ABSTRACT
Emerging SARS-CoV-2 sublineages continue to cause serious COVID-19 disease, but most individuals have not received any COVID-19 vaccine for >1 year. Assessment of long-term effectiveness of bivalent COVID-19 vaccines against circulating sublineages is important to inform the potential need for vaccination with updated vaccines. In this test-negative study at Kaiser Permanente Southern California, sequencing-confirmed BA.4/BA.5- or XBB-related SARS-CoV-2-positive cases (September 1, 2022 to June 30, 2023), were matched 1:3 to SARS-CoV-2-negative controls. We assessed mRNA-1273 bivalent relative (rVE) and absolute vaccine effectiveness (VE) compared to ≥2 or 0 doses of original monovalent vaccine, respectively. The rVE analysis included 20,966 cases and 62,898 controls. rVE (95%CI) against BA.4/BA.5 at 14–60 days and 121–180 days was 52.7% (46.9–57.8%) and 35.5% (−2.8–59.5%) for infection, and 59.3% (49.7–67.0%) and 33.2% (−28.2–68.0%) for Emergency Department/Urgent Care (ED/UC) encounters. For BA.4/BA.5-related hospitalizations, rVE was 71.3% (44.9–85.1%) and 52.0% (−1.2–77.3%) at 14–60 days and 61–120 days, respectively. rVE against XBB at 14–60 days and 121–180 days was 48.8% (33.4–60.7%) and −3.9% (−18.1–11.3%) for infection, 70.7% (52.4–82.0%) and 15.7% (−6.0–33.2%) for ED/UC encounters, and 87.9% (43.8–97.4%) and 57.1% (17.0–77.8%) for hospitalization. VE and subgroup analyses (age, immunocompromised status, previous SARS-CoV-2 infection) results were similar to rVE analyses. rVE of mRNA-1273 bivalent vaccine against BA.4/BA.5 and XBB infections, ED/UC encounters, and hospitalizations waned over time. Periodic revaccination with vaccines targeting emerging variants may be important in reducing COVID-19 morbidity and mortality.
KEYWORDS: Bivalent, XBB, BA.4/BA.5, BA.4, BA.5, omicron, COVID-19, mRNA-1273, vaccine effectiveness, durability
Introduction
As of October 7, 2023, COVID-19 has caused more than 6.4 million hospitalizations and 1.1 million deaths in the United States.1 Although monovalent COVID-19 vaccines against the original SARS-CoV-2 variants were highly effective in preventing SARS-CoV-2 infections and severe outcomes, their effectiveness decreased over time due to waning immunity and emergence of immune evasive omicron variants.2 To address this concern, updated bivalent COVID-19 vaccines containing equal amounts of the original variant and omicron BA.4/BA.5 mRNA were developed. On August 31, 2022, Moderna and Pfizer mRNA bivalent COVID-19 vaccines were authorized in the United States for adults aged ≥18 years who had received at least two monovalent doses,3 and by April 18, 2023, bivalent COVID-19 vaccines were authorized for all individuals aged ≥6 months.4
Early post-authorization studies demonstrated improved effectiveness of bivalent COVID-19 vaccines against COVID-19 outcomes during BA.4/BA.5 predominance compared to original monovalent COVID-19 vaccines.5 However, immune evasive omicron sublineages emerged, including XBB, against which several in vitro studies found lower neutralizing activity compared with that against previous omicron sublineages after receipt of bivalent vaccine.6 Data on the effectiveness of bivalent COVID-19 vaccines against XBB-related sublineages indicated that receipt of bivalent vaccine provided moderately improved protection against COVID-19 compared with receipt of no COVID-19 vaccines or original monovalent vaccines only.7,8 However, protection waned over as little as 2–6 months, likely due to both time since vaccination and the replacement of BA.4/BA.5 by BQ.1 and XBB-related sublineages during that time. Subsequently, updated monovalent COVID-19 vaccines targeting XBB-related sublineages on September 11, 2023, for persons aged ≥6 months were authorized.9
Despite continued high infection rates and 641,838 hospitalizations and 53,961 deaths from COVID-19 in the first 9 months of 2023, most of the US population has not received any COVID-19 vaccine in ≥1 year.1 Since only 17% of US individuals received a bivalent vaccine dose, concerns exist that uptake of the updated monovalent XBB vaccine may also be low.10,11 Hence, data on the durability of bivalent COVID-19 vaccine effectiveness against COVID-19 outcomes with currently circulating variants are needed to inform regulatory agencies, healthcare providers, and individuals of the potential importance of receiving an updated monovalent XBB vaccine.12 Therefore, we evaluated the absolute and relative effectiveness and durability of mRNA-1273 bivalent vaccine against a range of outcomes with sequencing-confirmed omicron BA.4/BA.5- and XBB-related sublineages.
Methods
Study setting
Kaiser Permanente Southern California (KPSC) is a large integrated healthcare system serving over 4.8 million socio-demographically diverse members at 15 hospitals and associated medical offices across Southern California. Comprehensive electronic health records (EHRs) used for this study included demographic information, vaccinations, diagnoses, laboratory tests, procedures, and pharmacy records. External COVID-19 vaccinations were imported into members’ EHRs daily, including from the California Immunization Registry, to which all COVID-19 vaccinations must be reported within 24 hours,13 and by member self-report (with valid documentation). The study was approved by the KPSC Institutional Review Board.
Laboratory methods
Molecular diagnostic testing for SARS-CoV-2 is available to KPSC members on request for any reason and for diagnostic purposes. Specimens were primarily collected using nasopharyngeal/oropharyngeal swabs (for symptomatic or asymptomatic individuals) or saliva (for asymptomatic individuals). Specimens were tested using RT-PCR TaqPath COVID-19 High-Throughput Combo Kit (ThermoFisher Scientific). Random samples of SARS-CoV-2 positive specimens were sent weekly for whole genome sequencing (WGS), as described previously.14–16
Study design
We used a test-negative case–control design to assess the relative vaccine effectiveness (rVE) and absolute vaccine effectiveness (VE) of mRNA-1273 bivalent vaccine as part of a regulatory commitment from Moderna to multiple health authorities. Cases included individuals with specimens collected between September 1, 2022, to June 30, 2023, that tested positive by SARS-CoV-2 RT-PCR and were sent for whole genome sequencing; controls were selected from those with a negative SARS-CoV-2 RT-PCR test and no SARS-CoV-2 positive molecular or antigen test, COVID-19 diagnosis code, or antiviral treatment during the same period. Cases and controls were included if they were aged ≥6 months with ≥12 months of KPSC membership before the specimen collection (index) date or since ≥3 months of age (for ascertainment of exposure status and covariates). Individuals were excluded if they received a COVID-19 bivalent vaccine other than mRNA-1273 bivalent vaccine, received any COVID-19 vaccine <14 days before the index date or <52 days prior to the bivalent COVID-19 vaccine (≥8 weeks with a 4-day grace period), or if they had a history of COVID-19 (SARS-CoV-2–positive molecular or antigen test, COVID-19 diagnosis code, or antiviral treatment) ≤90 days before the index date. For cases or controls with more than one test meeting all criteria, only the first eligible positive or negative SARS-CoV-2 RT-PCR test was included in the analysis, respectively. Controls were randomly selected and matched 3:1 to cases by age (≤5, 6–17, 18–44, 45–64, 65–74, and ≥75 years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian and other/unknown) and index date (±10 days). These matching variables were chosen as they were related to both outcome and exposure; matching on index date (specimen collection date) was particularly important since the risk of infection and probability of vaccination varied over time. Matching was conducted separately for the rVE and VE analytic cohorts so that separate confounding adjustment and analyses could be conducted.
