Abstract
Several groups have reported that certain herpesvirus envelope proteins do not remain on the surface of cells that express them but rather are internalized by endocytosis in a recycling process. The biological function of membrane protein endocytosis in the virus life cycle remains a matter of speculation and debate. In this report, we demonstrate that some, but not all, membrane proteins encoded by the alphaherpesvirus pseudorabies virus (PRV) are internalized after reaching the plasma membrane. Glycoproteins gE and gB are internalized from the plasma membrane of cells, while gI and gC are not internalized efficiently. We show for gE that the cytoplasmic domain of the protein is required for endocytosis. While the gI protein is incapable of endocytosis on its own, it can be internalized when complexed with gE. We demonstrate that endocytosis of the gE-gI complex and gB occurs early after infection of tissue culture cells but that this process stops completely after 6 h of infection, a time that correlates with significant shutoff of host protein synthesis. We also show that gE protein internalized at 4 h postinfection is not present in virions formed at a later time. We discuss the differences in PRV gE and gI endocytosis compared to that of the varicella-zoster virus homologs and the possible roles of glycoprotein endocytosis in the virus life cycle.
Pseudorabies virus (PRV) is a member of the alphaherpesvirus subfamily, which includes the human pathogens herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) as well as varicella-zoster virus (VZV). PRV is the causative agent of Aujeszky’s disease in its natural host, swine, but is also capable of causing lethal disease in a variety of animals (5, 44). PRV encodes at least 10 glycoproteins found in virion envelopes (32). Two of these, gE and gI, have been shown to be important for virulence and spread of the virus in all animal models tested (2, 6, 7, 9, 22, 24, 25, 29, 31, 33, 44, 47, 53). PRV gE and gI exhibit Fc receptor binding activity for swine immunoglobulin G but not for immunoglobulin G from other species (13, 58). gE and gI form a hetero-oligomer that facilitates the maturation and intracellular transport of both proteins to the plasma membrane of cells (53, 58). Unlike the gE protein of feline herpesvirus (35), however, PRV gE and gI can each reach the cell surface independent of each other’s expression, albeit with lower efficiency (reference 53 and unpublished observations).
Recently, several groups have reported the endocytosis of virally encoded glycoproteins from the plasma membrane of cells (1, 28, 38, 39, 41, 45, 48, 57). In the herpesvirus family, the VZV gE and gI proteins (1, 38, 39, 57) and the human cytomegalovirus (HCMV) gB protein (41) have been shown to be internalized in both transfected and infected cells. Internalization of the VZV gE protein is dependent upon a YAGL motif located in the cytoplasmic tail of the protein, while endocytosis of the VZV gI protein requires a dileucine-type motif (ML) also located in its cytoplasmic tail. YXXL and dileucine motifs interact directly with the endocytosis machinery to mediate internalization of proteins in clathrin-coated pits (30, 37, 52). Accordingly, the VZV gE protein colocalizes with clathrin-coated vesicles and with the transferrin receptor during internalization (39).
We have used a genetic approach to demonstrate that PRV gE, a type I membrane protein, can be resolved into three distinct functional domains: a 428-amino-acid extracellular domain, a 26-amino-acid hydrophobic transmembrane domain, and a 123-amino-acid, highly charged cytoplasmic domain (49). The gE cytoplasmic domain is not required for gE-mediated anterograde spread in the rat eye model, but it is essential for virulence. Animals infected with PRV mutants expressing truncated forms of gE live longer and have fewer symptoms than animals infected with wild-type virus. Moreover, gE protein lacking the cytoplasmic tail is no longer incorporated into viral particles, suggesting that this cytoplasmic domain also contains signals required for incorporating the gE protein into virion envelopes.
In this report, we demonstrate another function of the cytoplasmic domain of PRV gE: endocytosis of the gE-gI complex. We demonstrate that gE and the gE-gI complex, but not gI alone, were internalized from the plasma membrane of transfected cells. We also show that the gE-gI complex and the gB protein were internalized from the plasma membrane of infected cells early in infection. However, internalization of viral membrane proteins could not be detected after 6 h of infection. This inhibition correlated with the time of shutoff of host cell protein synthesis and occurred well before significant release of virus into the medium. Thus, endocytosis of viral glycoproteins appears to be an early event and may not play a major role in late events in the virus life cycle.
MATERIALS AND METHODS
Virus strains and cells.
PRV strain Becker (PRV Be) and the isogenic strains PRV 25 and PRV 26 (encoding anchored and secreted gE, respectively) and their revertants have been previously described (49, 53). All PRV strains were propagated in PK15 (pig kidney) cells. Cells were grown in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), while viral infections were performed in DMEM supplemented with 2% FBS.
Plasmids.
The gE gene was excised from pRT24 (49) with DraI and PmlI, blunt ended with Klenow fragment (New England Biolabs), and subcloned into pBluescript KS+ (Stratagene) that had been cut with EcoRV. The resulting plasmid was named pAK15. pAK15 was then digested with EcoRI and SalI to release the gE gene. This fragment was cloned into pBabePuro (36) (kindly provided by T. Shenk, Princeton University) that had also been digested with EcoRI and SalI. This plasmid was called pIB2. pIB2 was then digested with EcoRI and SalI to release the gE gene. This fragment was subsequently cloned into a pcDNA1/Amp vector (Invitrogen) to create plasmid pMT2.
The gI gene was released from pRT24 by using the restriction enzymes CspI and DraI. The CspI end was filled in by using Klenow fragment, and the fragment was then cloned into pBluescript KS+ that had been cut with EcoRV. This plasmid was called pAK19. The gI gene was isolated from pAK19 by cutting with EcoRI and SalI and ligated into an EcoRI-SalI-cut pBabePuro plasmid, creating plasmid pIB1. The gI gene was then transferred to pcDNA1/Amp by using the same EcoRI-SalI sites. This final vector was called pMT1. The pcDNA1/Amp vector contains a cytomegalovirus immediate-early promoter for expression of the cloned genes.
Antisera.
The monoclonal antibody specific for gE when it is complexed with gI (1/14) and polyclonal goat antisera to gB (284) and gC (282) have been previously described (15, 43, 46). The monoclonal antibody pool to gE (M133, M156, and M138) was kindly provided by T. Ben-Porat. Rabbit polyclonal anti-gI and -gE sera were generous gifts from K. Bienkowska-Szewczyk (University of Gdansk). Fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit or donkey anti-mouse immunoglobulin G and idocarbocyanine-conjugated donkey anti-goat immunoglobulin G were purchased from Jackson Laboratories.
