Abstract
Background and Objectives
Occlusion of the artery of Percheron (AOP) produces bilateral thalamic infarction classically leading to deficits of arousal. This nonspecific presentation complicates the diagnosis of acute ischemic stroke. We sought to describe the spectrum of clinical presentation, diagnostic neuroimaging findings, and outcomes in AOP infarction (AOPi).
Methods
We conducted a keyword search of our health system's neuroimaging database from 2014 to 2022 to identify patients with AOPi. We abstracted patient demographics, clinical presentation, neuroimaging findings, acute treatment, and modified Rankin Scale (mRS) scores (at baseline, 3 months, and 12 months). We used descriptive statistics to report our findings.
Results
Our initial keyword search identified 192 potential AOPi cases. Fifteen cases of AOPi were confirmed and included in our study (8 female [53%], median age 65 years [interquartile range (IQR): 59.5–79.5], median presenting NIHSS 6 [IQR: 2–22]). Common clinical findings on presentation were systolic blood pressure (SBP) > 140: 12 patients (80%); decreased level of consciousness (LOC): 11 patients (73%); diplopia: 8 patients (57%); disorientation: 6 patients (42%); dysarthria: 4 patients (28%); and acute memory/cognitive disturbance: 3 patients (21%). Twelve cases (80%) presented to the emergency department (ED). Median time from symptom onset to ED arrival was 774.5 minutes (IQR: 202.25–3789.0), 4 cases (27%) arrived within 4.5 hours, and one patient (7%) received intravenous thrombolysis. The median time from ED arrival to stroke diagnosis was 519.0 minutes (IQR: 227.5–1307). Head CT was only diagnostic when obtained >570 minutes from time last known well; MRI was diagnostic at all time points. Rates of functional independence (mRS ≤2) at baseline, 3 months, and 12 months were 64%, 21%, and 18%, respectively.
Discussion
The diagnosis of stroke was considerably delayed in patients with AOPi, and only one patient received IV thrombolysis. SBP >140, impaired consciousness, and diplopia were the most common findings at presentation. CT was often nondiagnostic, but MRI demonstrated bilateral thalamic infarct in all cases. AOPi caused considerable long-term morbidity. Clinicians should maintain a high degree of suspicion for AOP stroke and consider thrombolysis in appropriately selected patients.
Introduction
First described by Gérard Percheron in 1973,1 the artery of Percheron (AOP) is a vascular variant in which a single artery arising from the posterior cerebral artery supplies blood to the bilateral paramedian thalamus.2,3 Occlusion of the AOP is rare, representing just 0.17% of ischemic strokes.4 AOP infarction (AOPi) may disrupt the reticular activating system, a neural network that mediates consciousness, resulting in disordered arousal and cognitive deficits.5,6 AOPi may also cause ischemia of the midbrain through the superior mesencephalic (rubral) arteries,2,7 primarily manifesting as ophthalmoplegia. Thus, AOPi produces an atypical, under-recognized stroke syndrome characterized by a decreased level of consciousness (LOC) that may be accompanied by gaze palsy and memory impairment.3,8,9
The differential diagnosis for an acute decrease in LOC is broad, including infection, intoxication, metabolic disturbances, delirium, and neurologic diseases such as stroke.10 Therefore, AOPi represents a diagnostic challenge which may delay presentation, diagnosis, and treatment. Better characterization of the clinical syndrome and long-term outcomes of AOPi could improve recognition and rates of treatment with intravenous thrombolysis. Prompt clinical recognition and directed neuroimaging investigation may represent modifiable steps toward improving stroke outcomes in this population.
Existing case series have defined AOPi neuroimaging patterns and long-term neuropsychiatric outcomes.2,9 Our study focuses on the early clinical presentation of AOPi including the time to arrival and diagnosis, spectrum of neurologic deficits, and acute neuroimaging findings, in addition to assessing long-term functional outcomes.
Methods
We retrospectively identified ischemic stroke patients with isolated AOPi through a broad keyword search of a university hospital radiology database, which was mined using the search terms “artery of Percheron” OR “Percheron” OR “bithalamic” OR “bilateral thalamic” OR “bilateral paramedian thalamic” OR “bilateral thalamus” AND “infarct” OR “ischemia” OR “stroke”. All 192 cases identified through these search terms were screened to confirm the presence of neuroimaging findings consistent with AOPi. Patients with AOPi who also had concomitant nonpunctate infarcts that could have affected clinical presentation or functional outcome were excluded.
