Abstract
The nuclear pore complex (NPC) is the sole mediator of nucleocytoplasmic transport. Despite great advances in understanding its conserved core architecture, the peripheral regions can exhibit considerable variation within and between species. One such structure is the cage-like nuclear basket. Despite its crucial roles in mRNA surveillance and chromatin organization, an architectural understanding has remained elusive. Using in-cell cryo-electron tomography and subtomogram analysis, we explored the NPC’s structural variations and the nuclear basket across fungi (yeast; S. cerevisiae), mammals (mouse; M. musculus), and protozoa (T. gondii). Using integrative structural modeling, we computed a model of the basket in yeast and mammals that revealed how a hub of Nups in the nuclear ring binds to basket-forming Mlp/Tpr proteins: the coiled-coil domains of Mlp/Tpr form the struts of the basket, while their unstructured termini constitute the basket distal densities, which potentially serve as a docking site for mRNA preprocessing before nucleocytoplasmic transport
Keywords: Nuclear Pore Complex, Nuclear Basket, Nuclear Transport, Chromatin Organization, mRNA processing, cryo-ET, Integrative Modeling, Mass-Spec
Introduction
The nuclear pore complex (NPC) is a massive macromolecular assembly in the nuclear envelope (NE), responsible for nucleocytoplasmic transport (1-4). It comprises hundreds of proteins of more than 30 different types, known as Nucleoporins (Nups). These Nups, present in copies ranging from 8 to 48, assemble into multiple rings stacked along the nuclear envelope (1-10). These include outer rings on the nuclear and cytoplasmic sides (nuclear ring, NR; and cytoplasmic ring, CR) and an inner ring (IR) located between them. Each ring generally consists of 8 repeating subunits. Phenylalanine-glycine (FG)-rich repeats present in multiple Nups emanate inward from these rings to form the NPC’s central channel, and interact with transport factors to enable nucleocytoplasmic transport (5, 11). Apart from the CR, IR, and NR, the NPC features another prominent module known as the nuclear basket, also referred to as the basket (12-18). The basket is believed to play roles in mRNA transport and chromatin organization (14, 18-24). In yeast, Nup1, Nup2, Nup60, and Mlp1/Mlp2 are the main components of the basket and are referred to as basket Nups (4, 24-26). Their mammalian counterparts include Nup153 (ortholog of Nup60), Nup50 (ortholog of Nup2), and Tpr (ortholog of Mlp1/2) (12, 13). The basket has been observed in electron microscopy (EM) and in cryo-electron tomography (cryo-ET) studies of biochemically isolated NEs and NPCs of many organisms (15-17, 24, 27), although the exact role of different Nups in the observed basket structures is not well defined. In these studies, the basket is described as an assembly consisting of eight struts emanating from the nuclear side of the NPC core that converge into distal densities, that could restructure and dilate to allow passage of large cargoes through the NPC (20). These studies also highlighted the need for further studies, including obtaining a 3D map of the basket in-cell and describing the molecular organization of basket Nups, along with exploring the structural dynamics of the basket and its neighboring peripheral NPC structures in-cell.
While the major observable features of the NPC, chiefly the basket and rings, have been known for decades in vertebrates, their organization and variability - from within a single cell to between species - has been largely undefined. However, recent work has highlighted that the NPC’s architecture may vary significantly both within and between species (5, 28). To explore the nature of such variations, we performed in-cell cryo-ET on NPCs of cells from three different and evolutionarily divergent eukaryotes: fungi (S. cerevisiae), mammals (M. musculus; mouse’ NIH3T3 cells), and parasitic protozoa (T. gondii)(Fig. S1A). The cells of these organisms were rendered amenable for in-cell cryo-ET through cryo-focused ion beam (FIB) milling (29-31), that produces lamellae thin enough for cryo-ET from vitrified cells (32). Our in-cell cryo-ET dataset represents one of the largest of its kind, comprising 1604 tilt-series. Through subtomogram analysis and 3D classification, we classified different variants of NPCs across these organisms, yielding maps of one mammalian NPC (mNPC), one parasitic NPC (pNPC), and two distinct yeast NPC (yNPC) variants in-cell. The prefixes ‘m,’ ‘p,’ and ‘y’ have been used to denote mammals (mouse; M.musculus), and parasitic protozoa (T. gondii), and yeast (S. cerevisiae), respectively. From these maps, we discerned the basket architecture in the mNPC and one yNPC variant, shedding light on how the basket is organized on the NPC. Subsequently, we performed integrative modeling, incorporating a vast array of biochemical data on basket-Nups along with our in-cell maps, to model the molecular architecture of the basket-Nups. These maps and models provided us with a structural blueprint of how the basket forms and functions within the NPC.
Results
A stable basket is associated with a double NR.
Our previous study revealed at least two populations of yNPCs within each cell: a major population with a single nuclear NR, and a lesser population carrying a double NR (5). We set out to further investigate the structural variants of NPCs in yeast in-cell using cryo-ET (Fig. S1A and methods). Through 3D classification of a large data set of in-cell NPCs, we found that ~73% of NPCs in yeast during its log-phase growth possess a single NR, while the remaining have a double NR, which is consistent with the proportions estimated from quantitative fluorescence imaging (Fig. 1A-C, Fig. S1B, C) (5).
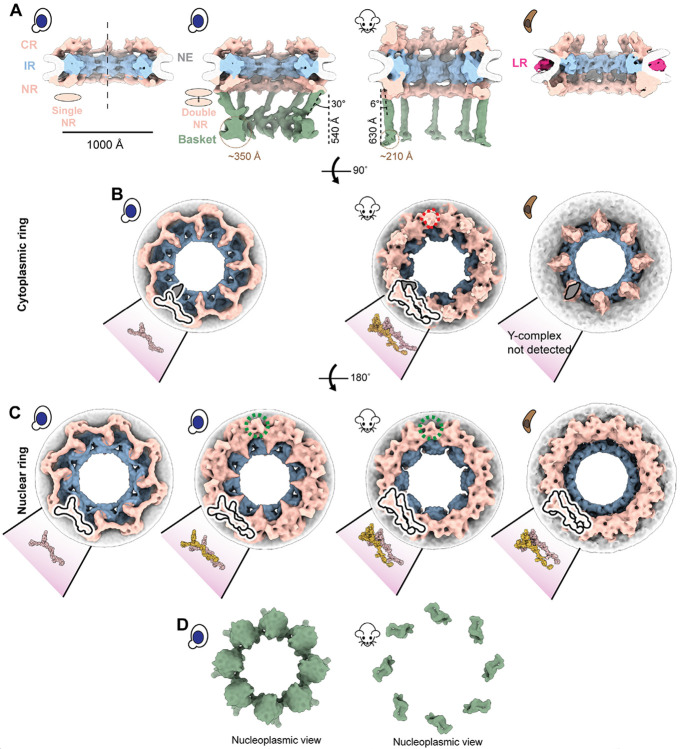
Fig. 1. A stable nuclear basket is bound to a double nuclear ring.
(A) Cross-sectional views along the central axis (dashed line) of the in-cell cryo-ET maps of yNPC with single and double NR variants, mNPC and pNPC. The nuclear basket is resolved for the mNPC and yNPC with a double NR. (B) Cytoplasmic views of the CR of yNPC with a single CR, mNPC with a double CR and pNPC with an incomplete CR. Also depicted are models of the single or double Y complex and the adjoining mRNA export platform, whose eight copies are arranged in head to tail orientation around the central axis to form the CR. Shown in dashed red lines is the region from which the cytoplasmic filaments emanate. (C) Nucleoplasmic view of the single and double NR of yNPC and double NR of mNPC and pNPC along with the models of the single or double Y complex. Shown in dashed green lines is the region in double NR from which the baske’s struts emanate. The models for yeast and mammalian CR/NR are from the PDB IDs: yNPC: 7N9F. mNPC/yNPC: 7R5J. (D) Nucleoplasmic views of the yeast and mammalian basket. CR: Cytoplasmic ring, IR: Inner ring, NR: Nuclear ring, NE: Nuclear envelope. LR: Lumenal ring.
The CR consists of eight subunits, each comprising one or two Y-complexes arranged in a head-to-tail orientation around the central axis passing through the center of the NPC (5, 6, 8, 33-35) (Fig. 1B, C). In addition to Y-complexes, the CR also has an mRNA export platform (5, 6, 8, 35-37) (Fig. 1B). The single NR also consists of eight Y-complexes, while the double NR carries sixteen such complexes in its two rings; the Y-complex rings proximal to and distal from the IR are referred to as the proximal and distal NR, respectively. The classification of the yNPCs into the single and double NR variants and their subsequent refinements gave us a more homogenous map of the single NR (devoid of any double NR densities). To date, a single NR is only observed in yNPCs (Fig. 1A, C). The IR and CR of the single and double NR variants are similar in stoichiometry and physical dimensions (Fig. S1B). Notably, a basket was observed only in the double NR variant in a dataset of more than 5100 NPC particles across 1449 tomograms from yeast, prompting the question of whether the single NR could support a stable and stoichiometric basket (Fig. 1A). However, it has previously been shown that most yNPCs, including both single and double NR forms, co-localize with basket components, with the exception of a class found only adjacent to the nucleolus that lack Mlp1/2 (23, 24), and that basket components in yeast are generally dynamic (5, 24, 38-40). Thus, we infer that basket components are more dynamic and so less well resolved in single NR NPCs (5, 38). Given that the basket was only resolved in the double NR variant in yeast we wondered if the mNPC, which always has a double NR (35) also has a discernible basket emanating from the double NR in a pattern similar to yNPC. Indeed, we also determined the architecture of the basket in mNPC and found it emanating similarly from its double NR (Fig. 1A, C). In an analogous manner to the basket and double NR, mNPC cytoplasmic mRNA export platforms emanate from the double CR, with an additional density likely representing the metazoan-specific Nup358 arrangement (41-43) (Fig. 1B).
A protozoan NPC has a double NR and an incomplete CR.
After observing the varying stoichiometries of the outer rings, we explored the diversity of these stoichiometries by performing in-cell cryo-ET and subtomogram analysis on NPCs of the protozoan parasite T. gondii, which diverged at least 1.5 billion years ago (44) and is responsible for toxo-plasmosis in humans (45). Surprisingly, this protozoan NPC (pNPC) features a double NR but has a minimal CR which is discontinuous, even more so than the disjointed CR observed in the fungi S. pombe (Fig. 1A-C, Fig. S2) (46). Focused refinement of the pNPC’s subunits revealed that the incomplete CR appears to lack a full-length Y-complex, consisting only of a complex resembling the mRNA export platform (and perhaps under which is a remnant core of the Y-complex) (Fig. 1B). To date, no biochemical data exists to further rationalize the architecture of the CR of pNPC. Notably, pNPC has the smallest diameter observed so far under normal, i.e., non-stress and in-cell conditions. Its lumenal ring (LR) is more prominent, unlike the LR of yNPC and mNPC which are closer to the NE and thus more difficult to resolve (Fig. 1A). The LR of the NPC separates from the NE upon the NPC’s contraction and becomes distinctly more visible, as was also observed for contracted isolated yNPCs and yNPCs in-cell under cellular stress (5, 46).
The basket consists of struts emanating from a double NR that end in a distal globular density.
