Abstract
Human liver organoids (HLOs) differentiated from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs) can recapitulate structure and function of human fetal liver tissues, thus, considered as a promising tissue model for liver diseases and predictive compound screening. Nonetheless, there are still several technical challenges to adopt HLOs in the drug discovery process, which include relatively long-term cell differentiation with multiple culture media (3 – 4 weeks) leading to batch-to-batch variation, short-term hepatic function after maturation (3 – 5 days), low assay throughput due to Matrigel dissociation and HLO transfer to a microtiter well plate, and insufficient maturity as compared to primary hepatocytes. To address these issues, expandable HLOs (Exp-HLOs) derived from human iPSCs were generated by optimizing differentiation protocols, which were rapidly printed on a 144-pillar plate with sidewalls and slits (144PillarPlate) and dynamically cultured for up to 20 days into differentiated HLOs (Diff-HLOs) in a 144-perfusion plate with perfusion wells and reservoirs (144PerfusionPlate) for in situ organoid culture and analysis. Dynamically cultured Diff-HLOs were generated robustly and reproducibly in the pillar/perfusion plate with higher maturity as compared to those in statically cultured HLOs by differentiating Exp-HLOs for 10 days. In addition, Diff-HLOs in the pillar/perfusion plate were tested with acetaminophen and troglitazone for 3 days to assess drug-induced liver injury (DILI) and then incubated in an expansion medium for 10 days to evaluate the recovery of the liver from DILI. The assessment of liver regeneration post injury is critical to understand the mechanism of recovery and determine the threshold drug concentration beyond which there will be a sharp decrease in the liver’s regenerative capacity. We envision that bioprinted Diff-HLOs in the pillar/perfusion plate could be used for high-throughput screening (HTS) of hepatotoxic compounds due to short-term differentiation of passage-able Exp-HLOs necessary, stable hepatic function after maturation, high reproducibility, and high throughput with capability of in situ organoid culture, testing, staining, imaging, and analysis.
Keywords: Regenerative human liver organoids, pillar plate, perfusion plate, dynamic organoid culture, organoid bioprinting, hepatotoxicity testing
Graphical abstract.

The overall process of dynamic liver organoid culture and in situ analysis in the 144PillarPlate/144PerfusionPlate for high-throughput hepatotoxicity assays.
Introduction
Sandwich-cultured, primary human hepatocytes (PHHs) have been used widely as the gold standard of an in vitro liver model for several decades for disease modeling and hepatotoxicity testing in preclinical studies1,2. Nevertheless, PHHs are expensive and difficult to obtain in large quantities for high-throughput screening (HTS) of compounds3. When PHHs are maintained under standard in vitro cell culture conditions over time, they rapidly lose liver specific functions4. In addition, PHHs show high donor-to-donor variability for hepatic functions, leading to irreproducible results and significant lab-to-lab variability.
To address the limited availability of PHHs, human liver organoids (HLOs) differentiated from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs) have been extensively studied because of their cellular structure and function strikingly similar to human fetal liver tissues5. In 2013 and 2015, Huch et al.6,7 generated biliary stem cell organoids by isolating and culturing EpCAM+ biliary cells from liver biopsy samples and demonstrated the generation of functional hepatocytes and cholangiocytes by further differentiation. In 2018, Hu et al.8 generated human hepatocyte organoids by the expansion of primary human hepatocytes. Nonetheless, HLOs derived from adult and pediatric liver biopsy samples had limited expansion capacity (up to 2 – 3 months) although fetal tissue-derived hepatocyte organoids did not show senescence. In addition, liver tissue-derived HLOs have limited potential in modelling complicated liver diseases that require multiple hepatic cell types such as hepatocytes, stellate cells, Kupffer cells, and endothelial cells9. Furthermore, variable efficiency of liver tissue-derived HLO generation raised the concern for patient-specific liver disease modeling and scale-up production of HLOs for compound screening.
On the other hand, HLOs derived from iPSCs demonstrated enhanced complexity and could potentially mimic the in vivo pathophysiology because iPSCs showed the ability to differentiate into multiple hepatic cell types. Several differentiation protocols have been developed to generate HLOs using iPSCs alone10–15 or iPSCs with other cell types16–18. For example, Ouchi et al.13 pioneered the generation of iPSC-derived multicellular HLOs consisting of parenchymal and non-parenchymal liver cell types for modeling steatohepatitis. They showcased inflammatory and fibrotic responses of steatohepatitis by 5-day treatment of HLOs with fatty acids. By using the same differentiation protocol, Shinozawa et al.15 demonstrated bile acid uptake by HLOs, which is a critical feature of functional bile canaliculi in the liver and performed large-scale compound screening for cholestatic liver injury by using live HLO imaging for bile acid uptake and viability. The differentiation of iPSCs into multicellular HLOs is robust, but it requires a month-long cell differentiation with several cell culture media. Although foregut cells differentiated from iPSCs can be cryopreserved, mature HLOs were difficult to passage and cryopreserve, which are critically necessary to shorten the differentiation and maturation time and enhance the throughput and reproducibility. In addition, mature HLOs gradually lose their hepatic functions over time, which could be a critical issue for reproducible organoid-based assays. For scale-up production of HLOs, foregut cells are embedded in Matrigel domes, which need to be physically or enzymatically dissociated to isolate and harvest mature HLOs. This process is labor-intensive and could lead to the loss of HLOs, damage to HLO structure and function, and batch-to-batch variation in their size. Harvested HLOs in 200 – 300 µm in diameter need to be dispensed in a microtiter well plate for organoid-based assays15, which could require an expensive liquid handling machine for high accuracy.
To resolve these issues, expandable human liver organoids (Exp-HLOs) were generated in the present work by differentiating day 15 early hepatic progenitor cells derived from iPSCs, which can be passaged and cryopreserved for scale-up production of HLOs. The Exp-HLOs, which contain bi-potent ductal cells, were expanded through cAMP activation (Forskolin), TGF-β inhibition (A8301), and Wnt signaling (R-spondin1). Subsequently, functional differentiated human liver organoids (Diff-HLOs) were generated by differentiating the Exp-HLOs. To enhance the maturity of Diff-HLOs, we optimized the differentiation medium and culture duration and incorporated dynamic HLO culture in a pillar/perfusion plate. A 144-pillar plate with sidewalls and slits (144PillarPlate) and a complementary 144-perfusion plate with perfusion wells and reservoirs (144PerfusionPlate) were manufactured by injection molding of polystyrene, which supported the uniform generation of Diff-HLOs in a dynamic condition as well as high-throughput assessment of drug-induced liver injury (DILI) and the recovery of the liver from DILI using Diff-HLOs. Thus, we envision that our approach could be a valuable addition to current in vitro hepatotoxicity assessment mainly focusing on live-dead viability of primary hepatocytes.
Materials and Methods
Fabrication of 144PillarPlate and 144PerfusionPlate
The 144PillarPlate, the 144PerfusionPlate, and the 384DeepWellPlate (Figure 4 and Supplementary Figure 1) were manufactured by injection molding of polystyrene (Bioprinting Laboratories Inc., Dallas, TX, USA). The 144PilllarPlate contains a 12 × 12 array of pillars (4.5 mm pillar-to-pillar distance, 11.6 mm pillar height, and 2.5 mm outer and 1.5 mm inner diameter of pillars), each pillar allowing to load up to 6 µL of cells suspended in hydrogel. The 144PerfusionPlate has a 12 × 12 array of perfusion wells (3.4, 3.4, and 11.9 mm well width, length and depth, and 4.5 mm well-to-well distance) with a 2 × 12 array of reservoirs (3.6, 30.3, and 15.4 mm reservoir width, length and depth, and 4.5 mm reservoir-to-reservoir distance), all connected by microchannels. Thus, the 144PerfusionPlate contains 12 fluidic channels, each channel consisting of 2 reservoirs (upper and lower) on both sides and 12 perfusion wells in a row connected by microchannels and allowing to load up to 2,000 µL of a cell growth medium for dynamic organoid culture. Flow simulation in the perfusion plate has been performed with SolidWorks software package (SolidWorks Research Standard and Simulation Premium 2022, MA, USA) with 1,300 µL of water at 10° tilting angle and 1 minute frequency of tilting angle change on a digital rocker19,20. The 384DeepWellPlate has a 16 × 24 array of deep wells (3.5, 3.5, and 14.7 mm well width, length and depth, and 4.5 mm well-to-well distance), each deep well allowing to load up to 80 µL of a cell growth medium for static organoid culture. The pillar plate with cells encapsulated in hydrogels can be sandwiched onto the perfusion plate or the deep well plate for dynamic and static culture of human organoids as well as in situ compound testing and organoid imaging.
Figure 4. The injection-molded pillar plate and the complementary perfusion plate for dynamic organoid culture.