Transfections.
Forty to fifty percent confluent PK15 cells seeded the night before onto coverslips were transfected by standard calcium phosphate transfection (17). Briefly, a total of 20 μg of precipitated DNA (either 10 μg of pMT1 or pMT2 alone and 10 μg of calf thymus DNA or 10 μg each of pMT1 and pMT2 for cotransfection) was incubated with the cells for 6 to 8 h, followed by a 15% glycerol shock for 3 min. The cells were rinsed two times with fresh DMEM plus 10% FBS and incubated overnight. Assays were performed 24 h after the addition of DNA.
Indirect immunofluorescence endocytosis assay.
Transfected or infected cells (multiplicity of infection [MOI] of 10) grown on coverslips were cooled to 4°C by rinsing three times with cold phosphate-buffered saline (PBS) on ice. Cooling the cells to 4°C inhibits endocytosis of cell surface molecules and allows one to label proteins on the cell surface. The cells were then incubated on ice for 30 min with primary antibody diluted in PBS–3% bovine serum albumin. After being rinsed three times with cold PBS, cells were shifted for various amounts of time to 37°C by the addition of prewarmed medium and placement into a 37°C incubator. At the indicated time points, the cells were fixed with 3.7% formaldehyde diluted in PBS–2.5 mM MgCl2 and permeabilized with 0.5% Igepal CA-630 (Sigma). The cells were then incubated with either idocarbocyanine-conjugated (gB) or FITC-conjugated (gI or gE) secondary antibody (1:100 in PBS–3% bovine serum albumin) for 30 min in a 37°C humidified chamber. Following three washes with PBS–2.5 mM MgCl2 and one wash with distilled water, the coverslips were mounted on microscope slides in Testog (Testog, Inc.). Single optical sections were taken through the center of the cells by using a Nikkon MRC600 confocal microscope mounted on an Optiphot II, which utilizes an argon-krypton laser.
Biotinylation-trypsinization endocytosis assays.
PK15 cells infected with PRV Be (MOI of 10) were cooled to 4°C by incubation on ice and rinsing three times with PBS-CM (PBS, 0.1 mM CaCl2, 1 mM MgCl2). The cells were then incubated for 30 min on ice in 1 mg of EZ-Link NHS-biotin (Pierce) per ml freshly diluted in biotinylation buffer (10 mM triethanolamine [pH 8.0], 150 mM NaCl, 4 mM CaCl2). The biotin was removed, and unreacted biotin was quenched by treatment with cold medium supplemented with 10 mM glycine for 10 min. The cells were then rinsed three times with cold PBS-CM, shifted to 37°C by the addition of prewarmed medium containing 10 mM glycine, and placed into a 37°C incubator for various times. The cells were then cooled again on ice and rinsed once with cold Hanks’ balanced salt solution before the addition of 1 mg of trypsin (Gibco-BRL) per ml in Hanks’ balanced salt solution. Trypsin digestion was performed on ice for 10 min, followed by rinsing the cells three times with medium containing 20% FBS and then twice with PBS containing 1 mg of chicken egg white trypsin inhibitor (Sigma) per ml. Cells were lysed by the addition of TNX buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100) containing 100 μg of phenylmethylsulfonyl fluoride (Sigma) per ml. Affinity purification of biotinylated proteins was performed with streptavidin-agarose (10 μl of resin/100 μl of extract) (Gibco-BRL) according to the manufacturer’s instructions with buffers previously described (53). The purified proteins were electrophoresed through a sodium dodecyl sulfate (SDS)–8% polyacrylamide gel and transferred to nitrocellulose membranes. Western blot analysis and enhanced chemiluminescence detection were performed as recommended by the manufacturer of SuperSignal (Pierce).
For isolation of biotinylated virions, cells were biotinylated at 4 h postinfection as described above. Medium containing 20% FBS was placed on the cells for an additional 12 h. The medium was then removed and cleared of cellular debris by centrifugation at 1,000 × g for 5 min. Virions were pelleted from the medium by centrifugation through a 7-ml 30% sucrose cushion in PBS (SW27 rotor, 23,000 rpm, 3 h). The resulting pellet was resuspended in medium by sonication and pelleted through a 1-ml 30% sucrose cushion (SW50.1 Ti rotor, 28,000 rpm, 90 min). The final pellet was resuspended in TNX buffer. Biotinylated virion proteins were affinity purified and analyzed as described above.
Host cell shutoff determination.
PK15 cells infected with PRV Be (MOI of 10) were pulsed for sequential 1-h time periods with [35S]cysteine plus [35S]methionine (NEN) (50 μCi/ml; specific activity, 11 μCi/μl) beginning with the second to third hour postinfection. Immediately following the 1-h labeling period, the cells were lysed in TNX buffer, and equal volumes of cell lysate were electrophoresed through an SDS–10% polyacrylamide gel prior to autoradiography.
Single-step growth determination.
PK15 cells were infected with PRV Be at an MOI of 10. Following a 1-h adsorption period at 37°C, the cells were rinsed for 1 min with citrate buffer (40 mM Na citrate, 10 mM KCl, 135 mM NaCl, pH 3.0) to inactivate any unabsorbed virus. The cells were then incubated with fresh medium supplemented with 2% FBS for various times. The medium was removed from the cells and frozen, while the cells were rinsed three times with PBS, scraped into medium, and frozen. Cells were exposed to three freeze-thaw cycles to release infectious intracellular virus, and both cell extracts and medium were sonicated prior to titration. Titers in all samples were determined in duplicate on monolayers of PK15 cells, and the average of each was taken.
RESULTS
Endocytosis in transfected cells.
