Abstract
Objective
To explore the correlation between radiomic features and the pathology of pure ground-glass opacities (pGGOs), we established a radiomics model for predicting the pathological subtypes of minimally invasive adenocarcinoma (MIA) and precursor lesions.
Methods:
CT images of 1521 patients with lung adenocarcinoma or precursor lesions appearing as pGGOs on CT in our hospital (The Third Affiliated Hospital of Sun Yat-sen University) from January 2015 to March 2021 were analyzed retrospectively and selected based on inclusion and exclusion criteria. pGGOs were divided into an atypical adenomatous hyperplasia (AAH)/adenocarcinoma in situ (AIS) group and an MIA group. Radiomic features were extracted from the original and preprocessed images of the region of interest. ANOVA and least absolute shrinkage and selection operator feature selection algorithm were used for feature selection. Logistic regression algorithm was used to construct radiomics prediction model. Receiver operating characteristic curves were used to evaluate the classification efficiency.
Results
129 pGGOs were included. 2107 radiomic features were extracted from each region of interest. 18 radiomic features were eventually selected for model construction. The area under the curve of the radiomics model was 0.884 [95% confidence interval (CI), 0.818–0.949] in the training set and 0.872 (95% CI, 0.756–0.988) in the test set, with a sensitivity of 72.73%, specificity of 88.24% and accuracy of 79.47%. The decision curve indicated that the model had a high net benefit rate.
Conclusion
The prediction model for pathological subtypes of MIA and precursor lesions in pGGOs demonstrated a high diagnostic accuracy.
Advances in knowledge:
We focused on lesions appearing as pGGOs on CT and revealed the differences in radiomic features between MIA and precursor lesions. We constructed a radiomics prediction model and improved the diagnostic accuracy for the pathology of MIA and precursor lesions.
Introduction
As of 2020, lung cancer is still one of the malignant tumors with the highest morbidity and mortality worldwide. 1 Lung adenocarcinoma is the most common histological subtype of lung cancer. 2,3 In 2011, atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma (IAC) were identified as pathological subtypes of lung adenocarcinoma. 4 In 2021, AAH and AIS were classified as precursor lesions to lung adenocarcinoma. 5 The 5-year disease-free survival rate of AAH and AIS after surgery can reach 100% and it can almost reach 100% in MIA. 6–11 Although these subtypes all have good prognoses, MIA with poorly differentiated invasive patterns, such as micropapillary or solid predominant pattern, is more likely to recur or develop metachronous adenocarcinoma, which requires stricter monitoring and additional treatment after operation. 12 Therefore, improving the predictive accuracy for pathological subtypes of MIA and precursor lesions is of great clinical significance for treatment and prognosis.
Most early lung adenocarcinomas appear as ground-glass opacities (GGOs) in CT. 13–15 GGO is defined as hazy increased opacity of lung, with preservation of bronchial and vascular margins. 16 According to the density and proportion of the solid components, GGOs can be divided into pure ground-glass opacities (pGGOs) and mixed ground-glass opacities (mGGOs). 17
Radiologists often use specific imaging features to distinguish among different pathological subtypes of lung adenocarcinoma. However, the imaging features might overlap between subtypes, limiting conventional imaging-based diagnosis. 12,18 Radiomics is a new interdisciplinary approach, first proposed by Lambin. 19 Aerts et al were the first to conduct a comprehensive analysis of lung cancer with radiomics. 20 Currently, radiomics has been increasingly widely used in the medical field. Researchers could extract a large number of high-dimensional imaging features from region of interest (ROI) on images obtained with imaging equipment such as CT and MRI and conduct large-scale and extensive mining of these quantitative data with machine learning, deep learning or other technologies to uncover more hidden information, thus helping improve diagnosis accuracy and promote personalized diagnosis and treatment. 21
Currently, a large number of radiomics studies for lung cancer have explored the distinction between pathological subtypes by radiomics. However, the difference between MIA and precursor lesions remains unclear because of the inclusion of an IAC group in most studies. Moreover, many studies included both pGGOs and mGGOs in one group. All these factors might impact the resulting radiomics model. 22–25
Therefore, we focused on lesions appearing as pGGOs on CT imaging and explored the correlation between radiomic features and pathology to reveal the radiomic differences between MIA and precursor lesions. We tried to improve the diagnostic accuracy of conventional imaging by constructing a radiomics prediction model to facilitate new possibilities for the development of precision medicine for MIA and precursor lesions.
Methods and materials
Research subjects
We reviewed the data from 1521 patients diagnosed with lung adenocarcinoma or precursor lesions at the Third Affiliated Hospital of Sun Yat-sen University from January 1, 2015 to March 1, 2021. All patients were included according to the following criteria.
Inclusion criteria: (1) had lesions treated with surgical resection and pathologically diagnosed as AAH, AIS or MIA; (2) underwent CT of the lung within 1 month before surgery; (3) did not receive antitumor therapy before surgery; (4) had lesions appeared as pGGOs on CT. (5) had peripheral lesions (Compared with central lesions, radiomics features extracted from peripheral lesions were less disturbed by the adjacent tissue); and (6) had complete clinical information that could be collected from the electronic medical record system.
