Abstract
Aggression, a sexually dimorphic behaviour, is prevalent in males and typically absent in virgin females. Following parturition, however, the transient expression of aggression in adult female mice protects pups from predators and infanticide by male conspecifics. While maternal hormones are known to elicit nursing, their potential role in maternal aggression remains elusive. Here, we show in mice that a molecularly defined subset of ventral premammillary (PMvDAT) neurons, instrumental for intermale aggression, switch from quiescence to a hyperexcitable state during lactation. We identify that the maternal hormones prolactin and oxytocin excite these cells through actions that include T-type Ca2+ channels. Optogenetic manipulation or genetic ablation of PMvDAT neurons profoundly affects maternal aggression, while activation of these neurons impairs the expression of non-aggression-related maternal behaviours. This work identifies a monomorphic neural substrate that can incorporate hormonal cues to enable the transient expression of a dormant behavioural program in lactating females.
Introduction
Aggression is a near-ubiquitous adaptive behaviour in animals and humans that serves multiple purposes and takes several forms, depending on the agent and context1–8. Intermale aggression is arguably the best studied form of aggression found in nature, serving to establish social hierarchy and, consequently, access to resources and mating opportunities. In contrast, aggressive encounters initiated by female mammals are typically absent, with some notable exceptions9–11. Female interactions with conspecifics, however, change dramatically after pregnancy and parturition, when lactating dams typically exhibit profound aggressive behaviour towards both males and females of the same species12. Indeed, maternal aggression is as indelible as other components of maternal behaviour, such as nursing and nest-building. This dramatic – yet reversible13 – phenotypical metamorphosis powerfully exemplifies a neurobiological challenge, specifically: How does the brain reconfigure to transiently enable the expression of a behaviour that is otherwise outside of the animal’s repertoire? The brain loci and mechanisms underlying maternal aggression,and the signals that drive this transition, are underinvestigated and remain ambiguous. A role for hormones that drive the maternal state has been proposed14–20, but the mechanisms at the cellular and network level have received scant attention. Importantly, it is not well understood if the neural modules that lead to the expression of aggression are shared between the two sexes, or exhibit sexual dimorphism. Finally, it is unknown if brain nodes that elicit certain maternal behaviours facilitate or impair the expression of other behaviours typical of the mother.
Here we address the above questions by focusing on the ventral premammillary nucleus (PMv), which is coextensive with the caudal two-thirds of the classically defined “hypothalamic attack area”21, and which has been implicated in social behaviour22–24 and intermale aggression25–28. This nucleus receives dense innervation from areas associated with sensory processing29, and projects30 to nuclei implicated in social behaviour31–34, aggression26,35–38, and reward27,39–42. Within the PMv, a subset of glutamatergic cells that express the dopamine transporter gene (PMvDAT neurons) have been specifically implicated in male agonistic behaviours25,27,28.
Here we identify PMvDAT cells as quiescent in the virgin female mouse brain, with a dramatic switch in their excitability during motherhood – excited by the hormones that surge during nursing and which facilitate the expression of maternal care. Specifically, prolactin (Prl) triggers excitatory pre- and post-synaptic changes, an effect mimicked by another maternal hormone, oxytocin (OT). Optogenetic manipulation and deletion of these neurons revealed that their activity is instrumental in the expression of maternal aggression. Finally, activation of PMvDAT neurons during a maternal care paradigm impairs the expression of pup retrieval and nursing, suggestive of a specialised role of PMv cells in prioritising a distinct behavioural expression at the expense of others.
The present study suggests that the transient sex-specific expression of an adaptive behaviour can result from state-dependent reversal of neuronal excitability.
Results
Low vs. high baseline activity of PMvDAT cells in virgin vs. postpartum females
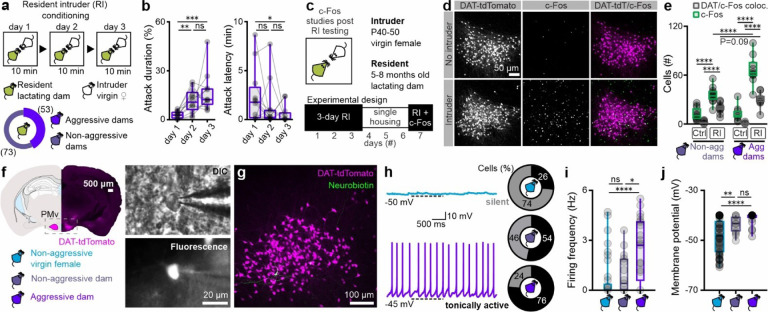
While maternal aggression is widespread in the animal kingdom, recent studies suggest substantial inter-individual variability in the expression of aggressive behaviour14,43. Furthermore, a number of variables impact on the expression of maternal aggression, including litter size44, pup presence45, and the intruder’s sex46 and behaviour47. To control for these factors, we used a three consecutive days resident-intruder (RI) paradigm to screen lactating dams for aggressive behaviour, using C57 or BALB/c juvenile virgin female mice (P40–50) as intruders and restricting litter size to four to six pups, on postnatal day three to five (P3–5). Under this paradigm, 42% of multiparous lactating dams expressed maternal aggression (Fig. 1a), with increased duration and decreased latency upon repeated exposure (Fig. 1b). The maternal aggression behaviours are similar to those observed in intermale aggression using the same paradigm27,42,48, and are in proportions consistent with earlier descriptions of maternal aggression49. C57 virgin female mice exposed to the same experimental design (Fig. S1a) did not exhibit aggression-related behaviours, including chasing, or biting: a similar observation to previous studies9,50.
Fig. 1 |. PMvDAT neurons activated by social encounters are electrically hyperexcitable in aggressive dams during the nursing state.
a Schematic representation of the experimental design used to identify lactating dams expressing maternal aggression (n=126 lactating mice). Lactating dams were between five and eight months of age.
b Quantification of maternal aggression traits identify increased attack duration (left; shown as the percentage of total session time) and decreased attack latency (right) with consecutive trials in the resident-intruder (RI) paradigm shown in Figure 1A (n=15 mice per group, RM one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value=0.0010).
c Schematic representation of the experimental design used to perform c-Fos studies following RI testing.
d Confocal images of c-Fos immunoreactivity in the PMv post-RI testing in the absence (top panels) or presence (bottom panels) of a juvenile female conspecific.
e Quantification of c-Fos and DAT-tdTomato positive cells in the PMv following control and social conditions, in lactating aggressive and non-aggressive mice (n=12 sections from 6 mice without intruder exposure, and n=10 sections from 5 mice with intruder exposure; comparisons performed using ordinary one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value<0.0001).
f Identification of PMvDAT neurons using the DAT-tdTomato mouse line to perform whole-cell patch clamp slice recordings from age-matched adult virgin female and lactating mice (five to eight months old).
g PMvDAT neurons were identified and reconstructed following the recording session using Neurobiotin-FITC.
h Example recordings from PMvDAT neurons (left) and group data in pie charts (right) from virgin female (blue) and lactating (purple) mice, exhibiting low (gray) vs. high (black) baseline spike discharge, respectively (n=53 and 21 recorded cells from 26 virgin female and 11 lactating dams, respectively).
i Quantification of firing frequency in PMvDAT cells in virgin females vs. lactating dams (n=53 vs. 21 cells, two-tailed unpaired t-test, P<0.0001).
j Quantification of membrane potential in PMvDAT cells in virgin females vs. lactating dams (n=53 vs. 21 cells, two-tailed unpaired t-test, P< 0.0001).
See also Fig. S1.
PMv neurons have been reported to activate in conjunction with an aggressive encounter in lactating rat dams24. To determine if this includes the PMvDAT neurons, we examined c-Fos immunoreactivity. Both the total number of c-Fos+ neurons, and the proportion of DAT+ cells containing c-Fos immunoreactivity, were increased following the expression of maternal aggression (Fig. 1c–e). Increased c-Fos immunoreactivity was also observed in DAT+ neurons in non-aggressive lactating dams (Fig. 1e) and in virgin female mice (in the estrus phase of the estrous cycle; Fig. S1a, b) following an encounter with an intruder. These observations are in agreement with reports in male rodents that PMvDAT neurons activate in variable degrees following different types of social encounters25,27.
A subpopulation of male laboratory rodents are non-aggressive27,42,48. Strikingly, PMvDAT neurons in male non-aggressor mice are significantly less electrically excitable than in aggressors27. This observation prompted the question of whether phenotypic differences in female mice (virgin, lactating non-aggressive, and lactating aggressive) correlate with a distinct electrical profile of PMvDAT cells51,52. Brain slice whole-cell patch-clamp recordings were performed on PMvDAT neurons, which were post-hoc reconstructed (Fig. 1f, g). Notably, PMvDAT cells from lactating dams were in a hyperexcited state compared to those from virgin females and non-aggressive lactating dams (Fig. 1h–j), exhibiting a greater proportion of discharging vs. quiescent cells (Fig. 1h), significantly higher firing frequency (Fig. 1i) and a more depolarised resting membrane potential (Fig. 1j).
