Abstract
BACKGROUND:
Adolescence is a unique period of psychosocial growth during which social adversity can negatively influence mental health trajectories. Understanding how adolescent social stress impacts males and females and why some individuals are particularly affected is becoming increasingly urgent. Social defeat stress models for adolescent male mice have been effective in reproducing some physical/psychological aspects of bullying. Designing a model suitable for females has proven challenging.
METHODS:
We report a version of the adolescent male accelerated social defeat stress (AcSD) paradigm adapted for females. Early adolescent C57BL/6J female mice (N = 107) were exposed to our modified AcSD procedure twice a day for 4 days and categorized as resilient or susceptible based on a social interaction test 24 hours later. Mice were then assessed for changes in Netrin-1/DCC guidance cue expression in dopamine systems, for inhibitory control in adulthood using the Go/No-Go task, or for alterations in dopamine connectivity organization in the matured prefrontal cortex.
RESULTS:
Most adolescent females showed protection against stress-induced social avoidance, but in adulthood, these resilient females developed inhibitory control deficits and showed diminution of prefrontal cortex presynaptic dopamine sites. Female mice classified as susceptible were protected against cognitive and dopaminergic alterations. AcSD did not alter Netrin-1/DCC in early adolescent females, contrary to previous findings with males.
CONCLUSIONS:
Preserving prosocial behavior in adolescent females may be important for survival advantage but seems to come at the price of developing persistent cognitive and dopamine deficiencies. The female AcSD paradigm produced findings comparable to those found in males, allowing mechanistic investigation in both sexes.
Adolescence is a unique and sensitive period during which adversity can greatly impact ongoing brain development and mental health trajectories. The ongoing changes in brain architecture and cognitive function, which are driven by the prolonged maturation of the prefrontal cortex (PFC) and gradual refinement in inhibitory control, render adolescents highly vulnerable to environmental challenges and more prone to developing psychiatric illness later in life (1–6). Adolescence is also an age of psychosocial growth when boys and girls spend more time socializing with peers than at any other age, and peers are their primary and most important source of appraisal (7). Unfortunately, peer victimization and cyberbullying are prevalent during adolescence (8–10) and can drastically increase internalizing disorders and psychiatric risk (10–15). However, there are important individual differences in response to social stress, with some adolescents being unaffected by social adversity and others who are susceptible being affected differently (16, 17).
Multiple factors, including age (11), influence individual differences in adolescent resilience and susceptibility to psychiatric consequences of stress (6, 18), with biological sex playing a major role. There are noticeable sex differences in the age of onset and prevalence of psychiatric disorders that emerge in adolescence, including depression and substance use disorder (19,20). Although females exposed to adversity in adolescence show a higher risk of developing mood disorders than males (21–24), sex-specific sensitivity to environmental threats seems to depend on the type of stressor and on the physiological and/or behavioral outcome examined. While females show elevated levels of salivary cortisol in response to social rejection, males react more to achievement challenges (25). Conversely, cyberbullying is associated with depression and anxiety in girls more than in boys, but it is linked to conduct disorders in boys to a greater extent than in girls (26). Understanding why/how social stress in adolescence affects males and females differently, in both the short- and long-terms, is critical and timely, considering the increasing incidence in peer victimization and depression in youth and the need to inform early detection, prevention, and intervention programs.
Modeling social stress in adolescent male and female rodents can provide insights regarding the dimorphic sensitivity to adversity. Social interactions in rodents are particularly important during adolescence, when social behaviors such as play and social exploration emerge for the first time (27–30). To reproduce certain physical and psychological aspects of the stress that is experienced by victims of bullying, researchers have used the chronic social defeat stress model in adolescent male rodents, in which a mouse (or rat) is subjected to repeated physical attacks and forced into submission by an aggressive mouse (31–42). Mice are then classified as social avoidant (susceptible) or nonsocial avoidant (resilient). We recently adapted the accelerated social defeat (AcSD) version of the chronic social defeat stress model to expose male mice to social stress during specific adolescent chronological periods (43,44) to be able to capture critical windows of vulnerability and assess possible molecular players (43–45). We found that AcSD specifically during early adolescence altered social behavior soon after exposure and had enduring detrimental consequences on impulse control (46,47). Although not all males exposed to AcSD showed impaired social behavior in adolescence, all defeated mice exhibited impulse control deficits in adulthood. This indicates that a socially avoidant phenotype is not a consistent measure of susceptibility to AcSD in adolescence and that there may be a tradeoff between protection against social deficits in adolescence and poor inhibitory control in adulthood (48). Indeed, all mice subjected to AcSD showed reduced expression of the Netrin-1 guidance cue receptor gene Dcc (46), which controls the protracted growth of dopamine axons from the nucleus accumbens (NAcc) to the PFC, an event that occurs in parallel to the gradual refinement of impulse control (49–51).
Because dominance hierarchies in C57BL/6J mice involve males fighting against males, but not females, building a model of adolescent social defeat stress in female mice has been challenging for many groups. Here we sought to overcome this limitation by modifying and adapting our adolescent AcSD male model to female mice. Using a combination of behavioral, molecular, anatomical, and cognitive measures, we were able to assess, for the first time, the short- and long-term impact of social defeat stress specifically in early adolescent female mice. We were also able to compare these effects to those that have been reported in male mice exposed to social stress during early adolescence. Previous findings have shown substantial sex differences in response to social stress in adolescent rats (52–57) and in sensitivity to the effects of drugs of abuse in mice (44). Therefore, we hypothesized divergent short and/or long-term outcomes of AcSD in female mice compared with the ones that we previously reported in males (46).
METHODS AND MATERIALS
Animals
Experiments were performed in accordance with the Canadian Council of Animal Care and approved by the McGill University and Douglas Hospital Animal Care Committee.
AcSD Stress Paradigm for Adolescent Female Mice
We used the AcSD stress paradigm (Figure 1A) that we used in our previous studies with male mice (46,47) but made modifications for early adolescent females. This paradigm consists of CD-1 screening and priming for aggressive behavior followed by the AcSD (see the Supplemental Methods and Materials in Supplement 1 for a full description). Briefly, during phase 1 of the priming and screening of CD-1 aggressor, an adult male C57BL/6J mouse is introduced to a male CD-1 mouse’s home for 3 consecutive days. During phase 2, an adult male C57BL/6J mouse is introduced to the CD-1’s home cage for only 30 seconds and is then immediately replaced by an early adolescent (postnatal day [PND] 24–25) female C57BL/6J mouse for 5 minutes. This priming is done twice a day (9:00 am and 2:00 pm) and lasts 3 to 4 days until a subset of CD-1 mice become consistently aggressive toward the adolescent female mice. Adolescent female mice used during the priming phase are not used in the rest of the experiment.
Figure 1.
