Abstract
The treatment for peritoneal malignancies has evolved over the years with the growing success of cytoreductive surgery combined with the use of hyperthermic intraperitoneal chemotherapy. Patients receiving this treatment are at risk for developing malnutrition not only due to the areas of tumor involvement but also due to the risk of undernutrition if nutritional interventions are not timely or fall short of their goal. Malnutrition leads to a gamut of health consequences. Understanding peritoneal malignancies, cytoreductive surgery with hyperthermic intraperitoneal chemotherapy, as well as the latest nutrition research may lead to a focus on the prevention or attenuation of the procedure’s associated malnutrition risk. Reducing the effects of malnutrition in these patients is the goal of the nutrition support practitioner.
Introduction
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is a complex oncological surgery procedure that has become a popular treatment option for peritoneal malignancies, with an estimated 29,000 to 41,000 patients eligible for CRS with HIPEC per year in the United States.1 Although primary peritoneal cancers, such as peritoneal mesotheliomas, are uncommon, the peritoneum (Figure 1) serves as a prevalent site for metastasis or peritoneal carcinomatosis, a manifestation of appendiceal, colorectal, gastric, and ovarian malignancies.2–6 Tumors from organs outside of the abdomen can also metastasize to the peritoneal cavity, such as breast cancers, lung malignancies, and melanomas, although the incidence of peritoneal carcinomatosis from these tumors is considered low.7 In the past, patients with peritoneal malignancies were presumed to have a terminal illness, and their treatment primarily involved palliative measures, often including the use of chemotherapy. The introduction of CRS and HIPEC led to significant survival rate improvement over the past three decades, and the combination of CRS and HIPEC progressively became the treatment of choice for those malignancies.8,9
Figure 1. The peritoneum: a cross-section of the abdomen shows the relationship between abdominal organs and the peritoneum (thicker lines).
Reprinted with permission from Betts JG, Young KA, Wise JA, et al. Anatomy and Physiology. Houston, TX: OpenStax; 2013. Available at: https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.
Patients who undergo surgery for peritoneal malignancies and general abdominopelvic cancer are often diagnosed with malnutrition.10,11 The prevalence of malnutrition in cancer patients worldwide is reported to range from 20% to more than 70%.12 Variations in prevalence are related to factors such as the patient’s age, the stage to which the cancer has progressed, and the specific type of cancer, with patients with gastrointestinal tract malignancies being at increased risk for malnutrition.13,14
A range of negative outcomes is associated with malnutrition in hospitalized patients, such as delayed wound healing, higher infection rates, prolonged length of stays (LOS), as well as increased morbidity, readmission, and mortality rates.15 Malnutrition can significantly affect the recovery of surgical oncology patients undergoing CRS with HIPEC.11 In addition, the procedure itself is associated with multiple long-term gastrointestinal-related effects such as nausea, vomiting, diarrhea, constipation, and loss of appetite, and those may lead to or exacerbate postoperative malnutrition.16
Understanding CRS and HIPEC and how malnutrition affects outcomes in patients undergoing this procedure is critical for development of institutional-specific strategies to optimize perioperative nutrition and recovery. This narrative review will discuss the CRS with HIPEC procedure, outcomes affected by nutritional status, and the current literature on postoperative nutrition management of CRS with HIPEC patients. Additionally, a case study will be presented with discussion of nutritional-related considerations.
Understanding Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
CRS and HIPEC were initially developed to treat pseudomyxoma peritonei, an uncommon condition usually associated with a perforated epithelial neoplasm of the appendix and marked by mucinous ascites that may progress eventually to a bowel obstruction among other complications.17 Today, the combination of CRS and HIPEC is considered by a number of experts one of the key surgical options for all peritoneal surface malignancies.18 CRS with HIPEC can be briefly described as the maximum removal of macroscopic disease within the abdomen, including resection of the involved organs, with subsequent delivery of heated chemotherapy directly into the abdominal cavity.19,20 This therapeutic approach targets both visible masses and microscopic residual disease. CRS involves an exploratory laparotomy with a comprehensive examination of the peritoneal cavity to evaluate the extent of the disease. The peritoneal cancer index (PCI) score is often used to assess the extent of peritoneal cancer throughout the peritoneal cavity, dividing the abdominal area into 13 regions, with each region given a score from 0 to 3 depending on the extent of disease present; a higher PCI is associated with a worse prognosis.21 The score is calculated during surgery but can be estimated on imaging or at a staging laparoscopy. Omentectomies, cholecystectomies, hysterectomies and bilateral oophorectomies, partial or total gastrectomies, and bowel resections, among other procedures, may be performed contingent on the extent of the disease. Visible tumor implants are removed. The completion of CRS is determined by the end of the surgery using the completeness of cytoreduction (CC) score, a reliable prognosticator. A complete CRS can be defined as CC-0 (visible complete tumor removal) or CC-1 (residual tumor nodules ≤2.5 mm), and those scores are associated with the most favorable outcomes.22
HIPEC may be used in select patients promptly following the completion of CRS. By directly instilling chemotherapy drugs into the peritoneal cavity, typically at a temperature of around 41–43 degrees Celsius, a higher concentration and a more uniform distribution of drugs can be achieved throughout the abdomen. Additionally, the association of hyperthermia with chemotherapy has synergistic effects, such as increased intracellular drug accumulation, reduced DNA repair, decreased drug detoxification, reduced cell proliferation, enhanced apoptosis, and improved tissue penetration. During treatment, the heated chemotherapy solution is administered into the peritoneum at a controlled flow rate through drains placed in the abdominal cavity and connected to an extracorporeal circuit. Table 1 lists the drugs commonly used for this treatment. The drugs may be used in combination with each other, in various dosage ranges, either as a singular initial dose or multiple doses divided throughout the drug administration period, which can range between 30 and 180 minutes. Total volume and flow rate can also vary, and different carrier solutions can be used. Both closed and open abdomen techniques are utilized for administration of HIPEC. After perfusion, the liquid is drained, and the surgeon ensures there are no complications or injuries before closing the abdominal wall.23
Table 1.
