Abstract
Objective:
To localize the distribution of regional nodes in recurrent/advanced breast cancer patients based on 18-fludeoxyglucose (FDG) positron emission tomography/CT (PET/CT) images and validate the coverage of clinical target volumes (CTVs) for regional nodes with current contouring guidelines.
Methods:
We enrolled 154 recurrent/advanced breast cancer patients with FDG-avid regional nodes who underwent PET/CT between January 2018 and June 2020. Involvement of lymph node regions including axillary lymph node level I-III (ALN-I, ALN-II, ALN-III), Rotter’s nodes (RN), medial supraclavicular (SC-M), lateral supraclavicular (SC-L) and internal mammary nodes (IMN) was recorded respectively. Coverage of the CTVs in different atlases and the locations of out-of-field were evaluated.
Results:
A total of 348 lymph node regions containing disease were identified, including ALN-I 109, ALN-II 46, ALN-III 36, RN 17, SC-M 68, SC-L 36 and IMN 36. Recurrent ALNs mainly located cranially and ventrally to the axillary vein (AV). Ipsilateral cervical nodes were simultaneously affected in 33/76 SC positive patients. RADCOMP (306/348) and RUIJIN (291/348) guidelines had higher coverage compared with RTOG (205/348) and ESTRO (202/348) guidelines (p < 0.001, respectively). In primary non-metastastic and recurrent patients, major missings located in SC-L (7/7, 17/17) and IMN (7/10, 15/19) for RTOG guideline while SC-L (7/7, 17/17) for ESTRO guideline (p < 0.001, respectively). Among recurrent patients, SC-M (22/31) was another major missing area for ESTRO guideline (p < 0.001).
Conclusion:
The current guidelines effectively cover most regional nodes in postoperative breast cancer patients. SC-L and IMN were the major missing regions. Recurrent ALNs were most often seen in cranial and ventral to the AV. The CTV of patients with clinically positive SC was recommended to extend up to the hyoid level. The CTVs should be adjusted based on risks of recurrence individually.
Advances in knowledge:
The difference of regional nodes delineation between current guidelines mainly located in SC and IMN regions. High axilla including subclavicular nodes and the RN above AV for recurrent patients and the region between cricoid and hyoid for positive SC patients should be meticulously contoured.
Introduction
Regional nodal irradiation (RNI) including internal mammary and medial supraclavicular region in addition to breast/chest wall irradiation has been shown to improve disease-free survival, even in moderate risk patients. 1–7 Data from the Z0011 trial indicates whole breast irradiation with high tangents did not compromise locoregional control or survival compared to axillary lymph node dissection (ALND) in early breast cancer patients with one or two positive sentinel nodes receiving breast conservative surgery. 8 AMAROS trial also suggests an equally excellent axillary control between ALND and axillary irradiation in positive sentinal lymph node (SLN) patients with a significant less lymphedema. 9
To standardize the clinical target volume (CTV) for breast cancer, the first contouring atlas was published in 2009 by the Radiation Therapy Oncology Group (RTOG). 10 Later in 2015, the European Society for Radiation Therapy and Oncology (ESTRO) released another guideline, mainly for early-stage patients. 11 In 2016, the Radiotherapy Comparative Effectiveness (RADCOMP) atlas was released as a reference for proton therapy. 12 Althogh these guidelines were published according to expert consensus and frequently used in daily practice, previous research showed inadequate coverage of CTVs exceeding the recommended boundaries. 13 Coverage comparison of all these guidelines in a single report was also seldomly seen.
Most of the previous studies identified the positive lymph nodes based on computed tomography (CT). 18-fludeoxyglucose (FDG) positron emission tomography/CT (PET/CT) is able to provide functional and anatomical information simultaneously compared with traditional imaging modalities such as CT, therefore can detect extra lymph node involvement when it is in normal morphological shape. 14–16
The aim of our study is to create a three-dimensional mapping of the distribution of regional nodes in patients with recurrent/advanced breast cancer based on FDG PET/CT images, so as to validate and compare the coverage by the CTVs definitions in different contouring guidelines.
Methods and materials
Patient
The medical history and image of consecutive 492 breast cancer patients including primary diagnosed before any treatment, reevaluation after neoadjuvant treatment, routine follow-up examinations after surgery, first time recurrent before any treatment and evaluation after recurrent treatment, who underwent FDG PET/CT between January 2018 and June 2020 in single center were reviewed. A total of 154 patients primary diagnosed or recurrent before any treatment identified of having FDG-avid regional nodes were enrolled. This study was approved by the Ethics Committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, and written informed consent was waived.
Mapping of regional nodes
The regional nodes were categorized into seven lymph node regions anatomically: axillary lymph node level I (ALN-I), level II (ALN-II), level III (ALN-III), Rotter’s nodes (RN), medial supraclavicular (SC-M), lateral supraclavicular (SC-L) and internal mammary nodes (IMN). We defined SC-M as the region posterior to the sternocleidomastoid, which correlated to the CTV of SC in RTOG guideline. SC-L was described as the region lateral to the sternocleidomastoid and posterior to the transverse process, with a similar definition of the CTV of Posterior Neck volume in RADCOMP atlas, which was also named as posterolateral SC and lateral SC in other studies. 17,18 All the FDG-avid regional nodes were mapped manually by one senior radiation oncologist and one nuclear medicine physician on a simulation CT scan of a reference female patient in a treatment position with both arms abducted overhead on a breast board and uploaded to the planning software (Eclipse 15.6, Varian Medical Systems, Palo Alto, CA). Considering the deformation of enlarged lymph nodes, we plotted the epicenter of each positive regional node as a circle with a diameter of 5 mm in order to minimize the distortion effect. 17–23 The CTVs for RNI were delineated by two senior radiation oncologists according to RTOG, ESTRO, RADCOMP and RUIJIN (which was developed by our hospital for patients requiring RNI in clincal trials and also used in daily practice, on the basis of RTOG, ESTRO guidelines and relevant literatures about breast cancer contouring) contouring guidelines blinded to the locations of FDG-avid regional nodes, whose delineation are detailed in Appendix (Table A1)and Figure 1.