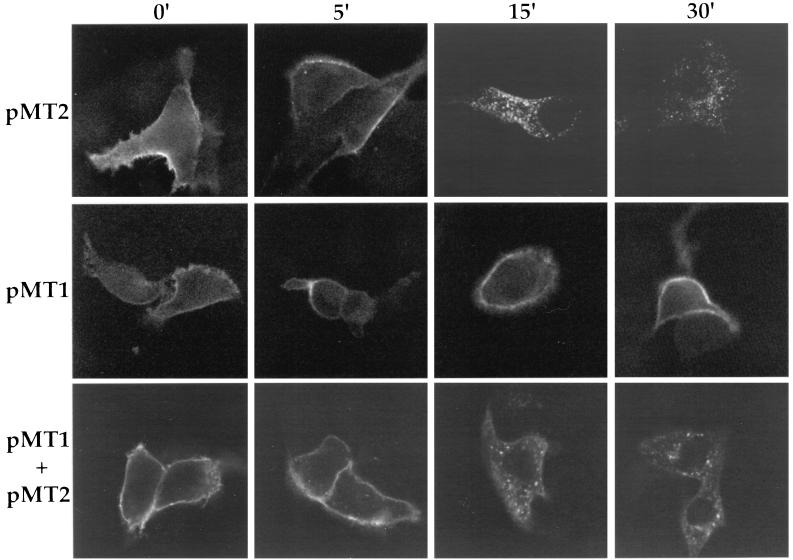
Our first objective was to determine if the PRV gE and gI proteins behaved like the VZV homologs and were internalized from the plasma membrane of transfected cells (1, 38, 39). PK15 cells were transfected with plasmids encoding gE (pMT2), gI (pMT1), or gE and gI (pMT2 plus pMT1), and endocytosis was assayed by indirect immunofluorescence and confocal microscopy as described in Materials and Methods. As shown in the top panels of Fig. 1 (pMT2), gE protein was found on the plasma membrane surface if the cells were not shifted to 37°C. After incubation of the cells for 15 min at 37°C, protein began to accumulate in small vesicles in the interior of the cells. After 30 min of incubation, the majority of the protein was found in vesicular structures inside the cells. The intensity and distribution of the protein did not change after 45 or 60 min of incubation at 37°C (data not shown). The middle panels of Fig. 1 (pMT1) show cells transfected with a plasmid encoding the gI protein alone. At 0 min, the protein was found on the cell surface. After a shift to 37°C for up to 30 min, the protein remained on the cell surface. The bottom panels of Fig. 1 (pMT1 plus pMT2) show cells transfected with both gE- and gI-expressing plasmids and reacted with an antibody that specifically recognized the gE-gI complex. At time zero, the complex was detected on the plasma membrane of the cell, and the complex was internalized rapidly after the shift to 37°C in a pattern similar to that for the gE protein alone in transfected cells. Like the VZV gE protein, PRV gE was rapidly internalized from the plasma membrane of transfected cells. In contrast to the VZV gI protein (38), however, PRV gI was not internalized in the absence of its binding partner, gE.
FIG. 1.
Endocytosis of transfected proteins. PK15 cells were transfected with either gE (pMT2), gI (pMT1), or both gE and gI (pMT1 + pMT2) for 24 h prior to an indirect immunofluorescence endocytosis assay. Endocytosis assays were performed with either a monoclonal antibody pool (M133, M156, and M138) that recognized gE (top panels), polyclonal rabbit antiserum to gI (middle panels), or a monoclonal antibody (1/14) that recognized gE when complexed with gI (bottom panels). Cells were incubated with the antibodies for 30 min on ice prior to being shifted to 37°C for the indicated times to allow internalization of cell surface proteins with bound primary antibody. The cells were then fixed, permeabilized, and incubated with FITC-labeled immunoglobulin G secondary antibodies to detect bound primary antibody.
Endocytosis in infected cells.
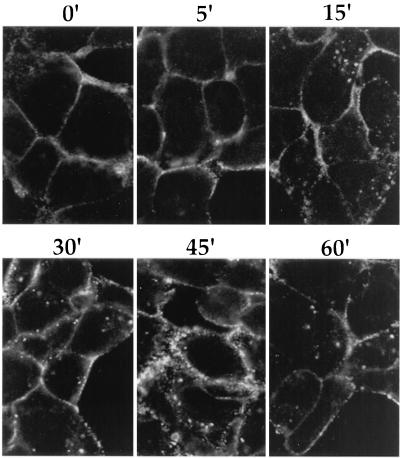
While gE was internalized in transfected cells, it was critical to understand what happens to the gE protein during viral infection. To address this question, we infected PK15 cells with a wild-type strain, PRV Be, at an MOI of 10. At 4 h postinfection, the cells were cooled and an indirect immunofluorescence endocytosis assay was performed with an antibody that recognized the gE-gI complex. As shown in the confocal images in Fig. 2, the gE-gI complex was found on the surface of cells when the cells were not shifted to 37°C. After incubation at 37°C, the proteins moved into the interior of the cell. Significant accumulation of protein in vesicles inside the cells was not seen until approximately 15 min after the shift, and the accumulation of gE-gI-containing vesicles increased up to 45 min after the temperature shift. At 60 min after the shift, the staining in the interior of the cell decreased. While the gE-gI complex was internalized from the plasma membrane of infected cells, this was somewhat less efficient than internalization observed in transfected cells (compare to Fig. 1, bottom panels).
FIG. 2.
Endocytosis of the gE-gI complex in cells infected with wild-type virus. PK15 cells were infected with PRV Be (wild type) at an MOI of 10 for 4 h. The cells were then cooled and incubated for 30 min on ice with a monoclonal antibody (1/14) that recognized gE when complexed with gI. The cells were then shifted to 37°C for the indicated times. The cells were fixed, permeabilized, and reacted with an FITC-labeled anti-mouse immunoglobulin G secondary antibody.
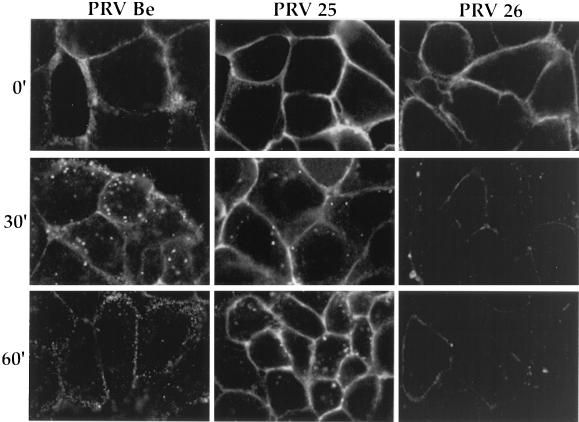
To investigate the role of the gE cytoplasmic tail domain in endocytosis of the protein, PK15 cells were infected with mutant viruses encoding either a transmembrane-anchored gE protein lacking the cytoplasmic tail (PRV 25, anchored gE) or a secreted gE protein (PRV 26, secreted gE). Figure 3 shows a comparison of gE-gI endocytosis in cells infected with PRV Be, PRV 25, or PRV 26 as detected with the antibody that recognized gE complexed with gI. The plasma membrane was brightly stained at time zero. After the shift to 37°C for 30 min, some anchored-gE–gI complex was internalized, but most of it remained on the cell surface. By 60 min, the majority of the complex remained on the cell surface, while most of the wild-type gE-gI complexes had been internalized. For the secreted-gE–gI complex, even though the gE protein is secreted, much of it remained on the cell surface of infected cells, presumably through its ability to oligomerize with gI (49). At time zero the plasma membrane was brightly stained. However, after the shift to 37°C for 30 or 60 min, bound primary antibody could no longer be visualized easily. This suggests that the complex may be disrupted by the binding of the antibody and subsequent shifting to 37°C or, alternatively, that it may be quickly internalized and rapidly degraded. Lack of internalization of the gE-truncated proteins was also confirmed with a biotinylation-trypsin assay, as described below (data not shown). These experiments demonstrate that a truncated gE protein lacking the cytoplasmic tail was not internalized efficiently from the plasma membrane of infected cells, suggesting that the cytoplasmic domain encodes signals required to engage the endocytosis machinery.