Chemotherapy agents used for intraperitoneal administration
| 5-Fluorouracil | Irinotecan |
| Carboplatin | Melphalan |
| Cisplatin | Mitomycin C |
| Docetaxel | Mitoxantrone |
| Doxorubicin | Oxaliplatin |
| Etoposide | Paclitaxel |
| Gemcitabine | Pemetrexed |
Data from Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist. 2005;10(2):112–122.
Early postoperative intraperitoneal chemotherapy (EPIC) is another treatment approach often used in combination with CRS and HIPEC in patients with peritoneal malignancies. EPIC consists of chemotherapy delivered via an intraperitoneal catheter for several days during the early postoperative period before adhesions develop.24 EPIC, however, is associated with increased morbidity, and the benefits are contingent upon the tumor origin and the specific pharmaceutical agent utilized. New research suggests that certain drugs traditionally used during EPIC, such as 5-fluorouracil, may have inferior efficacy than CRS with HIPEC, used without EPIC, for control of peritoneal metastases.25
Malnutrition in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
Cancer patients are at high risk for malnutrition, resulting from the concurrent effects of the malignant disease itself and its treatment. In 2013, Vashi et al investigated the relationship between baseline nutritional status and clinical outcomes in patients who had undergone CRS with HIPEC for peritoneal carcinomatosis.26 Their findings showed that baseline nutritional status, as evaluated by subjective global assessment (SGA), was predictive of patient LOS (well-nourished patients had an average LOS of 15 days, moderately malnourished patients an average LOS of 15.2 days, and severely malnourished patients an average LOS of 27.8 days; P=0.02), and survival (well-nourished patients survived an average of 22.4 months, with a 95% confidence interval [CI] of 18.7–26.1 months, compared to malnourished patients who survived an average of 10.4 months, with a 95% CI of 5.2–15.7 months; P=0.006). Malnutrition was similarly linked to increased LOS in patients undergoing CRS with HIPEC for peritoneal malignancy in a prospective observational study by Reece et al in 2019 (with a median LOS of 24 days [interquartile range of 16–30 days] compared to 15 days [interquartile range of 12–23 days] for well-nourished patients; P=0.006).11 The authors also utilized SGA to assess nutritional status and found that postoperative infections were more likely in malnourished patients when compared to their well-nourished counterparts (47% vs. 25%, respectively; P=0.025). BMI was not associated with major morbidity or hospital LOS, suggesting assessment of nutritional status should rely on more than BMI as a singular marker. Skeletal muscle mass depletion, assessed by van Vugt et al using CT-based muscle mass measurements, was associated with an increased risk of severe postoperative complications in patients undergoing CRS and HIPEC for colorectal peritoneal carcinomatosis.27 Sarcopenic patients were significantly more likely to undergo reoperations than the nonsarcopenic patients (25.6% vs. 12.1%, respectively; P=0.012).
The combination of CRS with HIPEC significantly improved survival rate; however, severe morbidity rates associated with the procedure are reported to occur in approximately 20.8% to 53.3% of cases.28–31 Admission for failure to thrive following CRS and HIPEC is strongly correlated with preoperative malnutrition.32 Recent data from Fernandez-Candela et al in patients who underwent CRS with and without HIPEC suggested that preoperative intake of immune-modulating nutrition oral supplements is an independent protective factor against severe morbidity when compared to patients not receiving immune-modulating nutrition oral supplements (with an odds ratio of 0.247; P=0.025).33 The significant role of perioperative nutrition on the treatment of patients undergoing CRS with HIPEC was also reinforced by Raspe et al and Cortes-Guiral et al when they summarized best practices and prehabilitation recommendations for patients undergoing CRS and HIPEC.34,35 Nutrition screening, protein provision of least 1.2–2.0 g protein/kg/d, and the use of oral nutritional supplements are recommended to all patients. For patients identified as malnourished, appropriate nutritional interventions, including enteral nutrition (EN) and parenteral nutrition (PN) preoperatively are recommended as needed. This approach aims to optimize their nutritional status before surgery to improve treatment outcomes.