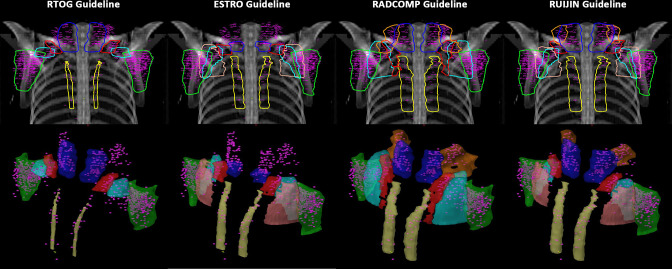
Figure 1.
Mappings of the FDG-avid regional nodes on the CT slices based on RTOG, ESTRO, RADCOMP and RUIJIN guidelines. 18F-FDG, 18-fludeoxyglucose; ESTRO, European Society for Radiation Therapy and Oncology; RADCOMP, Radiotherapy Comparative Effectiveness; RTOG, Radiation Therapy Oncology Group.
Analysis of coverage
The location of each involved node was defined as “inside” if completely or most (more than 50% volume of the 5 mm epicenter) within the CTVs and “outside” if entirely or most outside the CTVs. The coverage of each lymph node region was determined by lymph nodes within the same region. For example, if a patient had five FDG-avid regional nodes in the area of ALN-I, we defined the region as “inside” only if all these five regional nodes were within the boundary of CTV, otherwise defined as “outside”. All the coverage analyses in our study were based on lymph node regions rather than individual lymph nodes.
Statistical analysis
The variables were described by frequency and percentage, analyzed using χ2 test. Statistical significance was considered if p-value was <0.05. A Bonferroni-adjusted significance threshold of p = 0.008 (0.05/6) was used to compare the coverage of the four guidelines with each other. Statistical analyses were performed using SPSS 21.0 (IBM Corporation, Armonk, NY).
Results
Distribution of regional nodes
In 154 patients, 1184 FDG-avid regional nodes in 348 lymph node regions were identified, whose clinical characteristics are summarized in Table 1. Among these positive regional nodes, six enlarged nodes in six patients were more than 3 cm in diameter. Five of them were totally (the whole lymph node) within the CTVs in all four guidelines. The other one was completely beyond the caudal side of ALN-II CTV in RTOG guideline, while totally within the CTVs in the other three guidelines. Therefore, these six enlarged lymph nodes were also contained in our analysis. Pathological biopsy was confirmed in 140 regions (85 in ALN, 48 in SC and 7 in IMN). Figure 1 shows the locations of the FDG-avid nodes on the CT slices of the standard patients. Figure 2 illustrated a two-dimensional and three-dimensional overview of the regional nodes and CTVs. As shown in Table 2, the percentage of involvement in total population were: ALN-I 70.8%, SC-M 44.2%, ALN-II 29.9%, ALN-III, SC-L, IMN 23.4% each and RN 11.0% respectively.
Table 1.
Clinical characteristics of the 154 enrolled patients
| Characteristic | Total (n = 154) n (%) |
Primary
non-metastatic (n = 55) n (%) |
De-novo metastatic (n = 49) n (%) |
Recurrent
without RNI history (n = 35) n (%) |
Recurrent
with RNI history (n = 15) n (%) |
|---|---|---|---|---|---|
| Age | |||||
| ≤40 | 26 (16.9%) | 9 (16.4%) | 11 (22.4%) | 3 (8.6%) | 3 (20.0%) |
| >40 | 128 (83.1%) | 46 (83.6%) | 38 (77.6%) | 32 (91.4%) | 12 (80.0%) |
| Tumor laterality | |||||
| Left | 78 (50.7%) | 32 (58.2%) | 23 (46.9%) | 18 (51.4%) | 5 (33.3%) |
| Right | 76 (49.4%) | 23 (41.8%) | 26 (53.1%) | 17 (48.6%) | 10 (66.7%) |
| Histopathological type | |||||
| IDC | 138 (89.6%) | 51 (92.7%) | 47 (95.9%) | 27 (77.1%) | 13 (86.7%) |
| DCIS | 3 (2.0%) | 2 (3.6%) | 1 (2.0%) | 0 (0%) | 0 (0%) |
| ILC | 3 (2.0%) | 1 (1.8%) | 1 (2.0%) | 1 (2.9%) | 0 (0%) |
| Others | 10 (6.5%) | 1 (1.8%) | 0 (0%) | 7 (20.0%) | 2 (13.3%) |
| Breast surgery | |||||
| Breast-conserving surgery | 6 (3.9%) | 1 (1.8%) | 4 (8.2%) | 1 (2.9%) | 0 (0%) |
| Mastectomy | 46 (29.9%) | 2 (3.6%) | 0 (0%) | 30 (85.7%) | 14 (93.3%) |
| Reconstruction | 6 (3.9%) | 1 (1.8%) | 0 (0%) | 4 (11.4%) | 1 (6.7%) |
| No surgery | 96 (62.3%) | 51 (92.7%) | 45 (91.8%) | 0 (0%) | 0 (0%) |
| Axillary surgery | |||||
| ALND | 50 (32.5%) | 0 (0%) | 3 (6.1%) | 35 (100%) | 15 (100%) |
| SLN biopsy only | 7 (4.6%) | 4 (7.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| No surgery | 97 (63.0%) | 51 (92.7%) | 46 (93.9%) | 0 (0%) | 0 (0%) |
| Molecular type | |||||
| Luminal A | 25 (16.2%) | 10 (18.2%) | 7 (14.3%) | 4 (11.4%) | 4 (26.7%) |
| Luminal B | 56 (36.4%) | 21 (38.2%) | 18 (36.7%) | 11 (31.4%) | 6 (40.0%) |
| Her2-enriched | 26 (16.9%) | 10 (18.2%) | 8 (16.3%) | 6 (17.1%) | 2 (13.3%) |
| Triple-negative | 38 (24.7%) | 13 (23.6%) | 15 (30.6%) | 9 (25.7%) | 1 (6.7%) |
| Unknown | 9 (5.8%) | 1 (1.8%) | 1 (2.0%) | 5 (14.3%) | 2 (13.3%) |
| T stage | |||||
| T0 | 1 (0.7%) | 1 (1.8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| T1 | 18 (11.7%) | 8 (14.5%) | 3 (6.1%) | 6 (17.1%) | 1 (6.7%) |
| T2 | 85 (55.2%) | 31 (56.4%) | 27 (55.1%) | 18 (51.4%) | 9 (60.0%) |
| T3 | 11 (7.1%) | 4 (7.3%) | 7 (14.3%) | 0 (0%) | 0 (0%) |
| T4 | 24 (15.6%) | 10 (18.2%) | 11 (22.4%) | 2 (5.7%) | 1 (6.7%) |
| Tx | 15 (9.7%) | 1 (1.8%) | 1 (2.0%) | 9 (25.7%) | 4 (26.7%) |
| N stage | |||||
| N1 | 25 (16.2%) | 12 (21.8%) | 8 (16.3%) | 4 (11.4%) | 1 (6.7%) |
| N2 | 43 (27.9%) | 18 (32.7%) | 14 (28.6%) | 8 (22.9%) | 3 (20.0%) |
| N3 | 86 (55.8%) | 25 (45.5%) | 27 (55.1%) | 23 (65.7%) | 11 (73.3%) |
| M stage | |||||
| M0 | 69 (44.8%) | 55 (100%) | 0 (0%) | 12 (34.3%) | 2 (13.3%) |
| M1 | 85 (55.2%) | 0 (0%) | 49 (100%) | 23 (65.7%) | 13 (86.7%) |
ALND, axillary lymph node dissection; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; RNI, regional nodal irradiation; SLN, sentinal lymph node.