FIG. 3.
The cytoplasmic tail of gE is required for efficient endocytosis of the gE-gI complex from the plasma membrane of infected cells. PK15 cells were infected at an MOI of 10 with either PRV Be (wild type), PRV 25 (anchored gE), or PRV 26 (secreted gE) for 4 h. An indirect immunofluorescence assay was then performed as described in the legend to Fig. 2 with the monoclonal antibody (1/14) that recognized gE when complexed with gI.
Endocytosis of gE-gI is not antibody dependent.
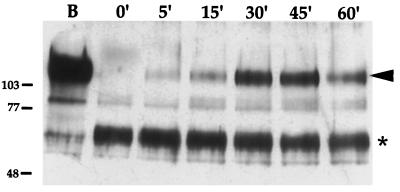
Antibody bridging and capping of glycoproteins through the mouse monoclonal antibody used to detect gE may have induced internalization of the gE-gI complex (13). To investigate this possibility, an endocytosis assay that did not rely on antibodies was performed by using biotinylation of cell surface proteins followed by mild trypsin digestion, as described in Materials and Methods. In this assay, only internalized proteins are protected from trypsin digestion and retain the biotin tag. As shown in Fig. 4, lane B, the full-length gE protein was efficiently biotinylated and could be visualized without trypsin digestion. When the cells were immediately treated with trypsin prior to the 37°C shift (0 min), the full-length, biotinylated gE protein was cleaved to a protease-resistant form. After a shift to 37°C, however, protected full-length gE protein could be visualized (5 to 60 min), with the most protection observed between 30 and 45 min of incubation at 37°C. This indicated that the gE protein had become protected from protease digestion via internalization and that internalization of gE was not antibody dependent. In these experiments, the mature form of gE was the predominant species biotinylated. However, we always observed a small amount of biotinylated immature gE precursor form. This may reflect the presence of precursor species on the plasma membrane or that a small amount of biotin reaches internal compartments by pinocytosis. The salient point of these experiments is the fate of the mature form after endocytosis.
FIG. 4.
Endocytosis of gE is not antibody dependent. PK15 cells were infected with PRV Be (wild type) at an MOI of 10 for 4 h. The cells were cooled, and cell surface proteins were biotinylated for 30 min on ice. The cells were either then lysed immediately (lane B), treated with trypsin immediately (0 min), or shifted to 37°C for the times indicated (5 to 60 min) prior to trypsin treatment. Biotinylated proteins were affinity purified from cell lysates by using streptavidin-agarose prior to Western blot analysis. The blot was probed with polyclonal rabbit antiserum against gE. Protected full-length gE is marked with an arrowhead, while a protease-resistant trypsin fragment is indicated by an asterisk. A biotinylated precursor form of gE is between the 77- and 103-kDa marks. Apparent molecular mass markers (kilodaltons) are indicated on the left.
Endocytosis of gB and gC.
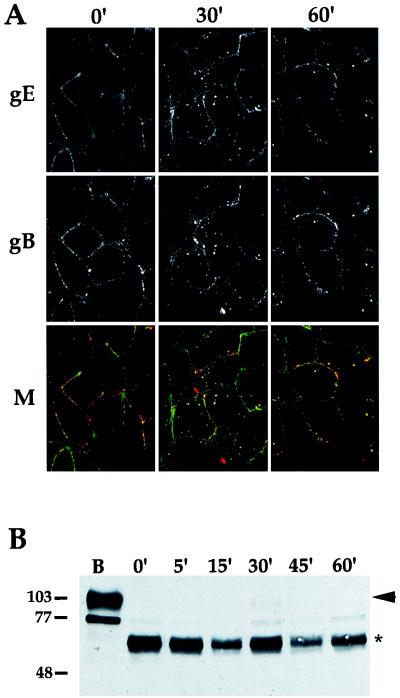
We next asked whether endocytosis was restricted to gE and determined whether other viral glycoproteins could be internalized from the plasma membrane of infected cells. To test this, we first looked at gB, another PRV membrane protein that contains a YQRL motif in its cytoplasmic tail (43). A confocal image of the gE-gI complex and the gB protein at 4 h postinfection is shown in Fig. 5A. At time zero, both the gE-gI complex and gB protein were observed on the plasma membrane of infected cells as punctate staining. The two proteins did not appear to completely colocalize, as staining of distinct regions was observed. After a shift to 37°C, both proteins were internalized and were seen in a vesicular staining pattern inside the cell. In every case in which gB was internalized, gB colocalized precisely with the gE-gI complex.
FIG. 5.
Internalization of gB and gC. (A) PK15 cells were infected with PRV Be (wild type) at an MOI of 10 for 4 h. An indirect immunofluorescence endocytosis assay was then performed as described in the legend for Fig. 2, using the monoclonal antibody (1/14) that recognized gE when complexed with gI (row gE) and a polyclonal goat antiserum (284) against gB (row gB). A merge of the two fields is shown in row M. The gE-gI complex is shown in green, and gB is shown in red. (B) Endocytosis of gC was determined by biotinylation-trypsin digestion as described in the legend to Fig. 4. The cells were either lysed immediately (lane B), treated with trypsin immediately (0 min), or shifted to 37°C for the times indicated (5 to 60 min) prior to trypsin treatment. Western blot analysis was performed with polyclonal goat antiserum to gC. Full-length gC protein is marked with an arrowhead, while the protease-resistant fragment is indicated by an asterisk. A biotinylated precursor form of gC is below the 77-kDa mark. Apparent molecular mass markers are indicated on the left in kilodaltons.