Exclusion criteria: (1) only underwent nodules pathological biopsy or bronchial biopsy, or the pathological diagnosis was uncertain (The heterogeneity of lung adenocarcinomas would make it difficult for biopsy to accurately reveal the pathology); (2) the margin of the lesion was too blurred to delineate the region of interest (ROI); (3) the quality of CT imaging was poor, with significant motion artifacts; and (4) had CT images failed to be analyzed by the artificial intelligence scientific research platform system due to missing images in sequences.
120 patients with 129 pGGOs were included (Figures 1 and 2).
Figure 1.
Flow chart of patient selection. AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity; ROI, region of interest.
Figure 2.
CT images of pGGOs. (a) A 43-year-old male with an MIA lesion in the outer segment of the right middle lobe that appeared as a round pGGO with a blurred margin, spiculation, vacuole sign and vascular convergence sign. (b) A 63-year-old female with an AIS lesion in the outer segment of the right middle lobe that appeared as a round pGGO with a blurred margin, spiculation and vascular convergence sign. AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity.
Research groups
129 pGGOs were divided into the AAH/AIS group (n = 72) and the MIA group (n = 57). Of the 120 patients, 9 patients had 2 pGGOs each.
Clinical data collection
Clinical information of the 120 included patients was collected from the electronic medical record system. These data included the patient’s name, gender, age, main symptoms, hospitalization number, examination number, pathology number, pathological diagnosis description, pathology type, date of pathology report, interval between the last CT scan before surgery and surgery, tumor TNM stage based on the eighth edition of the TNM classification for lung cancer, presence of invasion into the pleura, lymph nodes and blood vessels, surgical method and smoking history. The pathological diagnosis of each lesion, made by one pathologist, were reviewed and confirmed by another senior pathologist.
CT scan and imaging features acquisition
The CT scans were performed with a 320-slice Aquilion ONE CT scanner (Toshiba Medical Systems, Tokyo, Japan) and an IQon Spectral scanner (Philips Healthcare, Best, the Netherlands). During the scan, the patient was in a supine position, with arms raised and head advanced. All scans were performed at the end of inspiration during a breath hold without intravenous contrast. The CT protocol was as follows: 120 kV, automatic tube current modulation (180 mA-440 mA), iterative reconstruction technique, detector 160/40 mm, collimation 0.5/0.625 mm, pitch 1.5, matrix 512*512 and rotation time 0.5/0.27 s. The reconstructed axial layer thickness and layer distance were 1 mm.
The CT images were reviewed independently by two radiologists blinded to the pathological type. The results were confirmed by one senior radiologist. When observing the images, the lung window was set to a window level of −600 HU and a window width of 1600 HU and the mediastinal window was set to a window level of 40 HU and a window width of 300 HU.
ROI segmentation
The CT images were uploaded in original DICOM format to the Deepwise multimodal research platform (https: //keyan. deepwise. com, v. 1. 6. 2) for ROI segmentation and radiomic feature extraction. The semiautomatic segmentation method was used for ROI segmentation. 26,27 First, artificial intelligence technology was used for automatic segmentation. Second, one radiologist manually modified the segmentation. Third, another radiologist independently reviewed the modifications. If there were differences in opinions, a consensus decision would be reached through joint consultation. The results were confirmed by one senior radiologist. Feature extraction and quantification were performed after the acquisition of three-dimensional volume of interest (VOI).
Radiomic feature extraction and selection
High- or low-pass filter wavelet filter, Laplacian of gaussian filter with different λ parameters and other imaging transformations were applied for preprocessing in the original images. Filters, such as wavelets or Laplacian of gaussian filters, play an important part in emphasizing specific image characteristics such as edges and blobs. 28 Radiomic features were extracted from the original and preprocessed images, including first-order features, shape features, gray level co-occurrence matrix (GLCM) features, gray level run length matrix (GLRLM) features, gray level size zone matrix (GLSZM) features, gray level dependence matrix (GLDM) features and neighboring gray tone difference matrix (NGTDM) features.
2107 radiomic features were extracted from each VOI and all the features were standardized by Z-score (which was calculated by subtracting the average value, dividing by the standard deviation). Additionally, to correct for the scanner effect, the ComBat realignment method was used before selecting the features. 29 This approach has already been successfully validated for radiomic features obtained from CT 30,31 images of patients or phantom data in studies supporting the relevance of harmonization. Then, one-way ANOVA was used to select the features related to pathological subtypes and the features with p values less than 0.05 were used for subsequent analysis.
Last, to avoid the “curse of dimensionality”, which could lead to a large false-positive result, the least absolute shrinkage and selection operator (LASSO) feature selection algorithm was used to select the most informative radiomic features extracted from all VOIs. During the feature selection process, most of the covariate coefficients were reduced to zero and the variables that still had a non-zero coefficient after the shrinking process were selected to construct the pathological subtype prediction model.
Construction of radiomics model
We used the features selected by the LASSO algorithm to build the radiomics model. Logistic regression model was used to predict the pathological subtypes of MIA and precursor lesions in pGGOs. All 129 cases were divided into training set and test set at a ratio of 7:3. In the training set, the five-fold cross-validation method was used to select the best model and the test set was used to verify the efficiency of the model (Figure 3). The calculation of the RadScores can visually show the weights of different features in the radiomic model, as shown in Formula 1:
Figure 3.
Workflow diagram of radiomics procedure
Radscore = Sigmoid (β1 * x1 + β2 * x2 + β3 * x3 + … + βn * xn),
Sigmoid(x) = . β1, β2, β3, βn are the coefficients of each feature. x1, x2, x3, and xn are the values of radiomic features.
