Abstract
Objective:
This study aimed to develop a model to predict KRAS mutations in colorectal cancer according to radiomic signatures based on CT and clinical risk factors.
Methods:
This retrospective study included 172 patients with colorectal cancer. All patients were randomized at a 7:3 ratio into a training cohort (n = 121, 38.8% positive for KRAS mutation) and a validation cohort (n = 51, 39.2% positive for KRAS mutation). Radiomics features were extracted from single-slice and full-volume regions of interest on the portal-venous CT images. The least absolute shrinkage and selection operator (LASSO) algorithm was adopted to construct a radiomics signature, and logistic regression was applied to select the significant variables to develop the clinical-radiomics model. The predictive performance was evaluated by receiver operating characteristic curve (ROC) analysis, calibration curve analysis, and decision curve analysis (DCA).
Results:
1018 radiomics features were extracted from single-slice and full-volume ROIs. Eight features were retained to construct 2D (two-dimensional, 2D) radiomics model. Similarly, eight features were retained to construct 3D (three-dimensional, 3D) radiomics model. The area under the curve (AUC) values of the test cohort were 0.75 and 0.84, respectively. Delong test showed that the integrated nomogram (AUC = 0.92 in the test cohort) had better clinical predictive efficiency than 2D radiomics (p-value < 0.05) model and 3D radiomics model (p-value < 0.05).
Conclusion:
The 2D and 3D radiomics models can both predict KRAS mutations. And, the integrated nomogram can be better applied to predict KRAS mutation status in colorectal cancer.
Advances in knowledge:
CT-based radiomics showed satisfactory diagnostic significance for the KRAS status in colorectal cancer, the clinical-combined model may be applied in the individual pre-operative prediction of KRAS mutation.
1. Introduction
Colorectal cancer (CRC) is the fourth most common cancer globally. Its incidence and mortality have increased significantly over the years. 1 Therefore, the choice of the treatment plan for CRC has become a major clinical challenge. Molecular targeted therapy has gradually become a research hotspot in the comprehensive treatment of advanced cancer.
Rat sarcoma viral oncogene (RAS) mutations in CRC, represented by kirsten rat sarcoma viral oncogene (KRAS) mutations, account for approximately 50% 2 of CRC cancers and have an important influence on the choice of treatment plan. Studies have found that 3 KRAS mutant CRC patients are resistant to targeted drugs, chemotherapy combined with immunotherapy can improve their prognosis. Therefore, KRAS gene detection is helpful for selecting CRC patients who can benefit from EGFR (epidermal growth factor receptor)-targeted therapy drugs and it can also help select the most effective treatment method for KRAS mutant patients who are resistant to these targeted drugs.
Undoubtedly, DNA analysis of tumor tissues (local pathological biopsy) is the primary method used to determine the tumor gene phenotype. 4 However, the sample of tissue obtained by these methods is limited and it may not represent the whole tumor’s genotype. Therefore, these factors limit the application of invasive sampling for real-time monitoring of tumor biological characteristics.
Traditional medical imaging can capture macroscopic tumor imaging features that can reflect the changes of tumor genes, and it provides non-invasive, real-time, dynamic evaluation of the tumor biological characteristics, which has an important reference value for predicting KRAS mutation in CRC patients. Xu et al 5 found parameters derived from diffusion-weighted magnetic resonance imaging. In particular, the average apparent diffusion coefficient value and D value were significantly associated with the KRAS mutation status in CRC, but the thresholds of these diffusion-weighted imaging-related values used to distinguish the KRAS status are unclear and have not been widely accepted. Moreover, these traditional quantitative imaging parameters used to distinguish the KRAS state are easily affected by the relaxation time of the blood components and tissues, so the measurement will be different, resulting in a limited evaluation value. 6
Radiogenomics, as an emerging field, integrates imaging and genomics data and can be used to evaluate the correlation between the KRAS genetic phenotype and the quantitative imaging characteristics of tumors, which breaks through the limitations of traditional medical imaging. 7 Wu et al 8 used CT texture analysis to predict KRAS mutations in CRC and achieved moderate diagnostic efficiency (area under the curve, AUC = 0.719). In addition, some researchers 9,10 have also tried to incorporate machine learning and deep learning into research. They also tried to find the best combination of feature selection and classifier modeling methods to achieve the best efficiency. However, most of the previous studies paid too much attention to the selection of classifier models while ignoring the delineation of tumor focus target areas. Generally, in the past, the extraction of CT imaging features of CRC cancer was mostly performed on a single section of the largest cross-sectional diameter (2D) of the tumor and rarely on the entire tumor volume (3D). The reason for this methodology may be that manually delineating the full volume of the tumor necessitates considerable manpower and material resources, requiring researchers to have a solid imaging diagnosis level and spend a significant amount of time performing heavy radiomics feature calculations. These problems have restricted the development of CRC cancer imagingomics based on 3D images. However, intuitively speaking, because the full-volume-based 3D model includes the entire tumor lesion, it will provide more meaningful features for researchers to study and analyze compared to the 2D model that only studies the largest cross-sectional area. Machado et al 11 found that radiomics features can pre-operatively predict occult peritoneal metastasis of advanced gastric cancer. Compared with two-dimensional radiomics features, three-dimensional radiomics features have better predictive performance. Cui et al 12 non-invasively detected KRAS mutations in rectal cancer by extracting radiomics features from the T2 sequence in MRI and achieved higher accuracy from the model extracted from the 3D segmentation. The imaging omics model based on the 3D model may have comparable or even better performance than the imaging omics model of the 2D model.
The purpose of this study was to explore the value of CT imaging omics based on the different mapping methods of the tumor target area in the pre-operative prediction of KRAS gene mutation in CRC cancer and to compare the efficacy of predicting KRAS mutations based on the maximum cross-section and full volume characteristics of the tumor.