Endocytosis of gC at 4 h post infection was determined by using the biotinylation-trypsin assay described above, and the results are shown in Fig. 5B. Full-length mature gC protein was efficiently biotinylated (Fig. 5B, lane B). The biotinylated protein was cleaved to a protease-resistant fragment when incubated with trypsin without a shift to 37°C (0 min). Unlike for gE, however, barely any full-length gC protein was protected from trypsin digestion by incubation of the cells at 37°C (5 to 60 min). This indicated that gC was not internalized efficiently into the interior of the cells and protected from the protease.
Endocytosis is inhibited at 6 h postinfection.
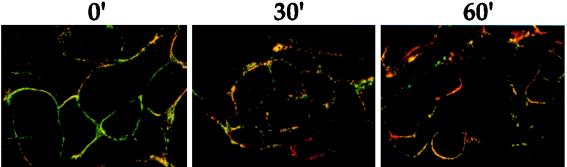
The next set of experiments was designed to determine if other viral proteins are internalized from the plasma membrane of infected cells. To facilitate detection, we performed endocytosis assays at a later point in infection when more viral proteins were expressed abundantly. As a control, a gE-gI complex and gB protein colocalization study was performed as described for Fig. 5A, except the experiment was initiated at 6 h postinfection. Figure 6 shows the confocal merged images of such an experiment. As before, both proteins were seen on the cell surface at time zero, but both proteins were more abundant at this time point than at 4 h postinfection. In contrast to the case at 4 h postinfection, neither the gE-gI complex nor gB protein appeared to be internalized after incubation of the cells at 37°C when examined at 6 h postinfection. These striking results were confirmed by using biotinylation-trypsin digestion assays (data not shown). Glycoprotein internalization was also not observed at 8 and 16 h postinfection and was less efficient at 5 h post infection as determined by indirect immunofluorescence assays (data not shown). Although glycoproteins were internalized efficiently at 4 h postinfection, endocytosis of the gE-gI complex and the gB protein was not observed at intermediate and late times of infection.
FIG. 6.
Endocytosis is inhibited at 6 h postinfection. Infections and endocytosis assays were performed at 6 h postinfection as described in the legend for Fig. 5. Internalization of the gE-gI complex and gB is shown as a merged image. The gE-gI complex is shown in green, and gB is shown in red.
Host cell shutoff and single-step growth determination.
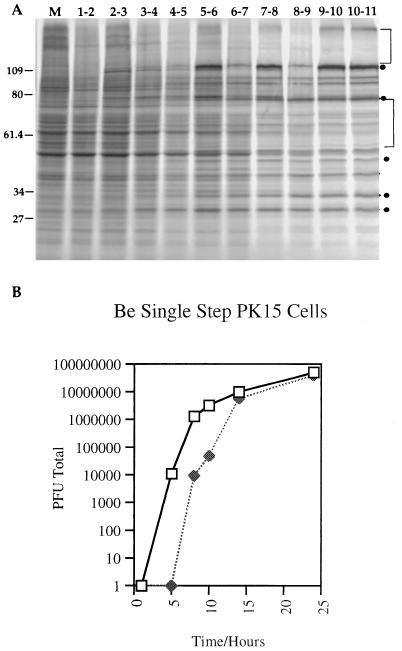
One explanation for the inhibition of endocytosis of glycoproteins could be that the expression of cellular proteins required for endocytosis was inhibited due to the host cell shutoff functions of PRV (4, 20, 21, 23, 42). To determine the time and extent of host cell shutoff, duplicate monolayers of PK15 cells were infected with PRV Be at an MOI of 10 and labeled with [35S]methionine and [35S]cysteine as described in Materials and Methods. The results are shown in Fig. 7A. Many bands representing host cell proteins were labeled until 5 h postinfection. Beyond 5 h, however, most of these host cell protein bands disappeared and more prominent viral protein bands appeared. This indicated that in our system host cell shutoff began to be obvious during the fifth hour of infection and most, if not all, host cell shutoff had occurred by the seventh hour of infection (see the legend to Fig. 7 for quantitation).
FIG. 7.
Host cell shutoff and single-step growth in PK15 cells. (A) PK15 cells infected with PRV Be at an MOI of 10 were labeled with [35S]methionine plus [35S]cysteine for 1-h periods beginning with the second hour of infection. After the labeling period, cell lysates were prepared and equal volumes of the lysates were analyzed on an SDS–10% polyacrylamide gel. Labeling periods are indicated at the top as the hour of infection that the labeling was performed. Quantitation by densitometry of the total amount of protein in the mock-infected lane compared to the total amount of protein in the 10- to 11-h-infected lane showed a decrease of 53% in total protein production. To correct for new viral proteins that were produced, regions containing host cell proteins only, such as the ones denoted by brackets, were quantitated. These measurements showed approximately a 55% reduction in host cell protein production at 6 to 8 h postinfection and a 67% reduction at 10 to 11 h postinfection. There was essentially no difference in shutoff between 7 to 8 and 10 to 11 h of infection. Representative viral bands are denoted by circles. The 109-kDa band was recovered in our extracts variably from experiment to experiment. Apparent molecular mass markers (kilodaltons) are indicated on the left. (B) PK15 cells were infected with PRV Be (wild type) at an MOI of 10. At the times indicated after infection, the medium was removed from the cells and frozen. The cells were then rinsed with PBS and scraped into medium before being frozen. Cell lysates underwent three freeze-thaw cycles to release intracellular virus. Both medium (closed symbols) and cell fractions (open symbols) were sonicated prior to determination of titers. Titers were determined on PK15 cells in duplicate as the total number of PFU produced, and the average of each was taken.
Endocytosis of viral glycoproteins has been proposed to be a mechanism to target the proteins for incorporation into virion particles (41, 56, 57). If so, then endocytosis would be predicted to occur during a time period when infectious viral particles were made and released. Accumulation of intracellular and extracellular infectious virus in PK15 cells was determined by single-step growth analysis of PRV Be. The results are depicted in Fig. 7B. Intracellular infectious virus was detected at 5 h postinfection, and this pool of intracellular virus increased exponentially until 10 h postinfection. The first infectious virion particles in the medium were detected at approximately 8 h postinfection. This pool of virus also increased exponentially, reaching a peak at 14 h postinfection. Thus, the majority of intracellular infectious virus and released infectious viral particles was recovered at times after which host cell shutoff was evident and the endocytosis of glycoproteins was no longer detectable.
Cell surface biotinylated gE protein is not incorporated into virion particles.