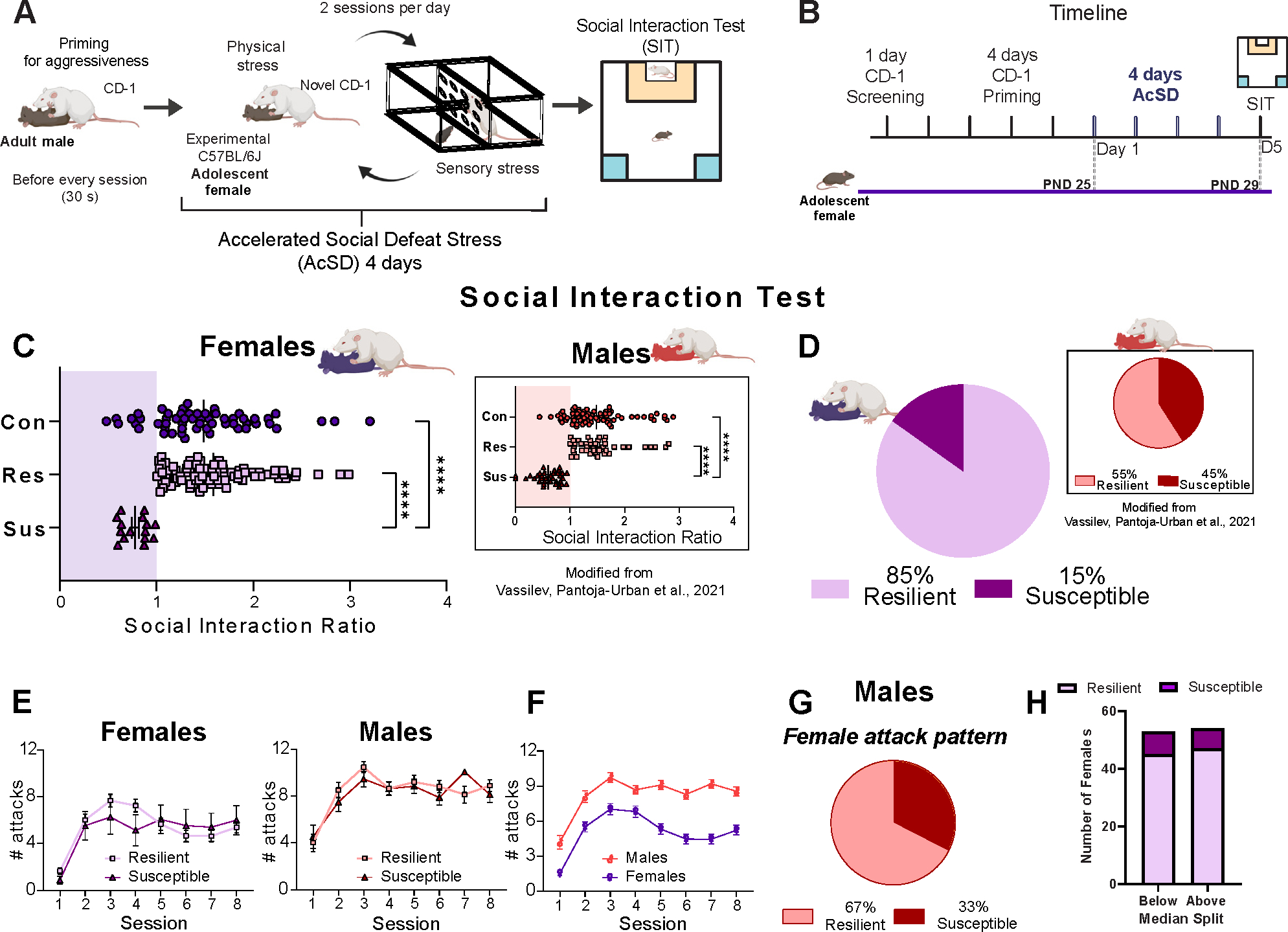
High proportion of resilient female mice after exposure to AcSD stress. (A) Graphic representation of AcSD stress paradigm for adolescent mice. (B) Timeline of AcSD stress in female mice. (C) SIT results after AcSD in female adolescent mice; significantly different at ****p < .0001. Inset: Data reproduced from Vassilev et al. (46) showing SIT after AcSD for adolescent male mice (significantly different at ***p < .001). (D) Proportion of resilient and susceptible female mice. The proportion of the resilient phenotype was higher in females than in males. Inset: Data reproduced from (46) showing that most males exposed to AcSD in adolescence showed resilience. (E) The number of attacks between resilient and susceptible mice is not significantly different in either females or males. (F) Female mice received fewer attacks than male mice during the AcSD. (G) Results from the AcSD experiment with adolescent males exposed to the “female” pattern of attacks. The resilient vs. susceptible proportion does not differ from the one that we found using the typical male AcSD protocol. (H) Median split on the cumulative number of received attacks in female mice. The proportion of females that were categorized as susceptible or resilient does not differ between the low and high received attack groups. All data are shown as mean ± SEM. AcSD, accelerated social defeat; con, control; PND, postnatal day; res, resilient; SIT, social interaction task; sus, susceptible.
The AcSD consists of 2 defeat sessions per day for a total of 4 consecutive days during which an adult C57BL/6J mouse is introduced into the CD-1 compartment for a period of 30 seconds (to prime the CD-1 for aggression) and is immediately replaced by an experimental female adolescent (PND 25) C57BL/6J mouse, which is left with the CD-1 for 10 minutes or until 10 attacks occur (Table 1). Control C57BL/6J adolescent females are housed in similar 2-compartment rat cages with a conspecific every day, and no physical interaction occurs between conspecific mice. Twenty-four hours after the last AcSD session, C57BL/6J adolescent mice are assessed in a social interaction task (SIT) to measure approach and/or avoidance behavior toward a novel adult male CD-1 social target (58,59). The amount of time spent (seconds) in the interaction zone is estimated when a target is absent and when the target is present. The amount of time between sessions 1 and 2 is approximately 1 minute. The social interaction ratio is calculated as the amount of time spent in the interaction zone with the target divided by the amount of time spent in the interaction zone without the target. Defeated mice with a ratio < 1.00 are classified as susceptible, and those with a ratio > 1.00 are classified as resilient (Figure 1B).
Table 1.
Definition of an Attack During the Accelerated Social Defeat Paradigm
| Attack Conditions | Description |
|---|---|
| 1 | An attack is defined as “when the CD1 mouse bites the C57BL/6J mouse, and the C57BL/6J mouse moves away in response to the bite.” |
| 2 | A bite is defined as “when the CD1 mouse places its teeth on any part of the C57BL/6J mouse’s body.” |
| 3 | Moving away is defined as “when the C57BL/6J mouse moves both of its hind paws from the position they were in before the CD1 engaged it.” |
| 4 | There must be ~2 seconds between separate attacks. If more than 1 bite occurs, 2 seconds apart, count as 1 attack. |
| 5 | If the CD1 mouse bites more than once in succession without a break (<~2 s apart), gently separate animals. |
| 6 | If the CD1 mouse bites and does not let go, gently separate animals. |
| 7 | If the C57BL/6J mouse becomes trapped in a corner or is pinned down by the CD1 mouse and cannot move away in response to a bite, gently separate animals with a ruler. |
| 8 | In attack conditions 5–7, count as 1 attack (up to separation). |
Elevated Plus Maze
Twenty-four hours after the SIT, females were tested in the elevated plus maze to assess risk taking–like behavior.
Go/No-Go Task
The Go/No-Go task was used to measure inhibitory control in adulthood as in (46,47,50,60). Responses to the Go cue were recorded as hits, and responses to the No-Go cue were recorded as commission errors and considered a measure of action impulsivity (61–63).
Molecular and Neuroanatomical Analysis
A description of Western blot, quantitative polymerase chain reaction, and stereology of PFC tyrosine hydroxylase immunofluorescence experiments is in the Supplemental Methods and Materials in Supplement 1.
Experimental Design and Statistical Analyses
Statistical analyses are described in Supplemental Methods and Materials in Supplement 1 and in Supplement 2.
RESULTS
The AcSD Model Adapted for Female Mice Reveals That Most Females Do Not Show Stress-Induced Susceptibility in Adolescence
The modified AcSD model (Figure 1A) leads to consistent attacks from the CD1 aggressor to the experimental adolescent female mice—as operationally defined in Table 1—across all 8 sessions. The majority (85%) of female mice that were exposed to AcSD did not display social avoidance in the SIT (i.e., resilient phenotype). This finding was replicated in 6 different cohorts (Figure S1 in Supplement 1) and is shown as pooled data (Extended data Figure 1C in Supplement 2). The proportion of resilience in females is significantly greater than the proportion of resilience that we reported in early adolescent males [55% from (46)] (Extended data Figure 1D in Supplement 2). Importantly, there was no difference in the number of received attacks between resilient and susceptible male and female groups (Extended data Figure 1E in Supplement 2). However, when comparing the number of attacks between females and males [using the male data derived from our published study (46)], we found that females received fewer attacks than males across all defeat sessions (Extended data Figure 1F in Supplement 2).
To test whether reduced physical harm in females could account for their increased resilience, we adopted 2 strategies. First, we reproduced the “female” pattern of attacks in a cohort of adolescent males using the limited-attacks strategy that we have described previously (46). This manipulation does not significantly alter the proportion of resilient (67%) versus susceptible (33%) phenotypes in males (Extended data Figure 1G in Supplement 2). Second, we performed a median split on the cumulative number of received attacks by the females used in all the experiments (N = 107). The proportion of females that were categorized as susceptible or resilient did not differ between the low and the high received attack groups (Extended data Figure 1H in Supplement 2). This was also true when we compared the proportion of susceptibility and resilience between the 75th and 25th percentile of the female attack distribution (Extended data Figure 1H in Supplement 2). These results showed that the AcSD model can be used to study the effects of social defeat stress in adolescence in both males and females and that females are less vulnerable to exhibiting social avoidance to an adult aggressor male, regardless of the number of attacks received. Moreover, an awake, behaving social target is necessary for susceptible defeated females to show social avoidance following adolescent AcSD (Figure S2 in Supplement 1).
To assess whether exposure to AcSD in adolescent females alters risk taking–like behavior, we tested them in the elevated plus maze 48 hours after exposure (Figure 2A). The percent of time spent in the open arms was similar across the control, resilient, and susceptible groups (Extended data Figure 2B in Supplement 2), and there was no correlation between the amount of time spent in the interaction zone with the target present in the SIT and the percentage of time spent in the open arms (Extended data Figure 2C in Supplement 2). In our previous studies with males, we found increased risk taking–like behavior in the elevated plus maze test in the resilient group compared with both the control and susceptible groups, and this change was positively correlated with social avoidance behavior (46).