Figure 2.
A two-dimensional and three-dimensional overview of the FDG-avid regional nodes based on RTOG, ESTRO, RADCOMP and RUIJIN guidelines. 18F-FDG, 18-fludeoxyglucose; ESTRO, European Society for Radiation Therapy and Oncology; RADCOMP, Radiotherapy Comparative Effectiveness; RTOG, Radiation Therapy Oncology Group.
Table 2.
Distribution of lymph node regions containing cancer in the 154 breast cancer patients
| Total (n = 154) n (%) |
Primary
non-metastatic (n = 55) n (%) |
De-novo metastatic (n = 49) n (%) |
Recurrent
without RNI history (n = 35) n (%) |
Recurrent
with RNI history (n = 15) n (%) |
|
|---|---|---|---|---|---|
| ALN-I | 109 (70.8%) | 54 (98.2%) | 47 (95.9%) | 4 (11.4%) | 4 (26.7%) |
| ALN-II | 46 (29.9%) | 15 (27.3%) | 23 (46.9%) | 8 (22.9%) | 0 (0%) |
| ALN-III | 36 (23.4%) | 10 (18.2%) | 19 (38.8%) | 6 (17.1%) | 1 (6.7%) |
| RN | 17 (11.0%) | 7 (12.7%) | 6 (12.2%) | 3 (8.6%) | 1 (6.7%) |
| SC-M | 68 (44.2%) | 12 (21.8%) | 25 (51.0%) | 21 (60.0%) | 10 (66.7%) |
| SC-L | 36 (23.4%) | 7 (12.7%) | 12 (24.5%) | 15 (42.9%) | 2 (13.3%) |
| IMN | 36 (23.4%) | 10 (18.2%) | 7 (14.3%) | 14 (40.0%) | 5 (33.3%) |
ALN-I, axillary lymph node level I; ALN-II, axillary lymph node level II; ALN-III, axillary lymph node level III; FDG, 18-fludeoxyglucose; IMN, internal mammary nodes; RN, Rotter’s nodes; RNI, regional node irradiation; SC-L, lateral supraclavicular; SC-M, medial supraclavicular.
The relationship between ALN distribution and axillary vein (AV) in primary and recurrent patients was shown in Figure 3. The ratio of patients with ALN involvement located cranially and caudally to the AV were 60 vs 101 in primary diagnosed patients and 16 vs 8 in patients with prior ALND (p = 0.006). Above the level of AV, ALNs were more commonly detected ventrally to AV rather than dorsally in both primary diagnosed and recurrent patients, with a ratio of 58 vs 11 and 14 vs 0, respectively. Among the 76 patients with SC involvement, 43.4% of them had their ipsilateral cervical nodes involved, typically, within the level between hyoid and cricoid. 54 positive IMNs were found in 36 patients, 35.2%, 42.6%, 20.4% and 1.9% of these involved nodes located in the first, second, third and fourth intercostal spaces respectively.
Figure 3.
Relationship between ALN distribution and AV in primary and recurrent patients. ALN, axillary lymph node; AV, axillary vein.
We divided the 154 patients into four groups based on surgery and radiation therapy (RT) history: primary non-metastatic/de-novo metastatic, recurrent with/without RNI history, characteristics shown in Table 1. Eight recurrent patients who received breast surgery without ALND and RNI were classified as primary non-metastatic/de-novo metastatic group considering their similar natural history. The different distribution of regional nodes in these four groups are listed in Table 2. Primary diagnosed patients had a higher involvement of ALNs with 98.1% vs 42.0% while SC and IMN were more frequently found in recurrent patients with 68.0% vs 40.4% and 38.0% vs 16.3% (p < 0.01, respectively).
Coverage of regional nodes in different guidelines
Table 3 listed the coverage of the seven lymph node regions based on RTOG, ESTRO, RADCOMP and RUIJIN atlases, respectively. The total coverage of the four contouring guidelines were 58.9% (205/348), 58.0% (202/348), 87.9% (306/348) and 83.6% (291/348) respectively. RADCOMP and RUIJIN guidelines had a significantly higher coverage compared with RTOG and ESTRO guidelines (p < 0.001, respectively). CTVs for SC-L were not contoured either in RTOG or ESTRO guidelines, the coverage of this region was 0% (0/36) in both atlases, significantly poorer than RADCOMP and RUIJIN guidelines (p < 0.001, respectively). For RTOG atlas, ALN-II and IMN were two major missing regions in comparison with the other three guidelines (p < 0.001, respectively), with a coverage of 30.4% (14/46) and 22.2% (8/36). SC-M was poorly covered in ESTRO atlas with a coverage of 33.8% (23/68), significantly lower than the other three atlases (p < 0.001, respectively). The majority of the regions were successfully covered by the RADCOMP and RUIJIN atlases, with no statistical significance between them.