The basket consists of eight struts, each ~100 Å thick, emanating from each of the eight subunits of the double NR at an angle from the central axis (30° for yBasket and 6° for mBasket), and terminating in a globular density referred to as the basket distal density (or basket ring), which is ~540 Å and ~630 Å away from the double NR in the yBasket and mBasket, respectively (Fig. 1A). The size of a single (one out of eight) basket distal density is ~350 Å for the yBasket and ~210 Å for the mBasket (Fig. 1A). In the yBasket, these densities are connected to form a ring with a diameter of ~760 Å. However, no connections between distal densities were resolved in the mBasket with an apparent distal diameter of ~1000 Å (Fig. 1A, D). These features of the basket bear broad similarities to those identified in isolated NEs and nuclei from various species (15, 16, 24, 27), except that the basket distal densities in isolated samples were mostly found to be connected, with their ring contracted unlike those in our in-cell maps (Fig. 1A, D) (15, 16, 24, 27).
The nuclear periphery exhibits an exclusion zone around the basket.
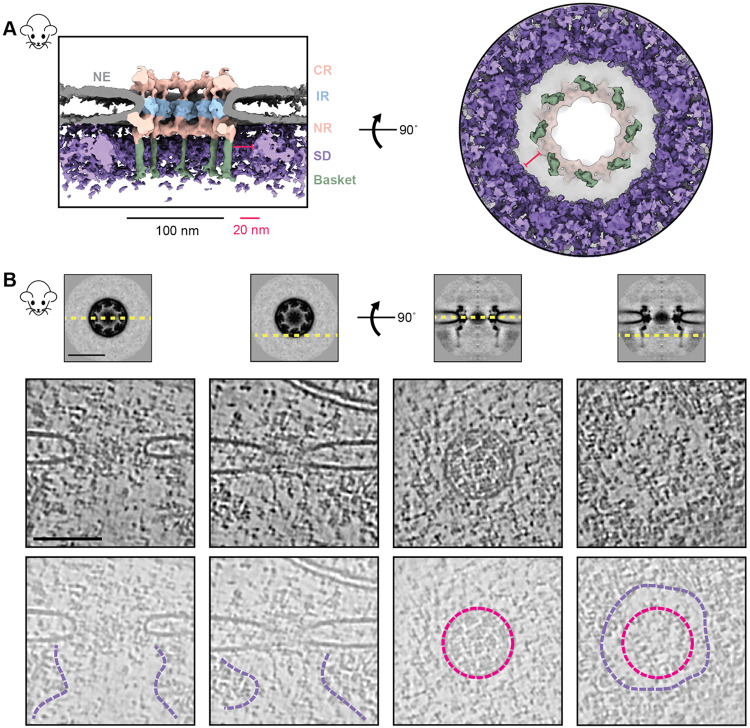
We leveraged the advantage of in-cell cryo-ET to examine the molecular environment in the proximity of NPCs with single and double NRs to explore why S. cerevisiae has two structural variants in NPCs and whether these variants had some specialized spatial distribution in the nucleus (Fig. S3A, B) (32, 47). In contrast to our expectation, in many cases, yNPCs with either a single or a double NR (those with a stable basket) were nevertheless found in similar environments, often adjacent to each other (Fig. S3A, B). One proposed role of the basket is to help create an exclusion zone around the NPC, presumably to streamline nucleocytoplasmic transport (21, 24). This hypothesis arose from observations of fixed and stained specimens in 2D electron micrographs (21, 24). Here, we generated a 3D average of the nucleoplasmic densities around the NPC to more clearly observe this zone and contextualize it with the basket structure. We used mNPCs, as they have morphologically better-defined heterochromatin than yeast (Fig. S3C). We generated a map of the mNPC, with a much larger box size, encompassing a significant portion of its surroundings, including nucleoplasmic regions (Fig. 2A). The map indeed revealed an exclusion zone around the mNPC and its basket on the nucleoplasmic side. This zone could also be seen directly in individual tomograms of mNPCs, which provide a clear depiction of this well-defined exclusion zone, which extends about 20 nm from the mBasket (Fig. 2A, B). Beyond the exclusion zone on the nucleoplasmic side, the densities likely represent lamina and chromatin, supporting the role of the NPC in chromatin organization (48, 49).
Fig. 2. Direct observation of the heterochromatin exclusion zone around the mNPC.
(A) The cross-sectional (left) and nucleoplasmic view (right) of the map of mNPC shows that molecular crowding (via surrounding densities; SD) around the mNPC is absent in the immediate vicinity of the basket (~20nm).(B) Tomogram slices of different regions around the NPC show the extent of this exclusion zone. Top: slices of the mNPC average used to depict viewing planes (yellow, dashed) in tomogram slices below. Middle and bottom: tomogram slices of an mNPC viewed parallel (left) and perpendicular (right) to the plane of the NE, as depicted in the top panel. Tomogram slices are duplicated in the bottom row to show annotated views. Lumenal rings (pink) and boundaries of the exclusion zone (purple) are indicated. CR: Cytoplasmic ring, IR: Inner ring, NR: Nuclear ring, NE: Nuclear envelope. SD: Surrounding densities. Scale bars: 100 nm unless stated otherwise.
The molecular architecture of the yeast and mammalian baskets.
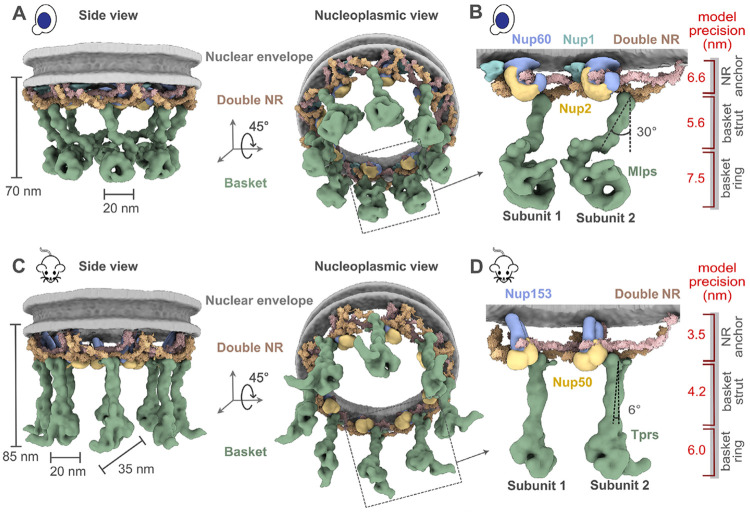
We used our iterative four-stage integrative approach to produce architectural maps of the yeast and mammalian baskets (Fig. 3 and Methods) (6, 50-54), based on subunit structure models, cryo-ET maps, chemical crosslinks, immuno-electron microscopy, coiled-coil propensities, sequence connectivity, excluded volume, and published data (Fig. 3 Stages 1,2) (SI Tables 1); a similar approach was used previously to determine the structure of the entire NPC (6). The modeling of the yBasket included basket Nups (yMlp, yNup1, yNup60, and yNup2, without their FG repeats) and NR Nups (yNup84 complexes), while the mammalian model consists of the basket Nups mTpr, mNup50, mNup153, and the NR mNup107 complexes (Fig. S4) (SI Tables 2, 3). The model optimizes the conformations and positions of these components while keeping the yNup84/mNup107 complexes fixed in their previously identified locations within our maps (Fig. 3 Stage 3) (SI Tables 2, 3). Before interpreting the models, we validated them using our standard assessment process (Fig. 3 Stage 4) (SI Tables 2, 3, Methods) (52, 54). Both models satisfy the data used to construct them (Fig. 3 Stage 1, Fig. S5, S6). In particular, the key input information, including the cryo-ET map, chemical croslinks, coiled-coil propensities, and subunit structure models is satisfied by a single cluster of structural solutions with an overall precision of 57 Å and 42 Å for yeast and mammalian, respectively (Fig. 3, Fig. S5, S6) (SI Tables 2, 3); the model precision is defined as the variability of the good-scoring solutions quantified by the average root-mean-square deviation (RMSD) of all solutions in the cluster. These precision estimates are considered when analyzing model features and comparing the two basket models.
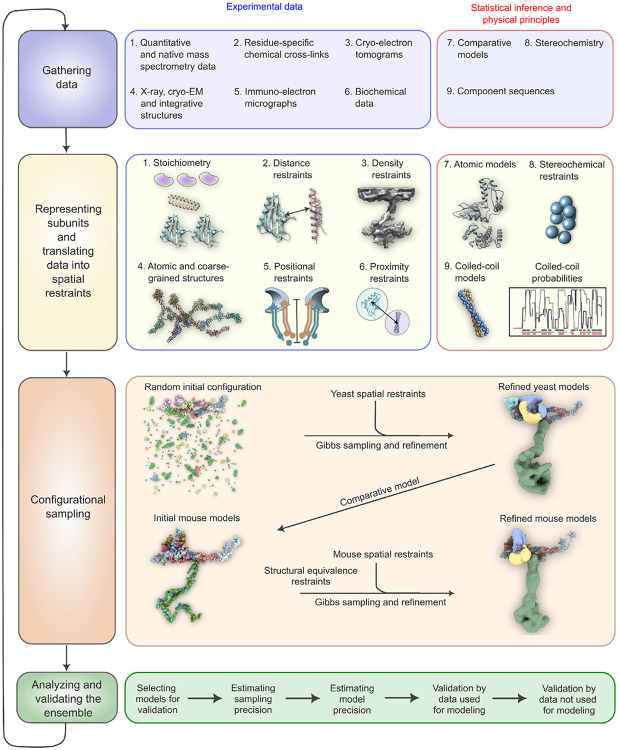
Fig. 3. The four-stage scheme for integrative modeling of the baskets.
Our integrative approach proceeds through four stages: (1) gathering data, (2) representing subunits and translating the data into spatial restraints, (3) configurational sampling to produce an ensemble of models that satisfies the restraints, and (4) analyzing and validating the ensemble. Stage 1 lists the experimental information used in this study for integrative modeling of baskets of yeast and mammals. Stage 2 lists representation and extracted spatial restraints obtained from information gathered in Stage 1. Stage 3 describes the configurational sampling to search for the models that satisfy the input information. A Gibbs sampling starting from a random initial configuration for the yBasket Nups generates the ensemble of good scoring models. The centroid model of the yBasket ensemble was used to model an initial mammalian basket. A similar Gibbs sampling with additional restraints from the mammalian data and structural equivalence restraint generates the ensemble of good scoring models. Stage 4 lists the model selection and validation protocol for the ensemble of good scoring configuration for both yeast and mammals.
The yeast and mammalian basket models are similar in topology but different in the overall shape.