Figure 2.
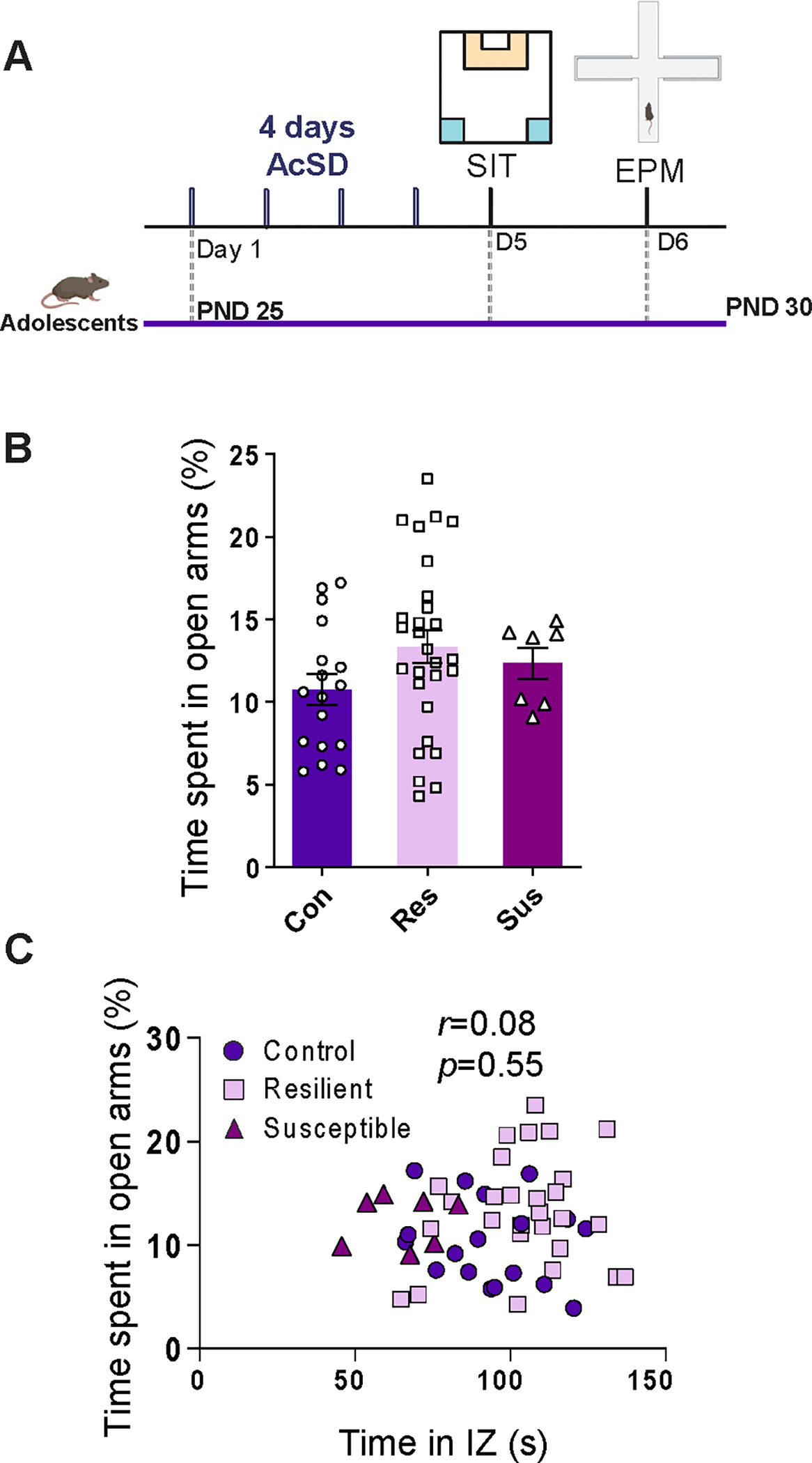
AcSD in adolescent female mice did not lead to changes in risk taking–like behaviors. (A) Experimental timeline. (B) AcSD in adolescent female mice did not lead to changes in risk taking–like behavior measured by the EPM test, p = .18. (C) There was no correlation between the amount of time spent in the open arms of the EPM and the amount of time spent in the IZ during the SIT in female mice, Pearson’s r53 = 0.08, p = .55. All data are shown as mean ± SEM. AcSD, accelerated social defeat; con, control; EPM, elevated plus maze; IZ, interaction zone; PND, postnatal day; res, resilient; SIT, social interaction task; sus, susceptible.
Social Stress in Adolescence Does Not Alter the Netrin-1/DCC Pathway in the Mesolimbic Dopamine System in Females
To determine whether AcSD in adolescence dysregulates the Netrin-1/DCC pathway in female mice, we measured Dcc messenger RNA (mRNA) expression in the ventral tegmental area—where 99% of dopamine neurons express Dcc (64)—and Netrin-1 protein levels in the NAcc 1 week later (Figure 3A). We found no difference in Dcc mRNA or Netrin-1 levels across the control, resilient, and susceptible groups (Extended data Figure 3B in Supplement 2). These findings contrast with the selective dysregulation of the Netrin-1/DCC pathway that we have observed in males exposed to AcSD during adolescence (46). Dcc mRNA is highly enriched in ventral tegmental area dopamine neurons, with no difference between the sexes in the percentage of dopamine neurons expressing this transcript (64).
Figure 3.
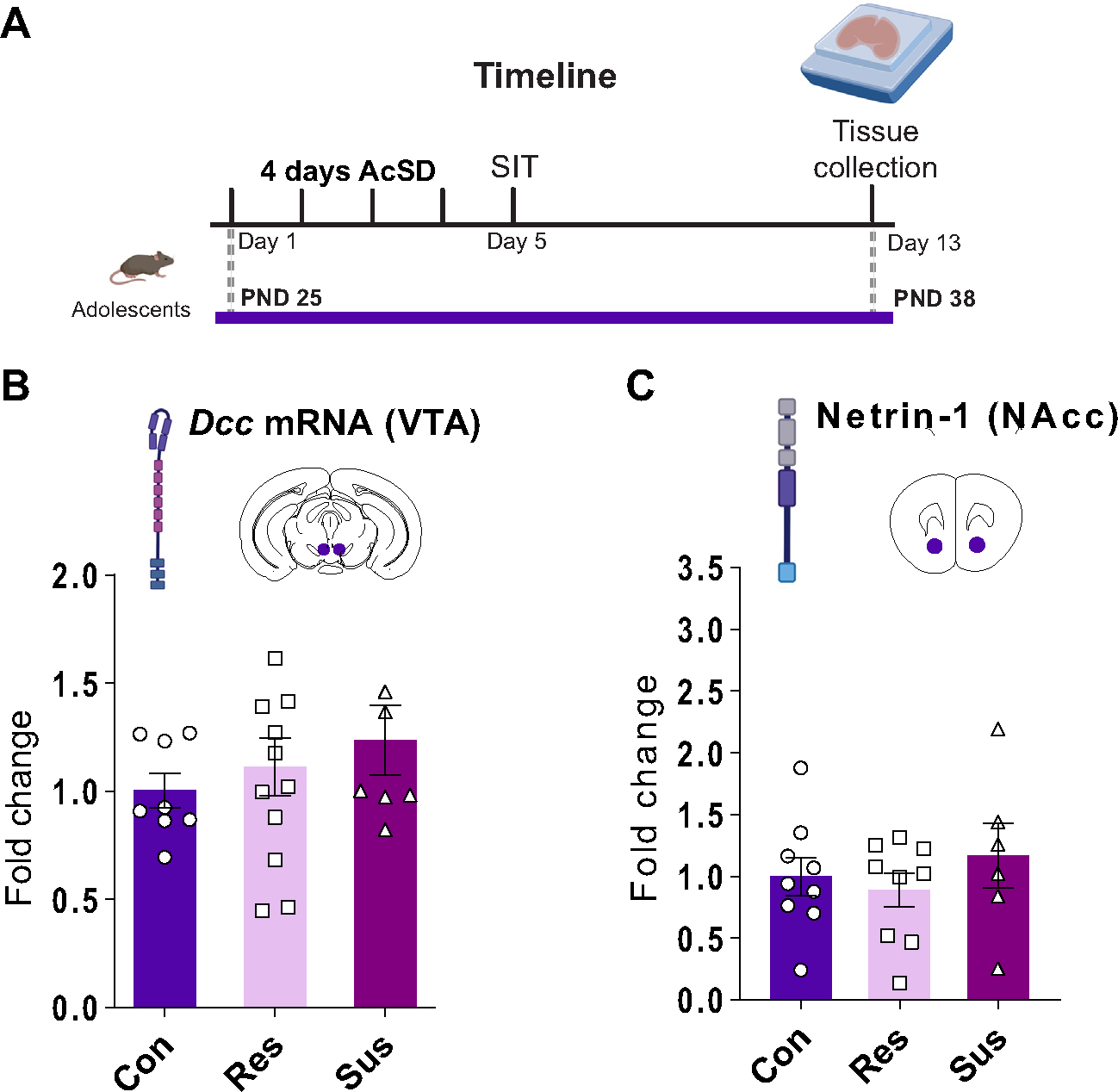
Following accelerated social defeat in adolescence, there were no changes in the Netrin-1/DCC system in female mice. (A) Experimental timeline. (B) Accelerated social defeat in adolescent female mice did not lead to changes in Dcc mRNA expression in the VTA. (C) Accelerated social defeat in adolescent female mice did not lead to changes in Netrin-1 protein levels in the NAcc. All data are shown as mean ± SEM. AcSD, accelerated social defeat; con, control; mRNA, messenger RNA; NAcc, nucleus accumbens; PND, postnatal day; res, resilient; SIT, social interaction task; sus, susceptible; VTA, ventral tegmental area.