Postoperative Nutrition in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
Nutrition support is frequently required for patients after CRS and HIPEC. The combination procedure is associated with a high incidence of prolonged ileus and consequential nil per os, or nothing by mouth (NPO).36 The procedure carries a considerable risk of other complications affecting the gastrointestinal tract, such as anastomotic leakage, fistulae, and perforations. The incidence of gastrointestinal morbidity after CRS with HIPEC varies between 4.5% to 19% and thus may further prolong inadequate oral or enteral intake and impact patients’ nutritional status.37,26 During the early postoperative period, patients may have to go one week or more without oral intake.38 Once patients are able to start an oral diet, it can take several weeks to meet their nutritional needs without nutrition support. Early start of EN or an oral diet postoperatively should be implemented when feasible. Since this patient population is usually either malnourished or at an elevated risk for malnutrition, Raspe et al recommends that early supplementary PN should be considered if delays greater than three days to start EN or an oral diet are anticipated.34 Cortes-Guiral et al suggests PN initiation when leaving the operating room until oral intake is sufficient to meet nutritional needs.35
Calculation of nutritional needs can be challenging in this population, as cancer patients are prone to experience changes in resting energy expenditure due to changes in body composition, systemic inflammation, tumor calorie demand, and possible brown adipose tissue activation.39 In the absence of more specific supportive evidence, if resting energy expenditure cannot be measured, determining nutritional needs with the general recommendation of 25–30 kcal/kg/d and 1.2–1.5 g protein/kg/d may grossly underestimate nutritional needs after CRS with HIPEC.12 The authors attempted to estimate protein needs for this population using urinary urea nitrogen. A positive nitrogen balance indicates that the patient’s protein intake has exceeded the amount of protein breakdown and excretion, resulting in a net gain of positive nitrogen in their body. In our sample of 83 patients, to achieve this positive nitrogen balance, we calculated that the patients would need approximately 1.4–2.6 g protein/kg/d (BMI dependent) or 2.3 g protein/kg of ideal body weight per day (unpublished data).40
Enteral Nutrition After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
Early EN has been associated with a reduced incidence of postoperative ileus and decreased LOS for malnourished patients with gastrointestinal cancer.41 Nevertheless, the benefits of the use of early EN after CRS and HIPEC are not well-documented in the literature.36,42 Placement of feeding access to facilitate early feeding after surgery was evaluated by Dineen et al in a retrospective evaluation of 41 patients who underwent CC-0 and CC-1 CRS with HIPEC for colorectal cancer with peritoneal carcinomatosis.36 Twenty-five patients had a feeding tube placement at the time of surgery: of these patients with feeding tubes, two had a gastrostomy and a separate jejunostomy placed, fifteen had gastrojejunostomies, and eight had gastrotomy tubes only. The majority of the patients (38 of the total 41) experienced weight loss, with no difference in the mean weight loss between patients with and patients without feeding tubes. In a multivariate analysis, the presence of a feeding tube (hazard ratio [95% CI]: 0.218 [0.091–0.518]), and a PCI>12 (hazard ratio [95% CI]: 0.297 [0.131–0.673]) was associated with a longer hospital LOS (18 days for the feeding tube group vs. 11 days for the group with no feeding tube, P<0.001). Sixty-day readmission rates were higher in patients with feeding tubes than those without feeding tubes (36% vs. 0% [P<0.01], respectively). It is important to note that the study had limitations, as it was a retrospective study and it was not designed to assess the effects of early enteral feeding; therefore, information regarding duration of nasogastric or gastric tube drainage, time of initiation of enteral and/or oral feeding, and calorie and protein intake were not documented.
Another more recent retrospective study compared nutritional status, postoperative complications, and hospital LOS between EN and PN in 51 patients that underwent CRS and HIPEC for pseudomyxoma peritonei.42 Patients on EN (n=25) and PN (n=26) were scheduled to receive formulas providing 30 kcal/kg, starting on postoperative day (POD) 2 or 3 for the enteral group and immediately after surgery for the parenteral group, until the patients recovered and were discharged. Hospital LOS (14 vs. 17 days respectively, P=0.008) and complication rates (25% vs. 36% respectively, P=0.03) were lower in the patients that received EN than those who received PN, suggesting a benefit toward providing EN. Nutritional status was also deemed superior in the EN group, with assessment parameters being BMI on POD 10 (21.27±2.87 for EN vs. 19.74±3.94 for PN, P=0.029), levels of absolute lymphocyte count (P<0.001), hemoglobin (P=0.016), and albumin (P<0.001). The number of days nutrition support was administered and the actual calorie and protein intake were not included on the reported data, challenging the conclusion that the results were exclusively associated with the nutritional intake delivery method. The utilization of non-specific parameters used for evaluation of nutritional status may also have skewed the results.
The combination of EN and PN was evaluated in patients undergoing total or subtotal gastrectomy and CRS with HIPEC for gastric cancer in the PERISCOPE I study.43 This prospective study included two cohorts: a dose escalating cohort with patients receiving an escalating dosage of docetaxel—a chemotherapeutic agent—across three doses, and an expansion cohort where patients received a single dose of docetaxel. EN via a surgical jejunostomy was started immediately after surgery, at 20 mL/hr in the dose escalating cohort vs. 10 mL/hr in the expansion cohort. The dose escalating cohort had EN increased every hour on POD 1 until estimated nutritional needs were met. In the expansion cohort, EN remained at 10 m L/hr until day 3 and was then increased to a maximum of 20 mL/hr until day 6, with PN starting on day 3 to meet nutritional needs. The EN rate was increased after day 6 if there was no ileus. The expansion cohort was associated with reduced serious adverse effects (P=0.021), respiratory complications (P=0.012), and ICU readmissions (50% for the dose escalating cohort vs. 9% for the expansion cohort; P=0.042). Based on these results, the authors implemented a specific nutrition protocol combining PN and early low-volume EN for the follow-up PERISCOPE II study.