Exposures
The primary exposure was receipt of mRNA-1273 bivalent vaccine (mRNA-1273.222 [original and omicron BA.4/BA.5]) following receipt of ≥2 doses of original monovalent mRNA COVID-19 vaccine (Pfizer, Moderna, or mixed) prior to the index date. For rVE analyses, the comparator exposure group comprised recipients of ≥2 doses of original monovalent mRNA vaccine who had not received COVID-19 bivalent vaccine prior to the index date. For VE analyses, the comparator group included individuals who had not received any COVID-19 vaccine prior to the index date.
Outcomes
Separate analyses were conducted for rVE and VE analyses against BA.4/BA.5-related (e.g., BA.4, BA.5, BQ.1 and BQ.1.1) and against XBB-related sublineages (XBB.1.5, XBB.1.16, XBB.1.9, and others). BA.2 was not included in the analyses due to low prevalence during the study period. Outcomes assessed included SARS-CoV-2 infection (any care setting), emergency department/urgent care (ED/UC) encounter occurring on or ≤7 days after the index date, COVID-19 hospitalization, and COVID-19 hospital death. COVID-19 hospitalizations were identified among cases with index dates ≤7 days prior to or during a hospitalization with confirmation by manual chart review performed by a physician investigator (B. K. A.) and trained chart abstractors to verify the presence of severe COVID-19 symptoms.
Covariates
Potential confounders were identified a priori based on the literature. Variables collected from EHRs at the index date included age, sex, self-reported race/ethnicity, socioeconomic status (Medicaid and neighborhood median household income), medical center area, and pregnancy status. Variables assessed before the index date included body mass index, smoking, Charlson comorbidity score, frailty index, chronic diseases, immunocompromised status, autoimmune conditions, and time since history of SARS-CoV-2 infection (based on available testing and diagnosis records from 3/1/2020 to the index date). To account for potential differences in care-seeking or test-seeking behaviors, healthcare utilization (virtual, outpatient, ED, and inpatient encounters), preventive care (influenza and other vaccinations, screenings, and wellness visits), and history of SARS-CoV-2 molecular tests were assessed. Additional covariates included month of specimen collection, specimen type, number of original monovalent COVID-19 vaccine doses prior to index date (mRNA and non-mRNA doses; for rVE analyses only) and any antiviral therapy (nirmatrelvir/ritonavir, molnupiravir, or remdesivir) ≤7 days after the index date.
Statistical analyses
Characteristics of cases and controls for each analysis were compared using the χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables. Absolute standardized difference (ASD) was calculated to assess the balance of covariates. Covariates described above with ASD > 0.1 were considered potential confounders and included in the adjusted models. Conditional logistic regression was used to estimate the adjusted odds ratios (OR) and 95% confidence intervals (CI) for vaccination with mRNA-1273 bivalent vaccine against BA.4/BA.5- and XBB-related SARS-CoV-2 infection, ED/UC encounters, COVID-19 hospitalization, and COVID-19 hospital death. We calculated rVE (%) and VE (%) as (1-OR) when OR was ≤1 and ([1/OR] − 1) when OR was >1. We also assessed rVE and VE by time since vaccination (14–60, 61–120, 121–180, and >180 days). Unconditional logistic regression adjusting for matching variables and potential confounders was used when matching was broken. Analyses were conducted using SAS 9.4 (SAS Institute, Cary NC).
Subgroup and sensitivity analyses
We conducted subgroup analyses by age group (≤17, 18–64, and ≥65 years), among immunocompromised individuals, and among individuals with a known history of SARS-CoV-2 infection. For sensitivity analysis, we used S-gene target failure (SGTF) status and calendar month as a proxy to assign SARS-CoV-2 lineage among cases that failed sequencing and had available SGTF data, as described previously.15,16 Positive specimens collected between September 1, 2022, and March 31, 2023, with SGTF were considered to be BA.4/BA.5-related sublineages, whereas positive specimens collected between February 1, 2023, and June 30, 2023, without SGTF were considered to be XBB-related sublineages. We also conducted rVE and VE sensitivity analyses against XBB.1.5 and against BA.4/BA.5- and XBB-related COVID-19 hospitalization and hospital death among those without antiviral treatment.
Results
The study identified 28,227 eligible cases and 90,043 eligible controls, of which 20,966 cases and 62,898 matched controls were included in the rVE analysis, and 10,336 cases and 31,008 matched controls were included in the VE analysis (Suplementary Figure S1). Among 28,227 specimens sent for sequencing, 54.1% were successfully sequenced (Supplementary Table S1, Supplementary Figures S2 and S3). Compared to specimens with successful sequencing, a greater proportion of those with sequencing failure were saliva specimens (18.3% vs 8.4%) and had cycle threshold values >27 (63.2% vs 2.0%). Most variants identified were BA.4/BA.5-related (65.0%) or XBB-related sublineages (30.6%).
We describe characteristics of cases and controls included in rVE (Table 1) and absolute VE (Suplementary Table S2) analyses. In the rVE analyses, median (IQR) age in years was 52 (37–65), 57.0% were female, and 48.5% were Hispanic. In the VE analysis, median (IQR) age in years was 41 (21–61) for cases and 40 (20–61) for controls; 52.8% of both cases and controls were female, and 47.8% were Hispanic. Since most BA.4/BA.5-related cases occurred in 2022, and most XBB-related cases occurred in 2023, the average follow-up time after vaccination was shorter for BA.4/BA.5-related outcomes than XBB-related outcomes (Supplementary Figure S3).
Table 1.