The yeast and mammalian basket models have almost identical orthologous protein compositions (SI Tables 2, 3). The mammalian model was calculated to resemble the yeast model as much as possible while satisfying all the available mammalian data (SI Tables 2, 3, Methods). The two models share a similar topological arrangement, albeit with notable differences in the overall shape of the basket (Fig. 4A, C). To facilitate comparison, we dissected the basket into three modules, including the NR anchor (yNup1, yNup2, and yNup60 for yeast; mNup50 and mNup153 for mammals), basket strut (yMlp; mTpr), and basket distal modules (yMlp; mTpr) (Fig. 4B, D). The model revealed the proximity of the NR anchor module to the central channel of the NPC, NE, and NR (Fig. 4) and their association with the proximal NR via yNup60/mNup153 (Fig. 5A-D) (24). The N-terminus (blue) and C-terminus of yMlp/mTpr (red) are situated in the basket distal density, while the intervening region extends toward the double NR, forming the basket strut module. The yMlps/mTprs interact with the distal NR via yNup84/mNup107, consistent with the demonstrated requirement of yNup84 for anchoring Mlps onto NPCs (Fig. 5E, F) (24). The presence of two binding sites for the basket Nups, one on each proximal and distal NR, places the yNup60/mNup153 and yMlps/mTprs in direct interaction. This stabilizing interaction is feasible only in the context of the double NR, highlighting its importance in assembling a stable and less dynamic basket structure. However, our model also highlights how the basket might assemble in a single NR, as yMlps and yNup60 can interact with NR proteins (yNup84, yNup85, ySeh1) independently (Fig. 5A-D). Given the estimated model precision, our models are consistent with previously reported interactions between Nup60 and Nup2 (via their Nup60N2BM domain) and Nup60 and Mlps (via their Nup60MBM domain) (26, 55, 56). The model also revealed the presence of the coiled-coil domains of yMlp/mTpr in the basket strut module (Fig. 5E, F). The rod-like basket strut module exhibited around a 20° tilt between the yeast and mammalian baskets, resulting in a relatively large difference in the radius of the ring formed by the basket distal density (Fig. 4) (26). The basket distal module in both baskets is approximately globular, occupying the distal end of the basket (Fig. 4B, D). As is generally the case, it is unclear whether the differences between the yeast and mouse basket models reflect the differences between species, experimental conditions for purification and structure determination, and/or functional states.
Fig. 4. Integrative structure models and precisions of yeast and mammalian nuclear baskets.
(A, C) Localization probability density for the yBasket (containing yMlp, yNup1, yNup60, yNup2 Nups) and mBasket (containing mTpr, mNup50, mNup153 Nups) obtained from the ensemble of good scoring models. A localization probability density map for a set of models is defined as the probability of observing a model component at any point in space. The yMlps/mTprs (green) are attached to the double NR and NE (light gray half-toroid) with a common interacting FG Nups yNup60/mNup153 (violet). (B, D) A close-up view of the two subunits of the yBasket and mBasket with model precision for different basket segments. Shown here are yMlp/mTpr (green), FG Nups yNup1 (cyan), yNup2/mNup50 (yellow), and yNup60/mNup153 (purple).
Fig. 5. Position of different nucleoporins in the basket model.
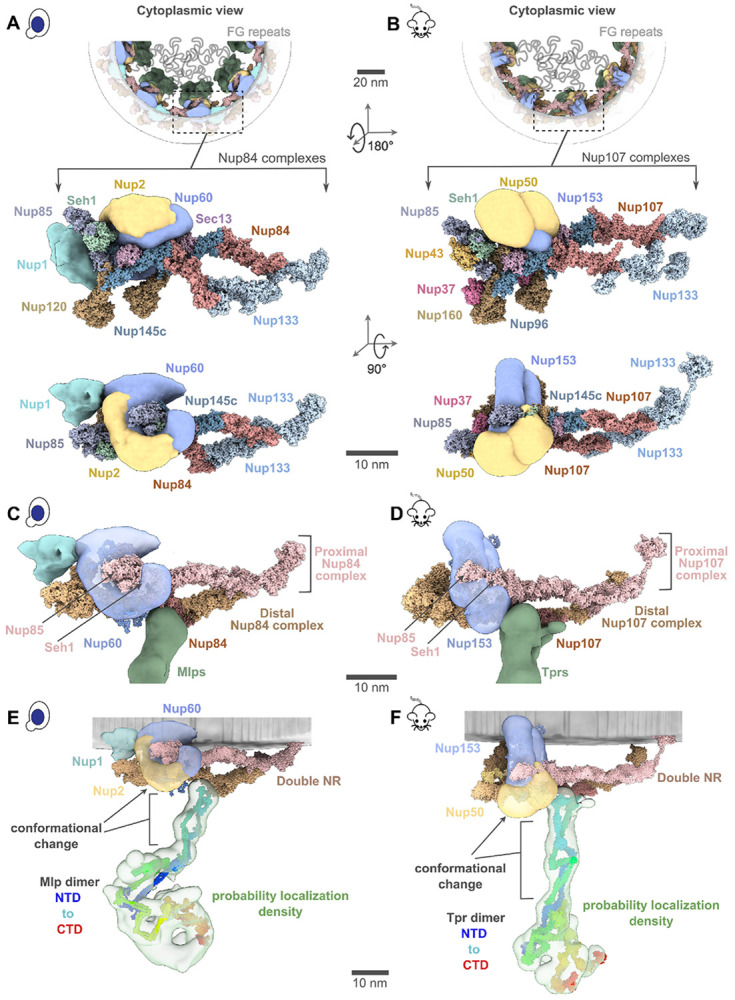
(A, B) Cytoplasmic view of the yeast and mammalian basket zooms into a Y-complexes (yNup84 complex for yeast and mNup107 complex for mammalian). Schematic representations of the FG repeats and anchoring positions are shown as curly lines (gray). Each double Y-complex’s zoomed image highlights individual Nups with two different views. Localization densities of the basket anchors, yNup1 (cyan), yNup2/mNup50 (dark yellow), and yNup60/mNup153 (violet), are shown relative to the Y-complex Nups. (C, D) Close-up inner view of the localization densities of the yNup60/mNup153 (violet) that contacts with the proximal nuclear ring (pink), whereas the localization densities of yMlp/mTpr dimer (green) contacts the distal nuclear ring (tan) of the NPC. yNup2/mNup50 not shown in this view. (E, F) The yMlp/mTpr dimer centroid models were colored from the N-terminus (blue) to the C-terminus (red) and shown embedded within their localization probability density (light green). The unidirectional arrows indicate local conformation change of yMlp/mTpr and yNup60/mNup153 between ybasket and mbasket models.
The FG regions in the NR anchor module face the central channel.
The yNup1 and yNup2 had not been included in the previous model of the NPC, due to the lack of data about their positions (5, 6, 50). In our models, the non-FG regions of FG Nups in the NR anchor module localize between the two copies of Nup85 in the proximal and distal yNup84/mNup107 complexes, with a precision of 6.6 nm (Fig. 4B, D and Fig. 5A, B). Both copies of yNup2 and yNup60 (and their mammalian orthologs) are proximal to each other as well as to several Nups in the double NR (Fig. 5A, B). The yNup1 is also positioned near yNup60, anchoring both to the nuclear envelope, consistent with previous mapping (55). This arrangement exposes the NR anchor module to the central channel. By virtue of the position of the globular anchor domain of the FG Nups, the FG anchoring sites are positioned such that the FG repeats face into the central channel, as is the case for previously localized FG repeat regions (5, 6, 57). This observation serves as further validation of our models, as this feature was not imposed on the models (Fig. 5A, B). The anchors are not sufficiently long to extend far into the struts or into the distal basket, indicating that the associated FG repeats remain localized to the proximal end of the basket and so spatially segregating transport from the initial docking processes occurring at the distal basket (58, 59).
The resilience of the basket architecture may arise from a combination of rigid and flexible modules.
It was proposed that the structural resilience of the NPC is achieved via an architecture that combines flexible and rigid modules, akin to the design of a suspension bridge (5, 6). Our models indicate that this architectural principle extends to the basket. Specifically, the apparently rigid components (SI Tables 2, 3) of the model include the subunits of the double NR (pink and tan, Fig. 5C, D), structured domains of the NR anchor module, and the basket strut module (green, Fig. 5C, D). The apparently flexible components (SI Tables 2, 3) of the model include the unstructured domains of the NR anchor module (violet, Fig. 5C, D) and the basket distal module (green, Fig. 4B, D). Our model indicates that the N-terminal amphipathic helices (Fig. S4C) within the yNup60/mNup153 of the NR anchor module may serve as critical anchors to the nuclear envelope (gray, Fig. 4) in full agreement with previous findings (26, 55). The flexible regions of the NR anchor module connect to the rigid double NR and basket strut module, mimicking the role of suspension cables that connect rigid columns and the roadway of a suspension bridge (55). The suspension bridge-like architecture may provide the necessary resilience of the basket while transporting large cargos.
Discussion
The array of stoichiometries we observe for the outer rings (CR+NR) across and within species, ranging from incomplete to 1 to 2, highlights the NPC’s modular construction and its structural plasticity, which allows it to easily adapt to gain or lose additional subcomplexes, presumably to confer alternate functionalities. T. gondii’s NPC appears to have the full mRNA export platform but an incomplete CR, suggesting that the full Y complex in the CR might be more dispensable than the mRNA export platform. While T. gondii’s CR is quite distinct, its IR is similar to other IRs, consistent with the differences among NPCs of various organisms being more pronounced in their outer rings rather than the IR, which appears to represent the most structurally and evolutionarily conserved module of the NPC (60). The NPCs with alternative stoichiometries of rings can be used to understand how the NPC’s parts are formed (e.g., we show here how the distal NR could help bind and stabilize the basket) and their dispensability (e.g., an NPC can function without a full length Y-complex on its CR, as shown here for T. gondii and has been shown for S. pombe (46)). Like the CR, the NR can also show different copy numbers. However, in the organisms examined so far, there is at least one complete NR as a necessary minimum for NPCs. Beyond this, many organisms can carry two NRs on either some or all of their NPCs; and Dictyostelium NPCs may usually carry 3 such NRs (61). The reason for this variability in NR copy number is not immediately evident; however, functional insights may be gained by examining the relationship between the NR architecture and that of the nuclear basket.
It should be noted that the term ’basket’, as traditionally defined and understood, refers to a stable architecture with struts. However, as we have shown and is consistent with observations here, the basket can be highly dynamic, and can also interact with many other proteins and indeed may have additional components, such as yPml39 / mZC3HC1 (56, 62). Various studies have established that much of the pool of nuclear basket components is dynamically associated with the yNPC (38); and, similar to yeast Nup1, Nup60, and Nup2 (39, 40), mammalian Nup153 and Nup50 are also dynamically associated with their respective NPCs (63). However, the major strut component in yeast (Mlp1/2) is dynamically associated (39, 40), whereas that in mammals (Tpr) seems more stably associated with the NPC, in agreement with the near ubiquitous appearance of the basket in mNPCs (above) (12, 13, 64). Moreover, we found that every double NR yNPC had a clearly associated basket, whereas the single NR NPCs did not have clear morphologically discernable baskets. However, in seeming contradiction, most yNPCs associate with basket components in vivo (6, 23-25, 38, 65). A reconciliation of this apparent contradiction can be made simply, as follows. We know that the majority of the yeast Mlp1/2 pool is also dynamic (above); we therefore suggest that for the single NR yNPCs, their association with basket components, including Mlp1/2, is transient, and furthermore that the basket components are more flexible and perhaps not stoichiometric (17), such that their presence is difficult to establish by in-cell cryo-EM. In contrast, we suggest that the double NR yNPCs, by virtue of their possession of an additional set of basket protein binding sites in their extra NR, can bind much more stably to Mlp1/2, thus accounting for the ubiquitous observation of basket struts in the double NR yNPCs. This idea also agrees with the observation, using scanning electron microscopy, of complete baskets on only a subset of yNPCs, although all had some strut-like nuclear filaments (17). We may also propose a function as to why yeast possess these minority double NR yNPC forms; while most yNPCs (with single NRs) utilize a rapidly reversible recruitment mechanism for the basket during mRNA export (38, 65), as masters of ’bet hedging’ (66) yeast keep in reserve an NPC subset with pre-assembled NPCs, perhaps to accommodate rapid changes in mRNA export or to ensure maintenance of epigenetic memory (67). In mNPCs, the presence of a double NR on essentially all NPCs ensures the stable association of Tpr and a morphologically recognizable basket.
