Introduction
Total mesorectal excision (TME) for rectal cancer can be performed open or with minimally invasive surgery (MIS) (laparoscopic TME (L-TME), robot-assisted TME (R-TME) and transanal TME (TaTME)). Initially, when improved short-term outcomes were reported for L-TME compared with open, MIS TME was widely adopted1–3. However, L-TME remained challenging due to anatomical restrictions of the bony pelvis and technical limitations of laparoscopy. R-TME was developed to overcome these ergonomic limitations including a stable platform with improved precision, 3D visualization and endo-wristed instrumentation.
Initial safety and feasibility of R-TME were reported to be non-inferior but not superior compared with open and L-TME in randomized controlled trials (RCTs)4–6. However, trial designs have been hindered by a number of factors, including variable operative experience within robotic surgery arms and primary outcomes that may not definitively demonstrate the technical and patient-centred benefits of R-TME for rectal cancer7.
Furthermore, as RCTs primarily focused on oncological outcomes, there has been less emphasis on other rectal cancer outcomes including patient-reported outcome measures (PROMs). For example, does increased technical precision offer significant benefit specifically in ‘high-risk’ cases (for example, in the setting of a threatened margin (circumferential resection margin (CRM))? Can robotic surgery optimize functional outcomes8,9? The aim of this international multicentre idea, development, exploration, assessment and long-term follow-up (IDEAL) stage 2b collaborative work is to evaluate robotic rectal cancer surgery in the context of the above questions. The IDEAL framework lays out a systematic pathway to evaluate the safety, efficacy, and effectiveness of new surgical procedures and complex interventions10,11.
Methods
Collaborative formation
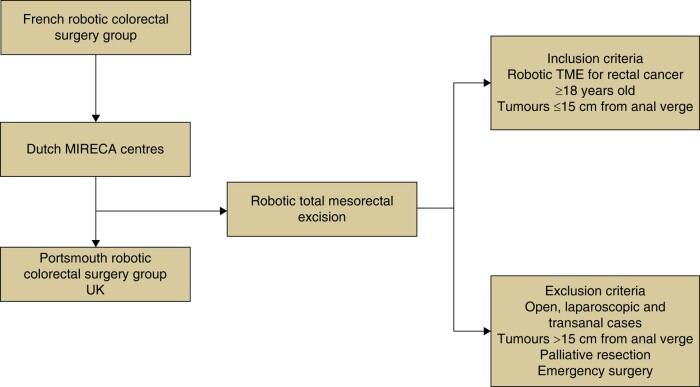
The EUREKA (Expert DUtch, FREnch and UK robotic rectal cAncer centres) collaborative was established to provide large volume ‘real-world’ data regarding robotic rectal cancer surgery. It was formed by colorectal surgeons working in high-volume robotic rectal cancer centres in France, The Netherlands and the UK. The data collection interval extends from 2013 to 2022. A full list of MIRECA and EUREKA collaborators is available in Supplementary materials, Data S1 & S2. The extended study protocol is available as Data S3.
Study design
The study design of the EUREKA collaborative studies will be in the format of IDEAL stage 2b as a bridge from single centre to large volume multicentre observational evaluation. The full recommended criteria for performing IDEAL stage 2b studies are summarized in Data S410,11. Studies will involve retrospective review of available data with prospective data analysis planned for future projects. It is expected that >2000 R-TME cases will be included.
Eligibility criteria
Overall, included patients in this work will have undergone surgery after the learning curve in included centres with the following inclusion criteria also applied:
Patients have undergone R-TME
Biopsy-confirmed rectal cancer
Aged 18 years or above
Rectal tumour located within 15 cm from the anal verge.
Extended exclusion criteria are available in Data S5.
Surgical interventions and perioperative oncological management
The standard oncological principles of TME were practised12. Choice of anastomosis and stoma use was decided based on individual patient and tumour characteristics. The following robotic surgery platforms were used in included cases: da Vinci Si (Intuitive Surgical, CA, USA) in earlier resections then subsequently da Vinci Xi (Intuitive Surgical, CA, USA). Depending on patient and tumour characteristics and institutional protocols an array of neoadjuvant and adjuvant therapies were utilized with further details in Data S313–15.