Characteristics of SARS-CoV-2 test-positive cases and test-negative controls with mRNA-1273 bivalent vaccine or ≥2 doses monovalent mRNA vaccines.
| Test Positive | Test Negative | ||
|---|---|---|---|
| N = 20,966 | N = 62,898 | ASD | |
| Age at specimen collection date, years | <0.01 | ||
| Mean (SD) | 51.58 (18.33) | 51.55 (18.63) | |
| Median | 52 | 52 | |
| Q1, Q3 | 37, 65 | 37, 65 | |
| Min, max | 0.7, 102 | 0.7, 105 | |
| Age at specimen collection date, years, n (%) | 0.00 | ||
| ≤5 | 123 (0.6%) | 369 (0.6%) | |
| 6–17 | 210 (1.0%) | 630 (1.0%) | |
| 18–44 | 7413 (35.4%) | 22,239 (35.4%) | |
| 45–64 | 7857 (37.5%) | 23,571 (37.5%) | |
| 65–74 | 2865 (13.7%) | 8595 (13.7%) | |
| ≥75 | 2498 (11.9%) | 7494 (11.9%) | |
| Sex, n (%) | 0.00 | ||
| Female | 11,954 (57.0%) | 35,862 (57.0%) | |
| Male | 9012 (43.0%) | 27,036 (43.0%) | |
| Race/ethnicity, n (%) | 0.00 | ||
| Non-Hispanic White | 4842 (23.1%) | 14,526 (23.1%) | |
| Non-Hispanic Black | 1763 (8.4%) | 5289 (8.4%) | |
| Hispanic | 10,160 (48.5%) | 30,480 (48.5%) | |
| Non-Hispanic Asian | 2968 (14.2%) | 8904 (14.2%) | |
| Other/Unknown | 1233 (5.9%) | 3699 (5.9%) | |
| Body mass index,a kg/m,2 n (%) | 0.11 | ||
| <18.5 | 351 (1.7%) | 1272 (2.0%) | |
| 18.5–<25 | 4378 (20.9%) | 13,618 (21.7%) | |
| 25 –<30 | 6180 (29.5%) | 18,983 (30.2%) | |
| 30–<35 | 4466 (21.3%) | 13,887 (22.1%) | |
| 35–<40 | 2408 (11.5%) | 7015 (11.2%) | |
| 40–<45 | 1022 (4.9%) | 3130 (5.0%) | |
| ≥45 | 727 (3.5%) | 2211 (3.5%) | |
| Unknown | 1434 (6.8%) | 2782 (4.4%) | |
| Smoking,a n (%) | 0.09 | ||
| No | 15,795 (75.3%) | 46,743 (74.3%) | |
| Yes | 4226 (20.2%) | 14,197 (22.6%) | |
| Unknown | 945 (4.5%) | 1958 (3.1%) | |
| Charlson comorbidity scoreb,c | 0.10 | ||
| Mean (SD) | 1.09 (1.94) | 1.29 (2.09) | |
| Median | 0 | 0 | |
| Q1, Q3 | 0, 1 | 0, 2 | |
| Min, max | 0, 17 | 0, 17 | |
| Charlson comorbidity score,b,c n (%) | 0.11 | ||
| 0 | 12,596 (60.1%) | 34,461 (54.8%) | |
| 1 | 3537 (16.9%) | 11,224 (17.8%) | |
| ≥2 | 4833 (23.1%) | 17,213 (27.4%) | |
| Frailty indexb,d | 0.10 | ||
| Mean (SD) | 0.12 (0.04) | 0.13 (0.04) | |
| Median | 0.11 | 0.12 | |
| Q1, Q3 | 0.10, 0.14 | 0.10, 0.14 | |
| Min, max | 0.05, 0.40 | 0.05, 0.40 | |
| Frailty index,b,d n (%) | 0.12 | ||
| Quartile 1 | 5689 (27.1%) | 15,277 (24.3%) | |
| Quartile 2 | 5607 (26.7%) | 15,324 (24.4%) | |
| Quartile 3 | 5102 (24.3%) | 15,901 (25.3%) | |
| Quartile 4, most frail | 4568 (21.8%) | 16,396 (26.1%) | |
| Chronic diseases,b n (%) | |||
| Kidney disease | 1796 (8.6%) | 6219 (9.9%) | 0.05 |
| Heart disease | 1165 (5.6%) | 4355 (6.9%) | 0.06 |
| Lung disease | 2602 (12.4%) | 9341 (14.9%) | 0.07 |
| Liver disease | 1084 (5.2%) | 3874 (6.2%) | 0.04 |
| Diabetes | 4093 (19.5%) | 13,484 (21.4%) | 0.05 |
| Immunocompromised, n (%) | 0.09 | ||
| Yes | 924 (4.4%) | 4032 (6.4%) | |
| HIV/AIDS | 75 | 293 | |
| Leukemia, lymphoma, congenital and other immunodeficiencies, Asplenia/hyposplenia | 386 | 1428 | |
| Organ transplant | 127 | 483 | |
| Immunosuppressant medications | 571 | 2725 | |
| Autoimmune conditions,b n (%) | 0.02 | ||
| Yes | 796 (3.8%) | 2641 (4.2%) | |
| Rheumatoid arthritis | 357 | 1203 | |
| Inflammatory bowel disease | 148 | 569 | |
| Psoriasis and psoriatic arthritis | 276 | 847 | |
| Multiple sclerosis | 43 | 111 | |
| Systemic lupus erythematosus | 64 | 241 | |
| Pregnant at specimen collection date, n (%) | 0.15 | ||
| Yes | 331 (1.6%) | 2497 (4.0%) | |
| 1st trimester | 73 | 204 | |
| 2nd trimester | 100 | 262 | |
| 3rd trimester | 158 | 2031 | |
| History of SARS-CoV-2 infectione, n (%) | 0.20 | ||
| Yes | 5721 (27.3%) | 23002 (36.6%) | |
| <180 days | 334 | 3949 | |
| 180–<360 days | 1859 | 8549 | |
| ≥360 days | 3528 | 10504 | |
| History of SARS-CoV-2 molecular test,e n (%) | 17,319 (82.6%) | 49,090 (78.0%) | 0.11 |
| Number of outpatient and virtual visits,b n (%) | 0.18 | ||
| 0 | 830 (4.0%) | 1779 (2.8%) | |
| 1–4 | 4966 (23.7%) | 11,533 (18.3%) | |
| 5–10 | 6027 (28.7%) | 17,022 (27.1%) | |
| ≥11 | 9143 (43.6%) | 32,564 (51.8%) | |
| Number of emergency department visits,b n (%) | 0.13 | ||
| 0 | 16,138 (77.0%) | 44,945 (71.5%) | |
| 1 | 3050 (14.5%) | 11,150 (17.7%) | |
| ≥2 | 1778 (8.5%) | 6803 (10.8%) | |
| Number of hospitalizations,b n (%) | 0.06 | ||
| 0 | 19,425 (92.7%) | 57,233 (91.0%) | |
| 1 | 1060 (5.1%) | 3992 (6.3%) | |
| ≥2 | 481 (2.3%) | 1673 (2.7%) | |
| Preventive care,b n (%) | 16,960 (80.9%) | 52,478 (83.4%) | 0.07 |
| Medicaid, n (%) | 2026 (9.7%) | 7529 (12.0%) | 0.07 |
| Neighborhood median household income, n (%) | 0.02 | ||
| <$40,000 | 352 (1.7%) | 1140 (1.8%) | |
| $40,000–$59,999 | 3445 (16.4%) | 10,066 (16.0%) | |
| $60,000–$79,999 | 4974 (23.7%) | 14,882 (23.7%) | |
| ≥$80,000 | 12,148 (57.9%) | 36,702 (58.4%) | |
| Unknown | 47 (0.2%) | 108 (0.2%) | |
| Medical center areaf | 0.14 | ||
| Month of specimen collection, n (%) | 0.10 | ||
| September to October 2022 | 3797 (18.1%) | 12,675 (20.2%) | |
| November to December 2022 | 8709 (41.5%) | 23,296 (37.0%) | |
| January to February 2023 | 3356 (16.0%) | 11,004 (17.5%) | |
| March to April 2023 | 2867 (13.7%) | 9411 (15.0%) | |
| May to June 2023 | 2237 (10.7%) | 6512 (10.4%) | |
| Specimen type, n (%) | 0.10 | ||
| Nasopharyngeal/oropharyngeal swab | 18262 (87.1%) | 56,730 (90.2%) | |
| Saliva | 2704 (12.9%) | 6168 (9.8%) | |
| Vaccination status, n (%) | 0.06 | ||
| Bivalent vaccinated | 3075 (14.7%) | 10,712 (17.0%) | |
| ≥2 doses monovalent mRNA | 17,891 (85.3%) | 52,186 (83.0%) | |
| Number of monovalent vaccines prior to index date,g n (%) | 0.10 | ||
| 2 doses | 4283 (20.4%) | 15,543 (24.7%) | |
| 3 doses | 12,075 (57.6%) | 33,989 (54.0%) | |
| ≥4 doses | 4608 (22.0%) | 13,366 (21.3%) | |
| Antiviral therapy within 7 days after the index date n (%) | N/A | ||
| Yes | 3844 (18.3%) | N/A | |
| Nirmatrelvir/ritonavir | 3779 | N/A | |
| Molnupiravir | 28 | N/A | |
| Remdesivir | 41 | N/A |
ASD = absolute standardized difference, N/A = not applicable.
