Abstract
Purpose of Review
Lack of consistent data and guidance have led to variations between clinicians in the management of pregnancy in women with multiple sclerosis (MS). Pregnant and/or lactating women are often excluded from clinical trials conducted in MS, and thus, the labeling for most disease-modifying therapies (DMTs) excludes use during pregnancy. This has led to heterogeneity in interpretation and labeling regarding the safety of DMTs during pregnancy and lactation and the required preconception washout periods. This review identifies key themes where there is conflicting information surrounding family planning and pregnancy in MS, focusing on the most common discussion points between physicians and patients during preconception planning, pregnancy, postpartum, and lactation. The goal was to inform the patient-physician conversation and provide best practice recommendations based on expert clinical expertise and experience.
Recent Findings
We outline the latest evidence-based data for DMT use during pregnancy and lactation, the effect of MS on fertility and fertility treatments, the risk of adverse pregnancy and delivery outcomes, the risk of postpartum relapse, and immunization and clinical imaging safety during pregnancy and breastfeeding.
Summary
Management of family planning and pregnancy in patients with MS requires the most current information. Health care providers should discuss family planning early and frequently with patients with MS, and partners where practicable. Because management of pregnant people with MS will often require a risk/benefit analysis of their needs, shared decision-making in family planning discussions is emphasized. Additional data are needed for specific and underrepresented populations with MS (e.g., single parents or those from the LGBTQ+ community) and those at risk of racial and socioeconomic disparities in care. Pregnancy registries and the design and conduct of clinical trials focused on pregnant and lactating patients should provide additional data to guide the ongoing management of patients with MS.
Introduction
Multiple sclerosis (MS) is typically diagnosed between the ages of 20 and 50 years.e1 Because most patients with MS are women (approximately 70%), diagnosis often coincides with patients' reproductive years.e1 Historically, women with MS have been discouraged from pregnancy;e2,e3 however, evidence suggests that pregnancy does not adversely affect the long-term course of MS and may even have a beneficial effect in MS, with overall better MS outcomes in patients who have been pregnant compared with those who have not.1 In the short term, pregnant women with MS often experience a substantial reduction in relapse rates, especially in the third trimester.e4 However, risk of postpartum disease rebound requires consideration and careful management. An increased risk of infection and preterm birth was noted in a study of 3,875 pregnant women with MS in US databases, with an unclear association between this risk and the use of disease-modifying therapies (DMTs); however, other pregnancy or fetal complications were not elevated in pregnant women with MS.e5
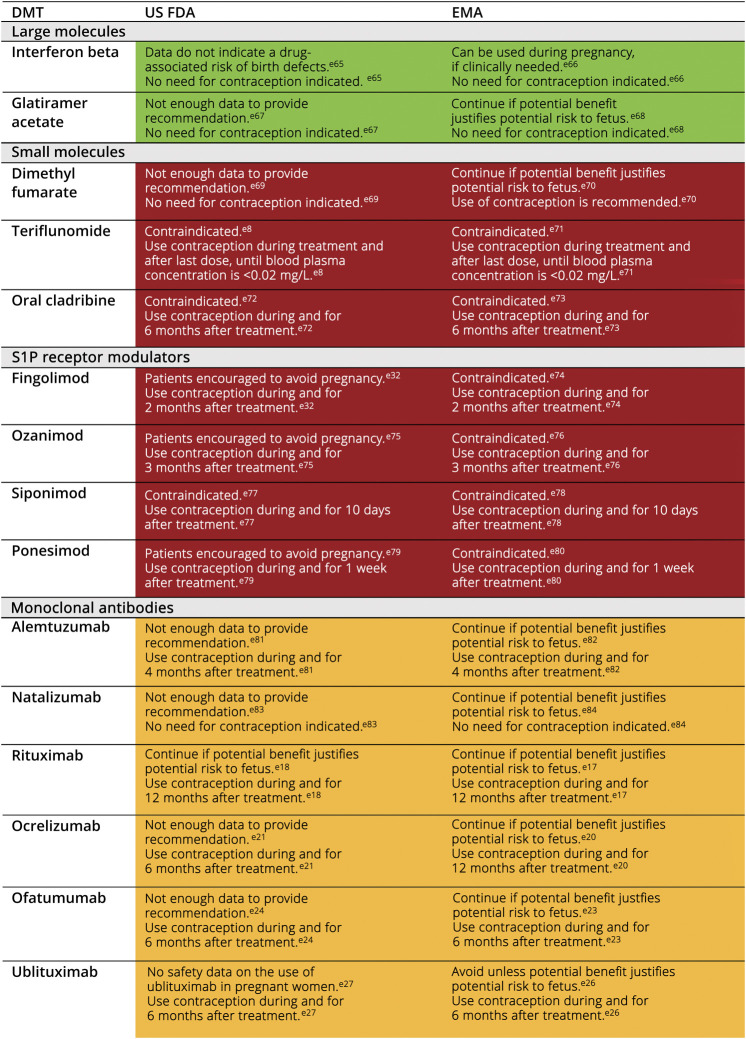
Although treatment paradigms in MS have evolved rapidly over the past decade, pregnant and lactating women have been excluded from phase 3 trials, and labeling for DMTs precludes their use during pregnancy (Figure 1; additional details are summarized in eTable 1, links.lww.com/CPJ/A500). Pregnancy safety evidence is often based on real-world data originating from pharmaceutical company postmarketing registries or claims databases.e5-e7 For some treatments, real-world data are often borrowed from other indications. For example, the pyrimidine synthesis inhibitor leflunomide, whose active metabolite is the MS DMT teriflunomide, is indicated for the treatment of rheumatoid arthritis. The safety of teriflunomide use during pregnancy, which is contraindicated, has been informed by pregnancy data from leflunomide.e8 Despite this, using DMTs to better control disease in young women, along with active treatment of patients prepregnancy, intrapregnancy, and postpregnancy, has resulted in a significant improvement in outcomes, with a decrease in the risk of disability, an improvement in quality of life, and more autonomy for pregnancy planning for women living with MS.e4,e9,e10
Figure 1. US FDA/EMA Guidelines for DMT Use During Pregnancy.
*See eTable 1 (links.lww.com/CPJ/A500) for further details and Health Canada guidelines. *Color coding represents the authors' interpretation of agency guidelines and literature to date. Green cells indicate that the DMT can be used during pregnancy; yellow cells indicate that the DMT may be used based on the balance of benefit and risk; red cells indicate that the DMT should not be used during pregnancy. DMT = disease-modifying therapy; EMA = European Medicines Agency; US FDA = US Food and Drug Administration; S1P = sphingosine-1-phosphate.