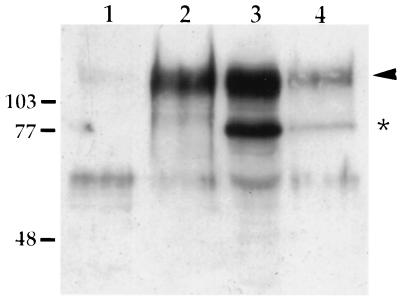
Although endocytosis of the gE-gI complex and gB protein was inhibited at 6 h postinfection, proteins that were internalized at 4 h postinfection may have remained in the cell for incorporation into virion particles later in infection. To test this, virions isolated from cells that had been biotinylated at 4 h postinfection were analyzed for the presence of biotinylated gE protein, as shown in Fig. 8. Although biotinylated gE protein could be detected in the cells 12 h after biotinylation (lane 4), little to no biotinylated gE protein could be detected in virions (lane 1). Lanes 2 and 3 show the total amount of gE protein in virion particles and cell extracts, respectively. To ensure that there was not an undetectable amount of biotinylated gE protein in the virions, 3.5 times more of the virion preparation was analyzed by affinity purification (Fig. 8, lane 1) as compared to the total amount of virions loaded in lane 2. Although gE was shown to be internalized from the plasma membrane of infected cells at 4 h postinfection, little internalized gE could be found in virion particles 12 h later.
FIG. 8.
Isolation of virions produced by cell surface-biotinylated cells. PK15 cells were infected with PRV Be (MOI = 10) for 4 h. The cell surface was then biotinylated in the absence of trypsin as described in the legend to Fig. 4. After biotinylation, the medium was replaced and the cells were incubated for an additional 12 h at 37°C. Virions were isolated from this medium by pelleting through a 30% sucrose cushion. Cell lysates were also collected at this time. Biotinylated protein was affinity purified from both cell lysates (lane 4) and purified virions (lane 1) as described in the text, and total cell (lane 3) and total virion (lane 2) fractions as well as the biotinylated fractions were analyzed by Western blotting with polyclonal rabbit antiserum to gE. Thirty-five percent of the isolated virions was analyzed for biotinylated gE protein (lane 1), 10% of the virions was analyzed for total gE protein content (lane 2), 20% of the cell lysate was analyzed for biotinylated gE protein, and 0.5% of the total cell extract was analyzed for total gE content. The mature form of gE is marked with an arrowhead, while the immature form is denoted by an asterisk. The band detected around 60 kDa is unrelated to gE. Molecular mass markers (kilodaltons) are indicated on the left.
DISCUSSION
The PRV gE protein is involved in several aspects of viral infection. Virus lacking gE has a reduced ability to spread from cell to cell in certain cultured cells and is defective in anterograde spread in some neuronal circuits (7, 24–27, 34, 53). Anterograde transneuronal spread in the rat eye model can be accomplished by viruses that express only the extracellular N-terminal portion of the protein (49). The gE protein also oligomerizes with gI, and as found for feline herpesvirus gE, the interaction domain is in the extracellular N-terminal domain of gE (35, 49). gE is also required for full expression of virulence of PRV in all animal models tested (3, 7, 22, 24, 29, 31, 44, 47). The cytoplasmic C-terminal domain of gE is required for full expression of gE-mediated virulence. In addition, this region is required for incorporation of gE into the viral envelope (49). Here we report that the cytoplasmic tail of gE is required for endocytosis of gE and the gE-gI complex.
Internalization of the gE protein from the plasma membrane occurred in cells transfected only with gE; thus, expression of other viral proteins was not required. Indeed, gE contains two YXXL motifs in its cytoplasmic tail (YTSL [amino acids 478 to 481] and YVSL [amino acids 517 to 520]) that direct endocytosis of other cell surface membrane proteins (1, 30, 37, 39, 51, 52). The PRV gI protein, however, was not able to be internalized from the plasma membrane on its own. This may not be surprising, as the cytoplasmic tail of gI contains no recognizable endocytosis motifs. However, cotransfection of gI with gE directed endocytosis of the gE-gI complex. Thus, PRV gI is functionally different during endocytosis than the VZV gI protein, which is rapidly internalized from the plasma membrane of transfected cells without the aid of gE (38). In fact, the PRV and VZV gE and gI proteins seem to have opposite roles in endocytosis, as the VZV gI protein increases the rate of internalization of the VZV gE protein, while the PRV gE protein directs endocytosis of the PRV gI protein. Nevertheless, the net result in both cases is internalization of the gE-gI complex. It is possible that gI modulates the rate or amount of internalization of gE when coexpressed with gE. However, the assays performed did not allow us to quantitate the amount of gE internalized. We are currently developing assays to quantitate endocytosis of the proteins.
A fundamental question concerns the role of endocytosis in the virus life cycle. We demonstrated that at 4 h postinfection, the gE-gI complex was internalized from the plasma membrane of PRV Be-infected cells, albeit at a lower rate than internalization of the complex from the plasma membrane of transfected cells. Internalization of the complex relied on the cytoplasmic tail of gE, as mutant viruses expressing secreted or membrane-anchored gE proteins showed diminished or no internalization of the gE-gI complex. Importantly, we showed that endocytosis was independent of antibody binding to gE through biotinylation of cell surface proteins followed by trypsin digestion. This procedure precluded potential antibody bipolar bridging and induced capping of glycoproteins that may have stimulated endocytosis. In addition, we found that another glycoprotein, gB, was internalized from the plasma membrane of infected cells at 4 h postinfection. The glycoproteins gI and gC do not have YXXL motifs. gI was not internalized without the aid of gE, and gC was not internalized from the plasma membrane of infected cells at any point postinfection. This suggested that endocytosis of glycoproteins may be a common feature of some, but not all, viral proteins.
We attempted to test other glycoproteins for their ability to be internalized. In doing so, we performed endocytosis experiments at 6 h postinfection, a time when most late membrane proteins are made in abundance. Much to our surprise, internalization of the gE-gI complex and the gB protein was inhibited at this time point. Similar negative results were obtained at 8, 10, 12, and 16 h postinfection. Inhibition of endocytosis correlated with the time of host cell shutoff in PK15 cells. Host cell shutoff was not evident at 4 h postinfection but was extreme by 6 and 8 h postinfection. This result implied that expression of proteins required for endocytosis may be shut off by viral infection. In addition, a viral protein expressed at later times may inhibit endocytosis or stabilize the gE-gI complex at the cell surface, preventing its internalization. Alternatively, the high expression level of the viral glycoproteins late in infection may simply overwhelm the endocytosis machinery. While further work is necessary to test these ideas, we do know that overproduction of gE and gI alone is not sufficient to block endocytosis. Internalization of gE-gI occurred in transfection experiments in which gE and gI were transcribed from the strong cytomegalovirus immediate-early promoter. The apparent concentration of gE and gI in these transfected cells as deduced by immunofluorescence was indistinguishable from that found in virus-infected cells.