Table 3.
Coverage of regional nodes based on RTOG, ESTRO, RADCOMP and RUIJIN guidelines
|
Total population
( n = 154) |
RTOG guideline
n (%) |
ESTRO guideline
n (%) |
RADCOMP guideline
n (%) |
RUIJIN guideline
n (%) |
| Total | 205/348 (58.9%) a1 | 202/348 (58.0%) a1 | 306/348 (87.9%) b1 | 291/348 (83.6%) b1 |
| ALN-I | 75/109 (68.8%) a2 | 65/109 (59.6%) a2 | 75/109 (68.8%) a2 | 75/109 (68.8%) a2 |
| ALN-II | 14/46 (30.4%) a3 | 42/46 (91.30%) b3 | 46/46 (100.0%) b3 | 42/46 (91.3%) b3 |
| ALN-III | 32/36 (88.9%) a4,b4 | 27/36 (75.0%) b4 | 35/36 (97.2%) a4 | 32/36 (88.9%) a4,b4 |
| RN | 17/17 (100.0%) a5 | 17/17 (100.0%) a5 | 17/17 (100.0%) a5 | 17/17 (100.0%) a5 |
| SC-M | 59/68 (86.8%) a6 | 23/68 (33.8%) b6 | 66/68 (97.1%) a6 | 64/68 (94.1%) a6 |
| SC-L | 0/36 (0.0%) a7 | 0/36 (0.0%) a7 | 34/36 (94.4%) b7 | 30/36 (83.3%) b7 |
| IMN | 8/36 (22.2%) a8 | 28/36 (77.8%) b8 | 33/36 (91.7%) b8 | 31/36 (86.1%) b8 |
|
Primary non-metastatic
( n = 55) |
RTOG guideline
n (%) |
ESTRO guideline
n (%) |
RADCOMP guideline
n (%) |
RUIJIN guideline
n (%) |
| Total | 31/61 (50.8%) a9 | 45/61 (73.8%) a9,b9 | 60/61 (98.4%) c9 | 55/61 (90.2%) b9,c9 |
| ALN-I | Not evaluated | |||
| ALN-II | 1/15 (6.7%) a10 | 14/15 (93.3%) b10 | 15/15 (100%) b10 | 14/15 (93.3%) b10 |
| ALN-III | 9/10 (90.0%) a11 | 8/10 (80.0%) a11 | 10/10 (100%) a11 | 9/10 (90.0%) a11 |
| RN | 7/7 (100%) a12 | 7/7 (100%) a12 | 7/7 (100%) a12 | 7/7 (100.0%) a12 |
| SC-M | 11/12 (91.7%) a13 | 8/12 (66.7%) a13 | 11/12 (91.7%) a13 | 11/12 (91.7%) a13 |
| SC-L | 0/7 (0%) a14 | 0/7 (0%) a14 | 7/7 (100%) b14 | 5/7 (71.4%) b14 |
| IMN | 3/10 (30.0%) a15 | 8/10 (80.0%) a15,b15 | 10/10 (100%) b15 | 9/10 (90.0%) b15 |
|
Recurrent (with/without RNI)
( n = 50) |
RTOG guideline
n (%) |
ESTRO guideline
n (%) |
RADCOMP guideline
n (%) |
RUIJIN guideline
n (%) |
| Total | 51/94 (54.3%) a16 | 44/94 (46.8%) a16 | 86/94 (91.5%) b16 | 83/94 (88.3%) b16 |
| ALN-I | 6/8 (75.0%) a17 | 5/8 (62.5%) a17 | 6/8 (75.0%) a17 | 6/8 (75.0%) a17 |
| ALN-II | 6/8 (75.0%) a18 | 7/8 (87.5%) a18 | 8/8 (100%) a18 | 7/8 (87.5%) a18 |
| ALN-III | 5/6 (83.3%) a19 | 4/6 (66.7%) a19 | 5/6 (83.3%) a19 | 5/6 (83.3%) a19 |
| RN | 4/4 (100%) a20 | 4/4 (100%) a20 | 4/4 (100%) a20 | 4/4 (100%) a20 |
| SC-M | 25/31 (80.6%) a21 | 9/31 (29.0%) b21 | 30/31 (96.8%) a21 | 29/31 (93.5%) a21 |
| SC-L | 0/17 (0%) a22 | 0/17 (0%) a22 | 15/17 (88.2%) b22 | 15/17 (88.2%) b22 |
| IMN | 4/19 (21.1%) a23 | 14/19 (73.7%) b23 | 17/19 (89.5%) b23 | 16/19 (84.2%) b23 |
ALN-I, axillary lymph node level I; ALN-II, axillary lymph node level II; ALN-III, axillary lymph node level III; ESTRO, European Society for Radiation Therapy and Oncology; IMN, internal mammary nodes; RADCOMP, Radiotherapy Comparative Effectiveness; RN, Rotter’s nodes; RTOG, Radiation Therapy Oncology Group; SC-L, lateral supraclavicular; SC-M, medial supraclavicular.
Individual analysis for primary/recurrent patients are also shown in Table 3. As ALN-I will be operated in primary non-metastatic patients, its coverage was not evaluated. Upfront treatment will be systemic therapy rather than RT in de-novo metastatic patients, coverage analysis of this group was not conducted either. In the primary diagnosed group, the major missing regions were ALN-II (14/15), SC-L (7/7) and IMN (7/10) for RTOG guideline, SC-L (7/7) for ESTRO guideline (p < 0.001, respectively). In the recurrent group, SC-L (17/17) and IMN (15/19) were frequently missed for RTOG atlas while SC-M (22/31) and SC-L (17/17) for ESTRO atlas (p < 0.001, respectively). As for the 23 positive regions in the 15 recurrent patients with RNI history, 15/23 (1 in ALN-III, 1 in RN, 10 in SC-M, 2 in SC-L and 1 in IMN) were located within the RT fields, 8/23 (4 in ALN-I and 4 in IMN) were outside the prior RT fields.