Bidirectional control of maternal aggression through PMvDAT neurons
Having established that PMvDAT neurons are in a hyperexcitable state in lactating dams compared to the non-aggressive virgin female mouse, we next performed activation, inactivation and deletion of these cells in order to investigate the effect of such manipulations in vivo on maternal aggression. Genetic modifications were introduced into PMvDAT neurons by stereotactic injection of Cre-dependent constructs within an AAV vector.
PMvDAT neurons in lactating dams were transduced with channelrhodopsin-2 (ChR2, Fig. 2a) and behaviour was recorded in RI trials in the presence of male and female conspecifics (Fig. 2b). Minimal control-condition maternal aggression was attained in this experimental design by introducing an adult intruder (typically five months old and 30–35 grams in weight), in agreement with previous literature53,54, and baseline behaviour was characterised by close investigation, but rare expressions of aggression (Fig. 2c). Optogenetic stimulation of PMvDAT cells resulted in significantly prolonged attack bouts appearing within seconds of initiation of photostimulation (Fig. 2c–e, Video S1). In marked contrast, photoactivation of PMvDAT neurons in virgin female mice did not lead to aggression, but rather an increased amount of close investigation of a conspecific (Fig. S1c–g). The latter experiment suggests that the ability of an activated PMvDAT population to trigger aggression is state-dependent.
Fig. 2 |. Manipulation of the PMvDAT neuron activity allows bidirectional control of maternal aggression.
a Bilateral Cre-dependent ChR2 transduction and fiber implants in the PMv of lactating dams (left) and confocal image of a sample section counterstained with the pan-neuronal marker NeuN, used to validate ChR2 expression and stereotactic implantation of optic fiber (ft) coordinates (right).
b Schematic of the experimental design used to perform ChR2 PMvDAT neuron photoactivation in the resident lactating dam during resident-intruder (RI) testing. Adult male and female intruders were used to minimise the levels of maternal aggression expressed in baseline conditions46,54.
c Sample behaviour raster plots at baseline (top) and during ChR2 stimulation (bottom) in a lactating dam during a RI test.
d Attack duration with and without photostimulation in lactating dams injected with eYFP or ChR2, during the RI test against adult male or adult female intruders (n=5–9 mice per group, ordinary one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value<0.0001).
e Attack latency with and without photostimulation in lactating dams injected with eYFP or ChR2, during the RI test against adult male or adult female intruders (n=5–9 mice per group, ordinary one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value=0.0027).
f Bilateral Cre-dependent eNpHR3 transduction and fiber implants in the PMv of lactating dams (left) and confocal image of a sample section counterstained with the pan-neuronal marker NeuN, used to validate eNpHR3 expression and stereotactic implantation of optic fiber (ft) coordinates (right).
g Schematic of the experimental design used to perform eNpHR3 PMvDAT neuron photoinhibition studies in the resident lactating dam during RI testing.
h Sample behaviour raster plots during baseline and eNpHR3 mediated photoinhibition in a lactating dam during a RI test.
i Quantification of aggression parameters (n=8 mice per group, two-tailed paired t-test, P<0.0001, P=0.0011, and P=0.0071 respectively).
j Quantification of the latency to an attack bout following a photoinhibition episode (n = 8 mice per group, two-tailed paired t-test, P=0.0131).
k Bilateral Cre-dependent taCasp3 transduction in the PMv of lactating dams (left) and sample confocal image sections following injection of eYFP (middle) vs. taCasp3 (right), used to validate the extent of the PMvDAT cell lesion.
l Schematic of the experimental design used to perform RI tests in lactating dams with complete or partial lesion of the PMvDAT cells.
m Sample behaviour raster plots in control vs. PMvDAT neuron lesioned lactating dams during a RI test.
n Quantification of aggression parameters (n=34 trials per group, 12 mice per group, two-tailed unpaired t-test, P<0.0001, P<0.0001, and P<0.0001 respectively). All but two mice were tested three times in the RI test. Two mice, one from each group, were tested twice in the RI test.
o Quantification of PMvDAT neurons in eYFP and taCasp3 injected mice (n=24 sections per group, duplicates from 12 mice per group, two-tailed un paired t-test, P<0.0001).
To determine the behavioural effect of inhibition of the PMvDAT population, these cells in lactating dams were transduced with halorhodopsin-3 (eNpHR3, Fig. 2f) and juvenile female intruders were used to facilitate high levels of maternal aggression in baseline conditions46,54 (Fig. 2g). Indeed, under these control conditions, lactating dams frequently engaged in sustained attacks. However, optogenetic inhibition of their PMvDAT neurons was followed by decreased attack bout duration and delayed initiation of subsequent attack episodes (Fig. 2h–j).
Lastly, genetic deletion of the PMvDAT neurons in lactating dams – sparing neighbouring DAT neuron populations (Fig. S2a, b) – and subsequent RI testing (Fig. 2k, l) dramatically decreased the attack frequency and duration and increased latency to attack (Fig. 2m–o), in agreement with an earlier report applying non-specific excitotoxic PMv lesions24. Together, these experiments establish a causal and state-dependent role for PMvDAT neurons in the expression of maternal aggression.
Maternal hormones excite PMvDAT neurons
Endocrine hallmarks of the postpartum female include a surge in the serum levels of specific hormones55–59. Among these hormones, the most well-documented maternal functions are served by Prl60, which rises in late pregnancy and remains elevated after birth in rodents61–63, facilitating maternal pup care behaviour and offspring survival64–66. Similar dynamics are shown by OT, which plays a parallel and complementary role in the mother’s care for offspring67–70. We next explored if these maternal hormones can affect the activity of PMvDAT neurons that the data above implicate in puerperal aggression.
Serum levels of Prl were found ten-fold higher in lactating mouse dams compared to virgin females at any phase of the estrous cycle (Fig. 3a), confirming earlier literature71. We next determined if this difference in circulating Prl corresponds to differential Prl receptor (Prl-R) activation in the PMv of virgin vs. lactating female mice. Indeed, phosphorylation of the Signal Transducer and Activator of Transcription 5 (pSTAT5), a key sensitive downstream mediator of Prl-R activation72–83, was significantly elevated in PMvDAT neurons in lactating females (Fig. 3b–d). These findings prompted the question of whether PMvDAT cells can be directly modulated by Prl.
Fig. 3 |. Maternal hormones activate PMvDAT neurons.
a Serum prolactin levels in virgin females across the estrous cycle (proestrus, estrus, met/diestrus) vs. lactating dams (n=4–9 mice per group, ordinary one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value=0.0007).
b Confocal images of pSTAT5 immunoreactivity in PMv, in virgin females in estrus vs. lactating dams (left). Overlap of pSTAT5 and DAT-tdTomato fluorescence along the Y axis, with 0 being the most ventral point of the confocal image and 1 the most dorsal (right).
c Quantification of pSTAT5 immunoreactive cells in the PMv of virgin females in estrus vs. lactating dams (n=10 sections per group, 5 mice per group, 2 sections per mouse, two-tailed unpaired t-test, P=0.0011).
d Quantification of DAT-tdTomato colocalising pSTAT5 immunoreactive cells in the PMv of virgin females in estrus vs. lactating dams (n=10 sections per group, 5 mice per group, 2 sections per mouse, two-tailed unpaired t-test, P=0.0015).
e Application of prolactin leads to a reversible depolarisation of PMvDAT neurons (n=7 cells from 5 mice, RM one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value=0.0016).
f Prolactin-induced changes in the action potential waveform of PMvDAT neurons (left). Quantification of action potential half-width and firing threshold in control (black) vs. prolactin (orange, n=15 action potentials in control vs. bath application of prolactin per cell, 7 cells from 5 mice, two-tailed paired t-test).
g (left) Comparison of post-inhibitory rebound bouts in control (TTX) vs. prolactin application (TTX + prolactin). Black traces represent the hyperpolarization induced by the highest absolute amplitude negative current pulse, with the effect of a smaller amplitude pulse illustrated in dark gray and the absence of any pulse in light gray. (middle) Quantification of the 1st post-inhibitory rebound in control (black) vs. prolactin (orange, n=11 cells per group collected from 6 mice, two-tailed paired t-test, P=0.0098). (right) Quantification of a 2nd post-inhibitory rebound probability in control (black) vs. prolactin (orange, n=11 cells per group collected from 6 mice, two-tailed paired t-test, P=0.0061).
h Identification of a prolactin-induced postsynaptic depolarisation on PMvDAT cells in current clamp (left, n=13 cells per group collected from 8 mice, two-tailed paired t-test, P<0.0001) and voltage clamp (middle, n=5 cells per group collected from 5 mice, two-tailed paired t-test, P=0.0078). Characterisation of the prolactin-induced current across the membrane voltage spectrum in PMvDAT neurons, with a low- and high-voltage component (right, n=7 cells collected from 4 mice).
