Abstract
Background and Objectives
Most published studies on the clinical utility of genetic testing for neuromuscular diseases (NMDs) focus on disease-specific cohorts and/or involve multiple centers. The aim of this study was to examine the clinical utility and diagnostic yield of genetic testing at a single, large neuromuscular center. Unlike previous studies, this study is unique in that it includes a broad array of patients at a single, large neuromuscular center, providing real-world data that may assist both neuromuscular specialists as well as general neurologists in decision-making regarding the need for genetic testing in patients with suspected NMDs.
Methods
Genetic testing results were reviewed for all patients who underwent testing through a single genetic testing company for NMDs in this single laboratory at a large neuromuscular center from 2015 to 2020. Retrospective chart reviews were performed to determine whether genetic testing results conferred a specific NMD diagnosis, including cases where a variant of uncertain significance (VUS) was identified.
Results
Genetic testing was pursued for 192 patients. A positive result, defined as a pathogenic mutation, a VUS, or both, was found in 77.1%. A definitive diagnosis was conferred in 35.9%. The most common testing indication was suspected neuropathy (53.3%), and the indication with the highest diagnostic yield was suspected myopathy (48.7%).
Discussion
This study provides further evidence of the clinical utility of genetic testing for NMDs in a real-world setting with over one-third of patients tested receiving a definitive diagnosis. Over time, genetic testing will continue to become increasingly accessible, cost-effective, and sensitive, which will lead to even more utilization.
Introduction
Neuromuscular diseases (NMDs) are a group of clinically and genetically heterogeneous disorders that affect skeletal muscle, the neuromuscular junction (NMJ), and/or peripheral nerves. Inherited NMDs include muscular dystrophies, congenital myopathies, myotonic dystrophies (DM), nondystrophic myotonias (NDM), congenital myasthenic syndromes, spinal muscular atrophy (SMA), hereditary neuropathies, and certain subtypes of motor neuron diseases (MND) such as amyotrophic lateral sclerosis (ALS), among others. Hundreds of monogenic, disease-causing mutations have been identified for NMDs, and the number continues to grow.1 It was previously estimated that 1 in 3,500 individuals have an inherited neuromuscular condition, but this is likely an underestimate based on the estimated of conditions such as Duchenne muscular dystrophy and SMA, so the real number may be closer to 1 in 1,000.2 Furthermore, many NMDs are associated with significant morbidity and mortality because of progressive loss of function, and they are costly for society and the health care system secondary to lost wages, health care utilization, and caregiving expenses.3 According to the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM), genetic testing plays a vital role in the accurate diagnosis of NMDs, is cost-effective because it directs management and avoids unnecessary testing, may lessen the psychological impact for the patient and family by confirming the diagnosis, may assist with family planning, and is a necessary first step in treating some conditions.4
It is now possible to obtain single gene testing, gene panel sequencing, or whole exome sequencing depending on the clinical scenario.1 The diagnostic yield of genetic testing in the diagnosis of NMDs varies depending on panel design and selection as well as patient population, but studies have shown a diagnostic yield range of 31%–49% for gene panel sequencing, with lower values for whole exome sequencing.1 In many cases, those who underwent whole exome sequencing likely already had a negative gene panel sequencing, so the pretest probability of a positive test was low. To date, most published studies on the clinical utility of genetic testing for NMDs focus on disease-specific cohorts and/or involve multiple centers.5-13
The aim of this study was to examine the clinical utility and diagnostic yield of genetic testing for suspected NMDs. Unlike previous studies, this study is unique in that it includes a broad array of patients in a single laboratory at a large neuromuscular center, providing real-world data that may assist both neuromuscular specialists as well as general neurologists in decision-making regarding the need for genetic testing in patients with suspected NMDs.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Institutional review board (IRB) approval was obtained from the Wake Forest IRB before initiation of this study. As this was a chart review study, patient informed consent was not requested from the IRB.
Data Availability
The principal author has full access to the data used in the analyses in this manuscript.
Methodological Protocol
The Wake Forest Neuromuscular Center has aligned itself with the AANEM and American Academy of Neurology and performs genetic testing for NMDs to confirm a molecular diagnosis or when the diagnosis remains uncertain.14 The medical records were reviewed for all patients who underwent genetic testing for suspected NMDs in the Wake Forest Neuromuscular Center from 2015 to 2020. The following were extracted from the medical record of each patient who underwent genetic testing: age, sex, testing indication, specific precurated gene panel ordered, specific genetic variants identified, and any variants of undetermined significance (VUS) identified. Retrospective chart reviews were performed to determine whether genetic testing results conferred a specific NMD diagnosis, including cases where a VUS was identified.