Location of the missing regional nodes in different guidelines
Directions and the trends of regional nodes located out-of-field by different CTV definitions are detailed in Table 4. Almost all missing ALN-I occurred laterally to the CTVs boundaries, with a rate of 31.2% (34/109) in all four guidelines. With 1 cm lateral expansion of the CTVs, the lateral missing rate would drop to 1.8% (2/109). Also for ALN-I, a posterior missing rate of 14.7% (16/109) was found with ESTRO guideline. The caudal border of the ALN-II CTV in RTOG guideline was axillary vessels, much limited than the other three guidelines, which was related to a caudal missing rate of 67.4% (31/46). The cranial border of the ALN-III CTV in ESTRO atlas was lower than the other guidelines, leading to a cranial missing of 19.4% (7/36). RN CTV were included as part of ALN-II/chest-wall CTVs in RTOG and RADCOMP guidelines, while defined as a separate CTV in ESTRO and RUIJIN atlas, their boundaries were almost identical and no missing lesions was found. The upper border of SC-M CTV in ESTRO guideline was the lowest among all the atlases, which was related to a cranial missing rate of 64.7% (44/68). The lesions in SC-L were completely missing (36/36) in both RTOG and ESTRO guidelines. All the missing part of IMNs per RTOG atlas occurred outside the boundaries of internal mammary vessel.
Table 4.
Locations of regional nodes outside RTOG, ESTRO, RADCOMP and RUIJIN guidelines
| ALN-I | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 75/109 (68.8%) | 34/109 (31.2%) | 1/109 (0.9%) a1 | 0/109 (0%) a2 | 34/109 (31.2%) a3 | ||||
| ESTRO guideline | 65/109 (59.6%) | 44/109 (40.4%) | 1/109 (0.9%) a1 | 16/109 (14.7%) b2 | 34/109 (31.2%) a3 | ||||
| RADCOMP guideline | 75/109 (68.8%) | 34/109 (31.2%) | 1/109 (0.9%) a1 | 0/109 (0%) a2 | 34/109 (31.2%) a3 | ||||
| RUIJIN guideline | 75/109 (68.8%) | 34/109 (31.2%) | 1/109 (0.9%) a1 | 0/109 (0%) a2 | 34/109 (31.2%) a3 | ||||
| ALN-II | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 14/46 (30.4%) | 32/46 (70.0%) | 4/46 (8.7%) a4 | 31/46 (67.4%) a5 | |||||
| ESTRO guideline | 42/46 (91.3%) | 4/46 (8.7%) | 4/46 (8.7%) a4 | 0/46 (0%) b5 | |||||
| RADCOMP guideline | 46/46 (100%) | 0/46 (0%) | 0/46 (0%) a4 | 0/46 (0%) b5 | |||||
| RUIJIN guideline | 42/46 (91.3%) | 4/46 (8.7%) | 4/46 (8.7%) a4 | 0/46 (0%) b5 | |||||
| ALN-III | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 32/36 (88.9%) | 4/36 (11.1%) | 1/36 (2.8%) a6 | 3/36 (8.3%) a7 | |||||
| ESTRO guideline | 27/36 (75.0%) | 9/46 (25.0%) | 7/36 (19.4%) a6 | 2/36 (5.6%) a7 | |||||
| RADCOMP guideline | 35/36 (97.2%) | 1/36 (2.8%) | 1/36 (2.8%) a6 | 0/36 (0%) a7 | |||||
| RUIJIN guideline | 32/36 (88.9%) | 4/36 (11.1%) | 1/36 (2.8%) a6 | 3/36 (8.3%) a7 | |||||
| RN | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 17/17 (100%) | 0/17 (0%) | |||||||
| ESTRO guideline | 17/17 (100%) | 0/17 (0%) | |||||||
| RADCOMP guideline | 17/17 (100%) | 0/17 (0%) | |||||||
| RUIJIN guideline | 17/17 (100%) | 0/17 (0%) | |||||||
| SC-M | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 59/68 (88.7%) | 9/68 (13.3%) | 1/68 (1.5%) a8 | 2/68 (2.9%) a9 | 6/68 (8.8%) a10 | 0/68 (0%) a11 | |||
| ESTRO guideline | 23/68 (33.8%) | 45/68 (66.2%) | 44/68 (64.7%) b8 | 2/68 (2.9%) a9 | 1/68 (1.5%) a10 | 3/68 (4.4%) a11 | |||
| RADCOMP guideline | 66/68 (97.1%) | 2/68 (2.9%) | 1/68 (1.5%) a8 | 0/68 (0%) a9 | 1/68 (1.5%) a10 | 0/68 (0%) a11 | |||
| RUIJIN guideline | 64/68 (94.1%) | 4/68 (5.9%) | 1/68 (1.5%) a8 | 2/68 (2.9%) a9 | 1/68 (1.5%) a10 | 0/68 (0%) a11 | |||
| SC-L | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 0/36 (0%) | 36/36 (100%) | not contoured in RTOG guideline | ||||||
| ESTRO guideline | 0/36 (0%) | 36/36 (100%) | not contoured in ESTRO guideline | ||||||
| RADCOMP guideline | 34/36 (94.4%) | 2/36 (5.6%) | 2/36 (5.6%) a12 | 0/36 (0%) a13 | 0/36 (0%) a14 | ||||
| RUIJIN guideline | 30/36 (83.3%) | 6/36 (16.7%) | 2/36 (5.6%) a12 | 2/36 (5.6%) a13 | 2/36 (5.6%) a14 | ||||
| IMN | Coverage of substructures | Directions of substructures outside the CTVs | |||||||
|
Inside
n (%) |
Outside
n (%) |
Cranial
n (%) |
Caudal
n (%) |
Anterior
n (%) |
Posterior
n (%) |
Lateral
n (%) |
Meidal
n (%) |
||
| RTOG guideline | 8/36 (22.2%) | 28/36 (77.8%) | 1/36 (2.8%) a15 | 5/36 (13.9%) a16 | 11/36 (30.6%) a17 | 13/36 (36.1%) a18 | |||
| ESTRO guideline | 28/36 (77.8%) | 8/36 (22.2%) | 1/36 (2.8%) a15 | 0/36 (0%) a16 | 4/36 (11.1%)a17,b17 | 4/36 (11.1%)a18,b18 | |||
| RADCOMP guideline | 33/36 (91.7%) | 3/36 (8.3%) | 1/36 (2.8%) a15 | 0/36 (0%) a16 | 2/36 (5.6%) b17 | 0/36 (0%) b18 | |||
| RUIJIN guideline | 31/36 (86.1%) | 5/36 (13.9%) | 1/36 (2.8%) a15 | 0/36 (0%) a16 | 2/36 (5.6%) b17 | 2/36 (5.6%) b18 | |||
ALN-I, axillary lymph node level I; ALN-II, axillary lymph node level II; ALN-III, axillary lymph node level III; ESTRO, European Society for Radiation Therapy and Oncology; IMN, internal mammary nodes; RADCOMP, Radiotherapy Comparative Effectiveness; RN, Rotter’s nodes; RTOG, Radiation Therapy Oncology Group; SC-L, lateral supraclavicular; SC-M, medial supraclavicular.