Parenteral Nutrition After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy
PN is often recommended after CRS with HIPEC due to expected prolonged ileus or other gastrointestinal complications that may delay or impede normal bowel function.35 In 2013, Vashi et al conducted a retrospective study to investigate the association between baseline nutritional status and clinical outcomes in patients undergoing CRS and HIPEC for peritoneal malignancies and receiving PN.26
Nutritional status was assessed using SGA (well-nourished: SGA-A; mildly-moderately malnourished: SGA-B; and severely malnourished: SGA-C). Sixty patients were included. Of those, 31 received PN. Baseline nutritional status was predictive of patients’ hospital LOS (SGA-A: 15 days, SGA-B: 15.2 days, and SGA-C: 27.8 days, P=0.02), and survival (SGA-A: 22.4 months, and SGA-B and SGA-C: 10.4 months, P=0.006). Hospital LOS was longer for patients receiving PN than those not receiving PN (19.4 days vs. 12.7 days, P=0.007). Additionally, their incidence of complications was higher for patients receiving PN (38.7% vs. 13.8%, P=0.03), and median survival time was shorter (14.3 months vs. 22.4, P=0.01). In a subgroup analysis within the SGA-A group to compare patients who received PN with the no-PN group, no statistically significant differences were found between the subgroups. As noted by the authors, conclusions regarding the role of PN in the outcomes could not be defined due to the retrospective nature of the study and the clinical and demographic differences between the PN and no-PN groups.
The use of PN as a standard of care after CRS with HIPEC was evaluated in a retrospective comparison of two centers with similar perioperative management protocols, which differed only by the placement of suprapubic urine bladder catheters, selective decontamination of the digestive tract, and the standard start of PN on POD 1 for all patients in one of the centers.44 Sixty-eight eligible patients from each center were compared. The institution with standard PN initiation on POD 1 had lower incidence of severe complications than the compared center that didn’t include immediate PN as part of their care protocol (11.8% vs. 26.4%, P=0.03). It is noteworthy that PN could not be avoided due to prolonged ileus in 54.4% of the patients from the compared facility. Calorie and protein intake were not reported or compared among the groups. Both groups received PN for an average of eight days.
The recommendation for PN to be initiated is often based on the expected duration of bowel rest. Swain et al analyzed duration of PN after CRS and HIPEC in 321 patients undergoing surgery in a peritoneal malignancy center with a routine standard protocol of initiation of PN the day after surgery.45 PN median duration was 9 days (ranged from 2–87 days). There was no significant difference in duration of postoperative PN among patients with different primary tumor sites. Thirteen percent of patients received PN for less than seven days. Among all patients, 6% were able to tolerate oral intake before POD 5, suggesting risk of PN overuse is minimal with a standard protocol to start on POD 1.
A recent systematic review and meta-analysis analyzed the impact of PN on the survival of patients with peritoneal carcinomatosis.46 Eight studies were included in the meta-analysis; however, only one involved patients after CRS and HIPEC. Patients receiving PN were found to have a survival benefit of 17.15 days (P=0.040) when compared with no PN. This difference persisted when data was analyzed with high quality studies only, resulting in a survival benefit of 29.17 days (P<0.001) in patients who received PN. It was noted by the authors that only one randomized controlled trial was included, and the lack of level one evidence was a limitation for interpretation of the results. The nature of retrospective studies prevents direct associations with specific complications and the lack of randomization in the study design introduces a susceptibility to selection bias, with PN initiation frequently relying on physician or patient preference. Retrospective studies can, however, provide valuable insights, especially when conducting a randomized controlled trial is not feasible.
Case Study
A 43-year-old female was admitted for scheduled CRS with HIPEC. Her past medical history included colonic mucinous adenocarcinoma for which she had undergone an open right colon resection 14 months prior to admission, followed by 12 cycles of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) chemotherapy. Two months prior to admission, an ultrasound-guided biopsy of an abdominal wall mass confirmed recurrence in at least two sites, and a decision was made to pursue CRS and HIPEC.
Her surgery lasted 12 hours and included: excision of tumor from the abdominal wall, exploratory laparotomy, greater omentectomy, lesser omentectomy, pelvic peritonectomy, hysterectomy and bilateral salpingo-oophorectomy, rectosigmoid colon resection with low anastomosis, right colon resection redo with anastomosis, partial gastrectomy (antral resection), excision of descending colon bowel lesion, and partial cystectomy with suture repair of the bladder. HIPEC with 25 mg of doxorubicin, 25 mg of mitomycin C, 680 mg of 5-fluorouracil, and 34 mg of leucovorin and EPIC with 1,360 mg of 5-fluorouracil were instilled into the peritoneal space for a 24-hour period after the closure of the abdomen. Five Jackson-Pratt drains were placed during surgery. Per the operative report, the patient’s small bowel appeared largely free of gross disease involvement, and no small bowel resection was performed. The CC score was 0, and the PCI was 14. The patient left the operating room and was transferred to the intensive care unit for observation after surgery. She was intubated, and a nasogastric tube was placed to continuous suction.
