2,5,6 Furthermore, metastatic tumours are more common than primary tumours, and metastasis occurs with 10% of tumours, which typically originate as primary pulmonary disease. 3,7 In addition, a heterogenous subgroup of pseudotumours, including thrombi, calcification, cysts or valvular nodules, have also been identified as cardiac masses. 8
According to a recent research letter, malignancies are usually found in the right heart chambers (28%), pericardium (32%), or pulmonary arteries (25%), which more often lead to associated pulmonary embolism and pericardial effusion, whereas benign masses are mainly located in the left heart and usually cause peripheral emboli. 9 Patients with benign tumours have been reported to experience a three-fold greater mean survival rate than those with malignancies. 9 Patients in the pseudotumour stratum were characterised by a history of congestive heart failure and also exhibited a mortality rate that was not negligible. 9
The therapeutic strategy for most cardiac tumours is complete resection. 10,11 With advances and developments in medical technologies, the rate of successful resection of cardiac masses has been increasing. 10 In malignant cases, additional postoperative chemotherapy or radiotherapy can be applied. 11 Such adjunctive therapies can significantly improve short-term prognosis, although long-term prognosis remains poor. 12–15 However, these additional therapies can lead to serious complications involving impairment of cardiac function. 15 Therefore, accurate initial identification of the nature of the cardiac mass, tumour extent, and involvement of any critical structures on non-invasive imaging are essential for establishing a management strategy and determining prognosis.
For ideal therapeutic management, it is essential to correctly identify the nature of the masses. 10 Echocardiography is the first-line imaging method, 16 while CT, or MRI is the second-line approach in clinical settings for characterising tumour morphology and location and surrounding structural information. 17,18 Nevertheless, these non-invasive imaging methods have limited utility in differentiating benign from malignant cardiac masses. As molecular imaging techniques, F-18 fludeoxyglucose positron emission tomography (18F-FDG PET), PET/CT, and PET/MRI can yield both anatomical and metabolic information. 19 However, published data regarding PET, PET/CT, and PET/MRI using 18F-FDG for cardiac masses are scarce; as such, their role in the assessment of cardiac masses has not been established. 19–22
The present meta-analysis assessed published data regarding comparison of the diagnostic performances of 18F-FDG PET and conventional imaging for characterisation of cardiac masses to obtain more evidence-based data and to provide reference for further studies.
Methods
The present study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (i.e. “PRISMA”) statement. 23
Data sources and search strategy
A structured approach was used to identify patient population, intervention(s), comparators, outcomes, and study design, in accordance with the PICOS criteria. 24 An electronic literature search of the English-language PubMed, Cochrane, and Embase databases, from inception of indexing through 31 July 2020, was performed. Additional studies identified as published references were also manually searched. The search algorithm was based on a combination of following terms: (1) “PET” OR “positron emission tomography” OR “positron emission tomography/computed tomography” OR “PET/CT” OR “positron emission tomography-computed tomography” OR “PET-CT” AND (2) “MRI” OR “magnetic resonance imaging” AND (3) “Computed tomography” OR “CT” AND (4) “Echocardiography” AND (4) “Cardiac mass” or “Cardiac tumour”.
Study selection
The inclusion criteria for potentially eligible studies were as follows: both of 18F-FDG PET and conventional imaging used to characterise cardiac masses in the same patients; the sensitivities and specificities for both 18F-FDG PET and conventional imaging were accessible in characterising cardiac masses or absolute numbers of true positive (TP), true negative (TN), false negative (FN), and false positive (FP) data were reported; and no overlapping data. Duplicate publications and those without original data, such as case reports, review articles, letters, and conference papers, were excluded.
The titles and abstracts of the retrieved articles were reviewed independently by two investigators who applied the above-mentioned selection criteria. Articles were rejected if they were clearly ineligible. The same two researchers then independently evaluated the full-text version of the included studies to determine their eligibility for inclusion.
Inclusion criteria
Studies that fulfilled the following PICOS criteria were eligible: patient population presenting with cardiac masses; imaging methods for characterisation included 18F-FDG PET/CT and conventional imaging modalities; final characterisation of cardiac masses was available as a reference standard; and comparative study design.
Studies were excluded if data could not be extracted using a 2 × 2 table, if the number of enrolled patients was <10, and if there were multiple published reports for the same study population. In the latter case, the most detailed or most recent publication was included.