In the past, an “escalation” treatment strategy has been used in MS.e11 With this approach, moderate-efficacy therapies with low side effect profiles are initiated early. If these therapies prove ineffective, treatment then escalates to high-efficacy therapies (HETs).e11 Notably, there is growing evidence supporting early initiation of HET for disease control instead.2-5 HET includes “induction” therapies (alemtuzumab, cladribine); lymphocyte trafficking therapies, including natalizumab and sphingosine-1-phosphate (S1P) receptor modulators (fingolimod, ozanimod, ponesimod, and siponimod); and B-cell–depleting therapies (ocrelizumab, ofatumumab, rituximab [used off-label for MS], and ublituximab). HET seems to represent a particularly effective way of managing inflammatory activity before and after pregnancy. It can therefore be expected that women with MS of childbearing potential will increasingly receive HETs as first-line therapy, and it is critical to educate clinicians about the safety of these medications during gestation and lactation.
Incomplete data have led to a lack of adequate guidance on how to best manage people of childbearing age with MS. The physician-patient conversation is essential for making individualized treatment decisions and must include the prepregnancy (planning in advance for anticipations of future pregnancies), pregnancy (whether first or subsequent pregnancy), lactation, and postpartum periods.e12,e13 The objective of this review was to inform the physician-patient conversation by clarifying recommendations and addressing conflicting evidence around family planning and pregnancy in women with MS, outlining the latest evidence-based data. We discuss the management of cisgendered women only because there is currently a lack of knowledge surrounding pregnancy in gender-diverse people with MS. We also provided an overview of the latest recommendations in the United States, Europe, and Canada on administration of DMTs in pregnancy and during breastfeeding.
Women of Childbearing Age Living With MS
Dispelling Family Planning Myths: Informing MS Treatment Selection
One of the key concerns regarding pregnancy in MS is patients not starting or discontinuing their treatment during pregnancy.e14 Some studies suggest that <25% of women received DMTs within 12 months before pregnancy.e15 Experts typically recommend that DMTs be initiated at diagnosis of MS and clinical stability should be established for ≥1 year before attempting to conceive.e12,e13,e16 Early MS treatment may prevent long-term neurologic disability, particularly when using a HET.5-7 Preconception use of DMTs whose pharmacodynamic effects outlast their pharmacokinetic effects (i.e., induction therapies and B-cell–depleting therapies) may reduce the incidence of relapses in the prepregnancy period while offering some protection from peripartum/postpartum relapses.8 However, factors such as advanced age, assisted reproductive technology use, or secondary autoimmune disease also need to be taken into account and can be a barrier to controlled timing of pregnancy.
When deciding on an MS therapy, factors such as the need for contraception, drug washout period, regulatory agency (US Food and Drug Administration [FDA], European Medicines Agency [EMA], Health Canada) guidelines, and safety information from clinical trials and pregnancy registries should be considered (Figure 1; additional details are summarized in eTable 1, links.lww.com/CPJ/A500). Some confusion around the ideal timing between DMT discontinuation and conception exists due to differences between expert consensus statements and (typically) a more conservative product labeling. Furthermore, guidelines and recommendations may differ based on approval rate, even for products in the same class with similar profiles. This is evident in the case of anti-CD20 therapies such as ocrelizumab, ofatumumab, ublituximab, and rituximab. For example, EMA, US FDA, and Health Canada guidelines for rituximab recommend that women do not conceive for 12 months after their last infusione17–e19; for ocrelizumab, the EMA also recommends 12 months,e20 but the US FDA and Health Canada recommend 6 monthse21,e22; and for a third similar product, ofatumumab, the EMA, US FDA, and Health Canada guidelines all recommend conception should not be attempted until 6 months after the last dose.e23–e25 Regarding the recently approved ublituximab, it is recommended that contraceptives are used during and for 6 months (US FDA) or 4 months (EMA) after the last dose (ublituximab is not yet approved in Canada).e26,e27
Most DMTs should be stopped before or upon a positive pregnancy test due to potential negative effects on the fetus (Figure 1; additional details are summarized in eTable 1, links.lww.com/CPJ/A500). By contrast, for the B-cell–depleting group of monoclonal antibodies (rituximab,e28 ocrelizumab,e13,e16,e29,e30 ublituximab, and ofatumumab), a reasonable interpretation of product half-lives and lack of placental transfer of immunoglobulins during the first trimester suggest that their pharmacokinetic and pharmacodynamic properties could be leveraged to optimize their therapeutic benefits during pregnancy. These antibodies require active transport through neonatal fragment crystallizable (Fc) receptors.9,10,e31 Fc receptors are not highly expressed during the first 20–22 weeks of pregnancy, limiting fetal exposure.9 This has led some experts to advise using effective contraception for just 3 months (sometimes less) after the last treatment. Furthermore, these parameters thereby provide a window of opportunity for discontinuing these agents in the case of an unplanned pregnancy.
A 2022 analysis of 2,020 MS pregnancies from the Roche safety database found that there was no increased risk of preterm birth, major congenital abnormalities, or other adverse outcomes with ocrelizumab exposure in utero (last infusion within 3 months before last menstrual period/during pregnancy), but numbers remained small.11 Due to the differences in agency guidelines and clinical practice recommendations, individual decisions in terms of timing of treatments before pregnancy depend on the MS clinician and the patient, but should be made with clear understanding of the currently known risks and benefits.
Some treatments have a known risk of disease reactivation on cessation, such as fingolimod and natalizumab.12,13 Fingolimod and natalizumab were among the most commonly used DMTs in patients who experienced relapses during pregnancy, based on findings from a retrospective analysis of 164 pregnancies from 2 registries.14 Given insufficient safety data for S1P receptor modulators during pregnancy and developmental toxicity displayed in animal studies with fingolimod at less than the recommended human dose,e32 these must be discontinued before attempting conception. Natalizumab should either be discontinued before conception or, as an alternative, continued with extended interval dosing during pregnancy up until 34 weeks of gestation, when there is greatest transplacental antibody transmission.8,9,e33 This decision is dependent upon the individual's benefit-risk tolerance because a recent study has shown that, although continuing natalizumab into pregnancy decreased relapse risk during pregnancy and postpartum,8 more than half of newborns exposed to natalizumab after the first trimester had transient hematologic abnormalities, including anemia and thrombocytopenia.15 B-cell–depleting monoclonal antibodies, which have lasting biologic activity, can be used as bridge therapies to stabilize disease activity prepartum, peripartum, or postpartum for patients discontinuing fingolimod or natalizumab.e13 This underscores the importance of pregnancy planning discussions with patients throughout the MS management process.
Best Practice Recommendations
When choosing an MS treatment, a shared decision-making process should be facilitated between patients, their partners (when possible and requested by the patient), and the clinician (Figure 2). Conversations about intent for family planning should happen at every visit and in the active decision-making phase should involve the patient's multidisciplinary team, including their neurologist, obstetric team, and primary physician.