The inhibition of endocytosis at later times after infection was not expected and must be considered when thinking of the function of this process in the virus life cycle. Internalization of glycoproteins from the plasma membrane may serve as a mechanism for incorporating mature glycoproteins into the final virion envelope (39, 41, 56, 57). The deenvelopment-reenvelopment model for acquisition of the final viral envelope proposed for PRV, VZV, and HCMV suggests that mature viral glycoproteins are acquired at the trans-Golgi network or at endosomal structures (8, 14, 16, 18, 41, 50, 56, 57). Thus, endocytosis of glycoproteins from the plasma membrane may deliver these proteins to organelles where final envelopment occurs. This hypothesis is consistent with our finding that truncated PRV gE proteins, unable to be internalized efficiently from the plasma membrane of infected cells, also were not incorporated into viral particles, despite their localization throughout much of the secretory pathway of the host cell (49). The inability to internalize viral glycoproteins correlated well with the exclusion from the viral envelope that we have previously noted. We have also noted, however, that the gI protein is incorporated into virion particles even when expressed with altered gE proteins that are defective in internalization (data not shown). This suggests that gI has another mechanism for incorporation into the virion envelope when gE is not able to direct its endocytosis. gC must also have another mechanism for incorporation into virions, as this protein is not internalized efficiently from the plasma membrane of infected cells yet is abundantly expressed in the viral envelope. The lack of internalization of any glycoprotein tested at 6 h postinfection in PK15 cells also suggests that endocytosis of glycoproteins may not be a major pathway followed for mature proteins destined for virion envelopes in these cells. Major accumulation of intracellular infectious viral particles began at 5 h postinfection and reached a plateau between 10 and 14 h postinfection in PK15 cells. While virion particles formed at the earliest times could contain newly endocytosed proteins, those enveloped at later time points probably lose access to viral glycoproteins on the cell surface, unless the proteins internalized at earlier times were set aside for later viral particle formation. This would require that after endocytosis from the plasma membrane, the proteins are targeted to an organelle and remain there until envelopment occurs. Zhu et al. have shown that the VZV gE protein is specifically targeted to the trans-Golgi network through a AYRV sequence in its cytoplasmic tail after internalization of the protein from the plasma membrane (57). Such a sequence is not found in the PRV gE protein.
We tested whether proteins internalized at 4 h postinfection could later be incorporated into virion particles by biotinylating the cell surface at 4 h postinfection and looking for biotinylated gE in virion particles 12 h later. While we were able to detect stable, biotinylated gE protein in the cells at this time, we could detect little biotinylated gE in virion particles. This suggests that even protein internalized at earlier times of infection is not specifically directed into virion particles with a high efficiency. There is evidence for HCMV that an internalized protein could remain in an intracellular organelle until envelopment. Radsak et al. showed through biotinylation that the HCMV gB protein was incorporated into viral particles after internalization of the protein from the plasma membrane of infected cells (41). As HCMV does not shut off host cell protein synthesis, endocytosis for envelopment remains a possibility. The role of endocytosis in infection of different cell types, particularly polarized cells, remains to be explored.
Although endocytosis of the gE-gI complex was inhibited at 6 h postinfection, endocytosis could still play a role during early stages of viral infection. We know that the cytoplasmic tail of gE is required both for endocytosis of the protein and for full expression of gE-mediated virulence. Perhaps early endocytosis of the gE-gI complex is responsible for the virulence phenotype. In one model, early in infection, the gE-gI complex could internalize a bound ligand, which when internalized causes the infected cell to express factors required for efficient virus replication or that stimulate a host response. Alternatively, internalization of the gE-gI complex could target delivery of the complex to an organelle or, in polarized cells, facilitate transcytosis of the internalized complex such that it is delivered to a cellular membrane that needs to marked early in infection. For example, the human immunodeficiency virus type 1 envelope gp120 glycoprotein is transcytosed from the apical side of MDCK cells to the basolateral side to mark the site of viral budding (28). Interruption of transcytosis through mutation of the endocytosis signal causes budding to occur from the apical side of the cells. In neurons, synaptic vesicle recycling shares several aspects with endocytosis (10). gE could function at sites of synaptic contact and may endocytose with synaptic vesicles.
To investigate the putative relationship between endocytosis of gE and gE-mediated virulence, we are constructing viruses carrying mutations in the YXXL sequences located in the cytoplasmic tail of gE. Such mutations should not only block endocytosis but also reduce virulence if the speculation has merit. Like the VZV and HSV-1 and -2 gE homologs, the cytoplasmic domain of PRV is phosphorylated (references 11, 12, 19, 40, 54, and 55 and our unpublished observations). Edson (11) showed by phosphopeptide mapping that PRV gE was phosphorylated on serine residues, most likely on the potential casein kinase I and II phosphorylation sites located in the cytoplasmic tail. The YAGL motif in VZV gE is also phosphorylated in rare dimeric forms of the protein (40). Although tyrosine phosphorylation of PRV gE has never been observed (reference 11 and our unpublished observations), phosphorylation-dephosphorylation at other sites could control trafficking of the protein. These YXXL motifs are also proposed to mediate the antibody-induced capping and shedding of viral glycoproteins (13), a phenomenon speculated to be involved in immune evasion. Further work to study gE targeting signals and phosphorylation should provide more insight into gE’s role in the pathogenesis of PRV.
ACKNOWLEDGMENTS
We thank Joe Goodhouse for his help and advice with the confocal images. We also thank K. Bienkowska-Szewcyzk for gE and gI antisera. Many thanks go to members of the Enquist lab for support and critical reading of the manuscript. R.S.T. also sincerely acknowledges M. Tomishima for his invaluable help.
