Abstract
Background and Objectives
Spontaneous intracranial hypotension (SIH) caused by a spinal CSF leak is a multisymptom syndrome, which can dramatically affect physical and mental health. However, systematic data on health-related quality of life (HRQoL) and mental health are scarce. We hypothesized that surgical treatment leads to significant and sustained improvements in HRQoL and mental health in patients with SIH.
Methods
In this single-center cohort study, we prospectively collected HRQoL and mental health data in patients undergoing surgical closure of a spinal CSF leak from September 2020 to November 2022. EuroQoL (EQ-5D-5L), including the health state index (EQ-Index) and the visual analog scale (EQ-VAS), measured HRQoL. The 21-item version of the Depression Anxiety Stress Scales (DASS-21) measured symptoms of mental health. Follow-ups were performed 3 and 6 months postoperatively. Primary outcome was the change in EQ-Index, EQ-VAS, and DASS-21 subscales. Secondary outcome was the impact of baseline depression symptoms on HRQoL outcomes following surgery.
Results
Seventy-four patients were included. EQ-VAS improved from 40 (interquartile range [IQR] 30–60) preoperatively to 70 (IQR 55–85) at 3 months and to 72 (IQR 60–88) at 6 months postoperatively (p < 0.001, respectively). EQ-Index increased from 0.683 (IQR 0.374–0.799) to 0.877 (0.740–0.943) at 3 months and to 0.907 (0.780–0.956) at 6 months postoperatively (p < 0.001, respectively). Depression, anxiety, and stress significantly improved after surgery. Preoperative depressive symptoms did not affect the HRQoL outcome.
Discussion
The severe impact of a spinal CSF leak on HRQoL and mental health significantly improved after closure of the leak. Higher levels of depressive symptoms do not predict worse outcomes and should not discourage invasive treatment. Further systematic evaluation of outcomes, with special regard to quality of life, is needed, as it allows a comparison of symptom burden between SIH and more familiar diseases as well as a comparison of different treatment modalities in future studies.
Introduction
Spontaneous intracranial hypotension (SIH) caused by a spinal CSF leak has an estimated incidence of approximately 4/100.000 per year with women more often affected.1,2 Besides the predominant clinical feature of orthostatic headache, a broad range of symptoms, including nausea, tinnitus, dizziness, visual symptoms, fatigue, and neck pain, are recognized as important manifestations of the syndrome.3,4 These symptoms profoundly affect patients' health-related quality of life (HRQoL). Many patients endure prolonged disease histories due to misdiagnosis and/or inadequate treatment leading to mental health issues and emotional challenges. However, mental health symptoms have not generally been investigated in patients with SIH. One recent patient cohort report with confirmed and suspected CSF leak suggested significant symptoms of depression and suicidality among patients with SIH.5 A better understanding of the mental health symptoms in SIH is necessary. By contrast, the diversity of physical and mental symptoms highlights that in SIH, metrics of suffering, impaired health, and the response to treatment must extend beyond headaches.
In cases of a proven spinal leak and failure of conservative management, often including epidural blood patching, microsurgical sealing of the leak is a safe and effective treatment. However, the existing literature primarily focuses on the successful closure of the leak6,7 or the improvement of headaches.8,9
Health-related quality of life (HRQoL) metrics have been previously used in a wide range of illnesses to capture the integrated impact of psychological and physical symptoms but have not been extensively reported in SIH. Importantly, HRQoL can also be compared between diverse diseases, e.g., SIH and multiple sclerosis or cancer, allowing policymakers to compare the needs of different patient populations and make informed decisions in allocating limited resources.
Furthermore, as treatments for SIH continue to evolve and improve, validated HRQoL outcomes following different treatments should shape treatment recommendations regarding choice of first-line therapy. This is particularly true in SIH where direct comparative trials comparing surgery with epidural patching or CSF venous fistula embolization are currently lacking and may be challenging to complete due to logistic barriers.
While epidural patching continues to be advocated as first-line treatment for SIH, surgery is increasingly a first-line alternative. Improvements in neuroimaging of SIH, including dynamic CT myelography, Digital Subtraction Myelography, and cone beam–assisted myelography, have resulted in rapid advances in the ability to identify the exact spinal location of CSF leak for many patients.10,11 These techniques have not only enhanced surgical efficacy by accurately locating the site of CSF leak but have simultaneously reduced morbidity by enabling minimally invasive surgical techniques.6 The outcomes of this approach have not been characterized with patient-oriented validated metrics, of which improvements in mental health and HRQoL are particularly important.
Owing to the presence of multiple symptoms and the potentially prolonged history of suffering before accurate diagnosis and treatment, emotional distress is often observed in SIH patients. Accentuated by individual and especially challenging cases, the clinical setting often leads to the impression of an increased prevalence of emotional challenges within the SIH population. In cases of high emotional distress, a subtle and ultimately unfounded premonition might exist that these patients might benefit less from surgery. However, there is no systematic evaluation of the effect of emotional distress on surgical outcome in SIH patients. In contrast to degenerative spinal surgery, where depressive symptoms are often considered a negative factor for the outcome,12 no data exist about the influence of emotional factors on outcome after surgery in SIH patients.
We hypothesized that surgical repair of CSF leaks leads to a significant and sustained improvement in HRQoL. By reporting outcomes of validated metrics of HRQoL, we establish a benchmark against which alternative treatments can be assessed in future research. Finally, we evaluated the response of mental health symptoms (depression, anxiety, and stress) and their potentially adverse influence on the outcome after surgical treatment of the underlying CSF leak.
