Abstract
Tva is the cellular receptor for subgroup A avian leukosis and sarcoma virus (ALSV-A). The viral interaction domain of Tva is determined by a 40-residue, cysteine-rich module closely related to the ligand binding domain of the human low-density lipoprotein receptor (LDLR). In this report, we examined the role of the LDLR-like module of Tva in envelope binding and viral infection by mutational analysis. We found that the entire LDLR module in Tva is essential for efficient binding to the viral envelope protein. However, the 17 N-terminal residues of this module can be deleted without affecting receptor function, suggesting that the major determinants for viral entry are located at the C terminus of the module. The effect on viral infection of many amino acid substitutions and deletions in the LDLR module is context dependent, suggesting that the residues important for viral entry are dispersed throughout the LDLR module. In addition, we found that all 27 mutations at residues D46, E47, and W48 greatly reduced envelope binding. These results are discussed in relation to a recently elucidated structure for an LDLR module.
Entry of an enveloped virus into its host is determined by a specific interaction between receptors on the cell surface and envelope glycoproteins on the virion. For most retroviruses, this process is pH independent; therefore, it has been hypothesized that for these viruses, receptor binding serves to trigger a series of conformational changes on the envelope protein required for viral-host membrane fusion. Thus, the receptor not only is likely to provide a binding site for the viral glycoprotein but also may initiate the conformational changes thought to be required for entry.
Infection of subgroup A avian leukosis and sarcoma virus (ALSV-A) is mediated by the cellular receptor, Tva, on avian cells (4, 23). Tva is a small, relatively simple protein with two functional isoforms, Tva 950 and Tva 800, that are produced by differential splicing (4). Tva 950 is tethered by a typical membrane-spanning domain near the carboxyl terminus of the protein, whereas Tva 800 appears to be anchored by a glycosylphosphatidylinositol tail. Within the extracellular region of both isoforms, near the amino terminus of Tva is a 40-residue, cysteine-rich motif that is closely related to a sequence in the low-density lipoprotein receptor (LDLR) (4). In LDLR, this module is repeated seven times and constitutes the ligand binding region of the protein (11, 22). The 40-residue LDLR-like module from Tva forms the ASLV-A interaction domain. It is sufficient for viral receptor function (19), and mutations within this module affect ASLV-A receptor function and envelope binding (5, 24, 25). A peptide corresponding to the 19 carboxyl-terminal residues of the Tva module inhibits ASLV-A entry, leading to the suggestion that this region comprises the receptor’s viral interaction domain (24).
Tva specifically binds to ALSV-A envelope protein (7, 13), induces conformational changes in EnvA (10, 14, 15), and when incorporated into viral particles can direct infection of ASLV-A envelope (EnvA)-expressing cells (2). Together these results strongly support a direct role for Tva in triggering ALSV-A envelope-mediated entry. Recently, a tryptophan residue (W48) near the carboxyl terminus of Tva’s LDLR module was reported to be specifically involved in triggering the conformational changes of ALSV-A envelope during viral entry (25). Additionally, W48 was proposed to be part of a common retroviral receptor motif consisting of an aromatic and adjacent acidic residue found in Tva and receptors for ecotropic murine leukemia virus and human immunodeficiency virus (17, 24).
To define further the requirements for Tva receptor function, we produced deletion and substitution mutations throughout the 40-residue LDLR module of Tva and analyzed separately the effect of these changes on EnvA binding and ASLV-A entry. We found that efficient EnvA binding requires an intact LDLR module since the majority of substitution or deletion mutations analyzed severely impaired envelope binding. However, the amino-terminal 17 residues of Tva’s LDLR module could be deleted without significant impact on viral receptor function, suggesting that while this region contributes to EnvA binding, determinants sufficient for ASLV-A entry localize to the carboxyl-terminal 23 residues of Tva’s LDLR module. Because of the proposed importance of residues 46 to 48 (DEW), an extensive mutational analysis of these three residues was also performed. Our analysis of 27 substitution mutants in residues 46 to 48 of Tva does not support the proposed model for a common motif in retroviral receptors, nor did we find evidence for a specific involvement of W48 in post-EnvA binding events. The effect of the mutations in Tva are discussed in relation to the recently elucidated structure for a module from the human LDLR (12).
MATERIALS AND METHODS
Cells and viruses.
Human embryonic kidney 293T cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% calf serum and 300 μg of Geneticin per ml. Quail QT6 cells were grown in medium 199 containing 10% tryptose phosphate broth, 5% fetal calf serum, and 1% chicken serum. HeLa cells were grown in a spinner flask in Joklik’s modified medium with 10% calf serum.
RCAS(A)AP viral stocks were generated as previously described (4). Vaccinia virus strains WR and vTF7-3 were obtained from Bernie Moss, National Institutes of Health. The vaccinia virus stocks were generated and titered as described previously (1).
DNA methodology.
All Tva mutants were generated in the quail Tva 950 background. Tva substitution and deletion mutants were constructed by using two rounds of PCR as previously described (19).
To facilitate mutagenesis of the LDLR motif of Tva, a unique BamHI site was introduced immediately upstream of the LDLR motif-coding region, producing the pTva cassette. Wild-type (wt) Tva 950 DNA was used as the template and amplified with four primers, OS26 (5′-TTAATTCAGATCTGGTACC-3′), OS185 (5′-ACCGGATCCGTTACCGGTCAC), OS183 (5′-ACCGGTAACGGATCCGGTAACGGTCT-3′), and OS186 (5′-AGGCTCGAGTCAGGAGAACAAGTCTGC-3′), in a two-step PCR. The amplified fragment was digested with restriction enzymes KpnI and XhoI and cloned into plasmid pcDNA 3, and the construct was confirmed by DNA sequencing. The introduced BamHI site does not alter the amino acid sequence of Tva.