Statistical analysis
All of the code was programmed in Python (v. 3.9.1). NumPy (v. 1.20.2) was used to preprocess the data and the scikit-learn framework (v. 0.20.3) was used to implement other machine learning algorithms. For logistic regression, the penalty was L1, C was 0.80335 and the solver was set to “liblinear”. Matplotlib (v. 3.1.0) was used to draw receiver operating characteristic (ROC) curves. IBM SPSS Statistics, v. 25.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis of general data. χ2 test was used to detect differences in categorical variables between groups. t test was used to detect differences in quantitative variables between groups. When p < 0.05, the difference was considered statistically significant. ROC curve was drawn to reveal the classification performance of the model in the training set and the test set. Evaluation indicators mainly included area under the curve (AUC), sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV). Decision curve was drawn to display the relationship between the benefits and risks brought by different cut-off points in different models. Analysis of the calibration curve was used to calibrate the model.
Result
Research groups
The training set (n = 90) included 50 cases of AAH/AIS and 40 cases of MIA. The test set (n = 39) included 22 cases of AAH/AIS and 17 cases of MIA. There was no statistical difference in data distribution between the two groups (p < 0.05).
Clinical characteristics
Among the 120 included patients, there were 34 male patients (28.33%) with an average age of 46.62 ± 12.39 and 86 female patients (71.66%) with an average age of 49.20 ± 11.51. The average and median intervals between the last CT scan before surgery and surgery were 11.20 ± 7.34 days and 10 days, respectively. There were 56 cases (46.67%) in TNM stage 0, 63 cases (52.50%) in stage I and one case (0.83%) in stage II. Only one case of MIA was accompanied by lymph node invasion. 61 patients (50.83%) underwent nodule resection, 43 (35.83%) underwent wedge/segment resection, and 16 (13.33%) underwent lobectomy. Most patients had no obvious symptoms (70.00%). The most common symptoms were cough, expectoration, hemoptysis and chest pain. Most of the patients had no history of smoking (92.50%). Except for TNM stage (p < 0.001), all clinical features were not significantly different between AAH/AIS and MIA (p > 0.05). (Table 1)
Table 1.
Clinical characteristics of 111 included patients with single pGGO
| Clinical characteristics | AAH / AIS (n = 62) | MIA (n = 49) | p | Cases |
|---|---|---|---|---|
| Gender | 0.056a | |||
| Male | 23 (37.10%) | 10 (20.41%) | 33 (29.73%) | |
| Female | 39 (62.90%) | 39 (79.59%) | 78 (70.27%) | |
| Age | 47.85 ± 11.40 | 49.43 ± 12.36 | 0.488b | 111 (100%) |
| TNM stage | <0.001a | |||
| 0 | 53 (85.48%) | 0 (0%) | 53 (47.75%) | |
| I | 9 (14.52%) | 48 (97.96%) | 57 (51.35%) | |
| II | 0 (0%) | 1 (2.04%) | 1 (0.90%) | |
| Invasion | 0.441a | |||
| Lymph node | 0 (0%) | 1 (2.04%) | 1 (0.90%) | |
| Surgery | 0.564a | |||
| Nodule resection | 34 (54.84%) | 24 (48.98%) | 58 (52.25%) | |
| Wedge/segment resection | 22 (35.48%) | 17 (34.69%) | 39 (35.14%) | |
| Lobectomy | 6 (9.68%) | 8 (16.33%) | 14 (12.61%) | |
| Symptoms | 0.309a | |||
| Absent | 46 (74.19%) | 32 (65.31%) | 78 (70.27%) | |
| Present | 16 (25.81%) | 17 (34.69%) | 33 (29.73%) | |
| Smoking history | 0.271a | |||
| None | 56 (90.32%) | 47 (95.92%) | 103 (92.79%) | |
| Yes | 6 (9.68%) | 2 (4.08%) | 8 (7.21%) |
AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity.
χ2 test.
t test.
Radiomic feature extraction and selection
2107 features were extracted from each VOI, including 414 first-order features, 14 shape features, 506 GLCM features, 368 GLRLM features, 368 GLSZM features, 322 GLDM features and 115 NGLDM features. 275 features were selected through ANOVA and were used in the final screening to further reduce the dimensionality of features. Finally, 18 features with nonzero coefficients were selected with LASSO. (Table 2, Supplementary Material 1)
Table 2.