2. Materials and methods
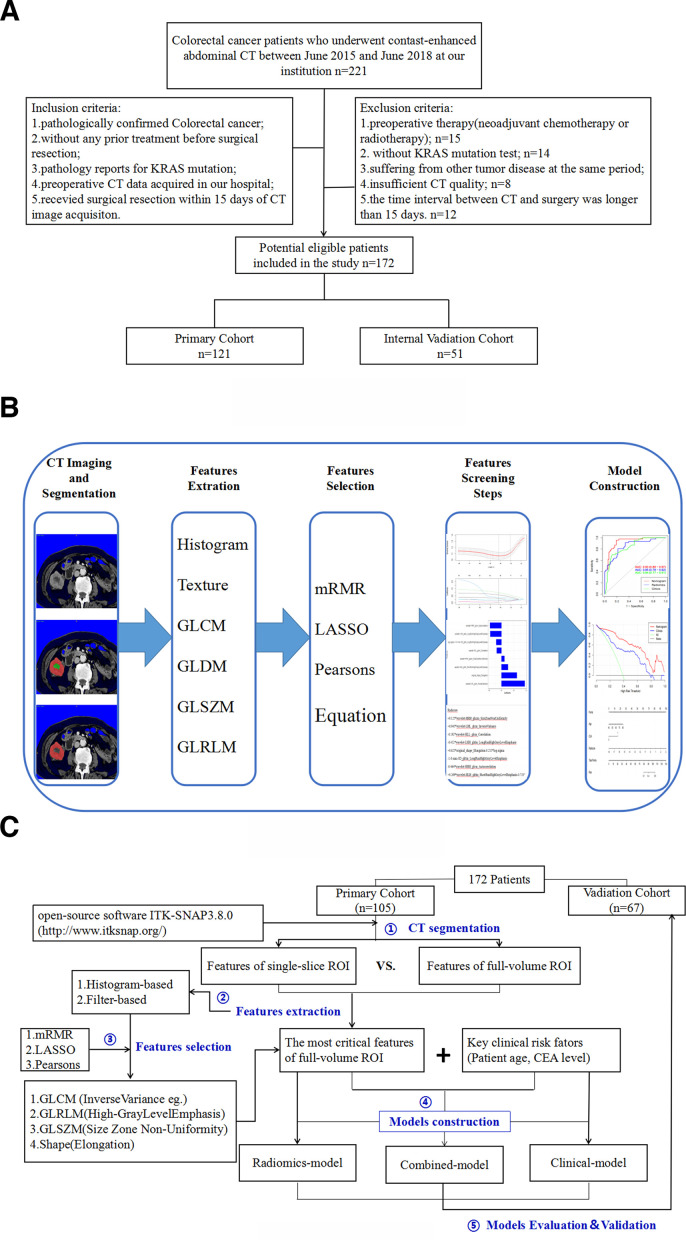
This retrospective study was approved by the ethics committee of the affiliated tumor hospital of nantong university, and the requirement for informed consent was waived. The patient recruitment pathway and patient inclusion and exclusion details are summarized in Figure 1a. The inclusion criteria were as follows:(1) patients with pathologically confirmed CRC; (2) patients who had undergone abdominal enhanced CT examination before any treatment; (3) patients who took KRAS mutation test; (4) patients with complete CT data sets and reconstructed images; (5) patients who had surgery within 15 days after contrast-enhanced CT examination. The exclusion criteria were as follows: (1) patients who had undergone pre-operative neoadjuvant chemotherapy or radiotherapy; (2) patients without KRAS mutation test; (3) patients suffering from another tumor disease during the same period; (4) patients with insufficient CT quality; (5) patients with a time interval between CT examination and surgery longer than 15 days. 172 patients with pathologically confirmed CRC were enrolled in the study.
Figure 1.
(a) Flow chart of the patients’ recruitment pathway. (b) Workflow of the radiomic analysis. (c) The process of model generation. GLCM, gray-level co-occurrence matrix; GLDM, gray-level dependence matrix; GLRLM, gray-level run-length matrix; GLSZM, gray-level size zone matrix.
2.1. Patients
The patients were enrolled and randomly divided into two data sets at a ratio of 7:3 (121 and 51 patients in the training and internal test data sets, respectively). Among patients in the training cohort, pathological assessment of surgical specimens confirmed that 74 patients were negative and 47 were positive for KRAS mutations. Similarly, in the test cohort, 31 patients were negative and 20 were positive for KRAS mutations. The predictive model for KRAS mutations was established using the training data set and assessed using the internal test data set.
Clinical pathological data were included for each patient enrolled in the group: ① clinical data included age, sex, and pre-operative carcinoembryonic antigen (CEA, normal level ≤5 ng ml−1); ② pathological data included primary tumor location, T stage, N stage, lymphatic vascular invasion, and peripheral nerve invasion.
2.2. Identification of the KRAS mutation status
The KRAS gene status of all cases was derived from the pathology department of our hospital. After the operation, the pelvic pathologist selected tumor specimens to ensure that the selected specimens had obvious tumor infiltration and then performed dicing, fixation, dehydration, paraffin embedding, and sectioning. DNA was extracted from paraffin-embedded pathological tissues with nucleic acid extraction reagents and then subjected to polymerase chain reaction (PCR) and amplification-blocking mutation system (ARMS) methods to analyze KRAS (exons 2, 3, 4). The mutation status of the gene was assessed.
2.3. CT image acquisition
Using the Siemens SOMATOM Sensation 64-slice spiral CT scanner, the scan range of the abdominal lesions was from the top of the diaphragm to the lower edge of L3, and the scan range of the middle and lower abdominal lesions was from the top of the diaphragm to the lower edge of the symphysis pubis. The scanning parameters are described inTable 1.
Table 1.
The scanning parameters were as follows
| Scanning parameters | Values |
|---|---|
| Tube voltage | 120 kV |
| Tube current | 90 ~ 160 mA |
| Layer thickness | 5 mm |
| Layer spacing | 5 mm |
| Thread pitch | 1.0 |
| Collimation | 1.0 mm |
| The contrast agent (iohexol) injection rate total dose | 350 mg ml−1
2.5 ~ 3.0 ml s−1 100 ml |
| Scanning time (portal vein phase) | 50 ~ 60 s |
2.4. ROI delineation
In this study, portal vein-phase CT images were retrieved from PACS for tumor segmentation, because the portal vein-phase was the optimal one for visualization of CRC cancer. Two tumor delineation methods were used for each included CRC cancer patient’s lesion [the total tumor volume of interest (VOI) and the largest single-slice ROI of the tumor]. Using ITK-SNAP software (ITK-SNAP, v. 3.4.0, www.itk-snap.org), the ROI of the tumor was manually segmented at the portal vein-phase CT images (5 mm slice thickness) 13 by the imaging Physician A with 8 years of experience in abdominal and pelvic imaging diagnoses. The tumor ROI is manually delineated layer by layer at the CT image (Supplementary Figure 1), and the full VOI is obtained. Similarly, the open source software package (ITK-SNAP, v. 3.4.0, www.itk-snap.org) is used for tumor single-slice segmentation. The imaging Physician A delineates the ROI on the largest slice of the portal vein-phase CT images (slice thickness of 5 mm) 7 manually. The definition of VOI and the largest single-slice ROI of the tumor is as follows: enhanced CT showed that the primary tumor was artificially delineated by an abnormal area that was unevenly enhanced along the venous phase and was different from the normal intestinal structure. In the process of VOI and the largest single-slice ROI delineation, the imaging physician tried to include all the tumor area and avoid intestinal gas, intestinal juice and feces in the intestines. In addition, another imaging expert B who has been engaged in abdominal and pelvic imaging diagnoses for more than 15 years checks and confirms the results of imaging expert A. If any objections are noted, the imaging experts discuss the issue to reach a consensus after discussion. During the image segmentation process, the two radiologists were blinded to the KRAS gene mutation information.