Prolonged ileus and inadequate nutritional intake were expected, and on POD 1, PN was initiated per hospital protocol with a multi-chamber PN bag via right internal jugular catheter inserted during surgery with tip placement confirmed by radiology. The patient was extubated and transferred out of the intensive care unit on POD 2, and a registered dietitian nutritionist completed a full nutrition assessment with recommendations to start a custom PN regimen that evening. Upon nutrition-focused physical assessment, the patient was found to have mild bilateral temporalis muscle wasting, some protrusion on the clavicle bone region, and generalized edema. She reported weight loss of approximately 10% of her usual body weight in a period of 6 months following her first surgery during chemotherapy treatment due to loss of appetite and persistent nausea and vomiting. Her weight recovery was slow and progressive, and she reported that prior to admission she was eating well, her body weight was stable for approximately two months, and she had no gastrointestinal symptoms. Her anthropometric data upon admission is shown in Table 2. Indirect calorimetry was not available at that time, and her estimated calorie and protein needs are shown in Table 3. She was not diagnosed with malnutrition upon admission due to reported adequate oral intake and weight gain; however, 100 mg of thiamine were added to her PN custom formulation for 5 days per expert opinion and experience with refeeding syndrome in cancer patients and electrolyte imbalances in patients after CRS with HIPEC. Labs and PN formulation adjustments can be found in Tables 4 and 5, respectively.
Table 2.
Anthropometric data—case study
| Admission weight | 69 kg |
| Ideal weight (Devine formula) | 58.1 kg |
| Admission BMI | 24.7 kg/m2 |
| Discharge weight | 66 kg |
| Discharge BMI | 23.6 kg/m2 |
Table 3.
Calculated calorie and protein requirements—case study
| Estimated needs | Total/day | |
|---|---|---|
| Calories | 30–35 kcal/kg | 2070–2415 kcal |
| Protein | 1.8–2.2 g/kg | 124–152 g |
Table 4.
Biochemical markers—case study
| Basic metabolic panel (reference) | POD 1 | POD 2 | POD 7 | POD 14 | POD 20 | POD 25 |
|---|---|---|---|---|---|---|
| Sodium (137–145 mmol/L) | 138 | 135 | 139 | 140 | 137 | 136 |
| Potassium (3.4–4.1 mmol/L) | 3.5 | 3.4 | 3.8 | 3.9 | 4.1 | 4.1 |
| Chloride (98–107 mmol/L) | 108 | 105 | 107 | 103 | 102 | 99 |
| CO2 (22–30 mmol/L) | 27 | 27 | 28 | 29 | 26 | 25 |
| Blood urea nitrogen (9–20 mg/dL) | 6 | 4 | 11 | 19 | 16 | 17 |
| Creatinine (0.66–1.50 mg/dL) | 0.38 | 0.44 | 0.37 | 0.55 | 0.47 | 0.40 |
| Glucose (70–140 mg/dL) | 120 | 122 | 114 | 108 | 117 | 107 |
| Calcium (8.4–10.2 mg/dL) | 8.1 | 7.6 | 8.3 | 8.6 | 9.1 | 9.4 |
| Magnesium (1.6–2.3 mg/dL) | 1.7 | 1.9 | 2.1 | 2.1 | 2.2 | 2.0 |
| Phosphorus (2.5–4.5 mg/dL) | 2.4 | 2.5 | 2.8 | 3.5 | 3.7 | 4.2 |
Note: Bolded numbers represent values outside of reference.
Table 5.
PN formulation adjustments—case study
| PN component | POD 1 | POD 2 | POD 7 | POD 14 | POD 20 | POD 25 |
|---|---|---|---|---|---|---|
| Total volume (mL) | 960 | 1,700 | 2,000 | 1,800 | 1,500 | 1,400 |
| Total kcal | 510 | 1,770 | 2,350 | 2,055 | 1,651 | 1,230 |
| Dextrose (g) | 100 | 180 | 350 | 275 | 215 | 150 |
| Amino acids (g) | 42.5 | 140 | 140 | 130 | 105 | 105 |
| ILE (g)(SO, MCT, OO, FO-ILEa) | 0 | 60 | 60 | 60 | 50 | 30 |
| Sodium (mEq) | 35 | 100 | 147 | 90 | 80 | 110 |
| Potassium (mEq) | 30 | 70 | 100 | 80 | 60 | 50 |
| Chloride (mEq) | 39 | 80 | 120 | 90 | 60 | 80 |
| Acetate (mEq) | 70 | 70 | 100 | 60 | 60 | 60 |
| Phosphorus (mmol) | 15 | 15 | 20 | 15 | 15 | 15 |
| Magnesium (mEq) | 5 | 8 | 10 | 12 | 8 | 8 |
| Calcium (mEq) | 4.5 | 10 | 10 | 8 | 5 | 5 |
| Thiamine (mg)b | 0 | 100 | 0 | 0 | 0 | 0 |
| Multivitamin injection (mL) | 0 | 10 | 10 | 10 | 10 | 10 |
| Trace elements injection (mL) | 0 | 1 | 1 | 1 | 1 | 1 |
ILE=intravenous lipid emulsion, SO=soybean oil, MCT=medium-chain triglycerides oil, OO=olive oil, FO=fish oil
30% soybean oil, 30% medium-chain triglycerides oil, 25% olive oil, 15% fish oil intravenous lipid emulsion.