Precurated gene panels were obtained through the Invitae Corporation (San Francisco, CA). The comprehensive neuromuscular disorders panel (up to 230 genes) was ordered for suspected skeletal muscle and neuromuscular junction disorders and the comprehensive neuropathies panel (up to 111 genes) for suspected peripheral nerve and motor neuron disorders.15
Testing indications included suspected neuropathy, myopathy, NMJ disease, or MND. All genetic testing was ordered by neuromuscular physicians. The determination of pathogenicity was made by the treating physician and was based on the American College of Medical Genetics and Genomics approach.16 For each pathologic mutation identified, the mutation was deemed causative or noncausative based on the interpretation of the treating physician. For each VUS identified, the treating physician considered the overall phenotype, electrodiagnostic tests, biopsy results, suspected inheritance pattern, genetic testing of first-degree relatives (when available), published literature and databases, and discussion with a genetic counselor to determine whether the VUS was likely pathogenic. The variant was then deemed causative, noncausative, or unknown based on the interpretation of the treating physician.
Results
Overview
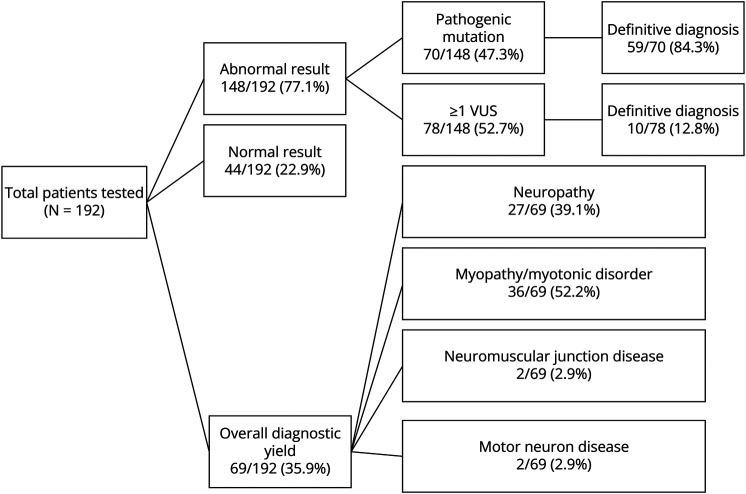
From 2015 to 2020, the Wake Forest Neuromuscular Center pursued genetic testing through Invitae for 192 patients from the approximately 28,000 patients seen. See stratified results in Figure and Table.
Figure. Genetic Testing Results.
Table.
Genetic Testing Results Regarding Testing Indication
| Testing indication | Total tests | Diagnostic yield by indication |
| Neuropathy | 106, 53.3% | 27, 25.5% |
| Myopathy or NDM | 76, 38.2% | 37, 48.7% |
| NMJ disease | 3, 1.5% | 1, 33.3% |
| MND | 15, 7.5% | 2, 13.3% |
Abbreviations: MND = motor neuron disease; NDM = nondystrophic myotonia; NMJ = neuromuscular junction.
A positive result was received in 148 (77.1%) patients, defined as a pathogenic mutation, VUS, or both. Normal genetic testing was identified in 44 (22.9%). A definitive diagnosis was made for 69, with an overall diagnostic yield of 35.9%. Of the 148 patients with a positive result, 70 (47.3%) had a pathogenic mutation with or without VUS, and 78 (52.7%) had one or more VUS. Of the 70 patients with a pathogenic mutation, 59 (84.3%) received a diagnosis. Some individuals had a single pathogenic mutation in a recessive gene which did not match their phenotype; thus, a specific diagnosis was not made on the single mutation alone. Of the 78 patients with one or more VUS, 10 (12.8%) received a diagnosis based on clinician interpretation and phenotypic correlation.
There were 27 neuropathies (39.1%), 36 myopathies or NDM (52.2%), 2 NMJ diseases (2.9%), and 2 MNDs (2.9%) determined through genetic testing. Of the 192 patients, 187 underwent a single round of testing. Five patients underwent multiple rounds of genetic testing for different indications. For the purpose of this study, multiple panels ordered for a single indication was defined as a single round of testing. In all, 199 genetic tests were performed.
Neuropathy
A suspected neuropathy was the genetic testing indication in 106 cases (53.3%). Genetic testing conferred a diagnosis in 27, with a diagnostic yield of 25.5%. Of these 27 diagnoses, 26 (96.3%) were neuropathies and 1 (3.7%) an MND.