i Application of the selective T-type Ca2+ current blocker, ML218, blocks the low-voltage component of the prolactin-induced current (PrlI). Application of prolactin does not induce an inward current in PMvDAT neurons pre-exposed to ML218 (left, n=6 cells per group collected from 4 mice, two-tailed paired t-test, P=0.5129). In the presence of ML218 the prolactin-induced current is composed only of a high-voltage component (middle, n=6 cells collected from 4 mice). Comparison of the low- (right, n=6–7 cells per group, two-tailed unpaired t-test, P=0.0042) and high-voltage (right, n=6–7 cells per group, two-tailed unpaired t-test, P=0.1384) components of the prolactin-induced current in the absence vs. presence of ML218.
j Electrophysiology recordings of spontaneous excitatory postsynaptic currents during application of gabazine (to block fast inhibitory synaptic transmission) and of Prl (n=8 cells collected from 4 mice).
k Oxytocin-immunoreactive fibers forming close appositions to PMvDAT neurons (n=5 mice).
l Oxytocin leads to a reversible depolarisation of PMvDAT neurons (n=7 cells per group, collected from 5 mice, RM one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value<0.0001).
m Application of the selective oxytocin receptor agonist, TGOT, leads to depolarisation of PMvDAT neurons (top, n = 5 cells per group, collected from 5 mice, two-tailed paired t-test, P=0.0274,). Under oxytocin receptor blockade, the oxytocin-induced depolarisation of PMvDAT neurons persists (bottom, n = 5 cells per group, collected from 5 mice, two-tailed paired t-test, P=0.0008).
n Identification of an oxytocin-induced postsynaptic depolarisation on PMvDAT cells in current clamp (left, n=8 cells per group collected from 4 mice, two-tailed paired t-test, P<0.0001). Characterisation of the oxytocin-induced current across the membrane voltage spectrum in PMvDAT neurons, with a low - and high -voltage component (right, n=8 cells collected from 4 mice).
Application of Prl to PMvDAT neurons during ex vivo patch clamp recordings yielded several electrophysiological changes, which can increase the excitability and output of these cells. Recordings were performed on brain slices collected from virgin female mice, in order to investigate the effect of the hormone on these cells without prior exposure to the long-term high Prl and OT concentrations associated with the pregnant/lactating state. Firstly, a prominent and reversible depolarisation, paralleled by increased discharge frequency, was seen (Fig. 3e), similar to observations from genetically unidentified PMv neurons84. Secondly, action potential duration was significantly prolonged (Fig. 3f), to levels that have been reported to increase neurotransmitter release by an order of magnitude85. Thirdly, the capacity for, and strength of, post-inhibitory rebound were augmented (Fig. 3g), key features of PMvDAT neurons, and implicated in their propensity for regenerative discharge27. The post-synaptic Prl-induced current in PMvDAT cells consisted of a low- and a high-voltage component (Fig. 3h). Application of the selective T-type low-threshold Ca2+ current blocker, ML218, abolished the low-voltage component (Fig. 3i). Lastly, we tested whether blocking the T-type Ca2+ channels impaired the post-inhibitory rebound and the Prl-induced potentiation of rebound excitation. Indeed, ML218 abolished the post-inhibitory rebound current and effect of Prl at below firing threshold membrane potential (data not shown).
Interestingly, the application of Prl also affected synaptic input into PMvDAT neurons, with an increase of both spontaneous excitatory (Fig. 3j) and inhibitory (data not shown) post-synaptic currents. A likely source of the increased excitatory synaptic currents is recurrent axon collaterals, as the glutamatergic PMvDAT population is densely interconnected27. These data reveal a wide repertoire of Prl actions on the membrane properties of PMvDAT neurons that together converge to increase excitability.
Oxytocin, beyond its role in milk ejection86–88, is most commonly discussed as an agent of maternal care and social bonding89,90. Previous studies have, however, also correlated increased serum OT levels to maternal aggression14,91,92. Yet, a cellular mechanism for how OT might promote attack behaviour in dams remains elusive. Immunofluorescence staining of the PMv identified close appositions between OT positive fibers and PMvDAT cell somata (Fig. 3k). This finding, and the presence of OT receptors (OT-R’s) in the PMv93, led us to evaluate the responsiveness of PMvDAT neurons to OT. Application of OT yielded a reversible depolarisation of PMvDAT neurons (Fig. 3l), similar to the effect of Prl (as above), as did application of the selective OT-R agonist, TGOT94,95 (Fig. 3m). However, in the presence of an OT-R antagonist (vasotocin [d(CH2)51, Tyr(Me)2, Thr4, Orn8, des-Gly NH29])93 a depolarisation of resting membrane potential was still observed when OT was applied. Thus, although available histochemical data indicate the expression of OT-R’s, but not vasopressin receptors in the PMv93,96–98, these pharmacological results suggest that vasopressin receptors99–104 may also contribute to the peptide’s action on PMvDAT neurons (Fig. 3m). The OT effect depended on a post-synaptic depolarisation, composed of low- and high-voltage components, similar to the Prl-induced current (Fig. 3n).
To investigate whether maternal hormones acting in the PMv are sufficient to trigger aggression in an inherently non-aggressive female, we performed a 28-day localised application of Prl, OT, or both peptide hormones (co-administration) unilaterally into the nucleus of virgin female adult mice. Two weeks after implantation, we performed a panel of tests to investigate changes in aggressive and/or alloparental behaviour, as well as other behavioural features (Fig. S3a). No attacks or changes in the degree of close investigation were observed in the RI test (Fig, S3b). Notably, however, local co-administration of maternal hormones in the PMv decreased sociability in the group (Fig. S3c) and, importantly, impaired alloparenting (Fig. S3d). Modest changes in locomotion, but not anxiety parameters, were identified (Fig. S3e, f).
Together, these experiments indicate that PMv-targetted application of maternal hormones in non-lactating females does not lead to the expression of aggression, suggestive of necessary upstream and/or downstream changes implemented in the brains of lactating dams, but does decrease sociability and pup retrieval performance.
Activation of PMvDAT neurons impairs maternal care
Aggression in dams is part of a behavioural program that turns on at parturition and declines by weaning46,105. This program includes a coincident initiation of pup care – such as nursing, grooming and crouching - and maternal aggression, aimed to inflict injury and deter infanticidal conspecifics or predators from attacking the pups105. In the final experiment, we tested whether pup care and maternal aggression can be triggered by common anatomical substrates, such as the PMv, or whether activation of defined brain loci/circuitry leads to the execution of dedicated/non-overlapping behavioural programs at the expense of other behaviours.
To investigate this issue, we tested the effect of optogenetic activation of PMvDAT neurons, in the absence of an intruder, during a pup retrieval test106,107. The pup retrieval test was performed with lactating dams expressing ChR2 or eYFP in PMvDAT neurons (Fig. 4a). Control (eYFP-expressing) dams retrieved all pups within one minute of the task initiation during photostimulation (Fig. 4b, d, e, Video S2). In contrast, photostimulated ChR2-expressing dams exhibited severely impaired pup retrieval behaviour and, in many cases, failed to retrieve any pups over the maximum test period of three minutes (Fig. 4b–e, Video S3). No aggression towards the pups was observed in any of the trials.
Fig. 4 |. Activation of PMvDAT neurons suppresses non-aggression-related behavioural programs.
a Schematic of the experimental design used to perform ChR2 PMvDAT neuron photoactivation during the pup retrieval test.
b Images from two photostimulation trials at the start (0 seconds) and 50 seconds post-trial initiation, with lactating dams expressing eYFP (left) and ChR2 (right) in PMvDAT neurons.
c Sample behaviour raster plots of two sequential trials without and with photostimulation in a ChR2-expressing lactating dam during the pup retrieval test.
d Pup retrieval duration with and without photostimulation in lactating dams injected with eYFP or ChR2, during the pup retrieval test (n=5 mice per group, ordinary one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value<0.0001).
e Percentage of pups retrieved with and without photostimulation in lactating dams injected with eYFP or ChR2, during the pup retrieval test (n=5 mice per group, ordinary one-way ANOVA with Tukey’s test for correcting for multiple comparisons, ANOVA P value<0.0001).
These data suggest that activation of these neurons promotes a specialised behavioural program – the one of maternal aggression – at the expense of other maternal behaviours. Thus, even in the absence of a competing intruder stimulus, normal nursing behaviour is inhibited when the PMvDAT population is activated.
Discussion
Maternal aggression is a well-established adaptive behaviour across species, remarkable for its sudden onset and reversibility in adulthood12,108. The neuronal substrate that underlies the transient aggressive state of the dam has remained elusive. In recent years, key elements of a brain architecture that drives intermale aggression have been identified26,27,109–112; some of these loci have also been implicated in the expression of aggression in females9,111,113. However, it cannot be automatically inferred that the same neurons drive maternal attacks, as the behaviour is manifested through distinct action patterns14,49,111. Importantly, a circuit-based model of maternal aggression needs to account for the transient nature of this trait.