Methods
This cohort study from a tertiary referral center was conducted using prospectively collected PROMs in SIH patients who underwent surgical closure of a spinal CSF leak between September 2020 and November 2022. The study population consisted of patients who met the ICDH-3 criteria for SIH13 and were linguistically and intellectually capable of completing the questionnaires were included. After the diagnostic work-up with confirmation of the type and location of the spinal CSF leak through dynamic myelography and/or dynamic CT myelography, patients completed the questionnaires preoperatively and postoperatively at 3 and 6 months, respectively. In case of a revision surgery, the 3-month and 6-month follow-up date was set according to the second surgery date. A digital PROM software (Heartbeat Medical, Berlin, Germany) with automated follow-up was used. Patients were contacted once in case of an incomplete follow-up; if they were still lost to follow-up, their data were censored at 3 months. Patient characteristics (age, sex, duration of symptoms), Bern SIH-Score,14 type of spinal CSF leak,15,16 and data concerning the perioperative course were analyzed retrospectively after prospective collection.
Standard Protocol Approvals, Registrations, and Patient Consents
The local Ethics Committee approved the study (22-1512-S1-retro). All patients provided informed consent to evaluate and publish their PROM data. The study followed the STROBE statement and guidelines.17
Questionnaires
EQ-5D-5L
The EuroQol five-dimension five-level questionnaire (EQ-5D-5L) score assesses the quality of life regardless of the underlying disease.18,19 It captures 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Respondents provide their level of impairment on a 5-point scale, which is then converted into a summary index score between 1 and <0 (EQ-5D-5L-Index, EQ-Index) representing the patient's overall health status. An EQ-Index value of 1 signifies a full and unimpaired health.20,21 The EQ-5D-5L includes a visual analog scale (EQ-VAS) that queries the current condition between the best imaginable health (100 points) and the worst imaginable health (0 points).
DASS-21
The 21-item version of the Depression Anxiety Stress Scales (DASS-21) assesses the 3 negative emotional states depression, anxiety, and stress.22,23 Every subscale is summed up to total scores ranging from 0–42, with higher scores indicating more symptoms. Specific cut-off values are used to classify the severity levels (normal—mild—moderate—severe—extremely severe) relative to the general population. In the depression subscale, scores <14 are categorized as “normal” and “mild.” However, it is essential to note that although “mild” indicates a level above the population mean, it does not necessarily imply a mild degree of depression, and it is still below a clinically manifest depression needing treatment. Therefore, scores <14 (“normal” and “mild”) are considered to be clinically acceptable conditions without intrinsic pathologic value and need for specific therapy.24,25
Statistics
Data analysis was performed using IBM SPSS Statistics (version 29) and Jamovi (version 2.3.21). As Shapiro-Wilk testing ruled out normal distribution for EQ-5D-5L and DASS-21 results on most follow-up dates, the median with interquartile range (IQR) was reported.Changes in the PROMs at the follow-up were analyzed using the Friedman test for nonparametric repeated measures with pairwise Conover post hoc comparisons. For subgroup analysis, DASS-21 depression subscores were dichotomized in favorable conditions (scores <14, “normal” and “mild”) and unfavorable conditions (scores ≥14, “moderate”, “severe”, and “extremely severe”). Correlations were rated through multiple linear regression on validation of standard premises. p values <0.05 were considered statistically significant.
Data Availability
Additional anonymized data not published in this article will be shared by request from any qualified investigator.
Results
Patient Demographics and Treatment
Between September 2020 and November 2022, 74 patients (46 female, 28 male) who underwent surgical treatment for a spinal CSF leak completed the scores preoperatively (Table 1). The main part of patients (63/74 = 85%) lived in Germany, and the remaining patients were distributed among various European countries (United Kingdom 2, Austria 2, Norway 2, Sweden 1, France 1, Italy 1, Denmark 1, Belgium 1). Subsequently, 82% (61/74) completed the follow-up assessment at 3 months postoperatively and 80% (59/74) at 6 months postoperatively.
Table 1.
Patient Characteristics
| n = 74 | % | |
| Female/male | 46/28 | 62/38 |
| Age in years | ||
| Median (IQR) | 45 (36–53) | |
| Range | 24–77 | |
| Duration of symptoms in months | ||
| Median (IQR) | 5 (2–14) | |
| Range | 0.5–157 | |
| Specific treatment before index surgery | ||
| None | 32 | 43 |
| Epidural blood patch | 39 | 53 |
| Unsuccessful surgery (externally) | 2 | 3 |
| Embolization of CSF-venous fistula | 1 | 1 |
| Bern SIH-Score | ||
| Median (IQR) | 5 (2–8) | |
| Type of spinal CSF leak | ||
| Ventral leak | 55 | 74 |
| Lateral leak | 16 | 22 |
| CSF-Venous fistula | 3 | 4 |
The median age was 45 years (IQR 36–53), and the median duration of symptoms before surgery was 5 months (IQR 2–14). The median Bern SIH-score was 5 (IQR 2–8).
Before the index surgery, 39/74 patients (53%) received at least one epidural blood patch, 2/74 had an unsuccessful surgical intervention at an external institution, 1/74 had an unsuccessful embolization of a CSF-venous fistula, and 32/74 (43%) had no specific treatment apart from conservative measures. Among the cohort, 55/74 patients (74%) had a ventral leak, 16/74 (22%) had a lateral leak, and 3/74 (4%) had a CSF-venous fistula.
