Many virus infections elicit vigorous host immune responses, both innate and acquired. The immune responses are frequently successful in controlling and then clearing the virus, using both cellular effectors such as natural killer (NK) cells and cytolytic T lymphocytes and soluble factors such as interferons (IFNs). However, some immune responses lead to pathologic changes or are unable to prevent the pathogen’s growth. This review will not be devoted to the different strategies viruses have taken to promote their transmission or survival but rather to one aspect of the innate immune response to infection: the role of nitric oxide (NO) in the antiviral repertoire. Recently, data from many laboratories, using both RNA and DNA viruses in experimental systems, have implicated a role for NO in the immune response. The data do not indicate a magic bullet for all systems but suggest that NO may inhibit an early stage in viral replication and thus prevent viral spread, promoting viral clearance and recovery of the host.
The earliest host responses to viral infections are nonspecific and involve the induction of cytokines, among them, IFNs and tumor necrosis factor alpha (TNF-α). Gamma IFN (IFN-γ) and TNF-α have both been shown to be active in many cell types and induce cascades of downstream mediators (reviewed in references 25, 34, and 41). Others have found that NO synthase type 2 (NOS-2, iNOS) is an IFN-γ-inducible protein in macrophages, requiring IRF-1 as a transcription factor (12, 17). We have observed that the isoform expressed in neurons, NOS-1, is IFN-γ, TNF-α, and interleukin-12 (IL-12) inducible (20). Thus, NOS falls into the category of IFN-inducible proteins, activated during innate immune responses.
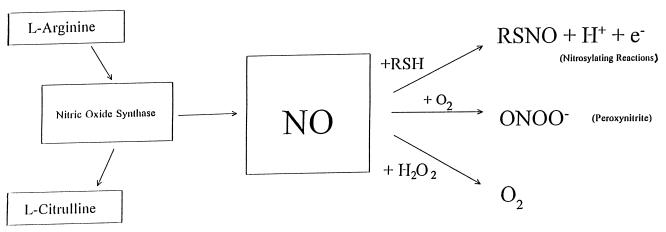
NO is produced by the enzymatic modification of l-arginine to l-citrulline and requires many cofactors, including tetrahydrobiopterine, calmodulin, NADPH, and O2. NO rapidly reacts with proteins or with H2O2 to form ONOO−, peroxynitrite, which is highly toxic (Fig. 1). NO also readily binds heme proteins, including Hb and its own enzyme.
FIG. 1.
Reaction of NO with proteins or H2O2 to form ONOO−.
This review will not include a great deal of detail about the biochemistry, pharmacology, and molecular biology of NOSs as there are many excellent review articles available (7, 10, 31, 41). However, to show the range of processes in which NOSs are involved, we will illustrate a few. NO was initially described as a physiological mediator of endothelial cell relaxation, an important role in hypotension (35, 42). An extension of this role is in penile erection (8). NO is central to long-term potentiation in neurons (32) and to activity in the biological clock, the suprachiasmic nucleus (13). There are three well-characterized isoforms of NOS, termed NOS-1, NOS-2, and NOS-3 (Table 1).
TABLE 1.
Isoforms of NOS
| Isoform | Other name(s) | Cellular expression | Regulation | Activity |
|---|---|---|---|---|
| NOS-1 | nNOS, ncNOS | Neurons; dystrophin complex of striated muscle | Ca2+ dependent; soluble; constitutively expressed but also inducible with cytokines (IFN-γ, IL-12, and TNF-α) | Short bursts of small-quantity NO |
| NOS-2 | iNOS | Macrophages; EBVa-transformed B cells; HeLa cells | Ca2+ independent; soluble; inducible with lipopolysaccharide, IFN-γ, and TNF-α | Long bursts of large-quantity NO |
| NOS-3 | eNOS, ecNOS | Endothelial cells; astrocytes | Ca2+ dependent; membrane bound; constitutively expressed but also inducible with cytokines; estrogen response element | Short bursts of small-quantity NO |
EBV, Epstein-Barr virus.
Immunologically, NOS activity, NOS-immunoreactive proteins, and mRNA have been found in autoimmune diseases, such as multiple sclerosis, associated with demyelinating lesions (11) and arthritic joints (40) and are thought to contribute to disease pathogenesis. NOS is frequently observed to be induced during the immune response (5). In contrast, in many intracellular bacterial and parasitic infectious diseases, NOS activity has been observed to be essential in eliminating pathogens such as Plasmodium falciparum (4).