When an NPC acquires a double NR, it not only becomes more stably associated with Mlp1/2; it also apparently becomes capable of more stably binding yNup1, yNup60 and yNup2, as the presence of the extra ring provides observed additional binding sites for these proteins on the NPC (Fig. 5A). The binding of these proteins to this afore-mentioned hub in the NE has been shown to be managed by posttranslational modifications (68, 69). Interestingly, a similar phosphorylation-driven mode of assembly and disassembly is found in flexible connector-containing Nups of cells that undergo NE breakdown, as we previously suggested (5).
Our cryo-ET analysis was not able to resolve a basket in the double NR-containing T. gondii NPCs, most likely due to the limited data set, and thus we cannot at this stage rule out the presence of a highly divergent basket structure or loss of the basket, as we were unable to identify obvious Tpr homologs in this organism. However, this may also suggest that the presence of a double NR might not be sufficient to sustain a highly stable basket assembly. Indeed, even in yeast and vertebrates the presence of a double NR does not guarantee the continued presence of a full stable basket, as it has been shown that under stress (70, 71) or under certain cellular states (68, 69, 72), the nuclear basket can dissociate from the NPC.
It was previously observed that the C-terminus of Mlp1 acts as a necessary transient docking site for messenger ribonucleoproteins (mRNPs) during mRNA export (59) and that the nucleocytoplasmic transport of large molecules through the NPC involves an increase in the basket ring radius (20). Our model rationalizes these observations as follows. The C-terminus of Mlps is located in the basket distal density. If the difference between the yeast and mammalian models was indicative of the basket structural dynamics, the dynamics of the basket would involve the movements of the basket subunit and conformational changes of anchor Nups (Fig. 5E, F) (24, 73). Moreover, the flexible linkers connecting the coiled-coil segments from the yMlps/mTprs dimer in the struts may also afford the flexibility to the basket to contract and expand as seen for other rings (5, 46). Such motions could account for the expansion and contraction of the basket seen during passage of large cargoes (20). A significant portion of the basket distal density in yeast remains unaccounted for in our integrative model, possibly indicating the presence of cargo, transport factors or other elements, which aligns with the observation that this density could be a docking site for cargo (Fig. 5E, F). Unlike the yNPC, the basket distal density in the mNPC is considerably smaller, and thus, a larger proportion of its density is accounted for in its models (Fig. 5E, F).
The sizes of many mRNPs range from ~200 to 600 Å, smaller than the diameters of the rings, including that of the basket, potentially allowing them to pass unaltered across the NPC (74, 75). However, some mRNPs can have elongated shapes or be much larger than the basket’s diameter (20, 74, 75); in these cases, their shape can be adjusted or remodeled to pass through the NPC, and the basket too can remodel as seen during passage of such large cargoes (20, 76). These adjustments or remodelings could commence while the mRNP is docked at the basket distal density. The role of the basket has primarily been discussed for nuclear export, not import. However, it would be interesting to explore what happens to the basket during import, including for large cargoes like HIV capsids that can pass through the NPC as intact entities (77).
Apart from chromatin exclusion, NPCs also influence chromatin organization through the context-specific localization of either active or repressed genes to the NPC (78-81). These localizations require basket Nups such as yNup1, yNup2, yNup60, mNup153, and Mlps, implying the baske’s critical role in these localizations (78-81). Similar to mRNA docking, these localizations may also occur at the basket distal density, consistent with reported interactions between the C-termini of Mlps (which we show to form the distal density) and complexes involved in genes localization to the NPC (82). Interestingly, chromatin organization is not only impacted by the presence of the basket but also by its systematic absence, as shown recently that basketless yNPCs are involved in a process of subtelomeric gene silencing (83).
Methods
Cell culture, vitrification and sample preparation.
W303 yeast cells were cultured in yeast extract peptone dextrose (YPD) media supplemented with adenine hemisulfate. These cells in the log-growth phase were collected and deposited on glow-discharged Quantifoil grids (R 2/1, Cu 200-mesh grid, Electron Microscopy Sciences), as described previously (5). The mouse fibroblasts cells (NIH3T3) were cultured at 37°C and 5% CO2 in DMEM with 10% fetal calf serum. Cells were seeded onto glow-discharged and Fibronectin-coated Quantifoil grids (R1/4, Au 200-mesh grid, Electron Microscopy Sciences). Following this seeding, the cells were cultured for 2 more hours on the grids to allow for their stable adherence onto the grid. In some cases, grids were micropatterned with 40 μm circles and treated with 100 nM jasplakinolide for a further two hours after seeding. The tachyzoites (T. gondii in rapid growth phase) were thawed out from liquid nitrogen and cultivated in human foreskin fibroblasts (HFFs) using Dulbecco’s modified Eagle’s medium (DMEM), with medium changes every 12 to 24 hours. To collect tachyzoites, trypsin-treated, parasite-infected HFFs were mechanically disrupted using a 27-gauge syringe, and the mixture was filtered to separate tachyzoites from HFF debris. The tachyzoites were then centrifuged, resuspended in DMEM with 30% FBS and 10% DMSO, and deposited on EM grids for vitrification, as described previously (84). Excess media was manually blotted from the back (opposite to the carbon film and seeded cells). Grids were plunge-frozen in a liquid ethane-propane mixture (50/50 volume, Airgas) using a custom-built vitrification device (Max Planck Institute for Biochemistry, Munich). Frozen grids were clipped into AutoGrids with a milling slot (Thermo Fisher Scientific) to allow milling at shallow grazing angles as described previously (31, 85). Cryo-FIB milling was performed in an Aquilos Dual-Beam (Thermo Fisher Scientific) as described previously (31, 85).
Tilt series acquisition.
Tilt series were acquired on the Titan Krios G3 (Thermo Fisher Scientific) at 300 keV with either a K2 detector and Quantum 968 LS post-column energy filter or a K3 Summit detector with 1067HD BioContinuum post-column energy filter in counting and dose fractionation modes (Gatan). The tilt-series parameters were as follows: tilt range: ± 45-60°, pixel size of 3.45 Å (yeast), 1.32 Å (mouse fibroblasts), 3.328 Å (T gondii), tilt increment: 3° (higher for some samples), effective defocus range: −2 to −11 μm, total fluence: ~100-180 e-/Å2. All image acquisition was done using SerialEM software (86, 87). For some tilt-series, parallel cryo-electron tomography (PACE-tomo) scripts were used (88). In total, 1449, 136, and 19 (total: 1604) tilt series were used for yeast, mouse, and T. gondii, respectively. This data set included 153 tilt-series of yeast from EMPIAR-10466 (8).
Subtomogram analysis.
Frames of the tilt images were motion-corrected using whole-frame motion and organized into stacks in WARP (89, 90). The motion-corrected tilt series were then aligned in AreTomo (91). The aligned tilt-series stacks were subsequently re-imported into WARP for CTF estimation, defocus handedness determination, and final reconstruction (89, 90). The CTF estimation and defocus handedness were manually inspected and further refined as needed. In tomograms, nuclear pores were manually picked in IMOD (92). For each pore, in addition to the coordinate of the pore’s center, an additional point approximately 50-100 nm on the cytoplasmic side was marked. The pores were oriented using these two points with the Dynamo dipole picking mode (93). The subtomograms of the pores, with these initial orientations, were generated in WARP at a pixel size of 10 Å. The total number of pores picked were ~5160 for yeast, ~220 for mouse, and ~50 for T. gondii, respectively. A small number of pore particles were used to generate a C8 symmetrized initial model in Relion (94). This initial model served as a reference for refining all the pore particles with C8 symmetry. The refinements were performed with local searches around the initial orientation (initial Euler angles), using the sigma_ang/rot/tilt/psi parameters to restrict the angular searches and prevent the pore particles from flipping. The term sigma_ang/rot/tilt/psi in Relion specifies the width of the Gaussian prior on the starting Euler angles. 3D classification (without alignments, simply referred to as classification), using C8 symmetry, was performed using a mask focused on the inner ring of the NPC to select good particles and discard bad ones. The selected NPC particles were refined further with C8 symmetry. For yeast, classification was performed using a mask focused on the nuclear ring to classify out NPCs with single and double NR, which accounted for ~77% and the remaining ~23% of total NPCs, respectively. The symmetry expansion was carried out to isolate subunits of the NPCs. These subtomograms of the subunits were then reconstructed at a pixel size of 10 Å in WARP. The relion_reconstruct was used to generate an average of these subtomograms for use as reference in the refinement of these subunits using a mask focused on the IR subunit. Following refinement, classification was performed, using the mask focused on the IR subunit, to select good subunits and discard bad ones. The refinements of the good subunits of IR, CR, NR, and the basket (as applicable) were then performed using their respective shape masks. All the refinements and classification of subunits were done without the use of symmetry. The total number of subunits used in the final refinements were ~28600 for yeast (out of which,~6600 were from the NPC with double NR), ~800 for mouse, and ~265 for T. gondii, respectively. The 0.143-cut-off criterion of the Fourier Shell correlations (FSC) between masked and independently refined half-maps was used to estimate all the reported resolutions (95). The maps of the subunits of these different rings were composited to generate the final map of the entire subunit of the NPC. This composite map was fit into the map of the whole NPC (of C8 symmetry) using Chimera’s fit-to-map tool (96), and then C8 symmetrized using relion_image_handler. The entire processing of the data from separate organisms was done completely separately and independently. The schematic of the entire workflow and resolution estimates is also shown in Figure S1. v3.1.1 of Relion was used for all steps involving Relion (94). v1.09 or v1.1.0-beta1 of WARP was used for all steps involving WARP (89, 90).
Pairwise distances amongst yNPCs and their radial distribution function [g(r)].
The coordinates of yNPCs with single or double NR in their tomograms were obtained following their subtomogram analysis. For each tomogram, pairwise distances among all yNPCs, as well as those with single and double NR, were calculated using these coordinates. These distances were then used to estimate the g(r) for each tomogram. The g(r) values from all the tomograms were averaged to generate the final g(r) shown in (Fig. S2B). It should be noted that these pairwise distances and their corresponding g(r) values are averages for all yNPCs and might not apply to small subsets of yNPCs. For instance, yNPCs near the nucleolus are likely to be less enriched in double NRs (with a stable basket). This observation comes from fluorescence imaging, which has shown that yNPCs near the nucleolus lack yMlps (one of the basket-Nups) and have a low level of NR-Nups, indicating a preference for single NR without the basket (5, 23, 38, 97).
Chemical cross-linking and MS (CX-MS) analysis of affinity-purified yeast NPCs.