(A) The 144PillarPlate with a 12 × 12 array of pillars for loading cells in hydrogels. (B) The 144PerfusionPlate with a 12 × 12 array of perfusion wells and a 2 × 12 array of reservoirs for supplying cell culture media. (C) The 144PillarPlate sandwiched onto the 144PerfusionPlate for dynamic cell culture. The 144PerfusionPlate contains 12 fluidic channels, each channel consisting of one upper reservoir (UR), one lower reservoir (LR), and a row of 12 perfusion wells connected by microchannels. (D) Volumetric flow rates in forward and backward directions in the 144PerfusionPlate coupled with the 144PillarPlate simulated with SolidWorks at 1300 μL cell culture medium per fluidic channel, 10° tilting angle, and 1 minute frequency of tilting angle change. The negative flow rates indicate the opposite direction of flow. (E) Average shear stress on the surface of the pillars by fluid flow simulated by SolidWorks.
iPSCs maintenance and differentiation into expandable human liver organoids (Exp-HLOs)
EDi029-A, a male human iPSC line (Cedar Sinai Biomanufacturing Center, USA), was maintained on growth factor reduced Matrigel (Corning; 354230) coated dishes under mTeSR™ Plus medium (Stemcell Technologies; 100–0276) and passaged using the StemPro™ EZPassage™ passaging tool (ThermoFisher; 23181–010) or ReLeSR™ human PSC selection and passaging reagent (Stemcell Technologies; 05872). The differentiation of iPSCs into foregut cells was performed using previously published protocols13,21. Briefly, at 70 – 80% confluency, iPSCs were harvested using Accutase (Gibco; A1110501), seeded on iMatrix-511 silk (Elixirgen Scientific; NI511) coated 6-well plate at a cell density of 1.3 × 106, and cultured using mTESR™ Plus medium supplemented with 10 µM Y27632 Rho-kinase (ROCK) inhibitor (Tocris; 1254). After 24 hours of culture, the iPSCs were differentiated into definitive endoderm using RPMI 1640 (Gibco; 22400089) supplemented with 50 ng/mL bone morphogenetic protein 4 (BMP4; Tocris; 314-BP) and 100 ng/mL activin A (Tocris; 338-AC) at day 1, 100 ng/mL Activin A and 0.2% knockout serum replacement (KSR; Gibco; 10828010) at day 2, and 100 ng/mL Activin A and 2% KSR at day 3. This was followed by foregut cell differentiation from day 4 – 6 using advanced DMEM/F12 (Gibco; 12634) with 2% B27 (Gibco; 17504), 1% N2 (Gibco; 17502), 10 mM HEPES (Gibco; 15630), 1% penicillin/streptomycin (Gibco; 15140), and GlutaMAX™ (Gibco; 35050) supplemented with 500 ng/mL fibroblast growth factor (FGF4; Peprotech; 100–31) and 3 mM CHIR99021 (R&D Systems; 4423). On day 7, foregut cells were dissociated using Accutase, resuspended in Matrigel (Corning; 356237) at a density of 750 cells/µL, and dispensed in a 24-well plate to form 50 µL Matrigel domes for culture, or cryopreserved using CryoStor® CS10 (Stemcell technologies; 07959) for later use. After gelation of Matrigel at 37°C for 10 – 12 minutes, foregut cells in the 24-well plate were cultured in DMEM base medium supplemented with 5 ng/mL recombinant human FGF basic/FGF2/bFGF (R&D systems; 233-FB), 10 ng/mL recombinant human VEGF-165 (Gibco; PHC9391), 20 ng/mL recombinant human EGF (R&D system; 236-EG), 0.5 µM A 83–01 (R&D Systems; 2939), 50 µg/mL L-ascorbic acid (Sigma; A4544), and the CEPT cocktail consisting of 50 nM chroman 1 (R&D systems; 7163), 5 µM emricasan (Selleckchem; S7775), 1x polyamine supplement (Sigma; P8482), and 0.7 µM trans-ISRIB (R&D systems; 5284). The medium volume used in the 24-well plate was 1 mL per well. The differentiation medium was changed every other day. On day 11, the differentiation medium was changed to DMEM base medium supplemented with 2 µM retinoic acid (Sigma; R2625) with medium change on every other day.
Starting from day 15, early hepatic progenitor cells was differentiated into expandable human liver organoids (Exp-HLOs) in an expansion medium (EM) consisting of advanced DMEM/F12 supplemented with 2% B27 supplement (without vitamin A), 1% N2 supplement, 10 mM HEPES, 1% GlutaMAX, 1% penicillin/streptomycin, 1 mM N-acetyl-L-cysteine (Sigma; A9165), 10 mM nicotinamide (Sigma; N0636), 10 nM recombinant human (Leu15)-gastrin I (Sigma; G9145), 50 ng/mL recombinant human EGF, 1 µg/mL R-Spondin1 (Rspo1) (R&D systems; 4645-RS/CF), 100 ng/mL recombinant human FGF10 (Peprotech; 100–26), 25 ng/mL recombinant human HGF (Peprotech; 100–39), 10 μM forskolin (R&D Systems; 1099), and 5 μM A83–01. The Exp-HLOs were culture for 1 – 2 weeks until these organoids retain the size of approximately 250 µm. Exp-HLOs were enzymatically dissociated into small clumps by treatment with 1x TrypLE™ Express Enzyme (ThermoFisher; 12605010) for 5 minutes at 37°C in the CO2 incubator. The dissociated cell clumps were either cryopreserved using Stem-Cellbanker DMSO free - GMP grade (Amsbio; 13926) for later use or mixed with undiluted Matrigel (Corning; 356237) for differentiation into Diff-HLOs. When cell clumps were thawed and cultured post cryopreservation, the EM was supplemented with the cryoprotectant CEPT cocktail for the first 4 days of culture.
Differentiation of passaged Exp-HLOs into Diff-HLOs
The dissociated cell clumps from Exp-HLOs were mixed with undiluted Matrigel (Corning; 356237) and dispensed at 50 μL Matrigel dome/well in a 24-well plate for differentiation into Diff-HLOs. The cell clumps in Matrigel dome were cultured in the EM supplemented with 25 ng/mL human active BMP7 recombinant protein (Gibco; PHC7204) initially for 3 – 6 days to regenerate and form Exp-HLOs using BMP7. After forming Exp-HLOs in approximately 200 μm diameter, the organoids were cultured in a differentiation medium (DM) consisting of advanced DMEM/F12 supplemented with 1% B27, 1% N2, 10 mM HEPES, 1% penicillin/streptomycin, and 1% GlutaMAX, 10 nM recombinant human (Leu15)-gastrin I, 100 ng/mL recombinant human FGF19 (Peprotech; 100–32), 25 ng/mL recombinant human hepatocyte growth factor (HGF; Peprotech; 100–39), 500 nM A8301 (R&D Systems; 2939), 10 μM notch inhibitor DAPT (Sigma; D5942), 25 ng/mL human active BMP7 recombinant protein, and 3 μM dexamethasone (Dex; Sigma; D4902) for 10 days with medium change on every other day.
Microarray bioprinting of cell clumps in Matrigel on the pillar plate
The cell clumps obtained from enzymatic dissociation of Exp-HLOs were mixed with undiluted Matrigel and printed on the 144PillarPlate at 4 µL cell clumps in Matrigel per pillar by using ASFA™ Spotter V6 (MBD Co., Ltd., South Korea). After Matrigel gelation at 37°C for 10 – 12 minutes, the 144PillarPlate with cell clumps was sandwiched onto a complementary 384DeepWellPlate containing 80 µL EM+BMP7 medium in each well for static HLO culture or coupled with the 144PerfusionPlate containing 1300 µL of EM+BMP7 medium per fluidic channel for dynamic HLO culture.
Gene expression analysis via RT-qPCR
HLOs in Matrigel were either collected manually by pipetting in cold PBS−/− or isolated from Matrigel using Cultrex organoid harvesting solution (R&D Systems; 3700–100-01) according to the manufacturer’s recommended protocol, which allows non-enzymatic depolymerization of Matrigel. In case of HLOs on the pillar plate, the pillar plate was sandwiched onto the deep well plate containing 80 µL of Cultrex organoid harvesting solution. The sandwiched plates were incubated for 30 minutes at 4°C and then centrifuged at 100 rcf for 10 minutes to detach the organoids. Total RNA was isolated from harvested cells by using the RNeasy Plus Mini Kit (Qiagen; 74134) following the manufacturer’s recommended protocol. cDNA was synthesized from 1 µg of RNA by following the protocol of the high-capacity cDNA reverse transcription kit (Applied Biosystems; 4368814). Real time PCR was performed using PowerTrack™ SYBR green master mix (Applied Biosystems; A46110) and forward/reverse primers from IDT Technology in the QuantStudio™ 5 Real-Time PCR System (Applied Biosystems; A28574). The cycle was run 40 times at 95°C denaturation for 30 sec, 58 – 62°C annealing for 45 sec, depending on primer pair, and 72°C extension for 30 sec. The primers used are listed in Supplementary Table 1. The expression level of target genes was normalized with that of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Whole mount immunofluorescence staining
The immunofluorescence staining was performed either by harvesting HLOs from Matrigel domes in the 24-well plate or with HLOs in situ on the pillar plate. In the case of Matrigel dome culture, Matrigel domes containing organoids were collected in cold dPBS−/− through pipetting into a 1.5 mL Eppendorf tube and then centrifuged to isolate HLOs at 300 x g for 4 minutes. The HLOs were fixed using 4% paraformaldehyde (PFA; ThermoFisher Scientific; J19943K2) for 2 hours at room temperature while gently rocking. The fixed HLOs were washed with dPBS−/− containing 0.1% (w/v) sodium borohydride twice for 15 minutes to reduce background due to free aldehyde. After washing, the HLOs were permeabilized with 500 µL of 0.5% Triton X-100 (Sigma; T8787) in dPBS−/− (i.e., permeabilization buffer) for 15 – 20 minutes twice at room temperature with gentle rocking. After permeabilization, HLOs were exposed to 500 µL of 5% normal donkey serum (NDS) in the permeabilization buffer (i.e., blocking buffer) for 1 hour at room temperature or overnight at 4°C with gentle rocking to prevent non-specific binding. For primary antibody staining, the HLOs were treated with 250 µL of 5 µg/mL primary antibody diluted in the blocking buffer for overnight at 4°C with gentle rocking. The HLOs were rinsed with 1 mL of the blocking buffer thrice for 30 minutes each at room temperature with gentle rocking to prevent non-specific binding. For secondary antibody staining, the HLOs were exposed to 500 µL of 5 µg/mL fluorophore-conjugated secondary antibody in the blocking buffer for 2 – 4 hours at room temperature with gentle rocking. The HLOs were stained with 500 µL of 0.5 µg/mL DAPI solution (ThermoFisher Scientific; 62248) in 1x dPBS−/− for 30 minutes at room temperature with gentle rocking. The HLOs were further washed with 1 mL of dPBS−/− twice to ensure the complete removal of unbound secondary antibody. Finally, the HLOs were transferred to a microscope cover glass (Fisher Scientific; 22266882) and treated with 25 μL of Visikol® Histo-M™ (Visikol; HM-30) to clear the organoids which also works as a mounting solution. The HLOs on the cover glass slide were covered by another cover glass from the top and imaged using a Zeiss LSM 710 confocal scanning microscope. For the HLOs on the pillar plate, all the immunofluorescence staining steps were performed by sandwiching the pillar plate with a 384DeepWellPlate containing 80 µL of respective solutions and incubating the sandwiched plates under the same conditions mentioned above. After the final wash with 80 μL dPBS−/− to remove unbound secondary antibody, the HLOs were treated with 35 µL of Visikol® Histo-M™ in a regular 384-well plate (ThermoFisher Scientific; 242757) for 1 hour at room temperature. At the time of imaging, the pillar plate containing stained HLOs was placed on the microscope cover glass. The specific names of primary and secondary antibodies used are listed in Supplementary Table 2 and 3, respectively.