In addition to thiamine in multivitamin injection.
On POD 7, the PN formulation reached the goal amount of dextrose. The nasogastric tube remained in place with suction output varying between 200 to 600 mL daily. On POD 9, a urinary urea nitrogen test revealed that nitrogen balance was in equilibrium, and no changes were made to the macronutrients in the PN formulation. Bowel function returned on POD 12, and a clear liquid oral diet was initiated. The dietitian clarified with the medical team the extension of the gastrectomy procedure prior to making determinations on the appropriate recommendations for a post-gastrectomy clear liquid diet or a standard clear liquid diet. The patient had an antral resection, and the pylorus remained in place. Risk of dumping syndrome was low, and a decision was made to recommend a standard clear liquid diet. The PN formulation was adjusted to accommodate for oral intake. On POD 15, the patient was started on a regular low fiber postoperative diet and had no nausea or vomiting symptoms. The next day, PN was discontinued. Oral intake was meeting less than 50% of patient’s calorie needs when the PN was discontinued, as the medical team expected a rapid oral intake and appetite improvement considering there was no small bowel resection during her CRS.
The patient had multiple emesis episodes on POD 17. The abdomen was distended, and the patient reported no bowel movements. There was concern for a rebound ileus. Although not often mentioned in the literature, the recurrence of ileus after the return of bowel function is recurrently observed in our clinical practice when providing care for CRS with HIPEC patients. The patient was made NPO, and PN was restarted with the same formulation she had been receiving prior to discontinuation. On POD 20, the patient’s bowel function started to return. An oral diet was resumed with a full liquid diet with high-calorie and high-protein oral supplements and advanced the next day to a regular low-fiber postoperative diet. Her anti-emetic regimen was also optimized. The PN formulation was adjusted appropriately in response to the patient’s gradual improvement in oral intake. Throughout the postoperative period, the patient was continuously encouraged and educated on strategies to increase calorie and protein intake. Additionally, guidance was provided on adhering to a low-fiber diet temporarily for prevention of bloating and abdominal discomfort.
In the period between POD 20 and 30, there was a concern for bladder leaks, as well as issues related to social work, both of which delayed discharge. The patient remained on an oral diet and PN due to inadequate oral intake. Supplemental EN was discussed; however, the patient declined. On POD 30, the PN was discontinued as the patient was meeting approximately 60% of her estimated calorie needs orally. She was discharged home the next day. The patient’s weight trend during hospitalization can be found in Figure 2.
Figure 2. Weight trend—case study.
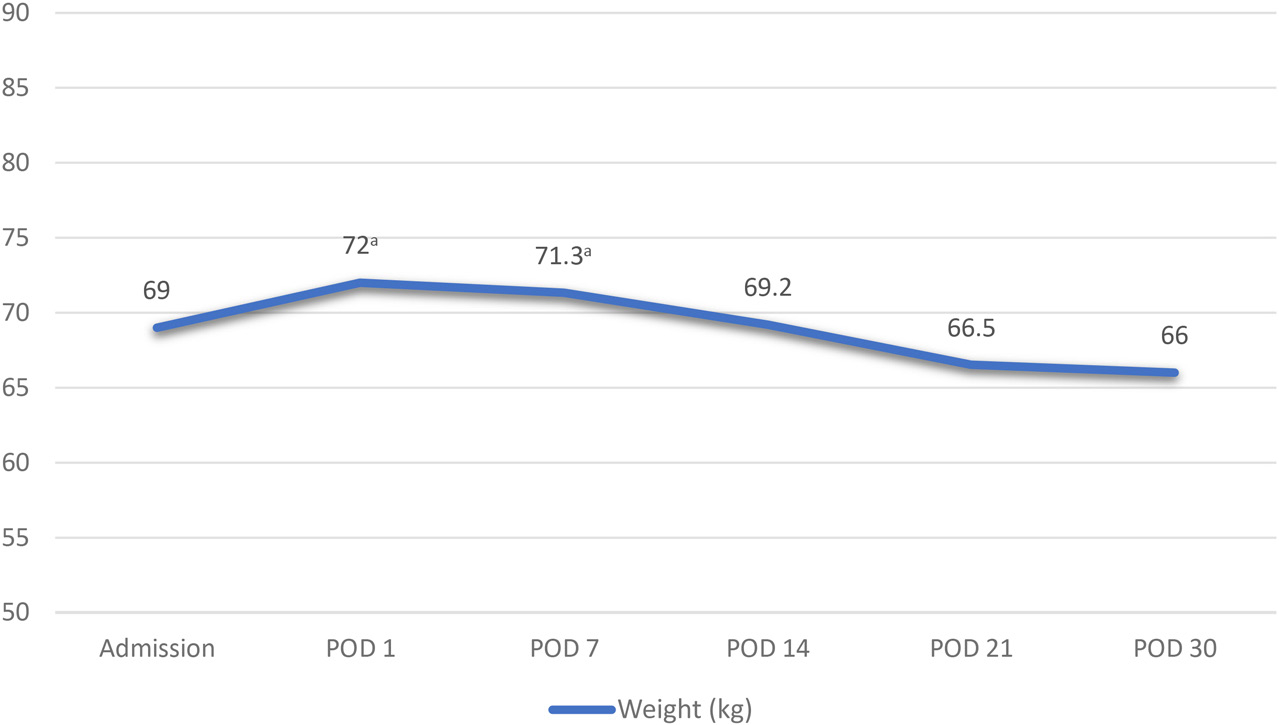
aEdema documented.
