CLINICAL VIGNETTE
A 15-year-old male with a past medical history of asthma is brought into the Emergency Department. He had been experiencing a respiratory illness over a week prior, for which he received a 2-day course of oral dexamethasone. He subsequently presented to the hospital disorientated, with a 5-day history of polydipsia, polyuria, nausea, emesis, diarrhea, and intermittent abdominal pain.
Upon initial assessment, he was found to be afebrile and normotensive (112/57 mmHg), but tachycardia (146 beats per minute) and tachypneic (32 breaths per minute), with an impaired level of consciousness (Glasgow coma score 12/15). His respiratory examination was within normal limits with no wheezing. He had a moderately delayed capillary refill time and dry mucus membranes. His abdomen was soft with mild generalized tenderness. Upon questioning, his mother reported his most recent weight as 108 kg from 2 weeks previous. In the Emergency Department, his weight was recorded as 102 kg (>99.9th percentile for sex and age) with a height of 175 cm (75th percentile), and body mass index of 33.3 kg/m2 (97th to 99.9th percentile).
A point-of-care glucometer read as ‘HI’ and initial venous blood gas revealed an isolated mild acidaemia, with a pH of 7.31 and bicarbonate of 26 mmol/L. Serum glucose was reported at 52 mmol/L, with minimal serum ketones (0.4 mmol/L). His biochemistry further revealed a serum sodium of 138 mmol/L (151 mmol/L corrected), potassium of 4 mmol/L and a chloride of 102 mmol/L, giving an anion gap of 14. With a urea of 12 mmol/L, the patient had an elevated calculated serum osmolarity of 340 mOsm/kg. The patient was diagnosed with diabetes and identified as being in hyperglycaemic hyperosmolar state (HHS). He required extensive fluid rehydration and electrolyte correction, and subsequently was initiated on an insulin infusion prior to transfer to the Paediatric Intensive Care Unit. His course was complicated by an acute kidney injury, with elevated creatinine at 187 µmol/L, and subsequent disseminated intravascular coagulopathy. He gradually recovered baseline renal function after a 6-day period in intensive care. The patient was transitioned to the general paediatric medicine ward for an additional 3 days, was then discharged on multiple daily injections of insulin, with a plan to wean off mealtime insulin and to initiate metformin.
LEARNING POINTS
The Canadian Paediatric Surveillance Program (CPSP) launched a 2-year study on June 1st 2023, with the goals of estimating the minimum annual incidence of HHS in children across Canada and better defining predisposing factors, as well as standards of management and outcomes. To learn more and to report cases which meet the definition, please visit: https://cpsp.cps.ca/surveillance/study-etude/hyperglycemic-hyperosmolar-state.
The prevalence of obesity and associated type 2 diabetes (T2D) amongst children and adolescents across Canada continues to rise. A recent report from Manitoba, one of the provinces most severely affected by paediatric obesity (1), demonstrated a greater than 20% increase in the prevalence of T2D amongst children and adolescents over the eight years leading up to 2018 (2). Rates of acute (e.g., HHS) and chronic complications associated with obesity and T2D have risen in concert as a direct result of this changing paediatric phenotype. Indeed, as many as 2% of all first presentations of patients registered to the Pediatric Diabetes Consortium T2D Clinic Registry were found to meet criteria for HHS (3).
HHS is a hyperglycaemic crisis distinct from diabetic ketoacidosis (DKA), which has traditionally been seen almost exclusively in adult T2D. The condition is defined by substantial hyperglycaemia (>33 mmol/L) and hyperosmolarity (serum osmolality of >320 or >330 mmol/L), with minimal or no acidosis (4,5). While a high anion gap acidosis is typical in DKA due to very high ketone production, anion gap in HHS can be somewhat variable. In paediatric HHS low levels of ketones may be present, along with high lactate which can contribute to slightly lower pH. Of note, the case definition applied in the present CPSP study does allow for the presence of a small amount of serum/urinary ketones provided bicarbonate is >15 mEq/L and pH is >7.25 venous or >7.3 arterial/capillary.