18 radiomic features included in the construction of radiomics model
| Radiomic features | Coef | Relative weight | p |
|---|---|---|---|
| lbp-3D-m2_firstorder_InterquartileRange | 1.0667 | 1.0000 | 0.001 |
| wavelet-HHL_gldm_LargeDependenceLowGrayLevelEmphasis | 0.7576 | 0.7103 | 0.003 |
| logarithm_glcm_InverseVariance | 0.5599 | 0.5249 | <0.001 |
| log-sigma-1–0 mm-3D_glrlm_RunEntropy | 0.3167 | 0.2969 | 0.001 |
| log-sigma-3–0 mm-3D_glszm_SizeZoneNonUniformity | 0.1975 | 0.1852 | 0.020 |
| wavelet-LLL_ngtdm_Strength | 0.1878 | 0.1760 | 0.001 |
| Wavelet-LLL_glszm_SizeZoneNonUniformityNormalized | 0.1411 | 0.1322 | <0.001 |
| square_firstorder_Minimum | 0.0683 | 0.0640 | 0.002 |
| gradient_glszm_LowGrayLevelZoneEmphasis | −0.0554 | −0.0520 | 0.001 |
| log-sigma-2–0 mm-3D_ngtdm_Strength | −0.0853 | −0.0799 | 0.025 |
| wavelet-HLL_glrlm_GrayLevelNonUniformityNormalized | −0.1698 | −0.1592 | 0.001 |
| log-sigma-2–0 mm-3D_glrlm_LongRunHighGrayLevelEmphasis | −0.1775 | −0.1664 | 0.002 |
| log-sigma-5–0 mm-3D_glcm_DifferenceEntropy | −0.245 | −0.2296 | 0.025 |
| squareroot_ngtdm_Contrast | −0.2776 | −0.2602 | 0.001 |
| gradient_ngtdm_Contrast | −0.2896 | −0.2715 | 0.008 |
| log-sigma-3–0 mm-3D_glszm_GrayLevelNonUniformity | −0.2909 | −0.2727 | 0.009 |
| log-sigma-2–0 mm-3D_firstorder_InterquartileRange | −0.3098 | −0.2904 | 0.003 |
| wavelet-HHL_glcm_SumEntropy | −0.3681 | −0.3451 | <0.001 |
Construction of radiomics model
We compared the performances of all models in the training set and test set. The logistic regression model was found to have the best performance, and its ROC curve were shown in Figure 4. The AUC was 0.884 (95% confidence interval (CI), 0.818–0.949) in the training set and 0.872 (95% CI, 0.756–0.988) in the test set. The model had a good classification performance in both the training set and the test set. The results verified that there was no overfitting in the radiomics prediction model. (Table 3, Figure 5)
Figure 4.

The ROC curves of radiomics prediction model for pathological subtypes of MIA and precursor lesions in pGGOs in the training set and the test set. MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity; ROC curve, receiver operating characteristic curve.
Table 3.
Evaluation indexes of diagnostic performance of radiomics prediction model for pathological subtypes of MIA and precursor lesions in pGGO
| AUC | 95% CI | Sensitivity | Specificity | Accuracy | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Training set | 0.8835 | (0.8181–0.9489) | 0.74 | 0.775 | 0.7556 | 0.8043 | 0.7045 |
| Test set | 0.8717 | (0.7555–0.9878) | 0.7273 | 0.8824 | 0.7947 | 0.8889 | 0.7143 |
AUC, area under the curve; CI, confidence interval; MIA, minimally invasive adenocarcinoma; NPV, negative predictive value; pGGO, pure ground-glass opacity; PPV, positive predictive value.
.
Figure 5.
The confusion matrix of radiomics prediction model for pathological subtypes of MIA and precursor lesions in pGGOs in the training set and the test set. MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity.
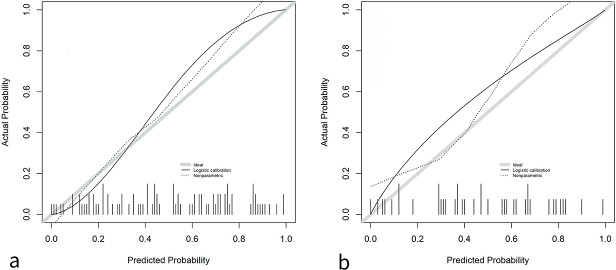
The decision curve is shown in Figure 6. The calibration curve is shown in Figure 7. In this study, we calibrated the model with the training and test sets and found that our prediction model performed well in correctly classifying AAH/AIS and MIA.
Figure 6.

The decision curves of radiomics prediction model for pathological subtypes of MIA and precursor lesions in pGGOs in the training set and the test set. MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity.
Figure 7.
The calibration curves of radiomics prediction model for pathological subtypes of MIA and precursor lesions in pGGOs in the training set and the test set. a Calibration curve of the training set. b Calibration curve of the test set. MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity.
The radiomics score of each lesion was calculated with Formula two shown in supplementary materials. (Figure 8, Supplementary Table 1)
Figure 8.

The Radscore of radiomics prediction model for pathological subtypes of MIA and precursor lesions in pGGOs in the training set and the test set. MIA, minimally invasive adenocarcinoma; pGGO, pure ground-glass opacity.
Discussion
In this study, we collected CT images of pGGOs diagnosed as AAH, AIS or MIA and we extracted radiomic features from them. Machine learning algorithms were used to construct the radiomics prediction model with high accuracy for differentiating MIA from precursor lesions.
During the progression from AAH, AIS, MIA to IAC, different pathological changes occur 32,33 that can be reflected on the CT images. Therefore, a large number of studies have explored the correlation between imaging features and the pathology of GGOs. 34,35
However, conventional imaging diagnosis is still insufficient for classifying pathological subtypes because it relies on visual observation, subjective discrimination and experience-based judgement. 25 Among the available imaging features, most can distinguish invasive adenocarcinoma from precursor lesions. Some are still difficult to distinguish between precursor lesions and MIA because there are many similar imaging features between the two pathological types.