Here we show that PMvDAT neurons, a key population in orchestrating intermale aggression28, are typically quiescent in the virgin female but undergo a dramatic shift to an active state during nursing and are required for the expression of maternal aggression. Stimulation of these cells disrupts maternal pup care, even in the absence of overt aggression. The hallmark hormonal agents of lactation, Prl and OT, can powerfully, and through multiple convergent mechanisms, stimulate these cells into discharge mode. These findings suggest a flexible neural framework for hormonally driven generation of adaptive aggression.
Mechanisms have been explored to explain the inherent – purportedly stable – aggression and non-aggression phenotypes of male animals, and one substrate has been found in the electrical excitability of PMvDAT neurons27. But behavioural and neural traits do not remain static over lifetime48. The present results show that PMvDAT cells are relatively hyperpolarised below firing threshold in virgin females, but enter a depolarised state of action potential discharge in nursing dams. Strikingly, this dichotomy closely mirrors the electrical properties of PMvDAT neurons in phenotypically aggressive and non-aggressive males38. These findings provide experimental support to the concept that sexual dimorphism in aggression does not result from fundamentally different wiring of the male and female brain (see Beach F. A., 1975114). Rather, a common framework, activated by adaptive cues, may underlie situationally relevant attack behaviour in both sexes. This organisation echoes the seminal demonstration that the neuronal substrate for male-like mating behaviour sequences also exists in the female mouse brain109,111,115. The present data expand this concept by showing not just the existence of such a “veiled circuit” for maternal aggression in the PMv, but that these neurons are also transiently mobilised when offspring needs to be defended to execute attacks until pups have left the nest. It remains to be determined if the different motor expressions of intermale and maternal aggression14,49,111 reflect the engagement of distinct downstream motor pattern generators.
Sex steroids underlie the brain adaptations during pregnancy, but post-partum maternalspecific physiology and behaviour have been largely attributed to pup cues116. Physical interactions with pups trigger hormone release in the dam, which serves as the interface to the brain. This is illustrated by the lactation reflex, where suckling on the nipple triggers the release of OT and Prl from the pituitary gland, driving milk production and ejection, respectively. In parallel, these hormones also initiate and maintain maternal care16,64,117. The induction of pSTAT5 in PMvDAT neurons during lactation (present findings) opens the possibility that Prl, and possibly also OT, may play a role in the initial excitability switch, but additional puerperal factors likely play a major role in this transition. Acutely, Prl increases discharge (by bringing them towards or beyond the firing threshold), amplifies the input-output ratio (by increasing action potential duration), and accelerates persistent activity (by promoting rebound bursting) in PMvDAT cells. A similar increase in excitability is induced by OT (possibly acting, at least in part, via vasopressin receptors), indicating a synergistic action of the two hormones. Collectively, this broad program of electrophysiological actions push PMvDAT neurons into the active state that they exhibit in phenotypically aggressive mice (38; present findings).
The roles of Prl and OT in maternal aggression remain poorly understood. Although these hormones are most commonly discussed in the context of reinforcing bonds between mother and child, there is also data pointing to their involvement in fighting off a potential threat to offspring14,15,17–19,118,119. A recent study proposed that Prl (acting in the ventrolateral subdivision of the ventromedial hypothalamus [VMHvl]) serves to dampen aggression120, seemingly at odds with the model suggested by our results. However, the nucleus-specific receptor deletion that was employed in the study may obscure the cumulative effect of the hormone in the brain on behaviour. Indeed, while stimulation of PMvDAT cells is a powerful inducer of attacks in the puerperal state (present data), chemogenetic activation of VMHvl Prl receptor neurons has no effect on maternal aggression120. Our study and Georgescu et al’s120 together indicate that Prl may be a powerful modulator of aggression in dams which merits further investigation. Our data further suggest that isolated action of Prl and OT in the PMv of virgin female mice is not sufficient to trigger aggression, but that additional circuit adaptations related to pregnancy and/or nursing are required to establish this behaviour in dams. This idea is also supported by the inability of photoactivating PMvDAT cells to elicit aggressive behavior in virgin female mice (Fig. S1c–g). Our findings favour a model where factors linked to pregnancy transform the PMvDAT cells into a hyperexcitable state, likely concomitant with reconfiguration of other elements of the aggression circuitry located further downstream, enabling them to translate PMv-driven stimulation into attack behaviour. With parturition, the surge in Prl and OT provide a powerful depolarising influence synergising with this primed state to push the circuit into hyperactivity and thus drive the full expression of aggression. Such a role for Prl and OT in maternal aggression would also account for the disappearance of this behaviour with weaning, when serum levels of these hormones quickly drop to pre-puerperal levels.
Finally, we show that stimulation of the PMvDAT neurons – the same paradigm that powerfully promoted aggressive behaviour in the resident-intruder test – does not elicit pup-directed aggression but markedly impairs parental care during a pup retrieval task. These data firstly suggest (as previously shown for the same population in males38) that activation of PMvDAT cells does not indiscriminately promote attacks against any available target, i.e. pups, but rather may be specific for juvenile and adult intruders. This property of context specificity distinguishes PMvDAT neurons from the well-characterised VMHvlESR1 cells, which drive attacks equally towards conspecifics or inanimate objects121. Secondly, it is clear that PMvDAT neurons do not constitute a hub for driving the full repertoire of maternal behaviours122. Rather, an intriguing speculation that these results invite is that parental care of offspring is actively suppressed during aggression orchestrated via the PMvDAT population. Indeed, in virgin females the infusion of OT and Prl into the PMv resulted in a disruption of alloparenting (Fig. S3). It should be noted, however, that the impairment of pup care following optogenetic PMvDAT activation does not automatically mean that this system plays a physiological role in suppressing nursing, but may impact the prioritisation of maternal behaviours; future work will need to explore this aspect in greater depth.
The data presented here reveal how the transient activation of a circuit can allow animals to reversibly access a behaviour for adaptive purposes. Our results suggest a model in which PMvDAT neurons are hormonally primed into a hyperexcitable state in the dam, greatly lowering the threshold for eliciting attacks against conspecifics. With the sudden appearance of an intruder, activation of these neurons may serve to prioritise attack over nursing to direct attention and resources to the most imminent threat to the pups’ survival.
Materials and methods
Animals.
All animal experiments had received approval from the local ethical board, Stockholms Norra Djurförsöksetiska Nämnd, and were performed in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC). Wild-type mice with C57BL/6J and BALB/c background were used, in addition to previously generated C57BL/6J Slc6aCre (DAT-Cre) knock-in123 and floxed-tdTomato mice (The Jackson Laboratory, strain datasheet – 007909). Animals were group-housed, up to five per cage, in a temperature (23°C) and humidity (55%) controlled environment, in a 12h light, 12h dark cycle with ad libitum access to food and water. Cages were changed on a weekly basis.
Adult virgin female, and lactating female, mice used in experiments were single housed for a period of two to four weeks, depending on the experimental design. Only multiparous lactating mice were used in the experimental group of lactating dams, typically following their third or fourth parturition.
Viral vectors.
For channelrhodopsin optogenetic studies animals were injected in the PMv with 400 nL of AAV5-EF1a-DIO-hChR2(H134R)-eYFP-WPRE-hGH (Addgene20298) 8.41 × 1012 genomic copies per mL. For halorhodopsin-mediated neuronal silencing, animals were injected with 400 nL of AAV5-EF1a-DIO-eNpHR3.0-eYFP-WPRE-hGH (Addgene26966) 7.02 × 1012 genomic copies per mL. The control groups in optogenetic were injected with 400 nL of AAV5-EF1a-DIO-eYFP-WPRE-hGH (Addgene27056) 5.82 × 1012 genomic copies per mL. The ChR2, eNpHR3 and eYFP AAV5 were prepared by the University of Pennsylvania Vector Core. For targeted cell ablation of PMvDAT neurons animals were injected in PMv with 300 nL of AAV5-flex-taCasp3-TEVp 2.9 × 1012 genomic copies per mL, and the AAV was prepared by the viral vector core at the University of North Carolina. Viral injections were performed bilaterally.
Stereotactic surgery and viral gene transfer.
Adult DAT-Cre mice 5–8 months old (sexually inexperienced) were stereotactically injected with a virus, and implanted with fiber implants when purposed for in vivo optogenetic behaviour experiments, and were individually housed for 2 weeks post-surgery. Animals were anaesthetised with isoflurane (1–5%) and placed in a stereotactic frame (David Kopf Instruments). Virus was injected into the PMv bilaterally using a pulled glass capillary (World Precision Instruments) by nanolitre pressure injection at a flow rate of 50 nL per min (Micro4 controller, World Precision Instruments; Nanojector II, Drummond Scientific). Stereotactic injection coordinates to target the PMv were obtained from the Paxinos and Franklin atlas124 (Bregma: −2.45 mm, midline ±0.6 mm, dorsal surface −5.5 mm). Ferrules and fiber-optic patch cords were purchased from Thorlabs and Doric Lenses, respectively. The virus-injected animals were housed individually during a 2-week recovery period, and then examined behaviourally and histologically.