Indications: A Bonferroni-adjusted significance threshold of p=0.008 (0.05/6) was used to compare the coverage between different guidelines. There was a statistically significant difference in proportions between groups with different marks (“a” vs “b”), while no significant difference was found between groups with the same mark (“a” vs “a”).
Discussion
In this retrospective study, we map the distribution of involved regional nodes in breast cancer patients based on PET-CT and classified them by lymph node regions rather than lymph nodes. It is also our first attempt to compare the compatibility of regional nodes natural history to CTV atlas in different guidelines. A detailed distribution of regional nodes in 154 patients with recurrent/advanced breast cancer was generated in our study, their trends of involvement was analyzed. A trend of more frequent axillary nodes involvement in primary diagnosed patients, more frequent supraclavicular and internal mammary nodes involvement in recurrent patients was found, which is in accordance with previous research. 13,19 In general, better coverage by RADCOMP and RUIJIN atlases than RTOG and ESTRO guidelines was confirmed in different lymph node regions. The coverage of CTVs in our study were in general lower than other studies, possible explanation is the different method of calculating regional nodes as “inside” the CTVs. 24 Regions as a unit other than individual lymph nodes can more reasonably reflect the coverage of target volumes in clinical practice.
ARMOS trial has shown axillary RT as effective as ALND with a lower tendency of lymphedema, 9 recommendation of ALND omission was therefore proposed by different guidelines and an increase rate of ALND omission was observed in clinical practice as well. 8 Thus, it is necessary to include low axilla as part of comprehensive nodal RT in case of high-risk patients without ALND. In all of the four atlases, inadequate coverage of ALN-I, mainly lateral border missing was found, which calls caution in today’s practice. Novikov et al demonstrated that only 82–85% SLNs in Level I was included in the CTVs of RTOG and ESTRO atlases, almost all missing happened in the lateral part. 25 In our study, the coverage of ALN-I could reach 98.2% with a 1 cm lateral expansion. Position of the arms might influence the coverage of lateral ALN-Is as well. 25 The different arm abduction for FDG PET/CT and CT simulation partly influenced the lateral missing rate of ALN-Is in our study. In IMRT treatment, an incidental irradiation is often associated with a therapeutic dose in this area, which will be substantial decreased with proton therapy. 26,27 This explains the importance of modifying CTV atlas for proton therapy in breast cancer RT, especially RNI.
In RTOG atlas, CTV of ALN-II was associated with a missing rate of 67.4% in our cohort. Beaton et al also found the coverage of ALN-II in RTOG atlas was only 68%. 20 In Gentile’s study, 80% of the lymph nodes in ALN-II exceeded the caudal border. 24 Borm et al reported many of the overlooked lesions by RTOG guideline located just below the caudal border. 13 However, the region caudal to the CTV of ALN-II in RTOG guideline, which are contoured in other three atlases, had an overlap with the CTV of chest wall in RTOG atlas. 10 Considering almost all patients indicated for RNI are also indicated for breast/chest wall irradiation, the missing part can therefore be covered in an integrated planning.
Contouring atlas in general, provides a standard reference in clinical practice. To better understand the natural history of regional nodes distribution in patients with different risk and prior treatment will be helpful in optimizing the CTV atlas with regard to individual clinical scenario. In our study, patients with ALN involvement at the caudal side of AV are much more common in primary diagnosed patients, while more involved nodes are found in the cranial side of AV in recurrent patients. We also found that almost all the involved ALNs superior to the level of AV located ventrally to the vessel, in both primary and recurrent patients. A three-dimensional mapping data of regional nodes metastases revealed hot spot distribution in similar trend. 13 Therefore, the high axilla including subclavicular nodes and the RN above that level should be the region that needs meticulous delineation and high-level of dosimetric coverage in patients after ALND, while the low axilla must also be well covered in patients with no surgical history. When contouring CTVs in high axilla, the area dorsal to the AV could probably be spared, which will be associated with reduced volume of normal tissue irradiation, markedly. Precise delineation of the axilla based on risks of recurrence individually is critical and whether the CTV volumes could be modified still needs further research.
As an essential part of RNI, the border of SC CTVs remains controversial. RTOG and ESTRO guidelines contour only the medial part of this region (SC-M), whereas RADCOMP and RUIJIN atlases also include the lateral and posterior area (SC-L). In our study, positive SC nodes located outside SC-M while within SC-L occurred in 23.4% patients. Besides, the cranial border of SC-M CTV in ESTRO atlas was subclavian artery, much lower than cricoid in the other three atlases, leading to a low coverage of only 33.8%. Borm et al also found a significant part of SC nodes located cranial to the SC CTV of ESTRO atlas but within RTOG atlas, 13 which was also reported by Beaton et al. 20 In DeSelm’s study, nearly half of the recurrences in SC were observed outside the CTVs of RTOG and ESTRO guidelines, 40% laterally and 14% posteriorly. 19 They also recommended to include clavicle inside the CTV of SC, as certain part of SC recurrences situated very close to the clavicle. In the studies of Chang et al, SC was the most poorly encompassed region compared with ALNs and IMN, 20% of the missing nodes located posterolateral to the anterior scalene muscle. 21,22 Jing et al reported RTOG guideline covered only 62.6% SC lesions, missing part occurred most beyond the lateral and posterior borders. 17 By extending the borders to natural anatomic barriers including trapezius muscle, clavicle bone and subclavicular muscles, which would be similar to the CTVs in RADCOMP and RUIJIN atlas, the coverage of SC nodes in their data would reach 96.1%. 17 Brown et al demonstrated the coverage of the posterior triangle and lateral low SC region should be considered in all patients with indications for RNI. 18 Beaton et al reported addition of the RADCOMP Posterior part to the CTVs of SC in RTOG and ESTRO guideline greatly improved the coverage of SC nodes. 20
Our cohort also showed 43.4% of simultaneous ipsilateral cervical node involvement in SC positive patients, two-thirds of these nodes located below the level of hyoid, similar finding was reported. 18,19 For patients with known SC lesions, extension of the CTV of SC to the level of hyoid should be recommended.