Patient demographics, baseline clinical characteristics, clinical presentation, and neuroimaging findings were abstracted manually from the electronic medical record and stored in a HIPAA-compliant REDCap database (Nashville, TN). Non-normally distributed variables were presented as medians (interquartile range [IQR]). In addition to routine demographics, we assessed clinical presentation for the following variables: systolic BP >140, level of consciousness, and pupillary unresponsiveness in all 15 patients. Other clinical features such as diplopia, dysarthria, and disorientation were assessed in 14 of the 15 patients because one patient was intubated at the time of initial presentation. The presence of cognitive impairment and deficits in memory or recall could only be assessed in subjects who were sufficiently alert to be examined or when a reliable witness reported these findings.
For patients presenting to the emergency department (ED), we used the electronic medical record to collect the following times: symptom onset, ED arrival, CT scan initiation, MRI scan initiation, and intravenous (IV) thrombolysis administration. We also documented the primary diagnosis after the initial evaluation. Length of stay was determined and reported as median (IQR) rounded to the nearest whole day. We recorded the modified Rankin Scale (mRS) at baseline, 3 months, and 12 months. The mRS is a commonly used functional assessment scale with scores ranging from 0 to 6.11
Neuroimaging studies including noncontrast head CT, head and neck CT angiogram, brain CT perfusion, and brain MRI (1.5 or 3.0 T) were reviewed by a fellowship-trained vascular neurologist (C.S.) and compared with the initial imaging findings reported by fellowship-trained neuroradiologists. A neuroradiologist (J.A.C.) adjudicated any disagreements between these interpretations. We recorded whether the scans were diagnostic for AOPi. When AOPi was visualized, we noted whether midbrain involvement was present and whether it was unilateral or bilateral.
Summary statistics were prepared in RStudio (Posit PBC, Vienna).
Standard Protocol Approvals, Registration, and Patient Consents
The study was completed with approval from our local institutional review board (study ID 00014617) that provided a waiver of informed consent.
Data Availability
The principal investigator (C.S.) takes full responsibility for the data, analyses and interpretations, and the conduct of the research. Dr. Streib has full access to all data and has the right to publish any and all data separate and apart from any sponsor.
Results
Search Results
Our initial keyword search of the radiology database identified 192 potential AOPi cases. After neuroimaging review, 174 cases were excluded because they did not have neuroimaging demonstrating acute ischemic stroke involving the bilateral paramedian thalamus. Eighteen consecutive cases of AOPi were identified, of which 2 cases were excluded for concomitant large stroke (one basilar occlusion with midbrain and posterior circulation infarcts and one right middle cerebral artery occlusion with a right parietal infarct). One additional patient was excluded because of mortality secondary to other medical illness during the initial hospitalization. Therefore, 15 patients with AOPi were included in our study.
Demographics
The median age of our cohort was 65 years (IQR: 59.5–79.5), and 8 (53%) were female. Cardiovascular risk factors were common, including HTN (11, 73%), smoker/former smoker (9, 60%), type II diabetes (5, 33%), and dyslipidemia (5, 33%), as summarized in Table 1. In addition, 2 patients had a history of stroke and one patient had a history of TIA. After completion of the stroke workup, the etiologies were felt to be small vessel disease: 40%; cardioembolism: 33%; large artery atherosclerosis: 13%; and hypercoagulability of malignancy: 13% (Table 2).
Table 1.
Patient Presentation Summary
| Patient | Age (sex) | Cardiovascular risk factors | NIHSS | Clinical presentation | SBP | LWK to arrival (minutes) | Code stroke (time to code) |
| 1 | 83 (F) | HTN, PFO, TIA, smoker | 11 | Obtundation after a fall | 172 | 688 | |
| 2 | 85 (F) | HTN, DM2, CAD, Afib, CHF, Valve replacement, CVA, smoker | 6 | Waxing and waning dysarthria and disorientation | 150 | 167 | |
| 3 | 63 (M) | HTN, DM2, PFO | 2 | Dizziness, diplopia, somnolence, memory disturbance | 89 | 7360 | |
| 4 | 70 (F) | HTN, HLD, Malignancy | 7 | Obtundation, eventually started on EEG | 171 | 45 | |
