Abstract
Introduction
Weaver syndrome (WS) is a rare autosomal dominant disorder characterized by distinctive facial features, pre- and post-natal overgrowth, macrocephaly, and variable developmental delay. The characteristic facial features are ocular hypertelorism, a broad forehead, almond-shaped palpebral fissures and, in early childhood, large, fleshy ears, a pointed “stuck-on” chin with horizontal skin creases, and retrognathia. Heterozygous pathogenic/likely pathogenic variants in the enhancer of zeste homolog 2 (EZH2) gene are responsible for WS.
Case Presentation
Here, we report a male patient with a heterozygous likely pathogenic variant in EZH2 gene who has tall stature, distinctive facial features, mild development delay, hypoxic-ischemic encephalopathy with a MRI finding of periventricular leukomalacia, gingival hypertrophy, and early onset high hypermetropia.
Conclusion
This case demonstrates the importance of reporting detailed molecular and clinical findings in patients to expand the genotypic and phenotypic findings of this rare syndrome.
Keywords: Weaver syndrome, EZH2, Overgrowth, Hypermetropia, Periventricular leukomalacia
Established Facts
-
•
Weaver syndrome (WS) is a rare autosomal dominant disorder characterized by distinctive facial features, pre- and post-natal overgrowth, macrocephaly, and variable developmental delay.
-
•
Heterozygous pathogenic/likely pathogenic variants in the enhancer of zeste homolog 2 (EZH2) gene are responsible for WS.
Novel Insights
-
•
We report a patient with a heterozygous mutation in the EZH2 gene with possible new clinical finding of gingival hypertrophy and prenatal polyhydramnios.
-
•
Our patient also had findings of periventricular leukomalacia and hypermetropia, which have been reported previously in 2 and 3 patients, respectively. The presence of gingival hypertrophy and prenatal polyhydramnios in our patients suggests that they may be a component of the syndrome, and further examination of more patients should be conducted.
Introduction
Weaver syndrome (WS) (MIM: 277590) is a rare syndrome first described by Weaver et al., in 1974. It is characterized by distinctive facial features, pre- and post-natal overgrowth, macrocephaly, and variable developmental delay [Weaver et al., 1974]. The most common clinical findings in WS are tall stature, which occurs in 90% of individuals, and intellectual disability, which is observed in 80% of individuals but is often mild. Both findings are nonspecific, and their use as distinctive clinical features is limited [Tatton-Brown et al., 2013]. In 2011, mutations in the enhancer of zeste homolog 2 (EZH2) gene were linked to WS. Although families with an autosomal dominant inheritance pattern for WS have been reported, this rarely occurs [Tatton-Brown et al., 2011; Gibson et al., 2012]. Fifty-six patients with genetically confirmed WS have been reported to date [Gibson et al., 2012; Tatton-Brown et al., 2011, 2013; Al-Salem et al., 2013; Usemann et al., 2016; Suri and Dixit, 2017; Lui et al., 2018; Turkkahraman et al., 2021; Oh et al., 2023]. An increased prevalence of neuroblastoma (NBL) has been reported in individuals with heterozygous pathogenic variants of EZH2. However, data are currently insufficient to determine the absolute risk. There is insufficient evidence of the increased risk of other malignancies [Villani et al., 2017; Tatton-Brown and Rahman, 2018]. Currently, clinical practice does not recommend tumor follow-up in individuals with WS. WS is diagnosed when a pathogenic or likely pathogenic heterozygous EZH2 variant is detected via genetic testing. Here, we report an EZH2 gene variant in a Turkish boy with clinical findings consistent with WS.
Case Report
The proband, a male infant, was the third child of third-degree consanguineous parents with no reported health problems. The proband’s mother had polyhydramnios after the 20th gestational week and had cholestasis after the 32nd gestational week. The proband was born at the 35th gestation week by cesarian section due to fetal distress. The proband’s weight and height at birth were 3,800 g (>97th percentile) and 55 cm (>97th percentile), respectively. The proband was transferred to an intensive care unit due to hypoxia after delivery. Because of the seizures during the neonatal period due to perinatal asphyxia, 4 mg/kg/day phenobarbital was started. Seizures did not recur after the neonatal period, and phenobarbital was stopped at the age of 6 months. The proband’s two older siblings had no reported health problems. At the age of 4.5 months the proband was referred to the pediatric genetic outpatient clinic because of dysmorphic features. On physical examination, his age-corrected weight, height, and occipitofrontal circumference were 8,200 g (1.74 SDS), 70 cm (2.97 SDS), and 41 cm (−0.28 SDS), respectively.