2.5. Feature selection and model construction
A total of 1018 radiomics features were extracted from single-slice and full-volume ROIs to quantify the intratumor heterogeneity. The following six features were extracted: first-order, GLCM, GLRLM, GLSZM, GLDM and shape. The feature descriptions and definitions can be found online (http://pyradiomics.readthedocs.io/en/latest/features.html). Among all the features, 80 features use Gaussian distribution (LoG, σ: 1, 2, 3, 5) filters and 688 features use wavelet processing conversion (LLL, LLH, LHH, LHL, HHH, HHL, HLH, HLL) filters. Among them, the first-order feature describes the distribution of pixel or voxel intensity in the ROI, and the morphological feature describes the shape and volume of the ROI. In addition, there are texture features that mainly reflect the gray distribution of the image and wavelet features that reflect the different frequency ranges of the original image. A Gaussian filter is used to detect the difference in image edge features and change in the gray area. The steps of this section are listed in Figure 1b and c. Since some of the features were redundant, we used the Pearson correlation coefficient to remove the redundant features.
We calculated inter- and ICCs for ROI-based imaging radiomics features from CT images of 30 randomly selected patients from the training cohort to assess the inter- and intracohort consistency of the feature extraction. The 2 ROIs of 30 patients (15 KRAS-present and 15 KRAS-absent CRC patients) were simultaneously delineated by the two physicians mentioned above (Physician A and expert B), and the features were extracted to observe the consistency between the cohorts. 1 week later, the ROIs were again delineated, and the features were extracted by Physician A to assess the intragroup agreement. An ICC greater than 0.75 suggested good agreement with the feature extraction.
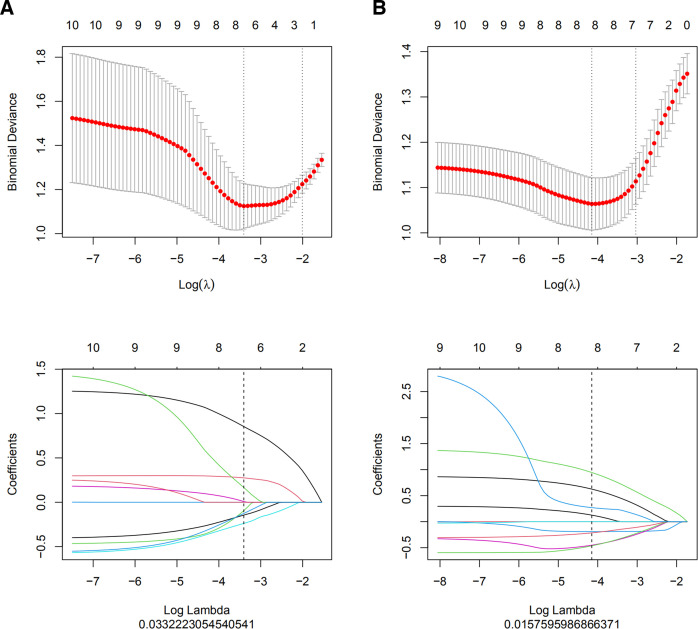
The best radiomics features were selected in three steps. First, to delineate the subjective differences in ROIs, robust radiomics features with intra- and intergroup ICCs > 0.75 were selected. Second, the maximal redundancy minimal relevance (mRMR) algorithm and LASSO regression were used to reduce the feature dimensionality. The optimal superparameter λ value of the LASSO regression model was screened using 10-fold cross-test. (Figure 2) Under the optimal λ value, the parameters with characteristic coefficients not equal to 0 were used as the features for the final construction of the radiomics model. Third, the radiomics score was the sum of the product of retained features and their corresponding coefficients, and the CT-based radiomics model was constructed.
Figure 2.
(a, b) Optimal hyperparameter λ values were selected using fold cross-test in the single-slice and full-volume LASSO regression models, with the lowest being the feature that best matched the true results. LASSO regression models identified single-slice and full-volume radiomics features with nonzero coefficients (a: single-slice; b: full-volume). LASSO, least absolute shrinkage and selection operator.
Before, we extracted the clinical features in the training group that had been confirmed to be significant by χ2 test (categorical variables) or t-test or Mann–Whitney U test (continuous variables); then, univariate logistic regression analyze was applied for exploring whether the features were discriminative between two groups (p-value < 0.05), and at last, we used multivariate logistic regression analyze to deal with these discriminative clinical features as well as radscore to construct a combined radiomics-clinical model and construct a predictive nomogram. In addition, a clinical predictor model using the significant clinical parameters without the addition of the radiomics score was also developed. For each predictor variable, we obtained a relative risk assessment probability (OR) with a 95% confidence interval (CI).
2.6. Statistical analysis
Statistical analysis was performed using SPSS software (v. 26.0IBM) and R software (v. 3.5.1: www. R-project.org). Univariate analysis evaluated patient clinical information (age, sex, tumor location, T stage, N stage, vascular invasion, nerve invasion, CEA levels, etc.). In the process, the t-test, the χ2 test, or the Mann–Whitney U test was used to compare the differences in these clinical variables between the KRAS positive and negative groups. Among them, continuous variables were compared using a t-test or a Mann–Whitney U test, and a χ2 test for categorical variables.