Cell cytometry analysis
Exp-HLOs in Matrigel were collected in cold PBS−/− and dissociated into single cells using Accutase for 5 – 8 minutes at 37° C in a CO2 incubator. The dissociated cells were collected in a 15 mL tube and fixed using 4% PFA for 15 minutes at room temperature. Subsequently, the cells were permeabilized with 0.25% Triton X-100 in PBS−/− for 15 minutes and blocked using 5% NDS in PBS−/− containing 0.1% Triton X-100 for 30 minutes at room temperature. The cells were then incubated with 2.5 µg/mL of EpCAM (SantaCruz; sc-25308) for 1 hours at room temperature, washed with the blocking solution thrice, and stained with 5 µg/mL of donkey anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor™ 488 (Invitrogen; A-21202) for 30 minutes at room temperature. After staining, the cells were washed with PBS−/− three times before flow cytometry analysis. UltraComp eBeads™ compensation beads (Fisher Scientific; 01–2222-41) stained with the same secondary antibody were used as a positive control for analysis. The assessment was performed using the Cytek Aurora spectral flow cytometer (Cytek® Biosciences) and analyzed with FlowJo 10.9.0 (Becton Dickinson & Company).
Measurement of cell viability
The viability of Exp-HLOs was analyzed by using the CellTiter-Glo® 3D cell viability assay kit (Promega; G9681) following the manufacturer’s recommended protocol. Briefly, the pillar plate with HLOs was sandwiched with an opaque white 384-well plate containing a mixture of 30 µL of the CellTiter-Glo® reagent and 10 µL of the cell culture medium in each well to measure cellular adenosine triphosphate (ATP) levels. To induce cell lysis, the sandwiched pillar/well plates were placed on an orbital shaker for 1 hour at room temperature. After cell lysis, the pillar plate was detached, and the lysis solution in the opaque white 384-well plate was left for 15 minutes at room temperature for stabilization. The luminescence signals were recorded using a microtiter well plate reader (BioTek® Cytation 5).
The viability of Exp-HLOs in a 24-well plate was also analyzed by using the Cell Counting Kit-8 (Dojindo; CK04). Briefly, the Exp-HLOs in the 24-well plate were treated with the CCK-8 solution diluted 10-fold in the EM without Rspo1 by mixing 30 μL of the CCK-8 solution with 270 μL of the EM without Rspo1 (EM-Rspo1) in each well. After 2.5 hours of treatment in the CO2 incubator, 50 μL of the supernatant was dispensed in triplicate in the opaque white 384-well plate. Subsequently, 500 μL of the fresh EM-Rspo1 was dispensed in the 24-well plate to culture the organoids continuously. The supernatant was left for 10 minutes at room temperature for stabilization, and the absorbance was measured at 450 nm by using the BioTek® Cytation 5 plate reader.
Measurement of CYP3A4 expression
The expression level of CYP3A4 was analyzed by using the P450-Glo™ CYP3A4 assay kit (Promega; V9001) and following the manufacturer’s recommended protocol. Rifampicin (Sigma; R3501) was used as an inducer of the CYP3A4 gene at the concentration of 25 µM. Briefly, on day 20, Diff-HLOs on the pillar plate were treated with rifampicin for 3 days with daily medium changes. After treatment, HLOs were incubated with luciferin IPA-substrate at a final concentration of 3 µM diluted in advanced DMEM/F12 for overnight at 37°C in the CO2 incubator. Following the overnight incubation, 25 µL of the culture medium from each well of the 384DeepWellPlate was transferred to the opaque white 384-well plate at room temperature, and 25 µL of luciferin detection reagent was added in each well to initiate a luminescent reaction. After 20 minutes of incubation at room temperature, luminescence was measured by using the BioTek® Cytation 5 plate reader.
Measurement of bile acid transport with cholyl-lysyl-fluorescein (CLF)
To analyze the function of bile acid transport in HLOs, the Diff-HLOs were washed with dPBS−/− and incubated in the DM containing 5 µM cholyl-lysyl-fluorescein (Corning; 451041). After overnight treatment at 37°C in the CO2 incubator, the Diff-HLOs were rinsed with dPBS−/− thrice and replaced with the DM without CLF. The Diff-HLOs were then imaged by using an automated fluorescence microscope (Keyence; BZ-X800E) equipped with a 20x objective lens. CLF is a fluorescein-labeled bile acid that can be transported into bile canaliculi by bile salt efflux pump (BSEP).
Testing model compounds with Diff-HLOs on the pillar plate
Day 20 Diff-HLOs on the pillar plate were exposed to varying concentrations of two hepatotoxic drugs, including acetaminophen (Sigma; A5000) and troglitazone (Sigma; T2573). The highest dosage tested was 10,000 μM for acetaminophen and 500 μM for troglitazone. Briefly, 4-fold serial dilutions of the highest dose of the drugs were performed in DMSO in a 384-well plate. Five dosages and one solvent-alone control (DMSO control) were prepared for each drug. The drug stock solutions in DMSO in the 384-well plate were 200-fold diluted with the DM and then dispensed in the 144PerfusionPlate (duplicates per dose) so that the final DMSO content is equal to 0.5% (v/v). The pillar plate with day 20 Diff-HLOs was then sandwiched with the 144PerfusionPlate containing the serially diluted drugs and incubated in the 5% CO2 incubator at 37°C for 3 days. The viability of HLOs was assessed with the CellTiter-Glo® 3D cell viability assay kit (Promega), and luminescence was measured by using the BioTek® Cytation 5 plate reader. Dose-response curves were generated using the luminescence values at varying dosages. To assess the recovery of the Diff-HLOs after drug treatment, the pillar plate with day 20 Diff-HLOs, exposed to the concentration of the drugs near to their IC50 value, were sandwiched with the 144PerfusionPlate containing the EM and incubated in the 5% CO2 incubator at 37°C for 10 days.
Calculation of the IC50 value
Since the background luminescence of completely dead cells (following treatment with 70% methanol for 1 hour) was negligible due to background subtraction, the percentage of live Diff-HLOs was calculated using the following equation:
where is the luminescence intensity of Diff-HLOs exposed to the drugs and is the luminescence intensity of fully viable Diff-HLOs (control).
To generate a conventional sigmoidal dose-response curve with response values normalized to span the range from 0% to 100% plotted against the logarithm of test concentration, we normalized the luminescence intensities of all Diff-HLO spots with the luminescence intensity of a 100% live Diff-HLO spot (Diff-HLOs incubated with no compound). We then converted the test drug concentrations to their respective logarithms using GraphPad Prism 9.3.1 (GraphPad Software, Inc., CA, USA). The sigmoidal dose-response curve (variable slope) and IC50 value (i.e., the concentration of drug where 50% of Diff-HLO viability is inhibited) were obtained using the following equation:
where is the midpoint of the curve, is the hill slope, is the logarithm of test concentration, and is the response (% live cells), starting from the top plateau (Top) of the sigmoidal curve to the bottom plateau (Bottom).
Statistical analysis
The statistical analysis was performed using GraphPad Prism 9.3.1. All the data were expressed as mean ± SD, with sample sizes specified in the figure legends where ‘n’ represents biological replicates. Student’s t-test was used for comparison between two groups, whereas one-way ANOVA was used for comparison among multiple groups. The statistically significant difference between the control and test groups was indicated by *** for p < 0.001, ** for p < 0.01, * for p < 0.05, and ns = not significant (p > 0.05).