During the treatment in our institution, no patient received a psychiatric or psychotherapeutic intervention or a new psychopharmacologic treatment. No changes to preexisting psychiatric medications were made.
All surgeries were performed in prone position using a posterior minimally invasive nonexpandable tubular approach. Ventral leaks were sealed using the intradural/extradural sandwich patching technique,6 lateral leaks were sealed by fibrin sealant patching with or without direct suturing, and CSF-venous fistulas were closed by direct thermocoagulation with or without clipping of the affected nerve root. Six patients (6/74 = 8%, 4 ventral and 2 lateral leaks) needed a second intervention, 2 because of an epidural hematoseroma, 3 because of a persisting or reopened leak in the same level, and 1 because of targeting the wrong level. No significant permanent neurologic deficits were observed in any of the cases.
EQ-5D-5L (EQ-Index and EQ-VAS)
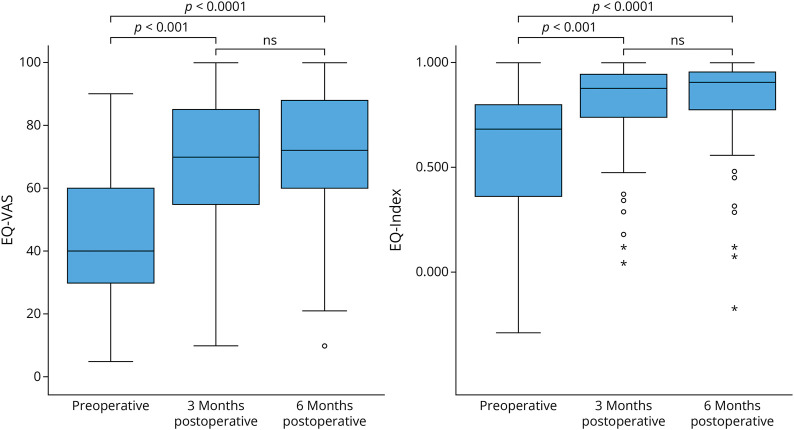
Before surgery, the quality of life scores was very low but showed significant improvement at 3 months following surgical closure of the leak (Table 2, Figure 1). The median EQ-Index increased from 0.683 (IQR 0.374–0.799) preoperatively to 0.877 (0.740–0.943) at 3 months postoperatively (p < 0.001) and further to 0.907 (0.780–0.956) at 6 months postoperatively (p < 0.001). Similarly, the median EQ-VAS improved from 40 (IQR 30–60) preoperatively to 70 (IQR 55–85) at 3 months (p < 0.001) and further improved to 72 (IQR 60–88) at 6 months postoperatively (p < 0.001).
Table 2.
Results of the Patient-Reported Outcome Questionnaires
| Preoperative | Postoperative | ||
| 3 mo | 6 mo | ||
| n | 74 | 61 | 59 |
| EQ-5D-5L | |||
| EQ-VAS | 40 (30–60) | 70 (55–85) | 72 (60–88) |
| EQ-Index | 0.683 (0.374–0.799) | 0.877 (0.740–0.943) | 0.907 (0.780–0.956) |
| DASS-21 | |||
| Depression | 10 (4–18) | 4 (0–12) | 6 (1–12) |
| Anxiety | 6 (2–12) | 2 (0–8) | 4 (0–8) |
| Stress | 14 (7–20) | 6 (2–12) | 8 (3–14) |
Results are reported as median and IQR.
Figure 1. Boxplots Representing the Health-Related Quality of Life Change Measured With the EQ-5L-5D Preoperatively, at 3 and 6 Months Postoperatively.
The EQ visual analog scale (0–100, EQ-VAS) and the EQ-Index score (<0–1, EQ-Index) both show a significant improvement after surgical closure of the leak. EQ-5D-5L = EuroQol five-dimension five-level questionnaire; EQ-Index = summary index score for health status of the EQ-5D-5L; EQ-VAS = visual analog scale for health status of the EQ-5D-5L.
DASS-21
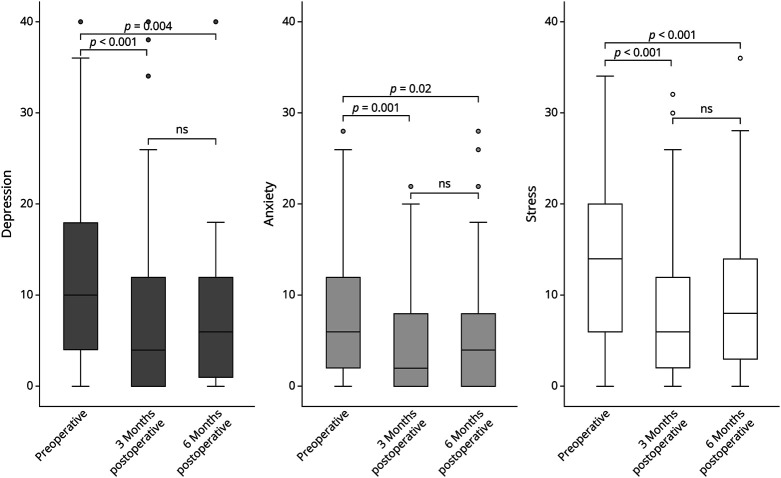
There was a significant decrease in all DASS-21 subscales from the preoperative assessment to the 3-month and 6-month follow-up, indicating a reduction in the burden of depression, anxiety, and stress (Table 2, Figure 2).