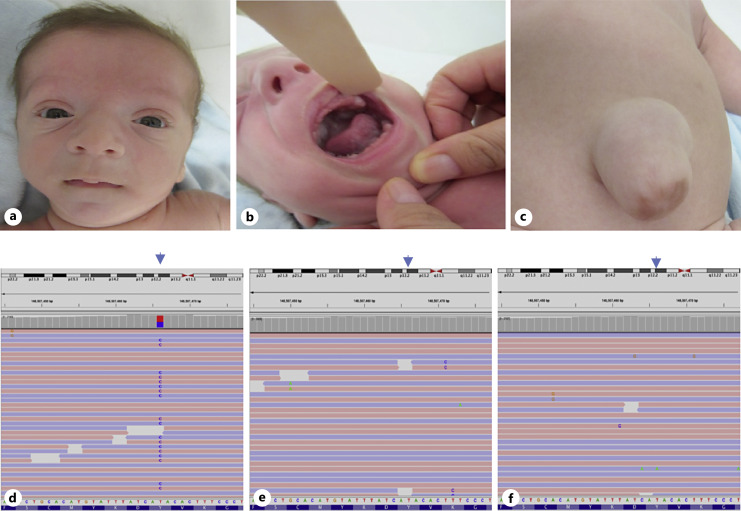
His physical examination at the age of 4.5 months revealed a short neck, large bifrontal diameter, flattened occiput, long philtrum, round face, micrognathia, prominent chin crease, hypertelorism, epicanthal folds, depressed nasal bridge, gingival hypertrophy, umbilical hernia, hydrocele, broad thumbs, large hands, prominent digit pads, deep palmar creases, and overriding toes and thin hair (Fig. 1). His neurological examination revealed axial hypotonia and appendicular hypertonia, increased deep-tendon reflexes. He had eye contact, social smiling, but no head control His ophthalmologic and audiometric evaluations were normal at the first evaluation. Abdominal ultrasonography and echocardiography were normal. Brain MRI revealed periventricular leukomalacia which was attributed to perinatal. At the age of 2 years and 10 months, his cognitive examination showed mild intellectual disability, and he was started in special education. At 3 years old high hypermetropia was detected, and he was prescribed glasses for vision correction. In clinical exome sequencing analyses, the EZH2 (NM_004456.5), c.1988A>G p.(Tyr663Cys) heterozygous missense variant was detected. Segregation analyses revealed that the variant is de novo (Fig. 1). Images of variants in the Integrative Genomics Viewer are shown in Figure 2. A different missense change in the same codon, NM_004456.5), c.1987T>A p.(Tyr663Asn) has been reported as a pathogenic variant [Tatton-Brown et al., 2011]. Considering the evidence of ACMG criteria (PM2, PP3, PM5, and PM6) the variant has been classified as likely pathogenic.
Fig. 1.
Picture of the patient at the age of 4 months (a–c). Facial features of the patient (a), gingival hypertrophy (b), umbilical hernia (c). The Integrative Genomics Viewer (IGV) (reverse strand) visualization of the EZH2 c.1988A>G variant in patient (d), in father (e), and mother (f).
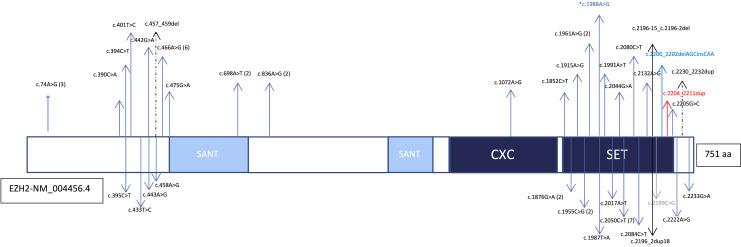
Fig. 2.
The protein domains of EZH2 and the representation of reported pathogenic variants. The number of variants is shown in parentheses. The variant detected in the present case is signified by an asterisk (*). Missense variants are indicated with blue arrows, inframe variants are signified by dashed arrows, stop codon variant are indicated with a gray arrow, a frameshift variant is shown with a red arrow, splice site variants are shown with black arrows.
Materials and Methods
Genomic DNA was extracted from EDTA-anticoagulated peripheral blood using a semi-automated robot according to Qiagen recommendations. Spectrophotometry (Nanodrop 2000, Thermo Scientific, USA) and flourometrics (Qubit v3.0, Thermo Fisher, USA) revealed the absorbance ratios for concentration and quality of the DNA samples were 260/280 nm and 260/230 nm. A capture-based Clinical Exome Solution Kit by SOPHiA GENETICS was used during preparation of the samples for next-generation sequencing.