CX-MS of Mlp1-PPX-PrA tagged, affinity purified, native, whole NPCs have been described in detail in (5, 6). To expand and complement these datasets with cross-links mapping exclusively to basket Nups fully assembled into the NPC, we used NPCs affinity purified using Dbp5-PPX-GFP and Gle1-PPX-PrA as the handles using a similar protocol, with the following modifications: After native elution, 1.0 mM disuccinimidyl suberate (DSS) was added and the sample was incubated at 25°C for 40 minutes with shaking (1,200 rpm). The reaction was quenched by adding a final concentration of 50 mM freshly prepared ammonium bicarbonate and incubating for 20 minutes with shaking (1,200 rpm) at 25°C. Crosslinked NPCs were pelleted by spinning for 20 minutes in a TLA-55 rotor (Beckman) at 25,000 rpm. The pelleted samples (~50 mg) were resuspended in 1xLDS with 25 mM DTT and incubated at 70°C for 10 minutes. Reduced samples were alkylated by adding a final concentration of 100 mM iodoacetamide and incubating in the dark at 25°C for 30 minutes, followed by the addition of an additional 25 mM DTT and further incubation for 15 minutes. Alkylated and reduced samples were denatured at 98°C for 10 minutes and then loaded into 4% SDS-PAGE Bis-Tris gel and run for 10 minutes at a constant 120 V to reduce the complexity of the sample. For in-gel digestion, the high-molecular-weight-region gel bands corresponding to cross-linked NPC proteins were sliced and proteolyzed by trypsin as previously described (6). In brief, gel plugs were crushed into small pieces and 5–10 μg of sequencing-grade trypsin (Promega) per ~100 μg protein were added. Trypsin was supplied in two equal additions and incubated with gel pieces at 37°C in 50 mM ammonium bicarbonate, 0.1% (w/v) Rapigest (Waters). After the first addition, the samples were incubated for 4 hours. After the second addition, the samples were incubated overnight. Peptides were extracted by formic acid and acetonitrile, and dried partially by vacuum centrifugation. To remove the hydrolytic insoluble by-products of Rapigest, the sample was centrifuged at 20,000 g for 10 min. The solution was transferred to another tube and then further dried by vacuum centrifugation. Peptides were separated into 6-7 fractions by high pH reverse phase fractionation in a pipet tip self-packed with C18 resin (ReproSil-Pur 120 AQ, 3μm, Dr. Maisch GmbH). Each peptide fraction was resuspended in 5% (v/v) methanol, 0.2% (v/v) formic acid and loaded onto an EASY-Spray column (Thermo Fisher Scientific, ES800, 15cm x 75mm ID, PepMap C18, 3mm) via an EASY-nLC 1200 (Thermo Fisher Scientific). The column temperature was set to 35°C. Using a flow rate of 300 nl/min, peptides were gradient-eluted (3–6% B, 0–6 min; 6–34% B, 6–97 min), where mobile phase B was 0.1% (v/v) formic acid, 95% (v/v) acetonitrile and mobile phase A was 0.1% (v/v) formic acid in water. An Orbitrap Fusion Lumos Tribrid (Thermo Fisher Scientific) was used to perform online mass spectrometric analyses. Full MS scans were performed at least every 5 s. As time between full scans allowed, ions with charge states +4 to +8 were fragmented by higher-energy collisional dissociation in descending intensity order with a maximum injection time of 800 msec. Both precursors and fragments were detected in the Orbitrap. The raw data were searched with pLink2 (98) with cysteine carbamidomethyl as a fixed modification and methionine oxidation as a variable modification. The initial search results were obtained using a default 5% false discovery rate (FDR) expected by the target-decoy search strategy. Spectra corresponding to basket components were selected and manually verified to ensure data quality (6).
Integrative modeling of the basket.
Coarse-grained structural models of the yeast and mouse baskets were computed using an integrative modeling approach (6, 50-54), based on information from varied experiments, physical principles, statistical preferences, and prior models (SI Table 1). The yBasket model includes the yMlp1/2, FG Nups (yNup1, yNup2, and yNup60), as well as the double NR Nups (yNup120, yNup85, yNup145c, ySec13, ySeh1, yNup84, and yNup133) (4, 24-26). The mBasket model includes the orthologs of yeast Nups (mTpr, mNup50, mNup153, mNup160, mNup85, mNup96, mSec13, mSeh1, mNup107, mNup133, mNup43, and mNup37) (12, 13). Modeling positioned the yMlp/mTpr and FG Nups relative to the fixed double nuclear ring; in addition, it optimized the conformations of the disordered Nup regions. The modeling protocol was scripted using the Python Modeling Interface (PMI) package version a41075a, which is a library for modeling macromolecular complex structures based on our open-source Integrative Modeling Platform (IMP) package version 2.19 (https://integrativemodeling.org) (52).
Stage 1: Gathering information.
The sequences of the basket Nups were obtained from the Uniprot database (99) (SI Tables 1, 2). Their stoichiometry in the yNPC was previously determined by quantitative mass spectrometry of the isolated yNPC complex (6) (SI Table 2). In total, 626 unique intra- and intermolecular DSS cross-links were previously identified using mass spectrometry (6, 24, 100). The cryo-ET map described here informed the overall shape of the basket and its anchoring on the double nuclear ring. The structural model of yNup84 complex of the double NR was previously determined by an integrative approach (5). The structural model of the yMlps was informed by the coiled-coil propensities and heptad repeat alignments and was generated using COCONUT software (101) (Fig. 3 stage 2 and Fig. S4A, B) (SI Table 1). The yNup2 structural model was obtained from the AlphagFold database version 4 (SI Table 2). Direct physical interactions between yNup60, yNup2, and yMlp1 were determined by in vitro binding assays (55) (SI Table 1). Previously determined immuno-electron microscopy images help localize the terminal domains of the yMlps (24). Similar information was used for mBasket modeling (12, 102) (Fig. 3 and Fig. S4) (SI Tables 1, 3).
Stage 2: Basket representation and spatial restraints.
Basket representation:
We modeled only a single subunit out of the eight subunits comprising the entire yBasket, mostly without explicitly considering the interfaces between the eight symmetry units in the yNPC. This simplification was possible because the explored positions and conformations of the basket components do not clash with each other across the symmetry unit interfaces, courtesy of their anchoring on the fixed double NR. The stoichiometries of yMlp1 and yMlp2 are ambiguous (6). Thus, we used two copies of poly-alanine per symmetry unit (yMlp), representing both yMlp1 and yMlp2; the yMlp length was set to that of yMlp1. The model also included a single copy of yNup1 and two copies of yNup2, yNup60, and the heptameric yNup84 complex. FG repeats were not included in the model. In total, the yBasket model consists of 21 protein subunits of 12 types (SI Table 2). A similar representation was used for mBasket modeling with two copies of mTpr, two copies of mNup50, mNup153, and the nonameric mNup107 complex (SI Tables 1, 3) (35, 103, 104). Thus, the mBasket model consists of 24 protein subunits of 12 types (SI Table 3). Each component was represented in a multiscale fashion to balance the accuracy of the formulation of restraints and the efficiency of structural sampling (Fig. S4C) (SI Tables 2, 3).
Spatial restraints on yeast and mouse baskets:
The subset of input information was converted to spatial The subset of input information was converted to spatial restraints for scoring alternative models (52). These restraints include upper bounds on pairs of crosslinked residues based on chemical crosslinks, the correlation coefficient between Gaussian Mixture Models of a model and the cryo-ET map, positional restraints on NTD/CTD domains of yMlps based on immuno-EM localizations, distance restraints between pairs of domains based on affinity co-purification data, positional restraints on residue segments predicted to lie within the nuclear envelope, connectivity restraints between consecutive pairs of beads in a subunit, excluded volume restraints between non-bonded pairs of beads (SI Table 2) (52). For the mBasket (SI Table 3), crosslinking and affinity copurification data were unavailable. However, we supplemented the remaining mBasket restraints with structural equivalence restraints; these distance restraints are designed to maximize the similarity between the mouse and yeast models across the aligned residues, subject to the satisfaction of the remaining restraints.
Stage 3: Structural sampling.
The initial positions and orientations of rigid bodies and flexible beads were randomized except for the double NR rigid body (SI Table 2), whose position was obtained by fitting into the cryo-ET map, ensuring accurate alignment with experimental cryo-ET maps (Fig. 3 stage 3) (SI Table 2). Structural sampling of rigid body positions and orientations as well as flexible bead positions, was performed using the Replica Exchange Gibbs Monte Carlo (MC) algorithm (SI Table 2) (105, 106). Each MC step consisted of a series of random transformations (i.e., rotations and translations) applied to the rigid bodies and flexible beads. The same sampling protocol was used for mBasket modeling, except that the starting structure mimicked the yBasket model (SI Table 3).
Stage 4: Analysis and validation.
Model validation followed four steps (54, 107): (i) selection of the models for validation, (ii) estimation of sampling precision (Fig. S5A), (iii) estimation of model precision, and (iv) quantification of the degree to which a model satisfies the information used and not used to compute it (SI Table 2, 3) (Fig. S5, Fig. S6) (54, 108).
Integrative modeling iterated through the four stages to find a set of models that satisfy our validation criteria listed above. In each iteration, we considered the input information, representation, scoring, and sampling guided by an analysis of models computed in the preceding iteration of the modeling. For example, the initial low precision of the yBasket model encouraged us to improve the resolution of the cryo-ET map by averaging a larger number of subtomograms; and the initial inability to find yBasket models that satisfied both cryo-ET and crosslinking data encouraged us to increase the resolution and flexibility of the coiled-coil representations, re-defining the coiled-coil segments (109), disorder predictions for FG Nups (99), and adding proximity restraints to some components.
Supplementary Material
Acknowledgments
We thank the members of the Villa, Sali, and Rout labs, as well as Chris Akey and Steve Ludke for their feedback and support. D.S. was supported by a Damon Runyon Post-doctoral Fellowship (DRG-2364-19) and is currently supported by the NIH’s K99 Pathway to Independence Award (K99AG080112). J.H. was supported by an EMBO long-term postdoctoral Fellowship (ALTF 871-2020). E.V. is an investigator of the Howard Hughes Medical Institute. We acknowledge the use of the UC San Diego cryo-EM facility, which was built and equipped with funds from UC San Diego and an initial gift from the Agouron Institute. This work was supported by an NSF MRI DBI 1920374 (to E.V.), U54 AI170856 (to E.V.), NIH P41 GM109824 (to M.P.R., B.T.C. and A.S.), NIH R01 GM083960 (to A.S.), NSF-1818129 and Spanish Ministerio de Ciencia e Innovacion PID2020-116404GB-I00 (to J.F.-M.), NIH R01 GM112108 and NIH GM117212 (to M.P.R.). We thank Prof. Ricardo Henriques’s Lab for LATEX template used for typesetting this document.
Footnotes
Competing interests
Authors have no conflicts of interest with the contents of this manuscript.