Consistent with previous studies, most of the IMN lesions located in the first three intercostal spaces. 19 In our study, the IMN CTV in RUIJIN atlas expands 7 mm to the RTOG guideline, which is further expanded by RADCOMP atlas, and the corresponding coverage was 86.1% and 91.7% respectively. Wang et al also reported that only 18.4% of IM-SLN central points were covered by the CTV of RTOG. 23 Jethwa et al reported an expansion of 4 mm based on the RTOG CTV could cover 90% of the lesions. 28 Davison et al demonstrated 93% of the metastatic IMNs would be encompassed with 5 mm expansion from the IM vessels. 29
With intensity-modulated radiation therapy (IMRT) and proton therapy in, dose conformity significantly improves in comparison with traditional three-dimensional conformal therapy. Therefore, the importance of accurate regional nodes delineation becomes increasingly important as it is associated with both high-risk area dose coverage and normal tissue sparing. Some of the missing part such as SC-L and IMN, could probably be covered by incidental irradiation in photon IMRT, nevertheless, with proton therapy, any anatomical part behind the Bragg peak will be well spared. This is the major reason explaining the larger definition of CTVs in the RADCOMP guidelines compared to RTOG and ESTRO atlases. 2,19,30
There exists inevitably selection bias in this retrospective study. Our cohort includes primary and recurrent patients, whose natural history varies. Validation of the results in our research is still needed in large-scale data. It should be noted that delineation guidelines are most applicable for adjuvant radiotherapy in operable patients. Recurrent and primary diagnosed advanced stage patients do provide a reference for natural history of regional nodes involvement, but certainly in different therapeutic background. For example, recurrence in the SC-L region was not commonly seen in N0-1 patients. 21 A previous IMN study turns out to be a practical way to optimize the CTV definition by analyzing its infiltration tendency. 31 The results of current study could also help to modify the existed international contouring guidelines based on different pre-treatment background and individual risk by mapping the comprehensive nodal involvement in recurrent and advanced patients. Besides, all the regional nodes were mapped manually in our study, deformable registration could be used in our future research to improve the accuray of anatomical and positional deviations.
In conclusion, the current guidelines effectively cover the majorities of regional nodes in post-operative breast cancer patients. SC-L and IMN were the major missing regions. Recurrent ALNs were most often seen in cranial and ventral to the AV. The CTV of patients with clinically positive SC was recommended to extend up to the hyoid level. The CTVs should be adjusted based on individual risks in clinical practice. CTV definition be different in proton therapy is reasonable subsequent to the disappearance of incidental irradiation with photon.
Supplementary Material
Contributor Information
Maochen Zhang, Email: zmc12182@rjh.com.cn, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
Lu Cao, Email: caolu_163@ymail.com, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
Jiayi Chen, Email: chenjiayi0188@aliyun.com, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
Biao Li, Email: lb10363@rjh.com.cn, Department of Nuclear Medicine, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
Xinyun Huang, Email: hxy12184@rjh.com.cn, Department of Nuclear Medicine, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
Gang Cai, Email: caigangcg@163.com, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
Cheng Xu, Email: xucheng60@126.com, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China .
REFERENCES
- 1. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials . Lancet 2005. ; 366: 2087 – 2106 . doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 2. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. . Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the british columbia randomized trial . J Natl Cancer Inst 2005. ; 97: 116 – 26 . doi: 10.1093/jnci/djh297 [DOI] [PubMed] [Google Scholar]
- 3. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. . Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. danish breast cancer cooperative group 82b trial . N Engl J Med 1997. ; 337: 949 – 55 . doi: 10.1056/NEJM199710023371401 [DOI] [PubMed] [Google Scholar]
- 4. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. . Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: danish breast cancer cooperative group DBCG 82c randomised trial . Lancet 1999. ; 353: 1641 – 48 . doi: 10.1016/S0140-6736(98)09201-0 [DOI] [PubMed] [Google Scholar]
- 5. Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. . Regional nodal irradiation in early-stage breast cancer . N Engl J Med 2015. ; 373: 307 – 16 . doi: 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poortmans PM, Collette S, Kirkove C, et al. . Internal mammary and medial supraclavicular irradiation in breast cancer . N Engl J Med 2015. ; 373: 317 – 27 . doi: 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 7. Thorsen LBJ, Offersen BV, Danø H, Berg M, Jensen I, Pedersen AN, et al. . DBCG-IMN: A population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer . JCO 2016. ; 34: 314 – 20 . doi: 10.1200/JCO.2015.63.6456 [DOI] [PubMed] [Google Scholar]
- 8. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. . Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis . JAMA 2017. ; 318: 918 . doi: 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donker M, van Tienhoven G, Straver ME, et al. . Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial . Lancet Oncol 2014. ; 15: 1303 – 10 . doi: 10.1016/S1470-2045(14)70460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White J, Tai A, Arthur D, et al. . RTOG breast cancer atlas . 2009. . Available from : https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE%3d&tabid=236