To randomly mutagenize residues D46, E47, and W48 of Tva, we designed three oligonucleotide primers: OS235 (5′-GCTGGTCCCGCAGCCCCAGNNGTCCCGCCCGTCGTC-3′), OS236 (5′-TGCGACGACGGGCGGNNCGAGTGGGGCTGCGGGACC-3′), and OS237 (5′-TGCGACGACGGGCGGGACGAGNNCGGCTGCGGGACCAGC-3′), where N represents all four bases. These primers were used in combination with OS183 (5′-ACCGGTAACGGATCCGGTAACGGTTCT-3′) and OS186 to amplify the Tva-coding region. Amplified DNA fragments were cloned into the pTva cassette as BamHI/SacII fragments. After transformation into Escherichia coli DH5α, plasmid DNA from each colony was isolated, and the DNA sequence of the BamHI/SacII region was determined by primer OS219 (5′-GGTAACGTGACCGGTAAC-3′).
To express a large quantity of ALSV-A envelope protein, a vaccinia virus expression system was used. A truncated gD-EnvA, lacking the membrane-spanning domain and the cytoplasmic tail (19a), was PCR amplified and cloned into plasmid pTM1. To facilitate purification of this envelope protein, a six-histidine tail was added to the carboxyl terminus. The gD-EnvA-His6 construct was used to produce recombinant virus with vaccinia virus strain WR by standard techniques, using thymidine kinase negative selection (1).
Protein expression and Western blotting.
Transfection and transient expression of Tva mutant DNAs in 293T and QT6 cells were done as described previously (4, 19). The Western blots were probed with an anti-Tva polyclonal antiserum as described previously (4).
A vaccinia virus expression system was used to produce gD-EnvA used in the enzyme-linked immunosorbent assays (ELISAs) according to a previously described protocol (1), with minor modification. A vaccinia virus encoding gD-EnvA-His6 gene under the control of T7 promoter was coinfected with virus vTF 7-3, which encodes T7 polymerase. Each virus was used to infect HeLa cells at a multiplicity of infection of 10. Twenty-four hours postinfection, infected cells were harvested by centrifugation and lysed in Triton lysis buffer (19). Expression of gD-EnvA-His6 was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using a monoclonal antibody, 1D3, against the gD epitope as previously described (20).
Infection.
Tva mutants were transiently expressed in 293T cells by CaPO4 transfection. Twenty-four hours after transfection, the cells were split into six-well plates for infection and 100-mm-diameter plates for analysis of protein expression. To determine the level of susceptibility conferred by a particular Tva mutant, a series of 10-fold dilutions of RCAS(A)AP was used to infect the transfected cells. Alkaline phosphatase (AP)-positive cells were detected and enumerated 48 h posttransfection as described previously (19).
Envelope binding assay.
The ability of Tva mutants to block soluble wt Tva (sTva) binding to envelope was measured by a blocking ELISA. In this assay, gD-EnvA-His6 was captured onto wells coated with monoclonal antibody 1D3. The wells were washed, and 10 μl of cell lysate containing wt Tva or mutant proteins was allowed to bind for 1 h at 4°C. After washing, 5 ng of biotin-labeled sTva was added, and the mixture was incubated at 4°C for an additional hour. After washing, bound sTva was detected by using streptavidin-horseradish peroxidase and 2,2′-azinobis(3-ethylbenzothiazolinesulfonic acid) (ABTS). Plates were read at room temperature in a Molecular Dynamics ELISA reader at 405 nm. Percent inhibition was calculated by the formula % inhibition = 100 − 100(S/T), where S is the test sample and T the total binding (no competitor). All binding experiments were performed at least three times in triplicate except for the wt Tva titration curve, which was performed twice in triplicate.
Immunofluorescence detection of Tva surface expression.
Tva mutants were transiently expressed in QT6 cells by CaPO4-mediated transfection. Twenty-four hours posttransfection, expression of wt or mutant proteins was detected by immunofluorescence as previously described (18), using a polyclonal anti-Tva antibody at a 1:250 dilution (4).
RESULTS
Substitution of regions from other LDLR-like repeats in Tva.
A 40-residue region of Tva which is highly homologous to the modules that comprise the human LDLR ligand binding domain is sufficient for ASLV-A receptor function (19). Figure 1A shows a cartoon of Tva’s LDLR-like module based on the recently reported disulfide bonding patterns of human LDLR modules 1, 2, and 5 (8, 9, 12). Three pairs of disulfide bonds connecting cysteines 1 and 3, 2 and 5, and 4 and 6 of Tva create a looped structure. To begin analyzing viral receptor function of the Tva LDLR motif, a series of conservative mutations of quail Tva 950 (4) was made by substituting residues between adjacent cysteines with the corresponding residues from other LDLR family members (Fig. 1B, C1C2, C2C3, C3C4, C4C5, and C5C6). For example, the residues between C2 and C3 of Tva (SEPPGAHGE) were replaced with those from a LDLR-like repeat of human heparin sulfate proteoglycan (HSGH) to create mutant C2C3. This strategy was taken for two considerations. First, we hypothesized that since other LDLR repeat-containing proteins do not function as ALSV-A receptors, then residues that determine the specificity of the Tva LDLR motif are likely to be nonconserved. Substitution of the nonconserved residues might enable identification of residues important for Tva viral receptor function. Second, it seems likely that conserved residues also play a structural role; therefore, alteration of these residues could abrogate folding. Thus, we initially avoided changes in the conservative residues.
FIG. 1.
Tva LDLR module and effects of homolog substitutions and deletions on viral receptor function. (A) Cartoon of the Tva LDLR module. Amino acids of the Tva LDLR module are labeled, and the proposed three disulfide bonds based on human LDLR repeats 1, 2, and 5 are indicated. The highly conserved residues found in most LDLR modules are shaded. (B) Sequences of the Tva LDLR module homolog substitution and deletion mutants. The functions of these mutants as viral receptors as assayed by infection with RCAS(A)AP are shown at the right. Values represent positive AP-staining cells per milliliter of viral stock [RCAS(A)-AP vector] used.