ROC curves were used to evaluate the diagnostic effect of radiomics prediction models based on two ROIs and radiomics nomograms in distinguishing KRAS mutation status. The Youden index was used to determine the best cut-off value in ROC analysis. Then, the AUC, sensitivity, specificity and accuracy were calculated. The decision curve analysis (DCA) method was used to evaluate the actual clinical utility of the model. The Wilcoxon test showed differences in the rad-score of the two radiomics models for differentiating between KRAS-present and KRAS-absent in both the training and test cohorts. Delong’ s test was used to obtain the p-value by calculating the variance and covariance of the AUC values of different ROC curves. When the p-value was less than 0.05, the difference between the AUC values of the two ROC curves was statistically significant. The Hosmer–Lemeshow (HL) test was used to provide a calibration curve to evaluate the consistency between the true and predicted results of KRAS mutations in CRC.
3. Results
3.1. Patient clinical characteristics
A total of 172 patients with pathologically confirmed CRC were included in the present study, including 98 men and 74 women, and the average age was 67.7 ± 11.6 years. The clinical baseline information of the patients is shown in Tables 2 and 3. Independent risk factor identification (multivariate logistic regression analysis) is shown in Table 4.
Table 2.
Demographic and clinical characteristics of patients with colorectal cancer of the 172 study patients
| Characteristic | Value |
|---|---|
| Female sex—no. (%) | 74 (43) |
| Age-yr | |
| Mean ± SD | 63 ± 8 |
| Range | 45–85 |
| Location—right. (%) | 106(62) |
| Lymphatic vascular invasion—no. (%) | 58 (34) |
| Peripheral nerve invasion—no.(%) | 38 (21) |
| Pretreatment CEA level >5 ng ml−1—no.(%) | 63 (37) |
CEA, carcinoembryonic antigen.
Table 3.
Demographic and clinical characteristic of patients with colorectal cancer in the training and test data set
| Characteristic | Training data set, n = 121 | p- value | Test data set, n = 51 | p- value | ||
|---|---|---|---|---|---|---|
| Wild-type
group (n = 74) |
Mutated
group (n = 47) |
Wild-type
group (n = 31) |
Mutated
group (n = 20) |
|||
| Age, mean ± SD, years | 63.1 ± 8.7 | 71.1 ± 5.4 | <0.05 | 64.2 ± 9.2 | 70.2 ± 4.7 | 0.007 |
| Sex
(male) (female) |
43 (58.1) | 27 (57.4) | 1.00 | 16 (51.6) | 12 (60.0) | 0.76 |
| 31 (41.9) | 20 (42.6) | 15 (48.4) | 8 (40.0) | |||
| Location (left) (right) |
28 (37.8) | 17 (36.2) | 0.73 | 11 (35.5) | 10 (50.0) | 1.00 |
| 46 (62.2) | 30 (63.8) | 20 (64.5) | 10 (50.0) | |||
| T
stage T1 |
3 (4.1) | 1 (2.1) | 0.66 | 0 (0.0) | 0 (0.0) | 1.00 |
| T2 | 5 (6.8) | 6 (12.8) | 2 (6.5) | 1 (5.0) | ||
| T3 | 23 (31.1) | 15 (31.9) | 7 (22.6) | 10 (50.0) | ||
| T4 | 43 (58.1) | 25 (53.2) | 22 (71.0) | 9 (45.0) | ||
| N
stage N0 |
46 (62.2) | 23 (48.9) | 0.09 | 19 (61.3) | 12 (60.0) | 0.72 |
| N1 | 19 (25.7) | 17 (36.2) | 7 (22.6) | 6 (30.0) | ||
| N2 | 5 (6.8) | 7 (14.9) | 5 (16.1) | 2 (10.0) | ||
| N3 | 4 (5.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| LVI
(negative) (positive) |
52 (70.3) | 31 (66.0) | 0.77 | 17 (54.8) | 12 (60.0) | 0.94 |
| 22 (29.7) | 16 (34.0) | 14 (45.2) | 8 (40.0) | |||
| PNI
(negative) (postive) |
57 (77.0) | 40 (85.1) | 0.39 | 25 (80.6) | 14 (70.0) | 0.59 |
| 17 (23.0) | 7 (14.9) | 6 (19.4) | 6 (30.0) | |||
| Pretreatment CEA level (0~5 ng ml−1 vs ≥5 ng ml−1) | 59 (79.7) | 19 (40.4) | <0.05 | 23 (74.1) | 8 (40.0) | 0.03 |
| 15 (20.3) | 28 (59.6) | 8 (25.9) | 12 (60.0) | |||
CEA, carcinoembryonic antigen.
Note: LVI means ‘Lymphatic Vessel Invasion’; PNI means ‘Peripheral nerve invasion’; Pretreatment CEA level means ‘preoperative carcinoembryonic antigen level’.
Table 4.
Independent risk factor identification (multivariate logistic regression analysis)
| Factors | Odds ratio | p-value | 95% confidence interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.11 | 0.012 | 1.02 | 1.21 |
| CEA | 13.98 | <0.01 | 3.69 | 52.98 |
| Rad score | 4.72 | <0.001 | 2.45 | 9.09 |
OR, odds ratio; 95 % CI, 95% confidence interval; CEA, carcinoembryonic antigen; A/G, albumin to globulin ratio
3.2. Feature selection and radiomics model establishment
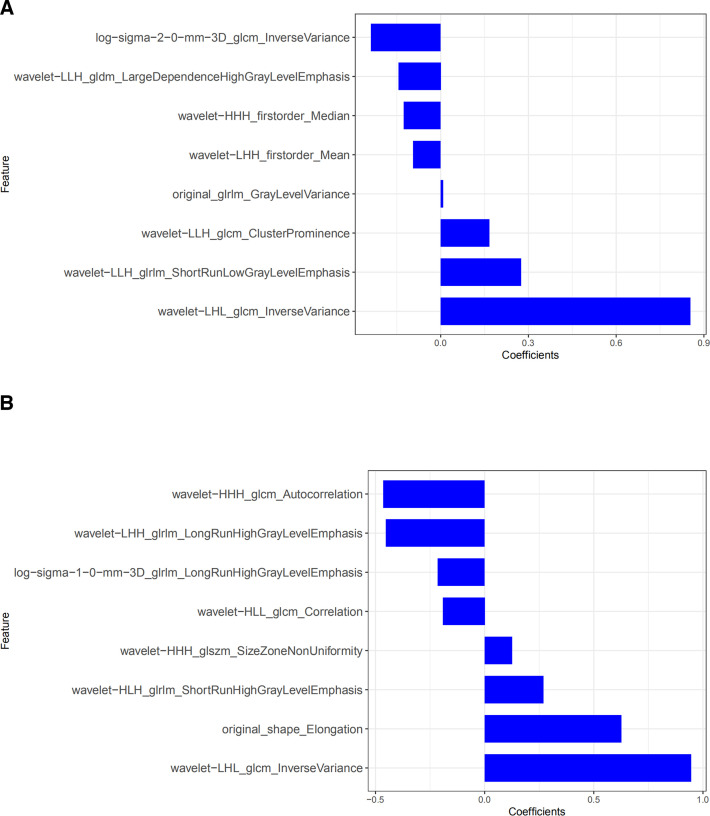
To prevent overfitting of the features, the data were dimensionally reduced. First, among the 1018 features extracted from each of the single-slice and full-volume ROIs, features with ICCs > 0.75 showed good inter- and intraobserver consistency. Second, the features with good consistency were screened using the mRMR method to eliminate redundant and irrelevant features in order to reduce the feature number to 30. LASSO regression analysis was then performed to optimize the subset (Figure 2). The selected features are shown in Figure 3. We obtained an candidate subset of two clinical features (age, pre-operative CEA level), 16 radiomics features of single-slice and full-volume ROI. (Figure 3) Then, radiomics features were identified in the radiomics signature and rad-score calculation formula as follows:
Figure 3.