Figure 2. Boxplots Representing the Change of the DASS-21 Subscales From Preoperatively to 3 and 6 Months Postoperatively.
All subscales (depression, anxiety, and stress) show a significant reduction in emotional distress postoperatively; the slight increase between 3 and 6 months is not statistically significant (n.s.).
Specifically, the scores for depression improved from 10 (IQR 4–18) preoperatively to 4 (IQR 0–12) at 3 months (p < 0.001) and to 6 (IQR 1–12) at 6 months postoperatively (p = 0.004). Anxiety scores improved from 6 (IQR 2–12) preoperatively to 2 (IQR 0–8) at 3 months (p = 0.001) and to 4 (IQR 0–8) at 6 months postoperatively (p < 0.02). Similarly, stress scores improved from 14 (IQR 7–20) preoperatively to 6 (IQR 2–12) at 3 months (p < 0.001) and to 8 (IQR 3–14) at 6 months (p < 0.001) postoperatively.
Following the 3-month assessment, no significant further changes were observed, and the slight increase of all subscores between 3 and 6 months was not statistically significant (p = 0.531 for depression, p = 0.292 for anxiety, and p = 0.513 for stress, respectively).
Depressive Symptom Load
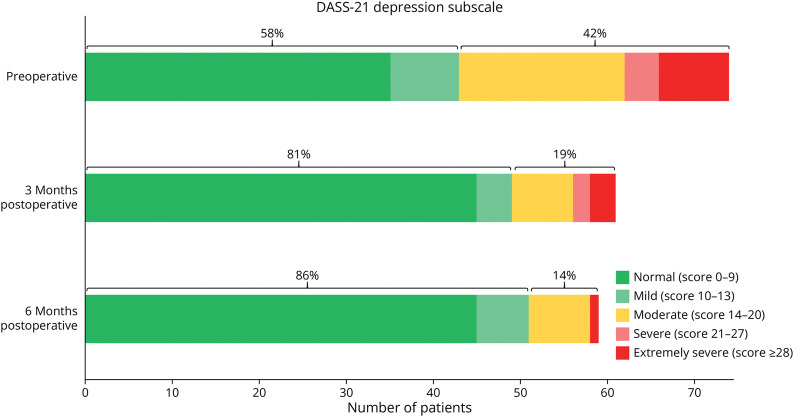
Preoperatively, 42% of patients (31/74) had a depression subscore of ≥14, indicating clinically relevant depressive symptoms, while 16% of patients (12/74) had a score of ≥ 21, indicating severe and extremely severe depressive symptoms (Figure 3). After surgery, the proportion of patients with unfavorable depression subscores decreased to 19% at 3 months and further to 14% at 6 months (p < 0.001, respectively). Severe and extremely severe depression subscores decreased to 8% at 3 months and 2% at 6 months (p < 0.001, respectively) (Figure 3).
Figure 3. Bar Graphs of the Depression Subscale of the DASS-21 Preoperatively and at 3 and 6 Months Postoperatively.
Scores <14 (“normal” and “mild”, green) are considered clinically acceptable conditions without intrinsic pathologic value. Scores ≥14 (“moderate”, orange, “severe”, and “extremely severe”, red) represent manifest depressive symptoms. Preoperatively, 42% of patients had a depression score of ≥14, and 16% had a score ≥21, indicating severe and extremely severe depressive symptoms. However, 6 months after surgery, only 14% had a score ≥14 and 2% had a score ≥21.
Preoperatively, higher depression subscores were significantly correlated with reduced quality of life, as measured by EQ-VAS and EQ-Index (Spearman's Rho −0.399 (p < 0.001) for EQ-VAS and −0.356 (p = 0.002) for EQ-Index, respectively).
There was no correlation between an unfavorable depression subscore (≥14) preoperatively and the EQ-VAS and EQ-Index following surgery; thus, a higher mental health burden did not hinder the improvement. Preoperatively, there is a significant difference between the favorable and unfavorable depression subgroups (p = 0.013 for EQ-VAS and p = 0.017 for EQ-Index, respectively). After 6 months, both subgroups reached a similar level in quality of life scores without significant difference (p = 0.128 for EQ-VAS and p = 0.076 for EQ-Index, respectively) (Figure 4). The extent of EQ-VAS and EQ-Index improvement was even higher in the subgroup with unfavorable depression subscores than favorable scores, although this difference did not reach statistical significance.
Figure 4. Line Graphs Comparing the Median Health-Related Quality of Life (EQ-VAS and EQ-Index) in Patients With a Favorable Depression Subscore (<14, Green Line) and an Unfavorable Depression Subscore (≥14, Red Line) at the 3-Month and 6-Month Follow-Up.