The pathophysiology of HHS differs from that of DKA in that there is a relative rather than absolute deficiency in insulin in the context of surging counter-regulatory hormones (Figure 1). This state drives increased hepatic production of glucose, in turn leading to osmotic diuresis, and profound dehydration (6). HHS is often thought to be preceded by an infectious insult in children (7,8), with thrombotic events and medications being recognized triggers in adult populations (9). Children with intellectual disabilities are overrepresented in paediatric HHS cohorts (42% in one case series) (10), suggesting that thirst recognition may be blunted, and ingestion of suitable rehydration solutions is variable in these children, thereby predisposing them to decompensation.
The majority of literature currently available on HHS in children is comprised of case reports or small series, which offer only a limited view of the range of presentations and event courses. The best available estimate of incidence and outcomes relating to HHS in children stems from a single analysis of the US Kids’ Inpatient Database (KID) between 1997 and 2009 (8). KID is a large-scale, publicly available paediatric inpatient care database created under and curated by the Healthcare Cost and Utilization Project. It was approximated that 3.2 cases of HHS occurred per million children and adolescents in 2009, a 52% increase in incidence from 1997. However, it is conceivable that cases of HHS in children are misdiagnosed as DKA or hyperosmolar DKA and the true incidence may be greater.
HHS has a high mortality rate in children and adolescents. Of the 18 cases reported in one review of the literature, 13 (i.e., 72%) ultimately ended in mortality (11). However, the KID study reported a mortality rate of 2.7% (8), suggesting that there is significant publication bias towards reporting of the most severe cases within the paediatric literature. Within the limited published literature on HHS in children, the state has been associated with a range of potentially life-threatening complications, including thrombosis, disseminated intravascular coagulation, rhabdomyolysis, renal injury or failure, cardiac arrhythmia, malignant hypertension-like syndrome, and cerebral edema or seizures (12–14).
Formal society guidelines on the management of HHS in children had been lacking until recently. The 2022 ISPAD guidelines are the first to focus on differentiating management of HHS and hyperosmolar DKA from that of normosmolar DKA (4). The guidance provided encourages prioritization of aggressive fluid rehydration in HHS and hyperosmolar DKA, inclusive of 20 mL/kg normal saline boluses repeated until restoration of perfusion, followed by maintenance fluids and replacement of an assumed 12% to 15% body water deficit and urine outputs over the subsequent 24 to 48 h. While insulin practices in hyperosmolar DKA mirror those of normosmolar DKA, infusions may not always be required in HHS, but are recommended at a lower rate of 0.025 to 0.05 units/kg/h should fluid rehydration be insufficient in complete restoration of normoglycaemia.
Figure 1.
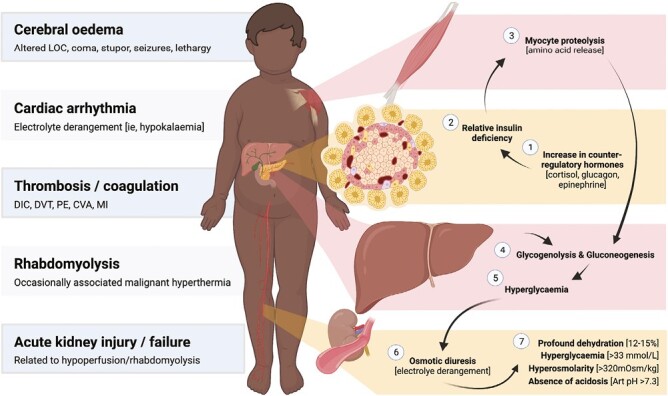
Pathophysiology, biochemical profile and known complications of hyperglycaemic hyperosmolar state in children. AKI acute kidney injury; ARF acute renal failure; Art pH arterial pH; CVA cerebrovascular accident; DIC disseminated intravascular coagulation; DVT deep vein thrombosis; LOC level of consciousness; MI myocardial infarct; PE pulmonary embolism.
ACKNOWLEDGEMENTS
We are grateful for the excellent work of Ms Melanie Laffin, previously Senior Manager, Canadian Paediatric Society, and Ms Christina Ricci, Epidemiologist, Public Health Authority of Canada, in progressing the current CPSP study to launch. Figure 1 was created with Biorender.com.