| 5 | 65 (M) | None | 2 | Somnolence, disorientation, dysarthria, diplopia | 201 | 4320 | |
| 6 | 90 (F) | HTN, HLD, CAD, Afib, smoker | 18 | Acute-onset unresponsiveness after an ERCP | 172 | a | |
| 7 | 59 (M) | HTN, DM2, HLD, smoker | 2 | Disorientation, somnolence, acute memory disturbance | 109 | 1487 | |
| 8 | 76 (F) | HTN | 6 | Disorientation and diplopia, later developed right-hand numbness | 171 | 3612 | |
| 9 | 72 (F) | Smoker | 2 | Woke up with disorientation, dysarthria, diplopia | 195 | 861 | Code Stroke (31 min) |
| 10 | 64 (M) | Malignancy | 26 | Found unresponsive after total knee replacement | 166 | a | |
| 11 | 58 (M) | HTN, DM2, HLD | 30 | Acute-onset unresponsiveness, diplopia reported by family | 205 | 490 | |
| 12 | 59 (F) | DM2, smoker | 37 | Intubated with low GCS, septic, dilated pupils, s/p mannitol | 167 | a | |
| 13 | 43 (M) | HTN, CVA, CNS vasculitis due to HIV, smoker | 2 | Dysarthria and diplopia, cognitive impairment | 137 | 214 | Code Stroke (18 min) |
| 14 | 84 (M) | HTN, HLD, OSA, smoker | 33 | Acute unresponsiveness, reported diplopia, pupils sluggish R>L while intubated | 150 | 89 | Code Stroke (85 min), tPA (92 min) |
| 15 | 60 (F) | HTN, smoker | 0 | Diplopia, abnormal taste, smell, ataxia, disorientation | 158 | 8640 | |
| Median (IQR) | 65.0 (59.5–79.5) | 6.0 (2.0–22.0) | 167.0 (150.0–172.0) | 774.5 (202.25–3789.0) |
Abbreviations: SBP = systolic blood pressure; ERCP = endoscopic retrograde cholangiopancreatectomy; FTH = Found to have; GCS = Glasgow Coma Scale; LWK = last well known; S/p = status post.
Did not present to the emergency department. Patients were already admitted for treatment of an unrelated diagnosis.
Table 2.
Clinical Outcomes
| Pt. | Etiology | Outcome comments | MRI | Midbrain infarct | LOS, dc | Premorbid mRS | 3-mo mRS | 12-mo mRS |
| 1 | Small vessel | Bilateral thalami | Yes (Unilateral) | 15 | 4 | 5 | 5 | |
| 2 | Cardioembolic | Bilateral thalamic | No | 2 | 3 | 3 | 3 | |
| 3 | Small vessel | Bilateral thalamic | Yes (bilateral) | 5 | 0 | 3 | 3 | |
| 4 | Hypercoagulability of malignancy | EEG monitored, received Narcan | Bilateral thalami, L parietal lobe, R cerebellar | No | 6 | 2 | 4 | 4 |
| 5 | Small vessel | Bilateral thalamus | No | 2 | 0 | 5 | 6 | |
| 6 | Cardioembolic | Bilateral thalamic | No | 7 | 1 | 1 | ||
| 7 | Small vessel | Bilateral thalamic | No | 10 | 0 | 0 | 1 | |
| 8 | Cardioembolic | Bilateral thalamic | Yes (unilateral) | 2 | 0 | 4 | 3 | |
| 9 | Small vessel | Code Stroke (No tPA) | Bilateral thalamus | No | 2 | 0 | 2 | |
| 10 | Hypercoagulability of malignancy | Received Narcan | Bilateral thalamic | No | 20 | 2 | 5 | 2 |
| 11 | Large vessel atherosclerosis | Bilateral thalamic | Yes (bilateral) | 21 | 0 | 4 | ||
| 12 | Cardioembolic | Received mannitol | Bilat thalami, punctate embolic | Yes (bilateral) | 27 | 5 | 6 | 6 |
| 13 | Large vessel atherosclerosis | Code Stroke (No tPA) | Bilateral thalamus | No | 2 | 4 | 4 | 4 |
| 14 | Cardioembolic | Stroke Code, Received tPA | Bilateral thalamus | No | 7 | 4 | 3b | 4 |
| 15 | Small vessel | Bilateral thalamus | No | 1 | 0a | |||
| Median (IQR) | 6c (2.0–12.0) | 1.5 (0–3.75) | 4 (3.0–4.75) | 4 (3.0–4.5) |
Data not included in calculation of median because follow-up mRS was not available.
Patient had knee injury at baseline that improved at the 3-mo mark, reducing mRS at that time.
Length of stay rounded to the nearest whole days
Clinical Presentation
On the initial assessment, common clinical findings included systolic blood pressure (SBP) >140 (12/15, 80%), decreased LOC (11/15, 73%), diplopia (8/14, 57%), disorientation (6/14, 42%), dysarthria (4/14, 28%), acute memory/cognitive disturbance (3/14, 21%), and abnormal pupil response (2/15, 13%), as depicted in Figure 1. Blood pressure, LOC, and pupillary response were evaluated in all patients, whereas diplopia, disorientation, and dysarthria could not be evaluated in a single patient who was intubated during the initial evaluation. Cognitive impairment and memory deficits were present in 3 subjects but could be reliably assessed in just 4 subjects whose LOC allowed for more comprehensive neurologic examination and history taking. Twelve cases (80%) presented to the ED while 3 patients were already admitted to the hospital for treatment of an unrelated diagnosis. The median initial NIHSS was 6 (IQR: 2–22). These data are summarized in Table 1.
