Abstract
Teredinibacter turnerae is a cultivable cellulolytic Gammaproeteobacterium (Cellvibrionaceae) that commonly occurs as an intracellular endosymbiont in the gills of wood-eating bivalves of the family Teredinidae (shipworms). The genome of T. turnerae encodes a broad range of enzymes that deconstruct cellulose, hemicellulose, and pectin and contribute to lignocellulose digestion in the shipworm gut. However, the mechanism by which symbiont-made enzymes are secreted by T. turnerae and subsequently transported to the site of lignocellulose digestion in the shipworm gut is incompletely understood. Here, we show that T. turnerae cultures grown on carboxymethyl cellulose (CMC) produce outer membrane vesicles (OMVs) that contain a variety of proteins identified by LC-MS/MS as carbohydrate-active enzymes with predicted activities against cellulose, hemicellulose, and pectin. Reducing sugar assays and zymography confirm that these OMVs retain cellulolytic activity, as evidenced by hydrolysis of CMC. Additionally, these OMVs were enriched with TonB-dependent receptors, which are essential to carbohydrate and iron acquisition by free-living bacteria. These observations suggest potential roles for OMVs in lignocellulose utilization by T. turnerae in the free-living state, in enzyme transport and host interaction during symbiotic association, and in commercial applications such as lignocellulosic biomass conversion.
Keywords: carbohydrate-active enzymes, CAZymes, lignocellulose degradation, OMVs, protein secretion, symbiosis, tonB-dependent receptors
Introduction
The biological degradation of lignocellulose, a primary component of wood and all plant biomass, is a critical process in the global carbon cycle and has potential applications in renewable energy and chemical production through biomass conversion (Ragauskas et al., 2014; Østby et al., 2020). Lignocellulose is a complex composite material composed primarily of microfibrils of cellulose, the most abundant organic polymer on Earth (Dahmen et al., 2019), embedded in a matrix of hemicellulose and lignin. Pectins may also be present in smaller quantities. However, due to its complexity, lignocellulose degradation requires a suite of specialized enzymes to convert its component polymers into accessible nutrients. This complexity precludes most organisms from utilizing lignocellulose and creates obstacles to bioconversion to renewable fuels or fine chemicals (Cragg et al., 2015).
One of the unique biological systems where efficient wood digestion occurs is found within shipworms, a group of marine bivalve mollusks of the family Teredinidae and the primary degraders of woody plant material in mangrove forests and in association with driftwood and marine woodfalls (Voight, 2015; Cragg et al., 2020). Shipworms harbor endosymbiotic bacteria in bacteriocytes confined to the gland of Deshayes, a tissue located within their gills (Distel et al., 1991). The genomes of these bacteria, primarily from the genus Teredinibacter, encode a wide variety of enzymes targeting lignocellulose (Distel et al., 2002; Yang et al., 2009; O’Connor et al., 2014; Altamia, Shipway, et al., 2020; Altamia et al., 2021) and express them within this gland (O’Connor et al., 2014; Sabbadin et al., 2018). The resulting bacterial enzymes are also found in the shipworm's cecum (O’Connor et al., 2014; Sabbadin et al., 2018; Altamia and Distel, 2022), a specialized organ that is the primary location of wood digestion for the shipworm and is nearly devoid of microbes (Betcher et al., 2012). This physical separation between the bacterial symbionts in the gill and wood digestion in the gut requires a transport mechanism for symbiont-made cellulolytic enzymes. Recently, a system of ducts called the ducts of Deshayes was shown to serve as an extracellular transport path for bacterial cellulases from the gills to the mouth of shipworms (Altamia and Distel, 2022). However, the mechanism by which enzymes produced by the intracellular bacteria move across multiple membranes to an external transport path is still unknown.
Bacteria use a variety of mechanisms to degrade lignocellulosic substrates in their environments. For example, polysaccharide-degrading bacteria, including the common rumen bacteria Bacteroides, Clostridia, and Fibrobacter, use the type IX secretion pathway (T9SS) to secrete large molecular-weight proteins, including multidomain carbohydrate-active enzymes (CAZymes) (Gharechahi et al., 2023). These enzymes may be released into the environment or bound to the cell envelope (McGavin et al., 1990; Cai et al., 1999; Yan and Wu, 2013). In addition to the secretion of soluble or membrane-bound enzymes, bacteria may export CAZymes by producing outer membrane vesicles (OMVs). OMVs are spherical buds that originate from the bacterial outer membrane and can contain various cellular components such as lipopolysaccharides, proteins, small molecules, and nucleic acids (Schwechheimer and Kuehn, 2015; Huang et al., 2022).
Recent studies have shown that OMVs can be highly enriched in CAZymes and retain activity capable of degrading cellulose and other plant biomass (Arntzen et al., 2017; Ichikawa et al., 2019; Salvachúa et al., 2020). The majority of research on OMVs has been done on pathogenic bacteria due to the capacity of OMVs to carry and deliver virulence factors and toxins into host cells (O’Donoghue and Krachler, 2016; Lynch and Alegado, 2017; Caruana and Walper, 2020). However, the role of OMVs in marine and other environments has been increasingly recognized, likely playing crucial roles in nutrient processing and ecological interactions (Frias et al., 2010; Biller et al., 2014, 2022; Fischer et al., 2019; Fadeev et al., 2023). Teredinibacter turnerae, unlike obligate intracellular symbionts, is also capable of free-living growth (Waterbury et al., 1983) and may directly acquire carbon from cellulose or other plant biomass in the marine environment (Naka and Haygood, 2023). Investigating the functionality of OMVs in T. turnerae could be pivotal to understanding their complex symbiotic interaction and the ecological impact of their dual lifestyle.
Here, we purify and characterize the protein composition of OMVs produced by T. turnerae during growth on water-soluble carboxymethyl cellulose (CMC). Proteins putatively involved in interactions with lignocellulose were identified by LC-MS/MS analysis and comparison to the Carbohydrate Active Enzyme Database (CAZy). Additionally, activity assays were used to show that OMVs produced by T. turnerae are capable of cellulose hydrolysis. These observations suggest that OMVs contribute to cellulose utilization by T. turnerae and may play essential roles in their metabolism in free-living and symbiotic states.