Results
Generation of passage-able Exp-HLOs
iPSCs were differentiated into day 15 early hepatic progenitor cells using retinoic acid (RA) by following the Ouchi protocol13. Since the early hepatic progenitor cells express LGR5 adult stem cell marker and EPCAM ductal epithelial marker, they were cultured in the expansion medium (EM) to form Exp-HLOs (Figure 1). The EM supports the expansion of bi-potent ductal cells by cAMP activation (using Forskolin), TGF-β inhibition (A8301), and Wnt signaling (R-spondin1 or Rspo1). In the first attempt for the generation of Exp-HLOs from day 15 hepatic progenitor cells, we encountered several issues ranging from slow formation to degradation of Exp-HLOs while passaging. Thus, we first optimized the EM to promote the expansion of LGR5+ stem cells using Noggin22,23 and Wnt signaling throughout the expansion (Supplementary Figure 2). In addition, R-spondin1 purchased from R&D Systems (Rspo1_R&D) in the EM was replaced with home-made R-spondin1 conditioned medium (Rspo1_HM) to evaluate the efficacy of Rspo1_HM. Furthermore, based on precedent works for critical growth factors necessary for HLO passage, we cultured Exp-HLOs in the EM without R-spo1 and EGF (EM – Rspo1 – EGF). As anticipated, Exp-HLOs were successfully generated in the EM containing either Rspo1_HM or Rspo1_R&D. Interestingly, the EM – Rspo1 – EGF was useful for the robust passage of Exp-HLOs. Thus, we compared the gene expression level of LGR5 adult stem cell marker and AFP hepatic progenitor marker in Exp-HLOs cultured in the EM with Rspo1 and without Rspo1 to better understand the role of Rspo1 (Supplementary Figure 3). Since Rspo1 is known to support the proliferation of adult stem cells by activating Wnt/β-catenin signaling24, Exp-HLOs cultured in the EM with Rspo1 showed higher level of LGR5 and AFP expression. With this result, the passage of Exp-HLOs were performed by mechanical dissociation using a pipette (Supplementary Figure 4) and enzymatic dissociation using TrypLE™ enzyme (Figure 1C). Since mechanical dissociation resulted in more variation in Exp-HLO size and fewer number of Exp-HLOs, we decided to use enzymatic dissociation for passage and cryopreservation. The Exp-HLOs were successfully passaged up to 10 passage numbers by enzymatic dissociation and demonstrated uniform morphology throughout the passages (Figure 1C). The Exp-HLOs showed bi-potential ductal epithelial in nature. The expression level of LGR5 adult stem cell marker, EPCAM ductal epithelial marker, SOX9 ductal marker, and AFP hepatic progenitor marker was maintained throughout the passages with few fluctuations, which were acceptable within experimental error ranges (Figure 1D). In addition, the EpCAM+ cell population was consistent within the range of 93 – 98% at different passage numbers (Figure 1E). The immunofluorescence staining of Exp-HLOs showed the expression of CK18 hepatocyte marker, EpCAM ductal epithelial marker, Ecad epithelial marker, along with no expression of ALB albumin, a functional biomarker of hepatocytes (Figure 1F). The uniformity of Exp-HLOs can be crucial to alleviate the issue of batch-to-batch variation in organoid generation, which was assessed both genotypically and phenotypically (Figure 1). Furthermore, the Exp-HLOs were cryopreserved and reseeded successfully to examine the feasibility of long-term storage (Supplementary Figure 5A). The morphology of Exp-HLOs did not change before and after cryopreservation. In addition, the gene expression level of critical biomarkers including LGR5, EPCAM, and SOX9 was maintained with some fluctuation potentially due to cryopreservation (Supplementary Figure 5B). The cryopreserved Exp-HLOs proliferated after 6 days of the lag phase for stabilization, which was measured by the Cell Counting Kit 8 (Supplementary Figure 5C).
Figure 1. Generation of expandable human liver organoids (Exp-HLOs):
(A) The differentiation protocol of iPSCs into Exp-HLOs and Diff-HLOs. (B) Bright-field image of whole Matrigel dome containing Exp-HLOs in a 24-well plate. (C) Morphology of Exp-HLOs at different passage numbers. Scale bars: 200 µm. (D) Changes in hepatic gene expression in Exp-HLOs at different passage numbers, including LGR5 adult stem cell marker, EPCAM ductal epithelial marker, SOX9 cholangiocyte marker, and AFP hepatic progenitor marker. n = 4. One-way ANOVA was performed for statistical analysis. (E) The percentage of subpopulation expressing EpCAM ductal epithelial marker in Exp-HLOs at different passage numbers obtained by flow cytometry analysis. (F) Immunofluorescence staining of Exp-HLOs showing the expression of CK18 hepatocyte marker, EpCAM ductal epithelial marker, Ecad epithelial marker, and ALB albumin marker. Scale bars: 50 µm, 100 µm, and 200 µm.
Differentiation of Exp-HLOs into Diff-HLOs
The Exp-HLOs were differentiated into Diff-HLOs using a differentiation medium (DM) containing notch inhibitor DAPT, HGF, and BMP7, as well as dexamethasone (Figure 2A). The Diff-HLOs were smaller and denser than Exp-HLOs and had canaliculi-like structure at the center (Figures 2B and 2C). The expression level of stem cell and hepatic genes, including LGR5 adult stem cell marker, ALB albumin marker, ASGR1 hepatocyte marker, EPCAM ductal epithelial marker, and AFP hepatic progenitor marker, in (I) iPSCs, (II) day 15 early hepatic progenitor cells, (III) Exp-HLOs at P0, and (IV) day 10 Diff-HLOs at P1 were measured by qPCR analysis (Figure 2D). The expression level of LGR5 and EPCAM genes was high in early hepatic progenitor cells and Exp-HLOs and decreased in Diff-HLOs. On the other hand, the expression level of ALB, ASGR1, and AFP genes was high in early hepatic progenitor cells and Diff-HLOs, indicating the enhancement of hepatic functions after differentiation. The immunofluorescence staining of day 10 Diff-HLOs showed the expression of ALB albumin marker, VM stellate cell marker, Ecad epithelial marker, and CK18 hepatocyte marker (Figure 2E). In addition, the Diff-HLOs demonstrated the capability of accumulating the fluorescently labeled bile acid in the lumen structure, indicating the function of BESP (Figure 2F). Furthermore, the Diff-HLOs showed the higher activity of CYP3A4 as compared to that of Exp-HLOs (Figure 2G). Finally, the expression level of hepatic genes, including ALB albumin marker, ASGR1 hepatocyte marker, SOX9 cholangiocyte marker, CD68 Kupffer cell marker, AFP hepatic progenitor marker, and drug metabolizing enzymes such as CYP3A4, UGT1A1, and SULT2A1, in (I) day 25 HLOs obtained by using Ouchi et al. protocol13 and (II) day 10 Diff-HLOs was compared (Figure 2H). Interestingly, the HLOs generated by the two protocols were comparable, with a marginal increase in the expression level of drug metabolizing enzymes such as CYP3A4 and UGT1A1 from our protocol.
Figure 2. Generation of differentiated human liver organoids (Diff-HLOs):
(A) The differentiation protocol of Exp-HLOs into Diff-HLOs by using the DM for 10 days. (B) Bright-field image of day 10 Diff-HLOs in Matrigel dome in a 24-well plate. Scale bar: 500 µm. (C) Representative morphology of day 10 Diff-HLOs. Scale bars: 200 µm. (D) Changes in hepatic gene expression in (I) iPSCs, (II) day 15 early hepatic progenitor cells, (III) Exp-HLOs at P0 and (IV) day 10 Diff-HLOs at P1, including LGR5 adult stem cell marker, ALB albumin marker, ASGR1 hepatocyte marker, EPCAM ductal epithelial marker, and AFP hepatic progenitor marker. n = 5. The significance of gene expression difference among the samples was analyzed using one-way ANOVA. (E) Immunofluorescence staining of day 10 Diff-HLOs showing the expression of ALB albumin marker, VM stellate cell marker, Ecad epithelial marker, and CK18 hepatocyte marker. Scale bars: 50 µm and 200 µm. (F) Uptake of fluorescein-labeled bile acid, cholyl-lysyl-fluorescein (CLF). Scale bars: 200 µm. (G) CYP3A4 activity in (I) Exp-HLOs and (II) day 10 Diff-HLOs measured by P450-Glo™ CYP3A4 luminescence assay kit. n = 9. The significance of CYP3A4 activity difference was analyzed by Student’s t test. (H) Comparison of hepatic gene expression in (I) day 25 HLOs obtained by using Ouchi et al. protocol and (II) day 10 Diff-HLOs, including ALB albumin marker, ASGR1 hepatocyte marker, SOX9 cholangiocyte marker, CD68 Kupffer cell marker, AFP hepatic progenitor marker, and drug metabolizing enzymes such as CYP3A4, UGT1A1, and SULT2A1. n = 4. The significance of gene expression difference was analyzed by Student’s t test.
To further improve the maturity of Diff-HLOs, the differentiation medium (DM) was optimized in the presence and absence of EGF and recombinant human oncostatin M (OSM; Peprotech; 300–10) (Figure 3A). Interestingly, the DM without EGF and with 20 ng/mL OSM (DM – EGF + OSM) further enhanced the maturity of Diff-HLOs as indicated by the considerably enhanced expression of hepatic biomarkers including ALB albumin, ASGR1 hepatocytes, and AFP hepatic progenitor cells (Figure 3B). It has been known that OSM induces hepatic maturation25,26, and thus has been supplemented in HLO maturation media previously. Similarly, EGF/EGF receptor (EGFR) is known to induce Notch1 that suppress hepatocyte commitment27.
Figure 3. Optimization of the differentiation medium (DM) of Diff-HLOs.