(a, b) Radiomics features and corresponding coefficients retained after dimensionality reduction by LASSO regression analysis. (a: retained eight single-slice features; b: eight full-volume features and their coefficients.) LASSO, least absolute shrinkage and selection operator.
Radscore(single-slice)=0.853*glcm_InverseVariance + 0.275*glrlm_Low-GrayLevelEmphasis-0.094*firstorder_Mean-0.238*glcm_InverseVariance + 0.008*glrlm_Gray-LevelVariance-0.144*gldm_High-GrayLevelEmphasis + 0.166*glcm_ClusterProminence-0.126*firstorder_Median-0.63”
Radscore(full-volume)=0.125*glszm_SizeZoneNon-Uniformity + 0.945*glcm_InverseVariance-0.192*glcm_Correlation-0.452*glrlm_High-GrayLevelEmphasis + 0.625*shape_Elongation-0.215*glrlm_LongRunHigh-GrayLevelEmphasis-0.464*glcm_Autocorrelation + 0.269*glrlm_High-GrayLevelEmphasis-0.733”
Wilcoxon test showed a significant difference between the radscores of KRAS+ (KRAS mutation positive) and KRAS- (KRAS mutation negative) in the radiomics model based on the optimal cut-off values (Figure 4). The radiomics signature incorporating the eight selected features of single-slice showed favorable discrimination with an AUC of 0.84 in the training cohort and 0.75 in the test cohort. Similiarly, the radiomics signature incorporating the eight selected features of full-volume showed favorable discrimination with an AUC of 0.85 in the training cohort and 0.84 in the test cohort (Figure 5) . In Figure 5, in the training cohort, the Delong test showed that the ROC curve (AUC) between the 2D radiomics model and the 3D radiomics model was not statistically significant (p-value = 0.74). In the test cohort, the ROC curve (AUC) between the 2D radiomics model and the 3D radiomics model was not statistically significant (p-value = 0.32). Compared with the 2D radiomics model based on the largest cross-section of the tumor, the 3D radiomics model based on the full volume of the tumor exhibited equal effectiveness in predicting KRAS mutations in CRC. The accuracy, sensitivity, specificity and AUC of the models are shown in Table 5. Finally, the patients were classified into a high-risk KRAS-mutated group [Rad-score(single-slice)≥−0.432 or Rad-score(full-volume)≥−0.486] or a low-risk KRAS-mutated group [Rad-score(single-slice)<−0.432 or Rad-score(full-volume)<−0.486].
Figure 4.
(a, b) Boxed scatter plots of single-slice and full-volume rad-score predicting KRAS-wild-type and KRAS-mutated CRC patients.(a: single-slice; b: full-volume). CRC, colorectal cancer.
Figure 5.

The ROC curve of the single-slice and full-volume radiomics models. AUC, area under the curve; ROC, receiver operating characteristic.
Table 5.
Performance of the models in the case of all the patients
| Model | Cut-off | Accuracy(95% CI) | Sensitivity | Specificity | PPV | NPV | AUC | |
|---|---|---|---|---|---|---|---|---|
| Clinical model | 0.19 | Training | 80% [0.73,0.88] | 0.70 | 0.87 | 0.78 | 0.82 | 0.84 |
| Test | 66% [0.52,0.79] | 0.50 | 0.77 | 0.58 | 0.70 | 0.77 | ||
| Single-slice radiomics model | −0.43 | Training | 78% [0.69,0.85] | 0.83 | 0.75 | 0.67 | 0.88 | 0.84 |
| Test | 65% [0.50,0.78] | 0.62 | 0.67 | 0.57 | 0.71 | 0.75 | ||
| Full-volume radiomics model | −0.49 | Training | 79% [0.71,0.86] | 0.81 | 0.78 | 0.70 | 0.87 | 0.85 |
| Test | 82% [0.69,0.92] | 0.90 | 0.77 | 0.72 | 0.92 | 0.84 | ||
| Combinded
model (clinical+full vol radiomics) |
−0.93 | Training | 87% [0.79,0.92] | 0.96 | 0.81 | 0.76 | 0.97 | 0.93 |
| Test | 84% [0.71,0.93] | 0.71 | 1.00 | 1.00 | 0.74 | 0.92 | ||
AUC, area under the curve; NPV, negative-predictive value; PPV, positive-predictive value.
When training the model, we used 10-fold cross-validation to determine the optimal hyperparameters of LASSO. We added 10-fold cross-validation to verify the reliability of the model, which is shown in Supplementary Figure 2.