Figure 1. Features of Acute Clinical Presentation.
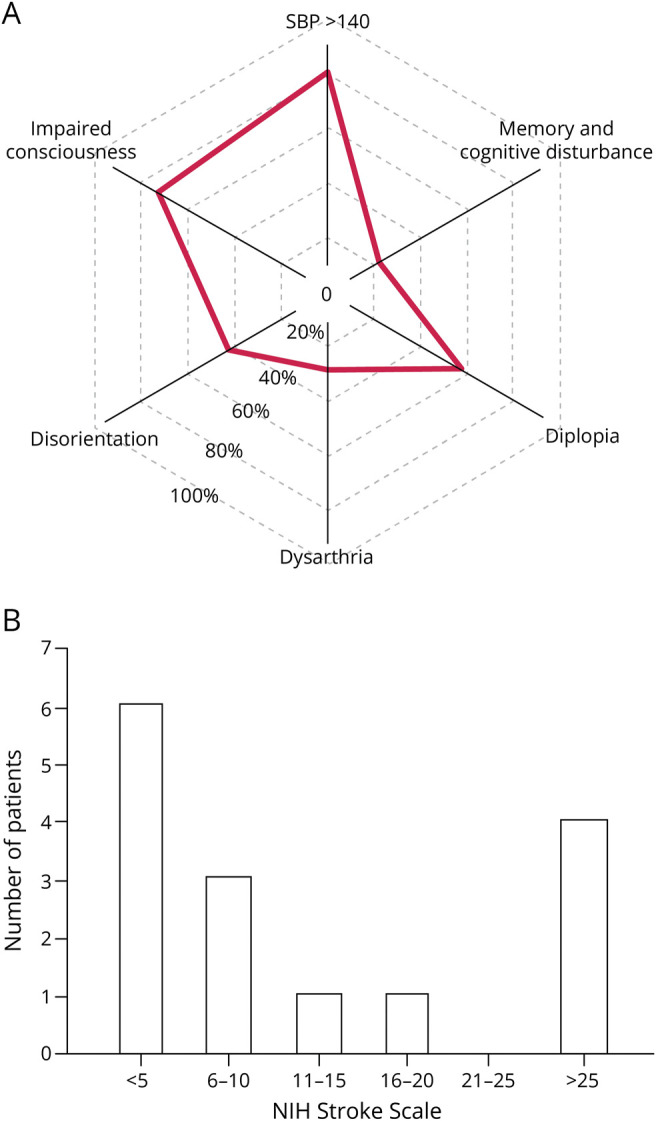
(A) Radar plot depicting the share of presenting symptoms among patients with an artery of Percheron occlusion. SBP >140: 12 patients (80%); decreased LOC: 11 patients (73%); diplopia: 8 patients (57%); disorientation: 6 patients (42%); dysarthria: 4 patients (28%); and acute memory/cognitive disturbance: 3 patients (21%). SBP, LOC, and pupils were assessed in all 15 patients; the remainder was reported in 14 patients because they could not be assessed in a single patient because of intubation. (B) Bar plot depicting the frequency of presentations stratified by NIHSS range. LOC = level of consciousness; SBP = systolic blood pressure.
Timing Metrics: Stroke Evaluation, Diagnosis, and Intervention in the ED
The following median (IQR) time metrics were observed for the 12 patients with AOPi who presented to the ED: symptom onset to ED arrival: 774.5 minutes (IQR: 202.25–3789.0); ED arrival to CT scan: 49.0 minutes (IQR: 36.0–106); and ED arrival until MRI: 519.0 minutes (IQR: 227.5–1307). Four patients (33%) presented to the ED within 4.5 hours, the conventional window for intravenous thrombolysis. Three patients (25%) had code stroke activations, which occurred 18, 31, and 85 minutes after ED arrival. A single patient received IV tenecteplase with a door-to-needle time of 92 minutes. Two code stroke patients did not receive thrombolysis; one was outside the conventional 4.5-hour time window (patient 9) and the other had minor deficits (patient 13) (Table 1). Among patients who presented to the ED, the admission diagnoses were stroke (58%), altered mental status (33%), and generalized weakness (8%). Among the 3 subjects who were already hospitalized before developing AOPi, one patient underwent EEG to evaluate for status epilepticus and another patient received mannitol for presumed intracranial pressure elevation before reaching the correct diagnosis.