Figure 2. Shared Decision-Making Pathway for Clinicians Treating Women of Childbearing Age.
DMT = disease-modifying therapy. For each decision step, learn the patient's goals, concerns, and lifestyle so that the most helpful information is shared.
If a patient expresses a desire to become pregnant, planned pregnancy should be recommended so that their MS can be well controlled before conception and DMTs adjusted before pregnancy to achieve this.e3 All patients should be advised about contraception during the treatment and washout periods because some DMTs may need to be discontinued for up to 6 months before conception (eTable 1, links.lww.com/CPJ/A500).
Family planning discussions should consider the many symptomatic treatments commonly prescribed for patients with MS to manage comorbid conditions such as anxiety, depression, fatigue, and mobility issues. These medications may need to be discontinued or the lowest dose used for the shortest possible time before conception and/or during pregnancy (eTable 2, links.lww.com/CPJ/A501). Furthermore, modafinil, which may be prescribed for fatigue, can reduce the effectiveness of hormonal contraceptives, so alternative contraceptive methods are recommended during modafinil treatment and for 1 month after modafinil discontinuation.e34
General guidelines for folic acid and prenatal vitamin supplementation should be followed as for all pregnancies; MS-specific considerations include vitamin D supplementation (refer to the Pregnancy and Childbirth sections), smoking cessation to help reduce the risk of disease progression, and sleep optimization.e2,e12
Fertility in MS
Dispelling Fertility Myths: Practical Considerations for Fertility Treatment
Based on claims data in the United States, women with MS may have a slightly higher risk (8.5% vs 8.1%; p = 0.0006) of being diagnosed with infertility, but are less likely to receive infertility treatment compared with women without MS.16 There is no difference in live birth outcomes with the use of fertility treatments between people with MS and healthy women.16 The causal relationship between infertility and MS is unclear because women often delay reproduction due to concerns associated with their MS diagnosise15 or may present to infertility clinics earlier than women without MS due to the need for optimization of conception windows. Current evidence suggests that MS in male individuals does not affect fertility or birth outcomes,17 but data are limited. As noted earlier, data are further limited in gender-diverse people.
Diverse fertility treatments are currently used to support fertility preservation and childbearing in individuals with MS. Recent studies have shown no significant change in annualized relapse rate (ARR) before and after fertility treatment18,19 and have not confirmed previous reports of elevated relapse risk with use of gonadotropin-releasing hormone agonists vs antagonists.20 Indeed, these modern cohorts have not reproduced findings from earlier, smaller, observational studies suggesting clinical and radiologic activity after fertility treatments.e35–37 In women with MS undergoing fertility treatment, those who achieved pregnancy and those remaining on DMT had lower risk or relapses.18,19,21
Best Practice Recommendations
The counseling provided to all patients with MS and their partners in the preconception period should address fertility and potential relapse risk of treatments for infertility to the patient. If a patient has been attempting to conceive for >6 months (regardless of age and following DMT washout period, as appropriate), fertility referral and counseling can be recommended to expedite the fertility evaluation and minimize time off DMT.
The use of DMTs that can be flexibly dosed around in vitro fertilization procedures may decrease the risk of relapse. If a patient is having their eggs cryopreserved, then there is no need to discontinue DMT use around this procedure. DMT discontinuation should only be required for egg transfer, with timing similar to that advised for conception.
Pregnancy
Dispelling Pregnancy Myths: Practical Considerations for Management
A higher rate of preterm birth has been noted for women with MS vs those without.e5,e12 MS does not seem to affect pregnancy outcomes, such as preeclampsia, chorioamnionitis, and postpartum hemorrhage.e5 Therefore, MS does not necessarily qualify a pregnancy as being high risk. Referral to maternal-fetal experts may, nonetheless, be recommended for guidance regarding treatment safety during pregnancy or other non–MS-related considerations. Furthermore, organizations such as the Organization of Teratology Information Specialists (OTIS) provide information on the use of numerous drugs during pregnancy and breastfeeding.e39
In most cases, there is a general decrease in risk of relapses during pregnancy, notably in the last trimester.22,23 This is often followed by a postpartum disease reactivation period or rebound mainly in the first 3 months after delivery.24-26 The magnitude of this reactivation/rebound likely depends on a patient's preconception MS activity, DMT before and after pregnancy, and breastfeeding practices.22,23 For further discussion on postpartum disease reactivation, refer to the Postpartum section of this review.
Disease-Modifying Therapies
Recommendations for DMTs during pregnancy are shown in Figure 1. Additional details are summarized in eTable 1 (links.lww.com/CPJ/A500). Some treatments may help to maintain disease stability during pregnancy (e.g., in severe or highly active MS where the benefit of MS treatment may outweigh the risk to the fetus). Of the DMTs currently used in the treatment of MS, interferon (IFN) beta and glatiramer acetate (GA) have the most evidence to support safe usage during pregnancy (based on extensive data from first trimester exposure).e12,e38 Data supporting use throughout the entire pregnancy exists mainly for GA.e40-e42 However, this large body of evidence may simply be a byproduct of the longer period of therapeutic use of IFN beta and GA, which were the first 2 DMTs to be approved for the treatment of MS in the 1990s,e43 and may not necessarily reflect the benefit-risk profile of IFN beta and GA during pregnancy. Use of IFN beta and GA during pregnancy is still not typically recommended.
For all other DMTs that are not contraindicated during pregnancy, there are still inadequate data regarding the developmental risk associated with their use, and treatment will therefore depend on the mother's benefit-risk tolerance (Figure 1; additional details are summarized in eTable 1, links.lww.com/CPJ/A500). It should be noted that, with the increasing use of HETs early after diagnosis, it is becoming more difficult to calculate a patient's risk of relapse off DMT and thus identify patients who would benefit from continuing DMT use into pregnancy.
Symptomatic Treatments
Symptomatic treatments should be reviewed before planned pregnancies, with the intention to wean patients off medications that are contraindicated in pregnancy (eTable 2, links.lww.com/CPJ/A501). Only a few treatments commonly prescribed for people with MS have supporting evidence for use during pregnancy (e.g., sertraline for depression, cephalexin and oral nitrofurantoin for urinary tract infections, and acyclovir and valacyclovir for viral infections; eTable 2).
Vitamin D
Women with MS are advised to take vitamin D during pregnancy.e12 Although vitamin D supplementation may not lower the risk of clinical relapse in patients themselves,27 maternal vitamin D deficiency (25(OH) vitamin D) during early pregnancy has been shown to nearly double the risk of offspring having MS compared with offspring of mothers who were not vitamin D deficient.28 Optimal dosing of vitamin D remains unknown,e44 but these authors recommend that dosage will depend on the vitamin D levels of the mother and should be agreed with the obstetrician, with supplemental dosing varying between 1,000 and 5,000 units per day to maintain serum levels between 30 and 100 ng/mL; supranormal serum levels should be avoided.