3.3. Clinical use (Nomogram construction and apparent performance)
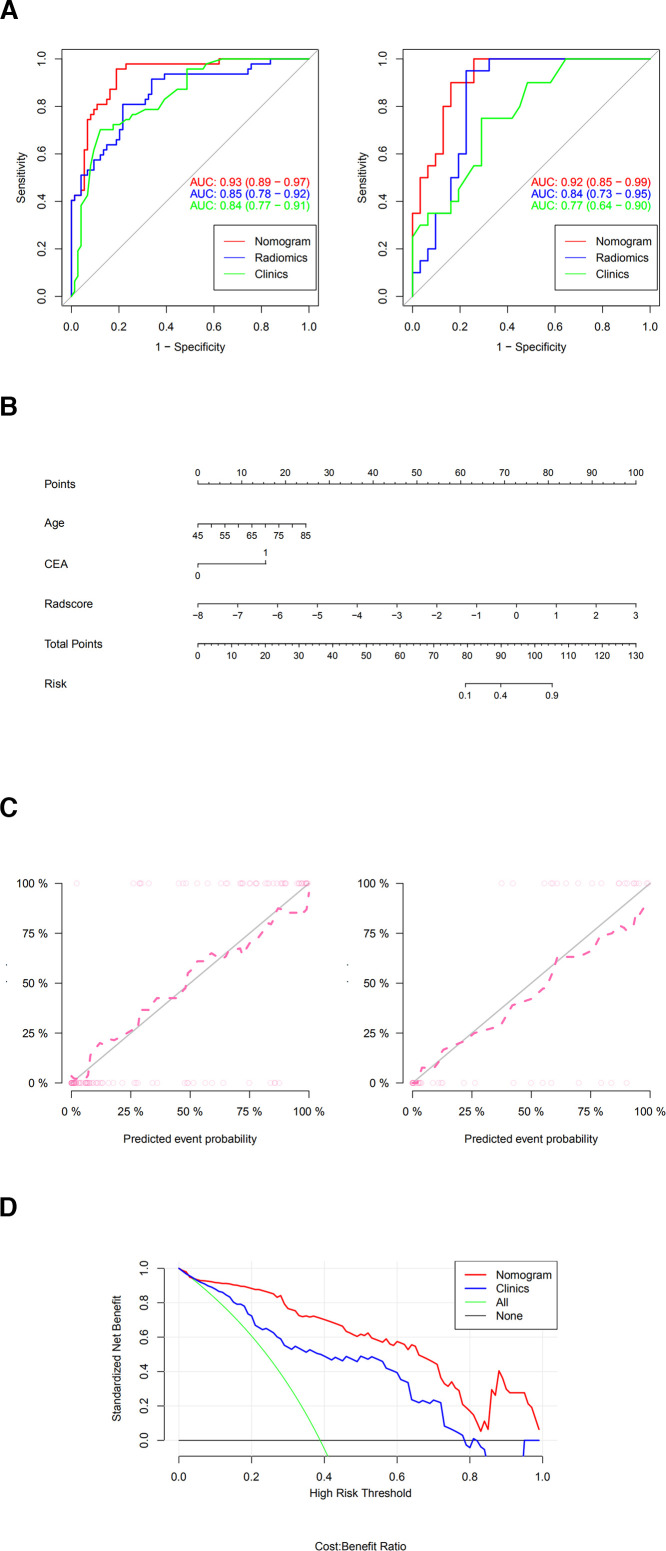
The radiomics features greatly help in the decision-making of the candidate model and increases the AUC value from 0.77 to 0.92 in the test cohort. (Table 5, Figure 6a). In Figure 6a, in the training cohort, the Delong test showed a statistically significant difference between the 3D radiomics-clinical model and the clinical model (p-value = 0.003). In the test cohort, a statistically significant difference was noted between the 3D radiomics-clinical model and the clinical model (p-value = 0.020). Compared with the clinical model, the combined radiomics-clinical model with radiomics features significantly improved the efficacy of predicting KRAS mutations in CRC (all p values < 0.05). Delong test also showed that the integrated nomogram (AUC = 0.92 in the test cohort) had better clinical predictive efficiency than 2D radiomics (p-value < 0.05) model and 3D radiomics model (p-value < 0.05). Based on this candidate model, we generated a clinical-radiomics nomogram based on full-volume of the tumor for model visualization (Figure 6b). The calibration curves of the integrated nomogram to predict KRAS mutation in the training and test cohorts demonstrated good agreement. Good calibration was observed, and the HL test showed that the data had goodness of fit (all p > 0.05) (Figure 6c). Then, two clinical features and eight radiomics features of full-volume were identified in the combined signature and rad-score calculation formula as follows:
Figure 6.
( a) ROC curve of full-volume radiomics models, clinical model, and the combined model.( b) The developed clinical-full-volume radiomics nomogram for predicting KRAS mutation status. (c) The calibration curves of the integrated nomogram to predict KRAS mutation in the training and test cohorts. (d) DCA of the clinical features and the nomogram (full-volume radiomics features and clinical features). CEA, carcinoembryonic antigen; DCA, decision curve analysis; ROC, reeiver operating characteristic.
Nomo-score=(Intercept)*−8.07 + Age*0.105 + CEA*2.638 + Radscore*1.55
With the addition of radiomic features, the combined clinical-radiomics DCAs achieved more clinical utility, especially the clinical DCA, which indicated that the nomogram based on the candidate model was a reliable clinical treatment tool for predicting KRAS mutation in CRC patients. DCA indicated that when the threshold probability for a patient is within a range from approximately 0.2 to 0.9, the clinical-radiomics nomogram adds more net benefit than the “treat” all or “treat none” strategies (Figure 6d)
The clinical utility of the clinical-radiomics nomogram established in this study is defined as follows: the clinical-radiomics nomogram established in this study is based on a multivariate logistic regression model according to the degree of influence of various variables in the model (age, pre-operative CEA level and rad-score based on the total tumor volume) on the KRAS mutation of CRC cancer, assigns scores to each value level of each influencing factor, and then sums the scores to obtain the total score. The predicted probability of KRAS mutation of the individual is calculated based on the function conversion relationship between the total score and the probability of KRAS mutation in CRC cancer, and the prediction model is finally displayed in a graphical manner. An example clinical utility of the nomogram is shown in Supplementary Figure 3.
4. Discussion
In this study, it was found that CT imaging features based on the maximum cross-section of the tumor and the whole volume of the tumor can pre-operatively predict KRAS mutations in CRC, and the radiomics model based on the whole volume of the tumor had the best performance (AUC = 0.85), which had better stability and reliability. Additional studies showed that the combined model based on tumor full-volume CT imaging features combined with key clinical factors was significantly superior to the prediction efficiency of the clinical model, and the AUC value was increased from 0.77 to 0.92. The prediction accuracy increased from 67 to 84%.