In our research, all the radiomic features included in the prediction model were first-order features and texture features, indicating that more differences existed in intensity rather than in morphology between AAH/AIS and MIA. Compared with AAH and AIS, MIA has more substantial components. In pGGOs, although MIA has more infiltration of tumor cells and solid components, these pathological changes are still not large enough to result in visible changes in CT images, which makes it difficult to distinguish MIA from precursor lesions in pGGOs based on imaging features. Xiang et al had a similar finding that the maximum diameter, clear and rough margins and air bronchogram could distinguish AAH from AIS/MIA, but could not distinguish AIS from MIA. The average CT value could distinguish MIA from AAH/AIS, but it could not distinguish AAH from AIS. 36 The difference in intensity might be difficult to detect in conventional imaging diagnosis, which shows the great significance and value of radiomics in improving the accuracy of pathological diagnosis.
Texture features can reveal the heterogeneity of tumors. Compared with precursor lesions, MIA lesions begin to appear with invasive components such as alveoli, papillae, micropapillae or solid components and tumor heterogeneity increases. Therefore, tumors with greater heterogeneity might be relatively more invasive. The difference in tumor heterogeneity is relatively difficult to be reflected by visible imaging features, but it can be manifested by texture features. It has been found that texture features can distinguish betwee benign and malignant lesions and degrees of invasiveness. Digumarthy et al found that the cluster shade of GLCM features could distinguish benign and malignant solid nodules. 37 Mao et al constructed a radiomics model with eight high-order texture features for predicting benign and malignant solid nodules that had high sensitivity and specificity. 25 Yang et al found that in pGGOs, the entropy of IAC was significantly higher than that of AIS and MIA. 38 Song et al found that sphericity and differential entropy of GLCM were predictive indicators of micropapillary pattern ≥5% in lung adenocarcinoma. 39 Gao et al found that in GGOs, GLCM features were significantly different between AIS/MIA and IAC. 40 Our study showed that texture features can distinguish AAH/AIS from MIA in pGGOs.
Wang et al explored the value of CT imaging features of pGGOs in differentiating MIA from precursor lesions. The lesion diameter and mean CT attenuation were significantly different between the two groups, with AUCs of 0.76 and 0.79, respectively. 18 In comparision, our radiomics model showed higher diagnostic accuracy, indicating the value of radiomics in assisting conventional imaging diagnosis of pathology. According to the decision curve, it has a high net benefit rate indicating great clinical application value. Therefore, the application of radiomics could overcome the limitations of conventional imaging diagnosis and improves accuracy. Radiomics makes it possible to extract comprehensive radiomic features from medical images and to apply more imaging information that is difficult to observe and analyze in conventional imaging diagnosis in the clinic. Additionally, the improvement in the accuracy of imaging diagnosis could make it possible to replace invasive examinations with noninvasive methods.
Currently, there has been a huge clinical demand for radiomics research and applications with broad clinical application prospects. Many studies have explored the application of radiomics in screening, diagnosis, treatment and prognosis assessment of lung cancer. To reduce the missed diagnosis rate in early lung cancer screening and improve the accuracy of diagnosis, Duan et al applied different kinds of algorithms to combine different aspects of medical information. They developed a three-layer diagnosis system. Artificial neural network (ANN) and decision tree C5.0 were used to construct three diagnostic subsystems based on epidemiology, clinical symptoms, biomarkers and radiomic features. 41 Mao et al constructed a radiomics model for predicting benign and malignant nodules with better accuracy than the ACR Lung Imaging Reporting and Data System (89.8% vs 76.5%). 25
GGOs are a common sign of lung cancer. Many studies have successfully used radiomics to improve the diagnostic accuracy of invasiveness. Xu et al constructed radiomics models for predicting AIS/MIA and IAC in pGGOs with random forest (RF) and support vector machine (SVM). The combined models showed better accuracy and sensitivity than the conventional model and radiomics model and a better performance than radiologists. 42 Cai et al constructed a prediction model for AIS/MIA and IAC with SVM. Compared with radiologists, radiomics models showed higher accuracy, sensitivity and specificity. 43 However, most of the studies focused on invasive adenocarcinoma and did not explain the difference between MIA and precursor lesions.
Since radiomics can be used to explore and reveal tumor heterogeneity, many studies have also used radiomics to evaluate the treatment and prognosis of lung cancer. Liu et al constructed risk prediction models for progression-free survival (PFS) and overall survival (OS) with SVM classifier, logistic regression classifier and gaussian Naïve Bayes classifier, thus providing a new method for predicting the clinical outcomes of advanced non-small cell lung cancer (NSCLC) patients treated with nivolumab. 44 Lee et al constructed clinical model, radiomics model and combined model to predict survival rates. The combined model had a better performance in assessing the prognosis of lung adenocarcinoma. 45
This study explored the radiomic features inside pGGOs. However, there is also an interaction between tumor cells and the surrounding environment. 46–48 In future research, we should try to incorporate perinodular radiomic features of pGGOs into the diagnostic model to improve the discrimination performance.
Although radiomics has shown great potential in clinical applications, it still faces many challenges and limitations. The biggest problem is the lack of reproducibility. Inconsistency in the collection and processing of imaging data, such as differences in imaging equipment types, scanning sequences, reconstruction thicknesses and segmentation methods, could cause differences between studies. 49,50 In our study, the CT images were acquired from two different scanners, which resulted in inter scanner differences among radiomic features and affected the reproducibility of our model. Preprocessing images and features helped reduce this effect to some extent. Additionally, our semiautomatic segmentation results might be affected by the subjectivity of radiologists. Moreover, the lack of a large sample, multi center sample and external test sets could affect the robustness of results and reduce the generalization ability of results. In our study, the sample size was relatively small and this was a single-center study with no external test set. These factors might affect the robustness of the results and reduce the generalization efficiency. In addition, we only included patients with confirmed pathological diagnoses after surgical resection, which might cause selection bias since the radiomic features of early-stage lesions only with biopsy results were not analyzed. Additionally, because we only included peripheral lesions, our model might not have good application for central lung adenocarcinoma.