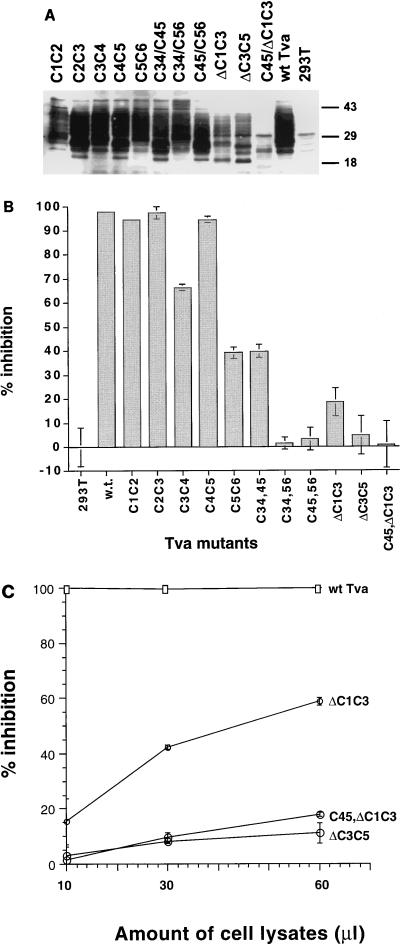
The receptor mutants (C1C2, C2C3, C3C4, C4C5, and C5C6) were transiently expressed in 293T cells, and the proteins were detected by Western blot analysis using a polyclonal antiserum against Tva. As shown in Fig. 2A, all five homolog substitution mutants were expressed well in 293T cells. The pattern of numerous bands seen for the mutants and wt Tva is characteristic for Tva and probably reflects differential modifications by N- and O-glycosylation.
FIG. 2.
Expression and EnvA binding properties of Tva LDLR module homolog substitution and deletion mutants. (A) Transient expression of Tva mutants in 293T cells. Cell lysates from 293T cells transiently transfected with Tva mutant plasmids were analyzed by SDS-PAGE and Western blot analysis using a polyclonal antiserum against Tva protein. 293T, mock-transfected cells. Sizes are indicated in kilodaltons. (B) EnvA binding activity of Tva mutants measured by blocking ELISA. Cell lysates (10 μl) from 293T cells transiently expressing the mutant Tva proteins were used in the blocking ELISA as described in Materials and Methods. Percent inhibition of labeled sTva binding was calculated as described in Materials and Methods. Percent inhibition for each sample is the average from three experiments, each performed in triplicate. Bars indicate standard deviation. (C) Relative envelope binding activities of Tva mutants ΔC1C3, ΔC3C5, and C45/ΔC1C3 with increasing amount of cell lysates measured by blocking ELISA, expressed as percent inhibition. Percent inhibition for each sample is the average from three experiments, each performed in triplicate. Bars indicate standard deviation.
Function of the mutant receptors was assessed by infection of transfected 293T cells with a recombinant ALSV-A vector carrying an AP reporter gene [RCAS(A)AP]. Because ALSV does not replicate in mammalian cells, this is a single-step infection assay and thus the number of AP-positive cells reflects the function of the receptor. The results are summarized in Fig. 1B. Infection of 293T cells expressing wt Tva and four Tva mutants (C1C2, C2C3, C4C5, and C5C6) resulted in very similar numbers of AP-positive cells. Therefore, it appears that these homolog substitution mutants do not significantly affect Tva viral receptor function. One exception is mutant C3C4, which in multiple independent experiments consistently functioned roughly fivefold less efficiently than wt Tva, indicating that this mutant is slightly defective.
Two possible alternative explanations for why the homolog substitutions did not affect Tva function were that the mutated residues are not involved in viral entry and that the mutated residues contribute to the envelope interaction site but individual alteration does not abrogate ASLV-A receptor function. To address the latter possibility, the substitution mutants C3C4, C4C5, and C5C6 were combined to create three mutants (C34/45; C34/56, and C45/56; [Fig. 1B]). Because of the deletion mutations described below, we concentrated our analysis on the nonconserved residues from C28 to the end of the LDLR motif. The C34/45, C34/56, and C45/56 proteins were expressed well in 293T cells and displayed the numerous bands characteristic of Tva; however, the pattern of modification for each mutant varies somewhat (Fig. 2A). The effect of the mutations on receptor function was again measured by assaying susceptibility to RCAS(A)AP. Combining the substitutions between the third and fourth or fourth and fifth cysteines (C34/45) had no significant effect on Tva function (Fig. 1B). In contrast, mutant C34/56 was unable to mediate viral infection in numerous experiments. Mutant C45/56 was also significantly impaired for receptor function, displaying roughly a 50-fold decrease compared to wt Tva. From these results, it appears that the nine nonconserved amino acids between the third cysteine (residue 28) and the end of the LDLR module of Tva are significant determinants of receptor function; however, their importance is manifested only when multiple residues are altered. This finding implies that the EnvA interaction site in Tva is composed of residues from discontinuous regions in the carboxyl-terminal half of the LDLR motif and suggests that numerous interactions contribute to receptor function.
Mutant C34/45 functioned similarly to wt Tva, yet one of the homolog substitution parents (C3C4) was slightly impaired in its ability to mediate infection. This discrepancy appears to be due to the fact that the C34/45 mutant has a wt leucine at residue 34, which was corrected during the overlap PCR protocol used for mutagenesis, while the parent C3C4 has an alanine at this position. Similar to data presented previously (24), this result suggests that L34 plays a role in EnvA recognition. In support of this conclusion, analysis of human LDLR repeat 4-Tva chimeras also identifies an important role for L34 in ASLV-A receptor function (21).