Data extraction
Basic information, including author(s), year of publication, country of origin, study design (i.e. prospective or retrospective), characteristics of the enrolled patients, and technical features was collected. Each study was analysed to retrieve the number of TP, TN, FP, and FN findings of 18F-FDG PET and conventional imaging for characterisation of cardiac masses according to the reference standard. Only studies providing such complete information were ultimately included in the final meta-analysis. 23
Methodological quality assessment
To minimise biases in the selection of studies and in the extraction of data, reviewers who were blinded to the journal, author, institution, and date of publication, independently selected articles based on the inclusion criteria and assigned scores to the study design characteristics. The anonymised data were prepared by independent review and reorganisation by two different assistant researchers.
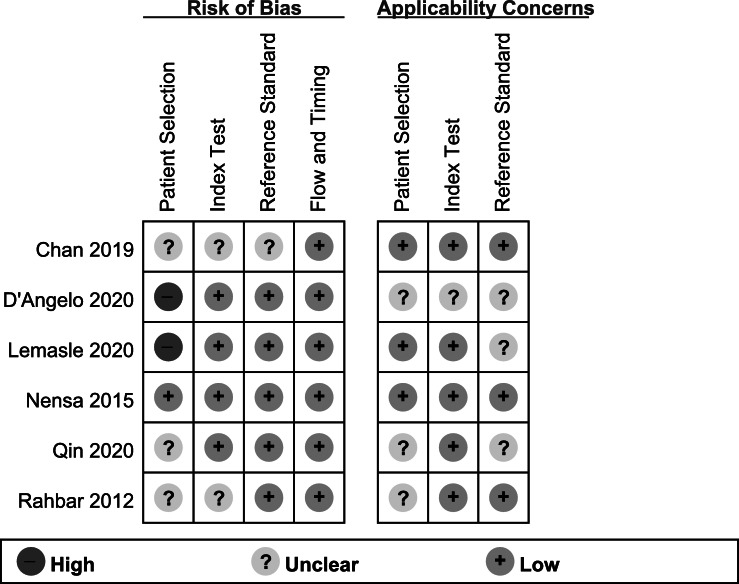
The quality of the examination results was examined using a standardised form based on the 15-item modified Quality Assessment of Diagnostic Accuracy Studies (QUADAS)−2 tool. According to the QUADAS-2 tool, overall risk of bias in patient selection was high in two (33.3%) studies, unclear in three (50%), and low in one (16.7%). Risk of bias in the index test was unclear in two (33.3%) studies and low in four (66.7%). Risk of bias in the reference standard test was unclear in one (16.7%) study and low in five (83.3%). Flow and timing in all six studies with low risk of bias. Overall applicability concerns were low (Figure 1).
Figure 1.
Risk of bias and applicability concerns summary for the assessment of study quality. Scores are based on the modified Quality Assessment of Diagnostic Accuracy Studies-2 for each included study. Symbols: (+), low risk of bias; (?), unclear risk of bias; (-), high risk of bias.
Two reviewers independently assessed each potentially eligible study and assigned a quality rating of “good,” “fair,” or “poor”. Quality assessment was based on following features: study design and presence of bias including selection, performance, recording, and reporting bias. Studies with high risk of bias were defined as poor quality, presence of moderate risk (did not affect the results) as fair quality, and those with minimal risk as good quality. Disagreements between the two authors were resolved by consensus discussion.
Data synthesis and analysis
Data were extracted from each eligible study. Categorical variables are expressed as frequencies or percentages, whereas continuous variables are expressed as mean unless indicated otherwise. Diagnostic performance, including sensitivity, specificity, and diagnostic odds ratio (DOR), are reported as point estimates with corresponding 95% confidence interval (CI). The ratio of the odds of positivity in a disease state relative to the odds of positivity in the non-disease state is expressed as DOR, with higher DOR values indicating better discriminatory test performance. 25 The I2 and the Cochrane Q test were used to assess between-study statistical heterogeneity based on random-effects analysis. 26 The bivariate random-effects model for analysis and pooling of the diagnostic performance measures across studies, as well as comparisons between different index tests were used. 27,28 The bivariate model evaluated pairs of logit transformed sensitivity and specificity from the studies, incorporating the association that may exist between sensitivity and specificity. The model was also used to create hierarchical summary receiver operating characteristic (SROC) curves and to estimate the area under the curve (AUC). 29 When statistical heterogeneity was substantial, meta-regression was performed to identify potential sources of bias. 30 Differences with a two-sided p ≤ 0.05 were considered to be statistically significant. Statistical analyses were performed using STATA v. 13.1 (StataCorp LLC, College Station, TX).