(A) Representative images of day 10 Diff-HLOs cultured in (I) the DM, (II) the DM without EGF, and (III) the DM without EGF and with OSM. Scale bars: 200 μm. (B) Comparison of hepatic gene expression in Diff-HLOs cultured in the three DM conditions, including ALB albumin marker, ASGR1 hepatocyte marker, SOX9 cholangiocyte marker, and AFP hepatic progenitor marker. n = 4. The significance of gene expression difference among the samples was analyzed using one-way ANOVA.
Dynamic culture of Diff-HLOs in the pillar/perfusion plate
To further enhance the maturity of HLOs, we adopted the engineering approach and dynamically cultured Diff-HLOs in the pillar/perfusion plate (Figures 4A and 4B). We hypothesized that organoid maturity could be enhanced when cultured in a fluidic system due to higher diffusion of nutrients and oxygen into the core of organoids and fluidic shear stress. To this end, the 144PillarPlate with a 12 × 12 array of pillars was introduced to loading Exp-HLOs in Matrigel on the pillars (Figure 4A). In addition, the complementary 144PerfusionPlate with a 12 × 12 array of perfusion wells and a 2 × 12 array of reservoirs was introduced to generate bi-directional flow of the differentiation medium under the pillars and culture organoids in a dynamic condition (Figure 4B). By sandwiching the 144PillarPlate with Exp-HLOs onto the 144PerfusionPlate, Diff-HLOs can be generated in 12 different fluidic channels, each channel consisting of one upper reservoir (UR), one lower reservoir (LR), and a row of 12 perfusion wells connected by microchannels (Figure 4C). Gravity-driven, fluid flow in forward and backward directions was generated in the 144PerfusionPlate coupled with the 144PillarPlate on a digital rocker (Supplementary Figure 6), which was simulated with SolidWorks at 1300 μL cell culture medium per fluidic channel, 10° tilting angle, and 1 minute of tilting angle change (Figure 4D). As a result, the maximum volumetric flow rates were 10 μL/seconds while the negative flow rates indicate the backward direction of flow. The volumetric flow rates in the pillar/perfusion plate are 100 – 1,000-fold faster than those obtained from conventional microfluidic devices and better mimic blood flow rates in large capillaries20. In addition, the average shear stress on the surface of the pillars by di-directional fluid flow simulated by SolidWorks was in the range of 0.2 – 0.5 dyne/cm2 (Figure 4E). The flow rate and shear stress can be controlled mainly by changing the tilting angle as demonstrated in our previous study20.
To demonstrate dynamic culture of Diff-HLOs in the pillar/perfusion plate, Exp-HLOs generated in Matrigel dome in the 24-well plate were enzymatically dissociated, isolated by centrifugation, resuspended in fresh Matrigel, and then printed on the 144PillarPlate by using the 3D bioprinter. The enzymatically dissociated cell clumps suspended on Matrigel was uniformly printed on the 144PillarPlate with the CV value of 10% (Supplementary Figure 7A). The 144PillarPlate with cell clumps was sandwiched onto the 144PerfusionPlate containing the EM supplemented with BMP7 and cultured for 6 days to form Exp-HLOs (Supplementary Figure 7B). The Exp-HLOs on the pillar plate were further cultured in the DM supplemented with OSM for up to 20 days in a dynamic condition in the perfusion plate (Supplementary Figure 7C). The Diff-HLOs became denser in morphology with the time of culture, and the expression level of critical hepatic biomarkers, including ALB, ASGR1, SOX9, CD68, AFP, CYP3A4, UGT1A1, and SULT2A1, was significantly enhanced over time (Supplementary Figure 7D). This is a significant advancement as iPSC-derived HLOs generated by Ouchi et al. protocol13 gradually lose their hepatic functions after maturation.
As compared to day 20 Diff-HLOs statically cultured in the 144PillarPlate/384DeepWellPlate, day 20 Diff-HLOs dynamically cultured in the 144PillarPlate/144PerfusionPlate showed denser cell morphology (Figures 5A and 5B). In addition, the expression level of critical hepatic biomarkers, including ALB, ASGR1, SOX9, VM, CD68, AFP, CYP3A4, UGT1A1, and SULT2A1, was significantly higher in dynamically cultured Diff-HLOs as compared to their static counterpart, illustrating the urgent need for high-throughput, dynamic organoid culture (Figure 5C). The immunofluorescence staining of day 20 Diff-HLOs cultured in static and dynamic conditions showed the expression of ALB albumin marker, CK18 hepatocyte marker, and Ecad epithelial marker (Figures 5D and 5E). The activity of one of the most representative drug metabolizing enzymes such as CYP3A4 in day 20 Diff-HLOs cultured in static and dynamic conditions was measured by using P450-Glo™ CYP3A4 luminescence assay kit (Figure 5F). As anticipated, CYP3A4 activity in dynamically cultured day 20 Diff-HLOs was significantly higher than that of their static counterpart. Interestingly, CYP3A4 activity in day 20 Diff-HLOs cultured in static and dynamic conditions was much higher than that of day 10 Diff-HLOs cultured in Matrigel dome (Figures 2G and 5F). This result indicates that the time of culture is critically important to enhance the maturity of Diff-HLOs, in addition to the dynamic culture.
Figure 5. Static and dynamic culture of Diff-HLOs on the pillar plate.
(A) Stitched (left) and representative (right) images of day 20 Diff-HLOs cultured in a static condition using the 144PillarPlate coupled with the 384DeepWellPlate. (B) Stitched (left) and representative (right) images of day 20 Diff-HLOs cultured in a dynamic condition using the 144PillarPlate coupled with the 144PerfusionPlate. (C) Comparison of hepatic gene expression in day 20 Diff-HLOs cultured in (I) static and (II) dynamic conditions, including ALB, ASGR1, SOX9, VM, CD68, CYP3A4, UGT1A1, SULT2A1, and AFP. n = 72. The significance of gene expression difference was analyzed by Student’s t test. (D) Immunofluorescence staining of day 20 Diff-HLOs cultured in a static condition. Scale bars: 50 µm. (E) Immunofluorescence staining of day 20 Diff-HLOs cultured in a dynamic condition. Scale bars: 50 µm and 200 µm. (F) CYP3A4 activity in day 20 Diff-HLOs cultured in (I) static and (II) dynamic conditions measured by P450-Glo™ CYP3A4 luminescence assay kit. n = 10. The significance of CYP3A4 activity difference was analyzed by Student’s t test.
Assessment of drug-induced liver injury (DILI) by using Diff-HLOs in the pillar/perfusion plate and the recovery of the liver from DILI
The 144PillarPlate with 144 replicates of Diff-HLOs was sandwiched onto the 144PerfusionPlate containing six different concentrations of a compound to obtain a dose-response curve with 24 replicates of Diff-HLOs per concentration (Figure 6A). Acetaminophen and troglitazone were used as model compounds for drug-induced liver injury (DILI) at the concentration range of 0 – 10,000 μM for acetaminophen and 0 – 500 μM for troglitazone (Figures 6B and 6C). After 3 days of dynamic compound treatment, the IC50 value obtained for acetaminophen was 4,060 μM whereas the IC50 value of troglitazone was 164.6 μM. Acetaminophen is a widely used over-the-counter (OTC) analgesic and antipyretic medication for relieving mild-to-moderate pain and fever. However, it can cause acute liver injury and death at high doses28. From recent studies, the IC50 value of acetaminophen was reported to be 4,286 µM with primary human hepatocytes (PHH) and 4,036 µM with tumor-derived hepatic cell line HepaRG29. Similarly, troglitazone was approved for type 2 diabetes, but already withdrawn from the market due to the cases of acute liver failure30. From recent studies, the IC50 value was reported to be 57.09 µM in PHH and 45.45 µM in HepaRG cells29. Thus, our results are comparable to those obtained from PHH and HepaRG cells.
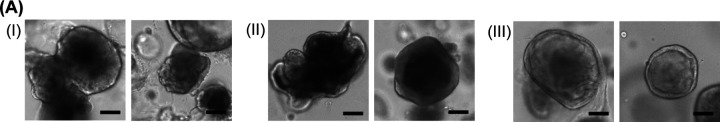
Figure 6. Assessment of hepatotoxicity with model compounds and liver recovery after compound treatment using day 20 Diff-HLOs in the pillar/perfusion plate:
(A) Schematic of compound dosage in the 144PerfusionPlate. (B) Representative images of Diff-HLOs after acetaminophen treatment for 3 days at the concentration range of 0 – 10,000 μM and its dose response curve. (C) Representative images of Diff-HLOs after troglitazone treatment for 3 days at the concentration range of 0 – 500 μM and its dose response curve. (D) Representative images of Diff-HLOs after 3 days of treatment with 5,000 μM acetaminophen (top panel) and after 10 days of recovery in the EM (middle and bottom panels). Scale bars: 200 μm. (E) Changes in inflammatory gene expression in (I) Diff-HLOs (control), (II) Diff-HLOs after 3 days of treatment with 5,000 μM acetaminophen, and Diff-HLOs after 10 days of recovery in the EM, including IL1, IL4, IL6, TNFA, IL10, TGFB, and HMOX1. n = 12. The significance of gene expression difference among the samples was analyzed using one-way ANOVA. (F) Representative images of Diff-HLOs after 3 days of treatment with 125 μM troglitazone (top panel) and after 10 days of recovery in the EM (middle and bottom panels). Scale bars: 200 μm. (G) Changes in inflammatory gene expression in (I) Diff-HLOs (control), (II) Diff-HLOs after 3 days of treatment with 125 μM troglitazone, and Diff-HLOs after 10 days of recovery in the EM, including IL1, IL4, IL6, TNFA, IL10, TGFB, and HMOX1. n = 12. The significance was analyzed by using one-way ANOVA.