Deletion mutants in the LDLR motif of Tva.
Next, we examined whether the entire LDLR motif of Tva was required for efficient viral receptor function by deletion analysis. The deletions in Tva were designed to maintain an even number of cysteine residues and thus the potential to form disulfide bonds. Mutant ΔC1C3 contains a deletion of the first 17 residues including the first two cysteine residues of the LDLR motif, while ΔC3C5 has a deletion of 13 residues including cysteines 3 and 4. An additional mutant, C45/ΔC1C3, combines the substitution mutant C4C5 with ΔC1C3 (Fig. 1B).
Unlike the substitution mutants described above, the level of expression of the Tva deletion mutants after transient transfection of 293T cells was much lower than that of wt Tva (Fig. 2A). It is possible that these mutant proteins are not detected well by the anti-Tva polyclonal antibody used. However, because the deletions are predicted to affect normal disulfide bond formation, it seems more likely that the deletions affected the expression of Tva. The ability of these mutants to mediate viral entry was examined by using RCAS(A)AP viruses to infect 293T cells transiently expressing the mutant proteins (Fig. 1B). Mutant ΔC1C3 was only slightly impaired as a viral receptor, displaying approximately 50% of wt Tva receptor activity in numerous experiments. The deletion encompassing the central region of the LDLR motif (ΔC3C5) had a more dramatic effect, reducing receptor function approximately 20-fold compared to wt Tva.
A mutant, C45/ΔC1C3, combining the amino-terminal deletion and the C4C5 homolog substitution decreased the receptor function of Tva more than 100-fold. Similar to the combined homolog substitution mutants discussed above, this result supports the hypothesis that the EnvA interaction domain of Tva is comprised of dispersed residues in the LDLR module encompassing both the C1C3 region and the C4C5 region. Alternatively, it is possible that the deleted and mutated regions are not directly involved in binding EnvA but affect folding of the module and thus presentation of the virus interaction site. Finally, although the three deletion mutants were expressed to significantly lower levels than wt Tva, it seems unlikely that decreased expression accounts for the defective receptor function since expression of wt Tva in transiently transfected cells at levels significantly below those of the deletion mutants resulted in full receptor function (5, 19a).
Effects of Tva mutations on envelope binding.
To directly analyze the effects of the mutations in Tva on envelope binding, we used an ELISA-based receptor-envelope binding assay and tested the ability of each of the mutants to block the binding of labeled sTva to EnvA. In this blocking assay, lysates from cells expressing the Tva mutants were first incubated with EnvA that had been captured on the ELISA plate; then after washing, 5 ng of labeled sTva was added. Therefore, if a Tva mutant can form a stable complex with EnvA, then binding of biotin-labeled sTva should be blocked.
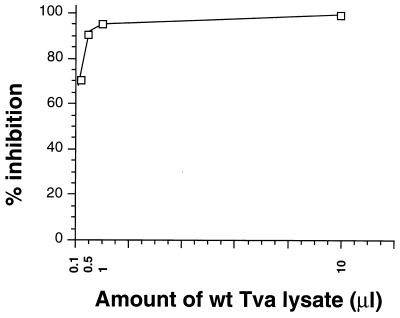
To determine the conditions for the binding inhibition experiments, lysates of transiently transfected 293T cells expressing wt Tva were used at various concentrations, and the range of inhibition was measured. We found that 10 μl of wt Tva lysate (Fig. 2A, wt Tva lane) could completely block labeled sTva binding to captured EnvA. Furthermore, as little as 0.1 μl of wt Tva lysate could block roughly 70% of sTva binding (Fig. 3). Based on these results, 5 ng of biotin-labeled sTva and 10 μl of each cell lysate were used to assess the relative EnvA binding capabilities of the Tva mutants. Using this amount of the mutant Tvas in the binding assay ensures that even low levels of binding capability should be detectable since the amount of receptor added is 100 times greater than the level of wt protein required for significant inhibition.
FIG. 3.
Analysis of EnvA binding by wt Tva by using a blocking ELISA. The ability of Tva in a cell lysate to bind EnvA was indirectly analyzed by determining the level of inhibition of labeled sTva binding. Increasing amounts of lysate from 293T cells expressing wt Tva were incubated with captured gD-EnvA before biotin-labeled sTva was added in the blocking ELISA protocol as described in Materials and Methods. Each experiment was performed in triplicate, and the standard deviation was less than 10% of the mean optical density reading.
Tva mutants C1C2, C2C3, and C4C5 inhibited sTva binding as efficiently as wt Tva (greater than 90% inhibition with 10 μl of lysate [Fig. 2B]), suggesting that these receptors display relatively normal EnvA binding activity. Mutant C3C4 appears to be partially impaired for EnvA binding since it inhibited sTva binding by approximately 65%. Mutants C5C6 and C34/45 displayed approximately 40% inhibition, while mutant ΔC1C3 inhibited binding by only 20%. None of these mutants with detectable EnvA binding were significantly impaired for receptor function (compare Fig. 1B and 2B). In contrast, mutants C34/56, C45/56, ΔC3C5, and C45/ΔC1C3 did not block sTva binding, suggesting that they do not stably associate with EnvA. These mutants, except C34/56, displayed some receptor function.
Since mutants ΔC1C3, ΔC3C5, and C45/ΔC1C3 appear to express much lower levels of receptor protein than either wt Tva or the other mutants, the effect of adding increasing amounts of the lysates containing these mutant proteins to the blocking assay was examined. For mutant ΔC1C3, there was a dose response to added lysate such that when 60 μl was added, sTva binding was inhibited by nearly 60%. In contrast, mutants ΔC3C5 and C45/ΔC1C3 displayed less than 20% inhibition when 60 μl of cell lysate was added (Fig. 2C). From these results, it appears that the deletion mutant ΔC1C3 retains significant EnvA binding activity; however, mutants ΔC3C5 and C45/ΔC1C3 have severely impaired envelope binding activities.