MRI and Gadolinium
Although MRI is considered safe for use during the entire pregnancy, the use of gadolinium-based enhancement should be limited to situations in which the benefit clearly outweighs the possible risk.e45 Gadolinium is water soluble and can cross the placenta into the amniotic fluid. The duration of fetal gadolinium exposure may be longer than expected because the amniotic fluid can be swallowed by the fetus and reenter fetal circulation.e45 Gadolinium is administered in its chelated form because free gadolinium is toxic, and increased duration of fetal exposure increases the potential for dissociation from the chelate.e45 Based on data from a Canadian health care database (2003–2015), the risks of stillbirths/deaths and congenital anomalies, neoplasm, and vision or hearing loss were found to be higher in pregnancies where the mother underwent gadolinium-enhanced MRI in the first trimester of pregnancy vs an unexposed cohort.e45 However, it should be noted that the unexposed cohort did not receive MRIs (presumably because they were not clinically necessary), and therefore the baseline populations for this comparison are different, with the unexposed cohort likely to be of better overall health than the exposed cohort.e45
Vaccination
Patients receiving B-cell–depleting therapies29,30 or S1P receptor modulators31 may have an attenuated immune response to vaccination, although routine vaccinations, including inactivated influenza and tetanus, diphtheria, and acellular pertussis, are still recommended to these and all patients during pregnancy (eTable 3, links.lww.com/CPJ/A502).
Best Practice Recommendations
Most DMTs need to be stopped before conception or as soon as pregnancy is confirmed in women with MS (Figure 1; additional details are summarized in eTable 1, links.lww.com/CPJ/A500). Shared decisions are therefore essential and treatment decisions should be based on the associated benefit-risk of the MS treatment and the individual's risk aversion and specific disease course. Similar consideration should be given to symptomatic MS treatments, which should also be reviewed for safety in pregnancy. Should accidental exposure to DMT arise after conception, referral to a maternal fetal medicine expert and fetal screening should be considered to advise on risk mitigation.
Although MRI is considered safe, IV gadolinium should be avoided in pregnancy.e45 The recommended immunization schedule for pregnant individuals should be followed in women with MS, with special consideration to their MS medication treatment regimen and with support from their obstetrical clinician (eTable 3, links.lww.com/CPJ/A502). To mitigate the possible attenuated immune response to vaccination, patients should consider getting fully vaccinated at least 2 weeks before starting S1P receptor modulator treatments.e46 Patients should receive live vaccines at least 4 weeks before and inactivated vaccines at least 2 weeks before starting B-cell–depleting treatments, such as rituximab, ocrelizumab, ofatumumab, and ublituximab.e18,e21,e24,e27 Due to the higher risk of severe illness, patients with MS not previously vaccinated are also advised to receive a COVID-19 vaccine or vaccine series, including modified series for patients who are B cell depleted or on S1P receptor modulators.e46,e47
Childbirth
Dispelling Childbirth Myths: Practical Considerations for Delivery
MS should not affect the mode of delivery except in cases of substantial disability.e12 In patients with MS with higher Expanded Disability Status Scale (EDSS) scores (≥3), cesarean rates are as high as 44%23 vs a general population rate of 31.8%e48; it is unknown whether this is due to MS itself or whether physicians perceived the mother as high risk and therefore took a conservative approach with delivery.e2 Cesarean sections do not seem to affect relapse rates after labor.32,33
Obstetrical anesthesia should be selected based on indications and patient preference and is considered safe for women with MS.e2,e12 Evidence from several studies indicates that MS disability and relapse rate do not worsen following spinal or epidural anesthesia, and satisfactory pain control is achieved.32-34
Evidence of the effect of MS on the baby's birth weight or gestational age is mixed. The birth weight of babies delivered by mothers with MS has been reported to be similar to,35 or lower than,e48 those delivered to mothers without MS. Although one study reported no difference in gestational age of babies delivered by mothers with MS and without MS,35 others have reported an increased risk of preterm delivery for women with MS.e5 MS does not seem to affect the baby's condition nor risk of malformation or stillbirth.e2,e5
Best Practice Recommendations
Women should be advised that MS does not contraindicate any type of obstetric anesthesia; the choice of anesthesia should be based on obstetric criteria.e2,e12 Women with severe spasticity or weakness in the pelvis and/or legs should be referred to a physiotherapist (preferably with a neurologic specialization) early in the pregnancy.e12 As part of the patient's multidisciplinary team, physiotherapists collaborate with obstetrician-gynecologists to optimize labor and delivery outcomes.
Abortion and Miscarriages
Dispelling Abortion Myths: Informing Treatment Decisions
Potential risks associated with pregnancy in patients with MS, and barriers to becoming a parent with MS, may result in patients being more likely to choose an elective abortion than healthy individuals, although this has not been directly investigated. One survey (involving 303 women with MS; 500 controls) reported more elective abortions in patients with MS than healthy controls (20% vs 12%; p = 0.005).36 In an MS registry study, there was no difference in abortion rates between DMT-exposed and non–DMT-exposed pregnancies; however, the number of pregnancies was low (142 pregnancies, 80 exposed to DMTs, in 120 women).37
Data on the effects of MS treatments on pregnancy loss are limited due to relatively low pregnancy rates in clinical trials, contraindications for many MS treatments during pregnancy, and the paucity of postmarketing surveillance program data. Nevertheless, some data report no increased rates of abortions or miscarriages with IFNs,38,39,e38 GA,39 natalizumab,39,40 and alemtuzumab.41
None of the standard drugs used to induce abortion in the first (mifepristone, misoprostol, and methotrexate) or second trimester (oxytocin, misoprostol, mifepristone, dinoprostone, and carboprost) have any contraindications, special warnings, or precautions specifically against use in patients with MS.e49-e54 If surgical abortion is required, the risks associated with anesthesia are comparable between people with and without MS.42
There is inconclusive evidence on the risk of disease rebound post abortion. Limited evidence indicates that elective and spontaneous abortion may cause disease rebound and worsen the disease course in patients with MS,43,44 although this was not supported in a more recent study.45 Given the clear data knowledge gaps around miscarriages in MS and the effects of MS treatments on pregnancy loss, programs such as the new MSBase pregnancy, neonatal outcomes, and women's health registry should help inform shared decision-making in the future.e55
Best Practice Recommendations
Patients should be reassured that MS does not increase the risk of spontaneous abortion or miscarriage.e12 There are no special precautions to drugs or procedures to induce abortion in patients with MS.42,e49-e55 Patients dealing with pregnancy loss should be referred for counseling to address grief and stress.