Results
Literature search and study selection
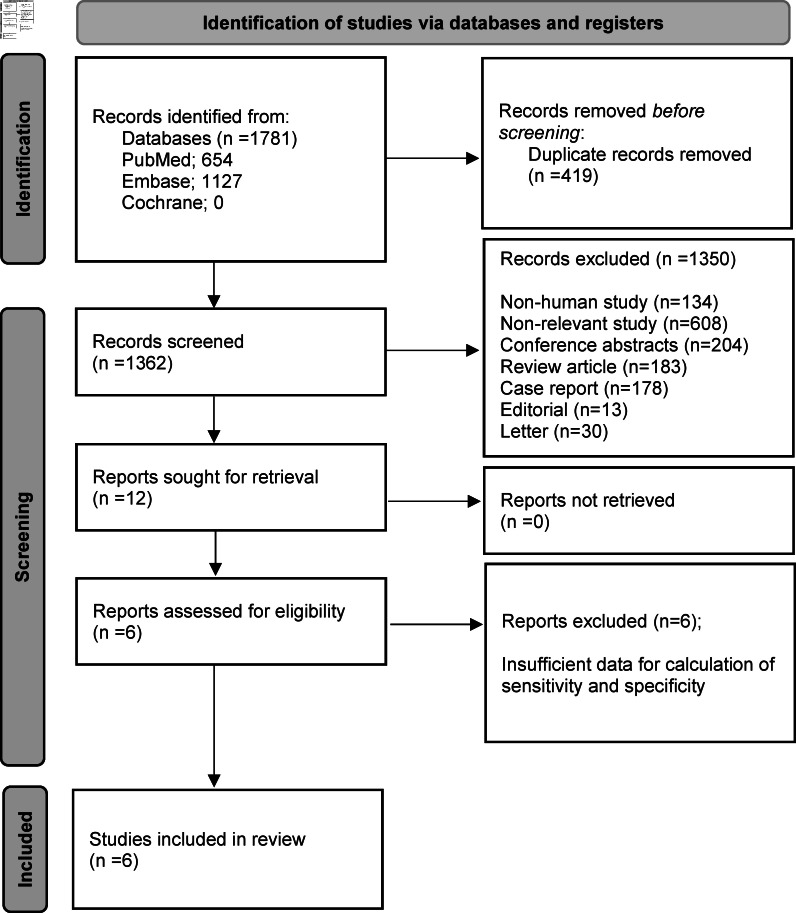
After a comprehensive computerised search was performed and references lists were extensively cross-checked, the search retrieved 1781 records, of which 419 were duplicate abstracts and excluded after reviewing the title and abstract. Additionally, 134 non-human studies, 608 non-relevant studies, 178 case reports, 204 conference abstracts, 13 editorials, 30 letters, and 183 review articles were excluded. The remaining 12 full text articles were assessed for eligibility, of which 6 were excluded due to insufficient data for the calculation of sensitivity and specificity of 18F-FDG PET and conventional imaging for characterisation of cardiac masses. Ultimately, therefore, six studies were selected and were eligible for systematic review and meta-analysis; no additional studies were found in screening the references of these articles. 21,31–36 Characteristics of the included studies are summarised in Table 1. A detailed flow diagram illustrating study selection for the current meta-analysis is shown in Figure 2.
Table 1.