Roles of residues D46, E47, and W48 in viral receptor function of Tva.
Residues D46, E47, and W48 near the carboxyl terminus of the Tva LDLR motif were previously proposed to be the critical determinants of Tva receptor function (24), and W48 was postulated to be involved in postbinding entry events (25). Because of the proposed critical role of these residues, a random mutagenesis strategy was used to alter each of these residues. The resulting mutants were examined for effects on EnvA binding and receptor function. Twenty-seven individual substitution mutations in residues D46, E47, and W48 of Tva were generated as described in Materials and Methods. The mutant receptors were transiently expressed in 293T cells by transfection and analyzed for Tva expression, ASLV-A receptor function, and EnvA binding as described above.
D46.
Seven substitutions for residue D46 were generated and included a basic (R), an aromatic (Y), three nonpolar (G, A, and I), and two polar (C and S) residues. All the mutants were expressed well in 293T cells compared to wt Tva (Fig. 4A).
FIG. 4.
Analysis of Asp46 mutants of Tva. (A) Expression of Tva Asp46 mutants in 293T cells analyzed by Western blotting with polyclonal serum to Tva. Sizes are indicated in kilodaltons. (B) Blocking ELISA (10 μl of each cell lysate) was performed to measure the EnvA binding activity of Tva Asp46 mutants, expressed as percent inhibition of labeled sTva binding. Percent inhibition for each sample is the average from three experiments, each performed in triplicate. Bars indicate standard deviation.
The binding affinity of each of the D46 mutants was analyzed by using the blocking ELISA with 10 μl of lysate as described above (Fig. 4B). EnvA binding was undetectable for most D46 mutants, suggesting that they do not stably associate with EnvA. However, one mutant, D46G, displayed a very low level of EnvA binding activity. These results demonstrate that there is little or no inhibition by any of the D46 mutants at levels of receptor protein more than 100-fold higher than that required for significant inhibition by wt Tva (compare Fig. 3 and 4B). Therefore, conservation of aspartic acid at this position appears to be critical for efficient EnvA binding by Tva.
The effect of the D46 mutations on Tva receptor function was assayed by using RCAS(A)AP as described above. Consistent with the EnvA binding results, most substitutions for residue D46 were functionally impaired (Table 1). Substitution of residue 46 with arginine, cysteine, or tyrosine reduced viral receptor function of Tva at least 1,000-fold, while alanine, isoleucine, and serine at same position affected viral infection 10- to 100-fold. However, mutation of residue 46 to glycine only slightly decreased receptor function despite the fact that this substitution appears to significantly reduce EnvA binding. Thus, while D46 appears to be important for receptor function, maintaining an acidic residue at this position is not essential for function.
TABLE 1.
Effects of mutations on Tva viral receptor function
| Residue | Mutation | AP+ cells/ml of virus (% of wt valuea) |
|---|---|---|
| D46 | G | 3.9 × 105 (71) |
| S | 2.8 × 104 (5) | |
| I | 1.9 × 104 (3) | |
| A | 2.6 × 103 (0.5) | |
| Y | 1.5 × 102 (0.03) | |
| C | 9 × 101 (0.02) | |
| R | 0 (0) | |
| E47 | F | 4.7 × 105 (85) |
| A | 3.9 × 105 (71) | |
| S | 3.2 × 105 (58) | |
| N | 1.6 × 105 (29) | |
| I | 1.4 × 105 (25) | |
| T | 7.4 × 104 (13) | |
| H | 4.9 × 103 (0.9) | |
| Y | 2.2 × 102 (0.04) | |
| R | 1.1 × 102 (0.02) | |
| P | 0 (0) | |
| W48 | L | 2.8 × 105 (51) |
| I | 2.6 × 105 (47) | |
| R | 1.7 × 105 (31) | |
| V | 8.9 × 104 (16) | |
| H | 2.3 × 103 (0.4) | |
| T | 1.3 × 103 (0.2) | |
| D | 3.4 × 102 (0.06) | |
| N | 2.9 × 102 (0.05) | |
| A | 2.8 × 102 (0.05) | |
| S | 2.2 × 102 (0.04) |
The value for wt Tva was 5.5 × 105.
E47.
Ten substitution mutants for E47 were generated and included two aromatic (Y and F), two basic (H and R), three nonpolar (A, I, and P), and three polar (T, S, and N) residues. All of these mutants were expressed in 293T cells at a level comparable to that of wt Tva (Fig. 5A). Like the D46 substitutions, most of the E47 mutants displayed little or no EnvA binding capability, inhibiting sTva binding by less than 10% when 10 μl of lysate was used (Fig. 5B). As discussed above, this result implies that these mutants are severely defective for EnvA binding compared to wt Tva. A single D47 mutant, E47F, retained detectable EnvA binding activity, inhibiting sTva binding by about 45% when 10 μl of lysate was used. These results suggest that maintenance of a glutamate at this position is important for high levels of EnvA binding activity.
FIG. 5.
Analysis of Glu47 mutants of Tva. (A) Expression of Tva Glu47 mutants in 293T cells analyzed by Western blotting with antiserum to Tva. Sizes are indicated in kilodaltons. (B) Blocking ELISA (10 μl of each cell lysate) was performed to assess the EnvA binding activity of Tva Glu47 mutants, expressed as percent inhibition. Percent inhibition for each sample is the average from three experiments, each performed in triplicate. Bars indicate standard deviation.