In this case study of nutrition support after CRS with HIPEC, the initiation of PN upon POD 1 deterred a lengthy inadequate nutritional intake in a patient with prolonged ileus. Early intervention prevented further decline in body weight and potentially averted the development of malnutrition and its related complications.
Conclusion
A number of studies have investigated the role of nutrition interventions and their impact on outcomes in patients undergoing CRS with HIPEC. The majority of those studies are retrospective in nature and have significant limitations. Considering the evidence available, there is a discernible association between malnutrition and outcomes in this population. Nutrition optimization seems to positively impact those outcomes. The prevailing evidence suggests a tendency to minimize prolonged periods of inadequate nutrition in order to avoid triggering or further exacerbating malnutrition, although the ideal method of nutritional delivery remains to be determined. Clinical nutrition professionals should be encouraged to strategize with the interdisciplinary team to proactively address the nutritional needs of patients undergoing CRS with HIPEC by conducting timely nutrition assessment and planning interventions to optimize patients’ perioperative nutritional status. This approach may play a pivotal role in improving clinical outcomes.
CPEU Codes
8.1.2: Integrates knowledge of biological, physical, and social sciences with knowledge of food and nutrition to make decisions related to nutrition care.
8.2.3: Integrates new knowledge of disease states and clinical conditions into practice.
Contributor Information
Silvia Figueiroa, Clinical Research Dietitian, National Institutes of Health, Bethesda, MD..
Annette Dourney, Clinical Nutrition Manager, MedStar Washington Hospital Center, Washington, DC..
References
- 1.Schuitevoerder D, Sherman SK, Izquierdo FJ, Eng OS, Turaga KK. Assessment of the surgical workforce pertaining to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the United States. Ann Surg Oncol. 2020;27(9):3097–3102. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH, Chang D. Cytoreductive surgery plus HIPEC with and without NIPEC for malignant peritoneal mesothelioma: a propensity-matched analysis. Ann Surg Oncol. 2021;28(12):7109–7117. [DOI] [PubMed] [Google Scholar]
- 3.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran B, Cecil T, Chandrakumaran K, Arnold S, Mohamed F, Venkatasubramaniam A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis. 2015;17(9):772–778. [DOI] [PubMed] [Google Scholar]
- 5.Sirody J, Kaji AH, Hari DM, Chen KT. Patterns of gastric cancer metastasis in the United States. Am J Surg. 2022;224(1):445–448. [DOI] [PubMed] [Google Scholar]
- 6.Lengyel E Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan M, Solon J, Chang KH, et al. Peritoneal metastases from extra-abdominal cancer – a population-based study. Eur J Surg Oncol. 2018;44(11):1811–1817. [DOI] [PubMed] [Google Scholar]
- 8.McQuellon RP, Loggie BW, Lehman AB, Russell GB, Fleming RA, Shen P, Levine EA. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10(2):155–162. [DOI] [PubMed] [Google Scholar]
- 9.Cashin PH, Dranichnikov F, Mahteme H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: cohort analysis of high volume disease and cure rate. J Surg Oncol. 2014;110(2):203–206. [DOI] [PubMed] [Google Scholar]
- 10.Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23(4):393–401. [DOI] [PubMed] [Google Scholar]
- 11.Reece L, Dragicevich H, Lewis C, et al. Preoperative nutrition status and postoperative outcomes in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2019;26(8):2622–2630. [DOI] [PubMed] [Google Scholar]
- 12.Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–1196. [DOI] [PubMed] [Google Scholar]
- 13.Wie GA, Cho YA, Kim SY, Kim SM, Bae JM, Joung H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010;26(3):263–268. [DOI] [PubMed] [Google Scholar]
- 14.Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38(2):196–204. [DOI] [PubMed] [Google Scholar]
- 15.Guenter P, Abdelhadi R, Anthony P, et al. Malnutrition diagnoses and associated outcomes in hospitalized patients: United States, 2018. Nutr Clin Pract. 2021;36(5):957–969. [DOI] [PubMed] [Google Scholar]
- 16.Balachandran R, Mogensen LZ, Christensen P, Thaysen HV, Iversen LH. Organ-specific adverse effects after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2022;29(9):6049–6083. [DOI] [PubMed] [Google Scholar]
- 17.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12(3):585–603. [DOI] [PubMed] [Google Scholar]
- 18.Auer RC, Sivajohanathan D, Biagi J, Conner J, Kennedy E, May T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: a clinical practice guideline. Curr Oncol. 2020;27(3):146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 2007;134:247–264. [DOI] [PubMed] [Google Scholar]
- 20.Kusamura S, Barretta F, Yonemura Y, et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery. JAMA Surg. 2021;156(3):e206363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durnford S, Boss L, Bell J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJA Educ. 2021;21(5):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugarbaker PH, Chang D. Incomplete cytoreduction of colorectal cancer peritoneal metastases: survival outcomes by a cytoreduction score. Visc Med. 2022;38(2):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macrì A, Fortugno A, Saladino E. Rationale and techniques of cytoreductive surgery and peritoneal chemohyperthermia. World J Gastrointest Oncol. 2011;3(12):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortés-Guiral D, Hübner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers. 2021;7(1):91. [DOI] [PubMed] [Google Scholar]