aDefined in the 2 years prior to specimen collection date.
bDefined in the year prior to specimen collection date.
cPossible range: 0–29.30
dPossible range: 0–1.31
eDefined based on all available medical records from March 1, 2020, to specimen collection date.
fFrequency and percent for the 19 medical center areas not shown.
gDefined based on all available vaccine records from December 11, 2020, to index date.
At 14–60 days since vaccination, rVE against BA.4/BA.5 was 52.7% (46.9–57.8%) for SARS-CoV-2 infection, 59.3% (49.7–67.0%) for ED/UC encounters, and 71.3% (44.9–85.1%) for COVID-19 hospitalization (Figure 1 and Supplementary Table S3). However, waning of rVE against BA.4/BA.5 and XBB was observed for all outcomes >120 days. The rVE against BA.4/BA.5 was 35.5% (−2.8–59.5%) for SARS-CoV-2 infection and 33.2% (−28.2–68.0%) for ED/UC encounters at 121–180 days since vaccination. Due to insufficient numbers, rVE could not be assessed against BA.4/BA.5 for COVID-19 hospitalization at 121–180 days and for all outcomes at >180 days. rVE against XBB at 14–60 days since vaccination was 48.8% (33.4–60.7%) for SARS-CoV-2 infection, 70.7% (52.4–82.0%) for ED/UC encounters, and 87.9% (43.8–97.4%) for COVID-19 hospitalization. rVE against XBB at 121–180 days since vaccination was −3.9% (−18.1–11.3%) for SARS-CoV-2 infection, 15.7% (−6.0–33.2%) for ED/UC encounters, and 57.1% (17.0–77.8%) for COVID-19 hospitalization. At >180 days since vaccination, point estimates of rVE against XBB were lower but had overlapping confidence intervals. Due to insufficient numbers, waning was not assessed against BA.4/BA.5 or XBB for COVID-19 hospital death; however, over the study period, confidence intervals for rVE against COVID-19 hospital death were wide and non-significant.
Figure 1.
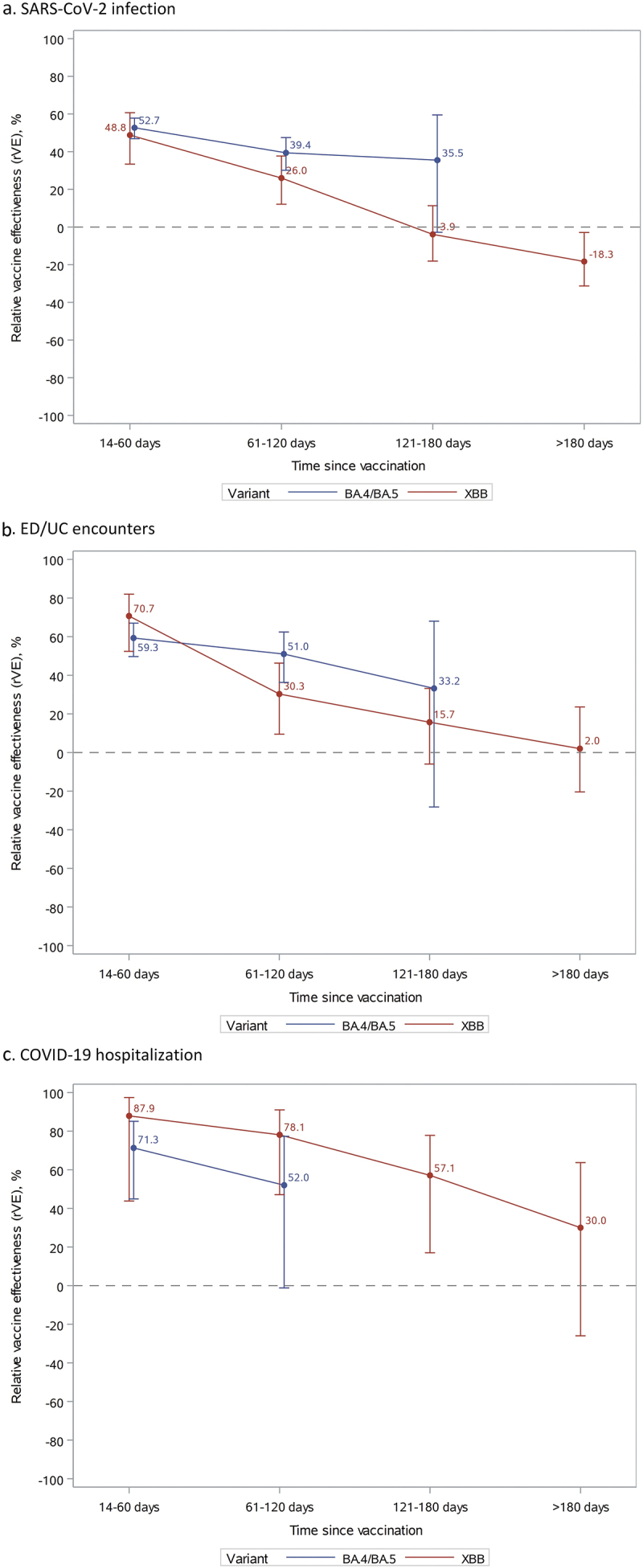
Vaccine effectiveness of mRNA-1273 bivalent vaccine vs ≥2 monovalent mRNA vaccines against SARS-CoV-2 infection, ED/UC encounters, or COVID-19 hospitalization by variant and time since vaccination.