The effects of the E47 mutants on virus receptor function did not correlate with the binding data. Consistent with the binding results, substitutions of glutamate at this position with four residues (H, Y, R, and P) either greatly reduced or abolished the viral receptor function of Tva (Table 1). Substitution by threonine reduced receptor function about 10-fold. In contrast, substitution of E47 by four residues (A, S, N, and I) either had no effect or caused only a two- to fourfold decrease in receptor function. Mutant E47F had the least effect on envelope binding and had no effect on Tva’s ability to mediate viral infection. From these results, it is evident that Tva receptor function does not require an acidic residue at this position and that it can be replaced with residues having quite divergent characteristics.
W48.
Ten substitution mutants of W48, including two basic (R and H), one acidic (D), four nonpolar (I, V, A, and L), and three polar (S, N, and T) residues, were analyzed. The expression level and pattern of modification of these mutants were similar to that of wt Tva (Fig. 6A).
FIG. 6.
Analysis of Trp48 mutants of Tva. (A) Expression of Tva Trp48 mutants in 293T cells analyzed by Western blotting with antiserum to Tva. Sizes are indicated in kilodaltons. (B) Blocking ELISA (10 μl of each cell lysate) was performed to assess the EnvA binding activity of Tva Trp48 mutants, expressed as percent inhibition. Percent inhibition for each sample is the average from three independent experiments, each performed in triplicate. Bars indicate standard deviation.
Like the mutations at D46 and E47, all 10 substitutions at W48 greatly decreased Tva’s EnvA binding activity. The level of inhibition of sTva binding by these mutants was consistently less than 10% in blocking ELISA (Fig. 6B). Thus, efficient EnvA binding appears to require a tryptophan residue at this position.
In contrast, a number of the W48 substitutions functioned efficiently as ALSV-A receptors (Table 1). Substitutions of residue W48 with three nonpolar residues (leucine, isoleucine, or valine) only marginally affected viral infection. Interestingly, W48 can be replaced with a basic residue (R) with only a threefold effect on receptor function. Substitutions with other charged or polar residues (H, T, D, N, and S) greatly decreased the viral infection, with the number of infected cells ranging from 200- to 1,000-fold lower than with wt Tva (Table 1). Finally, alanine was not well tolerated and decreased receptor function by 3 logs. From these data, it appears that hydrophobic residues and a bulky charged amino acid are functionally tolerated at this position whereas less bulky charged and nonpolar residues are not; however, as for the D46 and E47 positions, there is no strict amino acid requirement at this position.
Titration of selected DEW mutants in blocking ELISA.
To further assess the EnvA binding activities of the DEW mutants, a selected group of these mutants was analyzed over a range of input receptor concentration. These include mutants with high levels of receptor function (D46G, E47F, E47A, W48L, and W48R) and functionally impaired receptors (D46A, E47P, W48H, and W48A). As seen previously, there was a sharp response to input wt Tva such that with 1 μl of lysate EnvA, binding was saturated. Similarly, the inhibition by E47F appears to be responsive to the input amount of Tva; however, the highest level of inhibition achieved by E47F is significantly lower than for that of wt Tva when 100 times less receptor protein is used. None of the other eight mutants tested (D46A, D46G, E47A, E47P, W48A, W48H, W48L, and W48R) displayed detectable binding activity when the maximum amount of lysate (10 μl) was used (Fig. 7). These results suggest that even the most active DEW substitution mutant, E47F, has over 100-fold-lower EnvA binding activity compared to wt Tva and that the other substitutions at these three residues have significantly greater than a 100-fold decrease in EnvA binding. Thus, these three residues are critical for efficient envelope binding.
FIG. 7.
Effect of titration of selected Tva DEW mutants on EnvA binding. Various amounts of lysates from 293T cells expressing DEW mutants of Tva (0.1 to 10 μl) were used in the blocking ELISA. Percent inhibition of sTva binding to captured EnvA for each sample is the average from three independent experiments, each performed in triplicate. Bars indicate standard deviation.
Surface expression of Tva mutants by immunofluorescence.
We examined whether those Tva mutants that did not mediate efficient viral infection expressed Tva protein on the cell surface by immunofluorescence. Tva mutants C34/56, D46R, D46C, E47R, and E47P that either did not mediate viral infection or functioned extremely poorly as viral receptors in the infection assay (Fig. 1A and Table 1) were transiently expressed in QT6 cells. Cells were fixed with 95% ethanol–5% acetic acid either before (intracellular expression) or after (extracellular expression) a rabbit anti-Tva polyclonal antibody was incubated with the cells. Bound anti-Tva antibody was visualized by incubating fixed cells with a fluorescein isothiocyanate-conjugated secondary anti-rabbit antibody. Expression of both intracellular and extracellular wt Tva or Tva mutants was easily detected (not shown). Therefore, the defect in function for the mutants analyzed here was not caused by a lack of Tva surface expression, since previous experiments with wt Tva demonstrated that surface levels of Tva below the threshold of detection are sufficient for full receptor activity in transiently transfected cells (5) and avian fibroblasts (4).
DISCUSSION
The sequence requirements for ASLV-A envelope binding and viral receptor function of the LDLR module in Tva were examined by mutational analysis. Our results demonstrate that the sequences required for high-affinity binding to EnvA map throughout the 40-residue LDLR module in Tva. Deletion and substitution mutations in all regions of the Tva LDLR module affected EnvA binding. In contrast to the binding studies, ASLV-A infectivity assays demonstrate that the N-terminal 17 residues of the LDLR module are dispensable for viral entry (Fig. 1B), confirming a previous finding suggesting that the major determinants for infection localize to the carboxyl terminus of the cysteine-rich module (24). This N-terminal deletion and many substitution mutants in Tva exhibited greatly reduced EnvA binding activity, yet when transiently expressed, these receptors were functional for viral entry.