In the last 5 years, dozens of articles have been published which show some association between NO and viral infections both in vivo and in vitro. There have been three basic experimental strategies used to determine if NO functionally inhibits viral replication: (i) using NO donors such as sodium nitroprusside (SNP), S-nitroso-l-acetylpenicillamine (SNAP), or 3-morpholino-sydononimine (SIN-1) in vitro; (ii) using analogs of the substrate l-arginine, such as l-NMA, l-NAME, 7-nitroindazole, to inhibit enzyme activity in vitro or in vivo; (iii) infecting host strains of mice which are homozygously deficient (knockouts) in one of the NOS isoforms. Table 2 summarizes many of the findings in RNA and DNA viral systems.
TABLE 2.
Summary of published findings on the effect(s) of NO on viral infection
| System | In vitro | In vivo | Reference(s) |
|---|---|---|---|
| Poliovirus | Pretreatment of HeLa or U937 cells with NO donor glycerine trinitrate inhibited replication. Addition of the NOS inhibitor l-NMA did not block viral propagation. | NDa | 27 |
| Addition of the NO donor SNAP inhibited poliovirus replication in human poliovirus receptor-expressing murine neuroblastoma cells. | ND | 20 | |
| Theiler’s murine encephalomyelitis virus (picornavirus) | ND | Expression of mRNA or NOS-2 protein is not correlated with virus-induced demyelination. | 33 |
| CVB3 (picornavirus) | Treatment of HeLa cells with the NO donor SNAP inhibited both viral RNA synthesis and PFU yield. | ND | 45 |
| CVB3 induces NOS-2, which may be involved in cardiac pathology. | 28 | ||
| Rhinovirus (picornavirus) | Treatment of human respiratory epithelial cells with the NO donor NONOate inhibited the growth of rhinovirus. | ND | 39 |
| JEV (flavivirus) | NO generated by IFN-γ-treated RAW cells inhibited viral propagation in N18 neuroblastoma cells. Antagonized by N-MMA. vRNA synthesis inhibited. | Mortality was increased in mice treated with l-NAME. | 26 |
| Tick-borne encephalitis virus (flavivirus) | No inhibition by NO seen in cultures of murine macrophages. | Marginally increased survival of infected mice with aminoguanidine treatment. | 23 |
| MHV (coronavirus) | NO inhibited propagation of MHV OBLV60 in OBL21a cells. | Treatment of infected mice with the inhibitor aminoguanidine did not alter the course of infection in BALB/c mice. | 24 |
| ND | In persistently infected B6 mice, NOS-2 activity contributed to the development of demyelinating pathology. | 14 | |
| Lymphocytic choriomeningitis virus (arenavirus) | ND | There was a correlation of expression of NOS-2 and neuropathology in mice. | 9 |
| Influenza A/WSN virus | IFN-γ treatment of NB41A3 neuroblastoma cells cells (but not IFN-γ-induced NOS-1) inhibited viral replication. | ND | 20 |
| Borna virus (mononegavirus) | ND | NOS-2 expression correlated with neuropathology. | 2, 22 |
| Sindbis virus (alphavirus) | NO donors had no effect on viral replication in N18 neuroblastoma cells. | NO inhibitor treatment (l-NAME in water bottles) increased the mortality of infected mice but did not affect viral replication in vivo. | 44 |
| NO donors and IFN-γ-induced NOS-1 did not inhibit Sindbis virus growth in NB41A3 cells (IFN-γ did inhibit virus but not via NOS-1 activity). | 20 | ||
| VSV (rhabdovirus) | NO donor SNAP inhibited VSV replication in NB41A3 neuroblastoma cells as did activation of glutamate receptors with NMDA treatment. | ND | 6 |
| NOS-1 is induced by IFN-γ treatment of NB41A3 cells and inhibits VSV growth. NOS-1 is inhibited by l-NMA and 7-nitroindazole. | 7-Nitroindazole treatment of VSV-infected mice resulted in a 2-log increase in viral replication and abrogation of the beneficial effects of IL-12 treatment. | 20 | |
| Lactate dehydrogenase-elevating virus (arterivirus) | NO produced in macrophages is not responsible for the T-cell suppression observed in persistently infected mice. | ND | 38 |
| Friend MuLV (retrovirus) | Treatment of Mus dunni cells with three NO donors, SNP, SNAP, and SIN-1, diminished viral replication. | l-NMA treatment of infected mice resulted in increased viral load. | 3 |
| HIV gp120 (lentivirus) | gp120 coculture increased NO and inflammatory cytokine production by human glial cells. | ND | 19, 21 |
| HIV gp120 transgenic mice | ND | NO production and activation of NMDA-R contribute to neuropathology in transgenic mice. l-NMA inhibited neuropathology. | 36 |
| Hepatitis B virus (hepadnavirus) | ND | Transgenic mice showed decreased virus load, with CD4+ T-cell-produced IFN-γ inducing NOS in hepatocytes. | 15 |
| HSV-1 (herpesvirus) | IFN-γ-treated RAW cells produced NO which inhibited HSV-1 growth. | ND | 18 |
