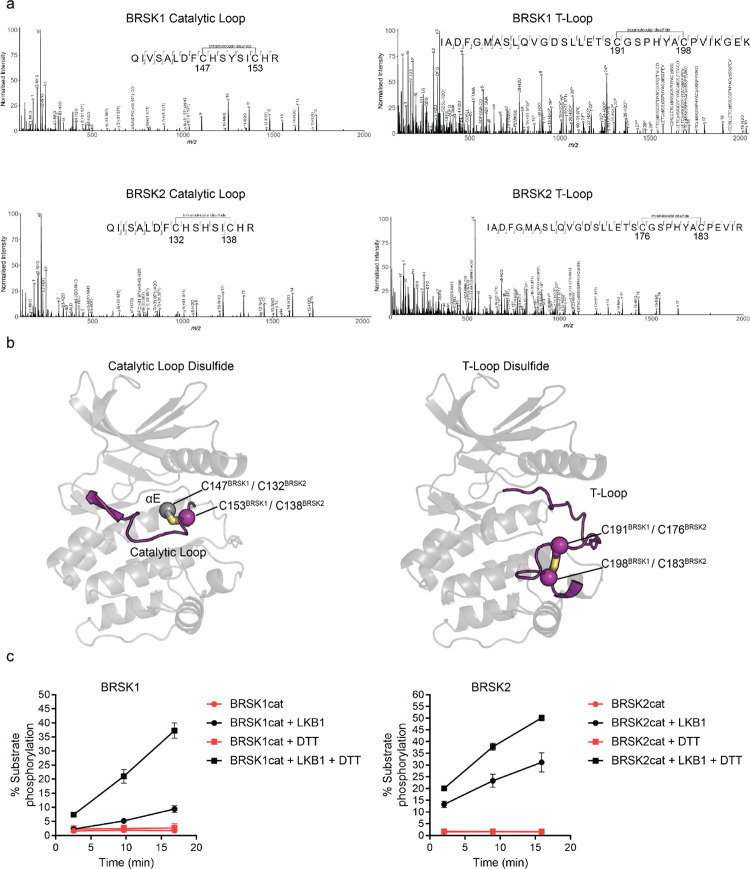
Figure 2:
Intramolecular disulfide bonds form in the kinase domains of BRSK1 and 2. (a) Full length BRSK1 and 2 were affinity-purified from HEK-293T cells and subjected to LC-MS/MS analysis. LC-MS/MS spectrum mapping revealed disulfide bridges formation between C147BRSK1 - C153BRSK1, C191BRSK1 - C198BRSK1, C132BRSK2 - C138BRSK2, and C176BRSK2 - C183BRSK2. Peptide coverage was 74 and 78 % for BRSK1 and BRSK2 respectively. (b) Alphafold structures demonstrating the location of disulfide bonds within the kinase domains of BRSK1 and BRSK2. (c) Real time phosphorylation of fluorescent AMARA peptide by the kinase domains of BRSK1 and 2 (100 ng) purified from E.coli. BRSK1 (29–358) and BRSK2 (14–341) were activated by incubation with LKB1 and assayed in the presence or absence of 1 mM DTT.