Receptor function was impaired for many mutants only when multiple regions of the LDLR module of Tva were altered. For example, a homolog substitution mutant affecting residues between cysteines 3 and 4 (C3C4) mediated infection approximately fivefold less efficiently than wt Tva. However, when the C3C4 mutant was combined with a mutant affecting residues between cysteines 5 and 6 (C5C6) which had no effect on function, the resulting mutant, C34/56, was unable to mediate viral infection. Since all of these receptor mutants are expressed on the cell surface, then the most straightforward interpretation of these findings is that discontinuous regions in Tva form the envelope binding site and that the homolog substitution mutations either affect the presentation of the binding site or, we believe more likely, alter critical residues within the binding site.
Previously, it was hypothesized that a 19-amino-acid peptide corresponding to the carboxyl-terminal end of the LDLR module, from cysteines 4 to 6 and including residues D46, E47, and W48, contained all of the structural and functional information of the Tva viral interaction site (24). While analysis of the N-terminal deletion described in this report is in agreement with this hypothesis, our results demonstrating effects on function and EnvA binding by mutations in other regions of Tva do not support this model (Fig. 1B, 2B, and 2C). Additionally, we have recently demonstrated that a single module from the human LDLR which contains residues D46, E47, and W48 and other elements proposed to be sufficient for receptor function (24) will not mediate infection or bind envelope until additional residues in the module are altered to conform to the Tva sequence (21). Interestingly, one region of Tva found to affect the function of the human LDLR module was the sequence between cysteines 1 and 3 of Tva. Thus, while these residues are dispensable for receptor activity in Tva, they can confer some level of function on an otherwise defective LDLR module or negatively affect an otherwise functional receptor as demonstrated by the C45/ ΔC1C3 mutant.
Analysis of a smaller set of Tva substitution mutants previously suggested that maintaining an aromatic residue at position 48 of Tva was crucial for efficient receptor function (25). Similar important aromatic residues in the human immunodeficiency virus and murine leukemia virus receptors CD4 and MCAT suggested that a common functional motif in these receptors consisted of an aromatic residue and an adjacent charged amino acid (17, 24). In contrast, our results analyzing a larger set of substitutions for W48 do not find an absolute requirement for an aromatic or even a hydrophobic residue at this position of Tva for high levels of receptor function. Similarly, although residues 46 and 47 in Tva are crucial for high levels of EnvA binding activity, they can be replaced by nonacidic residues without a significant effect on viral receptor function. Previous results had suggested that the requirement for an acidic residue at position 46 was absolute, while similar to our results, the requirement at position 47 was less stringent (24). Thus, our data from analysis of numerous Tva mutants at the DEW residues in Tva are not consistent with the previous hypothesis of a common functional motif in different retroviral receptors.
Substitutions of tryptophan 48 in Tva have also been reported to impair viral receptor function but not EnvA binding, leading to the suggestion that W48 may be involved in a postbinding step such as triggering conformational changes in EnvA (25). In our studies, we found that all 10 substitutions at this position produced severely diminished EnvA binding. Our analysis included the W48A substitution which was previously reported to dissociate binding from receptor function but that we found to be defective for both EnvA binding and function. The reason for these discrepant results may reflect differences in the assays used to measure envelope binding. The ELISA that we used evaluates monovalent binding of soluble receptor to a captured envelope protein, whereas the fluorescence-activated cell sorting assay used previously to test Tva-EnvA binding measures the avidity of a bivalent EnvA immunoadhesin for surface-expressed Tva (25). The bivalent nature of the immunoadhesin binding to Tva on the cell surface could underestimate decreases in Tva’s EnvA binding activity, especially if the effect of the mutation was on the dissociation rate of the receptor-envelope complex, since both EnvA molecules would have to dissociate simultaneously for the complex to detach from the cell surface. Similarly, mutant Tva proteins that do not associate stably with the viral envelope would not compete with wt Tva in the ELISA, possibly explaining the receptor mutants that function efficiently in infection but appear not to bind EnvA (D46G, W48L, W48I, etc.).
Recent structural analysis of module 5 from the human LDLR demonstrates that residues corresponding to D46 and E47 in Tva, as well as D36 and D40, are part of a calcium box involved in coordinating a calcium ion (6, 12). For module 5, coordination of Ca2+ is absolutely required for proper in vitro folding of the module (6). Furthermore, many of the point mutations in LDLR which abolish LDLR function and cause familial hypercholesterolemia replace one of these acidic residues (16). Given their conservation in Tva and all other LDLR-like modules, it seems likely that the corresponding residues of Tva also coordinate Ca2+ and thus are important for Tva protein folding. Therefore, it is surprising that our results and previous studies on Tva substitution mutants (24) do not display an absolute requirement for acidic amino acids at the D47 E48 positions for efficient viral receptor function. In addition, the ΔC3C5 mutant is deleted of two other acidic residues that coordinate the calcium ion and also two residues whose backbone carbonyl groups are also involved in coordination (12), yet this mutant expresses a functional, albeit impaired, receptor. Taken together, these data indicate that Tva can fold and function as a viral receptor under conditions where other LDLR modules are misfolded. In support of this view, mutation of individual cysteine residues in LDLR severely affects protein folding whereas similar mutants in Tva’s LDLR module have no effect on receptor function (5).
One possible explanation that reconciles our findings with the structure of the LDLR module is that with D46 or E47 mutations the majority of the Tva proteins are misfolded, but a fraction of the molecules adopt a native conformation suitable for binding EnvA. Folding of human LDLR module 5 in vitro suggests that a small percentage of the proteins exist in a native conformation even in mutants where residues in the calcium box are altered (6). Since extremely low surface levels of Tva are sufficient for full receptor function (24) and the Tva mutants are overexpressed in the infectivity assays used here, then it is likely that a small percentage of correctly folded Tva exists at the cell surface and that this correctly folded protein mediates the low levels of infection detected. This small amount of correctly folded Tva would not be readily detected in the EnvA binding assays. Thus, this model would account for the discrepant binding and infectivity seen with some of the Tva mutants.