| IFN-γ-treated and NMDA-activated NB41A3 cells produced NO which inhibited HSV-1 growth in neuroblastoma cells. | ND | 20 | |
| ND | NOS-2 contributes to HSV-1 pneumonia. l-NMA treatment of infected mice diminished pathology but increased viral yield. | 1 | |
| Epstein-Barr virus (EBV) (herpesvirus) | NO inhibited EBV Zta expression and thus latency. NO inhibited EBV-associated apoptosis of cells. | ND | 29 |
| MCMV (herpesvirus) | ND | l-NMA treatment of mice resulted in increased viral titers in the liver, but not the spleen, suggesting an organ-specific role for NOS-2 activity versus NK activity. | 43 |
| Vaccinia virus (poxvirus) | IFN-γ-treated RAW cells produced NO which inhibited vaccinia virus replication. | ND | 18 |
| Mechanism of viral inhibition was decreased in late viral protein synthesis. NOS-2 activity was antagonized by l-NMA treatment. Viral DNA synthesis was suppressed due to inhibition of viral ribonucleotide reductase. | ND | 16, 30 | |
| ND | NOS-2 was activated during vaccinia virus infection of mice but may not be essential for viral clearance. | 37 | |
| Ectromelia virus (poxvirus) | RAW cells treated with IFN-γ to induce NOS-2 produced lower yields of ectromelia virus. | Inhibition of NOS-2 with l-NMA treatment resulted in fulminating ectromelia virus infections of mice. | 18 |
ND, not determined.
In vitro, for most (but not all) viruses studied, prior activation of the cell to have enzyme activity before infection is associated with inhibition of viral replication. This has been accomplished by providing NO donors, by coculture with activated macrophages as a source of diffusing NO, or by directly activating NOS in cells with cytokines or through other cell surface receptors (e.g., the glutamate receptor, NMDA-R). This includes both DNA and RNA viruses, enveloped and encapsidated: all picornaviruses tested (20, 27, 28, 33, 39, 45), Japanese encephalitis virus (JEV) (26), mouse hepatitis virus (MHV) (24), vesicular stomatitis virus (VSV) (20), Friend murine leukemia virus (MuLV) (3), herpes simplex virus type 1 (HSV-1) (18, 20), vaccinia virus (16, 18, 30), and ectromelia virus (18).
There are several exceptions, some tested with positive controls of IFN-mediated viral inhibition (41), including influenza virus (20), Sindbis virus (20, 44), and tick-borne encephalitis virus (23) (note that another member of the Flaviviridae, JEV, is sensitive [26]). Thus, NO is not a magic bullet in vitro; however, it is very potent for many different viruses.
The mechanism of inhibition of viral replication in vitro is actively under investigation in many laboratories. It may be that there will be several different pathways involved, especially given the diversity of virus families which are sensitive or resistant. For coxsackievirus type B3 (CVB3) and JEV, RNA synthesis and protein synthesis are inhibited (26, 45). For VSV, very early protein synthesis is inhibited and the viral structural proteins are nitrosylated (40a). For vaccinia virus, late viral protein synthesis and DNA replication are inhibited (16, 30).
In some cases, NOS-2 is detectable in tissues from infected animals and may be attributable to activation of macrophages and microglia by IFN-γ or TNF-α. This has been associated with tissue pathology in several systems: CVB3 (28), borna virus (2, 22), MHV demyelination (14), human immunodeficiency virus (HIV) gp120 neuropathology (36), and HSV-1 pneumonia (1).
In vivo, the data on inhibition of viral replication tend to agree with the in vitro findings. That is, when a virus is very sensitive to NO in tissue culture, treatment of infected hosts with an inhibitor of the enzyme is associated with increased viral replication. This was found for VSV (20), Friend MuLV (3), HSV-1 (1), and ectromelia virus (18). An exception was observed with Sindbis virus, since two laboratories found that there was no in vitro sensitivity to NO (20, 44) but mice treated with an inhibitor succumbed more readily during central nervous system (CNS) infection (44); however, this drug was provided in drinking water, which may not have been palatable to sick mice. Exceptions to the correlation were observed for vaccinia virus and for MHV infection of the CNS, for which there was no effect on disease when mice were treated with an inhibitor of NOS (24, 37). In the murine cytomegalovirus (MCMV) system, treatment of mice with l-NMA suggested that NO played a more important role in controlling viral replication in the livers of mice, but NK cells were essential in the spleen for eliminating MCMV (43).