In addition to the assessment of DILI of model compounds, we evaluated the recovery of the liver from DILI using Diff-HLOs in the pillar/perfusion plate, which has been conventionally performed with animal models. Briefly, Diff-HLOs were dynamically treated for 3 days at a concentration of their IC50 value (e.g., 5,000 μM for acetaminophen and 125 μM for troglitazone) to induce severe liver organoid damage (Figures 6D and 6F). This was followed by dynamic culture of damaged Diff-HLOs in the EM for 10 days to regenerate HLOs. The EM induces the expansion of hepatic progenitor cells due to the presence of Rspo1, resulting in the regeneration of Diff-HLOs. After 10 days of recovery, we observed the expansion of Diff-HLOs, indicated by the appearance of circular organoids, which are Exp-HLOs (Figures 6D and 6F). Furthermore, epithelial cells are known to produce pro-inflammatory cytokines to initiate the acute sterile inflammatory response, resulting from damage-associated molecular patterns (DAMPs) recognized by pattern-recognition receptors (PRRs)31,32. Interestingly, pro-inflammatory mediators such as IL1, IL4, IL6, and TNFA as well as HMOX1 cellular stress marker were strongly expressed in Diff-HLOs after acetaminophen treatment (Figure 6E). In addition, the expression levels of anti-inflammatory mediators such as IL10 and TGFB were also enhanced, which are responsible to suppress inflammation, induce tissue remodeling, and facilitate to tissue homeostasis33. A similar trend of pro- and anti-inflammatory marker expression, except for IL6 and IL10, was observed for Diff-HLOs exposed to troglitazone (Figure 6G). Troglitazone, a thiazolidinedione drug, selectively binds to PPAR-γ and activates PPAR-γ, leading to the suppression of pro-inflammatory pathways, including IL6 production34. Since liver regeneration after DILI can be dose-dependent and significantly impaired beyond a certain dosage, the assessment of Diff-HLO recovery after compound treatment could provide a valuable insight into in vivo liver damage and regeneration while estimating a safe dosage of drugs for patient treatment35. In addition, Diff-HLOs could be useful for investigating inflammatory responses post DILI.
Discussion
In 2015, Huch et al. for the first time generated functional liver organoids by the expansion and differentiation of LGR5+ adult bile duct-derived bipotent progenitor cells obtained from human liver biopsy samples7. Since then, several protocols have been published to demonstrate the expansion and differentiation of functional liver organoids either by using human embryonic stem cells (ESCs)36,37 or induced pluripotent stem cells (iPSCs)36,38,39. However, the precedent studies mainly focused on the hepatocyte functions, including albumin secretion and the expression of drug metabolizing enzymes, while a few studies demonstrated the expression of cholangiocyte markers, indicating the presence of cholangiocyte-like cells in mature HLOs. None of the early studies focused on the cells of mesenchymal origin present in the liver, including Kupffer cells and stellate cells, along with endodermal hepatocytes and cholangiocytes necessary for modeling complex diseases such as fibrosis and alcoholic liver disease (ALD). One of the studies performed the co-culture of expandable hepatic organoids with human fetal liver mesenchymal cells isolated from fetal liver tissues and demonstrated the improvement in hepatic functions, including albumin secretion, urea production, and CYP3A4 activity37. In 2019, Ouchi et al. demonstrated the generation of multicellular HLOs composed of hepatocyte-, cholangiocyte-, stellate-, and Kupffer-like cells by the differentiation of patient-derived iPSCs13. Although the Ouchi protocol is robust and allows cryopreservation of foregut cells, it still requires 3 week-long differentiation and maturation steps, which may lead to batch-to-batch variation. In addition, mature HLOs gradually lose their hepatic functions over time, which could be a critical issue for high-throughput screening (HTS) of hepatotoxic compounds. For scale-up production of HLOs for compound screening, HLOs cultured in Matrigel domes need to be physically or enzymatically dissociated, which is labor-intensive and could lead to the loss of HLOs, damage to HLO structure and function, and batch-to-batch variation in their size. To resolve these issues and generate passage-able HLOs, we focused on day 15 early hepatic progenitor cells expressing LGR5 from the Ouchi protocol13 and modified the differentiation protocol used in Huch et al.7 The day 15 early hepatic progenitor cells were successfully differentiated and expanded into Exp-HLOs that were genotypically and phenotypically stable throughout 10 passages (Figure 1). The Exp-HLOs were further differentiated into Diff-HLOs with enhanced hepatic functions, along with the presence of ductal features as well as the expression of cellular biomarkers from mesenchymal origin (Figure 2). Although Diff-HLOs obtained from our modified protocol showed comparatively higher expression level of drug metabolizing enzymes such as CYP3A4 and UGT1A1 as compared to those in HLOs from Ouchi et al.13 protocol (Figure 2H), there was still a room for further improvement for HLO maturity and function. Among the few known compounds, the addition of OSM along with the removal of EGF in the DM improved the expression level of critical hepatic genes including ALB, ASGR1, and AFP in Diff-HLOs (Figure 3B).
One of the engineering approaches for improving the maturity of organoids is the incorporation of micro-/milli-fluidic devices introducing the fluid flow in the culture40. Several precedent studies demonstrated that the shear stress due to fluid flow resulted in improved viability, vascularization, and maturity of the organoids41–48. The improvement in organoid maturity could be due to the significant role of biophysical cues such as shear stress in the organoid development49,50 and increased oxygenation and exposure to the growth and differentiation factors in convective fluid flow transport44,51. Several companies have developed the state-of-the-art microfluidic organ-on-chip platforms, including Emulate’s Liver-Chip52, Javelin’s Liver Tissue Chip53, CN-Bio’s PhysioMimix® Liver Plate54, Nortis’s ParVivo™ Microfluidic Chip55, to develop more predictive in vitro human liver models. However, these systems are inherently low throughout and expensive to operate due to the requirement for tubes, pumps, and proprietary incubator systems. In addition, some of these platforms are specifically designed to mimic a certain aspect of liver function, rather than culturing whole liver organoids.
Currently, organoid culture, compound testing, and organoid imaging and analysis have been performed in different platforms. For example, iPSCs were differentiated into mature HLOs in Matrigel domes in a 24-well plate. For high-throughput compound testing with HLOs, Matrigel domes were dissociated physically or enzymatically to harvest HLOs, leading to low throughput due to manual steps involved. Harvested HLOs suspended in cell culture media were dispensed in a high-density microtiter well plate (e.g., 384-well plate) using a liquid handling system for cell-based assays15. This is one of key bottlenecks in organoid-based compound screening. Recently, Sun Bioscience developed Gri3D® microwell array plate56 for high-throughput organoid culture and imaging for compound testing. Although it is a significant advancement in the field, this platform only supports static culture of organoids, which don’t require ECM encapsulation. To address these issues, we designed the pillar/perfusion plate platform and demonstrated rapid cell loading, organoid culture, compound testing, and organoid imaging and analysis in a single system19,20,57–59. In the present work, we developed the 144PillarPlate that allowed to print 144 replicates of Exp-HLOs rapidly and reproducibly, which was coupled with the 144PerfusionPlate to generate 144 replicates of Diff-HLOs in 10 – 20 days of differentiation. Since HLOs were generated in a dynamic condition, there was no concern for the diffusion limitation of nutrients and oxygen, leading to high maturity of HLOs as compared to their static counterpart (Figure 5). In addition, unlike conventional iPSC-derived HLOs, Diff-HLOs showed higher maturity with longer time of culture in the DM (Supplementary Figure 7). Compound testing with Diff-HLOs and organoid staining were performed in the pillar/perfusion plate in high throughput (Figure 6).
In addition to testing DILI potential of the model compounds, we demonstrated the recovery of the liver post compound treatment using Diff-HLOs in the pillar/perfusion plate. Liver regeneration post liver damage is crucial to better understand the severity of DILI and can provide insights into patient survival after overdose and repair mechanisms of the liver, potentially leading to new treatment options for patients with high vulnerability to DILI. In addition, the mechanisms of liver repair and regeneration after DILI could be different from those after partial hepatectomy, which has been mainly focused on previous studies of liver regeneration60. Traditionally, animal models such as rat and mice have been used to study dose-dependent, liver regeneration after drug treatment61–63. Recently, one study has demonstrated the capability of hepatocyte-like liver organoids to recapitulate liver regeneration after acetaminophen treatment36. Nonetheless, the liver organoids used were at an immature expandable stage, thus less sensitive to metabolism-sensitive compounds such as acetaminophen.
Conclusions
In this study, we successfully generated passage-able and cryopreserve-able Exp-HLOs with genotypic and phenotypic stability throughout 10 passages, which were differentiated into functional Diff-HLOs in 10 – 20 days of culture. In addition, the pillar plate was introduced to print and load 144 replicates of Exp-HLOs per plate, which was coupled with the perfusion plate to generate 144 replicates of Diff-HLOs in a dynamic condition. Our 3D bioprinting approach of passage-able liver organoids in the pillar/perfusion plate enabled high-throughput organoid culture and compound testing in situ in a dynamic condition and addressed several technical challenges in the organoid field, which include user unfriendliness in dynamic organoid culture, high batch-to-batch variability, low throughput in organoid imaging, low organoid maturity, and high cost in organoid-based assays. The regenerative Diff-HLOs allowed to test drug-induced liver injury (DILI) with model compounds as well as the recovery of the liver post DILI, which has been tested with animal models traditionally. Thus, we envision that human organoids in the pillar/perfusion plate could be used for translational applications including high-throughput compound screening, disease modeling, and precision medicine with cells from patients.