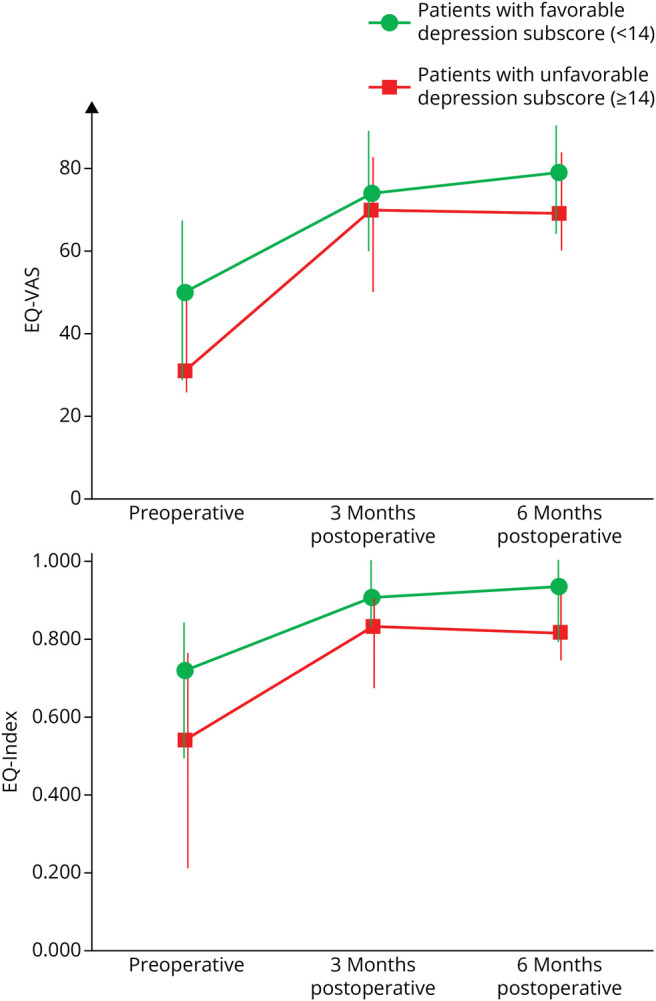
The whiskers represent the IQRs. Preoperatively, there is a significant difference between the subgroups (p = 0.013 for EQ-VAS and p = 0.017 for EQ-Index). After 6 months, both subgroups reach a similar level in quality of life scores without significant difference (p = 0.128 for EQ-VAS and p = 0.076 for EQ-Index). EQ-VAS and EQ-Index improvement is higher in the unfavorable subgroup but without reaching statistical significance. Thus, the effect of the treatment can be achieved irrespective of the extent of depressive symptoms before surgery. EQ-Index = summary index score for health status of the EQ-5D-5L; EQ-VAS = visual analog scale for health status of the EQ-5D-5L
Prior interventions (epidural blood patching, unsuccessful surgery, or unsuccessful embolization of a CSF-venous fistula) or the need for a second surgery had no significant impact on the postoperative outcome of the EQ-VAS, EQ-Index, or the DASS-21 subscales.
Discussion
This study represents the first systematic investigation and reports a significant improvement in health-related quality of life (HRQoL) and mental health among a large cohort of patients suffering from SIH after surgical closure of a spinal CSF leak. The 6-month follow-up period allowed for a reliable assessment, encompassing the emotional factors, as it goes far beyond the emotionally challenging perioperative period, which could otherwise confound the results.
Our results demonstrate a substantial and clinically relevant improvement in health-related quality of life measured by the EQ-5D-5L. The EQ-Index increased from 0.683 preoperatively to 0.877 at 3 months and further to 0.907 at 6 months postoperatively (Figure 1). Similarly, the mean EQ-VAS increased from 40 preoperatively to 70 at 3 months and further to 72 at 6 months postoperatively.
Previous studies in SIH patients after surgical treatment focus on the impact of headaches using the 6-item Headache Impact Test (HIT-6)8,26 that has an acceptable reliability and validity to evaluate the treatment response in patients with chronic or episodic headaches.27 However, with the myriad of possible symptoms, often exceeding the classic orthostatic headaches,3,4,28 SIH might very well be regarded as a polysymptomatic disease and not just as a headache disorder. Using HRQoL as an outcome parameter allows a comparison between SIH and a broader range of other diseases beyond different headache syndromes. This may prove striking for an unfamiliar policy maker or practitioner that conceives of SIH as primarily “a headache”—a symptom label that does not convey the profound impact of the symptoms in the syndrome.
Using the extensively used, standardized, and validated EuroQoL instrument allows the comparison of the impact of symptoms between SIH and other more familiar diseases. The preoperative median EQ-Index of 0.683 suggests the impact of SIH is comparable with multiple sclerosis (EQ-Index estimates ranging from 0.57 to 0.7829,30) or lung cancer (EQ-Index estimates ranging from 0.57 to 0.7431,32).
In the general German population aged between 30 and 50 years, the mean value of the EQ-VAS ranges from 87 to 91.33 We found a median EQ-VAS of 40 before treatment. This suggests a far greater impact of SIH on HRQoL than many neoplastic diseases. Patients with breast cancer before adjuvant chemotherapy report a EQ-VAS of 85,34 patients with advanced renal cell carcinoma before chemotherapy an EQ-VAS of 75,35 and patients with head and neck cancer before radiotherapy a EQ-VAS of 62.36
To our knowledge, only 2 previous reports, both published in 2022, have described quality of life in SIH. One report described HRQoL using the 15D instrument among a cohort of patients contacted 2–7 years after various treatments for SIH (surgical and/or epidural blood patching).37 However, owing to the study's design, the surgery's effect on HRQoL or the impact of untreated SIH on HRQoL could not be definitively assessed.
Another report described outcomes from 42 of 132 patients contacted in an SIH patient group who had remaining symptoms after various treatments and an average of 2 years following diagnosis.38 In that group, the mean EQ-VAS was 36.4. In our study, however, we measured HRQoL prospectively in a consecutive cohort of verified patients with SIH before surgery. Our finding of a median EQ-VAS of 40 preoperatively broadly agrees with and validates their cross-sectional data. It extends their work by addressing concerns that their data encoded responder bias—possibly capturing data from a subset of the most affected or least treatment responsive, patients. Stated simply, our data suggest that in the presence of SIH and the absence of treatment, HRQoL is devastated. This has important implications for practitioners having to decide if a given patient with suspected SIH and ambiguous imaging should be offered further invasive imaging or a trial of epidural patching. Future work should explore if such patients have similarly diminished HRQoL to identify the possible harm associated with a failure to reach a diagnosis and offer treatment in the setting of SIH.