Bibliography
- 1.Hampoelz Bernhard, Amparo Andres-Pons Panagiotis Kastritis, and Beck Martin. Structure and Assembly of the Nuclear Pore Complex. Annu. Rev. Biophys., 48:515–536, May 2019. [DOI] [PubMed] [Google Scholar]
- 2.Wente Susan R and Rout Michael P. The nuclear pore complex and nuclear transport. Cold Spring Harb. Perspect. Biol., 2(10):a000562, October 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz Thomas U. The structure inventory of the nuclear pore complex. J. Mol. Biol., 428(10 Pt A):1986–2000, May 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Daniel H and Hoelz André. The structure of the nuclear pore complex (an update). Annu. Rev. Biochem., 88:725–783, June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akey Christopher W, Singh Digvijay, Ouch Christna, Echeverria Ignacia, Nudelman Ilona, Varberg Joseph M, Yu Zulin, Fang Fei, Shi Yi, Wang Junjie, Salzberg Daniel, Song Kangkang, Xu Chen, Gumbart James C, Suslov Sergey, Unruh Jay, Jaspersen Sue L, Chait Brian T, Sali Andrej, Fernandez-Martinez Javier, Ludtke Steven J, Villa Elizabeth, and Rout Michael P. Comprehensive structure and functional adaptations of the yeast nuclear pore complex. Cell, 185(2):361–378.e25, January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Seung Joong, Fernandez-Martinez Javier, Nudelman Ilona, Shi Yi, Zhang Wenzhu, Raveh Barak, Herricks Thurston, Slaughter Brian D, Hogan Joanna A, Upla Paula, Chemmama Ilan E, Pellarin Riccardo, Echeverria Ignacia, Shivaraju Manjunatha, Chaudhury Azraa S, Wang Junjie, Williams Rosemary, Unruh Jay R, Greenberg Charles H, Jacobs Erica Y, Yu Zhiheng, Jason de la Cruz M, Mironska Roxana, Stokes David L, Aitchison John D, Jarrold Martin F, Gerton Jennifer L, Ludtke Steven J, Akey Christopher W, Chait Brian T, Sali Andrej, and Rout Michael P. Integrative structure and functional anatomy of a nuclearpore complex. Nature, 555(7697):475–482, March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosalaganti Shyamal, Kosinski Jan, Albert Sahradha, Schaffer Miroslava, Strenkert Daniela, Salomé Patrice A, Merchant Sabeeha S, Plitzko Jürgen M, Baumeister Wolfgang, Engel Benjamin D, and Beck Martin. In situ architecture of the algal nuclear pore complex. Nat. Commun., 9(1):2361, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allegretti M, Zimmerli C E, Rantos V, Wilfling F, Ronchi P, Fung H K H, Lee C W, Hagen W, Turonova B, Karius K, Bormel M, Zhang X, Muller C W, Schwab Y, Mahamid J, Pfander B, Kosinski J, and Beck M. In-cell architecture of the nuclear pore and snapshots of its turnover. Nature, 586(7831):796–800, 2020. [DOI] [PubMed] [Google Scholar]
- 9.Schuller Anthony P, Wojtynek Matthias, Mankus David, Tatli Meltem, Kronenberg-Tenga Rafael, Regmi Saroj G, Dip Phat V, Lytton-Jean Abigail K R, Brignole Edward J, Dasso Mary, Weis Karsten, Medalia Ohad, and Schwartz Thomas U. The cellular environment shapesthe nuclear pore complex architecture. Nature, 598(7882):667–671, October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amlacher Stefan, Sarges Phillip, Flemming Dirk, van Noort Vera, Kunze Ruth, Devos Damien P, Arumugam Manimozhiyan, Bork Peer, and Hurt Ed. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell, 146(2):277–289, July 2011. [DOI] [PubMed] [Google Scholar]
- 11.Terry L J and Wente S R. Flexible Gates: Dynamic Topologies and Functions for FG Nucleoporins in Nucleocytoplasmic Transport, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krull Sandra, Thyberg Johan, Björkroth Birgitta, Rackwitz Hans-Richard, and, Cordes Volker C. Nucleoporins as components of the nuclear pore complex core structure and tpr as the architectural element of the nuclear basket. Mol. Biol. Cell, 15(9):4261–4277, September 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordes V C, Reidenbach S, Rackwitz H R and Franke W W. Identification of protein p270/tpr as a constitutive component of the nuclear pore complex-attached intranuclear filaments. J. Cell Biol., 136(3):515–529, February 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bensidoun Pierre, Zenklusen Daniel, and Oeffinger Marlene. Choosing the right exit: How functional plasticity of the nuclear pore drives selective and efficient mRNA export. Wiley Interdiscip. Rev. RNA, 12(6):e1660, November 2021. [DOI] [PubMed] [Google Scholar]
- 15.Jarnik M and Aebi U. Toward a more complete 3-D structure of the nuclear pore complex. J. Struct. Biol., 107(3):291–308, December 1991. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg M W, and Allen T D. High resolution scanning electron microscopy of the nuclear envelope: demonstration of a new, regular, fibrous lattice attached to the baskets of the nucleoplasmic face of the nuclear pores. J. Cell Biol., 119(6):1429–1440, December 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiseleva Elena, Allen Terence D, Rutherford Sandra, Bucci Mirella, Wente Susan R, and Goldberg Martin W. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J. Struct. Biol., 145(3):272–288, March 2004. [DOI] [PubMed] [Google Scholar]
- 18.Strambio-De-Castillia Caterina, Niepel Mario, and Rout Michael P. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol., 11(7):490–501, July 2010. [DOI] [PubMed] [Google Scholar]
- 19.Kiseleva E, Goldberg M W, Daneholt B, and Allen T D. RNP export is mediated by structural reorganization of the nuclear pore basket. J. Mol. Biol., 260(3):304–311, July 1996. [DOI] [PubMed] [Google Scholar]
- 20.Kiseleva E, Goldberg M W, Allen T D and Akey C W. Active nuclear pore complexes in chironomus: visualization of transporter configurations related to mRNP export. J. Cell Sci., 111 ( Pt 2):223–236, January 1998. [DOI] [PubMed] [Google Scholar]
- 21.Krull Sandra, Dörries Julia, Boysen Björn, Reidenbach Sonja, Magnius Lars, Norder Helene, Thyberg Johan, and Cordes Volker C. Protein Tpr is required for establishing nuclear pore-associated zones of heterochromatin exclusion. EMBO J., 29(10):1659–1673, May 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kylberg Karin, Björk Petra, Fomproix Nathalie, Ivarsson Birgitta, Wieslander Lars, and Daneholt Bertil. Exclusion of mRNPs and ribosomal particles from a thin zone beneath the nuclear envelope revealed upon inhibition of transport. Exp. Cell Res., 316(6):1028–1038, April 2010. [DOI] [PubMed] [Google Scholar]
- 23.Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A and Nehrbass U. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell, 116(1):63–73, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Niepel Mario, Molloy Kelly R, Williams Rosemary, Farr Julia C, Meinema Anne C, Vecchietti Nicholas, Cristea Ileana M, Chait Brian T, Rout Michael P and Strambio-De-Castillia Caterina. The nuclear basket proteins mlp1p and mlp2p are part of a dynamic interactome including esc1p and the proteasome. Mol. Biol. Cell, 24(24):3920–3938, December 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strambio-de Castillia C, Blobel G, and Rout M P, Proteins connecting the nuclear pore complex with the nuclear interior. J. Cell Biol., 144(5):839–855, March 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mészáros Noémi, Cibulka Jakub, Mendiburo Maria Jose, Romanauska Anete, Schneider Maren, and Köhler Alwin. Nuclear pore basket proteins are tethered to the nuclear envelope and can regulate membrane curvature. Dev. Cell, 33(3):285–298, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck Martin, Förster Friedrich, Ecke Mary, Plitzko Jürgen M, Melchior Frauke, Gerisch Günther, Baumeister Wolfgang, and Medalia Ohad. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science, 306(5700):1387–1390, November 2004. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Martinez J and Rout M P. One Ring to Rule them All? Structural and Functional Diversity in the Nuclear Pore Complex. Trends Biochem. Sci., 46(7):595–607, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marko Michael, Hsieh Chyongere, Schalek Richard, Frank Joachim, and Mannella Carmen. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat. Methods, 4(3):215–217, March 2007. [DOI] [PubMed] [Google Scholar]
- 30.Rigort Alexander, Felix J B Bäuerlein, Villa Elizabeth, Eibauer Matthias, Laugks Tim, Baumeister Wolfgang and Plitzko Jürgen M. Focused ion beam micromachining of eukaryotic cells for cryoelectron tomography. Proc. Natl. Acad. Sci. U. S. A., 109(12):4449–4454, March 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner Felix R, Watanabe Reika, Schampers Ruud, Singh Digvijay, Persoon Hans, Schaffer Miroslava, Fruhstorfer Peter, Plitzko Jürgen, and Villa Elizabeth. Preparing samples from whole cells using focused-ion-beam milling for cryo-electron tomography. Nat. Protoc., 15(6):2041–2070, June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck Martin and Baumeister Wolfgang. Cryo-Electron tomography: Can it reveal the molecular sociology of cells in atomic detail? Trends Cell Biol., 26(11):825–837, November 2016. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Martinez Javier, Phillips Jeremy, Sekedat Matthew D, Diaz-Avalos Ruben, Velazquez-Muriel Javier, Franke Josef D, Williams Rosemary, Stokes David L, Chait Brian T, Sali Andrej and Rout Michael P. Structure-function mapping of a heptameric module in the nuclear pore complex. J. Cell Biol., 196(4):419–434, February 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley Kotaro, Knockenhauer Kevin E, Kabachinski Greg, and Schwartz Thomas U. Atomic structure of the Y complex of the nuclear pore. Nat. Struct. Mol. Biol., 22(5):425–431, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosalaganti Shyamal, Obarska-Kosinska Agnieszka, Siggel Marc, Taniguchi Reiya, Turoňová Beata, Zimmerli Christian E, Buczak Katarzyna, Schmidt Florian H, Margiotta Erica, Mackmull Marie-Therese, Hagen Wim J H, Hummer Gerhard, Kosinski Jan, and Beck Martin. AI-based structure prediction empowers integrative structural analysis of human nuclear pores. Science, 376(6598):eabm9506, June 2022. [DOI] [PubMed] [Google Scholar]
- 36.Gaik Monika, Flemming Dirk, von Appen Alexander, Kastritis Panagiotis, Mücke Norbert, Fischer Jessica, Stelter Philipp, Ori Alessandro, Bui Khanh Huy, Baßler Jochen, Barbar Elisar, Beck Martin, and Hurt Ed. Structural basis for assembly and function of the nup82 complex in the nuclear pore scaffold. J. Cell Biol., 208(3):283–297, February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Martinez Javier, Kim Seung Joong, Shi Yi, Upla Paula, Pellarin Riccardo, Gagnon Michael, Chemmama Ilan E, Wang Junjie, Nudelman Ilona, Zhang Wenzhu, Williams Rosemary, Rice William J, Stokes David L, Zenklusen Daniel, Chait Brian T, Sali Andrej, and Rout Michael P. Structure and function of the nuclear pore complex cyto-plasmic mRNA export platform. Cell, 167(5):1215–1228.e25, November 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bensidoun Pierre, Reiter Taylor, Montpetit Ben, Zenklusen Daniel, and Oeffinger Marlene. Nuclear mRNA metabolism drives selective basket assembly on a subset of nuclear pore complexes in budding yeast. Mol. Cell, 82(20):3856–3871.e6, October 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hakhverdyan Zhanna, Molloy Kelly R, Subbotin Roman I, Fernandez-Martinez Javier, Chait Brian T, and Rout Michael P. Measuring in vivo protein turnover and exchange in yeast macromolecular assemblies. STAR Protoc, 2(3):100800, September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onischenko Evgeny, Noor Elad, Fischer Jonas S, Gillet Ludovic, Wojtynek Matthias, Vallotton Pascal, and Weis Karsten. Maturation Kinetics of a Multiprotein Complex Revealed by Metabolic Labeling. Cell, 183(7):1785–1800.e26, December 2020. [DOI] [PubMed] [Google Scholar]