Acute Neuroimaging: CT, CT Angiogram, CT Perfusion, and MRI
Acute neuroimaging was available for 14 of 15 cases (93%). In a single case, the patient's MRI report was available, but the source imaging could not be reviewed. Of the remaining patients, all 14 underwent head CT as their initial imaging study. In 6 cases (43%), the initial head CT revealed evidence of bilateral thalamic infarction however, in each of these cases the head CT was obtained more than 14 hours after the last known-well (Table 3). Concomitant CT angiography was obtained in 8 cases but was unrevealing. A single brain CT perfusion scan was performed acutely. Although this scan was originally interpreted as normal, in retrospect, a perfusion deficit is visible in the bilateral paramedian thalamus where decreased CBF and increased Tmax are prominent (Figure 2, G–I). MRI was obtained at a median of 1,489 minutes from arrival; no patients underwent hyperacute MRI in the ED. MRI demonstrated classic bilateral paramedian thalamic infarct in all cases. Bilateral midbrain infarction was seen in 3 cases (21%) and unilateral involvement in another 2 cases (14%) (Table 2).
Table 3.
Acute Neuroimaging Results
| Patient | Head CT | CTA | CT perfusion | MRI | ||||
| Timinga | Evidence of AOPi | Timinga | Evidence of AOPi or vessel occlusion | Timinga | Evidence of AOPi | Timinga | Evidence of AOPi | |
| 1 | 1,073 | Yes | 1,990 | Yes | ||||
| 2 | 189 | No | 189 | No | 1,489 | Yes | ||
| 3 | 7,407 | Yes | 7565 | Yes | ||||
| 4 | 97 | No | 97 | No | 1426 | Yes | ||
| 5 | 4454 | Yes | 4454 | No | 4580 | Yes | ||
| 6 | 420 | No | 780 | Yes | ||||
| 7 | 1536 | Yes | 1536 | No | 1722 | Yes | ||
| 8 | 4191 | Yes | 4437 | Yes | ||||
| 9 | 890 | Yes | 890 | No | 945 | Yes | ||
| 10 | 42 | No | 708 | Yes | ||||
| 11 | 568 | No | 568 | No | 568 | Yes | 1252 | Yes |
| 12 | 72 | No | 72 | No | 1554 | Yes | ||
| 13 | 248 | No | 416 | Yes | ||||
| 14 | 127 | No | 127 | No | 1447 | Yes | ||
| 15 | 8916 | Yes | ||||||
| Median (IQR) | 494.0 (142.5–1420.25) | 378.5 (119.5–1051.5) | 1489.0 (1098.5–3213.5) | |||||
Minutes from symptom onset.
Figure 2. Acute Neuroimaging Findings.

(A–C) A depiction of an artery of Percheron infarct of the bilateral thalamic and left midbrain on axial and coronal views of a noncontrast head CT in 3 different patients from our cohort. (D–E) Diffusion-weighted imaging (1.5 T) MRI of the typical appearance of the artery of Percheron territory occlusion in a single patient. (F) MRI demonstrating “V-sign” or diffusion restriction of the pial surface of the midbrain. (G–I) CT perfusion (CTP) study from a study participant with cerebral blood flow (CBF), cerebral blood volume (CBV), and Tmax images depicting a perfusion defect consistent with acute ischemia in the territory of the artery of Percheron—a concomitant perfusion deficit in the left medial temporal lobe was related to intracranial atherosclerosis and did not produce associated symptoms or result in infarction. All pathologies are marked by a red arrow.
Length of Stay and Functional Outcomes
Median (IQR) length of stay for the initial hospitalization was 6.0 (2.0–12.0) days (rounded to nearest whole day). Fourteen patients had mRS data available at baseline and 3-month follow-up while 11 patients had mRS data available at 12 months. The rate of functional independence (mRS ≤2) at these time points was 64%, 21%, and 18%, respectively (Figure 3). Median (IQR) mRS was 1.5 (0–3.75) at baseline, 4 (3.0–4.75) at 3 months, and 4 (3.0–4.5) at 12 months.
Figure 3. Ordinal Plot Demonstrating the Change in mRS Between the Premorbid State, 3-Month Follow-Up, and 12-Month Follow-Up.