Conclusion
This study analyzed the correlation between radiomic features of pGGOs and pathological subtypes of AAH/AIS and MIA. A radiomics prediction model for pathology was established, manifesting high diagnostic accuracy. It can assist radiologists performing imaging diagnosis to improve the diagnostic efficiency for pathology and promote the development of personalized diagnosis and treatment and precision medicine.
Supplementary Material
Contributor Information
Yan-qiu Zhu, Email: woodwood-wang@live.com, Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Road, Tianhe District, Guangzhou, China .
Chaohui Liu, Email: liuchaohui0413@163.com, Department of Research Collaboration, R&D Center, Beijing Deepwise & League of PHD Technology Co. Ltd, Beijing, China .
Yan Mo, Email: moyan@deepwise.com, Department of Research Collaboration, R&D Center, Beijing Deepwise & League of PHD Technology Co. Ltd, Beijing, China .
Hao Dong, Email: donghao@deepwise.com, Department of Research Collaboration, R&D Center, Beijing Deepwise & League of PHD Technology Co. Ltd, Beijing, China .
Chencui Huang, Email: huangchencui@deepwise.com, Department of Research Collaboration, R&D Center, Beijing Deepwise & League of PHD Technology Co. Ltd, Beijing, China .
Ya-ni Duan, Email: dyn9539@163.com, Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Road, Tianhe District, Guangzhou, China .
Lei-lei Tang, Email: tangleileitll23@163.com, Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Road, Tianhe District, Guangzhou, China .
Yuan-yuan Chu, Email: chuyuanyuan2020@163.com, Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Road, Tianhe District, Guangzhou, China .
Jie Qin, Email: jason020@163.com, Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, No. 600 Tianhe Road, Tianhe District, Guangzhou, China .
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries . CA Cancer J Clin 2021. ; 71: 209 – 49 . doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Kim HK, Lee HY, Choi Y-L, Choi S-J, Choi H, Lee J, et al. . Assessment of intratumoral heterogeneity of oncogenic driver mutations in surgically-resected lung adenocarcinoma: implications of percutaneous biopsy-based molecular assay for targetdirected therapy . Anticancer Res 2014. ; 34: 707 – 14 . [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Riely GJ . New pathologic classification of lung cancer: relevance for clinical practice and clinical trials . J Clin Oncol 2013. ; 31: 992 – 1001 . doi: 10.1200/JCO.2012.46.9270 [DOI] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. . International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma . J Thorac Oncol 2011. ; 6: 244 – 85 . doi: 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Classification of Tumours Editorial Board. Thoracic Tumours WHO Classification of Tumours Geneva: : World Health Organization; . 2021. . Available from : https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Thoracic-Tumours-2021 [Google Scholar]
- 6. Wilshire CL, Louie BE, Horton MP, Castiglioni M, Aye RW, Farivar AS, et al. . Comparison of outcomes for patients with lepidic pulmonary adenocarcinoma defined by 2 staging systems: a North American experience . J Thorac Cardiovasc Surg 2016. ; 151: 1561 – 68 : S0022-5223(16)00168-9 . doi: 10.1016/j.jtcvs.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 7. . Li Y, Chen Z, Yu W, Zhang Y. Clinicopathology and prognosis of 489 patients with adenocarcinoma in situ and minimally invasive adenocarcinoma of lung . Zhong Guo Xiong Xin Xue Guan Wai Ke Lin Chuang Za Zhi 2017. ; 24( 6 ): 445 - 449 . [Google Scholar]
- 8. Gu J, Lu C, Guo J, Chen L, Chu Y, Ji Y, et al. . Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma . J Surg Oncol 2013. ; 107: 474 – 80 . doi: 10.1002/jso.23259 [DOI] [PubMed] [Google Scholar]
- 9. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA . Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification . J Thorac Oncol 2011. ; 6: 1496 – 1504 . doi: 10.1097/JTO.0b013e318221f701 [DOI] [PubMed] [Google Scholar]
- 10. Tsuta K, Kawago M, Inoue E, Yoshida A, Takahashi F, Sakurai H, et al. . The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations . Lung Cancer 2013. ; 81: 371 – 76 : S0169-5002(13)00269-9 . doi: 10.1016/j.lungcan.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 11. Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. . Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases . Mod Pathol 2011. ; 24: 653 – 64 . doi: 10.1038/modpathol.2010.232 [DOI] [PubMed] [Google Scholar]
- 12. Jia M, Yu S, Cao L, Sun PL, Gao H . Clinicopathologic Features and Genetic Alterations in Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma of the Lung: Long-Term Follow-Up Study of 121 Asian Patients . Ann Surg Oncol 2020. ; 27: 3052 – 63 . doi: 10.1245/s10434-020-08241-y [DOI] [PubMed] [Google Scholar]
- 13. . Zhu X, Li Y, Zhang X, Hu Z, Zhao Y, Zhang Z. The value of CT in the early diagnosis of ground-glass pulmonary nodules . Zhong Guo Lin Chuang Yi Sheng Za Zhi 2018. ; 46( 4 ): 435 - 437 . [Google Scholar]