Characteristics of the included studies
| Authors | Ref no | Year | Country | Study design | Analysis | Patient No | M/F | Age (Range) | F-18 FDG dose (MBq) | Conventional imaging | Diagnostic criteria of PET | Reference test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan AT | 26 | 2019 | USA | R | PB | 121 | 76/35 | 54 | 459 | CMR | VA | HP |
| D’Angelo EC | 27 | 2020 | Italy | R | PB | 60 | 37/23 | 59.4 | NR | CT | QA (SUVmax) | HP |
| Lemasle M | 28 | 2020 | France | R | PB | 119 | 70/49 | 58 | NR | CMR, CT, TEE | VA | HP |
| Nensa F | 29 | 2015 | Germany | P | PB | 20 | 7/13 | 57.5 (20–83) | 199 | CMR | QA (SUVmax) | HP |
| Qin C | 30 | 2019 | China | R | LB | 64 | 34/30 | 51.2 (33–67) | 5.5 MBq/kg | CT | QA (SUVmax) | HP |
| Rahbar K | 31 | 2012 | Germany | R | PB | 24 | 11/13 | 59 | 5 MBq/kg | NA | QA (SUVmax) | HP |
Analysis; PB, Patient-based: LB, Lesion-based
Conventional imaging; CMR, Cardiac magnetic resonance imaging: TEE, Transesophageal echocardiography
HP; Histopathology
NA; Not available
No; Number
NR; Not reported
QA; Quantitative analysis
Study design; R, Retrospective: P, Prospective
SUVmax; Maximal standardised uptake value
VA; Visual analysis
Figure 2.
Flow diagram illustrating the search strategy for eligible studies comparing the diagnostic performance of 18F-FDG PET and conventional imaging for the characterisation of cardiac masses. 18F-FDG PET, 18F-fludeoxyglucose positron emission tomography.
Study description, quality, and publication bias
Six studies were included in the current review and all analyses were based on per-patient data analysis. A total of 408 patients (235 male, 173 female) comprised the included population, with age ranging from 20 to 83 years. Of the six studies, one enrolled patients prospectively, 21 one performed the research retrospectively and prospectively, 36 and the remaining five enrolled patients retrospectively. 31–35 Three studies 21,31,33 used cardiovascular magnetic resonance as the conventional imaging modality, and three used CT, 32–34,36 and one used transesophageal echocardiography. 33 Two studies used visual analysis to interpret the 18F-FDG PET images. 31,33 Four studies used maximum standard uptake value (SUVmax) as the quantitative index for interpretation of 18F-FDG PET images. 21,32,34,35 All studies used histopathology as the reference standard for the characterisation of cardiac masses. The principal features of the six studies examined in the meta-analysis are summarised in Table 1. The test for publication bias, however, was not performed due to the small number of studies. 37
Comparison of diagnostic accuracies between 18F-FDG PET and conventional imaging modalities
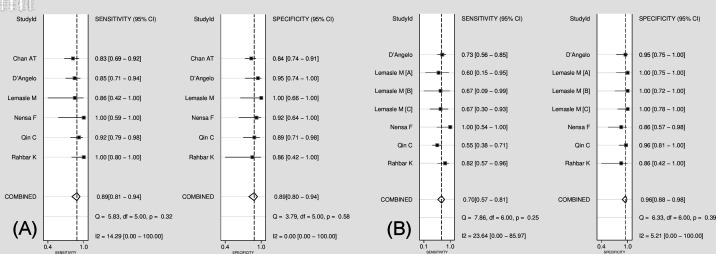
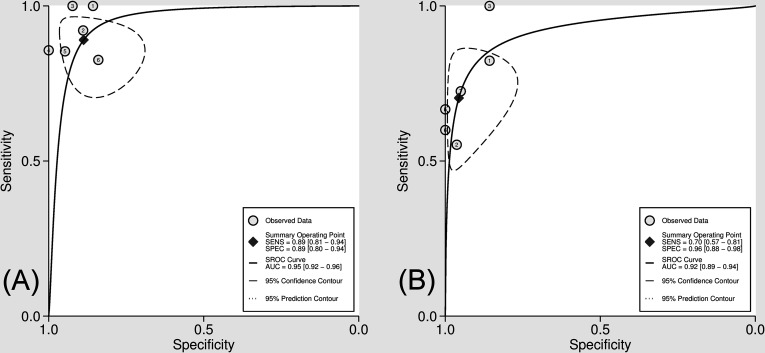
A comparison of the diagnostic performance between 18F-FDG PET and conventional imaging modalities for characterisation of cardiac masses from the six studies included in the current meta-analysis is presented in Figure 3. The pooled sensitivity of 18F-FDG PET was 0.89 (95% CI 0.81–0.94), without heterogeneity (I2 = 14.2 [95% CI 0–100]; p = 0.32), and a pooled specificity of 0.89 (95% CI 0.80–0.94) without heterogeneity (I2 = 0 [95% CI 0.0–100.0]; p = 0.58). Likelihood ratio (LR) syntheses revealed an overall positive LR+ of 7.9 (95% CI 4.3–14.6) and negative LR (LR-) of 0.12 (95% CI 0.07–0.22). The pooled DOR was 64 (95% CI 23–181). The pooled sensitivity of conventional imaging was 0.70 (95% CI 0.57–0.81), without heterogeneity (I2 = 23.6 [95% CI 0–85.9]; p = 0.25) and a pooled specificity of 0.96 (95% CI 0.88–0.98), without heterogeneity (I2 = 5.2 [95% CI 0–100]; p = 0.39). LR syntheses yielded an overall LR+ of 16.1 (95% CI 5.8–44.5) and LR- of 0.31 (95% CI 0.21–0.46) and the pooled DOR was 52 (95% CI 17–155). Figure 4 shows a hierarchical SROC curve indicating AUCs of 0.95 (95% CI 0.92–0.96) for 18F-FDG PET and 0.92 (95% CI 0.89–0.94) for conventional imaging in the characterisation of cardiac masses. Comparison of AUCs for 18F-FDG PET and conventional imaging revealed no statistical differences in the characterisation of cardiac masses (area difference 0.0116, SE; 0.0452; p = 0.7977).