Myopathy or Nondystrophic Myotonia
A suspected myopathy or NDM was the genetic testing indication in 76 cases (38.2%). Genetic testing conferred a diagnosis in 37, with a diagnostic yield of 48.7%. Of these 37 diagnoses, 36 (97.3%) were myopathies or NDM and 1 (2.7%) a NMJ disease.
Neuromuscular Junction Disease
A suspected NMJ disease was the genetic testing indication in 3 cases (1.5%). Genetic testing conferred a diagnosis in 1, with a diagnostic yield of 33.3%. This confirmed diagnosis was an NMJ disease.
Motor Neuron Disease
A suspected MND was the genetic testing indication in 15 cases (7.5%). Genetic testing conferred a diagnosis in 2, with a diagnostic yield of 13.3%. Of these 2 diagnoses, 1 (50%) was an MND and 1 (50%) a neuropathy.
Discussion
In this study, the overall diagnostic yield of genetic testing for NMDs at the Wake Forest Neuromuscular Center was 35.9%, which is consistent with previous studies demonstrating a yield of 31%–49% for gene panel sequencing.1
Even in the absence of disease-modifying therapy for most NMDs, a definitive genetic diagnosis is valuable for several reasons, including eligibility for future gene-targeted therapeutics, family planning, patient knowledge, and targeted disease management. For example, several patients in this study were diagnosed with an idiopathic myopathy before genetic testing and were then appropriately referred to cardiology or pulmonology on diagnosis of a specific type of muscular dystrophy.
It is noteworthy that 77.1% of patients overall had a genetic mutation identified, with more than half (52.7%) of the positive results being one or more VUS without a known pathogenic mutation. It can be challenging to decide how to classify results given the changing nature of VUS. When combined with clinical presentation and family history, a VUS can be used to make a definitive diagnosis. Even in the absence of diagnosis, identification of VUS may hold clinical value because it could contribute to a future diagnosis as research advances and new genetic conditions are discovered. The authors chose what was believed to be the most clinically relevant classifications, but other approaches, such as reclassifying some VUS results as “pathogenic,” would be reasonable too. These panels do expand over time, so there is possibility the yield may have evolved over time as well. Our study is not powered to detect a change over time. However, currently most VUS are incidental findings of minimal clinical significance.
This study highlights the clinical utility of genetic testing for suspected NMDs, specifically in a single laboratory at a large neuromuscular center. As genetic testing becomes increasingly cost-effective and accessible, even in cases of whole exome and genome sequencing, it becomes even more cost-effective to pursue genetic testing when clinical suspicion is high for an inherited NMD. Even in the case of VUS, genetic testing is much less expensive than it used to be, so in the long run, it likely is cost-effective.
This study did have some limitations. Most of the genetic testing at the Wake Forest Neuromuscular Center is performed primarily through one testing corporation. However, there are certain genetic tests that are sent to other laboratories, including most of those for SMA and repeat expansion disorders such as DM and the C9ORF72 subtype of ALS. Thus, the included data do not reflect the entirety of genetic testing at this center. Only the genetic testing performed through Invitae was included because it involved a broad range of testing through precurated gene panels, provided a web-based platform to facilitate this study, and identified patients who had undergone prior genetic testing. In addition, different providers likely had different diagnostic approaches. Genetic tests were ordered based on the diagnostic approach favored by each provider, which is not something we could quantify.
Larger, multicenter studies could provide more data on the clinical utility of genetic testing for inherited NMDs on a broader scale while also helping to account for differences in the clinical habits of individual providers and institutional practices. It would be worthwhile to include all genetic testing for NMDs, including those for repeat expansion disorders, to obtain a more complete data set. Other avenues for exploration include comparing the clinical utility of genetic testing for NMDs in a neuromuscular clinic patient population vs a community-based or general neurology practice. Finally, the clinical significance of individual VUS should continually be considered because variants are linked to more disease states.
This study provides further evidence of the clinical utility and diagnostic yield of genetic testing for NMDs in a real-world setting with over one-third of patients tested receiving a definitive diagnosis. Over time, genetic testing will continue to become increasingly accessible, cost-effective, and accurate, which will lead to even more utilization.
TAKE-HOME POINTS
→ A positive result, defined as either a pathogenic mutation, a variant of unspecified significance, or both, was found in 77.1%. A definitive diagnosis was conferred in 35.9%.
→ This study is unique in that it includes a broad array of patients at a single, large neuromuscular center, providing real-world data that may assist both neuromuscular specialists as well as general neurologists in decision-making regarding the need for genetic testing in patients with suspected neuromuscular diseases.
→ Over time, genetic testing will continue to become increasingly accessible, cost-effective, and sensitive, which will lead to even more utilization.