Experimental Procedures
Strain isolation, selection, and growth conditions
T. turnerae is an endosymbiont species with widespread occurrence among shipworm species (Distel et al., 2002; Altamia et al., 2014; Altamia, Lin, et al., 2020). Two strains were selected: T. turnerae T7901 (ATCC 39867), which was previously isolated from Bankia gouldi and was the first shipworm symbiont brought into pure culture (Waterbury et al., 1983; Distel et al., 2002; Yang et al., 2009); and T. turnerae SR01903, a closely related strain of T. turnerae (Altamia et al., 2014). T. turnerae SR01903 was isolated from the gills of a single specimen of Lyrodus pedicellatus found in naturally occurring driftwood collected by hand in shallow water in the Indian River Lagoon, Merit Island, FL. (N 28.40605 W 80.66034 ) on January 24, 2020. Gills were removed by dissection and homogenized in 1.0 ml of SBM medium (Waterbury et al., 1983) in an autoclave-sterilized glass dounce homogenizer. Homogenate was streaked onto a culture plate containing 1.0% Bacto agar shipworm basal medium (SBM) at pH 8.0 supplemented with 0.2% w/v powdered cellulose (Sigmacell Type 101; Sigma-Aldrich) and 0.025% NH4Cl. Plates were incubated at 30 °C until individual colonies could be observed. An individual colony was then picked and subjected to multiple rounds of restreaking from single colonies to ensure clonality. To identify the isolate, the 16S rRNA gene was PCR amplified, sequenced, and compared to published T. turnerae genomes. The complete genome of T. turnerae SR01903 was then sequenced (SRA number SRR28421271) and submitted to Genbank (Submission number SUB14332655, Bioproject PRJNA1090931, assembly accession number pending; assembly and annotation files provided in additional files for review but not for publication). For the experiments described herein, strains were initially propagated in 6 mL cultures in shipworm basal medium (Waterbury et al., 1983) supplemented with 0.025% NH4Cl and 0.2% carboxymethyl cellulose for 4 days before being diluted 1/250 in fresh media and harvested after 2 days (OD600 0.2-0.3). All cultures were incubated in a shaker incubator at 30 °C and 100 rpm. An overview of the procedures conducted in this research is presented in Figure 1.
Figure 1.
Methods used to isolate and characterize outer membrane vesicles from T. turnerae. Diagram showing: (A) bacterial culture, (B) OMV isolation based on differential and density gradient separation, (C) OMV visualization and size by TEM and NTA, proteome analysis by LC-MS/MS, and (D) detection of cellulase activity in purified OMV preparations by zymography and reducing sugar (DNS) assay. Created with BioRender.com.
Isolation of Outer Membrane Vesicles (OMVs)
Bacterial cells were separated from the culture supernatant by centrifugation at 5,000 x g for 20 minutes at 4 °C. The supernatant was then transferred to a fresh tube, and centrifugation was repeated to remove residual bacterial cells. The final supernatant was carefully collected without disturbing the remaining pellet and filtered through a 0.22 μm polyethersulfone filter as an additional precaution to remove cells. The putative OMVs were then pelleted from the filtrate by ultracentrifugation at 120,000 x g (T-647.5 rotor, Sorvall) for 90 minutes at 4 °C. For purification, the resulting pellet was resuspended in 0.1 M phosphate-buffered saline (PBS) and fractionated by bottom-up density gradient ultracentrifugation using Optiprep™ (iodixanol density gradient medium) as follows. The resuspended OMV-containing pellet was mixed with 60% iodixanol solution to a final density of 45% (w/v) and placed at the bottom of an ultracentrifuge tube. A discontinuous density gradient was generated using a syringe and G21 needle to deposit layers of 40%, 35%, 30%, 20%, and 10% iodixanol with a final layer of 0.25 mL 0.1 M PBS on top. The resulting gradient was then subjected to centrifugation for 16 hours at 150,000 x g and 4 °C (SW55 Ti rotor, Beckman-Coulter). Sample banding was visually observed at the interface of the 30% and 20% fractions (Supporting Information: Figure S1). All fractions were collected, and iodixanol was removed by passive diffusion dialysis (1,000 kDa MWCO) in exchanging buffer of 50 mM ammonium bicarbonate pH 8.3 at 4 °C. OMV samples were removed from the dialysis bag and concentrated under vacuum to 100 μL (SPD121P SpeedVac, Thermo Scientific). Particle visualization and characterization proceeded with the single 30% fraction.
Transmission electron microscopy (TEM)
Purified OMVs were diluted and absorbed onto 200 mesh formvar-treated and carbon-coated copper grids for 60 seconds. Samples were then fixed for 5 minutes in 4% glutaraldehyde in 0.1 M sodium cacodylate, and grids were stained with 1% aqueous uranyl acetate for 60 seconds and left to dry. OMVs were imaged in an FEI Tecnai T12 (Thermo Fisher) transmission electron microscope at 80 KV with an AMT bottom-mount camera.
Nanoparticle tracking analysis (NTA)
NTA was performed using the ZetaView® PMX-220 Twin (Particle Metrix) configured with 488 nm and 640 nm lasers with long wave-pass cut-off filters (500 nm and 660 nm, respectively) and a sensitive CMOS camera 640 x 480 pixels. Samples were diluted in 2 mL of 0.1 μm filtered deionized water (18 MΩ/cm) to obtain a particle concentration between 1 x 107 and 1 x 108 particles/mL. The instrument was set to a sensitivity of 80, a shutter speed of 100, and a frame rate of 30 frames per second. Each sample was measured at 11 different positions throughout the sample cell, with 1 cycle of reading at each position to have a minimum of 1,000 traces. If the number of traces was below 1,000 counts, some additional sample was flushed inside the sample cell, and the acquisition was repeated. Post-acquisition parameters were set to a minimum brightness of 20, a maximum size area of 1,000 pixels, a minimum size area of 10 pixels, and tracelength of 15 frames. Automated cell quality control was checked using high-quality deionized water. Camera alignment and focus optimization were performed using polystyrene Nanosphere™ 100 nm size standard beads. Data analysis was performed with ZetaView® 8.05.14 software provided by the manufacturer. Automated reports of the particle recordings across the 11 positions were manually checked, and any outlier position was removed to calculate particle concentration and distribution.