Only Resilient Females Show Inhibitory Control Deficits in Adulthood
We used the Go/No-Go task to assess the effect of AcSD in adolescence on inhibitory control in adulthood in female mice (Figure 4B). Resilient females exhibited a greater proportion of commission errors than controls in adulthood. This effect was evident in the last sessions of the task. Surprisingly, the proportion of commission errors in susceptible mice that showed social avoidance in adolescence was similar to that of control mice (Extended data Figure 4C in Supplement 2).
Figure 4.
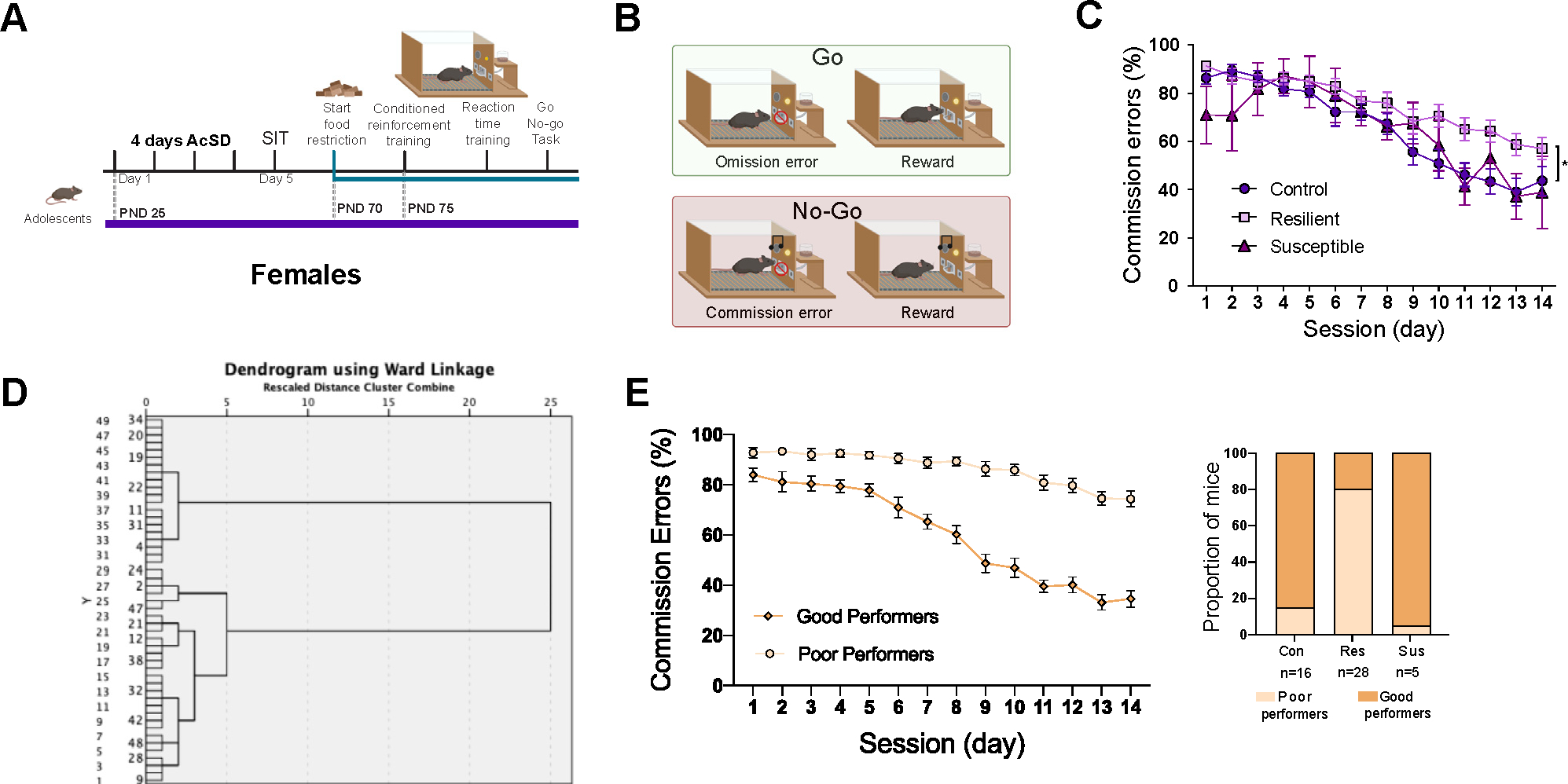
Only resilient female mice showed AcSD-induced deficits in impulse control in adulthood. (A) Experimental timeline. (B) Graphic representation of the Go/No-Go task. (C) Resilient female mice maintained a higher proportion of commission errors in comparison to control and susceptible mice, significantly different at *p < .05. (D) Dendrogram showing hierarchical clustering of subjects based on percentage of commission errors during 14 days of the Go/No-Go task. (E) Cluster analysis classified cases into one of two groups, good and poor performers, based on commission error scores. Most resilient females were classified in the poor performance cluster, compared with most control and susceptible females, which were classified in the good performer cluster. All data are shown as mean ± SEM. AcSD, accelerated social defeat; con, control; PND, postnatal day; res, resilient; SIT, social interaction task; sus, susceptible.
Next, because of the differences in sample sizes across groups and the large individual differences in action impulsivity in females, we performed an unbiased analysis of the No-Go performance data to classify them into good versus poor performers. We conducted agglomerative hierarchical clustering analysis using the Ward method on the proportion of commission errors. The dendrogram revealed 2 main clusters (Figure 4D) (k = 2) corresponding to good and poor performers in the No-Go trials (Figure 4E). The majority of both control (85%) and susceptible (95%) mice were classified in the good performance cluster. In contrast, defeated mice that showed resilience (80%) were mostly classified in the poor performance cluster (Extended data Figure 4E in Supplement 2). These findings corroborate that female mice that do not show AcSD-induced social avoidance in adolescence are vulnerable to enduring deficits in inhibitory control. Instead, socially avoidant defeated females show protection against developing deficits in action impulsivity in adulthood. These findings raise the intriguing possibility that preserving social behavior in adolescence occurs at the expense of developing cognitive dysfunction in adulthood (48).
AcSD in adolescence did not alter the proportion of hits in females, indicating that defeated and control mice engaged in the task equally (Figure S3 in Supplement 1). To rule out differences in motivation for food reward, we tested adult female mice on a progressive ratio task. We found no difference in progressive ratio performance across groups (Figure S4 in Supplement 1).
In females, stress-induced susceptibility in adolescence predicts vulnerability to cognitive deficits in adulthood, with “protection” in adolescence predicting vulnerability in adulthood. In our study with males, we showed that defeated males developed deficits in action impulsivity in adulthood regardless of their behavior in the SIT in adolescence (46). Furthermore, hierarchical clustering analysis of the proportion of commission errors data collected in our previous study with males (46) show that most male control mice were classified in the good performance cluster, but almost all defeated mice were classified in the poor performance cluster (Figure S5 in Supplement 1). Therefore, resilience and susceptibility to short- and long-term effects of AcSD in adolescence are specific to the behavioral outcome and vary as a function of sex.