Pastpartum
Dispelling Postpartum Myths: Managing Relapse
Postpartum relapses may occur in women with MS and can result in increased disability progression.46 One observational study of patients with MS from a tertiary care MS center reported that 48.8% of women relapsed within 6 months post partum.25 However, in a study of electronic health records from 2008 to 2016, only 26% of patients relapsed in the year after delivery.22 No increase in relapse rate in the first 3 months post partum was reported overall or in patients with suboptimally controlled disease before pregnancy. Relapse rates returned to prepregnancy rates 4–6 months post partum. Another recent study showed a lower ARR in the first 3 months post partum than previously reported, with only 17% of patients experiencing relapse during this period. Most patients did not experience relapse.26 Variability in reported relapse risk is likely due to differing study populations in terms of preconception levels of disease severity and disability.
There are limited data to guide management of disease relapses/rebounds in postpartum women with moderate or severe disability (most data exist for pregnancies in women with no or minimal disability).22,26 In a retrospective cohort study, patients with moderate to severe MS disability had a marked increase in relapse rate post partum (ARR of 1.22 in 3 months post partum), compared with preconception (ARR 0.59) and the third trimester of pregnancy (ARR 0.11).23 Those patients with higher EDSS scores preconception had a greater chance of postpartum relapse and worsening of disability than those with lower EDSS scores.23
The main risk factors of postpartum relapse include the following: younger maternal age,22 higher number of relapses before and during pregnancy, higher preconception disability (EDSS score),46,47 and lack of preconception DMT use,24 or discontinuation of DMTs known to induce rebound disease activity. DMT use during pregnancy (compared with no DMT use) has been associated with fewer relapses post partum in 4/7 studies; however, no difference in postpartum relapse rate with DMT use was found in the remaining 3 studies.48
There is no clear evidence to support use of IV corticosteroids or IV immunoglobulin to prevent postpartum disease relapses.49,50 Although pelvic floor physiotherapy is a standard recommendation for all postpartum women,e12 it is often underused when managing women with MS.51 An analysis of 142 pregnancies from women with MS showed that only 4.9% of postpartum women with a record of urinary incontinence were referred for pelvic floor physiotherapy, showing a lack of clinical education around physical therapy for postpartum women with MS.51
Best Practice Recommendations
During the first few months post partum, patients with MS should be closely monitored. Increased risk of postpartum depression in mothers and fathers with MS has been reported52,53; the multidisciplinary team should be made aware of this and offer support as required. Screening assessment for depressive symptoms using tools such as Edinburgh Postnatal Depression Scale or the Patient Health Questionnaire–2 should be encouraged.
Early reintroduction of DMTs post partum may be advised to reduce the risk of relapses. The effects of therapeutic lag on treatment effect should be considered, particularly in mothers with substantial disability or high relapse rates because therapeutic lag may be longer in these populations.54 Data on the benefit of IV corticosteroids or IV immunoglobulin in preventing postpartum relapses are lacking, so their use cannot be supported.49,50
Mothers should also be encouraged to undergo postpartum physiotherapy, including of the pelvic floor.e12 Pelvic floor physiotherapy is typically recommended to all postpartum women but can be particularly helpful in women with MS, who also experience bladder dysfunction as part of their disease symptomatology.e12
Due to the B-cell–depleting nature of anti-CD20 therapies, the US FDA, EMA, and Health Canada all recommend that infants of mothers exposed to ocrelizumab, ofatumumab, or ublituximab (US FDA, EMA only) during pregnancy are not vaccinated with live/live-attenuated vaccines before B-cell count recovery is confirmed. While nonlive vaccines can be administered to infants before recovery from B-cell depletion, the vaccine immune response should be assessed.e20–e27
Lactation
Dispelling Lactation Myths: Practical Considerations for Breastfeeding
Historically, data on the protective effect of breastfeeding on postpartum relapse have been mixed, partially due to heterogeneity in breastfeeding practices and study design.e56,e57 However, a 2020 systematic review and meta-analysis of 16 studies showed that breastfeeding, especially when conducted without supplementation, is protective against postpartum relapses.55 Indeed, women who breastfed had 37% lower odds of postpartum relapse compared with those who did not breastfeed or those who did not breastfeed exclusively.
Recommendations on DMT use during breastfeeding are shown in Figure 3. Further information on drugs not discussed here can be found on LactMed®, a peer-reviewed, online database containing information on drugs and other chemicals to which breastfeeding mothers may be exposed.e58 The decision to start or resume a DMT following birth in women at high risk of disease activity who are breastfeeding should be weighed against the known risks of the product during lactation. When resuming a DMT within the first 2 weeks post partum, patients may want to consider modifying premedications for the first infusion so that prolactin levels are not affected; for instance, diphenhydramine is recommended for mothers before each ocrelizumab infusion but can cause sedation and irritability in breastfed infants, and decrease milk supply after large/frequent doses.e21,e59
Figure 3. US FDA/EMA Guidelines for DMT Use During Breastfeeding.
*See eTable 1 (links.lww.com/CPJ/A500) for further details and Health Canada guidelines. *Color coding represents the authors' interpretation of agency guidelines and literature to date. Green cells indicate that the DMT can be used during breastfeeding; yellow cells indicate that the DMT may be used based on the balance of benefit and risk; red cells indicate that the DMT should not be used during breastfeeding. AE = adverse effect; DMT = disease-modifying therapy; EMA = European Medicines Agency; US FDA = US Food and Drug Administration; S1P = sphingosine-1-phosphate.
Women with MS may be able to breastfeed while on immunoglobulin G monoclonal antibody treatments, including ocrelizumab, natalizumab, and ofatumumab, with low risk to the infant (Figure 3). Monoclonal antibodies are detected at trace levels in milk and further are likely to be partially destroyed in the infant's gastrointestinal tract.e60 New data continue to support the use of anti-CD20 monoclonal antibodies during breastfeeding, with infants exposed to ocrelizumab and rituximab throughout breastfeeding showing normal growth and development with no unexpected severe or frequent infections.56 However, data on natalizumab are more limited. Further information on DMT safety during breastfeeding, including likely safety of first-line self-injectables such as IFNs and GA, can be found in eTable 1 (links.lww.com/CPJ/A500).