Figure 3.
Comparison of forest plot for pooled sensitivity and specificity for 18F-FDG PET (A) and conventional imaging (B) for the characterisation of cardiac masses. 18F-FDG PET, 18F-fludeoxyglucose positron emission tomography.
Figure 4.
Hierarchical summary receiver operating characteristic (SROC) curves for characterisation of cardiac masses 18F-FDG PET (A) and conventional imaging (B). 18F-FDG PET, 18F-fludeoxyglucose positron emission tomography.
Discussion
In the present study, we compared the diagnostic performance of 18F-FDG PET and other conventional imaging modalities, including MRI, CT, and echocardiography. All non-invasive cardiac imaging modalities demonstrate different efficacies in management strategies for cardiac masses. 18F-FDG PET yielded results comparable with conventional imaging modalities in discriminating between benign and malignant cardiac masses.
Echocardiography has been used as the first-line imaging modality for the evaluation of cardiac masses owing to its availability, non-invasiveness, and absence of any contrast medium or radiation exposure. 16 Additionally, onsite dynamic assessments can be performed using echocardiography. 16 However, echocardiography has a limitation for the evaluation of right atrial masses, probably due to a diverse spectrum of etiologies, 38 and in ventricular masses. 39 Global evaluation of entire cardiac structures and extracardiac lesions cannot be performed using echocardiography.
CT or MRI could provide further information about the location of masses and surrounding anatomy, and tissue characterisation, which may contribute to management decisions. 40 With improved soft tissue contrast and availability of extracardiac examination, both CT and MRI have demonstrated better diagnostic performance compared with echocardiography in identifying cardiac masses. 17,18 However, echocardiography, CT, and MRI are recommended by task force teams of American societies for cardiac assessment without distinction. 41
The nature of cardiac masses can affect prognosis and subsequent treatment strategies, with most benign masses having an excellent prognosis, whereas primary and secondary malignant cardiac masses result in poor outcomes. 18F-FDG is a biomarker of glucose metabolism of tumours, pseudotumours and normal cardiovascular structures, this information can only be obtained from PET. 35 As described in a study by Rahbar et al, which included 24 patients with cardiac tumours, PET using 18F-FDG was the most efficient modality in discriminating benign from malignant cardiac lesions. 35 18F-FDG PET/CT provides the most usable information associated with prognosis in both benign and malignant cardiac masses. 33
All non-invasive imaging methods played a useful role and affected decision-making in all patients in a study comparing multimodalities for cardiac masses, including echocardiography, CT, MRI, and 18F-FDG PET or PET/CT. 33 Our data also support that all included modalities were useful for the diagnosis of cardiac masses. Different imaging modalities can complement one another for more accurate diagnosis and inform physicians in designing efficient management strategies for patients.
Although, 18F-FDG PET/CT has emerged as a major diagnostic tool in the diagnosis of cardiac tumours, it is not useful for tumours that do not take up 18F-FDG, such as well-differentiated neuroendocrine tumours. 42 Other tracers, such as Ga-68-DOTATATE, are more useful in characterising cardiac metastases from neuroendocrine tumours. 43 Therefore, the study we performed—comparing imaging modalities—remains insufficient; as such, further study is needed to guide the most appropriate diagnostic pathway to evaluate cardiac masses.