Resilient Females in Adolescence Show a Depletion of Presynaptic Sites in PFC Dopamine Axons in Adulthood
To assess whether AcSD in adolescent females affects the developing dopamine system, we quantified the expanse of the dopamine projection to the cingulate, prelimbic, and infralimbic subregions of the PFC in adulthood. We also examined the number and density of dopamine varicosities in these regions (Figure 5A, B). There were no significant differences across groups in the volume that dopamine axons occupied in the cingulate, prelimbic, and infralimbic subregions (Extended data Figure 5C in Supplement 2). Alterations in the volume that PFC dopamine axons occupy are a proxy for changes in mesocortical dopamine axon growth in adolescence, which is a process mediated by DCC receptor signaling (50). The lack of AcSD-induced changes in this metric is consistent with the lack of changes in DCC receptor expression in dopamine neurons observed in adolescence. However, there is a trend suggestive of a reduction in the total number of dopamine varicosities in mice that display resilience in adolescence (Extended data Figure 5D in Supplement 2). In fact, dopamine varicosity density was reduced in adult females that displayed resilience to stress-induced social avoidance in adolescence (Extended data Figure 5E in Supplement 2). These findings contrast with our studies in males, in which defeated mice, regardless of social avoidance phenotype, showed both Dcc mRNA downregulation in dopamine neurons in adolescence and increased PFC dopamine input volume in adulthood (46).
Figure 5.
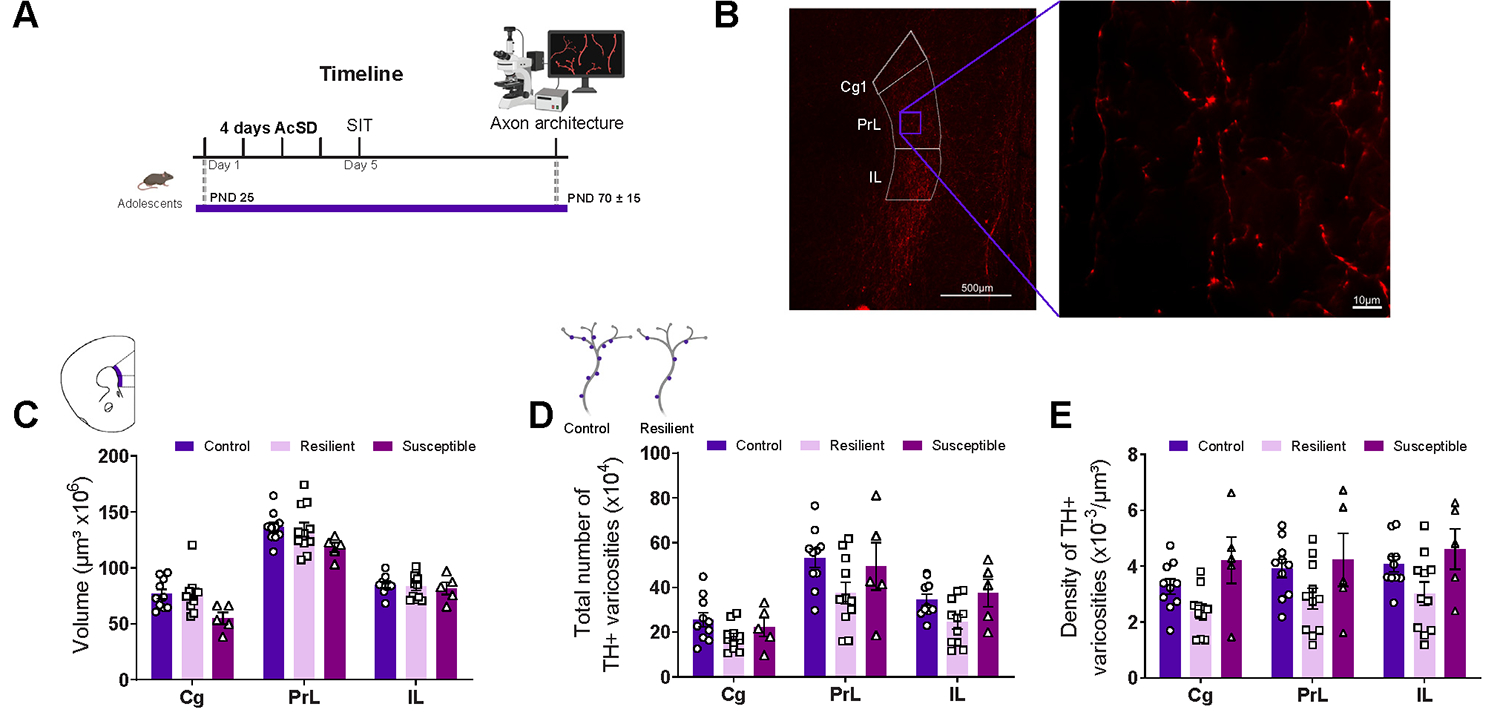
AcSD in adolescence depleted presynaptic sites from dopamine axons in the prefrontal cortex in adulthood, but only in resilient females. (A) Timeline of the experiments. (B) Photomicrographs show TH-immunolabeled dopamine axons in the Cg, PrL, and IL subregions of the prefrontal cortex (5× and 100× magnification). White arrows indicate examples of TH-positive varicosities. (C) There were no differences across groups in estimated dopamine input volume in any of the subregions. (D, E) Resilient female mice showed reduced (D) total number and (E) density of dopamine varicosities compared with control mice. All data are shown as mean ± SEM. AcSD, accelerated social defeat; Cg, cingulate; IL, infralimbic; PND, postnatal day; PrL, prelimbic; SIT, social interaction task; TH, tyrosine hydroxylase.
Social Stress in Adolescence Leads to Increased Body Weight in Defeated Females
Although body weight during the AcSD did not differ between control and defeated females, in adulthood, all defeated mice showed an increase in body weight compared with controls (Figure 6A). To examine changes in weight gain, we compared body weight on the last day of the social defeat and in adulthood and found a significant increase in all defeated versus control groups (Extended data Figure 6A in Supplement 2). These delayed effects of AcSD on body weight were not identified in our previous study with male mice (43) (see Figure S5 in Supplement 1), although alterations in male body weight have been reported using a different social stress procedure and later timing of exposure than the ones that were applied in our study (32).
Figure 6.
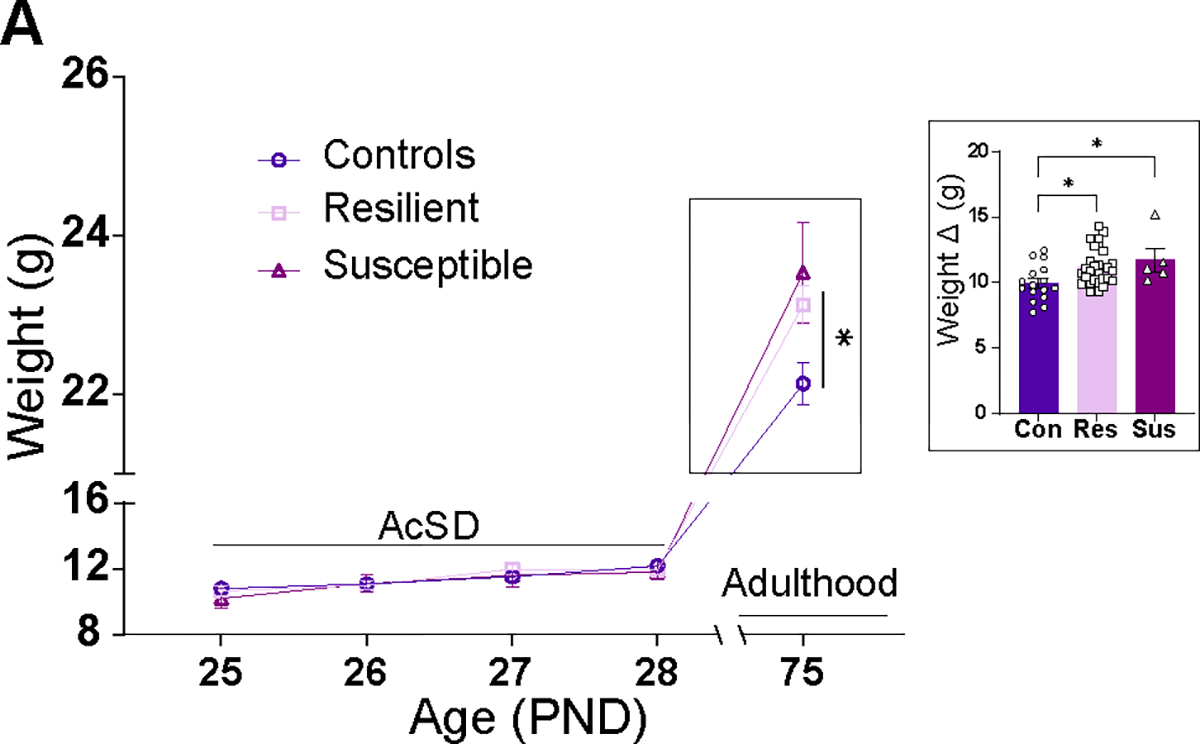
After AcSD, defeated female mice weighed more than the control mice. (A) Weights of females during AcSD and in adulthood. Inset: difference in weight between PND 75 and PND 28, showing that defeated mice gained more weight than controls; significantly different at *p < .05. All data are shown as mean ± SEM. AcSD, accelerated social defeat; con, control; PND, postnatal day; res, resilient; sus, susceptible.