Although there are knockout mice for each of the three isoforms of NOS, the experiments with two of the systems have not yet been published. We have found that CNS infection of mice with VSV, which replicates in neurons, requires NOS-1 for clearance of infection, recovery, and survival (20a). NOS-3-deficient mice resembled wild-type mice in their responses to the infection (20a). Lowenstein’s work with NOS-2-deficient mice indicates that CVB3 replicates to higher titers in knockout mice (28a).
What does this all mean? NO frequently is an important mediator in intracellular inhibition of viral replication, which results in lower viral yields and more efficient host clearance of the infection, hence recovery. NO is not the only intracellular inhibitor, because many of the IFN-inducible proteins block viral pathways (41). There is no clear-cut way of predicting if NO will have a role in viral clearance or pathogenesis. DNA and RNA viruses are both sensitive or resistant.
There are many pathogens which are not inhibited by NO; however, NO may also contribute to tissue damage, especially if substantial numbers of macrophages are activated, producing large quantities of NO, as in Borna disease (2, 22) or HSV-1 pneumonia (1). Since there are many enzyme inhibitors available, those diseases in which NOS-2 activity is detrimental may benefit from enzyme antagonism. Host organ tropism also does not predict the selectivity of this response. However, in the case of viral encephalitis due to infection with picornaviruses, rhabdoviruses, HSV-1, or JEV, for instance, activation of NOS-1 may be lifesaving.
ACKNOWLEDGMENTS
We gratefully acknowledge review of the manuscript by Ian M. Zitron.
We acknowledge the support of NIDCD grant 03536 and a Preclinical Research grant from the Genetics Institute.
REFERENCES
- 1.Adler H, Beland J L, Del-Pan N C, Kobzik L, Brewer J P, Martin T R, Rimm I. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS-2) J Exp Med. 1997;185:1533–1540. doi: 10.1084/jem.185.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike T, Weihe E, Schaefer M, Fu Z F, Zheng Y M, Vogel W, Schmidt H, Koprowski H, Dietzschold B. Effect of neurotropic virus infection on neuronal and inducible nitric oxide synthase activity in rat brain. J Neurovirol. 1995;1:118–125. doi: 10.3109/13550289509111016. [DOI] [PubMed] [Google Scholar]
- 3.Akarid K, Sinet M, Desforges B, Gougerot-Pocidalo M A. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J Virol. 1995;69:7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstey N M, Weinberg J B, Hassanali M Y, Mwaikambo E D, Manyenga D, Misukonis M A, Arnelle D R, Hollis D, McDonald M I, Granger D L. Nitric oxide in Tanzanian children with malaria: inverse relationship between severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barna M, Komatsu T, Reiss C S. Activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection. Virology. 1996;223:331–343. doi: 10.1006/viro.1996.0484. [DOI] [PubMed] [Google Scholar]
- 6.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredt D S, Snyder S H. Nitric oxide: a physiological messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 8.Burnett A L, Lowenstein C J, Bredt D S, Chang T S K, Snyder S H. Nitric oxide: a physiological mediator of penile erection. Science. 1992;257:401–404. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 9.Campbell I L, Samimi A, Chiang C-S. Expression of the inducible nitric oxide synthase: correlation with neuropathology and clinical features in mice with lymphocytic choriomeningitis. J Immunol. 1994;153:3622–3629. [PubMed] [Google Scholar]
- 10.Dawson T M, Dawson V L. Nitric oxide: actions and pathologic roles. Neuroscientist. 1994;1:9–20. [Google Scholar]
- 11.DeGroot C J A, Ruuls S R, Theeuwes J W M, Dijkstra C D, van der Valk P. Immunocytochemical characterization of the expression of inducible and constitutive isoforms of nitric oxide synthase in demyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol. 1997;56:10–20. doi: 10.1097/00005072-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2413. [PubMed] [Google Scholar]
- 13.Ding J M, Faiman L E, Hurst W J, Kuriashkina L R, Gillette M U. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzybicki D A, Kwack K B, Perlman S, Murphy S P. Nitric oxide synthase type II expression by different cell types in MHV-JHM encephalitis suggests distinct roles for nitric oxide in acute versus persistent infection. J Neuroimmunol. 1997;73:15–27. doi: 10.1016/S0165-5728(96)00159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 16.Harris N, Buller R M L, Karupiah G. Gamma interferon-induced nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh S I, Kimura T, Green S J, Mak T W, Taniguchi T, Vilcek J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 18.Karupiah G, Xie Q-w, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 19.Koka P, He K, Zack J A, Kitchen S, Peacock W, Fried I, Tran T, Yashar S S, Merrill J E. Human immunodeficiency virus 1 envelope protein induces interleukin 1, tumor necrosis factor α, and nitric oxide in glial cultures derived from fetal, neonatal, and adult human brain. J Exp Med. 1995;182:941–952. doi: 10.1084/jem.182.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu T, Bi Z, Reiss C S. Interferon-γ induced type 1 nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 20a.Komatsu, T., et al. Unpublished data.