Minimal levels of IV methylprednisolone are transferred into milk, so this treatment is not contraindicated while breastfeeding. Although concentration levels do not pose any threat to the infant, mothers can choose to wait 2–4 hours to breastfeed to limit infant exposure.e61
Previously, women may have been advised to interrupt breastfeeding after clinical imaging with a gadolinium-based contrast media.e62 However, data on the safety of gadolinium-based contrast media do not support this recommendation. Less than 0.04% of the IV dose of gadolinium-based contrast media is excreted into breast milk in the first 24 hours.57 Only 0.0004% of this dose is absorbed by the infant from the breast milk, which is much less than the permissible dose for IV use in neonates. Because of this, the American College of Radiology guidelines recommend that it is safe for mothers to continue breastfeeding after receiving gadolinium-based contrast media.e63 The taste of the milk may be altered after administration of a contrast agent.e64
Best Practice Recommendations
Treatment should not be delayed post partum in women whose inflammatory disease activity before pregnancy puts them at higher risk of postpartum active disease.e4 All patients with MS who wish to breastfeed should be encouraged to do so, when possible, with referral to a lactation consultant for support if warranted.
IV methylprednisolone and IV gadolinium are safe during breastfeeding.e61,e63 Immunoglobulin G monoclonal antibodies also do not readily transfer into breast milk.e60
Vaccine recommendations for women with MS who are breastfeeding should follow standard MS guidelines.58 Patients should be informed that, with some vaccines, maternal immunity can be transferred to breastfed infants (e.g., COVID-19 vaccination).59
Conclusion
Given that rates of pregnancy in women with MS are increasing, clinicians need to have the most up-to-date information to appropriately manage family planning and pregnancy in patients with MS. We have provided recommendations and an overview of regulatory body recommendations across 3 countries/regions to facilitate clinicians in achieving this aim. Family planning discussions should occur early and frequently with patients, and partners where appropriate and practicable. Although knowledge about the safety of treatments continues to expand, there remains uncertainty, with a critical role for shared decision-making between clinicians and patients when navigating risks and unknowns.
There is a crucial need for more comprehensive pregnancy registries and sponsored studies evaluating risks of DMTs in pregnancy and lactation. Additional key gaps include the lack of guidance for specific and underrepresented populations with MS (e.g., patients from the LGBTQ+ community), patients receiving fertility treatments for a number of other reasons (e.g., single parents), and patients at risk of racial and socioeconomic disparities in care. Further research into the specific needs of these populations will improve care for all patients with MS.
Acknowledgment
Medical writing support was provided by Francesca Sorrell, PhD, of Envision Pharma Inc. and was funded by Novartis Pharmaceuticals Corporation. The authors thank Ali Chappell for her contribution to early discussions regarding the conception of this manuscript. This manuscript was developed in accordance with Good Publication Practice (GPP 2022) guidelines.
Appendix. Authors
| Name | Location | Contribution |
| Edith L. Graham, MD | Department of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Riley Bove, MD, MMSc | Department of Neurology, UCSF Weill Institute for Neurosciences, San Francisco, CA | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Kathleen Costello, MS, CRNP | Can Do Multiple Sclerosis, Avon, CO | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Heidi Crayton, MD | Multiple Sclerosis Center of Greater Washington, Vienna, VA | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Dina A. Jacobs, MD | Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Suma Shah, MD | Department of Neurology, Duke University School of Medicine, Durham, NC | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Francesca Sorrell, PhD | Envision Pharma Group, Glasgow, UK | Drafting/revision of the article for content, including medical writing for content |
| Sharon S. Stoll, DO, MS | Department of Neurology, Yale School of Medicine, New Haven, CT | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Maria K. Houtchens, MD | Brigham Multiple Sclerosis Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA | Drafting/revision of the article for content, including medical writing for content; study concept or design |
Study Funding
This study was funded by Novartis Pharmaceuticals Corporation. Novartis Pharmaceuticals Corporation supported the development of this manuscript. Authors had full control of the content and made the final decision on all aspects of this publication.
Disclosure
E.L. Graham has received consulting and advisory board fees from Atara Biotherapeutics, Horizon Therapeutics, Novartis, Roche/Genentech, and Tavistock Life Sciences; has received research support from F. Hoffmann-La Roche Ltd.; and has received compensation for question writing from ACP MKSAP.; R. Bove has received research support from and/or served on advisory boards and/or steering committees for Alexion, Biogen, EMD Serono, Novartis, Roche/Genentech, Sanofi Genzyme, and TG Therapeutics.; K. Costello has served on advisory boards for Genentech, Janssen, and Novartis.; H. Crayton has served as a consultant for Biogen, Bristol Myers Squibb, EMD Serono, and Sanofi Genzyme and has received research support from Biogen, EMD Serono, Novartis, Roche/Genentech, and Sanofi Genzyme.; D.A. Jacobs has received consulting honoraria from Banner Life Sciences, Biogen, Bristol Myers Squibb, Cycle Pharma, EMD Serono, Genentech, Novartis, and Sanofi Genzyme and has received research support from Biogen, Genentech, and University of California, Los Angeles; S. Shah has received research support from Biogen and has served on advisory boards for EMD Serono and Novartis.; F. Sorrell is an employee of Envision Pharma Group.; S.S. Stoll has served on scientific advisory boards for Bristol Myers Squibb, F. Hoffmann-La Roche Ltd., Forepont Capital Partners, Genentech, Horizon Therapeutics, and TG Therapeutics; has received research support from BeCare MS Link and MedDay; has received compensation for consulting services, served on scientific advisory boards, and received speaker honoraria for Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Horizon Therapeutics, Novartis, Roche/Genentech, and Sanofi Genzyme; is Chief Executive Officer of Global Consultant MD; and serves on the steering committees of Horizon Therapeutics and Roche/Genentech.; M.K. Houtchens has served as a consultant for Biogen, EMD Serono, Genzyme, Novartis, and Roche/Genentech and has received research support from Biogen, EMD Serono, Genzyme, Novartis, and Roche/Genentech. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Keyhanian K, Davoudi V, Etemadifar M, Amin M. Better prognosis of multiple sclerosis in patients who experienced a full-term pregnancy. Eur Neurol. 2012;68(3):150-155. doi: 10.1159/000338847 [DOI] [PubMed] [Google Scholar]