DISCUSSION
Models of social defeat stress in adolescence have been used in male mice and in male and female rats to reproduce certain physical and psychological aspects of the stress experienced by victims of bullying and domestic violence (31–42,52–55,57,65–67). Here, we were able, for the first time, to assess short- and long-term effects of social defeat stress in early adolescent female mice using a modified adolescent male AcSD paradigm (46,47). Most females showed resilience against stress-induced social avoidance in adolescence, but in adulthood, these resilient females developed inhibitory control deficits and showed a depletion of presynaptic sites in PFC dopamine axons. In contrast, female mice that would typically be classified as susceptible based on their SIT profile were protected against impulse control deficits and reduced PFC dopamine connectivity in adulthood. While AcSD in early adolescence alters Dcc mRNA expression in dopamine neurons in males (46), this was not observed in females, suggesting that during this early adolescent developmental period, the Netrin-1/DCC guidance system is not involved in the behavioral changes that are observed in the female resilient group.
Social defeat stress during the same chronological window during adolescence affects males and females differently, with a higher percentage of susceptible males (45%) than females (15%), independent of the number of attacks received. Although the source of this difference is unknown, preserving sex-specific adolescent social behavior trajectories may be important to cope with an organism’s environmental demands (27,30). A study with rats showed that social exploration in males did not change across adolescence, but in females it peaked at approximately postnatal day 30–38 (29). The higher proportion of resilient females in our study may reflect a protective process aimed at maintaining a behavior that is relevant to females during this developmental stage. Favoring control levels of social behavior in adolescence, regardless of sex, must be important because rodents engage in social play and exploration in adolescence more than at any other age (27,29,56). Significantly more males exposed to the AcSD paradigm (or to chronic social defeat stress) in adulthood show susceptibility compared with those exposed in adolescence (46,58). This susceptibility is also greater in C57BL/6 female mice exposed to a modified version of the chronic social defeat stress paradigm in adulthood (68) or in California female mice exposed to defeat (65,66,69) than in female C57BL/6 mice subjected to AcSD in adolescence. Similar age-dependent effects have been described in female rats exposed to social defeat in adolescence versus adulthood (56). Social behaviors early in life seem to be protected whereas preserving cognitive processing is more prevalent at a later time point (46). Therefore, a resilient versus susceptible classification based on measures of performance at a specific age is problematic because protection and risk are domain specific and vary according to age and sex (16,17,70,71). We encourage caution against the use of social avoidance behavior as a main measure of stress vulnerability.
Facilitating social exploration in adolescent females seems to come at the expense of developing enduring cognitive deficits because most defeated resilient females showed poor adult impulse control. In contrast, females that showed susceptibility in adolescence performed at similar levels in the Go/No-Go task in adulthood as controls. In humans, a tradeoff has been shown between resilience to maladaptive behavioral outcomes in adolescence and increased vulnerability in other health domains, including metabolic disorders, in adulthood (72). Coping strategies in adolescence have been found to exert a sex-specific influence on the cognitive effects of adolescent stress (73,74). Why resilience to the detrimental effects of AcSD is specific to the behavioral outcome and why social protection may come at the price of developing adult cognitive impairment are thought-provoking questions that remain to be addressed. PFC dopamine plays a pivotal role in impulse control (75), which improves gradually from adolescence to adulthood, paralleling dopamine maturation (43,76,77). It is notable that in this study, we found that only females that were protected against social avoidance in adolescence and that developed cognitive impairment in adulthood showed impoverished adult PFC dopamine synaptic connectivity. The presence and absence of AcSD-induced social deficits in adolescence may be associated with a specific configuration of changes in mesocortical dopamine development, including structural changes in postsynaptic dopamine targets (35,78), and these alterations may be causally linked to adult cognitive phenotypes.
During adolescence, the PFC is still maturing and remains sensitive to environmental influence. The Netrin-1/DCC guidance cue pathway controls the extent of the mesocortical dopamine input in adolescence by promoting mesolimbic dopamine axon targeting in the NAcc, preventing them from growing ectopically to the PFC (45,50,60). Males and females show an identical target-dependent pattern of DCC receptor expression in mesocorticolimbic dopamine regions (44,79), suggesting that the overall role of the Netrin-1/DCC pathway in segregating mesolimbic and mesocortical projections is the same regardless of sex. AcSD in early adolescence downregulates Dcc levels and alters the extent of the dopamine input to the PFC, which is a proxy for ectopic mesolimbic dopamine axon growth to this region (46). However, in early adolescent females, the Netrin-1/DCC system is not altered in defeated mice, which is consistent with the lack of changes in the expanse of the dopamine input to the PFC (i.e., volume) in adulthood. The impact of social stress during this early adolescent period on the developing dopamine system appears to be sexually dimorphic and to involve divergent molecular mechanisms [see (55)]. Perhaps local expression of guidance cues in postsynaptic partners of PFC dopamine axons is involved, including changes in the SLIT/ROBO signaling, which has been shown to play a role in social stress susceptibility in adult female, but not male, mice using a model of chronic variable stress (80). It is also possible that the Netrin-1/DCC system in females is sensitive to AcSD but at a different adolescent age. Exposure to amphetamine during adolescence induces sex- and age-specific alterations in ventral tegmental area Dcc expression and in NAcc Netrin-1 (44). In our studies, chronological age between male and female mice overlaps, but their biological developmental trajectories may be quite different, considering that the timing of dopamine development in adolescence in rodents is highly sex specific (29,81–85). An interplay between stress and the developmental effects of gonadal hormones is also likely to be implicated, including in the female-specific increase in adult body weight [for example, see (83,86–89)]. Changes in stress hormones in defeated females may also be involved in the effects of stress on dopamine and cognitive development (53,68,90). Detailed longitudinal assessment of gonadal and stress hormone profiles elicited by AcSD will shed light on this question and guide future functional/mechanistic experiments.
Understanding how adolescent social stress affects neurodevelopment, both early and enduringly, and why only some individuals are susceptible is urgently needed considering the high prevalence of bullying in North America (91). Adolescent social stress models that capture and quantify individual differences in stress sensitivity can provide insights into this issue. Studies with rats have shown that adolescent females but not males show social avoidance following chronic stress (52). However, females have shown greater sensitivity to developing depression-like abnormal traits and learning and memory deficits (53,54). While chronic adolescent stress in male and female rats enhances adult immune reactivity, the underlying mechanisms and physiological consequences are sex specific (55,57). Our AcSD model could be used with adolescent male and female mice. We showed that exposure to social stress during the same chronological window in adolescence affected the developing mesocortical dopamine system and cognitive control differently in males and females and appears to involve sex-specific molecular processes. We propose that individual differences in the way that social stress affects sociability in adolescence are associated with a unique pattern of developmental dopamine signatures and that these changes may predispose to or protect against cognitive harm later in life. Indeed, social behavior in rats is critical for setting up synaptic connections in the developing PFC and for establishing cognitive processing in adulthood (92). Our study highlights the importance of conducting longitudinal preclinical studies and including both sexes.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Goat polyclonal Anti-Netrin-1 (ab122903) | Abcam | Cat. No. / ID: 126729 | |
| Antibody | Polyclonal Rabbit alpha-tubulin Ab | Cell Signaling | Cat. No. / ID: 2144S | |
| Antibody | Polyclonal AffiniPure Goat Anti-Rabbit IgG Alexa Fluor® 594 | Jackson Immuno | AB 2307325 | |
| Commercial assay/kits | miRNeasy Micro Kit | QIAGEN | Cat. No. / ID: 217084 | |
| Commercial assay/kits | Applied Biosystems High-Capacity cDNA Reverse Transcription kit | Applied Biosystems- Thermo Fisher Scientific | Cat. No. / ID: 4368814 | |
| Sequence-Based Reagent | Applied Biosystems™ TaqMan™ Gene Expression Assay | Applied Biosystems- Thermo Fisher Scientific | Cat. No. / ID: 4331182 | |
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Institute on Drug Abuse (Grant No. R01DA037911 [to CF]), Canadian Institutes of Health Research (Grant No. RGPIN-2020–04703 [to CF]), and Natural Sciences and Engineering Research Council of Canada (Grant No. FRN: 156272 [to CF]). AHP-U is supported by the National Council for Science and Technology/Consejo Nacional de Ciencia y Tecnología from México and Fonds de Recherche du Québec–Nature et Technologies-Merit Scholarship Program for foreign students.