Contributor Information
Paul M Ryan, Department of Paediatrics, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada.
Elizabeth A C Sellers, Department of Pediatrics and Child Health, Children’s Hospital Research Institute of Manitoba, University of Manitoba, Winnipeg, Manitoba, Canada.
Shazhan Amed, Division of Paediatric Endocrinology, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada.
Jill K Hamilton, Department of Paediatrics, The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada; Division of Endocrinology, The Hospital for Sick Children, Toronto, Ontario, Canada.
FUNDING
JKH receives unrestricted research funding from the Hospital for Sick Children and the University of Toronto with the Mead Johnson Chair in Child Nutrition. PMR received funding through the Elizabeth Arbuthnot Dyson Fellowship.
POTENTIAL CONFLICT OF INTEREST
JKH has participated in advisory boards for Novo Nordisk.
REFERENCES
- 1. Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC.. Childhood overweight and obesity trends in Canada. Health Promot Chronic Dis Prev Can 2016;36:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruth C, Sellers E, Chartrand C, et al. . Type 2 Diabetes in Manitoba. Winnipeg, MB: Manitoba Centre for Health Policy, 2020. [Google Scholar]
- 3. Klingensmith GJ, Connor CG, Ruedy KJ, et al. ; Pediatric Diabetes Consortium. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes 2016;17:266–73. [DOI] [PubMed] [Google Scholar]
- 4. Glaser N, Fritsch M, Priyambada L, et al. . ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2022;23:835–56. [DOI] [PubMed] [Google Scholar]
- 5. Diabetes Canada Clinical Practice Guidelines Expert Committee, Panagiotopoulos C, Hadjiyannakis S, Henderson M.. Type 2 diabetes in children and adolescents. Can J Diabetes 2018;42:S247–54. [DOI] [PubMed] [Google Scholar]
- 6. Guzman H, Lam DW-H.. Chapter 15—Diabetic emergencies: Ketoacidosis, hyperglycemic hyperosmolar state, and hypoglycemia. In: Shifrin AL, ed. Endocrine Emergencies. Philadelphia: Elsevier, 2022:167–82. [Google Scholar]
- 7. Murthy S, Sharara-Chami R.. Aggressive fluid resuscitation in severe pediatric hyperglycemic hyperosmolar syndrome: A case report. Int J Pediatr Endocrinol 2010;2010:379063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagdure D, Rewers A, Campagna E, Sills MR.. Epidemiology of hyperglycemic hyperosmolar syndrome in children hospitalized in USA. Pediatr Diabetes 2013;14:18–24. [DOI] [PubMed] [Google Scholar]
- 9. Kitabchi AE, Umpierrez GE, Murphy MB, et al. . Management of hyperglycemic crises in patients with diabetes. Diabetes Care 2001;24:131–53. [DOI] [PubMed] [Google Scholar]
- 10. Fourtner SH, Weinzimer SA, Levitt Katz LE.. Hyperglycemic hyperosmolar non-ketotic syndrome in children with type 2 diabetes*. Pediatr Diabetes 2005;6:129–35. [DOI] [PubMed] [Google Scholar]
- 11. Cochran JB, Walters S, Losek JD.. Pediatric hyperglycemic hyperosmolar syndrome: Diagnostic difficulties and high mortality rate. Am J Emerg Med 2006;24:297–301. [DOI] [PubMed] [Google Scholar]
- 12. Mercer S, Hanks L, Ashraf A.. Rhabdomyolysis in pediatric patients with diabetic ketoacidosis or hyperglycemic hyperosmolar state: A case series. Glob Pediatr Health 2016;3:2333794X16671391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price A, Losek J, Jackson B.. Hyperglycaemic hyperosmolar syndrome in children: Patient characteristics, diagnostic delays and associated complications. J Paediatr Child Health 2016;52:80–4. [DOI] [PubMed] [Google Scholar]
- 14. Kilbane BJ, Mehta S, Backeljauw PF, Shanley TP, Crimmins NA.. Approach to management of malignant hyperthermia-like syndrome in pediatric diabetes mellitus. Pediatr Crit Care Med 2006;7:169–73. [DOI] [PubMed] [Google Scholar]