At 14–60 days since vaccination, VE against BA.4/BA.5 was 29.3% (19.1–38.2%) for SARS-CoV-2 infection, 55.6% (43.4–65.1%) for ED/UC encounters, and 76.9% (52.7–88.7%) for COVID-19 hospitalization (Figure 2 and Supplementary Table S4). A similar waning trend was observed for VE against BA.4/BA.5 and XBB against all outcomes. At 120–180 days, VE against BA.4/BA.5 was negligible for SARS-CoV-2 infection and for ED/UC encounters and could not be assessed for COVID-19 hospitalization due to insufficient numbers. VE against XBB at 14–60 days was 21.2% (−4.6–40.7%) for SARS-CoV-2 infection, 60.4% (33.8–76.3%) for ED/UC encounters, and 93.4% (68.6–98.6%) for COVID-19 hospitalization. VE against XBB quickly became negligible for SARS-CoV-2 infection and ED/UC encounters; however, at 121–180 days and >180 days since vaccination, VE against XBB for COVID-19 hospitalization was 63.8% (20.1–83.6%) and 42.4% (−19.2–73.2%), respectively.
Figure 2.
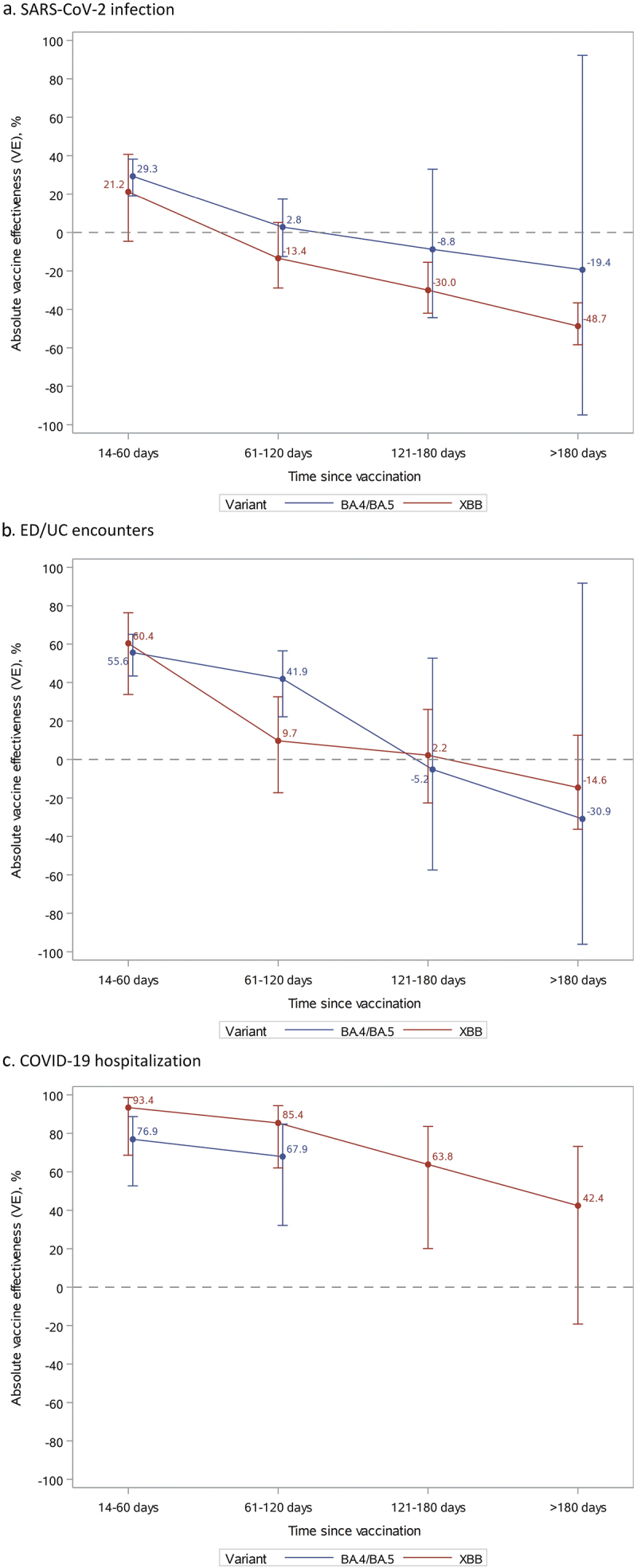
Vaccine effectiveness of mRNA-1273 bivalent vaccine vs unvaccinated against SARS-CoV-2 infection, ED/UC encounters, or COVID-19 hospitalization by variant and time since vaccination.
Results of subgroup analyses and sensitivity analyses generally followed the main results. rVE and VE in subgroup analyses by age group, immunocompromised status, and history of SARS-CoV-2 infection were consistent with the main results, although confidence intervals for subgroups analyses were wider (Figures 3 and 4 and Supplementary Tables S5–10). However, rVE against BA.4/BA.5 for ED/UC encounters was lower among immunocompromised individuals (12.1% [−33.1–48.4%]), and VE against BA.4/BA.5 for SARS-CoV-2 infection was higher among individuals with previous SARS-CoV-2 infection (51.8% [37.5–62.8%]). Results were similar for sensitivity analyses using SGTF data to assign unidentified subvariants, sensitivity analyses against XBB.1.5 specifically, and sensitivity analyses against BA.4/BA.5 and XBB for COVID-19 hospitalization and hospital death among those without antiviral treatment (Supplementary Tables S11–16).
Figure 3.
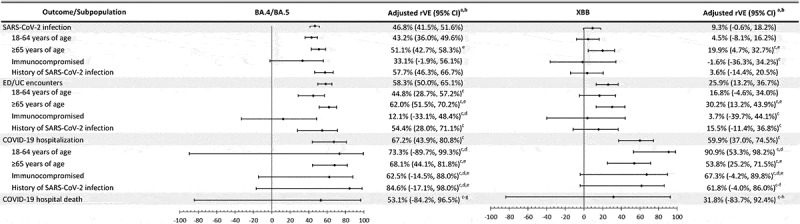
Vaccine effectiveness of mRNA-1273 bivalent vaccine vs ≥ 2 monovalent mRNA vaccines against infection and severe outcomes with SARS-CoV-2 variants, overall and by subpopulation.