- 41.Bley Christopher J, Nie Si, Mobbs George W, Petrovic Stefan, Gres Anna T, Liu Xiaoyu, Mukherjee Somnath, Harvey Sho, Huber Ferdinand M, Lin Daniel H, Brown Bonnie, Tang Aaron W, Rundlet Emily J, Correia Ana R, Chen Shane, Regmi Saroj G, Stevens Taylor A, Jette Claudia A, Dasso Mary, Patke Alina, Palazzo Alexander F, Kossiakoff Anthony A and Hoelz André. Architecture of the cytoplasmic face of the nuclear pore. Science, 376(6598):eabm9129, June 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Daniel H, Zimmermann Stephan, Stuwe Tobias, Stuwe Evelyn, and Hoelz André. Structural and functional analysis of the c-terminal domain of Nup358/RanBP2. J. Mol. Biol., 425(8):1318–1329, April 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassube Susanne A, Stuwe Tobias, Lin Daniel H, Danielle Antonuk C, Napetschnig Johanna, Blobel Günter and Hoelz André. Crystal structure of the n-terminal domain of Nup358/RanBP2. J. Mol. Biol., 423(5):752–765, November 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Timetree of Life. Oxford University Press, April 2009. [Google Scholar]
- 45.Matta Sumit K, Rinkenberger Nicholas, Dunay Ildiko R, and Sibley L David. Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol., 19(7):467–480, July 2021. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli Christian E, Allegretti Matteo, Rantos Vasileios, Goetz Sara K, Obarska-Kosinska Agnieszka, Zagoriy Ievgeniia, Halavatyi Aliaksandr, Hummer Gerhard, Mahamid Julia, Kosinski Jan, and Beck Martin. Nuclear pores dilate and constrict in cellulo. Science, 374(6573):eabd9776, December 2021. [DOI] [PubMed] [Google Scholar]
- 47.Mahamid J, Pfeffer S, Schaffer M, Villa E, Danev R, Cuellar L Kuhn, Forster F, Hyman A A, Plitzko J M, and Baumeister W. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science, 351(6276):969–972, February 2016. [DOI] [PubMed] [Google Scholar]
- 48.Tyagi Swati, Capitanio Juliana S, Xu Jiawei, Chen Fei, Sharma Rahul, Huang Jialiang, and Hetzer Martin W. High-precision mapping of nuclear pore-chromatin interactions reveals new principles of genome organization at the nuclear envelope. June 2023. [Google Scholar]
- 49.Boumendil Charlene, Hari Priya, Olsen Karl C F, Acosta Juan Carlos, and Bickmore Wendy A. Nuclear pore density controls heterochromatin reorganization during senescence. Genes Dev., 33(3-4):144–149, February 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alber Frank, Dokudovskaya Svetlana, Veenhoff Liesbeth M, Zhang Wenzhu, Kipper Julia, Devos Damien, Suprapto Adisetyantari, Karni-Schmidt Orit, Williams Rosemary, Chait Brian T, Rout Michael P, and Sali Andrej. Determining the architectures of macromolecular assemblies. Nature, 450(7170):683–694, November 2007. [DOI] [PubMed] [Google Scholar]
- 51.Rout Michael P and Sali Andrej. Principles for integrative structural biology studies, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russel Daniel, Lasker Keren, Webb Ben, Velázquez-Muriel Javier, Tjioe Elina, Schneidman-Duhovny Dina, Peterson Bret, and Sali Andrej. Putting the pieces together: integrative modeling platform software for structure determination of macromolecular assemblies. PLoS Biol., 10(1):e1001244, January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saltzberg Daniel, Greenberg Charles H, Viswanath Shruthi, Chemmama Ilan, Webb Ben, Pellarin Riccardo, Echeverria Ignacia, and Sali Andrej. Modeling Biological Complexes Using Integrative Modeling Platform, pages 353–377. Humana, New York, NY, 2019. [DOI] [PubMed] [Google Scholar]
- 54.Saltzberg Daniel J, Viswanath Shruthi, Echeverria Ignacia, Chemmama Ilan E, Webb Ben, and Sali Andrej. Using integrative modeling platform to compute, validate, and archive a model of a protein complex structure. Protein Sci, 30(1):250–261, January 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cibulka Jakub, Bisaccia Fabio, Radisavljević Katarina, Carrillo Ricardo M Gudino, and Köhler Alwin. Assembly principle of a membrane-anchored nuclear pore basket scaffold. Sci Adv, 8(6):eabl6863, February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunkel Philip, Iino Haruki, Krull Sandra, and Cordes Volker C. An evolutionarily conserved bimodular domain anchors ZC3HC1 and its yeast homologue pml39p to the nuclear basket. Mol. Biol. Cell, 34(5):ar40, May 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akey Christopher W, Echeverria Ignacia, Ouch Christna, Nudelman Ilona, Shi Yi, Wang Junjie, Chait Brian T, Sali Andrej, Fernandez-Martinez Javier, and Rout Michael P. Implications of a multiscale structure of the yeast nuclear pore complex. Mol. Cell, 83(18):3283–3302.e5, September 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fasken Milo B, Stewart Murray, and Corbett Anita H. Functional significance of the interaction between the mRNA-binding protein, nab2, and the nuclear pore-associated protein, mlp1, in mRNA export. J. Biol. Chem., 283(40):27130–27143, October 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green Deanna M, Johnson Christie P, Hagan Henry, and Corbett Anita H. The c-terminal domain of myosin-like protein 1 (mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export. Proc. Natl. Acad. Sci. U. S. A., 100 (3):1010–1015, February 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obado Samson O, Brillantes Marc, Uryu Kunihiro, Zhang Wenzhu, Ketaren Natalia E, Chait Brian T, Field Mark C and Rout Michael P. Interactome mapping reveals the evolutionary history of the nuclear pore complex. PLoS Biol., 14(2):e1002365, February 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann Patrick C, Kim Hyuntae, Obarska-Kosinska Agnieszka, Kreysing Jan Philipp, Andino-Frydman Eli, Cruz-Leon Sergio, Cernikova Lenka, Kosinski Jan, Turoňová Beata, Hummer Gerhard, and Beck Martin. Nuclear pores as conduits for fluid flow during osmotic stress. January 2024. [Google Scholar]
- 62.Gunkel Philip, Iino Haruki, Krull Sandra, and Cordes Volker C. ZC3HC1 is a novel inherent component of the nuclear basket, resident in a state of reciprocal dependence with TPR. Cells, 10(8), July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabut Gwénaël, Doye Valérie, and Ellenberg Jan. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol., 6(11):1114–1121, November 2004. [DOI] [PubMed] [Google Scholar]
- 64.Otsuka Shotaro, Tempkin Jeremy O B, Zhang Wanlu, Politi Antonio Z, Rybina Arina, Hossain M Julius, Kueblbeck Moritz, Callegari Andrea, Koch Birgit, Morero Natalia Rosalia, Sali Andrej, and Ellenberg Jan. A quantitative map of nuclear pore assembly reveals two distinct mechanisms. Nature, 613(7944):575–581, January 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saroufim M A, Bensidoun P, Raymond P, Rahman S, Krause M R, Oeffinger M and Zenklusen D. The nuclear basket mediates perinuclear mRNA scanning in budding yeast. J. Cell Biol., 211(6):1131–1140, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vermeersch Lieselotte, Cool Lloyd, Gorkovskiy Anton, Voordeckers Karin, Wenseleers Tom, and Verstrepen Kevin J. Do microbes have a memory? history-dependent behavior in the adaptation to variable environments. Front. Microbiol., 13:1004488, October 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brickner Jason H. Inheritance of epigenetic transcriptional memory through read-write replication of a histone modification. Ann. N. Y. Acad. Sci., 1526(1):50–58, August 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King Grant A, Wettstein Rahel, Varberg Joseph M, Chetlapalli Keerthana, Walsh Madison E, Gillet Ludovic C J, Hernández-Armenta Claudia, Beltrao Pedro, Aebersold Ruedi, Jaspersen Sue L, Matos Joao and Ünal Elçin. Meiotic nuclear pore complex remodeling provides key insights into nuclear basket organization. J. Cell Biol., 222(2), February 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meinema Anne C, Marzelliusardottir Anna, Mirkovic Mihailo, Aspert Théo, Lee Sung Sik, Charvin Gilles, and Barral Yves. DNA circles promote yeast ageing in part through stimulating the reorganization of nuclear pore complexes. Elife, 11, April 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carmody Sean R, Tran Elizabeth J, Apponi Luciano H, Corbett Anita H, and Wente Susan R. The mitogen-activated protein kinase slt2 regulates nuclear retention of non-heat shock mRNAs during heat shock-induced stress. Mol. Cell. Biol., 30(21):5168–5179, November 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zander Gesa, Hackmann Alexandra, Bender Lysann, Becker Daniel, Lingner Thomas, Salinas Gabriela, and Krebber Heike. mRNA quality control is bypassed for immediate export of stress-responsive transcripts. Nature, 540(7634):593–596, December 2016. [DOI] [PubMed] [Google Scholar]
- 72.Erez Neta, Israitel Lena, Bitman-Lotan Eliya, Wong Wing H, Raz Gal, Cornelio-Parra Dayanne V, Danial Salwa, Brodsly Na’ama Flint, Belova Elena, Maksimenko Oksana, Georgiev Pavel, Druley Todd, Mohan Ryan D, and Orian Amir. A non-stop identity complex (NIC) supervises enterocyte identity and protects from premature aging. Elife, 10, February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng Tiantian and Zilman Anton. Self-regulation of the nuclear pore complex enables clogging-free crowded transport. Proc. Natl. Acad. Sci. U. S. A., 120(7):e2212874120, February 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonneau Fabien, Basquin Jérôme, Steigenberger Barbara, Schäfer Tillman, Schäfer Ingmar B, and Conti Elena. Nuclear mRNPs are compact particles packaged with a network of proteins promoting RNA-RNA interactions. Genes Dev., 37(11-12):505–517, June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pacheco-Fiallos Belén, Vorländer Matthias K, Riabov-Bassat Daria, Fin Laura, O’Reilly Francis J, Ayala Farja I, Schellhaas Ulla, Rappsilber Juri, and Plaschka Clemens. mRNA recognition and packaging by the human transcription-export complex. Nature, 616(7958):828–835, April 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashkenazy-Titelman Asaf, Atrash Mohammad Khaled, Boocholez Alon, Kinor Noa, and Shav-Tal Yaron. RNA export through the nuclear pore complex is directional. Nat. Commun., 13(1):5881, October 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zila Vojtech, Margiotta Erica, Turoňová Beata, Müller Thorsten G, Zimmerli Christian E, Mattei Simone, Allegretti Matteo, Börner Kathleen, Rada Jona, Müller Barbara, Lusic Marina, Kräusslich Hans-Georg, and Beck Martin. Cone-shaped HIV-1 capsids are transported through intact nuclear pores. Cell, 184(4):1032–1046.e18, February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brickner Jason H and Walter Peter. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol., 2(11):e342, November 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cabal Ghislain G, Genovesio Auguste, Rodriguez-Navarro Susana, Zimmer Christophe, Gadal Olivier, Lesne Annick, Buc Henri, Feuerbach-Fournier Frank, Olivo-Marin Jean-Christophe, Hurt Eduard C and Nehrbass Ulf. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature, 441(7094):770–773, June 2006. [DOI] [PubMed] [Google Scholar]