In the case of the lactating dams group, sexually experienced female mice with prior maternal experience were placed in a cage with a male mouse, and were checked for plugs in the morning hours of the next four days125. Following identification of plugs, female mice were single housed, and surgeries were performed for AAV injections, and/or optic fiber implants, as above. The surgical procedures occurred within a week of plug identification. Female mice in which plug identification did not occur or was ambiguous were excluded from the study.
Optogenetics.
In optogenetic experiments, subjects were coupled via a ferrule patch cord to a ferrule on the head of the mouse using a zirconia split sleeve (Doric Lenses). The optical fiber was connected to a laser (447 nm for ChR2; 635 nm for eNpHR3; CNI-MLL-III-447-200-5-LED and CNI-MLL-III-635-200-5-LED, CNI lasers 200 mW) via a fiber-optic rotary joint (FRJ_1×1_FC-FC, Doric Lenses) to avoid twisting of the cable caused by the animal’s movement. After a testing session, DAT-Cre animals were uncoupled from the fiber-optic cable and returned to a housing room. The frequency and duration of photostimulation were controlled using custom written LabView software. Laser power was controlled by dialling an analogue knob on the power supply of the laser sources. Light power was measured from the tip of the ferrule in the patch cord before being installed in the brain (the ferrule-connector system) at different laser output settings, using an optical power and energy meter and a photodiode power sensor (Thorlabs). Upon identification of the fiber placement coordinates in brain tissue slides, irradiance (light intensity) was calculated using the brain tissue light transmission calculator based on (http://www.stanford.edu/group/dlab/cgi-bin/graph/chart.php) using laser power measured at the tip and the distance from the tip to the target brain region measured by histology. Animals showing no detectable viral expression in the target region and/or ectopic fiber placement were excluded from analysis. In some experiments, animals were photostimulated with a train of 473 nm light (20 Hz, 5 msec, 5 min) 45 min before perfusion in the absence of an intruder at an intensity which had evoked a behavioural phenotype in the final testing session. Brain sections were subsequently immunohistochemically labelled for c-Fos to identify optogenetically activated cells.
Behavioural tests.
Behavioural tests were performed at 2h post-initiation of the light phase and 2h prior to initiation of the dark phase. Mice were acclimated to the testing facility for 1 h prior to testing. Behaviours were recorded using a digital video recording unit. Behavioural annotations were performed manually, with conditions blinded to the experimenter performing the behavioural scoring.
Resident-intruder (RI) test.
RI tests were initiated at 2 to 4 weeks post-surgery and repeated weekly for 2 to 5 weeks. All dams used in the present study were multiparous and were used in behavioural experiments during their third or fourth litter. Mouse cages were not cleaned for a minimum of 3 days prior to the behavioural test. Intruders were individually introduced to a DAT-Cre mouse in a testing session in a random order with respect to sex, with a 5 min interval between intruders. The types of intruders were: juvenile females, adult females and adult males. Subjects involved in the RI test were exposed to 3 experimental days of the test, resulting in multiple measurements per resident.
ChR2-mediated activation in RI.
After the introduction of an intruder, a virus-injected animal was recorded for 3 min to assess baseline behaviour towards each intruder. The baseline recording was followed by photostimulation trials (3 min in duration) with varying irradiance (intensity), stimulation intervals, distance, orientation and recent behaviour history between the two animals at the onset of photostimulation. The AAV5-DIO-eYFP and AAV5-DIO-ChR2 injected experimental animals were processed in random order.
eNpHR3-mediated silencing in RI.
DAT-Cre lactating females expressing eNpHR3 or control eYFP were introduced to between one to four juvenile female intruders, in three acclimation sessions without photostimulation to assess baseline aggression as well as to augment aggressiveness. Testing sessions initiated with recordings in the absence of laser stimulation to assess baseline behaviour towards each intruder on the day of the experiment. In photostimulation trials, irradiance (intensity) ranging from 2.1 – 11.6 mW/mm2 was delivered continuously in varying intervals (typically 2–10 sec) depending on the phase of the resident-intruder interaction. To examine whether photostimulation during charging stopped escalation to attack, residents were photostimulated during body realignment to gain access to the back of the intruder.
Pup retrieval test.
Adult virgin female or lactating mice 5–8 months old were exposed to foster pups or to their own litter, respectively. The litter size per test is described in the individual tests/figures. Material for nest building was provided in each cage. The activity in the cage was video recorded, and maternal behaviours were scored by an experimenter blind to the experimental conditions.
Three-chambered sociability test.
The social approach apparatus was an open-topped box made of acrylic (60 cm in length × 40 cm in width × 20 cm in height), and divided into three chambers with two clear acrylic walls. Dividing walls had retractable doorways allowing access into each chamber. The wire cup used to contain the novel mice was made of cylindrical chrome bars spaced 1 cm apart (10 cm H; bottom diameter: 10 cm). Test mice were confined in the center chamber at the beginning of each phase. Exploration of an enclosed mouse or an empty wire cup was defined as when a test mouse oriented toward the cup with the distance between the nose and the cup less than 1 cm, or as climbing on the cup. The time spent in each chamber and time spent exploring enclosed novel mice or empty cups (the novel objects) were recorded from an overhead camera, and analyzed using Ethovision XT12.
Open field test (OFT).
OFTs were performed in a white acrylic glass box (45 × 45 × 40 cm) with an overhead lamp directed to the centre of the field, providing 120 lux of illumination on the floor of the arena. Each mouse was placed in the corner of the apparatus and locomotion parameters were recorded for 20 min.
Elevated plus maze (EPM).
The EPM test was performed using a polyvinyl chloride maze comprising a central part (5×5 cm), two opposing open arms (32.5×5 cm), and two opposing closed arms (32.5×5×32.5 cm). The apparatus was set to a height of 50 cm, and the open arms were provided with uniform overhead illumination of 6 lux. Mice were placed in the open arm point close to the centre facing the closed arms, and video recordings were performed for a total duration of 20 min.
Chronic administration of maternal hormones in the PMv.
For localised chronic administration of maternal hormones in the PMv, we performed cannula implantations using the Paxinos and Franklin atlas124 coordinates (Bregma: −2.45 mm, midline ±0.6 mm, dorsal surface −5.5 mm). Cannulae (0008663 Brain Infusion Kit 2) and osmotic minipumps (0000298 ALZET Model 2004) were purchased from ALZET, and prepared according to protocol.
Brain slice electrophysiology.
Acute slices of the mediobasal hypothalamus were prepared from adult DAT-tdTomato mice (own breeding). Slices were cut on a vibratome (Leica VT1000S) to 250 μm thickness and continuously perfused with oxygenated aCSF containing (in millimolar): NaCl (127), KCl (2.0), NaH2PO4 (1.2), NaHCO3 (26), MgCl2 (1.3), CaCl2 (2.4), and D-glucose (10), at near-physiological temperature (33±1°C) during recording. Each slice was exposed only to a single bath application of pharmacological compounds and was used for a single experiment. Whole-cell current- and voltage-clamp recordings were performed with micropipettes filled with intracellular solution containing (in millimolar), K-gluconate (140), KCl (10), HEPES (10), EGTA (10), and Na2ATP (2) (pH 7.3 with KOH). Recordings were performed using a Multiclamp 700B amplifier, a DigiData 1440 digitiser, and pClamp 10.2 software (Molecular Devices). Slow and fast capacitative components were semi-automatically compensated. Access resistance was monitored throughout the experiments, and neurons in which the series resistance exceeded 15 MΩ or changed ≥20% were excluded from the statistics. Liquid junction potential was 16.4 mV and not compensated. The recorded current was sampled at 20 kHz.
Reagents used in slice electrophysiology experiments; Neurobiotin™ tracer (Vector laboratories) was used in combination with Streptavidin, DyLight™ 405 conjugated (21831 Thermoscientific) or Avidin-FITC (43–4411 Invitrogen). TTX was purchased from Alomone Labs. Oxytocin nonapeptide, the OT-receptor (OT-R) agonist, (Thr4, Gly7)-oxytocin (TGOT), and the OT-R antagonist, vasotocin [d(CH2)51, Tyr(Me)2, Thr4, Orn8, des-Gly NH29], were purchased from Bachem. Prolactin CYT-321 was purchased from PROSPEC, and SR 95531 (Gabazine) and ML-218 hydrochloride were purchased from Tocris. OriginPro9 was used for electrophysiological data analysis.
Immunofluorescence.