Definitions
A full list of study variables examined is included as Data S6. Table 1 summarizes a limited list of definitions and pertinent terms used within this collaborative work.
Table 1.
Definitions
| Term | Study definition |
|---|---|
| Oncological | |
| Tumour level | Mid rectum 6–10 cm from anal verge |
| Low rectum </=5 cm from anal verge | |
| MR resection margins (including circumferential resection margin, CRM) | Negative: tumour >2 mm from resection margin |
| Threatened: tumour 1–2 mm from resection margin | |
| Positive: tumour <1 mm from resection margin | |
| Pathology resection margins (including CRM) | R0 tumour present >1 mm from margin |
| R1 tumour present within 1 mm from margin | |
| R2 tumour present at resection margin | |
| Tumour response, tumour regression grade | TRG1: complete response, no residual cancer |
| TRG2: small volume residual cancer | |
| TRG3: fibrosis outgrowing residual cancer | |
| TRG4: residual cancer outgrowing fibrosis | |
| TRG5: absence of regression changes | |
| EMVI | EMVI+ presence of extramural venous invasion |
| EMVI– absence of extramural venous invasion | |
| Subclassification: small/medium/large vessel | |
| TME quality12 | Complete: smooth intact mesorectum, no defects >5 mm, regular CRM, no coning |
| Near complete: no visible muscularis propria, irregular CRM and moderate coning | |
| Incomplete: defect down to muscularis propria, irregular CRM and coning | |
| Classification of local recurrence | Anterior/central/posterior/lateral |
| Clinical | |
| Clavien–Dindo classification16 | I: any deviation from normal postoperative course |
| II: requiring pharmacological treatment (including blood transfusion and TPN) | |
| IIIa, requiring surgical endoscopic or radiological intervention; IIIb: under GA | |
| IV: life-threatening complication requiring ICU management | |
| V: death | |
| Surgical site infection | Clinical evidence or microbiologically confirmed infection at the site of surgery |
| Pelvic sepsis | An umbrella term to cover anastomotic leak, pelvic abscess and peritonitis |
| Anastomotic leak | Loss in gastrointestinal continuity at the site of anastomosis, detected clinically, biochemically or radiologically |
| Anastomotic leak grading (ISREC classification)17 | A: subclinical (managed through observation or medication) |
| B: clinical (requiring radiological or transanal drainage) C: clinical (requiring re-laparotomy) |
|
| Timing of anastomotic leak | Early: < 30 days |
| Late: > 30 days | |
| Preoperative morbidity | Graded according to the ASA classification of physical health18 |
| Overall survival | Defined as being alive on follow-up |
| Disease-free survival | Defined as being alive without recurrent disease at follow-up |
| Local recurrence | Defined as tumour deposit located in the pelvic cavity, with pathologically proven adenocarcinoma, or growth on consecutive imaging if histopathological confirmation was absent |
| Systemic recurrence | Defined as any distant metastasis, either pathologically proven or as a lesion suspect for metastasis on imaging that showed growth on consecutive imaging |
MR, mesorectal; EMVI, extramural vascular invasion; TME, total mesorectal excision; TPN, total parenteral nutrition; GA, general anaesthesia; ISREC, international study group of rectal cancer.
Patient-reported outcome measures
In this study, quality of life will be reported using both the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CR29 and QLQ-C30 scores. Bowel function will be reported using the Low Anterior Resection Score (LARS). Urinary function will be reported using the International Prostate Specific Score (IPSS). Sexual function will be reported using the International Index of Erectile Function (IIEF-5) for men and Female Sexual Function Index (FSFI) for women.
Outcomes
The following broad project themes, in three main domains, will be investigated with a focus on complex high-risk cases and centrally placing patient-reported outcomes:
Cancer outcomes (for example quality of resection (CRM, R0), local recurrence, metastasis rate, disease-free survival (DFS), overall survival (OS))
Clinical outcomes (for example risk factors for anastomotic leak and pelvic sepsis following, definition of ‘high-risk’ patient (for example, male, high BMI, preoperative neoadjuvant therapy))
PROMs
Data sharing
A principal investigator from each participating centre is responsible for quality assurance of institutional level data. A Data Sharing Agreement (DSA) was generated. In order to protect patient privacy and adhere to general data protection regulation (GDPR) (2016) guidelines, all centres pseudonymize their data and retain the pseudonymization key at their own centre, effectively making any data transfer between sites completely anonymous.