BMI = body mass index, ED/UC = emergency department/urgent care, rVE = relative vaccine effectiveness.
aApplied conditional logistic models conditioned on matched pairs for overall and age-stratified populations. Models for immunocompromised and history of SARS-CoV-2 infection are unconditional logistic models, adjusted for age, sex, race/ethnicity, and month of specimen collection, in addition to the covariates listed below.
bAdjusted for days since last monovalent dose, time since history of SARS-CoV-2 infection, history of SARS-CoV-2 molecular test, number of outpatient and virtual visits, number of emergency department visits, BMI, Charlson comorbidity score, frailty index, pregnancy, medical center area, and number of monovalent doses prior to index date.
cMedical center area dropped due to lack of model convergence.
dBMI dropped due to lack of model convergence.
ePregnancy dropped due to lack of model convergence or not being applicable (for subgroup ≥65 years of age).
fFrailty index dropped due to lack of model convergence.
gCharlson comorbidity score dropped due to lack of model convergence.
hNumber of monovalent doses prior to index date dropped due to lack of model convergence.
Figure 4.
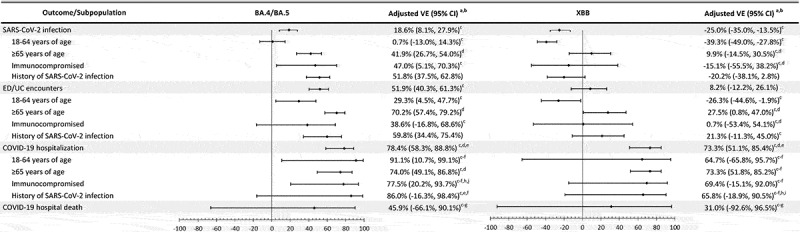
Vaccine effectiveness of mRNA-1273 bivalent vaccine vs unvaccinated against infection and severe outcomes with SARS-CoV-2 variants, overall and by subpopulation.
BMI = body mass index, ED/UC = emergency department/urgent care, VE = vaccine effectiveness.
aApplied conditional logistic models conditioned on matched pairs, for overall and age-stratified populations. Models for immunocompromised and history of SARS-CoV-2 infection are unconditional logistic models, adjusted for age, sex, race/ethnicity, and month of specimen collection, in addition to the covariates listed below.
bAdjusted for time since history of SARS-CoV-2 infection, history of SARS-CoV-2 molecular test, number of outpatient and virtual visits, BMI, pregnancy, specimen type, and medical center area.
cMedical center area dropped due to lack of model convergence.
dPregnancy dropped due to lack of model convergence or not being applicable (for subgroup ≥65 years of age).
eSpecimen type dropped due to lack of model convergence.
fBMI dropped due to lack of model convergence.
gTime since history of SARS-CoV-2 infection dropped due to lack of model convergence.
hHistory of SARS-CoV-2 molecular test dropped due to lack of model convergence.
iNumber of outpatient and virtual visits dropped due to lack of model convergence.
jMonth of specimen collection dropped due to lack of model convergence.
Discussion
We evaluated the effectiveness of mRNA-1273 bivalent COVID-19 vaccine against sequencing-confirmed BA.4/BA.5 and XBB-related sublineages in a large, socio-demographically diverse population. This is the first study to assess long-term bivalent VE against severe outcomes with sequencing-confirmed XBB-related sublineages. We found that rVE against infection and ED/UC encounters with BA.4/BA.5 or XBB was initially similar, but protection against infection with XBB waned and was minimal after 120 days. Although rVE against hospitalization for BA.4/BA.5 or XBB was high initially and waned more slowly than rVE against infection and ED/UC encounters, rVE against hospitalization for XBB declined to 30.0% (−26.0–63.7%) >180 days. These data highlight the need for periodic COVID-19 vaccination as protection wanes against infection and hospitalization, even when vaccine is well matched to circulating variants. Furthermore, these findings suggest that periodic adjustment of vaccines to better target emerging variants that can escape vaccine and infection-induced immunity may be beneficial.
Data on the effectiveness of BA.4/BA.5 bivalent vaccine against COVID-19 outcomes with XBB-related sublineages are limited. One study in an outpatient pharmacy setting found similar rVE against BA.4/BA.5 and XBB symptomatic infection for up to 3 months.17 An early study conducted in North Carolina by Lin and colleagues found an initial rVE against hospitalization of 68.3% at 2 weeks that decreased to 30.0% by 8 weeks during an interval when BA.4/BA.5 followed by XBB were predominant; in this study, time since vaccination and the emergence of increasingly evasive sublineages could have contributed to waning.7 While the estimated rVE against death through 7 weeks in Lin’s study (65.7% [19.7–85.3%]) was somewhat higher than the rVE against death with BA.4/BA.5 or XBB that we observed (53.1% [−84.2–96.5%] and 31.8% [−83.7–92.4%], respectively), our estimates were imprecise and included longer follow-up, potentially reflecting waning, although we were not able to stratify by time. More recently, Link-Gelles and colleagues found lower initial VE against hospitalization during a period that included BA.4/BA.5 and XBB (62% [57–67%] at 7–59 days post-vaccination) compared to our estimated initial VE against hospitalization with BA.4/BA.5 or XBB (76.9% [52.7–88.7%] and 93.4% [68.6–98.6%], respectively, at 14–60 days after vaccination) and greater waning than our observations, possibly because our analysis was limited to chart-confirmed COVID-19 hospitalizations.8,18
rVE and VE against infection with XBB reached statistically significant negative values at >180 and >120 days, respectively. However, negative vaccine effectiveness likely reflects the impact of bias rather than true negative biological effectiveness.19,20 Underreporting of prior infection could lead to underestimation of effectiveness and overestimation of waning as the number of controls with natural immunity to SARS-CoV-2 increases over time, biasing effectiveness estimates downward.21,22 In addition, increased healthcare-seeking behavior associated with vaccination may result in greater testing and detection of both past and current infection among vaccinees, reducing estimated effectiveness.23 Furthermore, vaccinated persons may have less infection-avoidant behavior and greater contacts than unvaccinated persons that may reduce estimated effectiveness.24–26 However, these biases have less impact on estimated effectiveness against more severe outcomes (e.g., hospitalization) for which healthcare seeking behavior and testing vary less by vaccination status. Similarly, VE against infection and ED/UC encounters but not hospitalization was found to be greater among adults ≥65 years of age compared to younger adults and may reflect increased likelihood of testing among older adults who are likely to experience worse illness than unvaccinated younger adults.27 This differential testing bias would likely reduce the absolute VE against infection and ED/UC encounters in younger adults compared to the absolute VE against infection and ED/UC encounters in older adults. Furthermore, for many outcomes, absolute VE appears to be lower than rVE. However, absolute VE compared members who received ≥2 monovalent vaccine doses and bivalent vaccine with unvaccinated members, while rVE was analyzed among members who received at least two doses of mRNA vaccine (e.g., ≥2 monovalent vaccine doses and bivalent vaccine vs. ≥2 monovalent vaccine doses). We believe that the lower absolute VE may be due to confounding, as unvaccinated persons likely have very different infection avoidance, healthcare seeking, and testing behaviors compared to persons who have received any COVID-19 vaccines. Such biases are likely to reduce absolute VE compared with rVE.