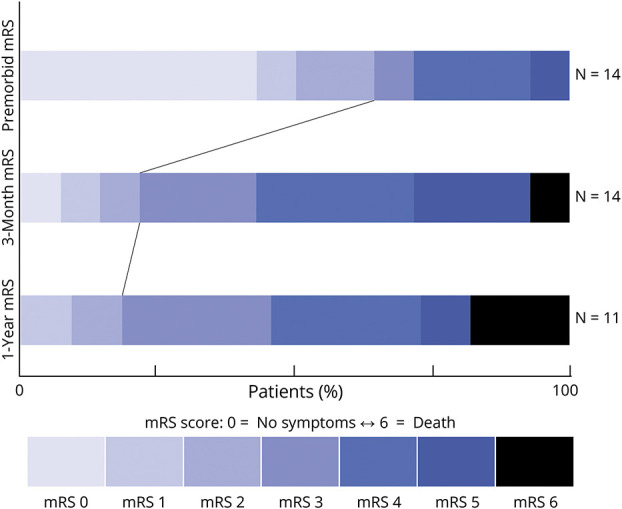
Independent functional status is demarcated by the thin line drawn between the mRS scores from different time points. mRS = modified Rankin Scale.
Discussion
The most common presenting symptoms in our AOPi cohort were SBP >140 (80%), decreased LOC (73%), diplopia (57%), and disorientation (42%). Although these findings might be considered nonspecific, acute neurologic changes accompanied by elevated blood pressure should always raise concern for an intracranial process, especially ischemic or hemorrhagic stroke.12 Similarly, a history of preceding or ongoing diplopia would be consistent with stroke, although directed history taking may be required to ascertain these symptoms in patients exhibiting decreased LOC. Intuitively, in AOPi these symptoms would also be abrupt in onset; however, we were unable to corroborate this within our cohort because detailed histories were often unavailable. The presence of known cardiovascular risk factors should further increase suspicion for AOPi in this setting. In our cohort, 14 of 15 subjects (93%) had known cardiovascular risk factors and 11 of 15 subjects (73%) had multiple risk factors; 3 of 15 subjects (20%) had a history of stroke or TIA.
Lack of “classic” stroke symptoms may have delayed subjects' presentation to the hospital.13,14 In our cohort, the median time to ED arrival was approximately 13 hours. This is more than double the time of the general stroke population, even in data collected before the NINDS trial, which considerably increased the level of urgency in stroke care.15 In addition, once at the hospital, the definitive diagnosis remained challenging. Only 3 of 15 patients with AOPi (20%) were immediately identified as having an acute ischemic stroke with consideration for emergent thrombolysis. In multiple cases (4/15, 27%), stroke consultation was delayed by alternative diagnoses or interventions including obtaining EEG, administration of Narcan, or treatment with mannitol. At the time of admission, only 58% of our cohort had been recognized as having an acute ischemic stroke.
The sensitivity of CT to detect acute ischemic stroke is limited.16 Given the nonspecific clinical presentation of many patients in our study, negative CT imaging did not facilitate the early diagnosis of AOPi or administration of intravenous thrombolysis. Hyperacute MRI is increasingly used for acute ischemic stroke diagnosis and treatment.17,18 Where available, emergent MRI could demonstrate AOPi to inform early diagnosis and management. Moreover, 6 patients in our cohort (40%) presented with wake-up stroke or stroke with unknown time of symptom onset; MRI in this setting could expand eligibility for IV thrombolysis through DWI-FLAIR mismatch.19 Although only one patient in our cohort was treated with IV thrombolysis and none underwent hyperacute MRI, there are strong existing data for DWI-FLAIR mismatch in the posterior circulation and general stroke population that can be reasonably extrapolated to patients with AOPi.20 We reported a single case in which CT perfusion identified AOPi corroborating previous publications21; however, the overall sensitivity remains unknown. Although CT perfusion can also be used for extended window thrombolysis, the core infarct and penumbral tissue volume thresholds were validated in medium and large vessel occlusion stroke22,23 and may be less applicable to AOPi.
Three patients with AOPi were initially evaluated acutely as a code stroke. Two patients were excluded from IV thrombolysis: one for minor symptoms (NIHSS = 2) and the other for being outside the conventional thrombolysis time window. One patient in our cohort received tenecteplase. This patient presented 1.5 hours after sudden-onset diplopia, which progressed to unresponsiveness requiring intubation (NIHSS 33) in the ED. The door-to-needle time was 92 minutes. Despite the patient's dramatic presentation, at the 3-month follow-up, the only residual symptom was occasional diplopia not causing further disability from baseline. An additional 2 patients were assessed in the conventional time window for thrombolysis, but a code stroke was not activated because of misdiagnosis. In these 2 cases, patient 2 was admitted with “altered mental status” and transferred to another institution for further care and patient 4 was placed on EEG to evaluate for nonconvulsive status epilepticus. The need for improved recognition and early intervention for patients with AOPi is supported by the poor long-term functional outcomes in our cohort, with just 18% of patients attaining functional independence 12 months post-stroke.