NextSeq 500 (Illumina, USA) was used. Data quality control, alignment, variant calling, and variant annotations were performed using the Sophia DDM analysis tool (version5.2). NCBI Build37 (hg19) was used as a reference library for human genome sequences. Variants located within ± 10 base pairs of targeted exons with a minimum read depth ×50 were selected. Variants outside these regions, those in homopolymer regions, and exonic variants with a variant fraction of less than 20% were considered false positives and were not analyzed. All variants were manually inspected using the Integrative Genomics Viewer visualization tool.
The 2015 the American College of Medical Genetics and Genomics (ACMG) standards and guidelines were used for interpretations of the variants, which were classified into 5 categories (benign, likely benign, variant of unknown significance, pathogenic, likely pathogenic) [Richards et al., 2015]. Common polymorphic variants with a minor allele frequency of more than 1% were excluded. Due to the lack of genome and exome databases for the Turkish population, 1000 genome projects, dbSNP ExAC, and GnomAD population frequency databases were used as control populations. Finally, the possible effects of the variants on protein function were determined by using in silico pathogenicity prediction tools such as SIFT, PolyPhen, Mutation Taster, and GERP based on the clinical findings of the patient.
Discussion
WS was first described by David Weaver, who reported 2 male cases in 1974 [Weaver et al., 1974]. Depending on the type of germline EZH2 pathogenic variants, there is a wide phenotypic spectrum ranging from tall stature to classic WS findings. The full phenotypic spectrum of EZH2 pathogenic variants is not yet clearly known. The comparison of the patient’s clinical findings with previously reported patients is shown in Table 1. Genotype-phenotype correlations were not described in these patients. Although available data are insufficient to determine the penetration of EZH2 germline mutations, penetration may be reduced in some pathogenic EZH2 variants given the phenotypic findings of reported patients [Tatton-Brown et al., 2013].The prevalence of WS is unknown because some cases have very mild clinical features and may escape clinical diagnosis. Identifying cases with new clinical and molecular findings is important due to the phenotypic variability of WS. The mechanism of how EZH2 mutations cause WS has yet to be described. To date, 56 mutations have been identified, most of which are clustering in the SET domain and missense mutations. Of the reported mutations, only 4 are truncating mutations reported in the last exon. A patient who presented with overgrowth and significant intellectual disability had a de novo 1.2 Mb microdeletion at 7q36.1 of EZH2 [Suri and Dixit, 2017]. All reported to date in EZH2 are shown in Figure 2. Here, we report on a male patient with a missense variation in EZH2 with clinical findings with WS diagnosis.
Table 1.
The comparison of the patient’s clinical findings with previously reported patients
| Clinical findings | Literature (Tatton-Brown et al., 2011; Gibson et al., 2012; Al-Salem et al., 2013; Tatton-Brown et al., 2013; Usemann et al., 2016; Suri and Dixit, 2017; Lui et al., 2018; Turkkahraman et al., 2021; Oh et al., 2023) | Present case |
|---|---|---|
| Patients, n | 56 | + |
| Gender | 33 females, 23 males | M |
| Mutation type | 49 missenses, 1 frameshift, 1 stop codon, 1 inframe duplication, 1 inframe deletion, 2 splice sites, 1 large deletion | Missense |
| Inheritance | 13 familial, 27 de novo, 16 NA | De novo |
| Tall stature | 49/54 | + |
| Macrocephaly | 22/46 | − |
| Hypertonicity/hypotonicity | 13/43, 22/47 | +/− |
| Mild intellectual disability | 25/51 | + |
| Moderate intellectual disability | 14/51 | − |
| Severe intellectual disability | 2/51 | − |
| Cranial MRI findings* | Ventriculomegaly: 6 individuals Pachy/polymicrogyria: 2 individuals Periventricular leukomalacia: 2 individuals Cerebellar anomalies: 2 individuals | Periventricular leukomalacia |
| Umbilical hernia | 21/44 | + |
| Soft and doughy skin | 19/37 | − |
| Tumor | 5/56** | − |
| Low-pitched cry in infants | 10/27 | − |
| Hydrocele | ||
| Bone age | 29/29 | NA |
| Other findings**** | Cardiac anomalies***, Café au lait macules (2), hemangioma (4), cleft palate (3), strabismus (3), hypermetropia (3), myopia (1), neonatal hypoglycemia (2), neonatal hypocalcemia (1), gastroesophageal reflux (1), hiatal hernia (1), hearing loss (3), cryptorchidism (1), hypospadias (1), scoliosis (9), talipes equinovarus (6), polydactyly (1), afebrile seizures (4) | Gingival hyperplasia Polyhydramnios Cholestasis Afebrile seizure |
NA, not available; +, present; −, absent.