Supplementary Material
Acknowledgments
This study was financially supported by the National Institutes of Health (NIDDK UH3DK119982, NCATS R44TR003491, and NIEHS R43ES035653) and the Ohio Third Frontier Commission (TVSF Phase IB and Phase II).
Abbreviations:
- LGR5
leucine-rich-repeat-containing G-protein-coupled receptor 5
- Exp-HLOs
expandable human liver organoids
- EM
expansion medium
- Diff-HLOs
Differentiated human liver organoids
- DM
differentiation medium
- DILI
drug-induced liver injury
References
- 1.Gomez-Lechon M, Donato M, Castell J, Jover R. Human Hepatocytes in Primary Culture: The Choice to Investigate Drug Metabolism in Man. Curr Drug Metab. 2004;5(5):443–462. doi: 10.2174/1389200043335414 [DOI] [PubMed] [Google Scholar]
- 2.Kwon SJ, Lee DW, Shah DA, Ku B, Jeon SY, Solanki K, Ryan JD, Clark DS, Dordick JS, Lee MY. High-throughput and combinatorial gene expression on a chip for metabolism-induced toxicology screening. Nat Commun. 2014;5:3739. doi: 10.1038/ncomms4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, Downey AD, Czerwinski M, Forster J, Ribadeneira MD, Gan L-S, LeCluyse EL, Zech K, Robertson P, Koch P, Antonian L, Wagner G, Yu L, Parkinson A. Effects of Prototypical Microsomal Enzyme Inducers on Cytochrome P450 Expression in Cultured Human Hepatocytes. Drug Metabolism and Disposition. 2003;31(4):421–431. doi: 10.1124/dmd.31.4.421 [DOI] [PubMed] [Google Scholar]
- 4.Gerets HHJ, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;28(2):69–87. doi: 10.1007/s10565-011-9208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam DTUH Dan YY, Chan Y-S, Ng H-H. Emerging liver organoid platforms and technologies. Cell Regeneration. 2021;10(1):27. doi: 10.1186/s13619-021-00089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huch M, Grompe M, Haft A, Clevers H, Dorrell C, Vries RG, Boj SF, van de Wetering M, Sato T, Sasaki N, Finegold MJ, Li VSW, Hamer K, van Es JH. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–250. doi: 10.1038/nature11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huch M, Gehart H, Van Boxtel R, Hamer K, Blokzijl F, Verstegen MMA, Ellis E, Van Wenum M, Fuchs SA, De Ligt J, Van De Wetering M, Sasaki N, Boers SJ, Kemperman H, De Jonge J, Ijzermans JNM, Nieuwenhuis EES, Hoekstra R, Strom S, Vries RRG, Van Der Laan LJW, Cuppen E, Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312. doi: 10.1016/j.cell.2014.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J, van den Born M, Zou C, Quirk C, Chiriboga L, Rice CM, Ma S, Rios A, Peters PJ, de Jong YP, Clevers H. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175(6):1591–1606.e19. doi: 10.1016/j.cell.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 9.Chang M, Bogacheva MS, Lou Y-R. Challenges for the Applications of Human Pluripotent Stem Cell-Derived Liver Organoids. Front Cell Dev Biol. 2021;9. doi: 10.3389/fcell.2021.748576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, Grompe M, Keller G, Kamath BM, Ghanekar A. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(8):853–861. doi: 10.1038/nbt.3294 [DOI] [PubMed] [Google Scholar]
- 11.Sampaziotis F, Cardoso de Brito M, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WTH, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NRF, Vallier L. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33(8):845–852. doi: 10.1038/nbt.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F, Wu D, Ren Y, Huang Y, Feng B, Zhao N, Zhang T, Chen X, Chen S, Xu A. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 2019;70(6):1145–1158. doi: 10.1016/j.jhep.2018.12.028 [DOI] [PubMed] [Google Scholar]
- 13.Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, Thompson W, Karns RA, Mayhew CN, McGrath PS, McCauley HA, Zhang R-R, Lewis K, Hakozaki S, Ferguson A, Saiki N, Yoneyama Y, Takeuchi I, Mabuchi Y, Akazawa C, Yoshikawa HY, Wells JM, Takebe T. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019;30(2):374–384.e6. doi: 10.1016/j.cmet.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramli MN Bin, Lim YS, Koe CT, Demircioglu D, Tng W, Gonzales KAU, Tan CP, Szczerbinska I, Liang H, Soe EL, Lu Z, Ariyachet C, Yu KM, Koh SH, Yaw LP, Jumat NHB, Lim JSY, Wright G, Shabbir A, Dan YY, Ng HH, Chan YS. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology. 2020;159(4):1471–1486.e12. doi: 10.1053/j.gastro.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 15.Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, Koike H, Maezawa M, Zhang R-R, Dunn A, Ferguson A, Togo S, Lewis K, Thompson WL, Asai A, Takebe T. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell–Derived Organoids. Gastroenterology. 2021;160(3):831–846.e10. doi: 10.1053/j.gastro.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang R-R, Ueno Y, Zheng Y-W, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. doi: 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- 17.Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017;21(10):2661–2670. doi: 10.1016/j.celrep.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Collin de l’Hortet A, Takeishi K, Guzman-Lepe J, Morita K, Achreja A, Popovic B, Wang Y, Handa K, Mittal A, Meurs N, Zhu Z, Weinberg F, Salomon M, Fox IJ, Deng C-X, Nagrath D, Soto-Gutierrez A. Generation of Human Fatty Livers Using Custom-Engineered Induced Pluripotent Stem Cells with Modifiable SIRT1 Metabolism. Cell Metab. 2019;30(2):385–401.e9. doi: 10.1016/j.cmet.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S, Kimura M, Shrestha S, Lewis P, Lee S, Cai Y, Joshi P, Acharya P, Liu J, Yang Y, Sanchez JG, Ayyagari S, Alsberg E, Wells JM, Takebe T, Lee M. A Pillar and Perfusion Plate Platform for Robust Human Organoid Culture and Analysis. Adv Healthc Mater. September 2023. doi: 10.1002/adhm.202302502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lekkala VKR, Kang S-Y, Liu J, Shrestha S, Acharya P, Joshi P, Zolfaghar M, Lee M, Jamdagneya P, Pagnis S, Kundi A, Kabbur S, Kim UT, Yang Y, Lee M-Y. A pillar/perfusion plate enhances cell growth, reproducibility, throughput, and user friendliness in dynamic 3D cell culture. bioRxiv. January 2023:2023.02.16.528892. doi: 10.1101/2023.02.16.528892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson WL, Takebe T. Generation of multi-cellular human liver organoids from pluripotent stem cells. In: Methods in Cell Biology. Vol 159. Academic Press Inc.; 2020:47–68. doi: 10.1016/bs.mcb.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi G, Simone PD, Ain R, Soares MJ, Wolfe MW. Noggin maintains pluripotency of human embryonic stem cells grown on Matrigel. Cell Prolif. 2009;42(4):425–433. doi: 10.1111/j.1365-2184.2009.00616.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugli N, Kamileri I, Keogh A, Malinka T, Sarris ME, Talianidis I, Schaad O, Candinas D, Stroka D, Halazonetis TD. R‐spondin 1 and noggin facilitate expansion of resident stem cells from non‐damaged gallbladders. EMBO Rep. 2016;17(5):769–779. doi: 10.15252/embr.201642169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moad HE, Pioszak AA. Reconstitution of R-Spondin:LGR4:ZNRF3 Adult Stem Cell Growth Factor Signaling Complexes with Recombinant Proteins Produced in Escherichia coli. Biochemistry. 2013;52(41):7295–7304. doi: 10.1021/bi401090h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyajima A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of Oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000;11(3):177–183. doi: 10.1016/S1359-6101(00)00003-4 [DOI] [PubMed] [Google Scholar]
- 26.Kamiya A, Kinoshita T, Miyajima A. Oncostatin M and hepatocyte growth factor induce hepatic maturation via distinct signaling pathways. FEBS Lett. 2001;492(1–2):90–94. doi: 10.1016/S0014-5793(01)02140-8 [DOI] [PubMed] [Google Scholar]
- 27.Kitade M, Factor VM, Andersen JB, Tomokuni A, Kaji K, Akita H, Holczbauer A, Seo D, Marquardt JU, Conner EA, Lee S-B, Lee Y-H, Thorgeirsson SS. Specific fate decisions in adult hepatic progenitor cells driven by MET and EGFR signaling. Genes Dev. 2013;27(15):1706–1717. doi: 10.1101/gad.214601.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acetaminophen. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. https://www.ncbi.nlm.nih.gov/books/NBK548162/. [Google Scholar]
- 29.Bouwmeester MC, Tao Y, Proença S, van Steenbeek FG, Samsom R-A, Nijmeijer SM, Sinnige T, van der Laan LJW, Legler J, Schneeberger K, Kramer NI, Spee B. Drug Metabolism of Hepatocyte-like Organoids and Their Applicability in In Vitro Toxicity Testing. Molecules. 2023;28(2):621. doi: 10.3390/molecules28020621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troglitazone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. https://www.ncbi.nlm.nih.gov/books/NBK548142/. [Google Scholar]
- 31.Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18(1):40–55. doi: 10.1038/s41575-020-0342-4 [DOI] [PubMed] [Google Scholar]