Proteomic analysis
OMVs were lysed and denatured in a solution containing 5% sodium dodecyl sulfate (SDS), 100 mM Tris (pH=8), 20 mM chloroacetamide, and 10 mM tris (2-carboxyethyl) phosphine hydrochloride and incubated at 90 °C for 10 minutes. Proteins were aggregated and isolated using Sera-Mag™ carboxylate-modified SpeedBeads (SP3 beads) and digested in 100 mM NH4HCO3 containing Trypsin (final concentration 6 ng/μL) overnight at 37 °C and 1,200 rpm. Digested samples were loaded onto C18 tips, and peptides were separated online using an 88-minute LC gradient (Evosep LC system). MS analysis was performed on a Q-Exactive HF-ti Hybrid Orbitrap mass spectrometer (Thermo Scientific). One full high-resolution MS spectrum was acquired with a resolution of 45,000, an AGC target of 3 x 106 with a maximum ion time of 45 ms, and a scan range of 400-1,500 m/z, followed by 20 HCD MS/MS scans with a resolution of 1,5000, an AGC target of 1 x 105 with a maximum ion time of 120 ms, NCE of 27, and isolation window of 2 m/z. The resulting MS/MS spectra were searched against the strain-specific and universal contaminant protein databases using Sequest within Proteome Discoverer 1.4. Identifications were filtered to include only high-confidence peptides based on a better than 1% false discovery rate (FDR) against a decoy database. Proteins were linked to peptides, and only proteins with >2 peptide spectral matches (PSM) with two unique peptides were kept (Supporting Information: Dataset S1).
Functional characterization of OMV proteins
Identified proteins were annotated against the Clusters of Orthologous Groups (COG) of proteins database (Galperin et al., 2021), and their subcellular locations were predicted using CELLO v2.5 (http://cello.life.nctu.edu.tw/) (Yu et al., 2006) and PSORTdb v4.0 (https://db.psort.org/) (Lau et al., 2021). Enrichment and functional associations between proteins were determined by STRINGdb v11 (https://string-db.org/) (Szklarczyk et al., 2019) and ShinyGO v0.8 (http://bioinformatics.sdstate.edu/go/) (Ge et al., 2020). Señgs were selected to use all coding sequences in the T7901 genome as background and a minimum FDR stringency of 1 x 10−5. We then established which OMV proteins are predicted to exhibit carbohydrate-active properties using the annotated T7901 genome in the Carbohydrate Active Enzymes Database (CAZy, https://cazy.org).
Enzymatic activity assays
The cellulolytic activity of proteins in OMV preparations was visualized and size fractionated by denaturing polyacrylamide gel electrophoresis (SDS-PAGE, 1.5 mm thickness, 9% acrylamide, and 0.2% CMC final concentration). For zymographic analysis, 14 μg of protein sample determined by Pierce 660 assay (Thermo Scientific) using bovine serum albumin as standard were heat denatured by boiling for 3 minutes in SDS without a reducing agent to facilitate recovery of activity after refolding and added to each lane. After electrophoresis, the SDS-PAGE gel was transferred to 500 mL of refolding buffer (20% isopropanol, 0.1% Triton X-100 in 1x PBS, pH 7.4) and gently shaken for 1 hour at room temperature. The gel was then incubated in fresh 1x PBS buffer, pH 7.4, for 16 hours at room temperature before being stained with 0.1% Congo Red in 1x PBS buffer, pH 7.4 for 1 hour. The zymogram was destained with 1 M NaCl overnight to visualize regions where cellulase activity removed CMC. Additionally, the production of reducing ends was measured by the colorimetric 3,5-dinitrosalicylic acid (DNS) method (Ghose, 1987). Reactions were carried out in 96-well plates containing 50 μL 1% CMC, 30 μL 50 mM citrate buffer, and 20 μL purified OMV suspension and incubated for 4 hours at 37 °C. Next, 900 μL of DNS was added to each reaction, and reactions were heated at 99 °C for 10 minutes. Absorbance was measured at 540 nm, and sugar concentration was calculated by comparison to a glucose standard curve. The enzyme activity unit was defined as the amount of enzyme that liberated 1 μmol of reducing sugar per minute.
Results
OMV isolation and characterization
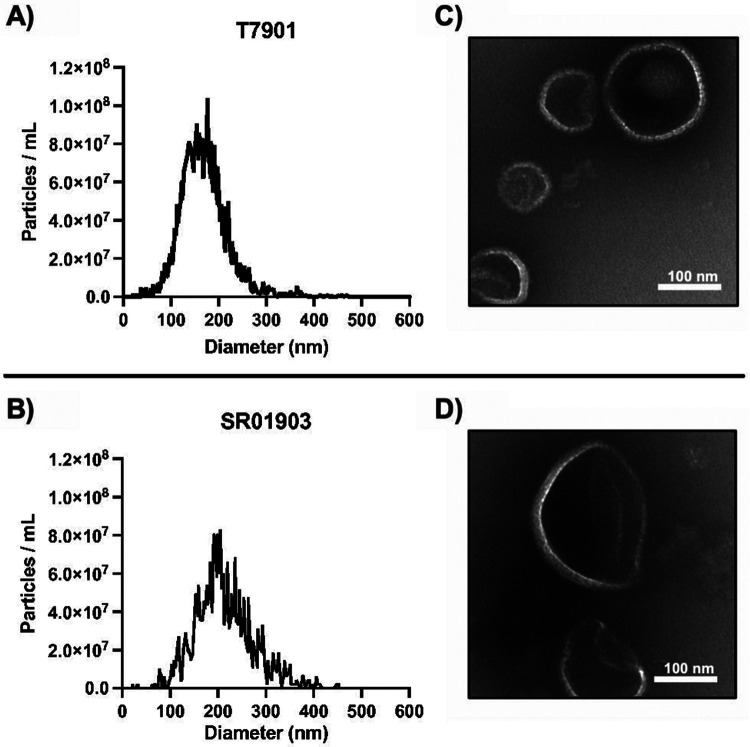
OMVs were isolated from cell-free supernatants of two closely related strains, T7901 and SR01903, by density gradient ultracentrifugation. The presence of OMVs was confirmed by NTA and TEM (Figure 2 A-D). The mean concentration of particles and average particle diameter size determined by NTA were 1.67 x 109 particles/mL and 162.5 ± 49.7 nm for T7901 and 1.30 x 109 particles/mL and 206.6 ± 61.7 nm for SR01903. TEM images (Figure 2 C-D) show that the putative OMV-enriched fractions from strains T7901 and SR01903 contain particles with size distribution and morphology typical of bacterial OMVs.
Figure 2.