- 14. National Lung Screening Trial Research Team. Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, et al. . Results of initial low-dose computed tomographic screening for lung cancer . N Engl J Med 2013. ; 368: 1980 – 91 . doi: 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Si M-J, Tao X-F, Du G-Y, Cai L-L, Han H-X, Liang X-Z, et al. . Thin-section computed tomography-histopathologic comparisons of pulmonary focal interstitial fibrosis, atypical adenomatous hyperplasia, adenocarcinoma in situ, and minimally invasive adenocarcinoma with pure ground-glass opacity . Eur J Radiol 2016. ; 85: 1708 – 15 : S0720-048X(16)30222-4 . doi: 10.1016/j.ejrad.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 16. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J . Fleischner Society: Glossary of Terms for Thoracic Imaging . Radiology 2008. ; 246: 697 – 722 . doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 17. Godoy MCB, Naidich DP . Overview and strategic management of subsolid pulmonary nodules . J Thorac Imaging 2012. ; 27: 240 – 48 . doi: 10.1097/RTI.0b013e31825d515b [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Wang L, Zhang W, Zhao H, Li F . Can we differentiate minimally invasive adenocarcinoma and non-invasive neoplasms based on high-resolution computed tomography features of pure ground glass nodules PLoS One 2017. ; 12( 7 ): e0180502 . doi: 10.1371/journal.pone.0180502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. . Radiomics: extracting more information from medical images using advanced feature analysis . Eur J Cancer 2012. ; 48: 441 – 46 . doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. . Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach . Nat Commun 2014. ; 5: 4006 . doi: 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. . Radiomics: the process and the challenges . Magn Reson Imaging 2012. ; 30: 1234 – 48 : S0730-725X(12)00220-2 . doi: 10.1016/j.mri.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan L, Fang M, Li Z, Tu W, Wang S, Chen W, et al. . Radiomics signature: a biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule . Eur Radiol 2019. ; 29: 889 – 97 . doi: 10.1007/s00330-018-5530-z [DOI] [PubMed] [Google Scholar]
- 23. Chen C-H, Chang C-K, Tu C-Y, Liao W-C, Wu B-R, Chou K-T, et al. . Radiomic features analysis in computed tomography images of lung nodule classification . PLoS One 2018. ; 13( 2 ): e0192002 . doi: 10.1371/journal.pone.0192002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu Y, Lu L, E L-N, Lian W, Yang H, Schwartz LH, et al. . Application of Radiomics in Predicting the Malignancy of Pulmonary Nodules in Different Sizes . AJR Am J Roentgenol 2019. ; 213: 1213 – 20 . doi: 10.2214/AJR.19.21490 [DOI] [PubMed] [Google Scholar]
- 25. Mao L, Chen H, Liang M, Li K, Gao J, Qin P, et al. . Quantitative radiomic model for predicting malignancy of small solid pulmonary nodules detected by low-dose CT screening . Quant Imaging Med Surg 2019. ; 9: 263 – 72 . doi: 10.21037/qims.2019.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qi L-L, Wu B-T, Tang W, Zhou L-N, Huang Y, Zhao S-J, et al. . Long-term follow-up of persistent pulmonary pure ground-glass nodules with deep learning-assisted nodule segmentation . Eur Radiol 2020. ; 30: 744 – 55 . doi: 10.1007/s00330-019-06344-z [DOI] [PubMed] [Google Scholar]
- 27. Qi L-L, Wang J-W, Yang L, Huang Y, Zhao S-J, Tang W, et al. . Natural history of pathologically confirmed pulmonary subsolid nodules with deep learning-assisted nodule segmentation . Eur Radiol 2021. ; 31: 3884 – 97 . doi: 10.1007/s00330-020-07450-z [DOI] [PubMed] [Google Scholar]
- 28.Mallat. . Mallat SG . A Theory for Multiresolution Signal Decomposition: The Wavelet Representation . IEEE Trans Pattern Anal Machine Intell 1989. ; 11: 674 – 93 . doi: 10.1109/34.192463 [DOI] [Google Scholar]
- 29. Fortin J-P, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. . Harmonization of cortical thickness measurements across scanners and sites . Neuroimage 2018. ; 167: 104 – 20 : S1053-8119(17)30931-X . doi: 10.1016/j.neuroimage.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahon RN, Ghita M, Hugo GD, Weiss E . ComBat harmonization for radiomic features in independent phantom and lung cancer patient computed tomography datasets Phys Med Biol . Phys Med Biol 2020. ; 65( 1 ): 015010 . doi: 10.1088/1361-6560/ab6177 [DOI] [PubMed] [Google Scholar]
- 31. Orlhac F, Frouin F, Nioche C, Ayache N, Buvat I . Validation of A Method to Compensate Multicenter Effects Affecting CT Radiomics . Radiology 2019. ; 291: 53 – 59 . doi: 10.1148/radiol.2019182023 [DOI] [PubMed] [Google Scholar]