The most common underlying stroke etiology was small vessel disease (40%) followed by cardioembolism (33% [Table 2]). Fisher et al. previously wrote that AOPi was most typically caused by cardioembolism, coining the phrase “top o’ the basilar embolism.”24 The discrepancy in our study findings may be in part due to the exclusion of 2 patients with cardioembolic AOPi because of the presence of multiple infarcts. We classified the stroke etiology as ‘small vessel disease’ when associated risk factors were present, a radiographic lacunar stroke was visible (0.3 mm–2.0 cm),24 and no alternative etiology was identified. Atherosclerotic disease can also cause AOPi through atheroembolism or branch artery atheromatous disease. Therefore, a complete stroke evaluation is necessary in AOPi to delineate optimal secondary prevention strategies.
Our findings are limited by small sample size and the lack of a natural control group. Still, the presence of elevated blood pressure or diplopia will often differentiate AOPi from other causes of decreased LOC (e.g., infectious, metabolic, or toxic) encountered in the ED setting. Another limitation relates to the use of mRS to ascertain functional outcomes. The mRS is not able to capture subtle neurologic deficits that may affect quality of life. Moreover, the mRS is nonlinear, and the accrual of additional disability in those with preexisting impairment may not be adequately reflected.25,26 Therefore, although we observed that patients with AOPi had dramatically worse 90-day mRS scores when compared with other large studies of posterior circulation stroke,27,28 these comparisons are limited by the baseline morbidity of our cohort. The NIHSS was used to assess stroke severity and may also be problematic. The NIHSS is known to be higher in patients with anterior circulation stroke29 and patients with decreased LOC. Finally, venous imaging was not routinely obtained; deep cerebral venous thrombosis could radiographically mimic AOPi; however, hyperintense T2 signal (edema) is typically more prominent than restricted diffusion, and the lesions often extend beyond the paramedian thalamus.30 Follow-up imaging would also show divergent courses between these 2 entities. Areas of future research could include volumetric analysis of AOPi with attention paid to the role of midbrain involvement and correlation with clinical presentation.
The diagnosis of AOPi was commonly delayed with only one of 15 patients receiving IV thrombolysis despite considerable associated long-term disability. Elevated blood pressure (SBP >140), diplopia, and decreased LOC were the most common presenting findings. Conventional stroke imaging with CT was typically unrevealing, whereas MRI was more definitive. Clinicians should maintain a high degree of suspicion for AOPi to facilitate timely diagnosis and stroke management including consideration of thrombolysis in appropriately selected patients.
Appendix. Authors
| Name | Location | Contribution |
| Salman S. Ikramuddin, MD | Department of Neurology, University of Minnesota, Minneapolis | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| John A. Coburn, MD | Midwest Radiology, Roseville, MN | Major role in the acquisition of data |
| Solmaz Ramezani, MD | Department of Neurology, University of Minnesota, Minneapolis | Major role in the acquisition of data |
| Christopher Streib, MD, MS | Department of Neurology, University of Minnesota, Minneapolis | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ The diagnosis of AOPi, a potentially disabling stroke syndrome, is often delayed, affecting acute stroke treatment.
→ The findings of elevated blood pressure and diplopia in patients presenting with an acutely decreased level of consciousness can help identify patients with AOPi.
→ Early, accurate diagnosis of AOPi may be facilitated by emergent MRI.
References
- 1.Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol. 1973;205:1-13. doi: 10.1007/BF00315956 [DOI] [PubMed] [Google Scholar]
- 2.Lazzaro NA, Wright B, Castillo M, et al. Artery of Percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010;31(7):1283-1289. doi: 10.3174/ajnr.A2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uz A. Variations in the origin of the thalamoperforating arteries. J Clin Neurosci. 2007;14(2):134-137. doi: 10.1016/j.jocn.2006.01.047 [DOI] [PubMed] [Google Scholar]
- 4.Macedo M, Reis D, Cerullo G, et al. Stroke due to Percheron artery occlusion: description of a consecutive case series from Southern Portugal. J Neurosciences Rural Pract. 2022;13(1):151-154. doi: 10.1055/s-0041-1741485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71(6):531-546. doi: 10.1097/NEN.0b013e3182588293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988;38(6):837-848. doi: 10.1212/wnl.38.6.837 [DOI] [PubMed] [Google Scholar]