Size, concentration, and visualization of OMVs isolated from T. turnerae culture supernatants after growth on carboxymethyl cellulose (CMC). (A-B) Size distribution and particle concentration for strains T7901 and SR01903, respectively. OMVs from SR01903 were slightly larger in average size than OMVs from T7901. (C-D) Isolated OMVs from strains T7901 and SR01903 were imaged using transmission electron microscopy. Scale bar = 100 nm.
OMV proteome analysis
The protein contents of OMVs from both strains were analyzed by LC-MS/MS. Peptide-generated data were used to search against T7901 and SR01903 annotated protein sequences to identify OMV proteins and their relative abundance. A cut-off of two or more unique predicted peptides per identified protein was used, resulting in the identification of 472 and 569 proteins for T7901 and SR01903, respectively. The Top 100 most abundant proteins identified in T7901 and SR01903 represented 81% and 74% of the total peptide content, respectively. Among those proteins, 71 were identified in both strains, indicating that the two strains produce a common core set of OMV proteins when grown under the examined culture condition. After protein identification, PSORTdb v4.0 (Lau et al., 2021) and CELLO v2.5 (Yu et al., 2006) were used to predict the subcellular location of all identified proteins. Outer membrane proteins comprised most (~75% and ~60%, respectively) of the OMV protein content of both T7901 and SR01903 (Figure 3 A). OMV proteins were clustered into orthologous groups (COG); 65% and 60% of OMV proteins for T7901 and SR01903 were assigned a single cluster, respectively. Most OMV proteins assigned to clusters in both strains were categorized as Cell Wall/Membrane/Envelope Biogenesis, Inorganic Ion Transport and Metabolism, and Carbohydrate Transport and Metabolism (Figure 3 B).
Figure 3.
T. turnerae OMV proteome profiles. (A) Relative protein abundance grouped by predicted subcellular location and (B) Clusters of Orthologous Groups (COG) functional categories of identified proteins. (C) Top 10 STRINGdb pathways (ranked by FDR) found in OMVs of T7901. Similar top pathways were found in OMVs of SR01903.
TonB-dependent receptors and glycosyl hydrolase activity are enriched in OMVs
To highlight functional associations, OMV protein content was analyzed using STRING (Szklarczyk et al., 2019) enrichment. We found significant enrichment of pathways involving TonB-dependent receptors (TBDR) and glycosyl hydrolase activity (Figure 4 C). The most abundant proteins were TBDRs, which constituted ~47% and ~44% of the OMV protein content in T7901 and SR01903, respectively. TBDRs are known to be deployed for the uptake of sugars from complex carbohydrates (Blanvillain et al., 2007; Pollet et al., 2021). Additionally, TonB knockouts of T. turnerae were shown to have lost the ability to grow on cellulose (Naka and Haygood, 2023), which suggests that the TonB system in T. turnerae is critical to cellulose utilization.
Figure 4.
Carbohydrate-active modules and observed cellulase activity of T. turnerae OMVs. (A) Heatmap profile of the relative abundance of the predicted carbohydrate-active enzymes found in OMVs. (B) Denaturing SDS-PAGE (left) and zymogram (right; CMC substrate) of isolated OMVs. (C) Histogram showing cellulase activity (CMC substrate) in Units/mg (as determined by DNS assay) for isolated OMVs and a purified cellulase enzyme cocktail (Cellulase R-10, Goldbio CAS# 9012-54-8). Bars indicate means (error bars: standard deviations of three replicates).
Efficient utilization of lignocellulose for growth often requires a cocktail of multiple enzymes targeting inner (endo-acting) and outer (exo-acting) polysaccharide glycosidic bonds and various bonds found in heteroxylans. In total, 39 and 48 predicted CAZymes were present, representing 30 and 29 different catalytic CAZy families and 8.3% and 8.4% of identified OMV proteins in T7901 and SR01903, respectively. Most identified CAZy modules belong to glycoside hydrolase families (GH) predicted to be involved in cellulose and hemicellulose degradation (Figure 4 A) and that include several hydrolytic activities: endo-glucanases (GH5, GH8, GH9, GH10, GH11, GH26, GH44, GH45, GH51, GH62), exo-glucanases (GH5, GH9, GH26GH43GH26, GH43), and beta-glucosidases (GH2, GH3, GH5, GH39). In addition, multiple enzymes were predicted to include non-catalytic carbohydrate-binding modules (CBMs), which can enhance enzyme activity by increasing accessibility to the substrate (Hervé et al., 2010).
Cellulase activity
Many OMV proteins were predicted to have or be associated with cellulolytic activity. We confirmed these by testing OMV suspensions for hydrolytic activity against CMC by zymography and reducing sugar DNS assay. In zymograms, clear zones indicating endoglucanase activity (Teather and Wood, 1982) were visualized between 95-130 kDa for both strains after 16-hour incubation (Figure 4 B). The production of reducing sugars from CMC was measured using DNS assay, and when normalized to total protein content, OMVs produced from SR01903 had the equivalent cellulolytic activity as a commercial soluble cellulase enzyme cocktail. Notably, OMVs produced from T7901 showed ~10x the activity of the commercial cellulase cocktail (Figure 4 C).
Discussion
T. turnerae secretes a broad array of carbohydrate-active enzymes within the host's gills and when grown in pure culture (Yang et al., 2009; O’Connor et al., 2014; Sabbadin et al., 2018). Genome sequence suggests these enzymes may be translocated across the inner membrane by Sec and Sec-independent (twin-arginine, Tat) pathways and through the outer membrane by the Type II (T2SS) generalized secretory pathway. The T. turnerae genome also encodes the complete Type VI secretion system (T6SS), which mediates many types of bacteria-bacteria and bacteria-host interactions (Yang et al., 2009). However, the role of OMVs in the transport of cellulolytic enzymes by T. turnerae has not been investigated. Here, we show that T. turnerae strains T7901 and SR01903 produce OMVs that contain diverse carbohydrate-active enzymes, including glycoside hydrolases, carbohydrate esterases, and polysaccharide lyase with predicted activity against cellulose, hemicellulose, and pectin. We further show that these OMV preparations can hydrolyze carboxymethyl cellulose (CMC) with specific activities comparable to or greater than that observed for a commercial purified cellulase enzyme cocktail (Figure 4 C).