- 25.Sugarbaker PH. After thirty years of experience with early postoperative intraperitoneal 5-fluorouracil now saying goodbye. Surg Oncol. 2022;42:101757. [DOI] [PubMed] [Google Scholar]
- 26.Vashi PG, Gupta D, Lammersfeld CA, Braun DP, Popiel B, Misra S, Brown KC. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutr J. 2013;12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vugt JL, Braam HJ, van Oudheusden TR, et al. Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2015;22(11):3625–3631. [DOI] [PubMed] [Google Scholar]
- 28.Aytin YE, Cakcak İE, Sağıroğlu T. The evaluation of morbidity in gastrointestinal tumor patients underwent cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC). Turk J Surg. 2023;39(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeenk RM, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei. Cancer Treat Rev. 2007;33(2):138–145. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Basave HN, Morales-Vasquez F, Ruiz Molina JM, et al. Morbidity and mortality of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: National Cancer Institute, Mexico City, Mexico. ISRN Oncol. 2011;2011:526384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canda AE, Sokmen S, Terzi C, et al. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20(4):1082–1087. [DOI] [PubMed] [Google Scholar]
- 32.Rieser CJ, Alvikas J, Phelos H, et al. Failure to thrive following cytoreduction and hyperthermic intraperitoneal chemotherapy: causes and consequences. Ann Surg Oncol. 2022;29(4):2630–2639. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Candela A, Calero A, Sánchez-Guillén L, et al. Effect of preoperative immunonutrition on postoperative major morbidity after cytoreductive surgery and hipec in patients with peritoneal metastasis. Nutrients. 2021;13(7):2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raspé C, Flöther L, Schneider R, Bucher M, Piso P. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2017;43(6):1013–1027. [DOI] [PubMed] [Google Scholar]
- 35.Cortés-Guiral D, Mohamed F, Glehen O, Passot G. Prehabilitation of patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Eur J Surg Oncol. 2021;47(1):60–64. [DOI] [PubMed] [Google Scholar]
- 36.Dineen SP, Robinson KA, Roland CL, et al. Feeding tube placement during cytoreductive surgery and heated intraperitoneal chemotherapy does not improve postoperative nutrition and is associated with longer length of stay and higher readmission rates. J Surg Res. 2016;200(1):158–163. [DOI] [PubMed] [Google Scholar]
- 37.Casado-Adam A, Alderman R, Stuart OA, Chang D, Sugarbaker PH. Gastrointestinal complications in 147 consecutive patients with peritoneal surface malignancy treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Int J Surg Oncol. 2011;2011:468698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arakelian E, Gunningberg L, Larsson J, Norlén K, Mahteme H. Factors influencing early postoperative recovery after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2011;37(10):897–903. [DOI] [PubMed] [Google Scholar]
- 39.Purcell SA, Elliott SA, Baracos VE, Chu QS, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70(11):1230–1238. [DOI] [PubMed] [Google Scholar]
- 40.Figueiroa S, Dourney A. Nitrogen balance in cytoreductive surgical patients. ASPEN Nutrition Science and Practice Conference: Phoenix, Arizona, March 23–26, 2019: Vars candidates, trainee awards, best of ASPEN (topic awards), international awards, abstracts of distinction, posters of distinction and other abstracts. JPEN J Parenter Enteral Nutr. 2019;43(3):445. [DOI] [PubMed] [Google Scholar]
- 41.Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358(9292):1487–1492. [DOI] [PubMed] [Google Scholar]
- 42.Kuang X, She G, Shi Y, Yang Z, Li J, Zhang Z. Enteral nutrition provides favorable postoperative outcomes for patients with pseudomyxoma peritonei: a retrospective study. Gland Surg. 2022;11(5):818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koemans WJ, Houwink A, van der Kaaij RT, et al. Perioperative management of gastric cancer patients treated with (sub)total gastrectomy, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy (HIPEC): lessons learned. Ann Surg Oncol. 2021;28(8):4647–4654. [DOI] [PubMed] [Google Scholar]
- 44.Elekonawo FMK, van der Meeren MMD, Simkens GA, de Wilt JHW, de Hingh IH, Bremers AJA. Comparison of 2 perioperative management protocols and their influence on postoperative recovery after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: standard parenteral nutrition, selective bowel decontamination and suprapubic catheters? Dig Surg. 2019;36(5):394–401. [DOI] [PubMed] [Google Scholar]
- 45.Swain DR, Yates AL, Mohamed F, Dayal SP, Tzivanakis A, Cecil TD, Moran BJ. Do patients undergoing cytoreductive surgery and HIPEC for peritoneal malignancy need parenteral nutrition? Pleura Peritoneum. 2018;3(4):20180123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong XS, Sultana R, Tan JW, et al. The role of total parenteral nutrition in patients with peritoneal carcinomatosis: a systematic review and meta-analysis. Cancers (Basel). 2021;13(16):4156. [DOI] [PMC free article] [PubMed] [Google Scholar]