Mice were anaesthetised with sodium pentobarbital (200 mg/kg, i.p., Sanofi-Aventis), then transcardially perfused with 10 mL Ca2+-free Tyrode’s solution (37°C) containing 0.2% heparin, followed by 10 mL fixative (4% paraformaldehyde and 0.4% picric acid in 0.16 M phosphate buffer (PBS), 37°C), then 50 mL ice cold fixative. Whole brains were dissected, immersed in ice cold fixative for 90 min then stored in 0.1M PBS (pH 7.4) containing 20% sucrose, 0.02% bacitracin and 0.01% sodium azide for three days, before freezing with CO2. Coronal sections were cut at a thickness of 14 μm on a cryostat (Microm) and thaw-mounted onto gelatin-coated glass slides. For indirect immunofluorescence staining (performed at room temperature unless otherwise specified), air-dried sections were washed in 0.01 M PBS for 30 min before incubation with primary antisera diluted in PBS containing 0.3% Triton X-100 and 1% BSA for 16 hours at 4°C. The slides were then washed for 30 min in PBS followed by 2h incubation with Alexa-488-conjugated donkey anti-rabbit secondary antisera (1:500; Invitrogen). Slides went through a final wash for 30 min in PBS and mounted with glycerol containing (2.5% DABCO; Sigma). This method was used with the following antibodies: NeuN was detected with primary antibody rabbit anti-NeuN (1:500; Cell Signalling, D4G40), eYFP was detected with chicken anti-GFP (1:500; Aves Labs, GFP-1020). Immunohistochemistry for pSTAT5: Prior to immunofluorescence staining, antigen retrieval was performed by incubating sections for 15 min in citric acid (pH 7.4) at 80°C, then cooled at room temperature for a further 30 min. After a 1% H2O2 Tris-buffered wash, sections were incubated in rabbit pSTAT5 primary antibody (pSTAT5 Tyr 694, Cat#: C11C5, 1:500; Cell Signaling Technology) for 72 hours at 4°C. Primary antibody incubation was followed by Alexa-488-conjugated donkey anti-rabbit secondary antisera (1:500; Invitrogen).
To perform c-fos immunostaining, mice were deeply anesthetised with sodium pentobarbital (as described above) and perfused transcardially with 4% (weight/vol) ice-cold paraformaldehyde in 0.1M PBS. Brains were post-fixed in the same solution and 40 μm-thick coronal slices were cut at the vibratome (Leica). Two PMv coronal sections per animal were selected to perform c-fos immunostaining as follows. Sections were washed in TBS (100 mM Tris-Cl, 150 mM NaCl, pH 7.5), incubated 1h at 25°C in 1% BSA-0.3% Triton X-100-TBS solution and then kept at 4°C in rabbit anti-c-fos antibody (sc-52 LotG1108, Santa Cruz Biotechnology) solution (1:200 in 1% BSA-TBS) overnight. After TBS washing, sections were incubated for 1h at 25°C in Alexa Fluor® 647 (Invitrogen) goat anti-rabbit secondary antibody (1:500 in 1% BSA-TBS).
Microscopy and cell-counting.
All brain slices were imaged by epifluorescence microscopy (ZEISS Imager M1) or confocal microscopy (Zeiss, LSM 800) for subsequent analysis. Brain areas were determined according to their anatomy using Paxinos and Franklin Brain Atlas124. For PMvDAT cell counts the entire PMv was cut, stained and counted. Quantification of c-fos staining was obtained by averaging the number of positive cells of right and left PMv in two brain sections (−2.46 and −2.54 mm from Bregma124). All counts were performed manually using Image J software and the annotator was blind to test conditions.
Randomization and blinding.
Behavioural data collection and analysis was performed blind to experimental conditions. Anatomy data analysis but not tissue collection was blinded. Electrophysiological data sampling and analysis was not blinded, with the exception of all whole-cell patch clamp datasets presented in Figure 1. Mice were first screened for expressing maternal aggression and then further assigned to groups for behavioural experiments.
Statistical analysis.
No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications2,11,14,30,31. Data met the assumptions of the statistical tests used, and were tested for normality and equal variance. All t-tests and one-way ANOVAs were performed using Graph Pad Prism software (Graphpad Software Inc.). The Tukey and Bonferroni posthoc tests were used as appropriate for one-way ANOVAs. Normality was determined by D’Agostino–Pearson normality test. Statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments
Members of the Broberger laboratory are thanked for advice and discussion during the preparation of this manuscript, as are the many investigators who originally developed the reagents and tools that made this study possible. S.S. received support from the Wenner-Gren Foundations and from NIH K99MH131754. The authors gratefully acknowledge support by a project grant from the Knut and Alice Wallenberg Foundation (2020.0054), a European Research Council Advanced Grant (TOGETHER 101021496), a Distinguished Professor Grant from the Swedish Research Council (021-00671), funding from Hjärnfonden (the Swedish Brain Foundation), and internal Stockholm University funds to C.B.
Footnotes
Competing interests
The authors declare no competing financial interests.
Data and code availability statement.
The data that support the findings of this study are available from the corresponding authors upon request. Analysis was performed with proprietary software specified in the Materials and Methods sections.
References
- 1.Maestripieri D. Costs and Benefits of Maternal Aggression in Lactating Female Rhesus Macaques. Primates; journal of primatology 35, 443–453 (1994). [Google Scholar]
- 2.Kent J.P. Maternal Aggression and Interindividual Distance in the Broody Hen (Gallus-Gallus). Behav Process 27, 37–44 (1992). [DOI] [PubMed] [Google Scholar]
- 3.Troisi A., Damato F.R., Carnera A. & Trinca L. Maternal Aggression by Lactating Group-Living Japanese Macaque Females. Hormones and behavior 22, 444–452 (1988). [DOI] [PubMed] [Google Scholar]
- 4.Olivier B., et al. Maternal Aggression in Rats. Aggressive Behav 11, 169–169 (1985). [Google Scholar]
- 5.Stockman E.R. Aggressive Experience and Maternal Aggression in the Mongolian Gerbil. Physiology & behavior 30, 319–321 (1983). [DOI] [PubMed] [Google Scholar]
- 6.Siegel H.I., Giordano A.L., Mallafre C.M. & Rosenblatt J.S. Maternal Aggression in Hamsters - Effects of Stage of Lactation, Presence of Pups, and Repeated Testing. Hormones and behavior 17, 86–93 (1983). [DOI] [PubMed] [Google Scholar]
- 7.Stjohn R.D. & Corning P.A. Maternal Aggression in Mice. Behavioral biology 9, 635–639 (1973). [DOI] [PubMed] [Google Scholar]
- 8.Figler M.H., Blank G.S. & Peeke H.V.S. Maternal aggression and post-hatch care in red swamp crayfish, Procambarus clarkii (Girard): The influences of presence of offspring, fostering, and maternal molting. Mar Freshw Behav Phy 30, 173–194 (1997). [Google Scholar]
- 9.Hashikawa K., et al. Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nature neuroscience 20, 1580–1590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rendon N.M. & Demas G.E. Bi-directional actions of dehydroepiandrosterone and aggression in female Siberian hamsters. Journal of experimental zoology. Part A, Ecological genetics and physiology 325, 116–121 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Been L.E., Moore K.M., Kennedy B.C. & Meisel R.L. Metabotropic Glutamate Receptor and Fragile X Signaling in a Female Model of Escalated Aggression. Biological psychiatry 79, 685–692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonstein J.S. & Gammie S.C. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neuroscience and biobehavioral reviews 26, 869–888 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Flannelly K.J. & Flannelly L. Time Course of Postpartum Aggression in Rats (Rattus-Norvegicus). Journal of comparative psychology 101, 101–103 (1987). [Google Scholar]
- 14.Bosch O.J. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos T R Soc B 368(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise D.A. & Pryor T.L. Effects of ergocornine and prolactin on aggression in the postpartum golden hamster. Hormones and behavior 8, 30–39 (1977). [DOI] [PubMed] [Google Scholar]
- 16.Bridges R.S., DiBiase R., Loundes D.D. & Doherty P.C. Prolactin stimulation of maternal behavior in female rats. Science 227, 782–784 (1985). [DOI] [PubMed] [Google Scholar]
- 17.Gleason P.E., Michael S.D. & Christian J.J. Brief report prolactin-induced aggression in female Peromyscus leucopus. Behavioral and neural biology 33, 243–248 (1981). [DOI] [PubMed] [Google Scholar]
- 18.Ferris C.F., et al. Oxytocin in the Amygdala Facilitates Maternal Aggression. Annals of the New York Academy of Sciences 652, 456–457 (1992). [DOI] [PubMed] [Google Scholar]
- 19.DeVries A.C., Young W.S. & Nelson R.J. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. Journal of neuroendocrinology 9, 363–368 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Bosch O.J. & Neumann I.D. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and behavior 61, 293–303 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Kruk M.R., et al. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain research 260, 61–79 (1983). [DOI] [PubMed] [Google Scholar]