In purified OMVs isolated from T. turnerae strains T7901 and SR01903, we detected representatives of 11 carbohydrate-binding module (CBM) families (CBM2, 5, 9, 10, 22, 35, 48, 50, 57, 85, and 91). Each of these families is known to bind cellulose or hemicellulose components, except for CBM50, which typically binds peptidoglycan or chitin. Additionally, catalytic modules representing 23 glycoside hydrolase (GH) families, four carbohydrate esterase (CE) families, and one polysaccharide lyase (PL) were detected. All of these GH families (GH2, 3, 5, 8, 9, 10, 11, 13, 23, 26, 28, 31, 35, 39, 43, 44, 45, 51, 62, 67, 94, 105, and 115) target bonds found within cellulose, hemicellulose or pectin, except GH23, which primarily acts on peptidoglycan. Finally, the four carbohydrate esterase (CE) families detected (CE1, 2, 15, and 20) target bonds found in hemicellulose, or bonds covalently linking hemicellulose to lignin. This combination of modules indicates a complete lignocellulose deconstruction system, including potential endo- and exo-activities against cellulose and hemicellulose backbones, debranching of hemicellulose sidechains, and hydrolysis of the resulting oligomers and monomers.
The fact that this wide array of activities was found in OMVs produced by cells of T. turnerae strains T7901 and SR01903 grown with cellulose as a sole carbon source indicates that the expression of genes active against other lignocellulose components was either constitutive or co-regulated with expression of genes targeting cellulose. The ability of T. turnerae to express these activities in the absence of induction by their specific target substrates is consistent with the observation that shipworm endosymbionts express a wide range of lignocellulolytic enzymes when growing within the gill bacteriocytes of the host where they have no direct contact with lignocellulose (O'Connor et al., 2014; Sabbadin et al., 2018). Notably, six of the catalytic module families detected in T. turnerae OMVs (CE11, 15, and GH9, 10, 11, and 45) were also detected in the cecum content of Bankia setacea in a previous investigation (O'Connor et al., 2014); however, it should be noted that this shipworm species hosts four Teredinibacter species, T. waterburyi (Altamia, Shipway, et al., 2020), T. franksiae, T. haidensis, and T. purpureus (Altamia et al., 2021) but does not harbor T. turnerae (O'Connor et al., 2014).
While diverse CAZymes are present in the OMV proteome of T. turnerae, they comprise less than 10% of the total protein content of OMVs produced under the conditions examined here. Interestingly, the most abundant proteins observed were identified as TonB-dependent receptors (TBDR), accounting for nearly half of the proteins detected. TBDR enrichment has been previously reported in OMVs of several Gram-negative bacteria (Veith et al., 2015; Zakharzhevskaya et al., 2017; Dhurve et al., 2022; Fadeev et al., 2023). TBDRs are outer membrane-associated proteins that bind and mediate the energy-dependent movement of siderophores and various nutrients, including carbohydrates that are too large to be taken up via transmembrane diffusion across the outer membrane (Silale and Van Den Berg, 2023). For example, in the marine bacterium Alteromonas macleodii, TBDR genes were selectively expressed in carbon- and iron-limiting conditions (Manck et al., 2020) and during polysaccharide utilization (Neumann et al., 2015). Recently, it was shown that two of T. turnerae's four TonB genes are essential for growth in iron-limiting conditions and for growth with cellulose as a sole carbon source. This dependence indicates that the TonB system is essential for both iron acquisition and cellulose catabolism by T. turnerae (Naka and Haygood, 2023).
Interestingly, in a proteomic examination of the shipworm Banka setacea, a TBDR was the only major symbiont-derived protein observed in the cecum content that was not associated with the decomposition of lignocellulose (O’Connor et al., 2014). This finding suggests the presence of symbiont outer membranes in the cecum. However, the cecum of shipworms has been shown by microscopic (Betcher et al., 2012; Pesante et al., 2021), transcriptomic (Sabbadin et al., 2018), and proteomic (O’Connor et al., 2014) methods to be nearly devoid of intact bacterial cells. These observations argue against the transport of whole bacterial cells from the gill to the cecum as suggested in (Pesante et al., 2021), but might suggest a role for OMVs in transporting lignocellulose degrading enzymes from the gill to the gut in shipworms.
Outer membrane vesicles are critical in bacterial interactions with many animal and plant hosts (Berleman and Auer, 2013; Lynch and Alegado, 2017). While most research has focused on pathogenic interactions, OMVs may also contribute to beneficial host-symbiont associations. For example, OMVs have been shown to mimic whole cells of Vibrio fisheri in the induction of specific aspects of light organ development in the Hawaiian bobtail squid Euprymna scolopes (Aschtgen et al., 2016). OMVs have also been shown to be produced by chemoautotrophic symbionts that occur in the trophosome of flatworms of the genus Paracatenula, where they play a critical role in provisioning the host with carbohydrates synthesized by the symbionts (Jäckle et al., 2019). In the human gut microbiome, members of Bacteroides have been shown to tailor OMV content to specific polysaccharides (Sartorio et al., 2023), and OMVs produced by human and ruminant gut bacteria, including Bacteroides, Fibrobacter, and Clostridium, communally degrade polysaccharides into nutrients available to the hosts (Rakoff-Nahoum et al., 2014; Arntzen et al., 2017; Gharechahi et al., 2023).
Outer membrane vesicles can also transport proteins across the plasma membranes of eukaryotic organisms (O’Donoghue and Krachler, 2016; Schorey et al., 2021; Toyofuku et al., 2023). While previous research has focused mainly on the role of OMVs in introducing bacterial molecules into eukaryotic cells, OMVs may also transport molecules produced by intracellular bacteria out of their eukaryotic host. For example, Salmonella enterica, which grows in vesicles within their host's cells, produces toxin-loaded OMVs that escape the infected host cells and deliver their toxic cargo to uninfected neighboring cells (O’Donoghue and Krachler, 2016). Similarly, OMVs produced by Mycobacterium tuberculosis are released from infected macrophages, exporting Mycobacterial lipoproteins and lipoglycans that affect the immune functions of neighboring uninfected host cells (Athman et al., 2015). Thus, OMV-mediated mechanisms could potentially explain the transport of cellulolytic proteins produced by T. turnerae in their intracellular location within the host's bacteriocytes to an extracellular location, allowing subsequent transport to the host's gut.