A major limitation of the present study was the considerable heterogeneity of the interpretation criteria for 18F-FDG PET for the definition of a positive PET scan in the included studies. Some studies adopted a positive result with visual evaluation according to differences in the intensity of the 18F-FDG uptake among primary masses or greater than the avidity in adjacent tissues. Other studies used different thresholds of SUVmax for quantitative indices for the positive interpretation of 18F-FDG PET scans. Moreover, in all meta-analyses, publication biases are a major concern because studies reporting significant findings are more likely to be published than those reporting non-significant results. Efforts made to minimise the risk of bias in the conduct of this review included having two researchers independently carrying out the inclusion and assessment processes.
Conclusion
18F-FDG PET and conventional imaging demonstrated comparable diagnostic performance in characterising cardiac masses. Nevertheless, further large-scale, prospective, multicentre studies are necessary to substantiate the diagnostic accuracies of 18F-FDG PET and conventional imaging for the characterisation of cardiac masses.
Contributor Information
Keunyoung Kim, Email: nmpnuh@gmail.com, Pusan National University College of Medicine, Pusan National University School of Medicine, Busan, South Korea .
Woo Seog Ko, Email: buisket@naver.com, Pusan National University College of Medicine, Pusan National University School of Medicine, Busan, South Korea .
Seong-Jang Kim, Email: growthkim@daum.net, Pusan National University College of Medicine, Pusan National University School of Medicine, Busan, South Korea .
REFERENCES
- 1. Amano J, Nakayama J, Yoshimura Y, Ikeda U . Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences . Gen Thorac Cardiovasc Surg 2013. ; 61: 435 – 47 . doi: 10.1007/s11748-013-0214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . McAllister HA, Fenoglio JJ. Tumors of the cardiovascular system: Armed Forces Institute of Pathology 1978. .
- 3. Lam KY, Dickens P, Chan A . Tumors of the heart. A 20-year experience with A review of 12,485 consecutive autopsies . Archives of Pathology & Laboratory Medicine 1993. ; 117: 10 . [PubMed] [Google Scholar]
- 4. Patel J, Sheppard MN . Pathological study of primary cardiac and pericardial tumours in a specialist UK centre: surgical and autopsy series . Cardiovasc Pathol 2010. ; 19: 343 – 52 . doi: 10.1016/j.carpath.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 5. Reynen K . Frequency of primary tumors of the heart . Am J Cardiol 1996. ; 77( 1 ): 107 . doi: 10.1016/s0002-9149(97)89149-7 [DOI] [PubMed] [Google Scholar]
- 6. Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, et al. . Primary cardiac tumors: early and late results of surgical treatment in 91 patients . Ann Thorac Surg 1999. ; 68: 1236 – 41 . doi: 10.1016/s0003-4975(99)00700-6 [DOI] [PubMed] [Google Scholar]
- 7. Chiles C, Woodard PK, Gutierrez FR, Link KM . Metastatic involvement of the heart and pericardium: CT and MR imaging . Radiographics 2001. ; 21: 439 – 49 . doi: 10.1148/radiographics.21.2.g01mr15439 [DOI] [PubMed] [Google Scholar]
- 8. Burke A, Tavora F . The 2015 WHO classification of tumors of the heart and pericardium . J Thorac Oncol 2016. ; 11: 441 – 52 . doi: 10.1016/j.jtho.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 9. Foà A, Paolisso P, Bergamaschi L, Rucci P, Di Marco L, Pacini D, et al. . Clues and pitfalls in the diagnostic approach to cardiac masses: are pseudo-tumours truly benign? Eur J Prev Cardiol 2022. ; 29: e102 – 4 . doi: 10.1093/eurjpc/zwab032 [DOI] [PubMed] [Google Scholar]
- 10. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T . Cardiac tumours: diagnosis and management . Lancet Oncol 2005. ; 6: 219 – 28 . doi: 10.1016/S1470-2045(05)70093-0 [DOI] [PubMed] [Google Scholar]