- 11. Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. . ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer . Radiother Oncol 2015. ; 114: 3 – 10 . doi: 10.1016/j.radonc.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 12. MacDonald SaC O . Breast contouring radcomp consortium I . 2016. . Available from : https://www.rtog.org/LinkClick.aspx?fileticket=eVB451KQ83M%3d&tabid=429
- 13. Borm KJ, Voppichler J, Düsberg M, Oechsner M, Vag T, Weber W, et al. . FDG/PET-CT-based lymph node atlas in breast cancer patients . Int J Radiat Oncol Biol Phys 2019. ; 103: 574 – 82 . doi: 10.1016/j.ijrobp.2018.07.2025 [DOI] [PubMed] [Google Scholar]
- 14. Groheux D, Giacchetti S, Espié M, Vercellino L, Hamy A-S, Delord M, et al. . The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study . J Nucl Med 2011. ; 52: 1526 – 34 . doi: 10.2967/jnumed.111.093864 [DOI] [PubMed] [Google Scholar]
- 15. Aukema TS, Straver ME, Peeters M-JTFDV, Russell NS, Gilhuijs KGA, Vogel WV, et al. . Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II-III breast cancer . Eur J Cancer 2010. ; 46: 3205 – 10 . doi: 10.1016/j.ejca.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 16. Chung HL, Shin K, Sun J, Leung JWT . Extra-axillary nodal metastases in breast cancer: comparison of ultrasound, MRI, PET/CT, and CT . Clin Imaging 2021. ; 79: 113 – 18 . doi: 10.1016/j.clinimag.2021.03.028 [DOI] [PubMed] [Google Scholar]
- 17. Jing H, Wang S-L, Li J, Xue M, Xiong Z-K, Jin J, et al. . Mapping patterns of ipsilateral supraclavicular nodal metastases in breast cancer: rethinking the clinical target volume for high-risk patients . Int J Radiat Oncol Biol Phys 2015. ; 93: 268 – 76 . doi: 10.1016/j.ijrobp.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 18. Brown LC, Diehn FE, Boughey JC, Childs SK, Park SS, Yan ES, et al. . Delineation of supraclavicular target volumes in breast cancer radiation therapy . Int J Radiat Oncol Biol Phys 2015. ; 92: 642 – 49 . doi: 10.1016/j.ijrobp.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 19. DeSelm C, Yang TJ, Cahlon O, Tisnado J, Khan A, Gillespie E, et al. . A 3-dimensional mapping analysis of regional nodal recurrences in breast cancer . Int J Radiat Oncol Biol Phys 2019. ; 103: 583 – 91 . doi: 10.1016/j.ijrobp.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaton L, Nica L, Tyldesley S, Sek K, Ayre G, Aparicio M, et al. . PET/CT of breast cancer regional nodal recurrences: an evaluation of contouring atlases . Radiat Oncol 2020. ; 15: 136 . doi: 10.1186/s13014-020-01576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang JS, Lee J, Chun M, Shin KH, Park W, Lee JH, et al. . Mapping patterns of locoregional recurrence following contemporary treatment with radiation therapy for breast cancer: A multi-institutional validation study of the ESTRO consensus guideline on clinical target volume . Radiother Oncol 2018. ; 126: 139 – 47 . doi: 10.1016/j.radonc.2017.09.031 [DOI] [PubMed] [Google Scholar]
- 22. Chang JS, Byun HK, Kim JW, Kim KH, Lee J, Cho Y, et al. . Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: validation of the ESTRO consensus guideline on target volume . Radiother Oncol 2017. ; 122: 24 – 29 . doi: 10.1016/j.radonc.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Wang W, Li J-B, Huo Z-W, Xu M, Qiu P-F, et al. . Definition of internal mammary node target volume based on the position of the internal mammary sentinel lymph nodes presented on SPECT/CT fusion images . Front Oncol 2019. ; 9: 1553 . doi: 10.3389/fonc.2019.01553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gentile MS, Usman AA, Neuschler EI, Sathiaseelan V, Hayes JP, Small W . Contouring guidelines for the axillary lymph nodes for the delivery of radiation therapy in breast cancer: evaluation of the RTOG breast cancer atlas . Int J Radiat Oncol Biol Phys 2015. ; 93: 257 – 65 . doi: 10.1016/j.ijrobp.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Novikov SN, Krzhivitskii PI, Melnik YS, Valitova AA, Bryantseva ZV, Akulova IA, et al. . Atlas of sentinel lymph nodes in early breast cancer using single-photon emission computed tomography: implication for lymphatic contouring . Radiat Oncol J 2021. ; 39: 8 – 14 . doi: 10.3857/roj.2020.00871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novikov S, Krzhivitskii P, Kanaev S, Krivorotko P, Ilin N, Melnik J, et al. . SPECT-CT localization of axillary sentinel lymph nodes for radiotherapy of early breast cancer . Rep Pract Oncol Radiother 2019. ; 24: 688 – 94 . doi: 10.1016/j.rpor.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gee HE, Moses L, Stuart K, Nahar N, Tiver K, Wang T, et al. . Contouring consensus guidelines in breast cancer radiotherapy: comparison and systematic review of patterns of failure . J Med Imaging Radiat Oncol 2019. ; 63: 102 – 15 . doi: 10.1111/1754-9485.12804 [DOI] [PubMed] [Google Scholar]
- 28. Jethwa KR, Kahila MM, Hunt KN, Brown LC, Corbin KS, Park SS, et al. . Delineation of internal mammary nodal target volumes in breast cancer radiation therapy . Int J Radiat Oncol Biol Phys 2017. ; 97: 762 – 69 . doi: 10.1016/j.ijrobp.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 29. Davidson T, Ben-David M, Galper S, Haskin T, Howes M, Scaife R, et al. . Use of 18f-FDG PET-CT imaging to determine internal mammary lymph node location for radiation therapy treatment planning in breast cancer patients . Pract Radiat Oncol 2017. ; 7: 373 – 81 . doi: 10.1016/j.prro.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 30. Chandra RA, Miller CL, Skolny MN, Warren LEG, Horick N, Jammallo LS, et al. . Radiation therapy risk factors for development of lymphedema in patients treated with regional lymph node irradiation for breast cancer . Int J Radiat Oncol Biol Phys 2015. ; 91: 760 – 64 . doi: 10.1016/j.ijrobp.2014.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, Qi W, Xu H, Zhang M, Han Y, Chen J, et al. . Infiltration tendency of internal mammary lymph nodes involvement in patients with breast cancer: anatomical characteristics and implications for target delineation . Radiat Oncol 2019. ; 14: 208 . doi: 10.1186/s13014-019-1412-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.