By contrast, we show the tremendous potential benefit when a diagnosis can be firmly established, and surgical treatment is offered at an experienced center. This is the first study to report prospectively acquired HRQoL and mental health outcomes in a large cohort of patients suffering from SIH before and after a specific single therapy—surgical closure of a spinal CSF leak. Our results demonstrate a significant and clinically relevant improvement of health-related quality of life measured. The mean EQ-VAS increased from 40 preoperatively to 70 at 3 months and further to 72 at 6 months postoperatively. By comparison, this is 3 times larger than the 7–12-point change in the EQ-VAS that is considered clinically relevant and perceived as beneficial by patients with cancer.39 Similarly, the EQ-Index increased from 0.683 preoperatively to 0.877 at 3 months and further to 0.907 at 6 months postoperatively (Figure 1). This improvement of >0.200 clearly exceeds the 0.08 that is considered clinically relevant in different forms of cancer39 or in multiple sclerosis.30 However, further studies are necessary to investigate whether the favorable improvement in HRQoL is mainly due to a reduction of the impact of headaches or rather due to a combination of various factors.
In an environment in which head-to-head studies of surgery, epidural patching, and CSF-venous embolization to treat SIH have not yet been accomplished, the HRQoL outcome data from a consecutive sample and the defined time points post-treatment reported here may form the best benchmark for future observational studies comparing these modalities competing for first-line status. As of this report, it is unclear if epidural patching, a treatment more frequently offered as first-line, can produce similar sustained improvements when evaluated prospectively with validated HRQoL metrics. If replicated with further research and in other centers, the current data may support a more widespread use of surgery as a safe and effective treatment option for spinal CSF leak.
While tinnitus, neck pain, and nausea are commonly referenced as nonspecific symptoms in the syndrome of SIH, mental health symptoms have not been listed or cited as prevalent parts of the syndrome.3,4
As a self-reported questionnaire, the DASS-21 is a practical and validated screening tool that is highly useful for monitoring symptom changes over time. Obviously, the depression subscale of the DASS-21 cannot replace a thorough psychological assessment and cannot independently diagnose depression. However, certain conclusions regarding the presence of depressive symptoms, as well as changes after an intervention, can be drawn.
We show that symptoms of depression, anxiety, and stress are highly prevalent in patients with SIH and dramatically improve following treatment. Depending on the publication, the prevalence of depressive symptoms in Germany is between 5% and 9%.40,41 In our study population, preoperatively, 42% had a depression score of ≥14, indicating clinically manifest depressive symptoms, and 16% had a score of ≥21, indicating severe and extremely severe depressive symptoms. However, 6 months after surgery, only 14% had a score ≥14 and 2% had a score ≥21 (Figure 3). This distribution at 6 months is almost equal to the prevalence in the average population, highlighting the positive effect of the surgery.
Although there was a slight nonsignificant deterioration in all DASS-21 subscales between the 3-month and 6-month follow-up, it is essential to note that there was still an overall improvement compared with the preoperative levels (Figure 2). The long-term trend of these changes and whether they represent a short-term fluctuation or a continuing pattern cannot be predicted at this stage. Therefore, we consider a regular and long-term follow-up of patients as essential and recommend a systematic and long-term follow-up in all dedicated centers.
The near normalization of depressive symptoms after surgery without special psychiatric or psychotherapeutic interventions might be interpreted as an indication that preoperative symptoms are reactive, and due to SIH, rather than primary and an independent disease. Future work should explore this further. If replicated, we suggest that our data warrant adding these nonspecific mental health symptoms to the legion of other neurologic symptoms that may, mistakenly, be thought of as primary and independent rather than be recognized more accurately as arising directly or indirectly from a CSF leak.
Finally, in degenerative spine surgery, the influence of preoperative anxiety and depression on overall postoperative improvement and patient satisfaction was intensively investigated. Most studies suggest that preoperative anxiety or depression is associated with worse surgical outcomes.42-44 In contrast to these studies in degenerative spine disease, our results show no correlation between the extent of preoperative depressive symptoms and the postoperative improvement in HRQoL. On the contrary, patients with higher depression subscores showed a higher relative improvement in their HRQoL (Figure 4) and a near normalization of their emotional distress. Our results do not support the assumption that depressive symptoms might be a negative predictor of the possible improvement in HRQoL after operative treatment. In cases of SIH, in which patients describe or demonstrate pronounced depressive symptoms, our data suggest that neither provider nor patient should be dissuaded from aggressively addressing a fixable cause of a CSF leak. HRQoL outcomes remain positive, and mental health outcomes are dramatically improved following surgery among patients with elevated levels of depressive symptoms preoperatively. These somewhat unexpected findings warrant replication before wide adoption.
This is a single-center analysis from a specialized tertiary center with a highly selected patient cohort, so a generalization of the results is not readily possible. A high experience in the diagnostic workup of spinal CSF leaks and in minimally invasive surgery is necessary to achieve similar results. Although PROM data were collected systematically, a small loss of follow-up is present. However, this study is the largest series of surgically closed CSF leaks focusing on HRQoL and emotional distress.