- 22.Cavalcante J.C., Bittencourt J.C. & Elias C.F. Female odors stimulate CART neurons in the ventral premammillary nucleus of male rats. Physiology & behavior 88, 160–166 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Yokosuka M., et al. Female-soiled bedding induced Fos immunoreactivity in the ventral part of the premammillary nucleus (PMv) of the male mouse. Physiology & behavior 68, 257–261 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Motta S.C., et al. Ventral premammillary nucleus as a critical sensory relay to the maternal aggression network. Proceedings of the National Academy of Sciences of the United States of America 110, 14438–14443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soden M.E., et al. Genetic Isolation of Hypothalamic Neurons that Regulate Context-Specific Male Social Behavior. Cell reports 16, 304–313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin D.Y., et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221−+ (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stagkourakis S., et al. A neural network for intermale aggression to establish social hierarchy. Nature neuroscience 21, 834−+ (2018). [DOI] [PubMed] [Google Scholar]
- 28.Chen A.X., et al. Specific Hypothalamic Neurons Required for Sensing Conspecific Male Cues Relevant to Inter-male Aggression. Neuron (2020). [DOI] [PubMed] [Google Scholar]
- 29.Cavalcante J.C., Bittencourt J.C. & Elias C.F. Distribution of the neuronal inputs to the ventral premammillary nucleus of male and female rats. Brain research 1582, 77–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canteras N.S., Simerly R.B. & Swanson L.W. Projections of the Ventral Premammillary Nucleus. Journal of Comparative Neurology 324, 195–212 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Sanchez M.M., Aguado F., Sancheztoscano F. & Saphier D. Effects of Prolonged Social-Isolation on Responses of Neurons in the Bed Nucleus of the Stria Terminalis, Preoptic Area, and Hypothalamic Paraventricular Nucleus to Stimulation of the Medial Amygdala. Psychoneuroendocrinology 20, 525–541 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Duque-Wilckens N., Steinman M., Grinevich V. & Trainor B. The Role of Oxytocin Neurons in the Bed Nucleus of the Stria Terminalis in Mediating Social Withdrawal. Biological psychiatry 81, S44–S45 (2017). [Google Scholar]
- 33.Lei K., Cushing B.S., Musatov S., Ogawa S. & Kramer K.M. Estrogen Receptor-alpha in the Bed Nucleus of the Stria Terminalis Regulates Social Affiliation in Male Prairie Voles (Microtus ochrogaster). PloS one 5(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jasnow A.M., Davis M. & Huhman K.L. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral neuroscience 118, 1052–1061 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Albert D.J., Walsh M.L., Gorzalka B.B., Mendelson S. & Zalys C. Intermale Social Aggression - Suppression by Medial Preoptic Area Lesions. Physiology & behavior 38, 169–173 (1986). [DOI] [PubMed] [Google Scholar]
- 36.Rosenblatt J.S., Hazelwood S. & Poole J. Maternal behavior in male rats: Effects of medial preoptic area lesions and presence of maternal aggression. Hormones and behavior 30, 201–215 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Scott N., Prigge M., Yizhar O. & Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519−+ (2015). [DOI] [PubMed] [Google Scholar]
- 38.Stagkourakis S., Perez C.T., Hellysaz A., Ammari R. & Broberger C. Network oscillation rules imposed by species-specific electrical coupling. eLife 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel H., et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology 110, 396–406 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Shin R. & Ikemoto S. Administration of the GABA(A) receptor antagonist picrotoxin into rat supramammillary nucleus induces c-Fos in reward-related brain structures. Supramammillary picrotoxin and c-Fos expression. BMC neuroscience 11(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikemoto S., Witkin B.M., Zangen A. & Wise R.A. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. Journal of Neuroscience 24, 5758–5765 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golden S.A., et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schino G., D’Amato F.R. & Troisi A. Maternal aggression in lactating female Japanese macaques: time course and interindividual variation. Can J Zool 82, 1975–1979 (2004). [Google Scholar]
- 44.Maestripieri D. & Alleva E. Maternal Aggression and Litter Size in the Female House Mouse. Ethology 84, 27–34 (1990). [Google Scholar]
- 45.Flannelly K.J. & Kemble E.D. The Effect of Pup Presence and Intruder Behavior on Maternal Aggression in Rats. B Psychonomic Soc 25, 133–135 (1987). [Google Scholar]
- 46.Haney M., Debold J.F. & Miczek K.A. Maternal Aggression in Mice and Rats Towards Male and Female Conspecifics. Aggressive Behav 15, 443–453 (1989). [Google Scholar]
- 47.Mos J., Olivier B. & Vanoorschot R. Maternal Aggression Towards Different Sized Male Opponents - Effect of Chlordiazepoxide Treatment of the Mothers and D-Amphetamine Treatment of the Intruders. Pharmacol Biochem Be 26, 577–584 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Stagkourakis S., Spigolon G., Liu G. & Anderson D.J. Experience-dependent plasticity in an innate social behavior is mediated by hypothalamic LTP. Proceedings of the National Academy of Sciences of the United States of America 117, 25789–25799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandelman R. Mice - Postpartum Aggression Elicited by Presence of an Intruder. Hormones and behavior 3, 23−+ (1972). [DOI] [PubMed] [Google Scholar]
- 50.Parmigiani S., Palanza P., Rodgers J. & Ferrari P.F. Selection, evolution of behavior and animal models in behavioral neuroscience. Neuroscience and biobehavioral reviews 23, 957–970 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Howard S.M., Gandelman R. & Rosenthal C. Isolation Potentiates the Aggression-Activating Property of Testosterone in Female Mice. Physiology & behavior 26, 971–972 (1981). [DOI] [PubMed] [Google Scholar]
- 52.Barkley M.S. & Goldman B.D. Testosterone-Induced Aggression in Adult Female Mice. Hormones and behavior 9, 76–84 (1977). [DOI] [PubMed] [Google Scholar]
- 53.Erskine M.S., Denenberg V.H. & Goldman B.D. Aggression in the lactating rat: effects of intruder age and test arena. Behavioral biology 23, 52–66 (1978). [DOI] [PubMed] [Google Scholar]
- 54.Flannelly K.J. & Flannelly L. Opponents’ size influences maternal aggression. Psychological reports 57, 883–886 (1985). [DOI] [PubMed] [Google Scholar]
- 55.Hallak M.D.M. & Hotra J.W. Maternal serum hormone concentrations in Long-Evans rats. J Trace Microprobe T 21, 103–110 (2003). [Google Scholar]
- 56.Dwyer C.M. & Lawrence A.B. Induction of maternal behaviour in non-pregnant, hormone-primed ewes. Anim Sci 65, 403–408 (1997). [Google Scholar]
- 57.Duenhoelter J.H. Maternal Hormone Measurements. Jama-J Am Med Assoc 248, 1109–1109 (1982). [Google Scholar]
- 58.Hansult C.D. & Kessler S. Role of Hormones in Maternal Aggression and Infant Care. Behavior genetics 7, 65–66 (1977). [Google Scholar]
- 59.Zarrow M.X., Denenberg V.H. & Gandelman R. Prolactin - Is It an Essential Hormone for Maternal Behavior in Mammal. Hormones and behavior 2, 343−+ (1971). [Google Scholar]
- 60.Riddle O. Prolactin in Vertebrate Function and Organization. Jnci-J Natl Cancer I 31, 1039−+ (1963). [PubMed] [Google Scholar]
- 61.Lhermite M. Gonadotropin and Prolactin Function during Pregnancy and Postpartum. Rev Fr Gynecol Obste 71, 159–165 (1976). [Google Scholar]
- 62.Moltz H., Levin R. & Leon M. Prolactin in Postpartum Rat - Synthesis and Release in Absence of Suckling Stimulation. Science 163, 1084-& (1969). [DOI] [PubMed] [Google Scholar]
- 63.Riesen J.W., Saiduddi S, Graves W.E., Tyler W.J. & Casida L.E. Pituitary Prolactin Activity in Postpartum Dairy Cows. Journal of animal science 25, 929-& (1966). [Google Scholar]
- 64.Numan M., Moltz H. & Leon M. Interference with Prolactin Release and Maternal Behavior of Female Rats. Hormones and behavior 3, 29-& (1972). [DOI] [PubMed] [Google Scholar]
- 65.Bridges R.S., Goldman B.D. & Bryant L.P. Serum Prolactin Concentrations and Initiation of Maternal-Behavior in Rat. Hormones and behavior 5, 219–226 (1974). [DOI] [PubMed] [Google Scholar]
- 66.Brown R.S.E., et al. Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proceedings of the National Academy of Sciences of the United States of America 114, 10779–10784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedersen C.A. & Prange A.J. Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America 76, 6661–6665 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedersen C.A., Ascher J.A., Monroe Y.L. & Prange A.J. Jr. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650 (1982). [DOI] [PubMed] [Google Scholar]