An intriguing alternative hypothesis for the apparent lack of effect of calcium box mutations on Tva function is that the residues at positions 46 and 47 have different roles, and thus different requirements, when Tva is free or bound to EnvA. This might explain the ability of residues which cannot participate in Ca2+ coordination to substitute for these acidic amino acids. Perhaps EnvA binding releases the Ca2+ and allows residues 46 and 47 of the receptor (and possibly residues 36 and 40 also) to participate in the ligand binding interaction. This model would explain why EnvA and a number of other LDLR module ligands contain clustered basic residues required for receptor binding (20). In addition, the affinity of Tva for EnvA (∼1 to 2 nM) is roughly 50 times that of the LDL module (∼70 nM) for calcium (6), suggesting that EnvA can displace the bound Ca2+. Future experiments examining the EnvA-Tva complex for wt and mutant receptors will be required to determine if any of these models are correct.
ACKNOWLEDGMENTS
We thank Joanne Crane for purified sTva, Jeffrey Haldorson for producing recombinant vaccinia virus expressing gD-EnvA-His6 protein, and David Cook for assistance in immunofluorescence. We thank members of the Bates laboratory for many useful discussions during the course of this study.
Research in the laboratory of P.B. is supported by grants from the National Institutes of Health (CA63531) and American Heart Association (95015200). L.R. was partially supported by training grant T32-NS07180 from the National Institutes of Health.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Balliet J W, Bates P. Efficient infection mediated by viral receptors incorporated into retroviral particles. J Virol. 1998;72:671–676. doi: 10.1128/jvi.72.1.671-676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balliet, J. W., J. Berson, C. D’Cruz, J. M. Gilbert, J. M. White, and P. Bates. Unpublished data.
- 4.Bates P, Young J A T, Varmus H E. A receptor for subgroup-A Rous sarcoma virus is related to the low-density-lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 5.Belanger C, Zingler K, Young J A T. Importance of cysteines in the LDLR-related domain of the subgroup A avian leukosis and sarcoma virus receptor for viral entry. J Virol. 1995;69:1019–1024. doi: 10.1128/jvi.69.2.1019-1024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blacklow S C, Kim P S. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nature Struct Biol. 1996;3:758–762. doi: 10.1038/nsb0996-758. [DOI] [PubMed] [Google Scholar]
- 7.Connolly L, Zingler K, Young J A T. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ASLV-A) blocks infection and binds directly to ALSV-A. J Virol. 1994;68:2760–2764. doi: 10.1128/jvi.68.4.2760-2764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly N L, Djordjevic J T, Kroon P A, Smith R. Three-dimensional structure of the second cysteine-rich repeat from the human low-density lipoprotein receptor. Biochemistry. 1995;34:14474–14481. doi: 10.1021/bi00044a025. [DOI] [PubMed] [Google Scholar]
- 9.Daly N L, Scanlon M J, Djordjevic J T, Kroon P A, Smith R. Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc Natl Acad Sci USA. 1995;92:6334–6338. doi: 10.1073/pnas.92.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damico R L, Crane J, Bates P. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc Natl Acad Sci USA. 1998;95:2580–2585. doi: 10.1073/pnas.95.5.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esser V, Limbird L E, Brown M S, Goldstein J L, Russell D W. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J Biol Chem. 1988;263:13282–13290. [PubMed] [Google Scholar]
- 12.Fass D, Blacklow S, Kim P S, Berger J M. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert J M, Bates P, Varmus H E, White J M. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup-A but not to subgroup-C envelope glycoprotein. J Virol. 1994;68:5623–5628. doi: 10.1128/jvi.68.9.5623-5628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert J M, Hernandez L D, Balliet J W, Bates P, White J M. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J Virol. 1995;69:7410–7415. doi: 10.1128/jvi.69.12.7410-7415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez L D, Peters R J, Delos S E, Young J A T, Agard D A, White J M. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J Cell Biol. 1997;139:1455–1464. doi: 10.1083/jcb.139.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobbs H H, Brown M S, Goldstein J L. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra S, Scott A G, Zavorotinskaya T, Albritton L M. Analysis of the murine ecotropic leukemia virus receptor reveals a common biochemical determinant on diverse cell surface receptors that is essential to retrovirus entry. J Virol. 1996;70:321–326. doi: 10.1128/jvi.70.1.321-326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rena S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J, Guo H, Du J, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Roles of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong L, Bates P. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor motif of Tva is sufficient to mediate viral entry. J Virol. 1995;69:4847–4853. doi: 10.1128/jvi.69.8.4847-4853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Rong, L., and P. Bates. Unpublished data.
- 20.Rong L, Edinger A, Bates P. Role of basic residues in the subgroup determining region of subgroup A avian sarcoma and leukosis virus envelope in receptor binding and infection. J Virol. 1997;71:3458–3465. doi: 10.1128/jvi.71.5.3458-3465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rong, L., K. Gendron, and P. Bates. Conversion of a human low density lipoprotein receptor ligand binding repeat to a virus receptor: identification of residues important for ligand specificity. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 22.Russell D W, Brown M S, Goldstein J L. Different combinations of cysteine-rich repeats mediate binding of low density lipoprotein receptor to two different proteins. J Biol Chem. 1989;264:21682–21688. [PubMed] [Google Scholar]
- 23.Young J A, Bates P, Varmus H E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zingler K, Belanger C, Peters R, Agard E, Young J A. Identification and characterization of the viral interaction determinant of the subgroup A avian leukosis virus receptor. J Virol. 1995;69:4261–4266. doi: 10.1128/jvi.69.7.4261-4266.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zingler K, Young J A T. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]