Packaging enzymes within OMVs or bound to OMV surfaces can have distinct advantages over their secretion as soluble proteins or proteins bound to the cell surface. For example, OMVs may protect proteins from degradation in the environment (Bonnington and Kuehn, 2014; Alves et al., 2016; Zingl et al., 2021), selectively concentrate proteins with specific functions (Orench-Rivera and Kuehn, 2021; Sartorio et al., 2023), and deliver proteins to remote locations in sufficient quantity to produce a desired effect without the dilution that would be experienced by soluble proteins (Toyofuku et al., 2023). These factors may be especially important for the degradation of complex substrates like lignocellulose, where the simultaneous delivery of specific sets of proteins in the correct proportions and spatial orientation can yield significant synergistic interactions that enhance efficiency (Park et al., 2014).
However, little is known about OMV production and its function in environmental lignocellulose degradation. Several investigations of lignocellulose-degrading microorganisms from soil suggest OMVs play critical roles in terrestrial plant biomass degradation. For example, when grown in the presence of lignin, Pseudomonas putida produces OMVs containing enzymes functionally active against lignin aromatic components (Salvachúa et al., 2020). Similarly, Trichoderma reesei, a filamentous fungus, produces OMVs containing a variety of cellulases when grown in the presence of cellulose (De Paula et al., 2019).
In addition to their potential roles in shipworm symbiosis and the environmental turnover of lignocellulose in terrestrial and aquatic environments, cellulolytic OMVs are of significant interest in converting plant biomass into renewable liquid fuels or fine chemicals (Thakur et al., 2023). A significant challenge in biomass conversion is understanding how cooperation among cellulases and associated enzymes can improve the design of efficient enzyme cocktails tailored to individual feedstocks (Østby et al., 2020). OMVs produced by T. turnerae and other lignocellulolytic organisms may represent nature-based solutions that reveal specific combinations, concentrations, and spatial organization of cooperating enzymes that significantly enhance lignocellulose degradation in nature and so might inspire the design of engineered lignocellulose deconstruction systems.
Supplementary Material
Acknowledgments
We would like to thank the PennVet Extracellular Vesicle Core SCR_022444 (Philadelphia, Pennsylvania) for work and assistance with OMV isolation and NTA analysis; the University of Maryland School of Medicine's and School of Dentistry's Electron Microscopy Core Imaging Facility - EMCIF (Baltimore, Maryland) for work and assistance with TEM imaging; and the NYU Langone's Proteomics Laboratory SCR_017926 (New York, New York) for work and assistance with mass spectrometry analysis.
Funding statement
Research reported in this publication was supported by the following awards to DLD: National Oceanic and Atmospheric Administration (NA19OAR0110303), Gordon and BeVy Moore Foundation (GBMF 9339), National Institutes of Health (1R01AI162943-01A1, subaward: 10062083-NE), and JHU/APL Internal Research and Development. The National Science Foundation (DBI 1722553) also funded some equipment used in this research.
Footnotes
Conflict of interest statement
The authors report no conflict of interest. The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication.
Data availability
The authors confirm that the data supporting this study's findings are available within the article and its supplementary materials. The mass spectrometric raw files are accessible at https://massive.ucsd.edu under accession MassIVE MSV000094250 and at https://www.proteomexchange.org under accession PXD050419.
References
- Altamia M.A. and Distel D.L. (2022) Transport of symbiont-encoded cellulases from the gill to the gut of shipworms via the enigmatic ducts of Deshayes: a 174-year mystery solved. Proc R Soc B 289: 20221478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamia M.A., Lin Z., Trindade-Silva A.E., Uy I.D., Shipway J.R., Wilke D.V., et al. (2020) Secondary Metabolism in the Gill Microbiota of Shipworms (Teredinidae) as Revealed by Comparison of Metagenomes and Nearly Complete Symbiont Genomes. mSystems 5: e00261–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamia M.A., Shipway J.R., Stein D., Betcher M.A., Fung J.M., Jospin G., et al. (2021) Teredinibacter haidensis sp. nov., Teredinibacter purpureus sp. nov. and Teredinibacter franksiae sp. nov., marine, cellulolytic endosymbiotic bacteria isolated from the gills of the wood-boring mollusc Bankia setacea (Bivalvia: Teredinidae) and emended description of the genus Teredinibacter. International Journal of Systematic and Evolutionary Microbiology 71:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamia M.A., Shipway J.R., Stein D., Betcher M.A., Fung J.M., Jospin G., et al. (2020) Teredinibacter waterburyi sp. nov., a marine, cellulolytic endosymbiotic bacterium isolated from the gills of the wood-boring mollusc Bankia setacea (Bivalvia: Teredinidae) and emended description of the genus Teredinibacter. International Journal of Systematic and Evolutionary Microbiology 70: 2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamia M.A., Wood N., Fung J.M., Dedrick S., Linton E.W., Concepcion G.P., et al. (2014) Genetic differentiation among isolates of Teredinibacter turnerae, a widely occurring intracellular endosymbiont of shipworms. Molecular Ecology 23: 1418–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves N.J., Turner K.B., Medintz I.L., and Walper S.A. (2016) Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci Rep 6: 24866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen M.Ø., Várnai A., Mackie R.I., Eijsink V.G.H., and Pope P.B. (2017) Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environmental Microbiology 19: 2701–2714. [DOI] [PubMed] [Google Scholar]
- Aschtgen M.-S., Wetzel K., Goldman W., McFall-Ngai M., and Ruby E. (2016) Vibrio fischeri - derived outer membrane vesicles trigger host development: OMV deliver signals in the squid/vibrio symbiosis. Cellular Microbiology 18: 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman J.J., Wang Y., McDonald D.J., Boom W.H., Harding C.V., and Wearsch P.A. (2015) Bacterial Membrane Vesicles Mediate the Release of Mycobacterium tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages. The Journal of Immunology 195: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleman J. and Auer M. (2013) The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environmental Microbiology 15: 347–354. [DOI] [PubMed] [Google Scholar]