- 32. Inamura K . Clinicopathological Characteristics and Mutations Driving Development of Early Lung Adenocarcinoma: Tumor Initiation and Progression . Int J Mol Sci 2018. ; 19( 4 ): E1259 . doi: 10.3390/ijms19041259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. . Wang S, Fu Z, Qiu J. Progress in the diagnosis of lung adenocarcinoma manifesting as ground glass nodule . Guo Ji Yi Xue Fang She Xue Za Zhi 2021. ; 44( 1 ): 67 - 71 . [Google Scholar]
- 34. Zhan Y, Peng X, Shan F, Feng M, Shi Y, Liu L, et al. . Attenuation and Morphologic Characteristics Distinguishing a Ground-Glass Nodule Measuring 5–10 mm in Diameter as Invasive Lung Adenocarcinoma on Thin-Slice CT . AJR Am J Roentgenol 2019. ; 213: W162 – 70 . doi: 10.2214/AJR.18.21008 [DOI] [PubMed] [Google Scholar]
- 35. Qi L, Xue K, Li C, He W, Mao D, Xiao L, et al. . Analysis of CT morphologic features and attenuation for differentiating among transient lesions, atypical adenomatous hyperplasia, adenocarcinoma in situ, minimally invasive and invasive adenocarcinoma presenting as pure ground-glass nodules . Sci Rep 2019. ; 9( 1 ): 14586 . doi: 10.1038/s41598-019-50989-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiang W, Xing Y, Jiang S, Chen G, Mao H, Labh K, et al. . Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring ≤10 mm on thin-section computed tomography . Cancer Imaging 2014. ; 14: 33 . doi: 10.1186/s40644-014-0033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Digumarthy SR, Padole AM, Rastogi S, Price M, Mooradian MJ, Sequist LV, et al. . Predicting malignant potential of subsolid nodules: can radiomics preempt longitudinal follow up CT Cancer Imaging 2019. ; 19: 36 . doi: 10.1186/s40644-019-0223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Y, Wang W-W, Ren Y, Jin X-Q, Zhu Q-D, Peng C-T, et al. . Computerized texture analysis predicts histological invasiveness within lung adenocarcinoma manifesting as pure ground-glass nodules . Acta Radiol 2019. ; 60: 1258 – 64 . doi: 10.1177/0284185119826536 [DOI] [PubMed] [Google Scholar]
- 39. Song SH, Park H, Lee G, Lee HY, Sohn I, Kim HS, et al. . Imaging phenotyping using radiomics to predict micropapillary pattern within lung adenocarcinoma . J Thorac Oncol 2017. ; 12: 624 – 32 : S1556-0864(16)33538-9 . doi: 10.1016/j.jtho.2016.11.2230 [DOI] [PubMed] [Google Scholar]
- 40. Gao C, Xiang P, Ye J, Pang P, Wang S, Xu M . Can texture features improve the differentiation of infiltrative lung adenocarcinoma appearing as ground glass nodules in contrast-enhanced CT Eur J Radiol 2019. ; 117: 126 – 31 : S0720-048X(19)30214-1 . doi: 10.1016/j.ejrad.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 41. Duan S, Cao H, Liu H, Miao L, Wang J, Zhou X, et al. . Development of a machine learning-based multimode diagnosis system for lung cancer . Aging (Albany NY) 2020; 12: 9840–54. 10.18632/aging.103249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu F, Zhu W, Shen Y, Wang J, Xu R, Qutesh C, et al. . Radiomic-Based Quantitative CT Analysis of Pure Ground-Glass Nodules to Predict the Invasiveness of Lung Adenocarcinoma . Front Oncol 2020. ; 10: 872 . doi: 10.3389/fonc.2020.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cai J, Liu H, Yuan H, Wu Y, Xu Q, Lv Y, et al. . A radiomics study to predict invasive pulmonary adenocarcinoma appearing as pure ground-glass nodules . Clin Radiol 2021. ; 76: 143 – 51 : S0009-9260(20)30446-3 . doi: 10.1016/j.crad.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 44. Liu C, Gong J, Yu H, Liu Q, Wang S, Wang J . A CT-Based Radiomics Approach to Predict Nivolumab Response in Advanced Non-Small-Cell Lung Cancer . Front Oncol 2021. ; 11: 544339 . doi: 10.3389/fonc.2021.544339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee G, Park H, Sohn I, Lee S-H, Song SH, Kim H, et al. . Comprehensive Computed Tomography Radiomics Analysis of Lung Adenocarcinoma for Prognostication . Oncologist 2018. ; 23: 806 – 13 . doi: 10.1634/theoncologist.2017-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu T, Dai Y . Tumor microenvironment and therapeutic response . Cancer Lett 2017. ; 387: 61 – 68 : S0304-3835(16)30015-5 . doi: 10.1016/j.canlet.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 47. Hanahan D, Coussens LM . Accessories to the crime: functions of cells recruited to the tumor microenvironment . Cancer Cell 2012. ; 21: 309 – 22 . doi: 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 48. Wu L, Gao C, Xiang P, Zheng S, Pang P, Xu M . CT-Imaging Based Analysis of Invasive Lung Adenocarcinoma Presenting as Ground Glass Nodules Using Peri- and Intra-nodular Radiomic Features . Front Oncol 2020. ; 10: 838 . doi: 10.3389/fonc.2020.00838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shafiq-Ul-Hassan M, Zhang GG, Latifi K, Ullah G, Hunt DC, Balagurunathan Y, et al. . Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels . Med Phys 2017. ; 44: 1050 – 62 . doi: 10.1002/mp.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He L, Huang Y, Ma Z, Liang C, Liang C, Liu Z . Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule . Sci Rep 2016. ; 6: 34921 . doi: 10.1038/srep34921 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.