Spontaneous intracranial hypotension (SIH) has a devastating effect on health-related quality of life, as measured by the EQ-5D-5L, and is associated to elevated levels of depression, anxiety, and stress, as measured by the DASS-21. Surgical closure of the spinal CSF leaks results in a substantial improvement in health-related quality of life and a significant reduction in emotional distress after a 6-month follow-up period. Higher depression subscores preoperatively are not a negative predictor of the potential improvement in health-related quality of life.
Appendix. Authors
| Name | Location | Contribution |
| Florian Volz, MD | Department of Neurosurgery, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Katharina Wolf, MD | Department of Neurosurgery, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Christian Fung, MD | Department of Neurosurgery, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Ian Carroll, MD | Stanford CSF Leak Headache Program, Stanford Headache Clinic, Stanford School of Medicine, CA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Claas Lahmann, MD | Department of Psychosomatic Medicine and Psychotherapy, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Niklas Lützen, MD | Department of Neuroradiology, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Horst Urbach, MD | Department of Neuroradiology, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Jan-Helge Klingler, MD | Department of Neurosurgery, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Jürgen Beck, MD | Department of Neurosurgery, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Amir El Rahal, MD | Department of Neurosurgery, Medical Center - University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
No author received financial support for the research, authorship, or publication of this article. There was no special funding for this study but research at the institution is supported by the Ministry of Research, Science and Arts Baden-Württemberg.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Schievink WI, Maya MM, Moser FG, Simon P, Nuño M. Incidence of spontaneous intracranial hypotension in a community: Beverly Hills, California, 2006-2020. Cephalalgia. 2022;42(4-5):312-316. doi: 10.1177/03331024211048510 [DOI] [PubMed] [Google Scholar]
- 2.Pradeep A, Madhavan AA, Brinjikji W, Cutsforth-Gregory JK. Incidence of spontaneous intracranial hypotension in Olmsted County, Minnesota: 2019-2021. Interv Neuroradiol. 2023:159101992311654. doi: 10.1177/15910199231165429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Antona L, Jaime Merchan MA, Vassiliou A, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: a systematic review and meta-analysis. JAMA Neurol. 2021;78(3):329-337. doi: 10.1001/jamaneurol.2020.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schievink WI. Spontaneous intracranial hypotension. N Engl J Med. 2021;385(23):2173-2178. doi: 10.1056/NEJMra2101561 [DOI] [PubMed] [Google Scholar]
- 5.Liaw V, Friedman D, McCreary M. Quality of life in patients with confirmed and suspected spontaneous intracranial hypotension (P3-12.002). In: Sunday, April 23. Lippincott Williams & Wilkins; 2023:3099. doi: 10.1212/WNL.0000000000203020 [DOI] [Google Scholar]
- 6.Beck J, Hubbe U, Klingler JH, et al. Minimally invasive surgery for spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension. J Neurosurg Spine. 2023;38:147-152. doi: 10.3171/2022.7.SPINE2252 [DOI] [PubMed] [Google Scholar]
- 7.Beck J, Raabe A, Schievink WI, et al. Posterior approach and spinal cord release for 360° repair of dural defects in spontaneous intracranial hypotension. Neurosurgery. 2019;84(6):E345-E351. doi: 10.1093/neuros/nyy312 [DOI] [PubMed] [Google Scholar]
- 8.Wang TY, Karikari IO, Amrhein TJ, Gray L, Kranz PG. Clinical outcomes following surgical ligation of cerebrospinal fluid-venous fistula in patients with spontaneous intracranial hypotension: a prospective case series. Oper Neurosurg (Hagerstown). 2020;18(3):239-245. doi: 10.1093/ons/opz134 [DOI] [PubMed] [Google Scholar]
- 9.Brinjikji W, Garza I, Whealy M, et al. Clinical and imaging outcomes of cerebrospinal fluid-venous fistula embolization. J Neurointerv Surg. 2022;14(10):953-956. doi: 10.1136/neurintsurg-2021-018466 [DOI] [PubMed] [Google Scholar]
- 10.Dobrocky T, Nicholson P, Häni L, et al. Spontaneous intracranial hypotension: searching for the CSF leak. Lancet Neurol. 2022;21(4):369-380. doi: 10.1016/S1474-4422(21)00423-3 [DOI] [PubMed] [Google Scholar]
- 11.Lützen N, Demerath T, Volz F, Beck J, Urbach H. Conebeam CT as an additional tool in digital subtraction myelography for the detection of spinal lateral dural tears. AJNR Am J Neuroradiol 2023;44(6):745-747. doi: 10.3174/ajnr.A7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strøm J, Bjerrum MB, Nielsen CV, et al. Anxiety and depression in spine surgery—a systematic integrative review. Spine J. 2018;18(7):1272-1285. doi: 10.1016/j.spinee.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 14.Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019;76(5):580-587. doi: 10.1001/jamaneurol.2018.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schievink WI, Maya MM, Jean-Pierre S, Nuño M, Prasad RS, Moser FG. A classification system of spontaneous spinal CSF leaks. Neurology. 2016;87(7):673-679. doi: 10.1212/WNL.0000000000002986 [DOI] [PubMed] [Google Scholar]
- 16.Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol. 2019;40(4):745-753. doi: 10.3174/ajnr.A6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 19.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EuroQol Research Foundation. EQ-5D-5L User Guide; 2019. Accessed August 10, 2023. euroqol.org/publications/user-guides [Google Scholar]