- Betcher M.A., Fung J.M., Han A.W., O’Connor R., Seronay R., Concepcion G.P., et al. (2012) Microbial Distribution and Abundance in the Digestive System of Five Shipworm Species (Bivalvia: Teredinidae). PLoS ONE 7: e45309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller S.J., Coe A., Arellano A.A., Dooley K., Gong J.S., Yeager E.A., et al. (2022) Environmental and taxonomic drivers of bacterial extracellular vesicle production in marine ecosystems, Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller S.J., Schubotz F., Roggensack S.E., Thompson A.W., Summons R.E., and Chisholm S.W. (2014) Bacterial Vesicles in Marine Ecosystems. Science 343: 183–186. [DOI] [PubMed] [Google Scholar]
- Blanvillain S., Meyer D., Boulanger A., Lautier M., Guynet C., Denancé N., et al. (2007) Plant Carbohydrate Scavenging through TonB-Dependent Receptors: A Feature Shared by Phytopathogenic and Aquatic Bacteria. PLoS ONE 2: e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington K.E. and Kuehn M.J. (2014) Protein selection and export via outer membrane vesicles. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1843: 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.J., Chapman S.J., Buswell J.A., and Chang S. (1999) Production and Distribution of Endoglucanase, Cellobiohydrolase, and β-Glucosidase Components of the Cellulolytic System of Volvariella volvacea, the Edible Straw Mushroom. Appl Environ Microbiol 65: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana J.C. and Walper S.A. (2020) Bacterial Membrane Vesicles as Mediators of Microbe – Microbe and Microbe – Host Community Interactions. Front Microbiol 11: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S.M., Beckham G.T., Bruce N.C., Bugg T.D., Distel D.L., Dupree P., et al. (2015) Lignocellulose degradation mechanisms across the Tree of Life. Current Opinion in Chemical Biology 29: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S.M., Friess D.A., Gillis L.G., Trevathan-Tackett S.M., Terrett O.M., Watts J.E.M., et al. (2020) Vascular Plants Are Globally Significant Contributors to Marine Carbon Fluxes and Sinks. Annu Rev Mar Sci 12: 469–497. [DOI] [PubMed] [Google Scholar]
- Dahmen N., Lewandowski I., Zibek S., and Weidtmann A. (2019) Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 11: 107–117. [Google Scholar]
- De Paula R.G., Antoniêto A.C.C., Nogueira K.M.V., Ribeiro L.F.C., Rocha M.C., Malavazi I., et al. (2019) Extracellular vesicles carry cellulases in the industrial fungus Trichoderma reesei. Biotechnol Biofuels 12: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurve G., Madikonda A.K., Jagannadham M.V., and Siddavattam D. (2022) Outer Membrane Vesicles of Acinetobacter baumannii DS002 Are Selectively Enriched with TonB-Dependent Transporters and Play a Key Role in Iron Acquisition. Microbiol Spectr 10: e00293–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel D.L., DeLong E.F., and Waterbury J.B. (1991) Phylogenetic characterization and in situ localization of the bacterial symbiont of shipworms (Teredinidae: Bivalvia) by using 16S rRNA sequence analysis and oligodeoxynucleotide probe hybridization. Appl Environ Microbiol 57: 2376–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel D.L., Morrill W., MacLaren-Toussaint N., Franks D., and Waterbury J. (2002) Teredinibacter turnerae gen. nov., sp. nov., a dinitrogen-fixing, cellulolytic, endosymbiotic gamma-proteobacterium isolated from the gills of wood-boring molluscs (Bivalvia: Teredinidae). International Journal of Systematic and Evolutionary Microbiology 52: 2261–2269. [DOI] [PubMed] [Google Scholar]
- Fadeev E., Carpaneto Bastos C., Hennenfeind J.H., Biller S.J., Sher D., Wietz M., and Herndl G.J. (2023) Characterization of membrane vesicles in Alteromonas macleodii indicates potential roles in their copiotrophic lifestyle. microLife 4: uqac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T., Schorb M., Reintjes G., Kolovou A., Santarella-Mellwig R., Markert S., et al. (2019) Biopearling of Interconnected Outer Membrane Vesicle Chains by a Marine Flavobacterium. Appl Environ Microbiol 85: e00829–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias A., Manresa A., De Oliveira E., López-Iglesias C., and Mercade E. (2010) Membrane Vesicles: A Common Feature in the Extracellular Matter of Cold-Adapted Antarctic Bacteria. Microb Ecol 59: 476–486. [DOI] [PubMed] [Google Scholar]
- Galperin M.Y., Wolf Y.I., Makarova K.S., Vera Alvarez R., Landsman D., and Koonin E.V. (2021) COG database update: focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Research 49: D274–D281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S.X., Jung D., and Yao R. (2020) ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36: 2628–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharechahi J., Vahidi M.F., Sharifi G., Ariaeenejad S., Ding X.-Z., Han J.-L., and Salekdeh G.H. (2023) Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environmental Research 229: 115925. [DOI] [PubMed] [Google Scholar]
- Ghose T.K. (1987) Measurement of cellulase activities. Pure and Applied Chemistry 59: 257–268. [Google Scholar]
- Hervé C., Rogowski A., Blake A.W., Marcus S.E., Gilbert H.J., and Knox J.P. (2010) Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Natl Acad Sci USA 107: 15293–15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Nieh M., Chen W., and Lei Y. (2022) Outer membrane vesicles (OMVs) enabled bio-applications: A critical review. Biotechnol Bioeng 119: 34–47. [DOI] [PubMed] [Google Scholar]
- Ichikawa S., Ogawa S., Nishida A., Kobayashi Y., Kurosawa T., and Karita S. (2019) Cellulosomes localise on the surface of membrane vesicles from the cellulolytic bacterium Clostridium thermocellum. FEMS Microbiology Letters 366: fnz145. [DOI] [PubMed] [Google Scholar]
- Jäckle O., Seah B.K.B., Tietjen M., Leisch N., Liebeke M., Kleiner M., et al. (2019) Chemosynthetic symbiont with a drastically reduced genome serves as primary energy storage in the marine flatworm Paracatenula. Proc Natl Acad Sci USA 116: 8505–8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau W.Y.V., Hoad G.R., Jin V., Winsor G.L., Madyan A., Gray K.L., et al. (2021) PSORTdb 4.0: expanded and redesigned bacterial and archaeal protein subcellular localization database incorporating new secondary localizations. Nucleic Acids Research 49: D803–D808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.B. and Alegado R.A. (2017) Spheres of Hope, Packets of Doom: the Good and Bad of Outer Membrane Vesicles in Interspecies and Ecological Dynamics. J Bacteriol 199:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manck L.E., Espinoza J.L., Dupont C.L., and Barbeau K.A. (2020) Transcriptomic Study of Substrate-Specific Transport Mechanisms for Iron and Carbon in the Marine Copiotroph Alteromonas macleodii. mSystems 5: e00070–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Lam J., and Forsberg C.W. (1990) Regulation and distribution of Fibrobacter succinogenes subsp. succinogenes S85 endoglucanases. Appl Environ Microbiol 56: 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H. and Haygood M.G. (2023) The dual role of TonB genes in turnerbactin uptake and carbohydrate utilization in the shipworm symbiont Teredinibacter turnerae. Appl Environ Microbiol 89: e00744–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A.M., Balmonte J.P., Berger M., Giebel H., Arnosti C., Voget S., et al. (2015) Different utilization of alginate and other algal polysaccharides by marine A lteromonas macleodii ecotypes. Environmental Microbiology 17: 3857–3868. [DOI] [PubMed] [Google Scholar]