- 11. Zhu D, Yin S, Cheng W, Luo Y, Yang D, Lin K, et al. . Cardiac MRI-based multi-modality imaging in clinical decision-making: preliminary assessment of a management algorithm for patients with suspected cardiac mass . Int J Cardiol 2016. ; 203: 474 – 81 . doi: 10.1016/j.ijcard.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 12. Patel SD, Peterson A, Bartczak A, Lee S, Chojnowski S, Gajewski P, et al. . Primary cardiac angiosarcoma - a review . Med Sci Monit 2014. ; 20: 103 – 9 . doi: 10.12659/MSM.889875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrich A, Cho SI, Billett H . Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns . Cancer 2011. ; 117: 581 – 89 . doi: 10.1002/cncr.25444 [DOI] [PubMed] [Google Scholar]
- 14. Cordioli E, Pizzi C, Bugiardini R . Left ventricular metastasis from uterine leiomyosarcoma . Cardiologia (Rome 1999. ; 44: 11 . [PubMed] [Google Scholar]
- 15. Abu Saleh WK, Ramlawi B, Shapira OM, Al Jabbari O, Ravi V, Benjamin R, et al. . Improved outcomes with the evolution of a neoadjuvant chemotherapy approach to right heart sarcoma . Ann Thorac Surg 2017. ; 104: 90 – 96 . doi: 10.1016/j.athoracsur.2016.10.054 [DOI] [PubMed] [Google Scholar]
- 16. Xia H, Gan L, Jiang Y, Tang Q, Zhang P, Tang X, et al. . Use of transesophageal echocardiography and contrast echocardiography in the evaluation of cardiac masses . Int J Cardiol 2017. ; 236: 466 – 72 . doi: 10.1016/j.ijcard.2017.01.073 [DOI] [PubMed] [Google Scholar]
- 17. Araoz PA, Mulvagh SL, Tazelaar HD, Julsrud PR, Breen JF . CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation . Radiographics 2000. ; 20: 1303 – 19 . doi: 10.1148/radiographics.20.5.g00se121303 [DOI] [PubMed] [Google Scholar]
- 18. Rathi VK, Czajka AT, Thompson DV, Doyle M, Tewatia T, Yamrozik J, et al. . Can cardiovascular MRI be used to more definitively characterize cardiac masses initially identified using echocardiography? Echocardiography 2018. ; 35: 735 – 42 . doi: 10.1111/echo.14017 [DOI] [PubMed] [Google Scholar]
- 19. Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T, et al. . Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT . J Nucl Med 2012. ; 53: 856 – 63 . doi: 10.2967/jnumed.111.095364 [DOI] [PubMed] [Google Scholar]
- 20. Shao D, Wang S-X, Liang C-H, Gao Q . Differentiation of malignant from benign heart and pericardial lesions using positron emission tomography and computed tomography . J Nucl Cardiol 2011. ; 18: 668 – 77 . doi: 10.1007/s12350-011-9398-4 [DOI] [PubMed] [Google Scholar]
- 21. Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Köhler J, et al. . Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study . J Nucl Med 2015. ; 56: 255 – 60 . doi: 10.2967/jnumed.114.147744 [DOI] [PubMed] [Google Scholar]
- 22. Yaddanapudi K, Brunken R, Tan CD, Rodriguez ER, Bolen MA . PET-MR imaging in evaluation of cardiac and paracardiac masses with histopathologic correlation . JACC Cardiovasc Imaging 2016. ; 9: 82 – 85 . doi: 10.1016/j.jcmg.2015.04.028 [DOI] [PubMed] [Google Scholar]
- 23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . Int J Surg 2021. ; 88: 105906 . doi: 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement . PLoS Med 2009. ; 6( 7 ): e1000097 . doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM . The diagnostic odds ratio: a single indicator of test performance . J Clin Epidemiol 2003. ; 56: 1129 – 35 . doi: 10.1016/s0895-4356(03)00177-x [DOI] [PubMed] [Google Scholar]
- 26. Thompson SG . Why sources of heterogeneity in meta-analysis should be investigated . BMJ 1994. ; 309: 1351 – 55 . doi: 10.1136/bmj.309.6965.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH . Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews . J Clin Epidemiol 2005. ; 58: 982 – 90 . doi: 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 28. Hamza TH, van Houwelingen HC, Stijnen T . The binomial distribution of meta-analysis was preferred to model within-study variability . J Clin Epidemiol 2008. ; 61: 41 – 51 . doi: 10.1016/j.jclinepi.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 29. Rutter CM, Gatsonis CA . A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations . Stat Med 2001. ; 20: 2865 – 84 . doi: 10.1002/sim.942 [DOI] [PubMed] [Google Scholar]