Recently, texture analysis can identify the histological and biological characteristics of tumors in addition to visual evaluation based on CT images. Previous studies have shown that CT-based imaging features can be used as a potential biomarker for predicting KRAS mutations in CRC. Miles et al 14 constructed a multifunctional imaging feature (including CT texture, blood flow and 18F-FDG PET/CT) to predict KRAS mutations, and its accuracy was as high as 90.1%. Although the accuracy of that model was higher than that of our study (the accuracy of the model in this study is only approximately 84%), only 33 patients participated in that study, which limits its clinical application. In addition, Yang et al 7 found that CT-based imaging features are related to KRAS/N-rat sarcoma viral oncogene (NRAS)/V-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations. The prediction efficiency and accuracy were similar to those of this study (the AUC was 0.85, the accuracy was 83%). However, that study defined positive cases as mutations in any one of KRAS/NARS/BRAF, which would be confusing in clinical applications. Recently, some studies 15,16 have used machine- or deep learning to establish a radiomics model that has the potential to pre-operatively predict KRAS mutations in CRC patients (the AUC of the deep learning radiomics models can reach 0.9). However, one of the challenges of machine- or deep learning models is that they require large training data sets. A limited number of suitable patients may lead to insufficient training data, which limits the reliability and stability of deep learning in predicting KRAS mutations.
This study was based on the pre-operative prediction of KRAS mutations in CRC patients. Because the center of a single-layer image mainly contains the necrotic region of the tumor, valuable information that could reflect the heterogeneity of the tumor is limited. 17 In addition, CRC has various growth forms, the tumor forms are usually irregular, and the invasive range of the tumors is often wide. Imaging analysis based on the whole tumor volume may provide richer and more comprehensive image information than single-layer cross-sections of tumors and can better reflect the heterogeneity of tumors. 18 Therefore, we compared the effectiveness of imaging omics models based on the largest tumor cross-section and the total tumor volume in predicting CRC KRAS mutations before surgery. The results show that the imaging radiomics features based on the full tumor volume exhibit better stability (AUC = 0.85 for the training cohort, AUC = 0.84 for the test cohort). Its prediction accuracy (82% for the test cohort), sensitivity (90%). and specificity (77%) were better than the prediction of KRAS mutations based on the single-slice imaging radiomics characteristics of the tumor (the accuracy in the verification cohort was 65%, the sensitivity was 62%, and the specificity was 67%). In this study, the 3D radiomics model was slightly better than the 2D radiomics model in predicting CRC KRAS mutations. However, regrettably, the Delong test revealed no statistically significant difference between the two predictive values (p > 0.05). The reason for the analysis may be that different doctors have great differences in the location of the lesion boundary when delineating the full volume of the tumor, which will generate more noise, overshadow the effective information, and interfere with the research results. 19 However, in view of the fact that the 3D radiomics model exhibits better prediction accuracy, sensitivity and specificity than the 2D omics model, the 3D radiomics characteristics and clinical risk factors are combined to establish a comprehensive nomogram. The results show that the nomogram is similar to the clinical model. Compared with the combined radiomics-clinical model with 3D radiomics features, the combined radiomics-clinical model significantly improved the efficacy of predicting KRAS mutations in CRC (p < 0.05).
To establish the radiomics model, this study used a LASSO regression model to select eight key radiomics features from candidate tumor single-layer and tumor full-volume radiomics features to improve the prediction performance of the imaging features. For small sample problems with a large number of radiomics features, there are many feature selection methods. For example, deep learning and convolutional neural networks have the disadvantages of feature redundancy and overfitting. Generally, when the number of variables is larger than the number of data points or there are too many unique values of discrete variables, it is possible to overfit. However, as an effective method for screening key features from mass radiomics features, LASSO not only satisfies the requirement of retaining the most significant radiomics features related to KRAS mutations but also overcomes the defects of other screening methods in small sample data. 20
Tumor tissue has unique spatial heterogeneity, and the complex spatial structure of tumor tissue images in all directions can be described in detail by using an optimized model. To show different levels of the image details, we used first-order features, morphological features and texture features to “interpret” the original data. This study found that after wavelet transformation, the imaging characteristics were closely related to the KRAS mutation status of CRC. Among the screened key radiomics features, 12/16 (75%) reserved parameters were all radiomics features after wavelet filtering. The variance in the GLCM reflected the best predictive ability. The reason for this may be that the radiomics features after wavelet filtering can quantitatively reflect the detailed differences of the image gray distribution between the gene wild-type and mutant patients. The greater the heterogeneity of the tumors in gene mutant patients, the greater the gray change and variance in the image to identify gene wild-type and mutant patients. These findings are similar to previous studies. He et al 10 established a radiomics model based on deep learning to predict KRAS mutations in CRC using four wavelet features and achieved good prediction efficiency (AUC = 0.818). Therefore, wavelet features may have a potential relationship with the KRAS genetic phenotype of tumors. In addition, this study also found that compared with the prediction of KRAS mutations in CRC by texture analysis alone, it may be better to combine imaging characteristics with key clinical factors. The results of this study showed that the combined model based on the imaging characteristics of the whole tumor volume combined with CEA and age level performed best for predicting KRAS mutations (AUC = 0.92 in the test cohort, 95% CI: 0.85–0.99).
There are some limitations of this study. First, because the sample size is small, the established model is prone to overfitting and instability. Second, the retrospective research might have inevitably caused bias in population selection. Third, our study only analyzed the KRAS mutation in CRC. The BRAFV600E mutation is a major marker of anti-EGFR in non-first-line treatment of metastatic CRC. However, its prevalence in CRC is low, so we have not accumulated enough BRAF mutation cases. Finally, this study is a single-center study, so we should expand the sample range and conduct multicenter studies in the future.
In conclusion, CT-based radiomics features can non-invasively and pre-operatively predict the KRAS mutation status of CRC patients. Moreover, the full-volume predictive model exhibits better efficacy than the single-slice predictive model. In addition, a comprehensive nomogram combining radiomics features and clinical predictors (CEA and age) can provide an intuitive and quantitative method for the clinical prediction of KRAS mutations in CRC. Therefore, the comprehensive nomogram can be used as an important clinical tool for the non-invasive prediction of KRAS mutations in patients with CRC.
Supplementary Material
Footnotes
Acknowledgments: The study was approved by the ethics committee of the Cancer Hospital Affiliated to
Nantong University, and the requirement of informed consent was exempted. Ting Xue: Data curation, Methodology, Writing - original draft, Software, Investigation. Hui Peng: Visualization, Investigation. Qiaoling Chen: Visualization, Investigation. Manman Li: Visualization, Investigation. Shaofeng Duan: Software, Formal analysis. Feng Feng: Conceptualization, Methodology, Writing - review & editing, Funding acquisition.