**Thirteen-year-old boy with pre-T cell non-Hodgkin’s lymphoma, 13-month-old boy with acute lymphoblastic leukemia and NBL, 4-year-old girl with NBL, 16-year-old girl with acute myeloid leukemia and secondary hemophagocytic lymphohistiocytosis, 7-month-old female with NBL.
***The numbers of individuals shown in parenthesis.
****Mitral valve prolapses in 1 patient, ventricular septal defect in 2 patients and patent ductus arteriosus in 1 patient, mitral insufficiency in 1 patient.
The clinical features associated with EZH2 are nonspecific. The classic WS signs are ocular hypertelorism, a broad forehead, almond-shaped palpebral fissures, and in early childhood, large, fleshy ears, a pointed “stuck-on” chin with horizontal skin creases, and retrognathia. Approximately half of the patients with mutations in EZH2 have classical WS facial features. The classic signs are primarily seen in patients less than 1 year of age. The proband had all classic findings of WS except an almond-shaped palpebral fissure. Tall stature (∼%90) and variable intellectual disability (∼%80) of affected individuals are the other most common findings, but both symptoms are nonspecific [Tatton-Brown et al., 2013]. Our patient had tall stature and mild intellectual disability. Specific WS findings include soft-doughy skin, a low-pitched voice, camptodactyly, and an umbilical hernia. Of these specific symptoms, our patient had only an umbilical hernia. Our patient had gingival hypertrophy postnatally and polyhydramnios prenatally. Our patient used 4 mg/kg/day phenobarbital 6 months for seizures due to perinatal asphyxia. Phenobarbital-induced gingival hypertrophy is rare. Gurbuz and Tan reported gingival enlargement due to the use of phenobarbital for more than 6 months [Gurbuz and Tan, 2010]. To our knowledge, these two findings have yet to be reported with WS. There is not enough information to discern whether there were prenatal and gingival findings in any of the 56 individuals previously described to have WS. Further investigations are needed to determine the relationship between these findings and the syndrome. Previously, hypermetropia was reported in 3 patients [Tatton-Brown and Rahman, 2018]. Our patient was diagnosed with high hypermetropia (+4 diopters or more) at the age of 3 years old. The prevalence of significant hypermetropia is rare, reported at a rate of 2.5% around 6 years of age [Mezer et al., 2015]. After combining our case with other previously reported cases of hypermetropia, the probability of hypermetropia in the syndrome is 7%. There is no clear information about the hypermetropia degrees of other reported patients. Although it is difficult to comment on whether it is an accidental finding or a part of the syndrome, we suggest that routine eye examination should be performed in the follow-ups of those with WS since high hyperopia was detected in the follow-ups while it was not detected in our patient at the initial examination.
Previously, 6 tumors have been reported in 5 of 56 affected individuals. Pre-T cell non-Hodgkins lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, and 3 NBLs have been associated with WS [Usemann et al., 2016; Tatton-Brown and Rahman, 2018; Oh et al., 2023]. The reported percentage of tumors is not small, especially for NBL, but data are insufficient to determine the absolute risk. A recent study reported NBL in a 6-month-old girl with the common NM_004456.4 (EZH2) c.2050C>T (p.Arg684Cys) mutation. Currently, clinical practice does not recommend tumor follow-up in individuals with WS. In our follow-up, we did not determine any tumors in the patient, but given the risk, follow-up for tumors is important for WS patients. Few reported cases include MRI imaging. One or more abnormalities were reported in 10 cases for which MRI was done. These anomalies include ventriculomegaly in 6 cases, pachy/polymicrogyria in 2 cases, periventricular leukomalacia in 2 cases, and cerebellar anomaly in 2 cases [Al-Salem et al., 2013; Tatton-Brown et al., 2013]. Additional information about birth history of these 2 periventricular leukomalacia patients was not defined. Our patient also had periventricular leukomalacia due to hypoxic-ischemic encephalopathy. As such, it is important to perform routine MRI to obtain more information on diagnosed WS patients.
It is often challenging to differentiate WS from other overgrowth syndromes, e.g., Sotos syndrome. Therefore, next-generation sequencing-targeted genetic analysis is crucial for making a definitive diagnosis. Even if the patient does not have classical facial features of WS, cases of overgrowth should be carefully assessed to determine if they are a form of WS. Here, we present a male patient with a de novo heterozygous missense variant in the EZH2 gene with tall stature, macrocephaly, distinctive facial features, periventricular leukomalacia, mild intellectual disability, prenatal polyhydramnios, gingival hypertrophy, and early onset high hypermetropia. Detailed reporting of clinical findings of more WS patients will increase awareness, and patients will be better managed.