Data management
To track all data entries the Research Data Management System (RDMS) of the University Medical Centre Groningen (UMCG) will be used to create a smaller database that will be used within this study and will fully comply with the GDPR Act, 2016. A secure digital link will be generated between the centres for data transfer. After the host (sponsoring) centre has congregated all the datasets and completed validation, verification and cleaning, the final dataset will be locked and password protected before being transferred back to the participating centres. All data queries will be submitted to and processed by the host (sponsoring) centre, and subsequently distributed to the correct participating centres. All data and documents will be archived on password-protected servers for at least 15 years by the creating party.
Statistical analysis
At a granular level, statistical analysis will be designed based on the research question of each individualized study. For each individual study, power calculations will be performed considering difference in independent means, an s.d. of 15, a power of 0.90 and a two-sided interval. Propensity score matching may be required to overcome institutional and geographical variation. Statistical significance will be defined as a P value <0.05.
Ethics and regulatory considerations
The EUREKA collaborative has received institutional review board (IRB) ethical approval from each of the participating centres for the individualized studies that have been defined and designed as part of this IDEAL stage 2b evaluation. Formal Clinical Transfer Agreements (CTA) and DSAs were completed prospectively for international data sharing.
Role of sponsor
The sponsor (University Medical Centre Groningen) will be responsible for monitoring that the data management Standard Operating Procedure (SOP) is followed as described and will have overall responsibility for implementing systems to ensure data quality and security.
Dissemination
The results of all studies performed by the EUREKA collaborative will be presented at relevant local, national and international scientific meetings, and will be submitted for publication in peer-reviewed journals.
Discussion
The EUREKA collaborative aims to deliver international multicentre outcome data following robotic rectal cancer surgery, from expert centres. An IDEAL 2b study exploring outcome data from high-volume specialized centres with experienced robotic surgeons can provide valuable data to both inform practice and future research and aid in the decision-making process with patients.
Fig 1.
Study flow chart
TME, total mesorectal excision.
Collaborators
G.J.D. van Acker (Haaglanden Medical Center, The Hague, The Netherlands); T.S. Aukema (Hagaziekenhuis, The Hague, The Netherlands); H.J. Belgers (Zuyderland Medical Center, Heerlen, The Netherlands); F.H. Beverdam (Fransiscus Gasthuis, Rotterdam, The Netherlands); J.G. Bloemen (Maastricht University Medical Center, Maastricht, The Netherlands); K. Bosscha (Catharina Hospital, Eindhoven, The Netherlands); S.O. Breukink (Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands); T.A. Burghgraef (University Medical Center Utrecht, Utrecht, The Netherlands); P.P.L.O. Coene (Maasstad Hospital Rotterdam, Rotterdam, The Netherlands); R.M.P.H. Crolla (Amphia Hospital, Breda, The Netherlands); P. van Duijvendijk (Gelre Hospital, Apeldoorn, The Netherlands); E.B. van Duyn (Medisch Spectrum Twente, Enschede, The Netherlands); I.F. Faneyte (ZGT, Almelo, The Netherlands); S.A.F. Fransen (Maastricht University Medical Center, Maastricht, The Netherlands); A.A.W. van Geloven (Tergooi Medical Center, Hilversum, The Netherlands); M.F. Gerhards (Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands); W.M.U. van Grevenstein (University Medical Center Utrecht, Utrecht, The Netherlands); K. Havenga (University Medical Center Groningen, Groningen, The Netherlands); I.H.J.T. de Hingh (Catharina Hospital, Eindhoven, The