- 21.Kong L-Y, Wilson B C, McMillan M K, Bing G, Hudson P M, Hong J-S. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell Immunol. 1996;172:77–83. doi: 10.1006/cimm.1996.0217. [DOI] [PubMed] [Google Scholar]
- 22.Koprowski H, Zheng Y M, Heber-Katz E, Fraser N, Rorke L, Fu Z F, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurological diseases. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreil T R, Eibl M M. Nitric oxide and viral infection: no antiviral activity against a flavivirus in vitro and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 24.Lane T E, Paoletti A D, Buchmeier M J. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J Virol. 1997;71:2202–2210. doi: 10.1128/jvi.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy D E. The house that JAK/STAT built. Cytokine Growth Factor Rev. 1997;8:81–90. doi: 10.1016/s1359-6101(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y-L, Huang Y-L, Ma S-H, Yeh C-T, Chiou S-Y, Chen L-K, Liao C-L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of NO on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Guerrero J A, Carrasco L. Effect of nitric oxide on poliovirus infection of two human cell lines. J Virol. 1998;72:2538–2540. doi: 10.1128/jvi.72.3.2538-2540.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowenstein C J, Allen G, Rose N, Snyder S H, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Lowenstein, C. J. Personal communication.
- 29.Mannick J B, Asano K, Izumi K, Kieff E, Stamler J S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus replication. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 30.Melkova Z, Esteban M. Inhibition of vaccinia virus DNA replication by inducible expression of nitric oxide synthase. J Immunol. 1995;155:5711–5718. [PubMed] [Google Scholar]
- 31.Nathan C, Xie Q-w. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 32.O’Dell T J, Hawkins R D, Kandel E R, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oleszak E L, Katsetos C D, Kuzmak J, Varadhachary A. Inducible nitric oxide synthase in Theiler’s murine encephalitis virus infection. J Virol. 1997;71:3228–3235. doi: 10.1128/jvi.71.4.3228-3235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Shea J J. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 35.Palmer R M, Ferrige A G, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 36.Raber J, Toggas S M, Lee S, Bloom F E, Epstein C J, Mucke L. Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: evidence for involvement of NMDA receptors and nitric oxide synthase. Virology. 1996;226:362–373. doi: 10.1006/viro.1996.0664. [DOI] [PubMed] [Google Scholar]
- 37.Rolph M S, Ramshaw I A, Rockett K A, Ruby J, Cowden W B. Nitric oxide production is increased during murine vaccinia virus infection, but may not be essential for virus clearance. Virology. 1996;217:470–477. doi: 10.1006/viro.1996.0141. [DOI] [PubMed] [Google Scholar]
- 38.Rowland R R R, Butz E A, Plagemann P G W. Nitric oxide production by splenic macrophages is not responsible for the T cell suppression during acute infection with lactate dehydrogenase-elevating virus. J Immunol. 1994;152:5785–5795. [PubMed] [Google Scholar]
- 39.Sanders S P, Sierkierski E S, Porter J D, Richards S M, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiraishi A, Didler J, Lotz M. The role of IRF-1 in synovitis and nitric oxide production. J Immunol. 1997;159:3549–3554. [PubMed] [Google Scholar]
- 40a.Srivastava, A., et al. Unpublished data.
- 41.Staeheli P. Interferon-inducible proteins and the anti-viral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 42.Stamler J S. A radical vascular connection. Nature. 1996;380:108–111. [PubMed] [Google Scholar]
- 43.Tay C H, Welsh R M. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucker P C, Griffin D E, Choi S, Bul N, Wesselingh S. Inhibition of nitric oxide synthase increases the mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaragoza C, Ocampo C J, Saura M, McMillan A, Lowenstein C J. Nitric oxide inhibition of coxsackie virus replication in vitro. J Clin Invest. 1997;100:1760–1767. doi: 10.1172/JCI119702. [DOI] [PMC free article] [PubMed] [Google Scholar]