- 32.Hora S, Wuestefeld T. Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives. Cells. 2023;12(17):2129. doi: 10.3390/cells12172129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian J, Jiao Y, Wang G, Liu H, Cao X, Yang H. Mechanism of TGF-β1 inhibiting Kupffer cell immune responses in cholestatic cirrhosis. Exp Ther Med. 2020;20(2):1541–1549. doi: 10.3892/etm.2020.8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luczak E, Wieczfinska J, Sokolowska M, Pniewska E, Luczynska D, Pawliczak R. Troglitazone, a PPAR-γ agonist, decreases LTC 4 concentration in mononuclear cells in patients with asthma. Pharmacological Reports. 2017;69(6):1315–1321. doi: 10.1016/j.pharep.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 35.Bhushan B, Apte U. Liver Regeneration after Acetaminophen Hepatotoxicity. Am J Pathol. 2019;189(4):719–729. doi: 10.1016/j.ajpath.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mun SJ, Ryu J-S, Lee M-O, Son YS, Oh SJ, Cho H-S, Son M-Y, Kim D-S, Kim SJ, Yoo HJ, Lee H-J, Kim J, Jung C-R, Chung K-S, Son MJ. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5):970–985. doi: 10.1016/j.jhep.2019.06.030 [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Wang X, Tan Z, Su Y, Liu J, Chang M, Yan F, Chen J, Chen T, Li C, Hu J, Wang Y. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29(12):1009–1026. doi: 10.1038/s41422-019-0242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akbari S, Sevinç GG, Ersoy N, Basak O, Kaplan K, Sevinç K, Ozel E, Sengun B, Enustun E, Ozcimen B, Bagriyanik A, Arslan N, Önder TT, Erdal E. Robust, Long-Term Culture of Endoderm-Derived Hepatic Organoids for Disease Modeling. Stem Cell Reports. 2019;13(4):627–641. doi: 10.1016/j.stemcr.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Im I, Jeon JS, Kang E-H, Lee H-A, Jo S, Kim J-W, Woo D-H, Choi YJ, Kim HJ, Han J-S, Lee B-S, Kim J-H, Kim SK, Park H-J. Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials. 2022;286(August 2021):121575. doi: 10.1016/j.biomaterials.2022.121575 [DOI] [PubMed] [Google Scholar]
- 40.Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6(5):402–420. doi: 10.1038/s41578-021-00279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Wang L, Guo Y, Zhu Y, Qin J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 2018;8(3):1677–1685. doi: 10.1039/c7ra11714k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Workman MJ, Gleeson JP, Troisi EJ, Estrada HQ, Kerns SJ, Hinojosa CD, Hamilton GA, Targan SR, Svendsen CN, Barrett RJ. Enhanced Utilization of Induced Pluripotent Stem Cell–Derived Human Intestinal Organoids Using Microengineered Chips. CMGH. 2018;5(4):669–677.e2. doi: 10.1016/j.jcmgh.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Wang H, Deng P, Chen W, Guo Y, Tao T, Qin J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip. 2018;18(23):3606–3616. doi: 10.1039/c8lc00869h [DOI] [PubMed] [Google Scholar]
- 44.Berger E, Magliaro C, Paczia N, Monzel AS, Antony P, Linster CL, Bolognin S, Ahluwalia A, Schwamborn JC. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip. 2018;18(20):3172–3183. doi: 10.1039/C8LC00206A [DOI] [PubMed] [Google Scholar]
- 45.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre J V., Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16(3):255–262. doi: 10.1038/s41592-019-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao T, Wang Y, Chen W, Li Z, Su W, Guo Y, Deng P, Qin J. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip. 2019;19(6):948–958. doi: 10.1039/C8LC01298A [DOI] [PubMed] [Google Scholar]
- 47.Cho AN, Jin Y, An Y, Kim J, Choi YS, Lee JS, Kim J, Choi WY, Koo DJ, Yu W, Chang GE, Kim DY, Jo SH, Kim J, Kim SY, Kim YG, Kim JY, Choi N, Cheong E, Kim YJ, Je HS, Kang HC, Cho SW. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun. 2021;12(1). doi: 10.1038/s41467-021-24775-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saglam-Metiner P, Devamoglu U, Filiz Y, Akbari S, Beceren G, Goker B, Yaldiz B, Yanasik S, Biray Avci C, Erdal E, Yesil-Celiktas O. Spatio-temporal dynamics enhance cellular diversity, neuronal function and further maturation of human cerebral organoids. Commun Biol. 2023;6(1). doi: 10.1038/s42003-023-04547-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137(9):1407–1420. doi: 10.1242/dev.024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vianello S, Lutolf MP. Understanding the Mechanobiology of Early Mammalian Development through Bioengineered Models. Dev Cell. 2019;48(6):751–763. doi: 10.1016/j.devcel.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 51.Sia J, Sun R, Chu J, Li S. Dynamic culture improves cell reprogramming efficiency. Biomaterials. 2016;92:36–45. doi: 10.1016/j.biomaterials.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ewart L, Apostolou A, Briggs SA, Carman C V., Chaff JT, Heng AR, Jadalannagari S, Janardhanan J, Jang K-J, Joshipura SR, Kadam MM, Kanellias M, Kujala VJ, Kulkarni G, Le CY, Lucchesi C, Manatakis D V., Maniar KK, Quinn ME, Ravan JS, Rizos AC, Sauld JFK, Sliz JD, Tien-Street W, Trinidad DR, Velez J, Wendell M, Irrechukwu O, Mahalingaiah PK, Ingber DE, Scannell JW, Levner D. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Communications Medicine. 2022;2(1):154. doi: 10.1038/s43856-022-00209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajan SAP, Sherfey J, Ohri S, Nichols L, Smith JT, Parekh P, Kadar EP, Clark F, George BT, Gregory L, Tess D, Gosset JR, Liras J, Geishecker E, Obach RS, Cirit M. A Novel Milli-fluidic Liver Tissue Chip with Continuous Recirculation for Predictive Pharmacokinetics Applications. AAPS J. 2023;25(6):102. doi: 10.1208/s12248-023-00870-x [DOI] [PubMed] [Google Scholar]
- 54.Docci L, Milani N, Ramp T, Romeo AA, Godoy P, Franyuti DO, Krähenbühl S, Gertz M, Galetin A, Parrott N, Fowler S. Exploration and application of a liver-on-a-chip device in combination with modelling and simulation for quantitative drug metabolism studies. Lab Chip. 2022;22(6):1187–1205. doi: 10.1039/D1LC01161H [DOI] [PubMed] [Google Scholar]
- 55.Chang S-Y, Voellinger JL, Van Ness KP, Chapron B, Shaffer RM, Neumann T, White CC, Kavanagh TJ, Kelly EJ, Eaton DL. Characterization of rat or human hepatocytes cultured in microphysiological systems (MPS) to identify hepatotoxicity. Toxicology in Vitro. 2017;40:170–183. doi: 10.1016/j.tiv.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 56.Iqbal A, Van Hul N, Belicova L, Corbat AA, Hankeova S, Andersson ER. Spatially segregated defects and IGF1 ‐responsiveness of hilar and peripheral biliary organoids from a model of Alagille syndrome. Liver International. 2024;44(2):541–558. doi: 10.1111/liv.15789 [DOI] [PubMed] [Google Scholar]
- 57.Acharya P, Joshi P, Shrestha S, Choi NY, Jeong S, Lee M-Y. Uniform cerebral organoid culture on a pillar plate by simple and reproducible spheroid transfer from an ultralow attachment well plate. Biofabrication. 2024;16(2):025005. doi: 10.1088/1758-5090/ad1b1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shrestha S, Lekkala VR, Acharya P, Kang S-Y, Vanga MG, Lee M-Y. Reproducible generation of human liver organoids (HLOs) on a pillar plate platform via microarray 3D bioprinting. bioRxiv. 2024:2024.03.11.584478. doi: 10.1101/2024.03.11.584478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acharya P, Shrestha S, Joshi P, Choi NY, Lekkala VR, Kang S-Y, Ni G, Lee M-Y. Dynamic culture of cerebral organoids using a pillar/perfusion plate for the assessment of developmental neurotoxicity. bioRxiv. 2024:2024.03.11.584506. doi: 10.1101/2024.03.11.584506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clemens MM, McGill MR, Apte U. Mechanisms and biomarkers of liver regeneration after drug-induced liver injury. In:; 2019:241–262. doi: 10.1016/bs.apha.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mangipudy RS, Chanda S, Mehendale HM. Tissue repair response as a function of dose in thioacetamide hepatotoxicity. Environ Health Perspect. 1995;103(3):260–267. doi: 10.1289/ehp.95103260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SPS, Apte U. Pro-Regenerative Signaling after Acetaminophen-Induced Acute Liver Injury in Mice Identified Using a Novel Incremental Dose Model. Am J Pathol. 2014;184(11):3013–3025. doi: 10.1016/j.ajpath.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bird TG, Müller M, Boulter L, Vincent DF, Ridgway RA, Lopez-Guadamillas E, Lu W-Y, Jamieson T, Govaere O, Campbell AD, Ferreira-Gonzalez S, Cole AM, Hay T, Simpson KJ, Clark W, Hedley A, Clarke M, Gentaz P, Nixon C, Bryce S, Kiourtis C, Sprangers J, Nibbs RJB, Van Rooijen N, Bartholin L, McGreal SR, Apte U, Barry ST, Iredale JP, Clarke AR, Serrano M, Roskams TA, Sansom OJ, Forbes SJ. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med. 2018;10(454). doi: 10.1126/scitranslmed.aan1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.