- O’Connor R.M., Fung J.M., Sharp K.H., Benner J.S., McClung C., Cushing S., et al. (2014) Gill bacteria enable a novel digestive strategy in a wood-feeding mollusk. Proc Natl Acad Sci USA 111: E5096–E5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue E.J. and Krachler A.M. (2016) Mechanisms of outer membrane vesicle entry into host cells: MicroReview - OMV entry into host cells. Cellular Microbiology 18: 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orench-Rivera N. and Kuehn M.J. (2021) Differential Packaging Into Outer Membrane Vesicles Upon Oxidative Stress Reveals a General Mechanism for Cargo Selectivity. Front Microbiol 12: 561863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby H., Hansen L.D., Horn S.J., Eijsink V.G.H., and Várnai A. (2020) Enzymatic processing of lignocellulosic biomass: principles, recent advances and perspectives. Journal of Industrial Microbiology and Biotechnology 47: 623–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Sun Q., Liu F., DeLisa M.P., and Chen W. (2014) Positional Assembly of Enzymes on Bacterial Outer Membrane Vesicles for Cascade Reactions. PLoS ONE 9: e97103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesante G., Sabbadin F., Elias L., Steele-King C., Shipway J.R., Dowle A.A., et al. (2021) Characterisation of the enzyme transport path between shipworms and their bacterial symbionts. BMC Biol 19: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet R.M., Martin L.M., and Koropatkin N.M. (2021) TonB-dependent transporters in the Bacteroidetes: Unique domain structures and potential functions. Molecular Microbiology 115: 490–501. [DOI] [PubMed] [Google Scholar]
- Ragauskas A.J., Beckham G.T., Biddy M.J., Chandra R., Chen F., Davis M.F., et al. (2014) Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 344: 1246843. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Coyne M.J., and Comstock L.E. (2014) An Ecological Network of Polysaccharide Utilization among Human Intestinal Symbionts. Current Biology 24: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbadin F., Pesante G., Elias L., Besser K., Li Y., Steele-King C., et al. (2018) Uncovering the molecular mechanisms of lignocellulose digestion in shipworms. Biotechnol Biofuels 11: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvachúa D., Werner A.Z., Pardo I., Michalska M., Black B.A., Donohoe B.S., et al. (2020) Outer membrane vesicles catabolize lignin-derived aromatic compounds in Pseudomonas putida KT2440. Proc Natl Acad Sci USA 117: 9302–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorio M.G., Pardue E.J., Scott N.E., and Feldman M.F. (2023) Human gut bacteria tailor extracellular vesicle cargo for the breakdown of diet- and host-derived glycans. Proc Natl Acad Sci USA 120: e2306314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey J.S., Cheng Y., and McManus W.R. (2021) Bacteria- and host-derived extracellular vesicles – two sides of the same coin? Journal of Cell Science 134: jcs256628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C. and Kuehn M.J. (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13: 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silale A. and Van Den Berg B. (2023) TonB-Dependent Transport Across the Bacterial Outer Membrane. Annu Rev Microbiol 77: 67–88. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., et al. (2019) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research 47: D607–D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R.M. and Wood P.J. (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43: 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M., Dean S.N., Caruana J.C., Walper S.A., and Ellis G.A. (2023) Bacterial Membrane Vesicles for In Vitro Catalysis. Bioengineering 10: 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku M., Schild S., Kaparakis-Liaskos M., and Eberl L. (2023) Composition and functions of bacterial membrane vesicles. Nat Rev Microbiol 21: 415–430. [DOI] [PubMed] [Google Scholar]
- Veith P.D., Chen Y.-Y., Chen D., O’Brien-Simpson N.M., Cecil J.D., Holden J.A., et al. (2015) Tannerella forsythia Outer Membrane Vesicles Are Enriched with Substrates of the Type Iti Secretion System and TonB-Dependent Receptors. J Proteome Res 14: 5355–5366. [DOI] [PubMed] [Google Scholar]
- Voight J.R. (2015) tiylotrophic bivalves: aspects of their biology and the impacts of humans. Journal of Molluscan Studies 81: 175–186. [Google Scholar]
- Waterbury J.B., Calloway C.B., and Turner R.D. (1983) A Cellulolytic Nitrogen-Fixing Bacterium Cultured from the Gland of Deshayes in Shipworms (Bivalvia: Teredinidae). Science 221: 1401–1403. [DOI] [PubMed] [Google Scholar]
- Yan S. and Wu G. (2013) Secretory pathway of cellulase: a mini-review. Biotechnol Biofuels 6: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.C., Madupu R., Durkin A.S., Ekborg N.A., Pedamallu C.S., Hostetler J.B., et al. (2009) The Complete Genome of Teredinibacter turnerae T7901: An Intracellular Endosymbiont of Marine Wood-Boring Bivalves (Shipworms). PLoS ONE 4: e6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Chen Y., Lu C., and Hwang J. (2006) Prediction of protein subcellular localization. Proteins 64: 643–651. [DOI] [PubMed] [Google Scholar]
- Zakharzhevskaya N.B., Vanyushkina A.A., Altukhov I.A., Shavarda A.L., Butenko I.O., Rakitina D.V., et al. (2017) Outer membrane vesicles secreted by pathogenic and nonpathogenic Bacteroides fragilis represent different metabolic activities. Sci Rep 7: 5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingl F.G., Thapa H.B., Scharf M., Kohl P., Müller A.M., and Schild S. (2021) Outer Membrane Vesicles of Vibrio cholerae Protect and Deliver Active Cholera Toxin to Host Cells via Porin-Dependent Uptake. mBio 12: e00534–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting this study's findings are available within the article and its supplementary materials. The mass spectrometric raw files are accessible at https://massive.ucsd.edu under accession MassIVE MSV000094250 and at https://www.proteomexchange.org under accession PXD050419.