- 21.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663-674. doi: 10.1007/s40273-018-0615-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry JD, Crawford JR. The short-form version of the depression anxiety stress scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(Pt 2):227-239. doi: 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- 23.Sinclair SJ, Siefert CJ, Slavin-Mulford JM, Stein MB, Renna M, Blais MA. Psychometric evaluation and normative data for the depression, anxiety, and stress scales-21 (DASS-21) in a nonclinical sample of U.S. adults. Eval Health Prof. 2012;35(3):259-279. doi: 10.1177/0163278711424282 [DOI] [PubMed] [Google Scholar]
- 24.Brumby S, Chandrasekara A, McCoombe S, Torres S, Kremer P, Lewandowski P. Reducing psychological distress and obesity in Australian farmers by promoting physical activity. BMC Public Health. 2011;11(1):362. doi: 10.1186/1471-2458-11-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales, 2nd ed. Psychology Foundation of Australia; 2015. [Google Scholar]
- 26.Volz F, Fung C, Wolf K, et al. Recovery and long-term outcome after neurosurgical closure of spinal CSF leaks in patients with spontaneous intracranial hypotension. Cephalalgia. 2023;43(8):3331024231196808. doi: 10.1177/03331024231195830 [DOI] [PubMed] [Google Scholar]
- 27.Haywood KL, Mars TS, Potter R, Patel S, Matharu M, Underwood M. Assessing the impact of headaches and the outcomes of treatment: a systematic review of patient-reported outcome measures (PROMs). Cephalalgia. 2018;38(7):1374-1386. doi: 10.1177/0333102417731348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheema S, Anderson J, Angus-Leppan H, et al. Multidisciplinary consensus guideline for the diagnosis and management of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry. 2023;94(10):835-843. doi: 10.1136/jnnp-2023-331166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones KH, Ford DV, Jones PA, et al. How people with multiple sclerosis rate their quality of life: an EQ-5D survey via the UK MS register. PLoS One. 2013;8(6):e65640. doi: 10.1371/journal.pone.0065640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohn CG, Sidovar MF, Kaur K, Zhu Y, Coleman CI. Estimating a minimal clinically important difference for the EuroQol 5-Dimension health status index in persons with multiple sclerosis. Health Qual Life Outcomes. 2014;12:66. doi: 10.1186/1477-7525-12-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan I, Morris S, Pashayan N, Matata B, Bashir Z, Maguirre J. Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the EORTC-QLQ-C30 in non-small cell lung cancer patients. Health Qual Life Outcomes. 2016;14:60. doi: 10.1186/s12955-016-0455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koide R, Kikuchi A, Miyajima M, et al. Quality assessment using EQ-5D-5L after lung surgery for non-small cell lung cancer (NSCLC) patients. Gen Thorac Cardiovasc Surg. 2019;67(12):1056-1061. doi: 10.1007/s11748-019-01136-0 [DOI] [PubMed] [Google Scholar]
- 33.Huber M, Reitmeir P, Vogelmann M, Leidl R. EQ-5D-5L in the general German population: comparison and evaluation of three yearly cross-section surveys. Int J Environ Res Public Health. 2016;13(3):343. doi: 10.3390/ijerph13030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg SM, O'Neill A, Sepucha K, et al. Quality of life following receipt of adjuvant chemotherapy with and without Bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer. JAMA Netw Open. 2022;5(2):e220254. doi: 10.1001/jamanetworkopen.2022.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):292-303. doi: 10.1016/S1470-2045(21)00693-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprave T, Gkika E, Verma V, Grosu AL, Stoian R. Patient reported outcomes based on EQ-5D-5L questionnaires in head and neck cancer patients: a real-world study. BMC Cancer. 2022;22(1):1236. doi: 10.1186/s12885-022-10346-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jesse CM, Häni L, Fung C, et al. The impact of spontaneous intracranial hypotension on social life and health-related quality of life. J Neurol. 2022;269(10):5466-5473. doi: 10.1007/s00415-022-11207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheema S, Joy C, Pople J, Snape-Burns J, Trevarthen T, Matharu M. Patient experience of diagnosis and management of spontaneous intracranial hypotension: a cross-sectional online survey. BMJ Open. 2022;12(1):e057438. doi: 10.1136/bmjopen-2021-057438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5(1):70. doi: 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Depression and Other Common Mental Disorders - Global Health Estimates. Published online 2015. Accessed April 29, 2023. who.int/publications/i/item/depression-global-health-estimates. [Google Scholar]
- 41.Hapke U, Cohrdes C, Nübel J. Depressive symptoms in a European comparison—results from the European health Interview survey (EHIS) 2. J Health Monit. 2019;4(4):57-65. doi: 10.25646/6227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y, Deng J, Yang M, et al. Does the preoperative depression affect clinical outcomes in adults with following lumbar fusion? A retrospective cohort study. Clin Spine Surg. 2021;34(4):E194–E199. doi: 10.1097/BSD.0000000000001102 [DOI] [PubMed] [Google Scholar]
- 43.Merrill RK, Zebala LP, Peters C, Qureshi SA, McAnany SJ. Impact of depression on patient-reported outcome measures after lumbar spine decompression. Spine. 2018;43(6):434-439. doi: 10.1097/BRS.0000000000002329 [DOI] [PubMed] [Google Scholar]
- 44.Sinikallio S, Aalto T, Airaksinen O, et al. Depression is associated with poorer outcome of lumbar spinal stenosis surgery. Eur Spine J. 2007;16(7):905-912. doi: 10.1007/s00586-007-0349-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional anonymized data not published in this article will be shared by request from any qualified investigator.