Conflict of Interest: The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding: Wu Jieping Medical Foundation; Fund No:320.6750
Contributor Information
Ting Xue, Email: 849638196@qq.com, Department of Radiology, Nantong University, Nantong, Jiangsu, PR China .
Hui Peng, Email: 1693264266@qq.com, Department of Radiology, Nantong University, Nantong, Jiangsu, PR China .
Qiaoling Chen, Email: 1372431158@qq.com, Department of Radiology, Nantong University, Nantong, Jiangsu, PR China .
Manman Li, Email: 380232471@qq.com, Department of Radiology, Nantong University, Nantong, Jiangsu, PR China .
Shaofeng Duan, Email: 18910063803@163.com, GE Healthcare China, Shanghai, China .
Feng Feng, Email: fengfeng@ntu.edu.cn, Department of Radiology, Affiliated Tumor Hospital of Nantong University, Nantong, Jiangsu, PR China .
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A . Cancer statistics, 2019, CA Cancer J . Clin 2019. ; 69: 7 – 34 . doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2. Lièvre A, Bachet J, Boige V, et al. . KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab . J Clin Oncol 2008. ; 26: 374 – 79 . doi: 10.1200/JCO.2007.12.5906 [DOI] [PubMed] [Google Scholar]
- 3. Sorich M, Wiese M, Rowland A . Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials, Ann . Ann Oncol 2015. ; 26: 13 – 21 . doi: 10.1093/annonc/mdu378 [DOI] [PubMed] [Google Scholar]
- 4. Sundström M, Edlund K, Lindell M, et al. . KRAS analysis in colorectal carcinoma: analytical aspects of Pyrosequencing and allele-specific PCR in clinical practice . BMC Cancer 2010. ; 10: 660 . doi: 10.1186/1471-2407-10-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Y, Xu Q, Sun H, Liu T, Shi K, Wang W . Could IVIM and ADC help in predicting the KRAS status in patients with rectal cancer Eur Radiol 2018. ; 28: 3059 – 65 . doi: 10.1007/s00330-018-5329-y [DOI] [PubMed] [Google Scholar]
- 6. Jo S, Kim S . Association between oncogenic RAS mutation and radiologic-pathologic findings in patients with primary rectal cancer, Quant . Quant Imaging Med Surg 2019. ; 9: 238 – 46 . doi: 10.21037/qims.2018.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang L, Dong D, Fang M, et al. . Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer . Eur Radiol 2018. ; 28: 2058 – 67 . doi: 10.1007/s00330-017-5146-8 [DOI] [PubMed] [Google Scholar]
- 8. Wu X, Li Y, Chen X, et al. . Deep learning features improve the performance of a radiomics signature for predicting kras status in patients with colorectal cancer, Acad . Acad Radiol 2020. ; 27: e254 - 62 . doi: 10.1016/j.acra.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Eresen A, Shangguan J, et al. . Preoperative prediction of perineural invasion and KRAS mutation in colon cancer using machine learning . J Cancer Res Clin Oncol 2020. ; 146: 3165 – 74 . doi: 10.1007/s00432-020-03354-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He K, Liu X, Li M, Li X, Yang H, Zhang H . Noninvasive KRAS mutation estimation in colorectal cancer using a deep learning method based on CT imaging . BMC Med Imaging 2020. ; 20( 1 ): 59 . doi: 10.1186/s12880-020-00457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang H, Chen Y, Zhang Y . ( n.d .). Comparison of clinical-computed tomography model with 2D and 3D radiomics models to predict occult peritoneal metastases in advanced gastric cancer . Abdom Radiol (NY . [DOI] [PubMed] [Google Scholar]
- 12. Cui Y, Liu H, Ren J, Du X, Xin L, Li D, et al. . Development and validation of a MRI-based radiomics signature for prediction of KRAS mutation in rectal cancer . Eur Radiol 2020. ; 30: 1948 – 58 . doi: 10.1007/s00330-019-06572-3 [DOI] [PubMed] [Google Scholar]
- 13. Tan X, Ma Z, Yan L, Ye W, Liu Z, Liang C . Radiomics nomogram outperforms size criteria in discriminating lymph node metastasis in resectable esophageal squamous cell carcinoma . Eur Radiol 2019. ; 29: 392 – 400 . doi: 10.1007/s00330-018-5581-1 [DOI] [PubMed] [Google Scholar]
- 14. Miles K, Ganeshan B, Rodriguez M, et al. . Multifunctional imaging signature for V-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations in colorectal cancer . J Nucl Med 2014. ; 55: 386 – 91 . doi: 10.2967/jnumed.113.120485 [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Eresen A, Shang J, et al. . Preoperative prediction of perineural invasion and KRAS mutation in colon cancer using machine learning . J Cancer Res Clin Oncol 2020. ; 146: 3165 – 74 . doi: 10.1007/s00432-020-03354-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang G, Chen L, Liu A, et al. . Comparable Performance of Deep Learning-Based to Manual-Based Tumor Segmentation in KRAS/NRAS/BRAF Mutation Prediction With MR-Based Radiomics in Rectal Cancer . Front Oncol 2021. ; 11: 696 – 706 : 696706 . doi: 10.3389/fonc.2021.696706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosman F . World Health Organization., International Agency for Research on Cancer, WHO classification of tumours of the digestive systemn, IARCPress . 2010. .
- 18. Lubner M, Stabo N, Lubner S, et al. . CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes . Abdom Imaging 2015. ; 40: 2331 – 37 . doi: 10.1007/s00261-015-0438-4 [DOI] [PubMed] [Google Scholar]
- 19. Meng L, Dong D, Chen X, et al. . 2D and 3D CT Radiomic Features Performance Comparison in Characterization of Gastric Cancer: A Multi-Center Study . IEEE J Biomed Health Inform 2021. ; 25: 755 – 63 . doi: 10.1109/JBHI.2020.3002805 [DOI] [PubMed] [Google Scholar]
- 20. Xu X, Wang H, Du P, et al. . A predictive nomogram for individualized recurrence stratification of bladder cancer using multiparametric MRI and clinical risk factors . J Magn Reson Imaging 2019. ; 50: 1893 – 1904 . doi: 10.1002/jmri.26749 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.