AHP-U, SR, and CF designed the research; AHP-U, SR, AM, and MG performed the research and acquired data; AHP-U, SR, GH, and MG analyzed the data; and AHP-U, SR, and CF wrote the paper.
We thank Dr. Philip Vassilev for helpful comments about the manuscript.
Footnotes
Data for male mice presented in Figure 1C–F and Figures S4 and S5 in Supplement 1 have been modified from our previous publication (43). Figure illustrations were created using templates from BioRender.com. Melina Jaramillo Garcia (Molecular and Cellular Microscopy Platform in the Douglas Hospital Research Centre) helped set up the imaging experiments and the analysis.
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2023.06.014.
Contributor Information
Andrea Harée Pantoja-Urbán, Integrated Program in Neuroscience, McGill University, Montreal, Québec, Canada; Douglas Mental Health University Institute, Montreal, Québec, Canada.
Samuel Richer, Douglas Mental Health University Institute, Montreal, Québec, Canada.
Amelie Mittermaier, Douglas Mental Health University Institute, Montreal, Québec, Canada.
Michel Giroux, Douglas Mental Health University Institute, Montreal, Québec, Canada.
Dominique Nouel, Douglas Mental Health University Institute, Montreal, Québec, Canada.
Giovanni Hernandez, Douglas Mental Health University Institute, Montreal, Québec, Canada.
Cecilia Flores, Douglas Mental Health University Institute, Montreal, Québec, Canada; Department of Psychiatry and Department of Neurology and Neurosurgery, McGill University, Montreal, Québec, Canada.
REFERENCES
- 1.Blakemore SJ, Robbins TW (2012): Decision-making in the adolescent brain. Nat Neurosci 15:1184–1191. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL (2015): Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Dev Psychopathol 27:477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tottenham N, Galván A (2016): Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev 70:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Angermeyer M, Anthony JC, DE Graaf RD, Demyttenaere K, Gasquet I, et al. (2007): Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 6:168–176. [PMC free article] [PubMed] [Google Scholar]
- 6.Paus T, Keshavan M, Giedd JN (2008): Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smetana JG, Robinson J, Rote WM (2015): Socialization in adolescence. In: Grusec JE, Hastings PD, editors. Handbook of Socialization: Theory and Research. New York: The Guilford Press, 60–84. [Google Scholar]
- 8.Lemstra M, Rogers M, Redgate L, Garner M, Moraros J (2011): Prevalence, risk indicators and outcomes of bullying among on-reserve first nations youth. Can J Public Health 102:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hymel S, Swearer SM (2015): Four decades of research on school bullying: An introduction. Am Psychol 70:293–299. [DOI] [PubMed] [Google Scholar]
- 10.Rijlaarsdam J, Cecil CAM, Buil JM, van Lier PAC, Barker ED (2021): Exposure to bullying and general psychopathology: A prospective, longitudinal study. Res Child Adolesc Psychopathol 49:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sourander A, Helstelä L, Helenius H, Piha J (2000): Persistence of bullying from childhood to adolescence—A longitudinal 8-year follow-up study. Child Abuse Negl 24:873–881. [DOI] [PubMed] [Google Scholar]
- 12.Oram S, Trevillion K, Feder G, Howard LM (2013): Prevalence of experiences of domestic violence among psychiatric patients: Systematic review. Br J Psychiatry 202:94–99. [DOI] [PubMed] [Google Scholar]
- 13.Stapinski LA, Bowes L, Wolke D, Pearson RM, Mahedy L, Button KS, et al. (2014): Peer victimization during adolescence and risk for anxiety disorders in adulthood: A prospective cohort study. Depress Anxiety 31:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lereya ST, Copeland WE, Zammit S, Wolke D (2015): Bully/victims: A longitudinal, population-based cohort study of their mental health. Eur Child Adolesc Psychiatry 24:1461–1471. [DOI] [PubMed] [Google Scholar]
- 15.Bowes L, Joinson C, Wolke D, Lewis G (2015): Peer victimisation during adolescence and its impact on depression in early adulthood: Prospective cohort study in the United Kingdom. BMJ 350:h2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood SK, Bhatnagar S (2015): Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress 1:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notaras M, van den Buuse M (2020): Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry 25:2251–2274. [DOI] [PubMed] [Google Scholar]
- 18.Beesdo K, Knappe S, Pine DS (2009): Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr Clin North Am 32:483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalsgaard S, Thorsteinsson E, Trabjerg BB, Schullehner J, PlanaRipoll O, Brikell I, et al. (2020): Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry 77:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, et al. (2014): A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 71:573–581. [DOI] [PubMed] [Google Scholar]
- 21.Heim C, Shugart M, Craighead WE, Nemeroff CB (2010): Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 52:671–690. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U (2012): Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd A, de Van de Velde SV, Vilagut G, de Graaf R, O’Neill S, Florescu S, et al. (2015): Gender differences in mental disorders and suicidality in Europe: Results from a large cross-sectional population-based study. J Affect Disord 173:245–254. [DOI] [PubMed] [Google Scholar]
- 24.Bale TL, Epperson CN (2015): Sex differences and stress across the lifespan. Nat Neurosci 18:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hankin BL, Mermelstein R, Roesch L (2007): Sex differences in adolescent depression: Stress exposure and reactivity models. Child Dev 78:279–295. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Boyle MH, Georgiades K (2018): Cyberbullying victimization and its association with health across the life course: A Canadian population study. Can J Public Health 108:e468–e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke AR, McCormick CM, Pellis SM, Lukkes JL (2017): Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci Biobehav Rev 76:280–300. [DOI] [PubMed] [Google Scholar]
- 28.Fairless AH, Dow HC, Kreibich AS, Torre M, Kuruvilla M, Gordon E, et al. (2012): Sociability and brain development in BALB/cJ and C57BL/6J mice. Behav Brain Res 228:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopec AM, Smith CJ, Ayre NR, Sweat SC, Bilbo SD (2018): Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun 9:3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, et al. (2007): Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One 2:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang GB, Zhao T, Muna SS, Bagalkot TR, Jin HM, Chae HJ, Chung YC (2013): Effects of chronic social defeat stress on behaviour, endoplasmic reticulum proteins and choline acetyltransferase in adolescent mice. Int J Neuropsychopharmacol 16:1635–1647. [DOI] [PubMed] [Google Scholar]
- 32.Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. (2014): Social defeat stress induces a depression-like phenotype in adolescent male C57BL/6 mice. Stress 17:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montagud-Romero S, Aguilar MA, Maldonado C, Manzanedo C, Miñarro J, Rodríguez-Arias M (2015): Acute social defeat stress increases the conditioned rewarding effects of cocaine in adult but not in adolescent mice. Pharmacol Biochem Behav 135:1–12. [DOI] [PubMed] [Google Scholar]
- 34.Burke AR, DeBold JF, Miczek KA (2016): CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: Prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology 233:2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iñiguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores-Ramirez FJ, et al. (2016): Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress 5:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Resende LS, Amaral CE, Soares RBS, Alves AS, Alves-dos-Santos L, Britto LRG, Chiavegatto S (2016): Social stress in adolescents induces depression and brain-region-specific modulation of the transcription factor MAX. Transl Psychiatry 6:e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez-Arias M, Montagud-Romero S, Rubio-Araiz A, Aguilar MA, Martín-García E, Cabrera R, et al. (2017): Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: Integrity of the blood-brain barrier. Addict Biol 22:129–141. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, Yuan S, Shao F, Wang W (2016): Adolescent social defeat induced alterations in social behavior and cognitive flexibility in adult mice: Effects of developmental stage and social condition. Front Behav Neurosci 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouri A, Ukai M, Uchida M, Hasegawa S, Taniguchi M, Ito T, et al. (2018): Juvenile social defeat stress exposure persistently impairs social behaviors and neurogenesis. Neuropharmacology 133:23–37. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa S, Miyake Y, Yoshimi A, Mouri A, Hida H, Yamada K, et al. (2018): Dysfunction of serotonergic and dopaminergic neuronal systems in the antidepressant-resistant impairment of social behaviors induced by social defeat stress exposure as juveniles. Int J Neuro-psychopharmacol 21:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Wang J, Zhang K, Zhao M, Ellenbroek B, Shao F, Wang W (2018): Effects of adolescent social stress and antidepressant treatment on cognitive inflexibility and BDNF epigenetic modifications in the mPFC of adult mice. Psychoneuroendocrinology 88:92–101. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Arias M, Navarrete F, Blanco-Gandia MC, Arenas MC, Bartoll-Andrés A, Aguilar MA, et al. (2016): Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addict Biol 21:87–97. [DOI] [PubMed] [Google Scholar]