Like all observational studies, our study has limitations. First, the results of our test-negative case–control study may not be generalizable to people who are not tested, including those with milder symptoms who may not seek testing in healthcare settings. However, the test-negative design reduces bias due to differences in care-seeking behavior since both test-positive cases and test-negative controls have been tested. While there may be residual bias due to factors that were not captured in EHR, such as mask use, social distancing, and hygiene practices, we attempted to reduce bias by adjusting for sociodemographic characteristics, prior healthcare utilization, SARS-CoV-2 testing and comorbidities. Second, although rapid antigen test results were included in the history of SARS-CoV-2 infection covariate, at-home positive rapid antigen test results that were not self-reported would be missed. Because both cases and controls had a PCR test performed at KPSC, we expect that the rate of under-reporting of at-home rapid antigen test results would be nondifferential between cases and controls, but it may have differed by vaccination status. Similarly, misclassification of vaccination status was possible but likely minimal as we captured external vaccine administrations from the California Immunization Registry. Third, limited sample size for subgroup analyses and rare outcomes, including COVID-19 hospital death, resulted in wide confidence intervals. Finally, due to the short interval between introduction of bivalent vaccine and the emergence of XBB sublineages, it was not possible to estimate effectiveness against hospitalization with BA.4/BA.5-related sublineages after 120 days.
This study of mRNA-1273 bivalent vaccine found adequate initial protection against infection with BA.4/BA.5- or XBB-related omicron sublineages that waned quickly against XBB, becoming minimal after 120 days.28 However, although initially high protection against hospitalization with BA.4/BA.5 or XBB decreased by 120 days, rVE and VE point estimates against XBB hospitalization remained moderately positive after 180 days although with confidence intervals that overlapped zero (30% [−26.0–63.7%] and 42.4% [−19.2–73.2%], respectively). Nonetheless, these data suggest the need for periodic vaccination and adjustment of vaccines to match circulating variants. However, only 17% of the US population received a bivalent COVID-19 vaccine and 4.5% have received the updated XBB.1.5 vaccine as of October 27, 2023. Consequently, most of the US population is either unvaccinated or has received monovalent vaccine ≥1 year ago, providing negligible protection against infection or severe disease. Greater awareness of disease activity and the effectiveness of updated XBB.1.5 vaccine against COVID-19 outcomes, particularly severe disease, may improve vaccine uptake, potentially reducing COVID-19 associated morbidity and mortality.29
Supplementary Material
Acknowledgments
The authors would like to acknowledge the following Kaiser Permanente Southern California staff: Maria Navarro, Elsa Olvera, Joy Gelfond, Jonathan Arguello, Diana Romero, Joanna Truong, Samuel Payan, Sierra Lewis, Brittany Brown, and Hiba Atif for their contributions in manual chart reviews of the electronic health records; and Elmer Ayala, Samantha Bayulot, Candice Beissel, Jared Davis, Sarbjit Kaur-Chand, Kourtney Kottmann, Samantha Quinones, Jose Rodriguez, Charanjot (Joe) Singh, Katy Taylor, Joanna Truong, and Vanessa Pan for technical and laboratory support in processing SARS-CoV-2 specimens. The authors would like to acknowledge HelixOpCo, LLC, for their WGS of SARS-CoV-2 specimens. The authors would also like to acknowledge the contributions by Moderna, Inc. staff: Christine Yu and Yamuna Paila. Editorial assistance was provided by Rachel Schultz, MA, ELS, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors. The authors thank the patients of Kaiser Permanente for their partnership with us to improve their health. Their information, collected through our electronic health record systems, leads to findings that help us improve care for our members and can be shared with the larger community.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by Moderna, Inc.
Disclosure statement
BKA, LQ, LSS, SQ, JET, GSL, JHK, YL, RB, JS, SKC, HST, MA, and HFT are employees of Kaiser Permanente Southern California (KPSC), contracted by Moderna, Inc. to conduct this study. KJB is an adjunct investigator at (KPSC). AF was an employee of (KPSC); currently an employee of SimulStat. MAM, EJA, CKZ, and TS are employees of and shareholders in Moderna, Inc. CAT was an employee of and a shareholder in Moderna Inc at the time of protocol development; currently an employee of AstraZeneca. BKA received funding from GlaxoSmithKline, Dynavax, Genentech, and Moderna unrelated to this manuscript. LQ received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. LSS received funding from GlaxoSmithKline, Dynavax, and Moderna unrelated to this manuscript. KJB received funding from GlaxoSmithKline, Dynavax, and Pfizer unrelated to this manuscript. SQ received funding from Dynavax unrelated to this manuscript. JET received funding from Pfizer and Moderna unrelated to this manuscript. GSL, JHK, and HFT received funding from GlaxoSmithKline and Moderna unrelated to this manuscript. HFT served on advisory boards for Janssen and Pfizer. AF received funding from Pfizer, GlaxoSmithKline, Gilead, and Moderna unrelated to this manuscript. YL received funding from GlaxoSmithKline, Pfizer, and Moderna unrelated to this manuscript. RB received funding from GlaxoSmithKline unrelated to this manuscript. JS received funding from Pfizer, Sanofi, and Intercept unrelated to this manuscript. SKC received funding from Pfizer, Bayer AG, and Pancreatic Cancer Action Network unrelated to this manuscript. HST received funding from GlaxoSmithKline, Pfizer, ALK, and Wellcome unrelated to this manuscript. MA received funding from Pfizer unrelated to this manuscript.
Author contributions
BKA, KJB, LQ, LSS, JHK, AF, MAM, CAT, and HFT conceived and designed the study. BKA, KJB, LQ, LSS, SQ, JET, GSL, JHK, AF, YL, RB, JS, SKC, HST, MA, MAM, EJA, CKZ, TS, CAT, and HFT were involved in acquisition, analysis, or interpretation of data. BKA and KJB developed the first draft of the manuscript. LQ, LSS, SQ, JET, GSL, JHK, AF, YL, RB, JS, SKC, HST, MA, MAM, EJA, CKZ, TS, CAT, and HFT were involved in the critical revision of the manuscript for important intellectual content. LQ, YL, JET, and SQ performed statistical analysis. CAT and HFT obtained funding for the study. GSL, LSS, and MAM provided administrative, technical, or material support. MAM, CAT, and HFT provided project supervision. All authors contributed to the writing of the manuscript and approved the final version for publication.
Data sharing statement
Individual-level data reported in this study involving human research participants are not publicly shared due to potentially identifying or sensitive patient information. Upon request to corresponding author [BKA], and subject to review and approval of an analysis proposal, KPSC may provide the deidentified aggregate-level data that support the findings of this study within 6 months. Anonymized data (deidentified data including participant data as applicable) that support the findings of this study may be made available from the investigative team in the following conditions: (1) agreement to collaborate with the study team on all publications, (2) provision of external funding for administrative and investigator time necessary for this collaboration, (3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and (4) agreement to abide by the terms outlined in data use agreements between institutions.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2335052
References
- 1.Centers for Disease Control and Prevention . COVID data tracker: trends. [accessed 2023 Oct 7]. https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- 2.Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, de Silva TI, Peacock SJ, Barclay WS, de Silva TI, Towers GJ, et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023. Mar;21(3):162–10. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes moderna, pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. 2022. [accessed 2023 Jun 17]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use.