Netherlands); C. Hoff (Medical Center Leeuwarden, Leeuwarden, The Netherlands); R. Hompes (Amsterdam University Medical Center, Amsterdam, The Netherlands); G. Kats (University Medical Center Groningen, Groningen, The Netherlands); J.W.A. Leijtens (Laurentius Hospital Roermond, Roermond, The Netherlands); M.F. Lutke Holzik (ZGT, Almelo, The Netherlands); J. Melenhorst (Maastricht University Medical Center, Maastricht, The Netherlands); M.M. Poelman (Fransiscus Gasthuis, Rotterdam, The Netherlands); A. Pronk (Diakonessenhuis, Utrecht, The Netherlands); A.H.W. Schiphorst (Diakonessenhuis, Utrecht, The Netherlands); J.M.J. Schreinemakers (Amphia Hospital, Breda, The Netherlands); C. Sietses (Hospital Gelderse Vallei, Ede, The Netherlands); A.B. Smits (St. Antonius Hospital, Nieuwegein, The Netherlands); I. Somers (Meander Medical Center, Amersfoort, The Netherlands); E.J. Spillenaar Bilgen (Rijnstate Hosptital, Arnhem, The Netherlands); H.B.A.C. Stockmann (Spaarne Gasthuis, Hoofddorp, The Netherlands); A.K. Talsma (Deventer Hospital, Deventer, The Netherlands); P.J. Tanis (Amsterdam University Medical Center, Amsterdam, The Netherlands); J. Tuynman (Amsterdam University Medical Center, Amsterdam, The Netherlands); G. Verdaasdonk (Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands); P. Verheijen (Meander Medical Center, Amersfoort, The Netherlands); F.A.R.M. Warmerdam (Zuyderland Medical Center, Heerlen, The Netherlands); H.L. van Westreenen (Isala, Zwolle, The Netherlands); D.D.E. Zimmerman (Elisabeth TweeSteden Hospital, Tilburg, The Netherlands).
Supplementary Material
Acknowledgements
C.A.F., R.D. and R.T.J.G. contributed equally to writing this protocol and share first authorship.
Contributor Information
Christina A Fleming, Department of Colorectal Surgery, Bordeaux Colorectal Institute, Clinique Tivoli, Bordeaux, France.
Rauand Duhoky, Department of Colorectal Surgery, Portsmouth Hospitals University NHS Trust and the University of Portsmouth, Portsmouth, UK.
Ritchie T J Geitenbeek, Department of Surgery, University Medical Centre Groningen, Groningen, The Netherlands.
Aurore Moussion, Clinical Research Department, Montpellier Cancer Institute (ICM), University of Montpellier, Montpellier, France.
Nabila Bouazza, Clinical Research Department, Montpellier Cancer Institute (ICM), University of Montpellier, Montpellier, France.
Jim Khan, Department of Colorectal Surgery, Portsmouth Hospitals University NHS Trust and the University of Portsmouth, Portsmouth, UK.
Eddy Cotte, Department of Digestive and Oncological Surgery, Lyon University Hospital, Lyon-Sud Hospital, Pierre-Bénite, France.
Anne Dubois, Department of Colorectal Surgery, Chu Estaing, Clermont-Ferrand, France.
Eric Rullier, Department of Digestive Surgery, Colorectal Unit, Haut-Lévêque Hospital, Bordeaux University Hospital, Pessac, France.
Roel Hompes, Department of Surgery, Academic Medical Centre, Amsterdam, The Netherlands.
Quentin Denost, Department of Colorectal Surgery, Bordeaux Colorectal Institute, Clinique Tivoli, Bordeaux, France.
Philippe Rouanet, Surgery Department, Montpellier Cancer Institute (ICM), University of Montpellier, Montpellier, France.
Esther C J Consten, Department of Surgery, University Medical Centre Groningen, Groningen, The Netherlands.
EUREKA collaborative:
G J D van Acker, T S Aukema, H J Belgers, F H Beverdam, J G Bloemen, K Bosscha, S O Breukink, T A Burghgraef, P P L O Coene, R M P H Crolla, P van Duijvendijk, E B van Duyn, I F Faneyte, S A F Fransen, A A W van Geloven, M F Gerhards, W M U van Grevenstein, K Havenga, I H J T de Hingh, C Hoff, R Hompes, G Kats, J W A Leijtens, M F Lutke Holzik, J Melenhorst, M M Poelman, A Pronk, A H W Schiphorst, J M J Schreinemakers, C Sietses, A B Smits, I Somers, E J Spillenaar Bilgen, H B A C Stockmann, A K Talsma, P J Tanis, J Tuynman, G Verdaasdonk, P Verheijen, F A R M Warmerdam, H L van Westreenen, and D D E Zimmerman
Funding
The authors have no funding to declare.