- 30. Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. . Empirical evidence of design-related bias in studies of diagnostic tests . JAMA 1999. ; 282: 1061 – 66 . doi: 10.1001/jama.282.11.1061 [DOI] [PubMed] [Google Scholar]
- 31. Chan AT, Fox J, Perez Johnston R, Kim J, Brouwer LR, Grizzard J, et al. . Late gadolinium enhancement cardiac magnetic resonance tissue characterization for cancer-associated cardiac masses: metabolic and prognostic manifestations in relation to whole-body positron emission tomography . J Am Heart Assoc 2019. ; 8( 10 ): e011709 . doi: 10.1161/JAHA.118.011709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D’Angelo EC, Paolisso P, Foa A, Bergamaschi L, Magnani I, Toniolo S, et al. . Diagnostic accuracy of cardiac computed tomography and 18f-fluorodeoxyglucose with positron emission tomography/computed tomography in cardiac masses . European Heart Journal 2020. ; 41( Supplement_2 . doi: 10.1093/ehjci/ehaa946.0294 [DOI] [PubMed] [Google Scholar]
- 33. Lemasle M, Lavie Badie Y, Cariou E, Fournier P, Porterie J, Rousseau H, et al. . Contribution and performance of multimodal imaging in the diagnosis and management of cardiac masses . Int J Cardiovasc Imaging 2020; 36: 971–81. 10.1007/s10554-020-01774-z [DOI] [PubMed] [Google Scholar]
- 34. Qin C, Shao F, Hu F, Song W, Song Y, Guo J, et al. . 18F-FDG PET/CT in diagnostic and prognostic evaluation of patients with cardiac masses: a retrospective study . Eur J Nucl Med Mol Imaging 2020; 47: 1083–93. 10.1007/s00259-019-04632-w [DOI] [PubMed] [Google Scholar]
- 35. Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T, et al. . Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT . J Nucl Med 2012. ; 53: 856 – 63 . doi: 10.2967/jnumed.111.095364 [DOI] [PubMed] [Google Scholar]
- 36. Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Köhler J, et al. . Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study . J Nucl Med 2015. ; 56: 255 – 60 . doi: 10.2967/jnumed.114.147744 [DOI] [PubMed] [Google Scholar]
- 37. Di Girolamo N, Winter A, Meursinge Reynders R . High and unclear risk of bias assessments are predominant in diagnostic accuracy studies included in cochrane reviews . J Clin Epidemiol 2018. ; 101: 73 – 78 . doi: 10.1016/j.jclinepi.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 38. Kuon E, Kreplin M, Weiss W, Dahm JB . The challenge presented by right atrial myxoma . Herz 2004. ; 29: 702 – 9 . doi: 10.1007/s00059-004-2571-7 [DOI] [PubMed] [Google Scholar]
- 39. Obeid AI, al Mudamgha A, Smulyan H . Diagnosis of right atrial mass lesions by transesophageal and transthoracic echocardiography . Chest 1993. ; 103: 1447 – 51 . doi: 10.1378/chest.103.5.1447 [DOI] [PubMed] [Google Scholar]
- 40. Takahashi A, Otsuka H, Harada M . Multimodal cardiovascular imaging of cardiac tumors . Annals of Nuclear Cardiology 2016. ; 2: 61 – 67 . doi: 10.17996/ANC.02.01.61 [DOI] [Google Scholar]
- 41. Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Dehmer GJ, et al. . ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the american college of cardiology appropriate use criteria task force, american association for thoracic surgery . American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons Journal of the American College of Cardiology 2019. ; 73: 488 – 516 . doi: 10.1007/s12350-019-01751-7 [DOI] [PubMed] [Google Scholar]
- 42. Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. . Detection of atherosclerotic inflammation by 68ga-dotatate pet compared to [18f]fdg pet imaging . J Am Coll Cardiol 2017. ; 69: 1774 – 91 . doi: 10.1016/j.jacc.2017.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kunz WG, Eschbach RS, Stahl R, Kazmierczak PM, Bartenstein P, Rominger A, et al. . Identification and characterization of myocardial metastases in neuroendocrine tumor patients using 68ga-DOTATATE PET-CT . Cancer Imaging 2018. ; 18: 34 . doi: 10.1186/s40644-018-0168-2 [DOI] [PMC free article] [PubMed] [Google Scholar]