- 4.U.S. Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes changes to simplify use of bivalent mRNA COVID-19 vaccines. 2023. [accessed 2023 Jun 17]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-changes-simplify-use-bivalent-mrna-covid-19-vaccines.
- 5.Tenforde MW, Weber ZA, Natarajan K, Klein NP, Kharbanda AB, Stenehjem E, Embi PJ, Reese SE, Naleway AL, Grannis SJ, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19–associated Emergency Department or urgent care encounters and hospitalizations among immunocompetent adults — VISION network, Nine States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2023. Mar 17;71(12):1637–46. doi: 10.15585/mmwr.mm7153a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller J, Hachmann NP, Collier AR, Lasrado N, Mazurek CR, Patio RC, Powers O, Surve N, Theiler J, Korber B, et al. Substantial neutralization escape by SARS-CoV-2 omicron variants BQ.1.1 and XBB.1. N Engl J Med. 2023. Feb 16;388(7):662–4. doi: 10.1056/NEJMc2214314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DY, Xu Y, Gu Y, Zeng D, Sunny SK, Moore Z.. Durability of bivalent boosters against Omicron Subvariants. N Engl J Med. 2023. May 11;388(19):1818–20. doi: 10.1056/NEJMc2302462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Link-Gelles R, Ciesla AA, Fleming-Dutra KE, Smith ZR, Britton A, Wiegand RE, Miller JD, Accorsi EK, Schrag SJ, Verani JR, et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection — increasing community access to testing program, United States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022. Dec 2;71(48):1526–30. doi: 10.15585/mmwr.mm7148e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration . FDA takes action on updated mRNA COVID-19 vaccines to better protect against currently circulating variants. 2023. [accessed 2023 Sept 21]. https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating.
- 10.Centers for Disease Control and Prevention . COVID data tracker: vaccination distribution and coverage. [accessed 2023 Jun 17]. https://covid.cdc.gov/covid-data-tracker/#vaccination-states-jurisdictions.
- 11.Bruxvoort KJ, Sy LS, Hong V, Lewin B, Qian L, Huang X, Holmquist KJ, Han B, Xu S. Factors associated with uptake of bivalent mRNA COVID-19 vaccines in a large US health care system. Vaccine. 2023. Nov 30;41(49):7460–8. doi: 10.1016/j.vaccine.2023.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu PJ, Zhou T, Santibanez TA, Jain A, Black CL, Srivastav A, Hung M-C, Kriss JL, Schorpp S, Yankey D, et al. COVID-19 bivalent booster vaccination coverage and intent to receive booster vaccination among adolescents and adults — United States, November–December 2022. MMWR Morb Mortal Wkly Rep. 2023. Feb 17;72(7):190–8. doi: 10.15585/mmwr.mm7207a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groom HC, Crane B, Naleway AL, Weintraub E, Daley MF, Wain K, Beth Kurilo M, Burganowski R, DeSilva MB, Donahue JG, et al. Monitoring vaccine safety using the vaccine safety datalink: assessing capacity to integrate data from immunization information systems. Vaccine. 2022. Jan 31;40(5):752–6. doi: 10.1016/j.vaccine.2021.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, Tian Y, Florea A, Aragones M, Tubert JE, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. Dec 15 2021;375:e068848. doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng HF, Ackerson BK, Bruxvoort KJ, Sy LS, Tubert JE, Lee GS, Ku JH, Florea A, Luo Y, Qiu S, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun. Jan 12 2023;14(1):189. doi: 10.1038/s41467-023-35815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022. May;28(5):1063–71. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, Wiegand RE, Accorsi EK, Verani JR, Shang N, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults - increasing community access to testing program, United States, December 2022-January 2023. MMWR Morb Mortal Wkly Rep. 2023. Feb 3;72(5):119–24. doi: 10.15585/mmwr.mm7205e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowe J, Andrews N, Kirsebom F, Ramsay M, Bernal JL. Effectiveness of COVID-19 vaccines against omicron and delta hospitalisation, a test negative case-control study. Nat Commun. 2022. Sep 30;13(1):5736. doi: 10.1038/s41467-022-33378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, Al-Khatib HA, Smatti MK, Hasan MR, Al-Kanaani Z, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022. Jun 2;13(1):3082. doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022. Apr 21;386(16):1532–46. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn R, Schrag SJ, Verani JR, Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in postmarketing evaluations of COVID-19 vaccines. Am J Epidemiol. 2022. Mar 24;191(5):800–11. doi: 10.1093/aje/kwac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein C, Nassereldine H, Sorensen RJD, Amlag JO, Bisignano C, Byrne S, Castro E, Coberly K, Collins JK, Dalos J. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023. Mar 11;401(10379):833–42. doi: 10.1016/s0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, Sharp GC, Sterne J, Palmer TM, Davey Smith G, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020. Nov 12;11(1):5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodner K, Knight J, Hamilton MA, Mishra S. Testing whether higher contact among the vaccinated can be a mechanism for observed negative vaccine effectiveness. Am J Epidemiol. 2023. Aug 4;192(8):1335–40. doi: 10.1093/aje/kwad055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henk K, Rosing F, Wolff F, Frenzel SB, van Dick R, Erkens VA, Häusser JA, Mojzisch A, Boer D. An examination and extension of the Peltzman effect during the COVID-19 pandemic. Curr Res Ecol Soc Psychol. 2023;4:100091. doi: 10.1016/j.cresp.2023.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falahi S, Mohamadi J, Sayyadi H, Pakzad I, Rashidi A, Naserifar R, Abdi J, Kenarkoohi A. COVID-19 vaccination, Peltzman effect and possible increase in high risk behaviors: a growing concern related to risk compensation and reduced compliance to public health protective measures after vaccines rollout. Infect Disord Drug Targets. 2022;22(8):8–12. doi: 10.2174/1871526522666220419133849. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017. Aug 24;35(36):4796–800. doi: 10.1016/j.vaccine.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Tokars JI, Patel MM, Foppa IM, Reed C, Fry AM, Ferdinands JM. Waning of measured influenza vaccine effectiveness over time: the potential contribution of leaky vaccine effect. Clin Infect Dis. 2020. Dec 17;71(10):633–41. doi: 10.1093/cid/ciaa340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coe AB, Elliott MH, Gatewood SBS, Goode JR, Moczygemba LR. Perceptions and predictors of intention to receive the COVID-19 vaccine. Res Social Adm Pharm. 2022. Apr;18(4):2593–9. doi: 10.1016/j.sapharm.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V.. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011. Mar 15;173(6):676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 31.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-Based frailty Index. J Gerontol A Biol Sci Med Sci. 2018. Jun 14;73(7):980–7. doi: 10.1093/gerona/glx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