This work was supported by NINDS grant 1RO133506 to L.W.E. and NIH grant 5T32GMO7312 to R.S.T.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 3.Banfield B W, Yap G S, Knapp A C, Enquist L W. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J Virol. 1998;72:4580–4588. doi: 10.1128/jvi.72.6.4580-4588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Porat T, Rakusanova T, Kaplan A S. Early functions of the genome of herpesvirus. Virology. 1971;46:890–899. doi: 10.1016/0042-6822(71)90089-4. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Porat T, Kaplan A S. Molecular biology of pseudorabies virus. In: Roizman B, editor. The herpesviruses. New York, N.Y: Plenum Publishing Corp.; 1985. pp. 105–173. [Google Scholar]
- 6.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card J P, Rinaman L, Lynn R B, Lee B H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two alphaherpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 10.De Camilli P. Molecular mechanisms in synaptic vesicle recycling. FEBS Lett. 1995;369:3–12. doi: 10.1016/0014-5793(95)00739-v. [DOI] [PubMed] [Google Scholar]
- 11.Edson C M. Phosphorylation of neurotropic alphaherpesvirus envelope glycoproteins: herpes simplex virus type 2 gE2 and pseudorabies virus gI. Virology. 1993;195:268–270. doi: 10.1006/viro.1993.1372. [DOI] [PubMed] [Google Scholar]
- 12.Edson C M, Hosler B A, Waters D J. Varicella-zoster virus gpI and herpes simplex virus gE: phosphorylation and Fc binding. Virology. 1987;161:599–602. doi: 10.1016/0042-6822(87)90157-7. [DOI] [PubMed] [Google Scholar]
- 13.Favoreel H W, Nauwynck J J, van Oostveldt P, Mettenleiter T C, Pensaert M B. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J Virol. 1997;71:8254–8261. doi: 10.1128/jvi.71.11.8254-8261.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs W, Klupp B G, Granzow H, Rhiza H J, Mettenleiter T C. Identification and characterization of the pseudorabies virus UL3.5 protein, which is involved in virus egress. J Virol. 1996;70:3517–3527. doi: 10.1128/jvi.70.6.3517-3527.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs W, Rziha H J, Lukacs N, Braunschweiger I, Visser N, Luetticken D, Schreurs C S, Thiel H J, Mettenleiter T C. Pseudorabies virus glycoprotein gI: in vitro and in vivo analysis of immunorelevant epitopes. J Gen Virol. 1990;71:1141–1151. doi: 10.1099/0022-1317-71-5-1141. [DOI] [PubMed] [Google Scholar]
- 16.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham R L, Van der Eb A S. A new technique for the assay of infection of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 18.Granzow H, Weiland R, Joens A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 20.Hamada C, Kaplan A S. Kinetics of synthesis of various types of antigenic proteins in cells infected with pseudorabies virus. J Bacteriol. 1965;89:1328–1334. doi: 10.1128/jb.89.5.1328-1334.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihara S, Feldman L, Watanabe S, Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983;131:437–454. doi: 10.1016/0042-6822(83)90510-x. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs L. Glycoprotein I of pseudorabies virus and homologous proteins in other alphaherpesvirinae. Arch Virol. 1994;137:209–228. doi: 10.1007/BF01309470. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan A S, Ben-Porat T. The incorporation of C14-labeled nucleosides into rabbit kidney cells infected with pseudorabies virus. Virology. 1960;11:12–27. doi: 10.1016/0042-6822(60)90053-2. [DOI] [PubMed] [Google Scholar]
- 24.Kimman T G, de Wind N, Oei-Lie N, Pol J M A, Berns A J M, Gielkens A L J. Contribution of single genes within the unique short region of Aujeszky’s disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 25.Kritas S K, Nauwynck H J, Pensaert M B. Dissemination of wild-type and gC-, gE- and gI-deleted mutants of Aujeszky’s disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995;76:2063–2066. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- 26.Kritas S K, Pensaert M B, Mettenleiter T C. Invasion and spread of single glycoprotein deleted mutants of Aujeszky’s disease virus (ADV) in the trigeminal nervous pathway of pigs after intranasal inoculation. Vet Microbiol. 1994;40:323–334. doi: 10.1016/0378-1135(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 27.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky’s disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 28.Lodge R, Lalonde J P, Lemay G, Cohen E A. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 1997;16:695–705. doi: 10.1093/emboj/16.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984;52:198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks M S, Ohno H, Kirchhausen T, Bonifacino J S. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–127. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 31.Mettenleiter T C, Lukacs N, Rziha H J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985;56:307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mettenleiter T C. Pseudorabies (Aujeszk’s disease) virus: state of the art. Act Vet Hung. 1994;42:153–177. [PubMed] [Google Scholar]
- 33.Mettenleiter T C, Zsak L, Kaplan A S, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies virus in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987;61:2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mijnes J D F, Lutters B C H, Vlot A C, van Anken E, Horzinek M C, Rottier P J M, de Groot R J. Structure-function analysis of the gE-gI complex of feline herpesvirus: mapping of gI domains required for gE-gI interaction, intracellular transport, and cell-to-cell spread. J Virol. 1997;71:8397–8404. doi: 10.1128/jvi.71.11.8397-8404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 38.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radsak K, Eickmann M, Mockenhaupt T, Bogner E, Kern H, Eis-Huebinger A, Reschke M. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch Virol. 1996;141:557–572. doi: 10.1007/BF01718317. [DOI] [PubMed] [Google Scholar]
- 42.Rakusanova T, Ben-Porat T, Kaplan A S. Effect of herpesvirus infection on the synthesis of cell-specific RNA. Virology. 1972;49:537–548. doi: 10.1016/0042-6822(72)90505-3. [DOI] [PubMed] [Google Scholar]
- 43.Robbins A K, Dorney D J, Wathen M W, Whealy M E, Gold C, Watson R J, Holland L E, Weed S D, Levine M, Glorioso J C, Enquist L W. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987;61:2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roizman B. Herpesviridae: a brief introduction. In: Fields B N, Knipe D M, editors. Fundamental virology. 2nd ed. New York, N.Y: Raven Press; 1991. pp. 841–847. [Google Scholar]
- 45.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 46.Ryan J P, Whealy M E, Robbins A K, Enquist L W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987;61:2251–2257. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rziha H J, Mettenleiter T C, Ohlinger V, Wittmann G. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology. 1986;155:600–613. doi: 10.1016/0042-6822(86)90220-5. [DOI] [PubMed] [Google Scholar]
- 48.Sauter M M, Pelchen-Matthews A, Bron R, Marsh M, LaBranche C C, Vance P J, Romano J, Haggarty B S, Hart T K, Lee W M F, Hoxie J A. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J Cell Biol. 1996;132:795–811. doi: 10.1083/jcb.132.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tooze J, Hollihshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 51.Trowbridge I S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3:634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 52.Trowbridge I S, Collawn J F. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 53.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao Z, Grose C. Unusual phosphorylation sequence in the gpIV (gI) component of the varicella-zoster virus gpI-gpIV glycoprotein complex (VZV gE-gI complex) J Virol. 1994;68:4204–4211. doi: 10.1128/jvi.68.7.4204-4211.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuckermann F S, Mettenleiter T C, Schreurs C, Sugg N, Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988;62:4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]