Statement of Ethics
This study was performed in accordance with the Declaration of Helsinki Principles. Written informed consent was obtained from the patients’ parents for publication of this case report and any accompanying images. Ethical approval was not required for this study in accordance with local/national guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors received no specific funding for this work.
Author Contributions
The authors confirm contribution to the paper as follows: all authors contributed to the conception and design of the work. Analyses and interpretation of results were performed by Yasemin Kendir-Demirkol. The first draft of the manuscript was written by Yasemin Kendir-Demirkol. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement
The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Al-Salem A, Alshammari MJ, Hassan H, Alazami AM, Alkuraya FS. Weaver syndrome and defective cortical development: a rare association. Am J Med Genet A. 2013;161A(1):225–7. 10.1002/ajmg.a.35660. [DOI] [PubMed] [Google Scholar]
- Gibson WT, Hood RL, Zhan SH, Bulman DE, Fejes AP, Moore R, et al. Mutations in EZH2 cause Weaver syndrome. Am J Hum Genet. 2012;90(1):110–8. 10.1016/j.ajhg.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuz T, Tan H. Oral health status in epileptic children. Pediatr Int. 2010;52:279–83. [DOI] [PubMed] [Google Scholar]
- Lui JC, Barnes KM, Dong L, Yue S, Graber E, Rapaport R, et al. EZH2 mutations found in the Weaver overgrowth syndrome cause a partial loss of H3K27 histone m ethyltransferase activity. J Clin Endocrinol Metab. 2018;103(2):1470–8. 10.1111/j.1442-200X.2009.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezer E, Meyer E, Wygnansi-Jaffe T, Haase W, Shauly Y, Biglan AW. The long-term outcome of the refractive error in children with hypermetropia. Graefes Arch Clin Exp Ophthalmol. 2015;253(7):1013–9. 10.1007/s00417-015-3033-z. [DOI] [PubMed] [Google Scholar]
- Oh I, Kim B, Lee J-S, Kim MJ, Cho SI, Park SS, et al. First Korean case of Weaver syndrome along with neuroblastoma and genetic confirmation of EZH2 variant. Lab Med Online. 2023;13(1):48–52. 10.47429/lmo.2023.13.1.48. [DOI] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and Genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–24. 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri T, Dixit A. The phenotype of EZH2 haploinsufficiency-1.2-Mb deletion at 7q36.1 in a child with tall stature and intellectual disability. Am J Med Genet A. 2017;173(10):2731–5. 10.1002/ajmg.a.38356. [DOI] [PubMed] [Google Scholar]
- Tatton-Brown K, Hanks S, Ruark E, Zachariou A, Duarte SDV, Ramsay E, et al. Germline mutations in the oncogene EZH2 cause Weaver syndrome and increased human height. Oncotarget. 2011;2(12):1127–33. 10.18632/oncotarget.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton-Brown K, Murray A, Hanks S, Douglas J, Armstrong R, Banka S, et al. Weaver syndrome and EZH2 mutations: clarifying the clinical phenotype. Am J Med Genet A. 2013;161A(12):2972–80. 10.1002/ajmg.a.36229. [DOI] [PubMed] [Google Scholar]
- Tatton-Brown K, Rahman N. EZH2-related overgrowth gene reviews®; 2018. [Google Scholar]
- Turkkahraman D, Sakarya ANP, Randa NC. A novel EZH2 gene variant in a case of Weaver syndrome with postaxial polydactyly. Am J Med Genet A. 2021;185(7):2234–7. 10.1002/ajmg.a.62189. [DOI] [PubMed] [Google Scholar]
- Usemann J, Ernst T, Schafer V, Lehmberg K, Seeger K. EZH2 mutation in an adolescent with Weaver syndrome developing acute myeloid leukemia and secondary hemophagocytic lymphohistiocytosis. Am J Med Genet A. 2016;170A(5):1274–7. [DOI] [PubMed] [Google Scholar]
- Villani A, Greer MLC, Kalish JM, Nakagawara A, Nathanson KL, Pajtler KW, et al. Recommendations for cancer surveillance in individuals with RASopathies and other rare genetic conditions with increased cancer risk. Clin Cancer Res. 2017;23(12):e83–90. 10.1158/1078-0432.CCR-17-0631. [DOI] [PubMed] [Google Scholar]
- Weaver DD, Graham CB, Thomas IT, Smith DW. A new overgrowth syndrome with accelerated skeletal maturation, unusual facies, and camptodactyly. J Pediatr. 1974;84(4):547–52. 10.1016/s0022-3476(74)80675-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.