- 2.He A, Merkel B, Brown JWL, et al. ; MSBase Study Group. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307-316. doi: 10.1016/s1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 3.Buron MD, Chalmer TA, Sellebjerg F, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology. 2020;95(8):e1041-e1051. doi: 10.1212/wnl.0000000000010135 [DOI] [PubMed] [Google Scholar]
- 4.Merkel B, Butzkueven H, Traboulsee AL, Havrdova E, Kalincik T. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev. 2017;16(6):658-665. doi: 10.1016/j.autrev.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Iaffaldano P, Lucisano G, Caputo F, et al. ; Italian MS Register. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574. doi: 10.1177/17562864211019574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landfeldt E, Castelo-Branco A, Svedbom A, Löfroth E, Kavaliunas A, Hillert J. The long-term impact of early treatment of multiple sclerosis on the risk of disability pension. J Neurol. 2018;265(3):701-707. doi: 10.1007/s00415-018-8764-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen CS, Flemmen HØ, Broch L, et al. Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol. 2021;12:693017. doi: 10.3389/fneur.2021.693017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh WZ, Widyastuti PA, Van der Walt A, et al. Natalizumab, fingolimod and dimethyl fumarate use and pregnancy-related relapse and disability in women with multiple sclerosis. Neurology. 2021;96(24):e2989-e3002. doi: 10.1212/wnl.0000000000012084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valor L, Ovalles-Bonilla JG, Hernández-Flórez D, López-Longo FJ. Treatment with monoclonal antibodies and pregnancy in women with systemic inflammatory diseases: a special situation. Reumatol Clin. 2016;12(6):359-360. doi: 10.1016/j.reuma.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 10.Ciobanu AM, Dumitru AE, Gica N, Botezatu R, Peltecu G, Panaitescu AM. Benefits and risks of IgG transplacental transfer. Diagnostics (Basel). 2020;10(8):583. doi: 10.3390/diagnostics10080583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oreja-Guevara C, Vukusic S, Pietrasanta C, et al. Pregnancy and infant outcomes in women receiving ocrelizumab for the treatment of multiple sclerosis. Mult Scler. 2022;28(3 suppl l):31. [Google Scholar]
- 12.Evangelopoulos ME, Miclea A, Schrewe L, et al. Frequency and clinical characteristics of multiple sclerosis rebounds after withdrawal of fingolimod. CNS Neurosci Ther. 2018;24(10):984-986. doi: 10.1111/cns.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809. doi: 10.1177/1756286419837809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alroughani R, Akhtar S, Zeineddine M, et al. Risk of relapses during pregnancy among multiple sclerosis patients. Mult Scler Relat Disord. 2019;34:9-13. doi: 10.1016/j.msard.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 15.Thiel S, Litvin N, Haben S, Ciplea AI, Gold R, Hellwig K. Disease activity and pregnancy outcomes after long-term exposure to natalizumab during pregnancy. Mult Scler. 2022;28(3 suppl l):32. [Google Scholar]
- 16.Houtchens MK, Edwards NC, Hayward B, Mahony MC, Phillips AL. Live birth rates, infertility diagnosis, and infertility treatment in women with and without multiple sclerosis: data from an administrative claims database. Mult Scler Relat Disord. 2020;46:102541. doi: 10.1016/j.msard.2020.102541 [DOI] [PubMed] [Google Scholar]
- 17.Lu E, Zhu F, Zhao Y, et al. Birth outcomes of pregnancies fathered by men with multiple sclerosis. Mult Scler. 2014;20(9):1260-1264. doi: 10.1177/1352458514521308 [DOI] [PubMed] [Google Scholar]
- 18.Romero-Pinel L, Bau L, Munoz-Vendrell A, et al. Disease activity in multiple sclerosis patients after assisted reproductive technology. Mult Scler. 2022;28(3 suppl l):465-466. [Google Scholar]
- 19.Graham EL, Bakkensen JB, Anderson A, et al. Inflammatory activity after diverse fertility treatments: a multicenter analysis in the modern multiple sclerosis treatment era. Neurol Neuroimmunol Neuroinflamm. 2023;10(3):e200106. doi: 10.1212/NXI.0000000000200106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mainguy M, Tillaut H, Degremont A, et al. Assessing the risk of relapse requiring corticosteroids after in vitro fertilization in women with multiple sclerosis. Neurology. 2022;99(17):e1916-e1925. doi: 10.1212/wnl.0000000000201027 [DOI] [PubMed] [Google Scholar]
- 21.Brzosko B, Thiel S, Gold R, Hellwig K. Low relapse risk under disease modifying treatment during ART in women with relapsing remitting multiple sclerosis. Neurology. 2018;90(15 suppl l):P4.356. [Google Scholar]
- 22.Langer-Gould A, Smith JB, Albers KB, et al. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology. 2020;94(18):e1939-e1949. doi: 10.1212/wnl.0000000000009374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrem BL, Anderson A, Conway S, et al. Peripartum disease activity in moderately and severely disabled women with multiple sclerosis. Mult Scler J Exp Transl Clin. 2022;8(2):20552173221104918. doi: 10.1177/20552173221104918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes SE, Spelman T, Gray OM, et al. ; MSBase study group. Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler. 2014;20(6):739-746. doi: 10.1177/1352458513507816 [DOI] [PubMed] [Google Scholar]
- 25.Houtchens M, Bove R, Healy B, et al. MRI activity in MS and completed pregnancy: data from a tertiary academic center. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e890. doi: 10.1212/nxi.0000000000000890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson A, Krysko KM, Rutatangwa A, et al. Clinical and radiologic disease activity in pregnancy and postpartum in MS. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e959. doi: 10.1212/nxi.0000000000000959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassard SD, Fitzgerald KC, Qian P, et al. High dose vitamin D3 supplementation does not reduce disease activity in relapsing remitting multiple sclerosis in a large randomized controlled trial. Mult Scler. 2022;28(3 suppl l):337-338. [Google Scholar]