- 43.Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R (2015): An integrative model of the maturation of cognitive control. Annu Rev Neurosci 38:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds LM, Hernandez G, MacGowan D, Popescu C, Nouel D, Cuesta S, et al. (2023): Amphetamine disrupts dopamine axon growth in adolescence by a sex-specific mechanism in mice. Nat Commun 14(1):4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds LM, Yetnikoff L, Pokinko M, Wodzinski M, Epelbaum JG, Lambert LC, et al. (2019): Early adolescence is a critical period for the maturation of inhibitory behavior. Cereb Cortex 29:3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vassilev P, Pantoja-Urban AH, Giroux M, Nouel D, Hernandez G, Orsini T, Flores C (2021): Unique effects of social defeat stress in adolescent male mice on the Netrin-1/DCC pathway, prefrontal cortex dopamine and cognition. eNeuro 8:0045–21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassilev P, Fonseca E, Hernandez G, Pantoja-Urban AH, Giroux M, Nouel D, et al. (2022): Custom-built operant conditioning setup for calcium imaging and cognitive testing in freely moving mice. eNeuro 9: 0430–21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brody GH, Yu T, Chen E, Miller GE (2020): Persistence of skin-deep resilience in African American adults. Health Psychol 39:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoops D, Reynolds LM, Restrepo-Lozano JM, Flores C (2018): Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence. eNeuro 5: 0372–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds LM, Pokinko M, Torres-Berrío A, Cuesta S, Lambert LC, Del Cid Pellitero EDC, et al. (2018): DCC receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence. Biol Psychiatry 83:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuesta S, Restrepo-Lozano JM, Popescu C, He S, Reynolds LM, Israel S, et al. (2020): DCC-related developmental effects of abused-versus therapeutic-like amphetamine doses in adolescence. Addict Biol 25:e12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bekhbat M, Mukhara D, Dozmorov MG, Stansfield JC, Benusa SD, Hyer MM, et al. (2021): Adolescent stress sensitizes the adult neuroimmune transcriptome and leads to sex-specific microglial and behavioral phenotypes. Neuropsychopharmacology 46:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourke CH, Neigh GN (2011): Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav 60:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyer MM, Shaw GA, Goswamee P, Dyer SK, Burns CM, Soriano E, et al. (2021): Chronic adolescent stress causes sustained impairment of cognitive flexibility and hippocampal synaptic strength in female rats. Neurobiol Stress 14:100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekhbat M, Howell PA, Rowson SA, Kelly SD, Tansey MG, Neigh GN (2019): Chronic adolescent stress sex-specifically alters central and peripheral neuro-immune reactivity in rats. Brain Behav Immun 76:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ver Hoeve ESV, Kelly G, Luz S, Ghanshani S, Bhatnagar S (2013): Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience 249:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pyter LM, Kelly SD, Harrell CS, Neigh GN (2013): Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun 30:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. [DOI] [PubMed] [Google Scholar]
- 59.Golden SA, Covington HE, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuesta S, Nouel D, Reynolds LM, Morgunova A, Torres-Berrío A, White A, et al. (2020): Dopamine axon targeting in the nucleus accumbens in adolescence requires Netrin-1. Front Cell Dev Biol 8:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bari A, Robbins TW (2013): Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol 108:44–79. [DOI] [PubMed] [Google Scholar]
- 62.Dalley JW, Robbins TW (2017): Fractionating impulsivity: Neuropsychiatric implications. Nat Rev Neurosci 18:158–171. [DOI] [PubMed] [Google Scholar]
- 63.Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT (2014): Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci 1327:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips RA, Tuscher JJ, Black SL, Andraka E, Fitzgerald ND, Ianov L, Day JJ (2022): An atlas of transcriptionally defined cell populations in the rat ventral tegmental area. Cell Rep 39:110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC (2015): Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur J Neurosci 42:3081–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trainor BC, Pride MC, Villalon Landeros RV, Knoblauch NW, Takahashi EY, Silva AL, Crean KK (2011): Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One 6:e17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yohn CN, Dieterich A, Bazer AS, Maita I, Giedraitis M, Samuels BA (2019): Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology 44:2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. (2018): A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinman MQ, Trainor BC (2017): Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Semin Cell Dev Biol 61:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Mücke-Heim IA, Urbina-Treviño L, Bordes J, Ries C, Schmidt MV, Deussing JM (2023): Introducing a depression-like syndrome for translational neuropsychiatry: A plea for taxonomical validity and improved comparability between humans and mice. Mol Psychiatry 28:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radley JJ, Herman JP (2023): Preclinical models of chronic stress: Adaptation or pathology? Biol Psychiatry 94:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor HA, Washington-Plaskett T, Quyyumi AA (2020): Black resilience – Broadening the narrative and the science on cardiovascular health and disease disparities. Ethn Dis 30:365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilhsson M, Svedberg P, Högdin S, Nygren JM (2017): Strategies of adolescent girls and boys for coping with school-related stress. J Sch Nurs 33:374–382. [DOI] [PubMed] [Google Scholar]
- 74.Snyder K, Barry M, Plona Z, Ho A, Zhang XY, Valentino RJ (2015): The impact of social stress during adolescence or adulthood and coping strategy on cognitive function of female rats. Behav Brain Res 286:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan R, Wang T, Zhou Q (2019): Elevated dopamine signaling from ventral tegmental area to prefrontal cortical parvalbumin neurons drives conditioned inhibition. Proc Natl Acad Sci U S A 116:13077–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen B, Luna B (2018): Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94:179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reynolds LM, Flores C (2021): Mesocorticolimbic dopamine pathways across adolescence: Diversity in development. Front Neural Circuits 15:735625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christoffel DJ, Golden SA, Russo SJ (2011): Structural and synaptic plasticity in stress-related disorders. Rev Neurosci 22:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manitt C, Mimee A, Eng C, Pokinko M, Stroh T, Cooper HM, et al. (2011): The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci 31:8381–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Zee YY, Lardner CK, Parise EM, Mews P, Ramakrishnan A, Patel V, et al. (2022): Sex-specific role for SLIT1 in regulating stress susceptibility. 8ijm iatry 91:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willing J, Cortes LR, Brodsky JM, Kim T, Juraska JM (2017): Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev Psychobiol 59:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koss WA, Belden CE, Hristov AD, Juraska JM (2014): Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse 68:61–72. [DOI] [PubMed] [Google Scholar]
- 83.Juraska JM, Willing J (2017): Pubertal onset as a critical transition for neural development and cognition. Brain Res 1654:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drzewiecki CM, Willing J, Juraska JM (2016): Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse 70:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willing J, Juraska JM (2015): The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience 301:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delevich K, Klinger M, Okada NJ, Wilbrecht L (2021): Coming of age in the frontal cortex: The role of puberty in cortical maturation. Semin Cell Dev Biol 118:64–72. [DOI] [PubMed] [Google Scholar]
- 87.Meaney MJ (1988): The sexual differentiation of social play. Trends Neurosci 11:54–58. [DOI] [PubMed] [Google Scholar]
- 88.Fulton S, Décarie-Spain L, Fioramonti X, Guiard B, Nakajima S (2022): The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab 33:18–35. [DOI] [PubMed] [Google Scholar]
- 89.Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ (2017): Adolescence and reward: Making sense of neural and behavioral changes amid the chaos. J Neurosci 37:10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabib S, Castellano C, Patacchioli FR, Cigliana G, Angelucci L, Puglisi-Allegra S (1996): Opposite strain-dependent effects of post-training corticosterone in a passive avoidance task in mice: Role of dopamine. Brain Res 729:110–118. [PubMed] [Google Scholar]
- 91.Biswas T, Scott JG, Munir K, Thomas HJ, Huda MM, Hasan MdM, et al. (2020): Global variation in the prevalence of bullying victimisation amongst adolescents: Role of peer and parental supports. EClinicalmedicine 20:100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bijlsma A, Omrani A, Spoelder M, Verharen JPH, Bauer L, Cornelis C, et al. (2022): Social play behavior is critical for the development of prefrontal inhibitory synapses and cognitive flexibility in rats. J Neurosci 42:8716–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