- 69.Watarai A., et al. The blockade of oxytocin receptors in the paraventricular thalamus reduces maternal crouching behavior over pups in lactating mice. Neuroscience letters 720, 134761 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Champagne F., Diorio J., Sharma S. & Meaney M.J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America 98, 12736–12741 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guillou A., et al. Assessment of lactotroph axis functionality in mice: longitudinal monitoring of PRL secretion by ultrasensitive-ELISA. Endocrinology 156, 1924–1930 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Gouilleux F., Wakao H., Mundt M. & Groner B. Prolactin Induces Phosphorylation of Tyr694 of Stat5 (Mgf), a Prerequisite for DNA-Binding and Induction of Transcription. Embo Journal 13, 4361–4369 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DaSilva L., et al. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Molecular and cellular endocrinology 117, 131–140 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Ali S. & Ali S. Prolactin receptor regulates Stat5 tyrosine phosphorylation and nuclear translocation by two separate pathways. Journal of Biological Chemistry 273, 7709–7716 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Lerant A., Kanyicska B. & Freeman M.E. Nuclear translocation of STAT5 and increased expression of Fos related antigens (FRAs) in hypothalamic dopaminergic neurons after prolactin administration. Brain research 904, 259–269 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Brockman J.L. & Schuler L.A. Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Molecular and cellular endocrinology 239, 45–53 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cave B.J., et al. Prolactin-induced activation of STAT5 within the hypothalamic arcuate nucleus. Neuroreport 16, 1423–1426 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Jaroenporn S., Nagaoka K., Ohta R., Watanabe G. & Taya K. Prolactin induces phosphorylation of the STAT5 in adrenal glands of Hatano rats during stress. Life sciences 85, 172–177 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Buntin J.D. & Buntin L. Increased STAT5 signaling in the ring dove brain in response to prolactin administration and spontaneous elevations in prolactin during the breeding cycle. General and comparative endocrinology 200, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown R.S.E., Kokay I.C., Herbison A.E. & Grattan D.R. Distribution of Prolactin-Responsive Neurons in the Mouse Forebrain. Journal of Comparative Neurology 518, 92–102 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Augustine R.A., et al. Prolactin regulation of oxytocin neurone activity in pregnancy and lactation. J Physiol-London 595, 3591–3605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown R.S.E., Herbison A.E. & Grattan D.R. Effects of Prolactin and Lactation on A15 Dopamine Neurones in the Rostral Preoptic Area of Female Mice. Journal of neuroendocrinology 27, 708–717 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Brown R.S.E., Piet R., Herbison A.E. & Grattan D.R. Differential Actions of Prolactin on Electrical Activity and Intracellular Signal Transduction in Hypothalamic Neurons. Endocrinology 153, 2375–2384 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Silveira M.A., et al. Acute effects of somatomammotropin hormones on neuronal components of the hypothalamic-pituitary-gonadal axis. Brain research 1714, 210–217 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Sabatini B.L. & Regehr W.G. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience 17, 3425–3435 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamikawa A. & Seko J. Physiological and pharmacological evaluation of oxytocin-induced milk ejection in mice. Experimental animals 69, 345–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y.F. & Hatton G.I. Milk ejection burst-like electrical activity evoked in supraoptic oxytocin neurons in slices from lactating rats. Journal of neurophysiology 91, 2312–2321 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Donker J.D., Koshi J.H. & Petersen W.E. The effect of exogenous oxytocin in blocking the normal relationship between endogenous oxytocic substance and the milk ejection phenomenon. Science 119, 67–68 (1954). [DOI] [PubMed] [Google Scholar]
- 89.Finkenwirth C., Martins E., Deschner T. & Burkart J.M. Oxytocin is associated with infant care behavior and motivation in cooperatively breeding marmoset monkeys. Hormones and behavior 80, 10–18 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Ross H.E. & Young L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in neuroendocrinology 30, 534–547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stohn J.P., et al. Increased aggression and lack of maternal behavior in Dio3-deficient mice are associated with abnormalities in oxytocin and vasopressin systems. Genes Brain and Behavior 17, 23–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bosch O.J., Meddle S.L., Beiderbeck D.I., Douglas A.J. & Neumann I.D. Brain oxytocin correlates with maternal aggression: Link to anxiety. Journal of Neuroscience 25, 6807–6815 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kremarik P., Freund-Mercier M.J. & Stoeckel M.E. Oxytocin and vasopressin binding sites in the hypothalamus of the rat: histoautoradiographic detection. Brain research bulletin 36, 195–203 (1995). [DOI] [PubMed] [Google Scholar]
- 94.Zaninetti M. & Raggenbass M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. The European journal of neuroscience 12, 3975–3984 (2000). [DOI] [PubMed] [Google Scholar]
- 95.Elands J., Barberis C. & Jard S. [3H]-[Thr4,Gly7]OT: a highly selective ligand for central and peripheral OT receptors. The American journal of physiology 254, E31–38 (1988). [DOI] [PubMed] [Google Scholar]
- 96.Vaccari C., Lolait S.J. & Ostrowski N.L. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139, 5015–5033 (1998). [DOI] [PubMed] [Google Scholar]
- 97.Young W.S., Li J., Wersinger S.R. & Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143, 1031–1039 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.In situ hybridization experiment against the Avpr1a mRNA across the C57BL/6J mouse brain.
- 99.Gruskos J., Goldwaser A. & Guarracino D.A. Competition of peptide hormone mimics with vasopressin and oxytocin for binding their target membrane receptors. Abstr Pap Am Chem S 244(2012). [Google Scholar]
- 100.Hibert M., et al. Functional architecture of vasopressin/oxytocin receptors. Journal of receptor and signal transduction research 19, 589–596 (1999). [DOI] [PubMed] [Google Scholar]
- 101.Zingg H.H. Vasopressin and oxytocin receptors. Bailliere Clin Endoc 10, 75–96 (1996). [DOI] [PubMed] [Google Scholar]
- 102.Manning M., Stoev S., Chan W.Y. & Sawyer W.H. Receptor-Specific Antagonists of Vasopressin and Oxytocin - a Current Perspective. Neurohypophysis : A Window on Brain Function 689, 219–232 (1993). [DOI] [PubMed] [Google Scholar]
- 103.Jard S., Elands J., Schmidt A. & Barberis C. Vasopressin and Oxytocin Receptors - an Overview. Progress in Endocrinology 1988, Vol 1 and 2 799, 1183–1188 (1988). [Google Scholar]
- 104.Martin P.J. & Schild H.O. Interaction of Oxytocin and Vasopressin with Tissue Receptors. Biochemical pharmacology 12, 139-& (1963). [Google Scholar]
- 105.St John R.D. & Corning P.A. Maternal aggression in mice. Behavioral biology 9, 635–639 (1973). [DOI] [PubMed] [Google Scholar]
- 106.Zimprich A., et al. Assessing Sociability, Social Memory, and Pup Retrieval in Mice. Current protocols in mouse biology 7, 287–305 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Bendesky A., et al. The genetic basis of parental care evolution in monogamous mice. Nature 544, 434–439 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moyer K.E. Kinds of aggression and their physiological basis. Communications in Behavioral Biology, p. 65–87. (1968). [Google Scholar]
- 109.Yang C.F., et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee H., et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Unger E.K., et al. Medial Amygdalar Aromatase Neurons Regulate Aggression in Both Sexes. Cell reports 10, 453–462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Falkner A.L., Grosenick L., Davidson T.J., Deisseroth K. & Lin D. Hypothalamic control of male aggression-seeking behavior. Nature neuroscience 19, 596–604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu M., Kim D.W., Zeng H. & Anderson D.J. Make war not love: The neural substrate underlying a state-dependent switch in female social behavior. Neuron 110, 841–856 e846 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beach F.A. Hormonal Modification of Sexually Dimorphic Behavior. Psychoneuroendocrinology 1, 3−+ (1975). [Google Scholar]
- 115.Kimchi T., Xu J. & Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Brunton P.J., Russell J.A. & Douglas A.J. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. Journal of neuroendocrinology 20, 764–776 (2008). [DOI] [PubMed] [Google Scholar]
- 117.Pedersen C.A. & Prange A.J. Induction of Maternal-Behavior in Virgin Rats after Intracerebroventricular Administration of Oxytocin. Proceedings of the National Academy of Sciences of the United States of America 76, 6661–6665 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bosch O.J. & Neumann I.D. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61, 293–303 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Erskine M.S., Barfield R.J. & Goldman B.D. Postpartum aggression in rats: II. Dependence on maternal sensitivity to young and effects of experience with pregnancy and parturition. J Comp Physiol Psychol 94, 495–505 (1980). [DOI] [PubMed] [Google Scholar]
- 120.Georgescu T., Khant Aung Z., Grattan D.R. & Brown R.S.E. Prolactin-mediated restraint of maternal aggression in lactation. Proceedings of the National Academy of Sciences of the United States of America 119(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin D., et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kohl J., et al. Functional circuit architecture underlying parental behaviour. Nature 556, 326–331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ekstrand M.I., et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America 104, 1325–1330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Franklin K.B.J. & Paxinos G. THE MOUSE BRAIN IN STEREOTACTIC COORDINATES, (2008).
- 125.Behringer R., Gertsenstein M., Nagy K.V. & Nagy A. Selecting Female Mice in Estrus and Checking Plugs. Cold Spring Harb Protoc 2016(2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request. Analysis was performed with proprietary software specified in the Materials and Methods sections.