- 7.Castaigne P, Lhermitte F, Buge A, Escourolle R, Hauw JJ, Lyon-Caen O. Paramedian thalamic and midbrain infarct: clinical and neuropathological study. Ann Neurol. 1981;10(2):127-148. doi: 10.1002/ana.410100204 [DOI] [PubMed] [Google Scholar]
- 8.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264-2278. doi: 10.1161/01.STR.0000087786.38997.9E [DOI] [PubMed] [Google Scholar]
- 9.Cetin FE, Kumral E, Dere B. The clinical and cognitive spectrum of artery of Percheron infarction: 1-year follow-up. Can J Neurol Sci. 2022;49(6):774-780. doi: 10.1017/cjn.2021.212 [DOI] [PubMed] [Google Scholar]
- 10.Patti L, Gupta M. Change in Mental Status. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 11.Suchaew H, Kleindorfer D, Khoury JC, et al. Deriving place of residence, modified rankin scale, and EuroQol-5D scores from the medical record for stroke survivors. Cerebrovasc Dis. 2021;500:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25(1):32-38. doi: 10.1016/j.ajem.2006.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanni JL, Casale JA, Koek AY, Espinosa del Pozo PH, Espinosa PS. Artery of Percheron infarct: an acute diagnostic challenge with a spectrum of clinical presentations. Cureus. 2018;10(9):3276. doi: 10.7759/cureus.3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso P, Manganotti P, Moretti R. Complex neurological symptoms in bilateral thalamic stroke due to Percheron artery occlusion. Vasc Health Risk Manag. 2017;13:11-14. doi: 10.2147/VHRM.S119395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barsan WG, Brott TG, Broderick JP, Haley EC, Levy DE, Marler JR. Time of hospital presentation in patients with acute stroke. Arch Intern Med. 1993;153(22):2558-2561. doi: 10.1001/archinte.153.22.2558 [DOI] [PubMed] [Google Scholar]
- 16.Smajlović D, Sinanović O. Sensitivity of the neuroimaging techniques in ischemic stroke. Med Arh. 2004;58(5):282-284. [PubMed] [Google Scholar]
- 17.Warach S, Chien D, Li W, Ronthal M, Edelman R. Fast magnetic resonance diffusion‐weighted imaging of acute human stroke. Neurology. 1992;42(9):1717-1723. doi: 10.1212/wnl.42.9.1717 [DOI] [PubMed] [Google Scholar]
- 18.Lutsep HL, Albers GW, Decrespigny A, Kamat GN, Marks MP, Moseley ME. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol. 1997;41(5):574-580. doi: 10.1002/ana.410410505 [DOI] [PubMed] [Google Scholar]
- 19.Thomella G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;389:611-622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 20.Forster A, Gass A, Kern R, Wolf ME, Hennerici MG, Szabo K. MR Imaging-guided intravenous thrombolysis in posterior cerebral artery stroke. AJNR Am J Neuroradiol. 2011;32(2):419-421. doi: 10.3174/ajnr.A2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebrahimi Y. Diagnosis of artery of Percheron stroke on CT perfusion. Acad J Stroke. 2020;2(1):31-33. [Google Scholar]
- 22.Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-1803. doi: 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 23.Leira EC, Muir KW. EXTEND trial: towards a more inclusive but complex thrombolysis. Stroke. 2019;50(9):2637-2639. doi: 10.1161/STROKEAHA.119.026249 [DOI] [PubMed] [Google Scholar]
- 24.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32(8):871-876. doi: 10.1212/wnl.32.8.871 [DOI] [PubMed] [Google Scholar]
- 25.Dijkland SA, Voormolen DC, Venema E, et al. Utility-weighted modified rankin scale as primary outcome in stroke trials: a simulation study. Stroke. 2018;49(4):965-971. doi: 10.1161/STROKEAHA.117.020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salwi S, Cutting S, Salgado AD, et al. Mechanical thrombectomy in patients with ischemic stroke with prestroke disability. Stroke. 2020;51(5):1539-1545. doi: 10.1161/STROKEAHA.119.028246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommer P, Posekany A, Serles W, et al. Is functional outcome different in posterior and anterior circulation stroke? Stroke. 2018;49(11):2728-2732. doi: 10.1161/STROKEAHA.118.021785 [DOI] [PubMed] [Google Scholar]
- 28.Lin SF, Chen CI, Hu HH, Bai CH. Predicting functional outcomes of posterior circulation acute ischemic stroke in first 36 h of stroke onset. J Neurol. 2018;265(4):926-932. doi: 10.1007/s00415-018-8746-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato S, Toyoda K, Uehara T, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70(24 Pt 2):2371-2377. doi: 10.1212/01.wnl.0000304346.14354.0b [DOI] [PubMed] [Google Scholar]
- 30.Gogineni S, Gupta D, Pradeep R, et al. Deep cerebral venous thrombosis-a clinicoradiological study. J Neurosci Rural Pract. 2021;12(3):560-565. doi: 10.1055/s-0041-1730109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The principal investigator (C.S.) takes full responsibility for the data, analyses and interpretations, and the conduct of the research. Dr. Streib has full access to all data and has the right to publish any and all data separate and apart from any sponsor.