Disclosure statement
E.C.J.C. and P.R. are proctors for Intuitive Surgical. J.S.K. is a proctor and trainer with Intuitive Surgical, and a trainer with Johnson & Johnson. All other co-authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Anonymized data can be made available from the corresponding authors following reasonable request. In compliance with what has been agreed to in the consortium agreement and informed consent, pseudonymized data will be made accessible to other researchers through dataverse.nl (with restricted access) if they comply with Dutch legislation and comply with any restrictions that the ethics committee might impose on the reuse. To do so, researchers will have to contact the EUREKA Steering Committee.
Author contributions
Christina Fleming (Conceptualization, Data curation, Methodology, Project administration, Resources, Visualization, Writing—original draft, Writing—review & editing), Rauand Duhoky (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing—original draft, Writing—review & editing), Ritchie Geitenbeek (Conceptualization, Data curation, Resources, Validation, Visualization, Writing—original draft, Writing—review & editing), Aurore Moussion (Conceptualization, Methodology, Project administration, Writing—original draft, Writing—review & editing), Nabila Bouazza (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Jim Khan (Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing), Eddy Cotte (Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing), Anne Dubois (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Writing—review & editing), Eric Rullier (Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing—review & editing), Roel Hompes (Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing—original draft, Writing—review & editing), Quentin Denost (Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing), Philippe Rouanet (Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing), and Esther Consten (Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing)
References
- 1. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC et al. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–218 [DOI] [PubMed] [Google Scholar]
- 2. Park JW, Kang S-B, Hao J, Lim S-B, Choi HS, Kim D-W et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:569–577 [DOI] [PubMed] [Google Scholar]
- 3. Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 2015;314:1356–1363 [DOI] [PubMed] [Google Scholar]
- 4. Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 2017;318:1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam B-H et al. Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg 2018;267:243–251 [DOI] [PubMed] [Google Scholar]
- 6. Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 2022;7:991–1004 [DOI] [PubMed] [Google Scholar]
- 7. Corrigan N, Marshall H, Croft J, Copeland J, Jayne D, Brown J et al. Exploring and adjusting for potential learning effects in ROLARR: a randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials 2018;19:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleming CA, Cullinane C, Lynch N, Killeen S, Coffey JC, Peirce CB et al. Urogenital function following robotic and laparoscopic rectal cancer surgery: meta-analysis. Br J Surg 2021;108:128–137 [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Choi G-S, Park JS, Park SY, Yang CS, Lee HJ et al. The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Color Dis 2018;20:O103–O113 [DOI] [PubMed] [Google Scholar]
- 10. Agha RA, Hirst A, Khachane A, McCulloch P. A protocol for the development of reporting guidelines for IDEAL stage studies. Int J Surg Protoc 2018;9:11–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg 2019;269:211–220 [DOI] [PubMed] [Google Scholar]
- 12. Nagtegaal I, van de Velde C, van der Worp E, Kapiteijn E. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 2002;20:1729–1734 [DOI] [PubMed] [Google Scholar]
- 13. Conroy T, Bosset J-F, Etienne P-L, Rio E, François É, Mesgouez-Nebout N et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702–715 [DOI] [PubMed] [Google Scholar]
- 14. Denost Q, Moreau J-B, Vendrely V, Celerier B, Rullier A, Assenat V et al. Intersphincteric resection for low rectal cancer: the risk is functional rather than oncological. A 25-year experience from Bordeaux. Color Dis 2020;22:1603–1613 [DOI] [PubMed] [Google Scholar]
- 15. Denost Q, Fleming CA, Burghgraef T, Celerier B, Geitenbeek R, Rullier E et al. An international multicenter prospective study evaluating the long-term oncological impact of adjuvant chemotherapy in ypN+ rectal cancer. Ann Surg 2022;277:299–304 [DOI] [PubMed] [Google Scholar]
- 16. Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, Rahbari NN et al. International Study Group of Rectal Cancer. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 2013;153:753–761 [DOI] [PubMed] [Google Scholar]
- 18. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth 2011;55:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data can be made available from the corresponding authors following reasonable request. In compliance with what has been agreed to in the consortium agreement and informed consent, pseudonymized data will be made accessible to other researchers through dataverse.nl (with restricted access) if they comply with Dutch legislation and comply with any restrictions that the ethics committee might impose on the reuse. To do so, researchers will have to contact the EUREKA Steering Committee.