Appendix. Authors
| Name | Location | Contribution |
| Suzahn E. Ebert, MD | Department of Neurology, University of Virginia | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| James B. Meiling, DO | Department of Physical Medicine and Rehabilitation, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| James B. Caress, MD | Department of Neurology, Wake Forest School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Rachana K. Gandhi Mehta, MD | Department of Neurology, Wake Forest School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Vanessa Baute Penry, MD | Department of Neurology, Wake Forest School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Araya Puwanant, MD | Department of Neurology, Wake Forest School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Michael S. Cartwright, MD, MS | Department of Neurology, Wake Forest School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Volk AE, Kubisch C. The rapid evolution of molecular genetic diagnostics in neuromuscular diseases. Curr Opin Neurol. 2017;30(5):523-528. doi: 10.1097/WCO.0000000000000478 [DOI] [PubMed] [Google Scholar]
- 2.Emery AE. Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord. 1991;1(1):19-29. doi: 10.1016/0960-8966(91)90039-u [DOI] [PubMed] [Google Scholar]
- 3.Larkindale J, Yang W, Hogan PF, et al. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve. 2014;49(3):431-438. doi: 10.1002/mus.23942 [DOI] [PubMed] [Google Scholar]
- 4.Kassardjian CD, Amato AA, Boon AJ, Childers MK, Klein CJ, AANEM Professional Practice Committee. The utility of genetic testing in neuromuscular disease: a consensus statement from the AANEM on the clinical utility of genetic testing in diagnosis of neuromuscular disease. Muscle Nerve. 2016;54(6):1007-1009. doi: 10.1002/mus.25387 [DOI] [PubMed] [Google Scholar]
- 5.Kuhn M, Glaser D, Joshi PR, et al. Utility of a next-generation sequencing-based gene panel investigation in German patients with genetically unclassified limb-girdle muscular dystrophy. J Neurol. 2016;263(4):743-750. doi: 10.1007/s00415-016-8036-0 [DOI] [PubMed] [Google Scholar]
- 6.Tousignant R, Trepanier A, Shy ME, Siskind CE. Genetic testing practices for Charcot-Marie-Tooth type 1A disease. Muscle Nerve. 2014;49(4):478-482. doi: 10.1002/mus.23991 [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa A, Mitsuhashi S, Miyata N, Nishino I. Targeted massively parallel sequencing and histological assessment of skeletal muscles for the molecular diagnosis of inherited muscle disorders. J Med Genet. 2017;54(2):104-110. doi: 10.1136/jmedgenet-2016-104073 [DOI] [PubMed] [Google Scholar]
- 8.Evila A, Arumilli M, Udd B, Hackman P. Targeted next-generation sequencing assay for detection of mutations in primary myopathies. Neuromuscul Disord. 2016;26(1):7-15. doi: 10.1016/j.nmd.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Savarese M, Di Fruscio G, Torella A, et al. The genetic basis of undiagnosed muscular dystrophies and myopathies: results from 504 patients. Neurology. 2016;87(1):71-76. doi: 10.1212/WNL.0000000000002800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chae JH, Vasta V, Cho A, et al. Utility of next generation sequencing in genetic diagnosis of early onset neuromuscular disorders. J Med Genet. 2015;52(3):208-216. doi: 10.1136/jmedgenet-2014-102819 [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Brady L, Shoffner J, Tarnopolsky MA. Next-generation sequencing to diagnose muscular dystrophy, rhabdomyolysis, and hyperCKemia. Can J Neurol Sci. 2018;45(3):262-268. doi: 10.1017/cjn.2017.286 [DOI] [PubMed] [Google Scholar]
- 12.Kitamura Y, Kondo E, Urano M, Aoki R, Saito K. Target resequencing of neuromuscular disease-related genes using next-generation sequencing for patients with undiagnosed early-onset neuromuscular disorders. J Hum Genet. 2016;61(11):931-942. doi: 10.1038/jhg.2016.79 [DOI] [PubMed] [Google Scholar]
- 13.Al-Ghamdi F, Darras BT, Ghosh PS. Spectrum of neuromuscular disorders with hyperCKemia from a tertiary care pediatric neuromuscular center. J Child Neurol. 2018;33(6):389-396. doi: 10.1177/0883073818758455 [DOI] [PubMed] [Google Scholar]
- 14.Narayanaswami P, Weiss M, Selcen D, et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2014;83(16):1453-1463. doi: 10.1212/WNL.0000000000000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Invitae Corporation. Test Catalog – Neurology [Invitae]; 2023. Accessed March 21, 2023. invitae.com/en/providers/test-catalog/neurology?tab=tests [Google Scholar]
- 16.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The principal author has full access to the data used in the analyses in this manuscript.