- 80.Ahmed Sara, Brickner Donna G, Light William H, Cajigas Ivelisse, McDonough Michele, Froyshteter Alexander B, Volpe Tom and Brickner Jason H. DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nat. Cell Biol., 12(2):111–118, February 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Light William H, Brickner Donna G, Brand Veronica R, and Brickner Jason H. Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory. Mol. Cell, 40(1):112–125, October 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luthra Roopa, Kerr Shana C, Harreman Michelle T, Apponi Luciano H, Fasken Milo B, Ramineni Suneela, Chaurasia Shyam, Valentini Sandro R, and Corbett Anita H. Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J. Biol. Chem., 282(5):3042–3049, February 2007. [DOI] [PubMed] [Google Scholar]
- 83.Choudhry Sanjeev K, Neal Maxwell L, Li Song, Navare Arti T, Van Eeuwen Trevor, Wozniak Richard W, Mast Fred D, Rout Michael P, and Aitchison John D. Nuclear pore complexes mediate subtelomeric gene silencing by regulating PCNA levels on chromatin. J. Cell Biol., 222(9), September 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Zhixun, Du Wenjing, Yang Jiong, Lai De-Hua, Lun Zhao-Rong, and Guo Qiang. Cryo-Electron tomography of toxoplasma gondii indicates that the conoid fiber may be derived from microtubules. Adv. Sci., 10(14):e2206595, May 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lam Vinson and Villa Elizabeth. Practical approaches for Cryo-FIB milling and applications for cellular Cryo-Electron tomography. Methods Mol. Biol., 2215:49–82, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mastronarde David N. SerialEM: A program for automated tilt series acquisition on tecnai microscopes using prediction of specimen position. Microsc. Microanal., 9(S02):1182–1183, August 2003. [Google Scholar]
- 87.Mastronarde David N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol., 152(1):36–51, October 2005. [DOI] [PubMed] [Google Scholar]
- 88.Eisenstein Fabian, Yanagisawa Haruaki, Kashihara Hiroka, Kikkawa Masahide, Tsukita Sachiko, and Danev Radostin. Parallel cryo electron tomography on in situ lamellae. Nat. Methods, 20(1):131–138, January 2023. [DOI] [PubMed] [Google Scholar]
- 89.Tegunov Dimitry and Cramer Patrick. Real-time cryo-electron microscopy data preprocessing with Warp. Nat. Methods, 16(11):1146–1152, November 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tegunov Dimitry, Xue Liang, Dienemann Christian, Cramer Patrick, and Mahamid Julia. Multi-particle cryo-EM refinement with M visualizes ribosome-antibiotic complex at 3.5 Å in cells. Nat. Methods, 18(2):186–193, February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng Shawn, Wolff Georg, Greenan Garrett, Chen Zhen, Faas Frank G A, Bárcena Montserrat, Koster Abraham J, Cheng Yifan, and Agard David A. AreTomo: An integrated software package for automated marker-free, motion-corrected cryo-electron tomographic alignment and reconstruction. Journal of structural biology: X, 6:100068, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kremer J R, Mastronarde D N, and McIntosh J R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol., 116(1):71–76, 1996. [DOI] [PubMed] [Google Scholar]
- 93.Castaño-Diez Daniel, Kudryashev Mikhail, and Stahlberg Henning. Dynamo Catalogue: Geometrical tools and data management for particle picking in subtomogram averaging of cryo-electron tomograms. J. Struct. Biol., 197(2):135–144, February 2017. [DOI] [PubMed] [Google Scholar]
- 94.Zivanov Jasenko, Nakane Takanori, Forsberg Björn O, Kimanius Dari, Hagen Wim Jh, Lindahl Erik, and Scheres Sjors Hw. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife, 7, November 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenthal Peter B and Henderson Richard. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol., 333(4):721–745, October 2003. [DOI] [PubMed] [Google Scholar]
- 96.Pettersen Eric F, Goddard Thomas D, Huang Conrad C, Couch Gregory S, Greenblatt Daniel M, Meng Elaine C, and Ferrin Thomas E. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem., 25(13):1605–1612, October 2004. [DOI] [PubMed] [Google Scholar]
- 97.Niepel M, Strambio-de Castillia C, Fasolo J, Chait B T, and Rout M P. The nuclear pore complex-associated protein, Mlp2p, binds to the yeast spindle pole body and promotes its efficient assembly. J. Cell Biol., 170(2):225–235, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Zhen-Lin, Meng Jia-Ming, Cao Yong, Yin Ji-Li, Fang Run-Qian, Fan Sheng-Bo, Liu Chao, Zeng Wen-Feng, Ding Yue-He, Tan Dan, Wu Long, Zhou Wen-Jing, Chi Hao, Sun Rui-Xiang, Dong Meng-Qiu, and He Si-Min. A high-speed search engine plink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nat. Commun., 10(1):3404, July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nucleic Acids Research and 2023. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res., 51(D1):D523–D531, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vallotton Pascal, Rajoo Sasikumar, Wojtynek Matthias, Onischenko Evgeny, Kralt Annemarie, Derrer Carina Patrizia, and Weis Karsten. Mapping the native organization of the yeast nuclear pore complex using nuclear radial intensity measurements. Proc. Natl. Acad. Sci. U. S. A., 116(29):14606–14613, July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soni Neelesh and Madhusudhan Mallur S. COCONUT: An analysis of coiled-coil regions in proteins. bioRxiv, 2024. [Google Scholar]
- 102.Frosst Phyllis, Guan Tinglu, Subauste Cecilia, Hahn Klaus, and Gerace Larry. Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export. J. Cell Biol., 156(4):617–630, February 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sali A and Blundell T L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol., 234(3):779–815, December 1993. [DOI] [PubMed] [Google Scholar]
- 104.Eswar Narayanan, Webb Ben, Marti-Renom Marc A, Madhusudhan M S, Eramian David, Shen Min-Yi, Pieper Ursula and Sali Andrej. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci., Chapter 2:Unit 2.9, November 2007. [DOI] [PubMed] [Google Scholar]
- 105.Shi Yi, Pellarin Riccardo, Fridy Peter C, Fernandez-Martinez Javier, Thompson Mary K, Li Yinyin, Wang Qing Jun, Sali Andrej, Rout Michael P, and Chait Brian T. A strategy for dissecting the architectures of native macromolecular assemblies. Nat. Methods, 12(12):1135–1138, December 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swendsen R H and Wang J S. Replica monte carlo simulation of spin glasses. Phys. Rev. Lett., 57(21):2607–2609, November 1986. [DOI] [PubMed] [Google Scholar]
- 107.Viswanath Shruthi, Chemmama Ilan E, Cimermancic Peter, and Sali Andrej. Assessing exhaustiveness of stochastic sampling for integrative modeling of macromolecular structures. Biophys. J., 113(11):2344–2353, December 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaake Robyn M, Echeverria Ignacia, Kim Seung Joong, Dollen John Von, Chesarino Nicholas M, Feng Yuqing, Yu Clinton, Ta Hai, Chelico Linda, Huang Lan, Gross John, Sali Andrej, and Krogan Nevan J. Characterization of an A3G-VifHIV-1-CRL5-CBF structure using a cross-linking mass spectrometry pipeline for integrative modeling of Host-Pathogen complexes. Mol. Cell. Proteomics, 20:100132, August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDonnell A V, Jiang T, Keating A E and Berger B. Paircoil2: improved prediction of coiled coils from sequence. Bioinformatics, 22(3):356–358, February 2006. [DOI] [PubMed] [Google Scholar]
- 110.Jumper John, Evans Richard, Pritzel Alexander, Green Tim, Figurnov Michael, Ronneberger Olaf, Tunyasuvunakool Kathryn, Bates Russ, Žídek Augustin, Potapenko Anna, Bridgland Alex, Meyer Clemens, Kohl Simon A A, Ballard Andrew J, Cowie Andrew, Romera-Paredes Bernardino, Nikolov Stanislav, Jain Rishub, Adler Jonas, Back Trevor, Petersen Stig, Reiman David, Clancy Ellen, Zielinski Michal, Steinegger Martin, Pacholska Michalina, Berghammer Tamas, Bodenstein Sebastian, Silver David, Vinyals Oriol, Senior Andrew W, Kavukcuoglu Koray, Kohli Pushmeet, and Hassabis Demis. Highly accurate protein structure prediction with AlphaFold. Nature, 596(7873):583–589, August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Varadi Mihaly, Anyango Stephen, Deshpande Mandar, Nair Sreenath, Natassia Cindy, Yordanova Galabina, Yuan David, Stroe Oana, Wood Gemma, Laydon Agata, Žídek Augustin, Green Tim, Tunyasuvunakool Kathryn, Petersen Stig, Jumper John, Clancy Ellen, Green Richard, Vora Ankur, Lutfi Mira, Figurnov Michael, Cowie Andrew, Hobbs Nicole, Kohli Pushmeet, Kleywegt Gerard, Birney Ewan, Hassabis Demis, and Velankar Sameer. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res., 50(D1):D439–D444, January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Yichen, Aksenova Vasilisa, Tingey Mark, Yu Jingjie, Ma Ping, Arnaoutov Alexei, Chen Shane, Dasso Mary, and Yang Weidong. Distinct roles of nuclear basket proteins in directing the passage of mRNA through the nuclear pore. Proc. Natl. Acad. Sci. U. S. A., 118(37), September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ori Alessandro, Banterle Niccolò, Iskar Murat, Andrés-Pons Amparo, Escher Claudia, Bui Huy Khanh, Sparks Lenore, Solis-Mezarino Victor, Rinner Oliver, Bork Peer, Lemke Edward A, and Beck Martin. Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines. Mol. Syst. Biol., 9(1):648, January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vollmer Benjamin, Lorenz Michael, Moreno-Andrés Daniel, Bodenhöfer Mona, Magistris Paola De, Astrinidis Susanne Adina, Schooley Allana, Flötenmeyer Matthias, Leptihn Sebastian, and Antonin Wolfram. Nup153 recruits the nup107-160 complex to the inner nuclear membrane for interphasic nuclear pore complex assembly. Dev. Cell, 33(6):717–728, June 2015. [DOI] [PubMed] [Google Scholar]
- 115.Goddard Thomas D, Huang Conrad C, Meng Elaine C, Pettersen Eric F, Couch Gregory S, Morris John H, and, Ferrin Thomas E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci., 27(1):14–25, January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kohn Jonathan E, Millett Ian S, Jacob Jaby, Zagrovic Bojan, Dillon Thomas M, Cingel Nikolina, Dothager Robin S, Seifert Soenke, Thiyagarajan P, Sosnick Tobin R, Hasan M Zahid, Pande Vijay S, Ruczinski Ingo, Doniach Sebastian and Plaxco Kevin W. Random-coil behavior and the dimensions of chemically unfolded proteins. Proc. Natl. Acad. Sci. U. S. A., 101(34):12491–12496, August 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kawabata Takeshi. Multiple subunit fitting into a low-resolution density map of a macromolecular complex using a gaussian mixture model. Biophys. J., 95(10):4643–4658, November 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.