- 28.Munger KL, Åivo J, Hongell K, Soilu-Hänninen M, Surcel HM, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish maternity cohort. JAMA Neurol. 2016;73(5):515-519. doi: 10.1001/jamaneurol.2015.4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. doi: 10.1212/wnl.0000000000010380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak F, Nilsson AC, Nielsen C, et al. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103251. doi: 10.1016/j.msard.2021.103251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigaut K, Kremer L, Fleury M, Lanotte L, Collongues N, de Seze J. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev Neurol (Paris). 2021;177(10):1237-1240. doi: 10.1016/j.neurol.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastò L, Portaccio E, Ghezzi A, et al. ; MS Study Group of the Italian Neurological Society. Epidural analgesia and cesarean delivery in multiple sclerosis post-partum relapses: the Italian cohort study. BMC Neurol. 2012;12:165. doi: 10.1186/1471-2377-12-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harazim H, Štourač P, Janků P, et al. Obstetric anesthesia/analgesia does not affect disease course in multiple sclerosis: 10-year retrospective cohort study. Brain Behav. 2018;8(9):e01082. doi: 10.1002/brb3.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu E, Zhao Y, Dahlgren L, et al. Obstetrical epidural and spinal anesthesia in multiple sclerosis. J Neurol. 2013;260(10):2620-2628. doi: 10.1007/s00415-013-7035-7 [DOI] [PubMed] [Google Scholar]
- 35.van der Kop ML, Pearce MS, Dahlgren L, et al. Neonatal and delivery outcomes in women with multiple sclerosis. Ann Neurol. 2011;70(1):41-50. doi: 10.1002/ana.22483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferraro D, Simone AM, Adani G, et al. Definitive childlessness in women with multiple sclerosis: a multicenter study. Neurol Sci. 2017;38(8):1453-1459. doi: 10.1007/s10072-017-2999-1 [DOI] [PubMed] [Google Scholar]
- 37.Ahmed SF, Almuteri ML, Al-Hashel J, Alroughani R. Pregnancy outcome in multiple sclerosis patients exposed to disease modifying therapies. Neurology. 2019;92(15 suppl l):P4.2-100. [Google Scholar]
- 38.Hellwig K, Duarte Caron F, Wicklein EM, Bhatti A, Adamo A. Pregnancy outcomes from the global pharmacovigilance database on interferon beta-1b exposure. Ther Adv Neurol Disord. 2020;13:1756286420910310. doi: 10.1177/1756286420910310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Leon S, Geissbühler Y, Sabidó M, Turkson M, Wahlich C, Morris JK. A systematic review and meta-analyses of pregnancy and fetal outcomes in women with multiple sclerosis: a contribution from the IMI2 ConcePTION project. J Neurol. 2020;267(9):2721-2731. doi: 10.1007/s00415-020-09913-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friend S, Richman S, Bloomgren G, Cristiano LM, Wenten M. Evaluation of pregnancy outcomes from the Tysabri® (natalizumab) pregnancy exposure registry: a global, observational, follow-up study. BMC Neurol. 2016;16:150. doi: 10.1186/s12883-016-0674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh J, Achiron A, Chambers C, et al. Pregnancy outcomes in patients with RRMS who received alemtuzumab in the clinical development program. Neurology. 2016;86(16 suppl l):S24.008. [Google Scholar]
- 42.Gerhart C. Use of regional anesthesia in patients with multiple sclerosis. Glob J Anesthes Pain Med. 2020;3(2):251-255. doi: 10.32474/GJAPM.2020.03.000158 [DOI] [Google Scholar]
- 43.Landi D, Ragonese P, Prosperini L, et al. Abortion induces reactivation of inflammation in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(12):1272-1278. doi: 10.1136/jnnp-2018-318468 [DOI] [PubMed] [Google Scholar]
- 44.Tong Y, Liu J, Yang T, et al. Influences of pregnancy on neuromyelitis optica spectrum disorders and multiple sclerosis. Mult Scler Relat Disord. 2018;25:61-65. doi: 10.1016/j.msard.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 45.Hradilek P, Meluzinova E, Zapletalova O, et al. Is pregnancy in MS patients safe and what is its impact on MS course? Real world evidence of 1533 pregnancies in Czech Republic. Mult Scler Relat Disord. 2021;59:103391. doi: 10.1016/j.msard.2021.103391 [DOI] [PubMed] [Google Scholar]
- 46.Portaccio E, Ghezzi A, Hakiki B, et al. ; MS Study Group of the Italian Neurological Society. Postpartum relapses increase the risk of disability progression in multiple sclerosis: the role of disease modifying drugs. J Neurol Neurosurg Psychiatry. 2014;85(8):845-850. doi: 10.1136/jnnp-2013-306054 [DOI] [PubMed] [Google Scholar]
- 47.Lehmann H, Zveik O, Levin N, Brill L, Imbar T, Vaknin-Dembinsky A. Brain MRI activity during the year before pregnancy can predict post-partum clinical relapses. Mult Scler. 2021;27(14):2232-2239. doi: 10.1177/13524585211002719 [DOI] [PubMed] [Google Scholar]
- 48.Hellwig K, Verdun di Cantogno E, Sabidó M. A systematic review of relapse rates during pregnancy and postpartum in patients with relapsing multiple sclerosis. Ther Adv Neurol Disord. 2021;14:17562864211051012. doi: 10.1177/17562864211051012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horvat Ledinek A, Brecl Jakob G, Jerše J, et al. Intravenous immunoglobulins for the prevention of postpartum relapses in multiple sclerosis. Mult Scler Relat Disord. 2020;38:101519. doi: 10.1016/j.msard.2019.101519 [DOI] [PubMed] [Google Scholar]
- 50.Leguy S, Lefort M, Lescot L, et al. COPP-MS: COrticosteroids during the Post-Partum in relapsing Multiple Sclerosis patients. J Neurol. 2022;269(10):5571-5581. doi: 10.1007/s00415-022-11215-7 [DOI] [PubMed] [Google Scholar]
- 51.Block VJ, Mestas O, Anderson A, et al. Underutilization of physical therapy for symptomatic women with MS during and following pregnancy. Mult Scler Relat Disord. 2021;48:102703. doi: 10.1016/j.msard.2020.102703 [DOI] [PubMed] [Google Scholar]
- 52.Razaz N, Tremlett H, Marrie RA, Joseph KS. Peripartum depression in parents with multiple sclerosis and psychiatric disorders in children. Mult Scler. 2016;22(14):1830-1840. doi: 10.1177/1352458516631037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eid K, Torkildsen ØF, Aarseth J, et al. Perinatal depression and anxiety in women with multiple sclerosis: a population-based cohort study. Neurology. 2021;96(23):e2789-e2800. doi: 10.1212/wnl.0000000000012062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roos I, Leray E, Frascoli F, et al. Determinants of therapeutic lag in multiple sclerosis. Mult Scler. 2021;27(12):1838-1851. doi: 10.1177/1352458520981300 [DOI] [PubMed] [Google Scholar]
- 55.Krysko KM, Rutatangwa A, Graves J, Lazar A, Waubant E. Association between breastfeeding and postpartum multiple sclerosis relapses: a systematic review and meta-analysis. JAMA Neurol. 2020;77(3):327-338. doi: 10.1001/jamaneurol.2019.4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson A, Poole S, Rowles W, et al. Anti-CD20 monoclonal antibody therapy after 59 pregnancies in women with neurological conditions: low breastmilk transfer and normal infant development in a multicenter cohort. Mult Scler. 2022;28(3 suppl l):30. [Google Scholar]
- 57.Kubik-Huch RA, Gottstein-Aalame NM, Frenzel T, et al. Gadopentetate dimeglumine excretion into human breast milk during lactation. Radiology. 2000;216(2):555-558. doi: 10.1148/radiology.216.2.r00au09555 [DOI] [PubMed] [Google Scholar]
- 58.Galati A, McElrath T, Bove R. Use of B-cell-depleting therapy in women of childbearing potential with multiple sclerosis and neuromyelitis optica spectrum disorder. Neurol Clin Pract. 2022;12(2):154-163. doi: 10.1212/CPJ.0000000000001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023eReferences are listed at links.lww.com/CPJ/A503. [DOI] [PMC free article] [PubMed] [Google Scholar]